Abstract
The brain responds differently to environmental and internal signals that relates to the stage of development of neural systems. While genetic and epigenetic factors contribute to a premorbid state, hormonal fluctuations in women may alter the set point of migraine. The cyclic surges of gonadal hormones may directly alter neuronal, glial and astrocyte function throughout the brain. Estrogen is mainly excitatory and progesterone inhibitory on brain neuronal systems. These changes contribute to the allostatic load of the migraine condition that most notably starts at puberty in girls.
Introduction
Brain plasticity, influenced by genetic, epigenetic and environmental factors, refers to the ability of the brain to adapt to altering levels of neural signals, inflammatory molecules, drugs and hormones. Hypothalamic hormones, affecting neural network functioning and ‘stability’, have significant effects on migraine. We attempt to integrate brain systems neuroscience with endocrine regulation through the hypothalamus that drives hormonal, sex and gender differentiation of migraine by focusing on the following topics: (1) Phenotypic Expression by Physiological Modulators in the Developing Migraine Brain where we summarize the evolution of migraine from children to adults, with an emphasis on puberty in girls; (2) Sex Hormones and Brain Function where we review the widespread expression of estrogen and estrogen receptors across the brain providing a target for estrogen mediated changes on brain function and behavior; (3) Sex and Brain-Related Changes in Migraine where we summarize morphometric and functional changes in women vs. men; (4) Hypothalamic role in hormonal regulation of Brain Dysmetria in Migraine where we highlight the role of the hypothalamus as a center for the control of gonadotropin release and autonomic function that are critical in migraine related changes in patients; (5) Hormonal Systems Modulate the “Set Point” for Migraine Attacks where we cover the multiple processes (e.g., cortical spreading depression, sleep, etc.) that are affected by hormones that may alter the threshold for migraine attacks; (6) Hormonal Allostatic Load in Migraine where we discuss the idea that repeated migraines contribute to a feed-forward maladaptive allostatic cascade on brain function; and (7) Future Directions where we provide suggestions for future research studies needed to investigate hormonal effects on migraine.
Phenotypic Expression by Physiological Modulators in the Developing Migraine Brain
With age, brain networks extend the scope of their anatomical interactions and functional integration (1, 2). This developmental change in functional connectivity, reflected by underlying structural gray and white matter changes (2, 3), are thought to involve segregation of local regions and integration of distant regions into disparate sub-networks (4). These changes are functionally important as the nervous system may respond differently to external stimuli and/or disease (e.g., migraine), depending on brain maturation. The phenotypical expression of migraine in young children is different from pre- and post-pubertal children and adults. The prevalence of migraine changes with age (5, 6), with significant increases at puberty in girls and decreases in post-menopausal women (Figure 1).
Figure 1. Sex and Age in Migraine.
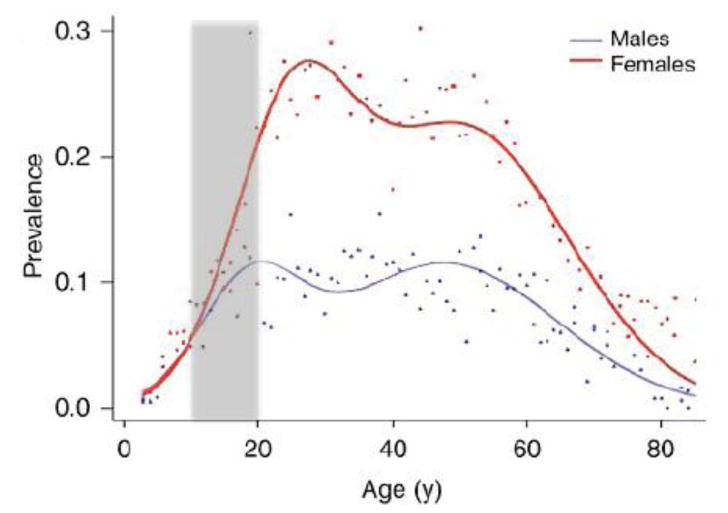
Migraine Prevalence (Adapted from (176) with permission): Prevalence measured over a 1-year period of self-reported and physician-diagnosed migraines. The prevalence in boys and girls is similar until puberty (approx. 10-11 yrs. of age) after which it diverges between the sexes with age. The prevalence is ∼6% in men and 15-17% in women (5). Note that the rate of increased prevalence shoots up in the teenage years and appears to decrease some years after menopause. The prevalence is in line with other reported data in children and adults (177, 178). A comprehensive report on the prevalence in children has been reported (177) with one group of children aged 3-11 yrs. reported. (179).
In infants, when networks that define resting state are still developing (7, 8), migraine could be associated with infantile colic, facial pallor, irritability, sleep disturbance or mood changes (9). Since anti-migraine treatment may improve infantile colic (10), it is often referred to as ‘abdominal migraine’ and as such may be considered as behavioral representation of the level of brain development (i.e., a correlation of networks that may define the behavioral phenotype). Along this line, functional connectivity in the cortex of infants showed thalamocortical connections that may underlie the unusual presentation of what is believed to be migraine in very young children (11-16).
In prepubertal children, migraine occurs in 3-10% (17) with no difference between boys and girls (18, 19). In this age group, periodic symptoms such as benign paroxysmal torticollis, benign paroxysmal vertigo, abdominal migraine, and cyclic vomiting syndrome become more frequent (20-22), potentially due to more mature brainstem effectors. In contrast, in post-pubertal children, the hypothalamus is thought to reset its hormonal (e.g., gonadotropin releasing hormone) and neural (e.g., autonomic) systems (Figure 2), which in turn may make females more susceptible to migraine (23, 24). Puberty-related changes in brain function are not restricted to the hypothalamus (25). Puberty, which begins between the ages of 8-14 years in girls and 9-15 years in boys, is associated with pulsatile release of gonadotropin releasing hormone (GnRH) from the hypothalamus, and peak cortical gray matter (26) and white matter (27) volume. In the context of migraine, there are ample examples of sex differences in brain structure (28-30) and brain function, such as default mode brain connectivity, language, and visual systems (31, 32) (see Figures 3A-D).
Figure 2. Hormonal Changes with Puberty Drives Alterations in Brain Networks.
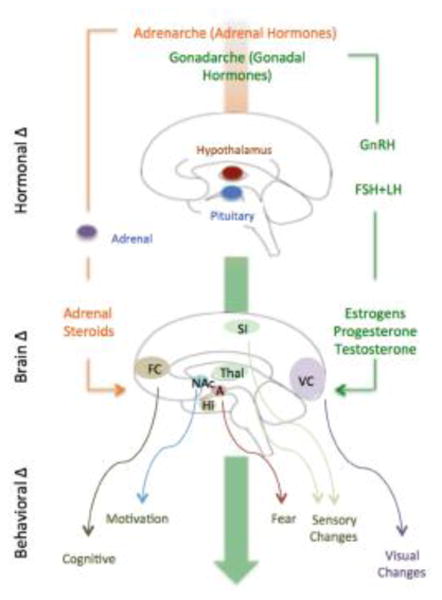
(see (25)). The two main hormonal systems that become active at puberty: (1) Gonadotrophic-Hypothalamic-Pituitary-Gonadal Axis: (shown on the right) that is initiated by pulsatile release of gonadotropin-releasing hormone (GnRH) in the hypothalamus and release of follicle stimulating hormone (FSH) and luteinizing hormone (LH) in the pituitary. Subsequent release of gonadal (testes or ovaries) sex steroid hormones (estrogen, progesterone, or testosterone) has direct effects on neurons and consequently neuronal networks. (2) Hypothalamic-Pituitary- Adrenal Axis: (shown on the left). This axis is the primary circuit that initiates, regulates, and terminates a stress response.
Figure 3. Examples of Sex and Age Differences in Brain Organization in Children vs. Adults.
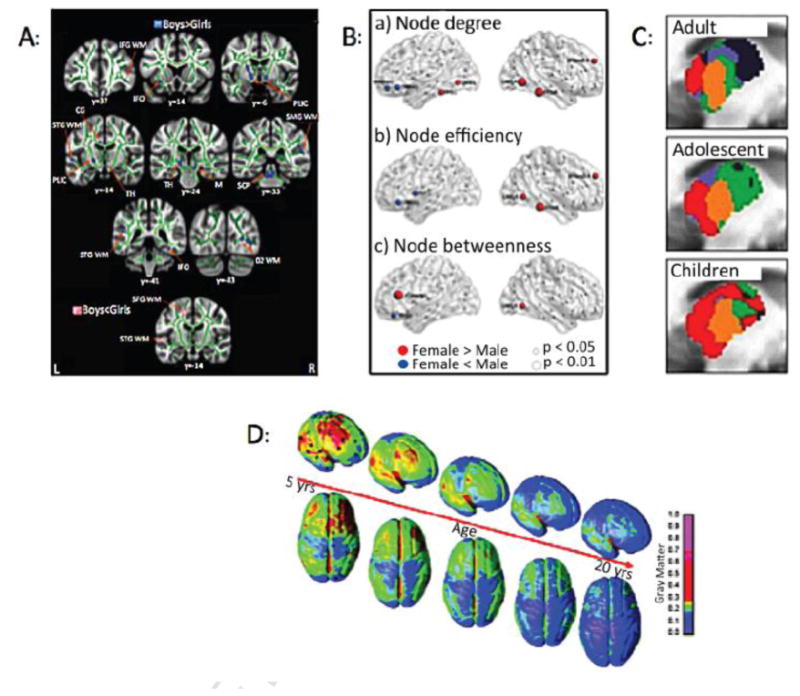
A: Sex Differences in White Matter Changes: (From (28) with permission). The figure shows differences in fractional anisotropy (FA) and MD controlling for age in girls and boys. Blue = FA b > g in a number of areas including PLIC - posterior limb of the internal capsule; SCP – superior cerebellar peduncle; IFO – inferior fronto-occipital fasciculus; and Pink = MD b < g in the SFG -superior frontal gyrus; STG – superior temporal gyrus; Green = mean FA skeleton.
B: Effect of Sex on Nodal Connectivity: The sex-related differences were seen predominantly in regions involved in the default mode network, language, and visual systems. (From (32) with permission).
C: Thalamic Functional Organization with Age: The figure shows thalamic functional organization differences in children, adolescents, and adults. For example, in children and adolescents, thalamo-temporal interactions involve a greater proportion of the anterior and middle thalamus (red) with frontal interactions involving less of the anterior thalamus. In contrast, thalamo-frontal interactions (blue) become more connected later in life. Somatosensory functional organization is shown in orange and remains relatively stable. (From (180) with permission).
D: Gray matter Changes in Children and Young Adults: LTP-like and LTD-like plasticity were large in young subjects but substantially smaller in elderly subjects (181), suggesting that younger brains are more susceptible to migraine. (From (182) with permission).
In the adult brain, women are more affected by migraine than men, and neuroendocrine drivers are thought to act as major modulators (33). In the 2011 National Health Interview Survey, 16.6% of adults > 18 years reported having migraine or other severe headaches in the last 3 months, and the prevalence was shown to be highest in females 18-44 years and lowest in males >75 years (34). Figure 4 illustrates the relationship to migraine frequency/prevalence, hormonal changes (hypothalamic, pituitary and gonadal) and brain changes across the menstrual cycle. Brain imaging studies have shown significant differences in gray and white matter, resting state functional connectivity, task-related neural activity, and brain chemistry between females and males (30, 35-38). Intriguingly, there is also evidence of brain alterations across the menstrual cycle in females. For example, gray matter volume peaks were found during ovulation compared to follicular and luteal cycle phases (39). Other findings suggest gray matter and white matter fluctuations in brain regions related to emotion and cognition (40-42) across the menstrual cycle phase. Additional evidence for the influence of sex steroid hormones on brain structure emerges from studies that have shown that women using a hormonal birth control method have greater gray matter volumes in prefrontal cortices, pre- and post-central gyri, parahippocampal/fusiform gyri, and temporal regions as compared to naturally cycling women (43).
Figure 4. Estrogen, Estrogen Receptors in the Brain and in the Trigemino-Vascular system.
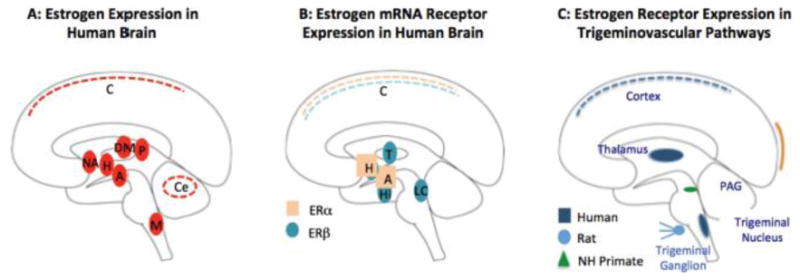
A: Estrogen Expression in the Human Brain: Areas noted in solid red are those expressing high levels of aromatase (the precursor for estrogen) based on in-vivo PET imaging using an estrogen specific ligand [N-methyl-11C] voroxole (60). Key: A = Amygdala; C = cortex; Ce = cerebellum; H = Hypothalamus; DM = Dorsomedial Nucleus of the thalamus; NA = Nucleus Accumbens; P = Pulvinar.
B: Estrogen mRNA Expression in the Human Brain: (183). Some brain areas have high expression of the alpha (ERα) subunit (H = Hypothalamus and A = Amygdala), others have high expression of the beta (ERβ) subunit (T = thalamus, Hi = hippocampus, and LC = locus ceruleus), while areas such as the cortex have a lower expression of both subunits.
C: Estrogen Receptor Expression in Sensory Pain Pathways: The trigeminal nucleus and thalamus contain high estrogen receptor levels in humans (183), in the trigeminal ganglion in rats (184) and in the periaqueductal gray (PAG) in non-human primates (185). It is postulated that estrogen and estrogen receptors in the sensory pathways may alter sensitivity to nociception in these neurons (186).
Furthermore, there is evidence in healthy female volunteers that pain intensity, pain unpleasantness, and functional brain activity in response to noxious stimuli fluctuate over the course of the menstrual cycle (45-47), which likely contributes to the allostatic load (48) and thus the increased susceptibility to migraine attacks may be related to migraine prevalence changes observed in women (49-51). With increasing age, changes in the neuroendocrine axis resulting from a loss of ovarian function leads to initial cycle deregulation, and, eventually, to the post-menopause state (52). Migraine usually improves in post-menopausal women (53), which is potentially due to low estrogen and high follicle-stimulating hormone (FSH) levels (54).
Sex Hormones and Brain Function
Sex hormones (estrogen, progesterone or testosterone) alter brain function. Estrogens can modulate neuronal activity electrophysiologically and morphologically, potentially through estrogen receptors that are widely distributed throughout the brain, with high concentrations in the hypothalamus (55) (see Figure 4). Examples of alterations in morphology include changes of hippocampal spines (56-58) and their related circuits (59). Estradiol biosynthesis takes place in neurons throughout the brain (viz., hypothalamus, basal forebrain, cerebral cortex, hippocampus, thalamus, cerebellum, and brainstem) and is catalyzed by the enzyme aromatase, which is also implicated in estrogen synthesis (60). Importantly, many of the brain areas associated with estradiol biosynthesis are involved in migraine (61-64). Positron emission tomography (PET) approaches have been used to measure estrogen (via the aromatase inhibitor [11C]vorozole) in human subjects (65). Of note, vorozole binding levels are high in (1) the pulvinar thalamus, a region implicated in increased sensory sensitivity to stimuli in migraine (61), (2) the nucleus accumbens, an area involved in reward and aversion (66, 67), and (3) the amygdala, a region involved in fear and anxiety (68). Thus, estrogen may modulate these brain areas, potentially contributing to migraine related behaviors of allodynia, mood changes, and dietary cravings. Intriguingly, some of these brain structures (e.g., the amygdala) show changes across the menstrual cycle, specifically, an increase in gray matter volume in the dorsal part of the left amygdala during the premenstrual phase compared with the late follicular phase (42). In addition, increases in the hippocampal volume and decreases in the dorsal basal ganglia volume have been observed in the post-menstrual phase (69). Taken together, gonadal hormonal feedback to the hypothalamus and other brain regions (70, 71) has significant impact on behaviors or neurological adaptations through specific neurotransmitters (72, 73). One of such system is the serotoninergic system (74). Serotonin-producing neurons are found in the mid- and hindbrain regions, and project to forebrain, limbic, diencephalic (rostral 5-HT nuclei), and the spinal cord (caudal 5-HT nuclei) (75), all of which contain both estrogen and progesterone receptors. Thus, aside from changes that may influence migraine circuits per se, estrogen-5-HT interactions may influence behaviors including mood (76, 77).
However, the influence of sex hormones on brain function is not limited to estrogen. Progesterone also has “multiple non-reproductive functions in the central nervous system to regulate cognition, mood, inflammation, mitochondrial function, neurogenesis and regeneration, myelination, and recovery from traumatic brain injury” (78). Similarly to estrogen, progesterone has effects on diverse brain systems beyond the hypothalamus and research data supports that estrogen and progesterone have opposite effects on neuronal excitability (79). For example, neuronal activity during seizures is amplified by estrogen, whereas progesterone and its metabolites have anticonvulsant effects (80). Thus, sex steroids may contribute to functional processing (including state of excitability) in the brain by acting through steroid receptors, which are dispersed throughout the brain (81).
Clinical observations suggest that testosterone may also play a role in migraine. First, it has been demonstrated that testosterone and its synthetic derivatives may improve migraine in both men and women (82, 83). Second, it has been shown that males treated with gonadotropins for infertility experienced a marked improvement in migraine and migraine with aura attacks (84). Mechanistically, this finding may be related to the suppression of cortical spreading depression (CSD) by androgens in mice (85).
Sex and Brain-Related Changes in Migraine
Brain alterations in migraineurs compared to healthy individuals have repeatedly been reported (62, 63, 86-89). Our group has previously shown prominent differences in brain structure and function of migraineurs compared to healthy controls, including the provocative finding that female migraineurs exhibit alterations in the precuneus and insula compared to male migraineurs (90, 91) (see Figure 5). With the knowledge that gonadal hormones are capable of altering brain circuits that regulate emotions in humans (92), we found that responses to noxious heat stimuli are distinctly segregated in men and women migraineurs and, therefore, proposed that this segregation is due to enhanced activation of the so-called ‘emotional circuits’ in women (90). This study suggests that the female brain is differentially affected by the disease state, with sex steroids as the prominent modulators (see Figure 6).
Figure 5. Alterations in Brain Sex and Migraine.
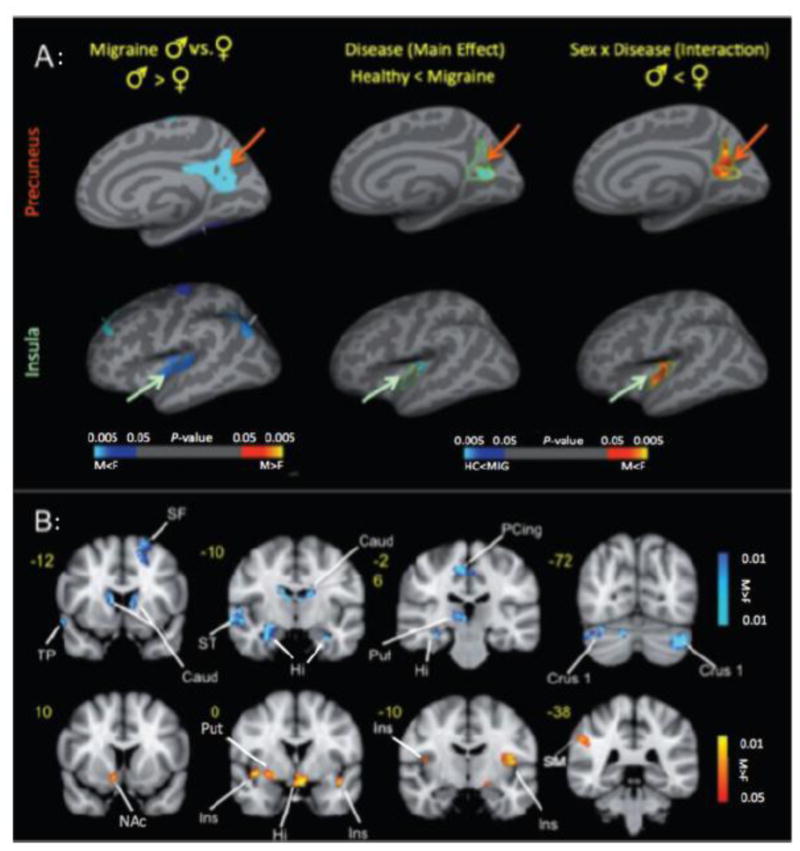
A: Cortical Thickness Changes: (Right) Significant clusters from vertex-wise cortical thickness comparisons conducted on female versus male healthy subjects (left column) and female versus male migraine patients (right column). Blue–light blue colors represent areas with thicker cortex in female versus male and red–yellow colors represent areas with thicker cortex in male versus male in each of the cohorts. (Middle and Left) Significant clusters from vertex-wise cortical thickness comparisons conducted on all of the subjects (migraine male and female and healthy control male and female) to determine the main effect (disease) effect and interaction effect (sex × disease). The disease effect (blue–light blue color map) and sex × disease interaction (red–yellow color map) are shown for (A) insula and (B) precuneus.
B: Effect of Pain across Sex: Contrast analysis of the male versus female migraine group in response to the pain threshold +1°C stimuli. Women had significantly (P < 0.05, corrected) greater activation in regions associated with emotional processing compared to men. Key: Caud = caudate; F = female; Hipp = hippocampus; Hypoth = hypothalamus; Ins = insula; L = left; M = male; NAc = nucleus accumbens; PCing = posterior cingulate; Pulv = pulvinar; Put = putamen; R = right; SF = superior frontal; SM = somatosensory cortex; ST = superior temporal. (Adapted from (90) with permission).
Figure 6. Differential Responses to Heat Pain across Menstrual Phase.
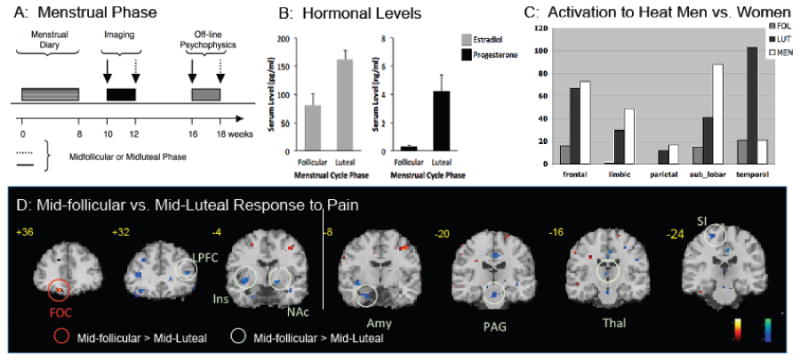
A: Data Collection: Ten men and 10 women were enrolled into the study. Using diaries, women provided a 3-month history of their menstrual cycle. Five women were scanned in the mid follicular phase first and 5 in their mid-luteal phase first.
B: Hormonal Levels: Serum levels were obtained form estradiol and progesterone during both phases of their cycles (i.e., follicular and luteal) at the time of scanning.
C: Differences in Regions in Men and Women: The histograms show volume of activation for women (mid-follicular - gray; mid-luteal - black;) and men (white). Note that activation volumes (in response to a 46°C stimulus to the arm) were similar for men and mid-luteal women in all areas except the temporal.
D: Differences between Mid-luteal and Mid-follicular Women: Significant activations are noted in the statistical maps overlaid on coronal images. In the mid-follicular phase (shown in red), women had greater activations in the Gob compared to the mid-luteal phase. In the mid-luteal phase (shown in green), women had greater activations in the LPF, Ins, NAc, Amy, PAG, Thal, and S1 compared to the mid-follicular phase (From P.A.I.N. Group, Unpublished Observations).
Key: FOC = frontal orbital cortex; LPF = lateral prefrontal cortex; Ins = insula; NAc = nucleus accumbens; Amy = amygdala; PAG = periaqueductal gray; Thal = thalamus; S1 = primary somatosensory cortex. periaqueductal gray; Thal = thalamus; S1 = primary somatosensory cortex.
Hypothalamic Role in Hormonal Regulation of Brain Dysmetria in Migraine
As noted by Facchinetti and colleagues, there is “Hypothalamic resetting at puberty and the sexual dimorphism of migraine” (24). Prior to puberty there are no sex differences in the occurrence of migraine (93). After the onset of the menarche, however, the prevalence of migraine is higher in girls than in boys and appears to be associated with the menstrual cycle in nearly 50% of attacks (94, 95). Aside from clear-cut menstrual migraine, fluctuations in estrogen and other hypothalamic hormones may contribute to a lowered threshold for migraine susceptibility through the menstrual cycle; a concept supported by the observation that mean plasma estrogen and progesterone levels are significantly higher in migraine patients for most of the menstrual cycle compared to controls, with the biggest differences found in the late luteal phase (96). Mechanistically, the increased prevalence of migraine at puberty may be driven by resetting of hypothalamic neuroendocrine circuits that determine sexual dimorphism (24). In the context of migraine, resetting of hypothalamic hormones can also alter the trigeminovascular system; the main neural pathway involved in migraine (97). Possible mechanisms include enhanced excitability and sensitization of neurons through estrogen-driven mismatch in homeostatic gene regulation and the resultant mitogen-activated membrane hyperexcitability (98). A meta-analysis of estrogen polymorphism studies indicates that two variants, ESR-1 594G> A and 325C> G, increase the risk for migraine 40-60% (99). Challenging that study, however, recent evidence indicates that aromatase polymorphisms (CYP19A1 rs10046 and CYP19A1 rs4646) also confer a risk for migraine and its protective effect, respectively, and may be more significant than estrogen polymorphisms (100). The latter hypothesis is supported by a twin study indicating that environmental and genetic factors have comparable contributions to migraine (101).
Hypothalamus, Gender, and Migraine
A large number of studies suggest that altered sex hormones cause structural instability and hypothalamic dysfunction (102) that appear as abnormal activation during migraine (23, 103, 104). Such dysfunction may explain the link between migraine and hypothalamic-regulated circadian rhythms, such as timekeeping, gonadal hormones, and cortisol secretion. Furthermore, homosexual men have a higher incidence of migraine (7.2 vs. 15.5%) (105), lower testosterone levels (106), increased suprachiasmatic nucleus volume (107), and decreased interstitial nucleus volume of the anterior hypothalamus number 3 (INAH3) compared to heterosexual men, but volumetric similarities (in the different hypothalamic nuclei) with women (108).
Gonadotropin Releasing Hormone
GnRH is secreted in a pulsatile fashion from the hypothalamus (109), initiating a cascade of events that may affect brain function (Figure 7). The pattern of GnRH release changes markedly at puberty, with significant physiologic changes in brain and body in girls and boys. Prior to puberty, secretion of leptin and kisspeptin evokes the release of GnRH in limited amounts. During mid-puberty, GnRH is available in low/minimal amounts during the day and higher levels at night, and continues to increase in late puberty, prior to the classic levels observed at the end of puberty (post-menarche). In the latter, the baseline shows a large pulse at the time of ovulation after which the range of release remains larger during the luteal phase compared to the follicular phase (110). Estrogen negatively regulates tonic GnRH synthesis except at the time of the preovulatory surge in GnRH through receptors on GnRH cells. In accordance with previous evidence linking estrogen to migraines (111), these changes in estrogen could be related to the increase in migraine prevalence in relation with puberty and ovulation.
Figure 7. Migraine Frequency, Hormones, and Brain Changes and Across the Menstrual Cycle.
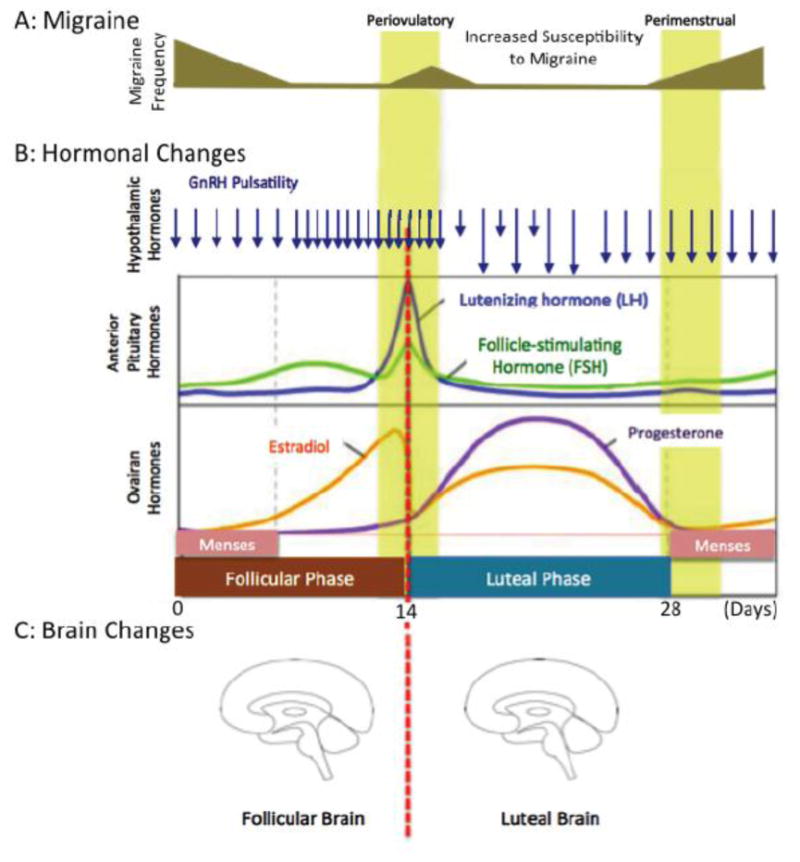
A: Incidence of Migraine across the Menstrual Cycle: More than 1/5 female migraineur aged 30-34 years have migraine in ≥50% of menstruations (187). Data suggest that migraine is most prevalent in the first few days at the onset of menses and the first few days at the beginning of the cycle (111). Falling levels of estrogen seem to contribute to migraine attacks and rising levels (perhaps associated with progesterone) protect from migraine attacks. Estrogen withdrawal is associated with increased migraine attacks (188). In addition, levels of estrogen and progesterone may be higher in migraine patients compared with controls (96). Increased excitability of neurons (72) and migraine risk may occur during the menstrual cycle as a result of neuromodulation by factors such as BDNF (brain derived neurotropic factor) that is induced by estrogen. Progesterone is thought to decrease BDNF excitability. Note that the model correlates with migraine prevalence across the cycle.
B: Hormonal Changes Across the Cycle: GnRH pulsatility changes across the menstrual cycle. Estradiol and Progesterone variation is dependent on menstrual phase (189).
C: Brain Changes Across the Cycle: Behavioral changes across the menstrual cycle (reviewed in (190)) are supported by imaging studies (191). Dramatic functional (192, 193) and morphological (e.g., increased amygdala volume in the luteal phase vs. follicular phase (42) or increased hippocampal and decreased in basal ganglia volume in the follicular phase (69)) changes are observed across the menstrual phase.
GnRH controls the release of hormonal waves (estradiol peaks in the late follicular phase; progesterone peaks in the mid-luteal phase) through increases in luteinizing hormone (LH) and FSH in the mid-cycle (around day 14) (see Figure 7). Sex hormones (estradiol and progesterone) and their releasing factors (FSH, LH) decrease to basal levels around day 28 with baseline (flat) levels for estrogen and progesterone observed in the first 7 days for estrogen and 14 days for progesterone that all contribute to a hormonal ‘dysequilibrium’. Thus, the undulating changes in gonadal hormones in the mid-luteal phase may contribute to neuronal excitability around days 14 and 28 of the cycle. Specifically, there is an increase in excitability after the pre-ovulatory estrogen surge and during the mid-luteal rise in estrogen levels, coincidentally when migraine risk appears to increase (72).
Female Sex Hormones and the Trigemino-Vascular System
Sex steroid hormones such as estrogen are known to alter the responsiveness of the system at the level of the dura (112, 113), peripheral nerve (114), trigeminal ganglion (115, 116), trigeminal nucleus (117-119), thalamus (120), cortical systems (121), and descending modulatory systems (122). Estrogen receptors are located in the trigeminal nucleus in humans (123) and in the periaqueductal gray (a region involved in pain modulation) in rhesus monkeys, thus highlighting the importance of species-specific research in terms of understanding estrogen function in humans (124). Preclinical data also suggest that estrogens may be important in regulating sensitization of trigeminal neurons through modulation of mediators such as calcitonin gene-related peptide (125) (see Figure 4).
The complexity of the interaction of gonadal hormones and pain processing (103) is illustrated by studies that show that oscillations in hormonal levels during the 5 phases of the menstrual cycle (menstrual, follicular, ovulatory, luteal, and premenstrual) influence experimental sensitivity to thermal pain (126) in healthy women (see Figure 6) and to experimental muscle pain in women with dysmenorrhea (127). Other studies that show that changes in pressure, electrical, and cold pain thresholds, which occur over the menstrual cycle with higher thresholds to pressure and electrical pain stimuli on day 22 and to cold on day 14, are not correlated with changes in gonadal hormones (128).
Positron emission tomography (PET) studies indicate that sex influences brain expression of dopamine (129, 130), serotonin (131), neurokinin 1 (132, 133), and opioids (134). Autoradiography studies in rodents further indicate sex differences in AMPA, kainate, and NMDA (135) as well as noradrenergic transmission (136). Moreover, these systems interact with the estradiol cycle, providing a neurochemical milieu that women may be more susceptible to migraine attacks. Furthermore, female rats are reportedly more sensitive to orofacial pain compared to male rats (but not in non-facial regions) (137). Accordingly, these findings highlight the importance of sexual dimorphisms in brain neuropeptides and innervation that may be relevant for migraine pain.
Hormonal Systems Modulate the “Set Point” for Migraine Attacks
A set point is defined as the point at which a variable physiological state (e.g., body temperature or weight) tends to stabilize. As migraine depends on a number of conditions, including environmental (e.g., barometric changes), genetic, and physiological factors, the susceptibility of an actual migraine attack may be determined by the relative functional status of each system. We have previously suggested that initiation of migraine is determined by elements such as CSD, oscillatory susceptibility, and insufficient modulation of nociceptive inputs by the periaqueductal gray, and/or vascular drives (48, 138). Specifically, we propose that, in the susceptible subject, the onset of migraine must coincide with a point in the cyclic rhythmicity of brainstem activity that is intended to maintain homeostasis. In the context of this article, we propose that the hypothalamus may also be involved in defining a ‘migraine set point’ through its ability to modulate spontaneous and evoked activities in trigeminal nucleus neurons (139) (see Figure 8). Dynamic changes of hypothalamic, pituitary, and gonadal hormones may alter brain function at a cellular (e.g., receptor), anatomical (e.g., dendritic spine growth and/or hydration status (140), and functional (e.g., neural circuit) level (see Figure 8). Table 1 summarizes set point perturbators thought to contribute to altered stability of biological systems (allostasis) and derange networks in migraine-susceptible brains.
Figure 8. Brain Tone and Migraine Threshold: A Model for Hormonal Stressors and Migraine Activation base on Alterations in Migraine “set point”.
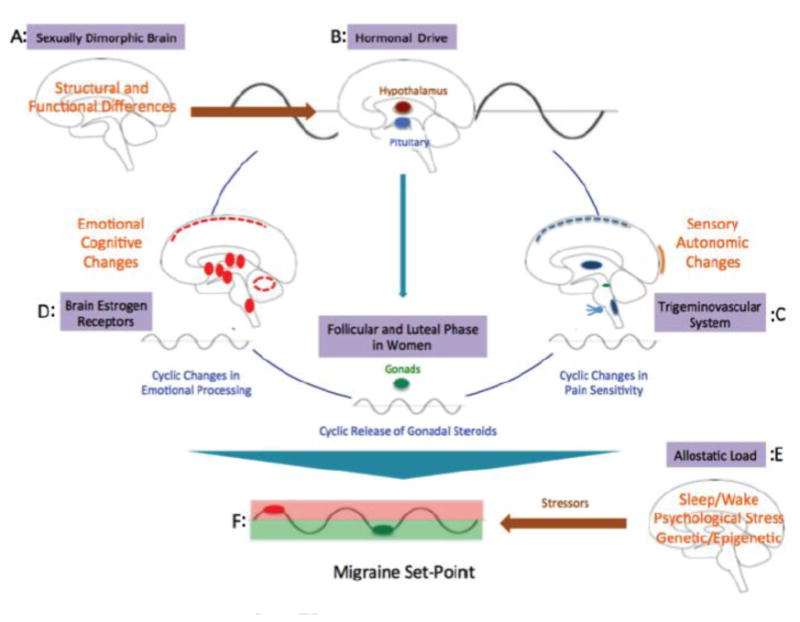
A: Sexually Dimorphic Brain: Significant sex differences in brain structure have been reviewed elsewhere (194). Women have larger volumes in the frontal and paralimbic cortices; men in the hypothalamus and amygdala.
B: Hormonal Drive: In healthy women and men hormonal control is primarily through hypothalamic release of GnRH that drives the menstrual cycle through FSH and LH to release estrogen and progesterone in women. The cyclicity alters stability of neural systems (primarily sensory, emotional and cognitive – see below).
C: Alterations in Sensory and Autonomic Systems by Estrogen: Pain thresholds alter over the menstrual cycle. Autonomic testing over the menstrual cycle has been reported to show differences across the menstrual cycle (195, 196). Sympathetic nervous activities are predominant in the luteal phase (197). Thus, cyclicity of physiological processes is also altered over the menstrual cycle.
D: Alterations in Emotional and Cognitive Systems by Estrogen: Women have a higher prevalence of anxiety and depression that may relate to alterations or the integration of cognitive and emotional processes (191).
E: Allostatic Load: Allostatic load, internal and external (environmental) challenges or perturbations that result in an organism to respond to these challenges in order to maintain stability (allostasis). The burden of stress may lead to alterations in the brain and body and exacerbation of the disease state (198). Such changes (e.g., genetic constitution, sleep wake, diet, medications, psychological stressors) have also been considered to be important in the migraine state (47).
F: Migraine Set Point: In migraineurs, the state of brainstem tone may be unstable or less robust than in healthy individuals by prophylactic and may thus be susceptible to activation of the cascade of networks that trigger the migraine attack by normal afferent nociceptive signals (red dot), which would be inhibited in healthy individuals. Genetic, physiological, pharmacological, social, and other interactions define migraineurs' susceptibility. When processes are in synchrony (i.e., a harmonic or repetitive frequency), the model suggests that the migraine potential is sub-threshold (red circle); however, when these are altered either in magnitude, phase or duration, the system becomes unstable and the migraine threshold is exceeded (48).
Table 1. Perturbators of Set Point as Contributors to Altered Stability of Biological Systems (Allostasis) in Migraine Patients.
|
Estrogen
The correlation between migraine onset and falling estrogen levels during the menstrual cycle is seen in estrogen withdrawal (141). In menstrual migraine falling levels of estrogen seem to correlate with migraine onset. Estrogen acts predominantly as excitatory on neuronal circuits and it seems therefore counterintuitive that estrogen withdrawal induces migraine. While the underlying mechanisms remain unknown it is possible that estrogen alters neuronal excitability (142) in brain regions implicated in migraine pathophysiology such as the hypothalamus (143) and hippocampus (144). Estrogens are also present in brain modulatory systems such as the periaqueductal gray (145) and excitatory effects of estrogens are likely to act on GABA-ergic neurons that enhance PAG outputs. Accordingly, low estrogen levels may reduce activity in pathways that inhibit pain processing. Nevertheless, the relationship between estrogen withdrawal and migraine is clearly more complex since estrogen supplements do not always prevent menstrual migraine (146).
Cortical Spreading Depression
CSD, a well defined process in migraine pathophysiology, is a slowly propagated wave of depolarization followed by suppression of brain activity (147). Two well-studied hormones that may influence CSD include testosterone (85) and estrogen (148). Regarding testosterone, the susceptibility to CSD in a mouse model of familial hemiplegic migraine has been shown to be reversed by orchiectomy (85). Regarding estrogen, it is interesting to note that menstrual migraines, coinciding with low levels of estrogen, are usually not preceded by aura (149), the clinical manifestation of CSD. This observation is supported by findings that show no difference in estradiol level in migraine patients with aura compared to migraine patients without aura (148).
Immunological/Cytokine and other Modulators of Neuronal Function
Given that the susceptibility to migraine is cyclical, one must take into consideration cyclical changes in both hormonal level and immune cell functions, such as, mast cells, cytokines, and microglia. (1) Mast Cells. Meningeal mast cells (150), which are rich in molecules that facilitate inflammation and activate pain fibers in the dura (e.g., histamine, serotonin, heparin) (151) (152), are stimulated by neuropeptides such as calcitonin gene-related peptide (CGRP), neurotensin (NT), pituitary adenylate cyclase activating peptide (PACAP), and substance P (SP). Once secreted, mast cell products can enter the central nervous system via transgranulation (153) and affect brain areas such as the hippocampus (154), a region first described by our group to be implicated in the migraine process (63). Mast cells are also present in the thalamus and hypothalamus (155), providing access from the perivascular space into multiple brain regions. Mast cells also synthesize GnRH (155), a neuroendocrine hypothalamic hormone that regulates gonadotropin release in the pituitary and modulates neuronal activity in the central nervous system (156, 157). (2) Cytokines. Several lines of evidence support cytokines role in migraine: (a) migraine patients have higher serum IL-1beta and IL-6 levels, and lower IL-10 levels than healthy subjects (158); (b) the trigeminal ganglia of a transgenic mouse model of familial hemiplegic migraine contains high level of TNF-α, IL-1β, IL-6, and IL-10 (159); (c) menstruation is an inflammatory process (160); and (d) increased levels of peripheral and central cytokines can alter neuronal activity in the central nervous system (161) (162, 163). (3) Microglia. Brain microglia are involved in a number of functions including communication with astrocytes, neurons, endothelium, and leukocyte (164). In the context of migraine, brain microglia exhibit estrogen receptors (165), become activated during CSD, and release cytokines, which in turn can activate peripheral and central neurons (166).
Hormonally-mediated Alterations in Physiological Parameters
Estrogens affect a number of physiological processes including the sleep-wake cycle, body temperature, and food intake. (1) Sleep-Wake Cycle. Insomnia is the most common sleep disorder in headache patients (167). Paradoxically, sleep frequently may stop migraine (168). It has been suggested that sleep alterations are part of the migraine attack itself (169). In support of this hypothesis, under conditions that control for sleep/wake, light/dark, activity, position, and nutritional cues, there is no circadian rhythm of LH, FSH, or FAS in women during the early follicular phase (170). However, recently, a gene mutation in casein kinase iδ has been linked to sleep (advanced sleep phase) and familial migraine disorders (171), suggesting a potential link between sleep and hypothalamus that may lead to secondary effects of altered hormonal regulation. (2) Body Temperature. A less noted correlation may exist between the body temperature cyclicity and the cyclicity of migraine. Given that the hypothalamus regulates the biological rhythm of core body temperature, sleep and energy expenditure (23), it is also likely to coordinate between them, potentially through neurons that contain orexin, melanin concentrating hormone, and histamine (172). Accordingly, it may be reasonable to speculate that migraine may be associated not only with disturbed sleep or fasting but also with body temperature alterations (173). This concept may provide novel explanation to the low occurrence of migraine onset at bedtime and high occurrence of migraines between 4 and 6 AM, when the body temperature begins to rise. (3) Obesity. Estrogen contributes to the regulation of the metabolism (174) and may influence body weight (175), usually exerting protective functions, perhaps by acting as a leptin-mimetic (176). Migraine is associated with obesity in adults and children (177-179) and low leptin levels (174).
Dynamic changes in hypothalamic, pituitary, and gonadal hormones may alter brain function at a cellular (e.g., receptor), anatomical (e.g., dendritic spine growth and/or hydration status (140)), and functional (e.g., neural circuit) level (see Figure 8 and Table 1).
Hormones and Allostatic Load in Migraine
The pulsatile release of hypothalamic hormones leads to the stimulation of gonadal steroids that alter migraine susceptible brains, particularly estrogens in women. In susceptible individuals, the hormonal rhythm produces either increased or decreased sensitivity to the phase at which a migraine may be triggered (49). Repeated migraine attacks, precipitated by an altered brain state or hormonal stress may lead to allostatic load (i.e., increased effects on brain systems as a result of repeated stressors). The concept of allostatic load as it applies to migraine has been reviewed elsewhere (48). Figure 9 summarizes the McEwen concept of how alterations of homeostasis lead to allostatic load (180), how hormonal load contributes to allostatic load (181), and how the two lead to alterations in brain functions (181). An example of the effect of allostatic load changing migraine frequency is the resolution of menstrual migraine that leads to a reversal of chronic migraine to episodic migraine (182).
Future Directions
Migraine processing is not limited to ictal attacks but a prolonged process that may involve pre-ictal (e.g., prodrome), ictal (headache, nausea), and post-ictal (e.g., fatigue) phenomena. Inflammation has been a consistent theme in migraine in humans (183-185) and animal models (159). We suggest that there are a number of important routes of research that may help us better understand how hormones affect the migraine brain. For example, it is important for future research to analyze the relationship between brain function and hormonal changes in migraine. Additionally, there is a need for basic science in translational medicine, as translating findings from animal models to the human condition has been a challenge. In the migraine field, there are a number of “animal models”, however, most use male animals (e.g., (113, 186)), which confounds sex differences in migraine. Another approach to study the influence of hormones is to investigate short term and long term effects of contraceptive use on migraine, as there is compelling evidence that oral contraceptive alter brain function (187, 188) and structure in regions such as the prefrontal cortex, pre- and post-central gyri, parahippocampal and fusiform gyri, and temporal regions (43). However, the impact of oral contraceptives on brain function and structure in migraine susceptible individuals remains unknown. Finally, brain alterations that occur during puberty and headache point to the potential value of studying the relationship among age, hormones, genomic expression, and brain structure and function as it may improve our understanding of how puberty in girls exacerbates migraine and how these early changes may persist later into life.
Conclusions
Hormonal fluctuations are prominent during puberty and are known to alter behavior, and, particularly affective dimensions (29). These changes produce dramatic phenotypic alterations in brain structure and function (189). Steroidal hormones alter neural circuits during adolescence, a time of ongoing neural development (190). When pathological alterations in brain systems exist as a result of genetic, epigenetic or other reasons, the surge essentially acts to enhance the expression of the condition. Migraine is a preeminent example.
Disruptions of homeostasis normally require allostatic processes to normalize the biological process. In healthy pubertal girls and women, there is an ongoing oscillation of changes throughout their reproductive lives. The lack of hormonal stability, afforded to healthy men, is a process that may include hyperexcitability in neurons and networks throughout the brain. Allostasis is a normal physiological adaptation to a stressor. When stressors become pathological and lead to a feed-forward cascade, allostasis is no longer preserved (i.e., allostatic load). Migraine can therefore be considered a model disease of allostatic load and overload in women (48), in which vulnerability to the hormonal cyclicity enhances the predisposition to migraine.
Box 1: Gender and Sex Definitions.
When discussing differences between men and women, terminology is important. Here, we use the term “sex” in reference to the biologically based differences, whereas the term “gender” refers to socially based phenomena. Biological sex exerts a major influence on one's gender ide qntity, but the terms are not interchangeable. As discussed more extensively by Greenspan et al (44): “…. in any statistical analysis of human subjects, the dichotomous variable sex (male vs. female) is confounded with the social construct of gender. That is, in human studies in which the dependent measure is pain report, group differences are likely to be attributable to both sex and gender. Therefore, both constructs should be examined when possible in order to understand their relative contribution to differences in pain between men and women.”
Acknowledgments
This work has been supported by NIH grants to DB from NINDS (K24 NS064050; R01NS056195; R01NS073997) and NCCAM (R21AT007530) and RB (R37 NS079678; R01NS069847).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu K, Taki Y, Sato K, Kinomura S, Goto R, Okada K, et al. Age-related changes in topological organization of structural brain networks in healthy individuals. Human brain mapping. 2012;33(3):552–68. doi: 10.1002/hbm.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, et al. Functional brain networks develop from a “local to distributed” organization. PLoS computational biology. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Power JD, Fair DA, Schlaggar BL, Petersen SE. The development of human functional brain networks. Neuron. 2010;67(5):735–48. doi: 10.1016/j.neuron.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel AC, Power JD, Petersen SE, Schlaggar BL. Development of the brain's functional network architecture. Neuropsychology review. 2010;20(4):362–75. doi: 10.1007/s11065-010-9145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart WF, Shechter A, Rasmussen BK. Migraine prevalence. A review of population-based studies. Neurology. 1994;44(6 Suppl 4):S17–23. [PubMed] [Google Scholar]
- 6.Merikangas KR. Contributions of epidemiology to our understanding of migraine. Headache. 2013;53(2):230–46. doi: 10.1111/head.12038. [DOI] [PubMed] [Google Scholar]
- 7.Smyser CD, Snyder AZ, Neil JJ. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. NeuroImage. 2011;56(3):1437–52. doi: 10.1016/j.neuroimage.2011.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, et al. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104(39):15531–6. doi: 10.1073/pnas.0704380104. Epub 2007/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romanello S, Spiri D, Marcuzzi E, Zanin A, Boizeau P, Riviere S, et al. Association between childhood migraine and history of infantile colic. JAMA : the journal of the American Medical Association. 2013;309(15):1607–12. doi: 10.1001/jama.2013.747. [DOI] [PubMed] [Google Scholar]
- 10.Katerji MA, Painter MJ. Infantile migraine presenting as colic. Journal of child neurology. 1994;9(3):336–7. doi: 10.1177/088307389400900325. [DOI] [PubMed] [Google Scholar]
- 11.Fransson P, Aden U, Blennow M, Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cerebral cortex. 2011;21(1):145–54. doi: 10.1093/cercor/bhq071. [DOI] [PubMed] [Google Scholar]
- 12.Fransson P, Metsaranta M, Blennow M, Aden U, Lagercrantz H, Vanhatalo S. Early development of spatial patterns of power-law frequency scaling in FMRI resting-state and EEG data in the newborn brain. Cerebral cortex. 2013;23(3):638–46. doi: 10.1093/cercor/bhs047. [DOI] [PubMed] [Google Scholar]
- 13.Omidvarnia A, Fransson P, Metsaranta M, Vanhatalo S. Functional Bimodality in the Brain Networks of Preterm and Term Human Newborns. Cerebral cortex. 2013 doi: 10.1093/cercor/bht120. [DOI] [PubMed] [Google Scholar]
- 14.Hartley C, Slater R. Neurophysiological measures of nociceptive brain activity in the newborn infant - the next steps. Acta paediatrica. 2013 doi: 10.1111/apa.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagmann P, Grant PE, Fair DA. MR connectomics: a conceptual framework for studying the developing brain. Frontiers in systems neuroscience. 2012;6:43. doi: 10.3389/fnsys.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakatani K, Chen S, Lichty W, Zuo H, Wang YP. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early human development. 1999;55(3):229–36. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- 17.Barnes NP. Migraine headache in children. Clinical evidence. 2011 2011. [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein M, Chen TC. The epidemiology of disabling headache. Advances in neurology. 1982;33:377–90. [PubMed] [Google Scholar]
- 19.Waters WE, O'Connor PJ. Epidemiology of headache and migraine in women. Journal of neurology, neurosurgery, and psychiatry. 1971;34(2):148–53. doi: 10.1136/jnnp.34.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arruda MA, Guidetti V, Galli F, Albuquerque RC, Bigal ME. Childhood periodic syndromes: a population-based study. Pediatric neurology. 2010;43(6):420–4. doi: 10.1016/j.pediatrneurol.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 21.Cuvellier JC, Lepine A. Childhood periodic syndromes. Revue neurologique. 2010;166(6-7):574–83. doi: 10.1016/j.neurol.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Winner P. Migraine-related symptoms in childhood. Current pain and headache reports. 2013;17(8):339. doi: 10.1007/s11916-013-0339-6. [DOI] [PubMed] [Google Scholar]
- 23.Alstadhaug KB. Migraine and the hypothalamus. Cephalalgia : an international journal of headache. 2009;29(8):809–17. doi: 10.1111/j.1468-2982.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 24.Facchinetti F, Sgarbi L, Piccinini F. Hypothalamic resetting at puberty and the sexual dimorphism of migraine. Functional neurology. 2000;15(Suppl 3):137–42. [PubMed] [Google Scholar]
- 25.Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Human brain mapping. 2010;31(6):926–33. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature neuroscience. 1999;2(10):861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 27.Barnea-Goraly N, Menon V, Eckert M, Tamm L, Bammer R, Karchemskiy A, et al. White matter development during childhood and adolescence: a cross-sectional diffusion tensor imaging study. Cerebral cortex. 2005;15(12):1848–54. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 28.Herting MM, Maxwell EC, Irvine C, Nagel BJ. The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cerebral cortex. 2012;22(9):1979–92. doi: 10.1093/cercor/bhr246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ladouceur CD, Peper JS, Crone EA, Dahl RE. White matter development in adolescence: the influence of puberty and implications for affective disorders. Developmental cognitive neuroscience. 2012;2(1):36–54. doi: 10.1016/j.dcn.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peper JS, Hulshoff Pol HE, Crone EA, van Honk J. Sex steroids and brain structure in pubertal boys and girls: a mini-review of neuroimaging studies. Neuroscience. 2011;191:28–37. doi: 10.1016/j.neuroscience.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Sprenger T, Borsook D. Migraine changes the brain: neuroimaging makes its mark. Current opinion in neurology. 2012;25(3):252–62. doi: 10.1097/WCO.0b013e3283532ca3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu K, Taki Y, Sato K, Hashizume H, Sassa Y, Takeuchi H, et al. Topological organization of functional brain networks in healthy children: differences in relation to age, sex, and intelligence. PloS one. 2013;8(2):e55347. doi: 10.1371/journal.pone.0055347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nappi RE, Nappi G. Neuroendocrine aspects of migraine in women. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2012;28(Suppl 1):37–41. doi: 10.3109/09513590.2012.651931. [DOI] [PubMed] [Google Scholar]
- 34.Smitherman TA, Burch R, Sheikh H, Loder E. The prevalence, impact, and treatment of migraine and severe headaches in the United States: a review of statistics from national surveillance studies. Headache. 2013;53(3):427–36. doi: 10.1111/head.12074. [DOI] [PubMed] [Google Scholar]
- 35.Allen EA, Erhardt EB, Damaraju E, Gruner W, Segall JM, Silva RF, et al. A baseline for the multivariate comparison of resting-state networks. Frontiers in systems neuroscience. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu CC, Kuo TB, Yang CC. Effects of estrogen on gender-related autonomic differences in humans. American journal of physiology Heart and circulatory physiology. 2003;285(5):H2188–93. doi: 10.1152/ajpheart.00256.2003. [DOI] [PubMed] [Google Scholar]
- 37.Sacher J, Neumann J, Okon-Singer H, Gotowiec S, Villringer A. Sexual dimorphism in the human brain: evidence from neuroimaging. Magnetic resonance imaging. 2013;31(3):366–75. doi: 10.1016/j.mri.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 38.Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(45):15034–43. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, et al. Changes in brain size during the menstrual cycle. PloS one. 2011;6(2):e14655. doi: 10.1371/journal.pone.0014655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Bondt T, Jacquemyn Y, Van Hecke W, Sijbers J, Sunaert S, Parizel PM. Regional gray matter volume differences and sex-hormone correlations as a function of menstrual cycle phase and hormonal contraceptives use. Brain research. 2013;1530:22–31. doi: 10.1016/j.brainres.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 41.De Bondt T, Van Hecke W, Veraart J, Leemans A, Sijbers J, Sunaert S, et al. Does the use of hormonal contraceptives cause microstructural changes in cerebral white matter? Preliminary results of a DTI and tractography study. European radiology. 2013;23(1):57–64. doi: 10.1007/s00330-012-2572-5. [DOI] [PubMed] [Google Scholar]
- 42.Ossewaarde L, van Wingen GA, Rijpkema M, Backstrom T, Hermans EJ, Fernandez G. Menstrual cycle-related changes in amygdala morphology are associated with changes in stress sensitivity. Human brain mapping. 2013;34(5):1187–93. doi: 10.1002/hbm.21502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pletzer B, Kronbichler M, Aichhorn M, Bergmann J, Ladurner G, Kerschbaum HH. Menstrual cycle and hormonal contraceptive use modulate human brain structure. Brain research. 2010;1348:55–62. doi: 10.1016/j.brainres.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 44.Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi JC, Park SK, Kim YH, Shin YW, Kwon JS, Kim JS, et al. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105(1):120–7. doi: 10.1097/00000542-200607000-00021. [DOI] [PubMed] [Google Scholar]
- 46.de Leeuw R, Albuquerque RJ, Andersen AH, Carlson CR. Influence of estrogen on brain activation during stimulation with painful heat. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2006;64(2):158–66. doi: 10.1016/j.joms.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Veldhuijzen DS, Keaser ML, Traub DS, Zhuo J, Gullapalli RP, Greenspan JD. The role of circulating sex hormones in menstrual cycle-dependent modulation of pain-related brain activation. Pain. 2013;154(4):548–59. doi: 10.1016/j.pain.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron. 2012;73(2):219–34. doi: 10.1016/j.neuron.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 49.Borsook D, Burstein R. The enigma of the dorsolateral pons as a migraine generator. Cephalalgia : an international journal of headache. 2012;32(11):803–12. doi: 10.1177/0333102412453952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351–3. doi: 10.1212/01.wnl.0000133134.68143.2e. [DOI] [PubMed] [Google Scholar]
- 51.Stewart WF, Lipton RB, Chee E, Sawyer J, Silberstein SD. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55(10):1517–23. doi: 10.1212/wnl.55.10.1517. [DOI] [PubMed] [Google Scholar]
- 52.Hall JE. Neuroendocrine changes with reproductive aging in women. Seminars in reproductive medicine. 2007;25(5):344–51. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 53.Neri I, Granella F, Nappi R, Manzoni GC, Facchinetti F, Genazzani AR. Characteristics of headache at menopause: a clinico-epidemiologic study. Maturitas. 1993;17(1):31–7. doi: 10.1016/0378-5122(93)90121-w. [DOI] [PubMed] [Google Scholar]
- 54.Wang SJ, Fuh JL, Lu SR, Juang KD, Wang PH. Migraine prevalence during menopausal transition. Headache. 2003;43(5):470–8. doi: 10.1046/j.1526-4610.2003.03092.x. [DOI] [PubMed] [Google Scholar]
- 55.Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. Journal of neurobiology. 1998;36(3):357–78. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 56.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, et al. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Frontiers in neural circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ooishi Y, Kawato S, Hojo Y, Hatanaka Y, Higo S, Murakami G, et al. Modulation of synaptic plasticity in the hippocampus by hippocampus-derived estrogen and androgen. The Journal of steroid biochemistry and molecular biology. 2012;131(1-2):37–51. doi: 10.1016/j.jsbmb.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 58.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, et al. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. Journal of neurochemistry. 2007;100(4):950–67. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 59.Cyr M, Calon F, Morissette M, Di Paolo T. Estrogenic modulation of brain activity: implications for schizophrenia and Parkinson's disease. Journal of psychiatry & neuroscience : JPN. 2002;27(1):12–27. [PMC free article] [PubMed] [Google Scholar]
- 60.Biegon A, Kim SW, Alexoff DL, Jayne M, Carter P, Hubbard B, et al. Unique distribution of aromatase in the human brain: in vivo studies with PET and [N-methyl-11C]vorozole. Synapse. 2010;64(11):801–7. doi: 10.1002/syn.20791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Thalamic sensitization transforms localized pain into widespread allodynia. Annals of neurology. 2010;68(1):81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia : an international journal of headache. 2012;32(8):607–20. doi: 10.1177/0333102412445622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maleki N, Becerra L, Brawn J, McEwen B, Burstein R, Borsook D. Common hippocampal structural and functional changes in migraine. Brain structure & function. 2013;218(4):903–12. doi: 10.1007/s00429-012-0437-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moulton EA, Burstein R, Tully S, Hargreaves R, Becerra L, Borsook D. Interictal dysfunction of a brainstem descending modulatory center in migraine patients. PloS one. 2008;3(11):e3799. doi: 10.1371/journal.pone.0003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lidstrom P, Bonasera TA, Kirilovas D, Lindblom B, Lu L, Bergstrom E, et al. Synthesis, in vivo rhesus monkey biodistribution and in vitro evaluation of a 11C-labelled potent aromatase inhibitor: [N-methyl-11C]vorozole. Nuclear medicine and biology. 1998;25(5):497–501. doi: 10.1016/s0969-8051(98)00009-2. [DOI] [PubMed] [Google Scholar]
- 66.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(Suppl 1):122–32. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(45):17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Talarovicova A, Krskova L, Kiss A. Some assessments of the amygdala role in suprahypothalamic neuroendocrine regulation: a minireview. Endocrine regulations. 2007;41(4):155–62. [PubMed] [Google Scholar]
- 69.Protopopescu X, Butler T, Pan H, Root J, Altemus M, Polanecsky M, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18(10):985–8. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 70.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behavioral neuroscience. 2012;126(1):4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacological reviews. 2010;62(2):155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scharfman HE, MacLusky NJ. Estrogen-growth factor interactions and their contributions to neurological disorders. Headache. 2008;48(Suppl 2):S77–89. doi: 10.1111/j.1526-4610.2008.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cellular and molecular neurobiology. 1996;16(3):325–44. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia : an international journal of headache. 2007;27(11):1293–300. doi: 10.1111/j.1468-2982.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 75.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in neuroendocrinology. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 76.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biological psychiatry. 1998;44(9):839–50. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 77.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behavioral and cognitive neuroscience reviews. 2005;4(1):43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 78.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, et al. Progesterone receptors: form and function in brain. Frontiers in neuroendocrinology. 2008;29(2):313–39. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2011;32(Suppl 1):S31–5. doi: 10.1007/s10072-011-0532-5. [DOI] [PubMed] [Google Scholar]
- 80.Beyenburg S, Stoffel-Wagner B, Bauer J, Watzka M, Blumcke I, Bidlingmaier F, et al. Neuroactive steroids and seizure susceptibility. Epilepsy research. 2001;44(2-3):141–53. doi: 10.1016/s0920-1211(01)00194-2. [DOI] [PubMed] [Google Scholar]
- 81.Joels M. Steroid hormones and excitability in the mammalian brain. Frontiers in neuroendocrinology. 1997;18(1):2–48. doi: 10.1006/frne.1996.0144. [DOI] [PubMed] [Google Scholar]
- 82.Calton GJ, Burnett JW. Danazol and migraine. The New England journal of medicine. 1984;310(11):721–2. doi: 10.1056/nejm198403153101114. [DOI] [PubMed] [Google Scholar]
- 83.Lichten EM, Bennett RS, Whitty AJ, Daoud Y. Efficacy of danazol in the control of hormonal migraine. The Journal of reproductive medicine. 1991;36(6):419–24. [PubMed] [Google Scholar]
- 84.Arango O, Bielsa O, Pascual-Calvet J, Herrero M, Gelabert-Mas A. Disappearance of migraine crises in two patients with male infertility treated with human chorionic gonadotropin/human menopausal gonadotrophin. Revista de neurologia. 1996;24(132):977–9. [PubMed] [Google Scholar]
- 85.Eikermann-Haerter K, Baum MJ, Ferrari MD, van den Maagdenberg AM, Moskowitz MA, Ayata C. Androgenic suppression of spreading depression in familial hemiplegic migraine type 1 mutant mice. Annals of neurology. 2009;66(4):564–8. doi: 10.1002/ana.21779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu J, Zhao L, Nan J, Li G, Xiong S, von Deneen KM, et al. The trade-off between wiring cost and network topology in white matter structural networks in health and migraine. Experimental neurology. 2013;248:196–204. doi: 10.1016/j.expneurol.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 87.Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM, et al. Structural brain changes in migraine. JAMA : the journal of the American Medical Association. 2012;308(18):1889–97. doi: 10.1001/jama.2012.14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–17. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 89.Xue T, Yuan K, Cheng P, Zhao L, Zhao L, Yu D, et al. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR in biomedicine. 2013;26(9):1051–8. doi: 10.1002/nbm.2917. [DOI] [PubMed] [Google Scholar]
- 90.Maleki N, Linnman C, Brawn J, Burstein R, Becerra L, Borsook D. Her versus his migraine: multiple sex differences in brain function and structure. Brain : a journal of neurology. 2012;135(Pt 8):2546–59. doi: 10.1093/brain/aws175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guidetti V, Lucchese F, Bellini B. Is the migrainous female brain different? Some new evidence. Brain : a journal of neurology. 2012;135(Pt 8):2311–3. doi: 10.1093/brain/aws191. [DOI] [PubMed] [Google Scholar]
- 92.van Wingen GA, Ossewaarde L, Backstrom T, Hermans EJ, Fernandez G. Gonadal hormone regulation of the emotion circuitry in humans. Neuroscience. 2011;191:38–45. doi: 10.1016/j.neuroscience.2011.04.042. [DOI] [PubMed] [Google Scholar]
- 93.Mavromichalis I, Anagnostopoulos D, Metaxas N, Papanastassiou E. Prevalence of migraine in schoolchildren and some clinical comparisons between migraine with and without aura. Headache. 1999;39(10):728–36. doi: 10.1046/j.1526-4610.1999.3910728.x. [DOI] [PubMed] [Google Scholar]
- 94.Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA : the journal of the American Medical Association. 2006;295(15):1824–30. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- 95.Karli N, Baykan B, Ertas M, Zarifoglu M, Siva A, Saip S, et al. Impact of sex hormonal changes on tension-type headache and migraine: a cross-sectional population-based survey in 2,600 women. The journal of headache and pain. 2012;13(7):557–65. doi: 10.1007/s10194-012-0475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Epstein MT, Hockaday JM, Hockaday TD. Migraine and reporoductive hormones throughout the menstrual cycle. Lancet. 1975;1(7906):543–8. doi: 10.1016/s0140-6736(75)91558-5. [DOI] [PubMed] [Google Scholar]
- 97.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annual review of physiology. 2013;75:365–91. doi: 10.1146/annurev-physiol-030212-183717. [DOI] [PubMed] [Google Scholar]
- 98.Welch KM, Brandes JL, Berman NE. Mismatch in how oestrogen modulates molecular and neuronal function may explain menstrual migraine. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2006;27(Suppl 2):S190–2. doi: 10.1007/s10072-006-0599-6. [DOI] [PubMed] [Google Scholar]
- 99.Schurks M, Rist PM, Kurth T. Sex hormone receptor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia : an international journal of headache. 2010;30(11):1306–28. doi: 10.1177/0333102410364155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ghosh J, Joshi G, Pradhan S, Mittal B. Potential role of aromatase over estrogen receptor gene polymorphisms in migraine susceptibility: a case control study from North India. PloS one. 2012;7(4):e34828. doi: 10.1371/journal.pone.0034828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gervil M, Ulrich V, Kaprio J, Olesen J, Russell MB. The relative role of genetic and environmental factors in migraine without aura. Neurology. 1999;53(5):995–9. doi: 10.1212/wnl.53.5.995. [DOI] [PubMed] [Google Scholar]
- 102.Baroncini M, Jissendi P, Catteau-Jonard S, Dewailly D, Pruvo JP, Francke JP, et al. Sex steroid hormones-related structural plasticity in the human hypothalamus. NeuroImage. 2010;50(2):428–33. doi: 10.1016/j.neuroimage.2009.11.074. [DOI] [PubMed] [Google Scholar]
- 103.Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. 2007;47(10):1418–26. doi: 10.1111/j.1526-4610.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 104.Geraud G, Donnet A. Migraine and hypothalamus. Revue neurologique. 2013;169(5):372–9. doi: 10.1016/j.neurol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Cochran SD, Mays VM. Physical health complaints among lesbians, gay men, and bisexual and homosexually experienced heterosexual individuals: results from the California Quality of Life Survey. American journal of public health. 2007;97(11):2048–55. doi: 10.2105/AJPH.2006.087254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loraine JA, Ismail AA, Adamopoulos DA, Dove GA. Endocrine function in male and female homosexuals. British medical journal. 1970;4(5732):406–9. [PMC free article] [PubMed] [Google Scholar]
- 107.Swaab DF, Hofman MA. An enlarged suprachiasmatic nucleus in homosexual men. Brain research. 1990;537(1-2):141–8. doi: 10.1016/0006-8993(90)90350-k. [DOI] [PubMed] [Google Scholar]
- 108.LeVay S. A difference in hypothalamic structure between heterosexual and homosexual men. Science (New York, NY. 1991;253(5023):1034–7. doi: 10.1126/science.1887219. [DOI] [PubMed] [Google Scholar]
- 109.Smith JT. Sex steroid regulation of kisspeptin circuits. Advances in experimental medicine and biology. 2013;784:275–95. doi: 10.1007/978-1-4614-6199-9_13. [DOI] [PubMed] [Google Scholar]
- 110.Marshall JC, Eagleson CA. Neuroendocrine aspects of polycystic ovary syndrome. Endocrinology and metabolism clinics of North America. 1999;28(2):295–324. doi: 10.1016/s0889-8529(05)70071-2. [DOI] [PubMed] [Google Scholar]
- 111.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67(12):2154–8. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 112.Gupta S, Villalon CM, Mehrotra S, de Vries R, Garrelds IM, Saxena PR, et al. Female sex hormones and rat dural vasodilatation to CGRP, periarterial electrical stimulation and capsaicin. Headache. 2007;47(2):225–35. doi: 10.1111/j.1526-4610.2006.00526.x. [DOI] [PubMed] [Google Scholar]
- 113.Boes T, Levy D. Influence of sex, estrous cycle, and estrogen on intracranial dural mast cells. Cephalalgia : an international journal of headache. 2012;32(12):924–31. doi: 10.1177/0333102412454947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rowan MP, Berg KA, Milam SB, Jeske NA, Roberts JL, Hargreaves KM, et al. 17beta-estradiol rapidly enhances bradykinin signaling in primary sensory neurons in vitro and in vivo. The Journal of pharmacology and experimental therapeutics. 2010;335(1):190–6. doi: 10.1124/jpet.110.167445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liverman CS, Brown JW, Sandhir R, Klein RM, McCarson K, Berman NE. Oestrogen increases nociception through ERK activation in the trigeminal ganglion: evidence for a peripheral mechanism of allodynia. Cephalalgia : an international journal of headache. 2009;29(5):520–31. doi: 10.1111/j.1468-2982.2008.01755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu LH, Li N, Liu CY, Ma B. Estrogen altered facial mechanical pain threshold and trigeminal P2X3 receptor expression. Neuro endocrinology letters. 2011;32(6):811–5. [PubMed] [Google Scholar]
- 117.Martin VT, Lee J, Behbehani MM. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache. 2007;47(4):552–63. doi: 10.1111/j.1526-4610.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 118.Amandusson A, Blomqvist A. Estrogen receptor-alpha expression in nociceptive-responsive neurons in the medullary dorsal horn of the female rat. European journal of pain. 2010;14(3):245–8. doi: 10.1016/j.ejpain.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 119.Puri J, Bellinger LL, Kramer PR. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. Journal of cellular physiology. 2011;226(12):3169–80. doi: 10.1002/jcp.22671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Reed WR, Chadha HK, Hubscher CH. Effects of 17beta-estradiol on responses of viscerosomatic convergent thalamic neurons in the ovariectomized female rat. Journal of neurophysiology. 2009;102(2):1062–74. doi: 10.1152/jn.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eikermann-Haerter K, Kudo C, Moskowitz MA. Cortical spreading depression and estrogen. Headache. 2007;47(Suppl 2):S79–85. doi: 10.1111/j.1526-4610.2007.00818.x. [DOI] [PubMed] [Google Scholar]
- 122.Blurton-Jones MM, Roberts JA, Tuszynski MH. Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. The Journal of comparative neurology. 1999;405(4):529–42. [PubMed] [Google Scholar]
- 123.Fenzi F, Rizzzuto N. Estrogen receptors localization in the spinal trigeminal nucleus: an immunohistochemical study in humans. European journal of pain. 2011;15(10):1002–7. doi: 10.1016/j.ejpain.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 124.Vanderhorst VG, Terasawa E, Ralston HJ., 3rd Estrogen receptor-alpha immunoreactive neurons in the brainstem and spinal cord of the female rhesus monkey: species-specific characteristics. Neuroscience. 2009;158(2):798–810. doi: 10.1016/j.neuroscience.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gupta S, McCarson KE, Welch KM, Berman NE. Mechanisms of pain modulation by sex hormones in migraine. Headache. 2011;51(6):905–22. doi: 10.1111/j.1526-4610.2011.01908.x. [DOI] [PubMed] [Google Scholar]
- 126.Barbosa Mde B, Guirro EC, Nunes FR. Evaluation of sensitivity, motor and pain thresholds across the menstrual cycle through medium-frequency transcutaneous electrical nerve stimulation. Clinics. 2013;68(7):901–8. doi: 10.6061/clinics/2013(07)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Iacovides S, Baker FC, Avidon I, Bentley A. Women with dysmenorrhea are hypersensitive to experimental deep muscle pain across the menstrual cycle. The journal of pain : official journal of the American Pain Society. 2013;14(10):1066–76. doi: 10.1016/j.jpain.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 128.Teepker M, Peters M, Vedder H, Schepelmann K, Lautenbacher S. Menstrual variation in experimental pain: correlation with gonadal hormones. Neuropsychobiology. 2010;61(3):131–40. doi: 10.1159/000279303. [DOI] [PubMed] [Google Scholar]
- 129.Pohjalainen T, Rinne JO, Nagren K, Syvalahti E, Hietala J. Sex differences in the striatal dopamine D2 receptor binding characteristics in vivo. The American journal of psychiatry. 1998;155(6):768–73. doi: 10.1176/ajp.155.6.768. [DOI] [PubMed] [Google Scholar]
- 130.Laakso A, Vilkman H, Bergman J, Haaparanta M, Solin O, Syvalahti E, et al. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biological psychiatry. 2002;52(7):759–63. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- 131.Parsey RV, Oquendo MA, Simpson NR, Ogden RT, Van Heertum R, Arango V, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain research. 2002;954(2):173–82. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 132.Engman J, Ahs F, Furmark T, Linnman C, Pissiota A, Appel L, et al. Age, sex and NK1 receptors in the human brain -- a positron emission tomography study with [(1)(1)C]GR205171. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2012;22(8):562–8. doi: 10.1016/j.euroneuro.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 133.Nyman MJ, Eskola O, Kajander J, Vahlberg T, Sanabria S, Burns D, et al. Gender and age affect NK1 receptors in the human brain - a positron emission tomography study with [18F]SPA-RQ. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2007;10(2):219–29. doi: 10.1017/S1461145706006572. [DOI] [PubMed] [Google Scholar]
- 134.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. The American journal of psychiatry. 1999;156(6):842–8. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 135.Palomero-Gallagher N, Bidmon HJ, Zilles K. AMPA, kainate, and NMDA receptor densities in the hippocampus of untreated male rats and females in estrus and diestrus. The Journal of comparative neurology. 2003;459(4):468–74. doi: 10.1002/cne.10638. [DOI] [PubMed] [Google Scholar]
- 136.Johnson AE, Nock B, McEwen BS, Feder HH. Alpha 1- and alpha 2-noradrenergic receptor binding in guinea pig brain: sex differences and effects of ovarian steroids. Brain research. 1988;442(2):205–13. doi: 10.1016/0006-8993(88)91505-3. [DOI] [PubMed] [Google Scholar]
- 137.Dominguez CA, Kouya PF, Wu WP, Hao JX, Xu XJ, Wiesenfeld-Hallin Z. Sex differences in the development of localized and spread mechanical hypersensitivity in rats after injury to the infraorbital or sciatic nerves to create a model for neuropathic pain. Gender medicine. 2009;6(Suppl 2):225–34. doi: 10.1016/j.genm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Maleki N, Becerra L, Borsook D. Migraine: maladaptive brain responses to stress. Headache. 2012;52(Suppl 2):102–6. doi: 10.1111/j.1526-4610.2012.02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Robert C, Bourgeais L, Arreto CD, Condes-Lara M, Noseda R, Jay T, et al. Paraventricular hypothalamic regulation of trigeminovascular mechanisms involved in headaches. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(20):8827–40. doi: 10.1523/JNEUROSCI.0439-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Streitburger DP, Moller HE, Tittgemeyer M, Hund-Georgiadis M, Schroeter ML, Mueller K. Investigating structural brain changes of dehydration using voxel-based morphometry. PloS one. 2012;7(8):e44195. doi: 10.1371/journal.pone.0044195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology. 1975;25(3):239–44. doi: 10.1212/wnl.25.3.239. [DOI] [PubMed] [Google Scholar]
- 142.Kelly MJ, Ronnekleiv OK. Control of CNS neuronal excitability by estrogens via membrane-initiated signaling. Molecular and cellular endocrinology. 2009;308(1-2):17–25. doi: 10.1016/j.mce.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee AW, Kyrozis A, Chevaleyre V, Kow LM, Devidze N, Zhang Q, et al. Estradiol modulation of phenylephrine-induced excitatory responses in ventromedial hypothalamic neurons of female rats. Proc Natl Acad Sci U S A. 2008;105(20):7333–8. doi: 10.1073/pnas.0802760105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zadran S, Qin Q, Bi X, Zadran H, Kim Y, Foy MR, et al. 17-Beta-estradiol increases neuronal excitability through MAP kinase-induced calpain activation. Proc Natl Acad Sci U S A. 2009;106(51):21936–41. doi: 10.1073/pnas.0912558106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loyd DR, Murphy AZ. Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. Journal of chemical neuroanatomy. 2008;36(3-4):216–26. doi: 10.1016/j.jchemneu.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Macgregor EA, Hackshaw A. Prevention of migraine in the pill-free interval of combined oral contraceptives: a double-blind, placebo-controlled pilot study using natural oestrogen supplements. The journal of family planning and reproductive health care / Faculty of Family Planning & Reproductive Health Care, Royal College of Obstetricians & Gynaecologists. 2002;28(1):27–31. doi: 10.1783/147118902101195974. [DOI] [PubMed] [Google Scholar]
- 147.Charles AC, Baca SM. Cortical spreading depression and migraine. Nature reviews Neurology. 2013 doi: 10.1038/nrneurol.2013.192. [DOI] [PubMed] [Google Scholar]
- 148.Nagel-Leiby S, Welch KM, Grunfeld S, D'Andrea G. Ovarian steroid levels in migraine with and without aura. Cephalalgia : an international journal of headache. 1990;10(3):147–52. doi: 10.1046/j.1468-2982.1990.1003147.x. [DOI] [PubMed] [Google Scholar]
- 149.MacGregor A. Migraine associated with menstruation. Functional neurology. 2000;15(Suppl 3):143–53. [PubMed] [Google Scholar]
- 150.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiological reviews. 1997;77(4):1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 151.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain research Brain research reviews. 2005;49(1):65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 152.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1-2):166–76. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilhelm M, Silver R, Silverman AJ. Central nervous system neurons acquire mast cell products via transgranulation. The European journal of neuroscience. 2005;22(9):2238–48. doi: 10.1111/j.1460-9568.2005.04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nautiyal KM, Dailey CA, Jahn JL, Rodriquez E, Son NH, Sweedler JV, et al. Serotonin of mast cell origin contributes to hippocampal function. The European journal of neuroscience. 2012;36(3):2347–59. doi: 10.1111/j.1460-9568.2012.08138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pang X, Letourneau R, Rozniecki JJ, Wang L, Theoharides TC. Definitive characterization of rat hypothalamic mast cells. Neuroscience. 1996;73(3):889–902. doi: 10.1016/0306-4522(95)00606-0. [DOI] [PubMed] [Google Scholar]
- 156.Gopinath A, Andrew Tseng L, Whitlock KE. Temporal and spatial expression of gonadotropin releasing hormone (GnRH) in the brain of developing zebrafish (Danio rerio) Gene expression patterns : GEP. 2004;4(1):65–70. doi: 10.1016/s1567-133x(03)00149-2. [DOI] [PubMed] [Google Scholar]
- 157.Check JH. The interrelationship of sleep, biologic clocks, neurotransmitters, gonadotropins and pubertal development. Clinical and experimental obstetrics & gynecology. 2013;40(1):7–14. [PubMed] [Google Scholar]
- 158.Uzar E, Evliyaoglu O, Yucel Y, Ugur Cevik M, Acar A, Guzel I, et al. Serum cytokine and pro-brain natriuretic peptide (BNP) levels in patients with migraine. European review for medical and pharmacological sciences. 2011;15(10):1111–6. [PubMed] [Google Scholar]
- 159.Franceschini A, Vilotti S, Ferrari MD, van den Maagdenberg AM, Nistri A, Fabbretti E. TNFalpha levels and macrophages expression reflect an inflammatory potential of trigeminal ganglia in a mouse model of familial hemiplegic migraine. PloS one. 2013;8(1):e52394. doi: 10.1371/journal.pone.0052394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Evans J, Salamonsen LA. Inflammation, leukocytes and menstruation. Reviews in endocrine & metabolic disorders. 2012;13(4):277–88. doi: 10.1007/s11154-012-9223-7. [DOI] [PubMed] [Google Scholar]
- 161.Guyon A, Massa F, Rovere C, Nahon JL. How cytokines can influence the brain: a role for chemokines? Journal of neuroimmunology. 2008;198(1-2):46–55. doi: 10.1016/j.jneuroim.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 162.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairments in long-term potentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(8):2974–81. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, et al. Exposure to acute stress induces brain interleukin-1beta protein in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(6):2239–46. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hanisch UK. Microglia as a source and target of cytokines. Glia. 2002;40(2):140–55. doi: 10.1002/glia.10161. [DOI] [PubMed] [Google Scholar]
- 165.Mor G, Nilsen J, Horvath T, Bechmann I, Brown S, Garcia-Segura LM, et al. Estrogen and microglia: A regulatory system that affects the brain. Journal of neurobiology. 1999;40(4):484–96. doi: 10.1002/(sici)1097-4695(19990915)40:4<484::aid-neu6>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 166.Grinberg YY, Milton JG, Kraig RP. Spreading depression sends microglia on Levy flights. PloS one. 2011;6(4):e19294. doi: 10.1371/journal.pone.0019294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rains JC. Optimizing circadian cycles and behavioral insomnia treatment in migraine. Current pain and headache reports. 2008;12(3):213–9. doi: 10.1007/s11916-008-0037-y. [DOI] [PubMed] [Google Scholar]
- 168.Higashiyama M, Monden T, Ogawa M, Matsuura N, Murotani M, Kawasaki Y, et al. Immunohistochemical study on pancreatic secretory trypsin inhibitor (PSTI) in gastric carcinomas. American journal of clinical pathology. 1990;93(1):8–13. doi: 10.1093/ajcp/93.1.8. [DOI] [PubMed] [Google Scholar]
- 169.Niederberger U, Gerber WD, Schiffer N. Sleeping behavior and migraine. An evaluation by daily self-reports. Schmerz. 1998;12(6):389–95. doi: 10.1007/s004829800038. [DOI] [PubMed] [Google Scholar]
- 170.Klingman KM, Marsh EE, Klerman EB, Anderson EJ, Hall JE. Absence of circadian rhythms of gonadotropin secretion in women. The Journal of clinical endocrinology and metabolism. 2011;96(5):1456–61. doi: 10.1210/jc.2010-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Brennan KC, Bates EA, Shapiro RE, Zyuzin J, Hallows WC, Huang Y, et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Science translational medicine. 2013;5(183):183ra56, 1–11. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacological reviews. 2006;58(1):46–57. doi: 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- 173.Ordas CM, Cuadrado ML, Rodriguez-Cambron AB, Casas-Limon J, del Prado N, Porta-Etessam J. Increase in body temperature during migraine attacks. Pain medicine. 2013;14(8):1260–4. doi: 10.1111/pme.12145. [DOI] [PubMed] [Google Scholar]
- 174.Rettberg JR, Yao J, Brinton RD. Estrogen: A master regulator of bioenergetic systems in the brain and body. Frontiers in neuroendocrinology. 2013 doi: 10.1016/j.yfrne.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain research. 2010;1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fuente-Martin E, Garcia-Caceres C, Morselli E, Clegg DJ, Chowen JA, Finan B, et al. Estrogen, astrocytes and the neuroendocrine control of metabolism. Reviews in endocrine & metabolic disorders. 2013 doi: 10.1007/s11154-013-9263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bigal ME, Lipton RB, Holland PR, Goadsby PJ. Obesity, migraine, and chronic migraine: possible mechanisms of interaction. Neurology. 2007;68(21):1851–61. doi: 10.1212/01.wnl.0000262045.11646.b1. [DOI] [PubMed] [Google Scholar]
- 178.Peterlin BL, Rapoport AM, Kurth T. Migraine and obesity: epidemiology, mechanisms, and implications. Headache. 2010;50(4):631–48. doi: 10.1111/j.1526-4610.2009.01554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Verrotti A, Di Fonzo A, Agostinelli S, Coppola G, Margiotta M, Parisi P. Obese children suffer more often from migraine. Acta paediatrica. 2012;101(9):e416–21. doi: 10.1111/j.1651-2227.2012.02768.x. [DOI] [PubMed] [Google Scholar]
- 180.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22(2):108–24. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 181.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiology of aging. 2002;23(5):921–39. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 182.Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache. 2008;48(8):1186–93. doi: 10.1111/j.1526-4610.2008.01176.x. [DOI] [PubMed] [Google Scholar]
- 183.Cseh A, Farkas KM, Derzbach L, Muller K, Vasarhelyi B, Szalay B, et al. Lymphocyte subsets in pediatric migraine. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2013;34(7):1151–5. doi: 10.1007/s10072-012-1218-3. [DOI] [PubMed] [Google Scholar]
- 184.Guzel I, Tasdemir N, Celik Y. Evaluation of serum transforming growth factor beta1 and C-reactive protein levels in migraine patients. Neurologia i neurochirurgia polska. 2013;47(4):357–62. doi: 10.5114/ninp.2013.36760. [DOI] [PubMed] [Google Scholar]
- 185.Oikari LE, Stuart S, Okolicsanyi RK, Cox HC, Dixit S, Lea RA, et al. Investigation of lymphotoxin alpha genetic variants in migraine. Gene. 2013;512(2):527–31. doi: 10.1016/j.gene.2012.09.116. [DOI] [PubMed] [Google Scholar]
- 186.Greco R, Tassorelli C, Mangione AS, Smeraldi A, Allena M, Sandrini G, et al. Effect of sex and estrogens on neuronal activation in an animal model of migraine. Headache. 2013;53(2):288–96. doi: 10.1111/j.1526-4610.2012.02249.x. [DOI] [PubMed] [Google Scholar]
- 187.Rumberg B, Baars A, Fiebach J, Ladd ME, Forsting M, Senf W, et al. Cycle and gender-specific cerebral activation during a verb generation task using fMRI: comparison of women in different cycle phases, under oral contraception, and men. Neuroscience research. 2010;66(4):366–71. doi: 10.1016/j.neures.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 188.Mareckova K, Perrin JS, Nawaz Khan I, Lawrence C, Dickie E, McQuiggan DA, et al. Hormonal contraceptives, menstrual cycle and brain response to faces. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in neuroendocrinology. 2005;26(3-4):163–74. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 190.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature neuroscience. 2004;7(10):1040–7. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]


