Abstract
Previous studies show that children who are sensitive to the bitter taste of 6-n-propylthiouracil (PROP) report more frequent intake of sweets and less frequent intake of meats (savory fats) relative to children who are PROP insensitive. Laboratory studies are needed to confirm these findings. In this study, seventy-nine 4- to 6-year-olds from diverse ethnicities attended four laboratory sessions, the last of which included a palatable buffet consisting of savory-fats (e.g. pizza), sweet-fats (e.g. cookies, cakes), and sweets (e.g. juices, candies). PROP phenotype was classified by two methods: 1) a common screening procedure to divide children into tasters and nontasters, and 2) a three-concentration method used to approximate PROP thresholds. Height and weight were measured and saliva was collected for genotyping TAS2R38, a bitter taste receptor related to the PROP phenotype. Data were analyzed by General Linear Model ANOVA with intake from savory fats, sweet-fats, and sweets as dependent variables and PROP status as the independent variable. BMI z-score, sex, age, and ethnicity were included as covariates. Adjusted energy intake from the food group “sweets” at the test-meal was greater for tasters than for nontasters. PROP status did not influence children’s adjusted intake of savory-fats, but BMI z-score did. The TAS2R38 genotype did not impact intake at the test-meal. At a palatable buffet, PROP taster children preferentially consumed more sweets than nontaster children, while heavier children consumed more savory fats. These findings may have implications for understanding differences in susceptibility to hyperphagia.
Keywords: Bitter taste, PROP phenotype, Eating behavior, Children, Obesity
Introduction
Although recent trends show that the prevalence rates of childhood obesity may be reaching a plateau, the percentage of children in the United States who are overweight or obese is still a public health concern (Ogden, Kit, & Flegal, 2012). One reason for the continued high rates of this disease is diet. Recent surveys show high discretionary fat and added sugar intake among children, with the most common foods in 2- to 18-year-olds reported as grain based desserts, pizza, and soda (Reedy & Krebs-Smith, 2010). Excess consumption of added sugars in adolescents is associated with increased blood lipid abnormalities (Welsh, Sharma, Cunningham, & Vos, 2011), and reducing the intake of added sugars can improve metabolic function (Davis et al., 2007). Developing strategies to curb these dietary trends in childhood is important because dietary patterns learned during this time track into adulthood (Mikkila, Rasanen, Raitakari, Pietinen, & Viikari, 2005; Nicklaus, Boggio, Chabanet, & Issanchou, 2005) where they can contribute to lifelong metabolic problems.
One of the primary reasons for excess intake of added sugars and discretionary fats among children is taste. Children tend to eat what they like (Birch, 1979; Drewnowski, 1997), and one of the strongest factors driving liking in children is sweetness (Sullivan & Birch, 1990). Liking for sweet taste is innate across humans and present before birth (Mennella & Beauchamp, 1998). Sweet liking is also highest during childhood and decreases into adulthood (Desor & Beauchamp, 1987; Liem & de Graaf, 2004). Strong preferences for sweet foods during childhood have been suggested to function as cues to direct humans to sources of calories, particularly during times of growth (Coldwell, Oswald, & Reed, 2009; Drewnowski, 2000). However, the strong genetic and developmental drives toward sweet taste may be maladaptive if they predispose children to excess energy intake in an environment that is highly obesogenic. For this reason, characterizing genetic markers that can be used to identify children who are prone to overconsumption of sweets could lead to improved ability to target dietary interventions effectively.
One genetic marker that has previously shown evidence of a relationship to sweet liking is the inherited taste sensitivity to bitter thiourea compounds, like PROP and phenylthiocarbamide (PTC). The ability to taste PROP/PTC is a well-described phenotype related most closely to genetic variation at the TAS2R38 gene that encodes a bitter taste receptor (Kim et al., 2003). There are three polymorphisms in the TAS2R38 gene that contribute to two common haplotypes depending on the amino acids present at positions 49, 262, and 296. The AVI haplotype confers insensitivity to PTC/PROP while the PAV haplotype is responsive to PTC/PROP. In Europeans, the AVI haplotype occurs at a frequency of 0.47, while PAV occurs at a frequency of 0.49. In addition, there are rare haplotypes (e.g. AAI, PVI) that have also been found to contribute to intermediate PROP/PTC sensitivity (Bufe et al., 2005; Kim et al., 2003). With respect to phenotype, individuals can be classified as non-, medium or supertasters, depending on sensitivity to the bitter taste of these compounds (Bartoshuk, 1994). While PROP was used as a surrogate for PTC for many years, recent studies demonstrate that the ability to taste PROP might be due not only to TAS2R38, but also to variation at other gene(s) (Bufe et al., 2005; Hayes, Bartoshuk, Kidd, & Duffy, 2008). The gustin gene, which is involved with taste cell development and proliferation, is one possibility (Calo et al., 2011). Gustin is a trophic factor known to play a role in early development of taste cells. Padiglia and colleagues reported that a polymorphism in the gustin gene at rs2274333 leads to a modification in the protein that is necessary for full functionality. Supertasters are more likely than nontasters to have the fully functional form of this protein (Padiglia et al., 2010). This, along with increased fungiform papillae density (Delwiche, Buletic, & Breslin, 2001), may partly explain why PROP tasters have higher responsiveness to a wide range of oral tastes and textures than nontasters [for review see (Tepper, 2008)].
With respect to the relationship between liking of sweets and PROP status, studies in adults and children have produced conflicting evidence. In some studies with adults, PROP tasters were more likely to be sweet dislikers than nontasters (Looy & Weingarten, 1992; Peterson, Bartoshuk, & Duffy, 1999). However, in a follow-up study, Duffy and Bartoshuk reported that liking of sweet-fats, fruits, and “natural sweets” (e.g. banana, honey) was inversely related to PROP intensity rating in women, but the opposite was found in men, suggesting that other covariates might moderate this relationship (Duffy & Bartoshuk, 2000). In children, Looy and Weingarten found similar relationships between PROP status and sweet liking in children as they found in adults (Looy & Weingarten, 1992). However, Mennella and colleagues found that children who had bitter sensitive genotypes (tasters) actually preferred sweeter concentrations of sucrose and more sugary cereals compared with children who were bitter insensitive (nontasters) (Mennella, Pepino, & Reed, 2005). In addition, Keller and Tepper (Keller & Tepper, 2004) showed that pre-school children who were PROP tasters reported greater intake of sweet snacks (e.g. cookies, candy, soda) than children who were nontasters. Still other studies that have investigated reported daily food intake in 7- to 13-year-old children have shown no relationship between PROP phenotype and intake of sweets (Obrien, Feeney, Scannell, Markey, & Gibney, 2013). The discrepancies in this area warrant additional investigation, particularly with studies collecting direct observations of eating behaviors. Because sweet preferences in children are higher than in adults (Mennella, Lukasewycz, Griffith, & Beauchamp, 2011), it is possible that the relationships between sweet preference and PROP status might vary across development as well. In addition, because sweet concentration is positively related to sweet liking, even at very high sweet concentration (de Graaf & Zandstra, 1999; Zandstra & de Graaf, 1998), it is conceivable that a heightened perception of sweetness in childhood might be related to increased liking and intake of sweetened foods during this period. No studies have actually assessed children’s intake of sweetened foods under controlled laboratory conditions to determine associations with PROP status. Furthermore, because of the amount of conflicting information across studies, additional investigation is warranted.
In addition to proposed differences in the liking and reported intake of sweets, several studies have also identified relationships between PROP status and perception and liking of fat. Tepper and Nurse originally reported that nontasters had lower ability to discriminate differences between high- and low-fat salad dressings compared with medium and super-tasters (Tepper & Nurse, 1997), but despite this, nontasters preferred the high-fat dressing (Tepper & Nurse, 1998). In addition, Hayes and Duffy reported that nontasters preferred higher concentrations of both fat and sweet compared with tasters to achieve maximal liking in sweetened dairy products (Hayes & Duffy, 2008). Follow-up studies from our lab (Keller, 2012; Liang et al., 2012) and Stewart and colleagues (2010, 2011) have reported that both less ability to discriminate fat (Liang et al., 2012) and less oral response to fatty acids (Stewart et al., 2010, 2011) are associated with greater fat preference, intake, and increased body weight. Therefore, it is possible that decreased fat discrimination could be a mediator between the inverse association reported between PROP sensitivity and body weight status reported in some studies (Keller et al., 2010; Keller & Tepper, 2004; Tepper, 1999; Tepper et al., 2008; Tepper & Ullrich, 2002). The mechanisms underlying the relationships between PROP status, fat discrimination, fat intake and body weight are still undetermined, but follow-up investigations in children should provide additional information.
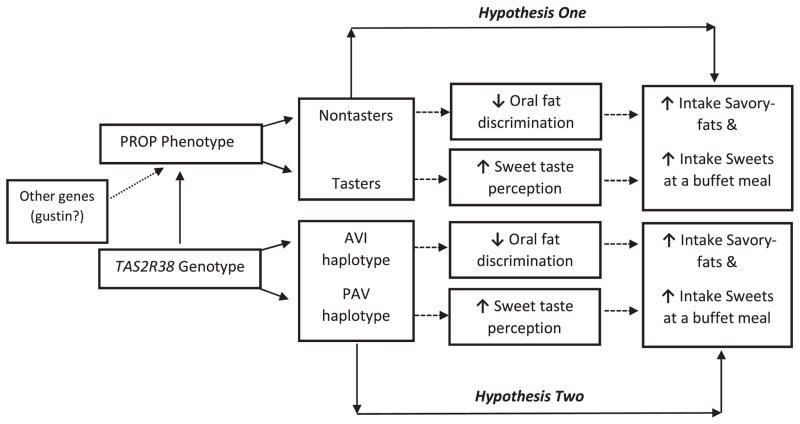
The objective of this study was to follow up on findings originally reported by Keller and Tepper (2004) using parentally reported questionnaires that found that taster children consumed more sweets, while nontaster children consumed more savory fats (e.g. meats, cheeses, etc.). First, we tested children’s intake at an ad libitum palatable buffet containing sweets, sweet-fats, and savory fats to determine whether taster and nontaster children would show different intake patterns. We predicted (Hypotheses One in Fig. 1) that taster children would consume more energy from sweets, while nontaster children would consume more savory-fats. Second, we genotyped children at TAS2R38 to determine the relationship between PTC taste receptor genotype and children’s intake at the buffet meal. We predicted (Hypothesis Two in Fig. 1) that children with bitter sensitive genotypes would also consume more sweets and less savory-fats at the meal than children with bitter insensitive genotypes.
Fig. 1.
Diagram of the study hypotheses. The first hypothesis tests the relationship between PROP status and intake of sweets and savory-fats at a palatable buffet meal. It is predicted that nontaster children will have increased intake of savory-fats in part due to decreased oral fat discrimination (not measured). It is also predicted that taster children will have increased intake of sweets at the meal, in part mediated by increased sweet taste perception (not measured). The second hypothesis tests the relationship between TAS2R38 genotype and intake of sweets and savory-fats. We predict that children with the AVI (nontaster) genotype will consume more savory-fats at the meal, while children with the PAV (taster) genotype will consume more sweets at the meal.
Methods
Participants
Children (n = 79) enrolled in this study were between 4 and 6 years old (mean ± SD = 5.04 ± 0.78). Parents self-reported the ethnicity of their children as African-American (42.5%), Hispanic/Latino (31.3%), Caucasian (12.5%), Asian (2.5%) or “other” (11.3%). Approximately 40% of the children were boys. Average BMI z-score for children was 1.00 ± 1.02, corresponding to the 85th BMI-forage percentile. This study was approved by the Institutional Review Board of St. Luke’s Roosevelt Hospital Center. Parents consented to allow their children to participate.
Study design
This study consisted of four, 1-hour sessions that took place at dinner, between 4:00 pm and 6:00 pm, and a fifth session where body composition was measured using dual-energy X-ray absorptiometry (DXA). The overall purpose of the study was to investigate the relationship between taste genetics and children’s eating behavior, measured by a series of laboratory test-meals. The first test session included a standard ad libitum meal, along with a series of taste and food preference assessments. The test-meals at visits two and three were high- or low-fat versions of the same foods. The full details of these visits are reported in two recent studies (Keller, Olsen, Kuilema, Meyermann, & Belle, 2013; Olsen, van Belle, Meyermann, & Keller, 2011). The last meal was designed to elicit excess consumption by offering children a range of palatable, energy-dense foods including sweets, sweet-fats, or savory-fats. The foods served and portion sizes provided at the last meal are listed in Table 1. If children finished a portion of food, they were offered additional servings.
Table 1.
Foods served and serving sizes provided at the buffet test-meal.
| Food | Serving size | Sugar content per 100 (g) or 100 mL for beverages | % kcals from carbohydrate | % kcals from fat | % kcals from protein |
|---|---|---|---|---|---|
| Savory-fats | |||||
| Cheese bagel bites | 4 pieces (~145 g) | 3.4 | 65 | 21 | 14 |
| Cheese pizza rolls | 4 pieces (~80 g) | 3.4 | 50 | 36 | 14 |
| Chicken nuggets | 5 nuggets (~105 g) | 1.0 | 55 | 45 | 26 |
| Mozzarella sticks | 4 sticks (~100 g) | 1.0 | 35 | 45 | 15 |
| Potato chips | 28 g | 0 | 39 | 60 | 1 |
| Sweet-fats | |||||
| Chocolate chip cookies | 3 cookies (~35 g) | 32.3 | 54 | 45 | 1 |
| Mini-brownies | 4 mini-brownies (~60 g) | 41.7 | 50 | 47 | 3 |
| Chocolate cupcakes | 2 mini-cupcakes (~50 g) | 46.8 | 64 | 33 | 3 |
| Donut holes | 4 holes (~50 g) | 36.5 | 57 | 39 | 4 |
| Whole-fat chocolate milk | 245 g (1 cup) | 10a | 60 | 20 | 20 |
| Sweets | |||||
| Red licorice | 4 pieces (~50 g) | 38 | 94 | 3 | 3 |
| Fruit leather | 1 package (~20 g) | 45 | 90 | 10 | 0 |
| Gummy candies | 42 g | 45 | 92 | 0 | 8 |
| Fruit candies | 42 g | 35 | 90 | 10 | 0 |
| Fruit punch | 235 g (1 cup) | 8a | 100 | 0 | 0 |
Sugar content of beverages is calculated based on a 100 mL serving. All other foods are calculated based on a 100 g serving.
Measurement of covariates
On the first visit, parents completed questionnaires to assess demographics, income, ethnicity, and dietary habits for themselves and their children. Anthropometric measures (weight and height) were performed by a trained researcher. Children were weighed and measured in stocking feet and light clothing on a standard balance scale and stadiometer, respectively. Height and weight were converted to BMI kg/m2 and BMI z-scores were calculated using The Centers for Disease Control and Prevention conversion program (Cole, Bellizzi, Flegal, & Dietz, 2000).
PROP taster status
We used similar procedures to those outlined by Mennella et al. (2005) to classify PROP taster status. In brief, children were given 10 mL samples of PROP in distilled water at concentrations of 56 μmol/L, 180 μmol/L, and 560 μmol/L in ascending order of concentration. Children were instructed to “sip and spit” the solution (described as a “drink” to the child) and give it to Big Bird (a well-known character from the show Sesame Street ©) if it tasted like “water” or “nothing.” If the drink tasted “bad,” “yucky,” or “bitter,” children were instructed to give it to Oscar the Grouch (another Sesame Street © character) so he could throw it in his trash can. Once children successfully detected PROP in a sample, they did not continue tasting the additional samples. Based on the child’s ability to taste the 56 μmol solution (a common screening solution used in previous studies) (Burd, Senerat, Chambers, & Keller, 2013; Keller et al., 2010; Keller, Steinmann, Nurse, & Tepper, 2002; Keller & Tepper, 2004), we classified children into “tasters” and “nontasters” for the primary study analyses. In addition, we performed exploratory analyses based on a three-group PROP classification to determine if there was a dose–response relationship between PROP sensitivity and intake of sweets and savory-fats at the meal. Based on the lowest concentration when children first detected PROP, children were classified into three groups: 1) children who first detected PROP at the lowest concentration, 2) children who first detected PROP at the medium concentration, or 3) children who first detected PROP at the highest concentration or who never detected PROP in any of the solutions.
DNA collection and genotyping
Genotyping was performed at three polymorphic sites in the TAS2R38 gene: rs713598 (A49P), rs1726866 (V262A), and rs10246939 (I296V). Saliva samples of approximately 4 mL total volume were collected and purified according to manufacturer’s instructions with Oragene DNA Self Collection Kits (DNA Genotek Inc, Ontario, Canada). In cases where it was difficult to get a child to produce enough saliva, the researcher encouraged them by racing to see who could “fill their container” first. In some cases, sugarless gum was provided to the child to enhance the flow of saliva. Extracted DNA products were quantified via UV spectrophotometry and run on a 2% agarose gel to ensure successful purification.
PCR was performed to amplify DNA fragments in 20 μl reaction volumes with 100 ng genomic DNA, 1 × reaction buffer (Boehringer Mannheim, Mannheim, Germany) containing [MgCl2] 1.5 mmol/1, 0.25 mmol/1 each dNTP, 100 ng of each PCR primer (available upon request), and 1 U Taq polymerase. All thermocycling was performed with 35 cycles of denaturation for 94°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s. Amplicons of ~200 base pair were amplified from genomic DNA using a biotin labeled primer (available upon request) and subsequently purified using streptavidin beads (Amersham Biosciences, Uppsala, Sweden). Genotyping of A49P, V262A, and I296V was performed by pryosequencing according to the manufacturer’s recommended protocol (PSQ96 Biotage, Westborough, MA).
Although there are three variant sites in TAS2R38, they are in strong linkage disequilibrium (Kim et al., 2003) so analyses were based upon results at the A49P site for ease of comparing to groupings used by Mennella et al. (2005). The cohort of children tested was ethnically diverse and rare haplotypes are known to occur more frequently in children of African descent (Campbell et al., 2011). To ensure that the incidence of rare haplotypes in the sample was not impacting the reported relationships, we performed the primary study analyses in children who had rare haplotypes separately from those who did not. The relationships between PTC genotype, PROP phenotype, and intake of sweets, sweet-fats, and savory-fats at the test-meal was similar in both groups of children. For this reason, we chose to report data as a function of children’s genotype at A49P only. Children who were homozygous for the bitter-insensitive allele at A49P were classified throughout as AA, those who were heterozygous for the bitter insensitive allele were classified as AP, and those who were homozygous for the bitter sensitive allele were classified as PP. However, in the present study, we combined the AP and PP groups for the primary analyses because these genotypes are most likely to be bitter sensitive or “tasters.” In addition, exploratory analyses were done treating each genotype separately and the AP and PP groups did not differ by main outcome variables, so we combined these groups for the present analyses.
Test-meal procedures
Children attended each test session at least 2 hours fasted, with a parent or guardian, in most cases, the mother. The first 30 min of each test-session were used to acclimate the child to the laboratory and complete taste and food preference assessments that have been reported elsewhere (Keller et al., 2013; Olsen et al., 2011). Test-meals were completed during the last half of each session. The purpose of this meal was to assess children’s susceptibility to overeating highly palatable foods that were sweet, sweet-fat, or savory-fat in predominant flavor quality. All foods and beverages at the meals were served without brand packaging, in plain plastic containers, and were prepared prior to the visit. Prior to the meal, children were given explicit instructions on the kinds of foods available and were told they could eat as much or as little as they wanted. Children were also told ahead of time that they had 30 min to eat and could ask for additional servings of anything provided. During the actual meal, a researcher was seated at the table to assist the child and to provide additional servings if needed. The researcher read a nonfood related book to the child while they ate. This provided a consistent distraction across all children and avoided the unrealistic situation of having the child eat alone in the laboratory. Parents were seated in an adjacent waiting room, unable to hear or see the child.
Items served at the test-meal were selected because they were highly palatable and familiar to most children this age. Prior to the experiment, the foods were divided into three categories: sweets (e.g. red licorice, gummies, and sweetened beverages), sweet-fats (e.g. cookies, brownies, doughnuts), and savory-fats (e.g. pizza, mozzarella sticks, chips). Foods in the savory-fat or sweet-fat category had either savory or sweet as their predominant flavor characteristic and contained 20% calories from fat or greater. Items in the sweet food category were primarily sweet tasting and contained less than 1 g of fat per serving. Items, serving sizes, and nutrient information are listed in Table 1.
Test-meal nutrient analysis
Total energy for each of the meals was computed as the difference between the pre- and post-weights of all items. Food label information was used to calculate total calories. Energy intakes from individual food items (e.g. chicken nuggets, candies) were summed to create intake of sweets, savory-fats, and sweet-fats for final analyses.
Statistical analysis
We determined means and standard deviations for continuous variables and frequencies for categorical variables. We computed Pearson’s correlations between continuous variables and nonparametric correlations between categorical variables.
The primary study aims were tested with Independent samples t-tests and General Linear Model Analysis of Variance Analysis (ANOVA) to determine the difference in children’s intake of sweets, sweet-fats, and savory-fats as a function of the predictor variables, PROP status and TAS2R38 genotype. Primary analyses were done with the two group PROP classification (taster versus nontaster). Exploratory analyses were done with the three-group PROP classification as predictor variables in order to explore the dose–response relationship between PROP sensitivity and test-meal intake. For the primary analyses, covariates were selected by identifying variables that were associated with the dependent variables at a cutoff of p ≤ 0.10 and by including other variables that were theoretically associated with the outcomes. Because of the strong association between children’s BMI z-score and total energy intake at the test-meal (r = 0.4; p < 0.001), child weight status (BMI z-score) was included in all final models. Child age and sex were also associated with some of the dependent test-meal variables, so these were included as covariates in all final models. In addition, ethnicity was associated with children’s intake at the test-meal, but only when dummy coded in the GLM model as “Caucasian versus other.” To account for any differences due to ethnic background, ethnicity (Caucasian versus other) was included in final GLM models. The results of all models are included after adjusting for covariates. When appropriate, Bonferroni tests were used to test multiple comparisons. SPSS version 20.0 (SPSS, Inc, Chicago, IL) was used for all analyses, all tests were two-tailed, and the cut-off for statistical significance was p < 0.05. All continuous variables are reported as means ± standard deviations (SD).
Results
Descriptive analyses
Out of 79 children, 21 (26.6%%) were overweight (BMI-for-age 85th–95th%) and 21 (26.6%) were obese (BMI-for-age ≥ 95th%). Twenty-nine percent (n = 23) of the children were PROP nontasters and 56 (71%) were tasters, a breakdown that agrees with previous studies in children (Anliker, Bartoshuk, Ferris, & Hooks, 1991; Keller et al., 2002, 2010; Keller & Tepper, 2004). Parents of the children ranged in BMI from 17.1 to 53.3 kg/m2, with mean ± SD equal to 29.0 ± 7.0 kg/m2. Approximately 32% of parents were overweight and an additional 36% were obese. Results from t-tests showed no differences in the participant characteristics reported in Table 2 as a function of PROP taster status.
Table 2.
Descriptive statistics as a function of PROP taster status.
| Categorical variablea | Nontasters (n = 23) | Taster (n = 56) | All children (n = 79) |
|---|---|---|---|
| Sex | % (n) | % (n) | % (n) |
| % Male | 34.8 (8) | 41.1 (23) | 39.2 (31) |
| % Female | 65.2 (15) | 58.9 (33) | 60.8 (48) |
| Ethnicity | |||
| % African-American | 47.8 (11) | 39.3 (22) | 41.8 (33) |
| % Caucasian | 8.8 (2) | 14.3 (8) | 12.7 (10) |
| % Hispanic/Latino | 21.7 (5) | 35.7 (20) | 31.6 (25) |
| % Asian | 0.0 (0) | 3.6 (2) | 2.5 (2) |
| % “Other” | 21.7 (5) | 7.1 (4) | 11.4 (9) |
| Family income | |||
| % ≤$20,000 per year | 30.4 (7) | 41.1 (23) | 38.0 (30) |
| % >$20,000 per year | 69.6 (16) | 58.9 (33) | 62.0 (49) |
| Continuous variable | Mean ± SD | Mean ± SD | Mean ± SD |
|---|---|---|---|
| Age (years) | 5.22 ± 0.74 | 5.00 ± 0.78 | 5.06 ± 0.77 |
| BMI z-score | 1.04 ± 1.16 | 1.04 ± 0.91 | 1.04 ± 0.98 |
There were no differences in the descriptive characteristics as a function of PROP phenotype.
TAS2R38 genotype was unable to be determined for three children due to failures of the test kits or inadequate saliva samples for a failure rate of 4%. Genotype at rs713598 is reported for 76 children. Sixteen (21.1%) of the children had the GG genotype at rs713598 (bitter insensitive homozygotes), 32 (42%) were CG (bitter sensitive heterozygotes), and 28 (37%) were CC (bitter sensitive homozygotes). The breakdown of PROP nontasters and tasters varied by PTC genotype according to Chi-square analysis (χ = 13.3; p < 0.001) (see Table 3). From herein, these groups will be referred to as AA, AP, and PP respectively. TAS2R38 genotype predicted PROP phenotype in 76.3% of participants, while the remainder showed discordance in these measures. These breakdowns are similar to those reported by Mennella et al. (2005) and in a previous study from our laboratory (Keller et al., 2010). Results from t-tests showed no differences in baseline participant characteristics (presented in Table 4) as a function of TAS2R38 genotype at rs173598.
Table 3.
Relationship between PROP taster status and TAS2R38 genotypea.
| PROP taster status | Genotype at SNP A49P
|
||
|---|---|---|---|
| AA (GG at rs713598) | AP (CG at rs713598) | PP (CC at rs713598) | |
| Nontasters | 10 | 9 | 3 |
| Tasters | 6 | 23 | 25 |
χ = 13.3; p < 0.001.
Table 4.
Descriptive characteristics as a function of TAS2R38 genotype at A49P.
| Categorical variablea | AA – Homozygous bitter insensitive (n = 16) | AP/PP Heterozygous and homozygous bitter sensitive (n = 60) |
|---|---|---|
| % (n) | % | |
| Sex | ||
| % Male | 31.3 (5) | 40.0 (24) |
| % Female | 68.8 (11) | 60.0 (36) |
| Ethnicity | ||
| % African-American | 60.0 (9) | 38.3 (23) |
| % Caucasian | ~6.7 (1) | 11.7 (7) |
| % Hispanic/Latino | 20.0 (3) | 35.0 (21) |
| % Asian | ~6.7 (1) | 1.7 (1) |
| % “Other” | ~6.7 (1) | 13.3 (8) |
| Family income | ||
| % ≤$20,000 per year | 31.3 (5) | 41.7 (25) |
| % >$20,000 per year | 68.7 (11) | 58.3 (35) |
| Continuous variable | Mean ± SD | Mean ± SD |
|---|---|---|
| Age (years) | 4.94 ± 0.85 | 5.07 ± 0.75 |
| BMI z-score | 1.14 ± 0.83 | 1.03 ± 0.97 |
There were no differences in the descriptive characteristics as a function of TAS2R38 genotype.
Intake of individual items at the test-meal
Results from t-tests showed no differences in children’s intake of the individual savory-fat food items as a function of PROP status (p-values from 0.2 to 0.9) or TAS2R38 genotype (p-values from 0.5 to 0.9). Likewise, there were no differences in children’s intake of individual sweet food items as a function of PROP status (p-values from 0.3 to 0.9) or TAS2R38 genotype (p-values from 0.4 to 0.9). Despite the fact that PROP status did not predict intake of the individual food items in the savory-fat or sweet categories, nontasters averaged between 3 and 20 kcals more than tasters for each of the savory-fat items, while tasters averaged between 3 and 20 kcals more than nontasters for each of the sweet food items.
Only two of the sweet-fat food items differed by PROP phenotype or TAS2R38 genotype. According to t-tests, PROP nontasters consumed more calories from donut holes than tasters did (t = 2.1; p < 0.05), and this remained significant after adjusting for covariates in GLM analyses (F = 4.1; p ≤ 0.05). Moreover, t-tests showed that children with AP or PP genotypes consumed more chocolate chip cookies at the test-meal than children who had the AA genotype (t = −2.0; p ≤ 0.05); however, this was no longer significant in the adjusted GLM models (p = 0.07).
Hypothesis one. Impact of PROP status on intake of savory-fats, sweet-fats, and sweets
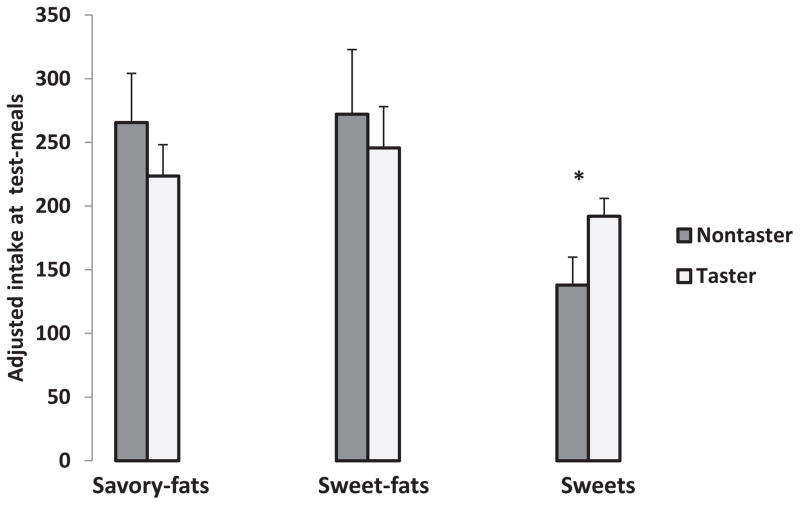
On average, children consumed 236.1 ± 197.9 kcals from savory-fats, 253.7 ± 244.8 kcals from sweet-fats, and 175.7 ± 108.0 kcals from sweets at the test-meal. According to the GLM, PROP taster status did not predict total energy intake at the test-meal (p = 0.95), but BMI z-score did [F(1,71) = 13.7; p < 0.001]. After adjusting for age, sex, ethnicity, and BMI-z-score, PROP status predicted consumption of the sweets group only, [F(1,71) = 4.2; p < 0.05]. Adjusted energy intakes at the meal for sweets were 192.0 ± 14.0 and 137.9 ± 22.0 for tasters and nontasters, respectively (Fig. 2).
Fig. 2.
Intake of savory-fats, sweet-fats, and sweets at a palatable buffet test-meal in 4- to 6-year-old children who were classified as PROP tasters (n = 56) and nontasters (n = 23) using a common screening procedure. Children who were PROP tasters consumed more kcals from sweet foods compared with PROP nontasters (p < 0.05). This remained significant after adjusting for ethnicity, age, sex, and BMI z-score. Intake of sweet-fats and savory-fats did not differ as a function of PROP taster status.
The relationship between PROP status and intake of savory fats was not significant (p = 0.30), but children’s BMI z-score did predict intake of savory fats at the meal [(F(1,71) = 12.0; p ≤ 0.001)]. According to t-tests, overweight children consumed about 100 kcals more from savory-fats compared with nonoverweight children [286.7 ± 227.8 kcals and 188.1 ± 152.5 kcals for overweight and nonoverweight, respectively; (t = −2.2; p < 0.05)].
None of the covariates included in the GLM models (ie. taster status, BMI z-score, ethnicity, age, sex) predicted children’s intake of sweet-fats at the test-meal.
Additional exploratory analyses related to hypothesis one
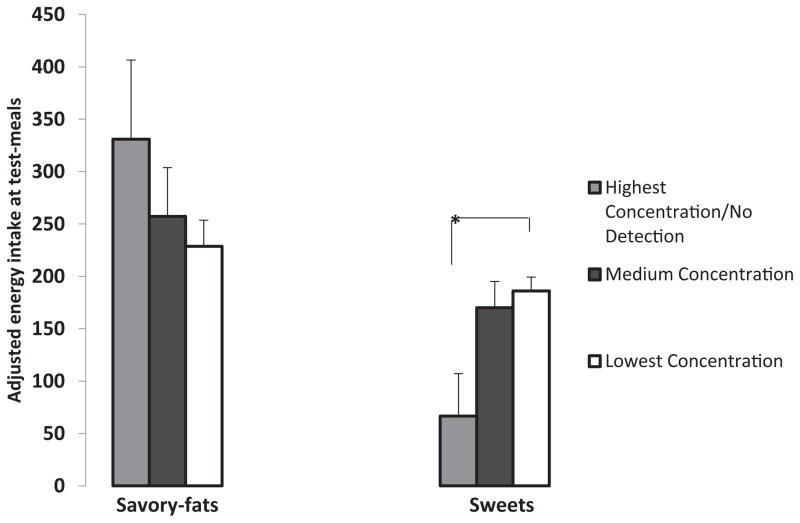
For exploratory purposes, we did additional analyses using the three-group PROP classifications in order to see if there were dose–response relationships between PROP status and intake of sweet foods. There were six children who only tasted PROP at the highest concentration (n = 1) or did not taste PROP at all (n = 5), even at the highest concentration offered. All of these children were AVI homozygotes or AVI heterozygotes (paired with one of the rare PTC haplotypes, PVI or AVV). Average BMI z-score for these children was 1.8 ± 0.9 (over the 95th% BMI-for-age). Exploratory analyses done to show the relationship between intake of sweets and savory-fats at the meal as a function of the three-group PROP classification showed a dose–response relationship between PROP taste sensitivity and intake of sweets and savory-fats. The six children who had the lowest PROP sensitivity consumed over 100 kcals more from savory fats than children who had higher PROP sensitivity, but PROP status was not significant in this model (p = 0.41). Intake of sweets at the test-meal differed depending on which concentration of PROP the children detected according to adjusted GLM models [F(2,68) = 4.1; p < 0.05]. Bonferroni tests for multiple comparisons showed that children who had the lowest PROP sensitivity had lower intake of sweets at the meal than children who had the highest PROP sensitivity (p < 0.01) (Fig. 3).
Fig. 3.
Intake of savory-fats and sweets at a palatable buffet test-meal in 4- to 6-year-old children who were classified into three groups with respect to their ability to taste PROP: 1) children who tasted at the highest concentration or never tasted it (Highest/Never), 2) children who tasted the medium PROP concentration (Medium), and 3) children who tasted the lowest PROP concentration (Lowest). PROP taster status was associated with intake of sweets after adjusting for ethnicity, age, sex, and BMI z-score. Bonferroni tests for multiple comparisons showed that children who were the least sensitive to PROP (Highest/Never) consumed more sweets than children who tasted PROP at the lowest concentration (p < 0.05). Children in the Highest/Never group also consumed about 125 kcals more from savory fats than children who tasted PROP at the medium and lowest concentrations, but in this model, only BMI z-score (p < 0.005) was significant.
Hypothesis two. Impact of TAS2R38 on intake of savory-fats, sweet-fats, and sweets
There were no differences in intake of savory-fats, sweet-fats, and sweets as a function of TAS2R38 genotype at A49P. Children who had the AA genotype consumed 248.2 ± 211.9 kcals, 228.3 ± 287.6 kcals, and 162.1 ± 118.8 kcals from savory-fats, sweet-fats, and sweets, respectively. Children who had the AP or PP genotype consumed 224.7 ± 185.3 kcals, 266.5 ± 238.7 kcals, and 176.1 ± 108.7 kcals for savory-fats, sweet-fats, and sweets, respectively. TAS2R38 genotype did not predict total energy intake at the test-meal (p = 0.83), but BMI z-score did [F(1,71) = 10.8; p < 0.005].
Discussion
The objective of this study was to determine the association between PROP taster status, TAS2R38 genotype, and intake of sweets, sweet-fats, and savory-fats at a palatable buffet meal administered in the laboratory. Previous reports from Keller and Tepper (2004) and Mennella et al. (2005) have reported greater intake and liking of sweet foods in PROP taster compared with nontaster children. In addition, Keller and Tepper (2004) reported that nontaster children consumed more savory fats (e.g. meats, cheeses, and nuts) compared with taster children. The present study provides evidence to suggest that during childhood, the PROP taster phenotype is associated with a greater intake of sweets in a short-term, laboratory paradigm. On the other hand, intake of savory-fats was strongly predicted by children’s weight status. Given that previous reports in children have demonstrated that some nontasters may also be more prone to obesity (Burd et al., 2013; Keller et al., 2010; Keller & Tepper, 2004), the causal pathway between PROP insensitivity, dietary intake of savory fats, and body weight requires future investigation.
Mennella et al. (2005) reported that children who had genotypes associated with higher bitter sensitivity liked beverages with higher sugar content and reported greater use of sugars on cereal compared with children who had bitter insensitive genotypes. In the present study, we reported supportive relationships when classifying children by PROP phenotype, but not by TAS2R38 genotype. The reason for these inconsistencies across studies is not known. In the Mennella study (Mennella et al., 2005), ~63% of the sample was African-American and the remainder were Caucasian, while our cohort was predominantly African-American (37%) and Hispanic/Latino (31%). Differences in ethnic background have been reported to influence sensitivity to PTC, with Africans and Asians showing a higher proportion of tasters than Caucasians (Bartoshuk, 1994; Guo, Shen, Wang, & Zheng, 1998). In addition, there is a higher prevalence of rare TAS2R38 haplotypes in individuals of African descent (Campbell et al., 2011; Pepino & Mennella, 2005). Higher numbers of African-Americans in Mennella et al. (2005) could have driven the relationships between TAS2R38 genotype and sweet liking, particularly because African-Americans have been reported to have higher sweet preferences (Pepino & Mennella, 2005; Schiffman, Graham, Sanly-Miller, & Peterson-Dancy, 2000). However, the fact that we showed different relationships depending on whether children were classified by PTC genotype or PROP phenotype reinforces important distinctions between these two measures. While PROP was used as a surrogate for PTC for many years, recent studies have shed light on key differences between the two compounds. Hayes et al. (2008) noted that PROP status predicted response to several common tastants (e.g. quinine, sucrose, and citric acid) while TAS2R38 did not. In addition, while some have reported modest relationships between TAS2R38 and diet-related outcomes (Colares-Bento et al., 2012; Feeney, O’Brien, Scannell, Markey, & Gibney, 2011), other studies have found no relationship with weight status or diet (Ooi, Lee, Law, & Say, 2010; Tepper et al., 2008). On the other hand, the PROP phenotype has been implicated in dietary patterns and body weight in a number of studies [for review see (Tepper, 2008)].
In relation to previous findings linking the PROP phenotype to eating behaviors, it’s important to note that there have been inconsistencies in this literature as well (Baranowski et al., 2011; Catanzaro, Chesbro, & Velkey, 2013; Feeney, O’Brien, Scannell, Markey, & Gibney, 2014; Mattes, 2005; Obrien et al., 2013). One could conclude that some of the present findings contribute to these inconsistencies (e.g. our lack of PROP related differences in savory-fat intake). These discrepancies across studies are not fully understood, but what seems reasonable to conclude is that both dietary patterns and body weight are complex phenotypes influenced by multiple environmental and genetic variables. Studies that have reported relationships with PROP status have most often been reported in demographically homogeneous populations (Tepper et al., 2008; Tepper, Neilland, Ullrich, Koelliker, & Belzer, 2011; Tepper & Ullrich, 2002) and/or have adjusted for several environmental or individual characteristics that are related to dietary patterns and body weight (Burd et al., 2013; Keller et al., 2002, 2010; Keller & Tepper, 2004; Tepper et al., 2008, 2011; Tepper & Ullrich, 2002). Furthermore, dietary patterns can be challenging to measure, particularly in large sample sizes where questionnaires are most often used. Several of the studies that have reported relationships between PROP status and eating behavior have assessed food intake under controlled laboratory conditions Shafaie, Koelliker, Hoffman, & Tepper, 2013; Tepper, Neilland, Ullrich, Koelliker, & Belzer, 2011), and this may also help to explain some of the aforementioned inconsistencies.
In addition to differences in intake of sweets, we also predicted differences in intake of savory-fats at the test meal between PROP phenotypic groups. In our previous work, nontaster children reported more frequent dietary intake of meats, cheeses, and other savory fats compared with taster children (Keller & Tepper, 2004). In addition, adult studies also show that nontasters like higher fat salad dressings than tasters (Tepper & Nurse, 1998) and may also have higher levels of obesity (Tepper, 1999; Tepper et al., 2008; Tepper & Ullrich, 2002). The mechanisms underlying these relationships are still being determined. In the present study, children with the lowest PROP sensitivity consumed over 100 kcals more from savory fats than children with higher PROP sensitivity; however, this relationship was not significant when body weight was included in the model. Given previous relationships in children demonstrating that some nontasters have higher BMIs, particularly if they live in environments with access to many unhealthy food options (Burd et al., 2013), it is probable that the relationships between PROP status, diet, and body weight are confounded. Larger studies that are designed prospectively are needed to disentangle these relationships. It is worth noting that children’s BMI z-scores were a robust predictor of total energy intake at the meal, but intake from savory fats clearly drove this relationship. The savory fats used in this study were the main meal options (e.g. chicken nuggets, pizza bagels). One might assume that overweight children would be more likely to consume sweets, but this was not the case.
The present study contributes to evidence gathered previously (Keller & Tepper, 2004; Mennella et al., 2005) to support the hypothesis that the ability to taste the bitter thiourea compound PROP is related to liking and intake of sweet taste, but this relationship might change across development. We propose three mechanisms to explain this. First, it is well established that children have higher preferences for sweet taste than adults (de Graaf & Zandstra, 1999; Mennella et al., 2011; Zandstra & de Graaf, 1998). Zandstra and de Graaf (1998) tested children, adolescents, and adults and reported that during childhood, the relationship between sucrose concentration in an orange beverage and perceived liking was the strongest. Heightened sweet preferences during childhood may help direct the child to sources of calories during times of growth (Coldwell et al., 2009). PROP tasters may have increased perception of sweetness in sweetened foods, and this may correspond to increased liking and preferential intake of these foods compared with nontasters. Second, sweetness is known to mask the perception of bitter in foods, and can be used to improve palatability of some vegetables (Capaldi & Privitera, 2008; Green, Lim, Osterhoff, Blacher, & Nachitigal, 2012; Sharafi, Hayes, & Duffy, 2013). PROP taster children might be more sensitive to bitter tastes in foods and, thus, might use sweetness more frequently than nontasters to mask these tastes. Over time, repeated exposures to sweetness could contribute to learned or acquired preferences for sweeter foods in the diet. Third, it is possible that variation in another gene that was not measured may be related to both PROP taster status and intake of sweets. These possibilities will need to be tested in future studies.
There were several strengths and limitations to this study. First, the sample size was small, particularly because the effects were driven by children who had the lowest PROP sensitivity and there were only six children in this group. A benefit of smaller cohorts, however, is that the phenotyping of both PROP status and food intake can be done more rigorously. As part of this rigorous phenotyping, we assessed food intake by measuring how much children ate at a palatable buffet meal. Single meal studies have been critiqued because they may not be representative of the diet under real world conditions (Blundell et al., 2010). However, we are reasonably certain that the test-meal used in this study was an accurate measure of children’s tendency to overeat because the correlations between energy intake, BMI z-score, and both fat free mass and percent adiposity (assessed by DXA) were high (p ≤ 0.001 for all, data not shown). Due to children’s young age, we used a simplified method to assess PROP thresholds that is age-appropriate, but it is not as reliable as the Harris–Kalmus method to assess threshold (Harris & Kalmus, 1949). The fact that our phenotyping method lacked sensitivity was a limitation. In addition, the high ethnic diversity in the sample was both a limitation and a strength. Allele frequency at TAS2R38 differs as a function of ethnicity (Campbell et al., 2011) and it is not known if this impacts the relationship between genotype and phenotype. The incidence of rare haplotypes in the current study was high (~14%), but we did not have an adequate sample size to test the effects of the different haplotypes separately on children’s test-meal intake. That said, there have been few studies looking at the relationship between PROP phenotype, PTC genotype, and eating behavior in ethnically diverse children. Therefore, this study adds to the literature.
Conclusion
The present findings demonstrate that at a laboratory-based, palatable buffet meal, PROP taster children preferentially consume sweets while heavier children tend to consume more savory fats. No relationship was found between the PTC genotype and intake of these food groups at the test-meal. These findings may have implications for understanding differences in susceptibilities to overconsumption of added sugars and discretionary fats. Prospective studies are needed to clarify how the PROP taste phenotype impacts sweet perception and liking across development.
Footnotes
Acknowledgments: The study was funded by the Career Development Award (Keller) K01DK068008 and the New York Obesity Research Center grant DK26687. Additional funding provided by DK524311 and DK26687 (Chung).
References
- Anliker JA, Bartoshuk LM, Ferris AM, Hooks LD. Children’s food preferences and genetic sensitivity to the bitter taste of 6-n-propylthiouracil (PROP) The American Journal of Clinical Nutrition. 1991;54:316–320. doi: 10.1093/ajcn/54.2.316. [DOI] [PubMed] [Google Scholar]
- Baranowski T, Baranowski JC, Watson KB, Jago R, Islam N, Beltran A, et al. 6-n-propylthiouracil taster status not related to reported cruciferous vegetable intake among ethnically diverse children. Nutrition Research (New York, NY) 2011;31:594–600. doi: 10.1016/j.nutres.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM. PTC/PROP tasting. Anatomy, psychophysics, and sex effects. Physiology & Behavior. 1994;6:1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Birch LL. Preschool children’s food preferences and consumption patterns. Journal of Nutrition Education. 1979;11:189–192. [Google Scholar]
- Blundell J, de Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, et al. Appetite control. Methodological aspects of the evaluation of foods. Obesity Reviews. 2010;11:251–270. doi: 10.1111/j.1467-789X.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufe B, Kuhn C, Reed DR, Tharp CD, Slack JP, Kim UK, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Current Biology: CB. 2005;15:1–20. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd C, Senerat A, Chambers EC, Keller KL. PROP taster status interacts with the built environment to influence children’s food acceptance and body weight status. Obesity (Silver Spring, Md) 2013;21:786–794. doi: 10.1002/oby.20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo C, Padiglia A, Zonza A, Corrias L, Contu P, Tepper BJ, et al. Polymorphisms in TAS2R38 and the taste bud trophic factor, gustin gene co-operate in modulating PROP taste phenotype. Physiology & Behavior. 2011;104:1065–1071. doi: 10.1016/j.physbeh.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Campbell MC, Ranciaro A, Froment A, Hirbo J, Omar S, Bodo JM, et al. Evolution of functionally diverse alleles associated with PTC bitter taste sensitivity in Africa. Molecular Biology and Evolution. 2011;29:1141–1153. doi: 10.1093/molbev/msr293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi ED, Privitera GJ. Decreasing dislike for sour and bitter in children and adults. Appetite. 2008;50:139–145. doi: 10.1016/j.appet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Catanzaro D, Chesbro EC, Velkey AJ. Relationship between food preferences and PROP taster status of college students. Appetite. 2013;68:124–131. doi: 10.1016/j.appet.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Colares-Bento FC, Souza VC, Toledo JO, Moraes CF, Alho CS, Lima RS, et al. Implication of the G145C polymorphism (rs713598) of the TAS2r38 gene on food consumption by Brazilian older women. Archives of Gerontology and Geriatrics. 2012;54:e13–e18. doi: 10.1016/j.archger.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Coldwell SE, Oswald TK, Reed DR. A marker of growth differs between adolescents with high vs. low sugar preference. Physiology & Behavior. 2009;96:574–580. doi: 10.1016/j.physbeh.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide. International survey. BMJ (Clinical Research Ed) 2000;320:1240–1243. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf C, Zandstra EH. Sweetness intensity and pleasantness in children, adolescents, and adults. Physiology & Behavior. 1999;67:513–520. doi: 10.1016/s0031-9384(99)00090-6. [DOI] [PubMed] [Google Scholar]
- Davis JM, Ventura EE, Shaibi GQ, Weigensberg MJ, Spruijt-Metz D, Watanabe RM, et al. Reduction in added sugar intake and improvement in insulin secretion in overweight Latina adolescents. Metabolic Syndrome and Related Disorders. 2007;5:183–193. doi: 10.1089/met.2006.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche JF, Buletic Z, Breslin PA. Relationship of papillae number to bitter intensity of quinine and PROP within and between individuals. Physiology & Behavior. 2001;74:329–337. doi: 10.1016/s0031-9384(01)00568-6. [DOI] [PubMed] [Google Scholar]
- Desor JA, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiology & Behavior. 1987;39:639–641. doi: 10.1016/0031-9384(87)90166-1. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Taste preferences and food intake. Annual Review of Nutrition. 1997;17:237–253. doi: 10.1146/annurev.nutr.17.1.237. [DOI] [PubMed] [Google Scholar]
- Drewnowski A. Sensory control of energy density at different life stages. The Proceedings of the Nutrition Society. 2000;59:239–244. [PubMed] [Google Scholar]
- Duffy VB, Bartoshuk LM. Food acceptance and genetic variation in taste. Journal of the American Dietetic Association. 2000;100:647–655. doi: 10.1016/S0002-8223(00)00191-7. [DOI] [PubMed] [Google Scholar]
- Feeney EL, O’Brien SA, Scannell AGM, Markey A, Gibney E. Genetic and environmental influences on liking and reported intakes of vegetables in Irish children. Food Quality and Preference. 2014;32:253–263. [Google Scholar]
- Feeney E, O’Brien S, Scannell A, Markey A, Gibney ER. Genetic variation in taste perception. Does it have a role in healthy eating? The Proceedings of the Nutrition Society. 2011;70:135–143. doi: 10.1017/S0029665110003976. [DOI] [PubMed] [Google Scholar]
- Green BG, Lim J, Osterhoff F, Blacher K, Nachitigal D. Taste mixture interactions. Suppression, additivity, and the predominance of sweetness. Physiology & Behavior. 2012;101:731–737. doi: 10.1016/j.physbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SW, Shen FM, Wang YD, Zheng CJ. Threshold distributions of phenylthiocarbamide (PTC) in the Chinese population. Annals of the New York Academy of Sciences. 1998;855:810–812. doi: 10.1111/j.1749-6632.1998.tb10664.x. [DOI] [PubMed] [Google Scholar]
- Harris H, Kalmus H. The measurement of taste sensitivity to phenylthiocarbamide (PTC) Annals of Eugenics. 1949;15:24–31. doi: 10.1111/j.1469-1809.1949.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chemical Senses. 2008;33:255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Duffy VB. Oral sensory phenotype identifies level of fat and sugar required for maximal liking. Physiology & Behavior. 2008;95:77–87. doi: 10.1016/j.physbeh.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL. Genetic influences on oral fat perception and preference. Journal of Food Science. 2012;77:S143–S147. doi: 10.1111/j.1750-3841.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- Keller KL, Olsen A, Kuilema L, Meyermann K, Belle CV. Predictors of parental perceptions and concerns about child weight. Appetite. 2013;62:96–102. doi: 10.1016/j.appet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL, Reid AR, MacDougall M, Cassano H, Song JL, Deng L, et al. Sex differences in the effects of bitter thiourea sensitivity on body weight in 4–6 year-old children. Obesity (Silver Spring, Md) 2010;18:1194–1200. doi: 10.1038/oby.2009.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002;38:3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- Keller KL, Tepper BJ. Inherited taste sensitivity to 6-n-propylthiouracil in diet and body weight in children. Obesity Research. 2004;12:904–912. doi: 10.1038/oby.2004.110. [DOI] [PubMed] [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299:1221–1224. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Liang LC, Sakimura J, May D, Breen C, Driggin E, Tepper BJ, et al. Fat discrimination. A phenotype with potential implications for studying fat intake behaviors and obesity. Physiology & Behavior. 2012;105:470–475. doi: 10.1016/j.physbeh.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem DG, de Graaf C. Sweet and sour preferences in young children and adults. Role of repeated exposure. Physiology & Behavior. 2004;83:421–429. doi: 10.1016/j.physbeh.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Looy H, Weingarten HP. Facial expressions and genetic sensitivity to 6-n-propylthiouracil predict hedonic response to sweet. Physiology & Behavior. 1992;52:75–82. doi: 10.1016/0031-9384(92)90435-5. [DOI] [PubMed] [Google Scholar]
- Mattes RD. 6-n-propylthiouracil taster status. Dietary modifier, marker, or misleader? In: Dekker M, editor. Genetic variation in taste sensitivity. New York: CRC press; 2005. pp. 245–250. [Google Scholar]
- Mennella JA, Beauchamp GK. Early flavor experiences. Research update. Nutrition Reviews. 1998;56:205–211. doi: 10.1111/j.1753-4887.1998.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Mennella JA, Lukasewycz LD, Griffith JW, Beauchamp GK. Evaluation of the Monell forced-choice, paired-comparison tracking procedure for determining sweet taste preferences across the lifespan. Chemical Senses. 2011;36:345–355. doi: 10.1093/chemse/bjq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennella JA, Pepino Y, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:216–222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkila V, Rasanen L, Raitakari OT, Pietinen P, Viikari J. Consistent dietary patterns identified from childhood to adulthood. The cardiovascular risk in Young Finns Study. The British Journal of Nutrition. 2005;93:923–931. doi: 10.1079/bjn20051418. [DOI] [PubMed] [Google Scholar]
- Nicklaus S, Boggio V, Chabanet C, Issanchou S. A prospective study of food variety seeking in childhood, adolescence and early adult life. Appetite. 2005;44:289–297. doi: 10.1016/j.appet.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Obrien SA, Feeney EL, Scannell AG, Markey A, Gibney ER. Bitter taste perception and dietary intake patterns in Irish children. Journal of Nutrigenetics and Nutrigenomics. 2013;6:43–58. doi: 10.1159/000348442. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA: The Journal of the American Medical Association. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A, van Belle C, Meyermann K, Keller KL. Manipulating fat content of familiar foods at test-meals does not affect intake and liking of these foods among children. Appetite. 2011;57:573–577. doi: 10.1016/j.appet.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SX, Lee PL, Law HY, Say YH. Bitter receptor gene (TAS2R38) P49A genotypes and their associations with aversions to vegetables and sweet/fat foods in Malaysian subjects. Asia Pacific Journal of Clinical Nutrition. 2010;19:491–498. [PubMed] [Google Scholar]
- Padiglia A, Zonza A, Atzori E, Chillotti C, Calo MC, Tepper BJ, et al. Sensitivity to 6-n-propylthiouracil (PROP) is associated with gustin (CA6) gene polymorphism, salivary zinc and BMI in humans. The American Journal of Clinical Nutrition. 2010;92:539–545. doi: 10.3945/ajcn.2010.29418. [DOI] [PubMed] [Google Scholar]
- Pepino MY, Mennella JA. Factors contributing to individual differences in sucrose preference. Chemical Senses. 2005;30:i319–i320. doi: 10.1093/chemse/bjh243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JM, Bartoshuk LM, Duffy VB. Intensity and preference for sweetness is influenced by genetic taste variation. Journal of the American Dietetic Association. 1999;99:A98. [Google Scholar]
- Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. Journal of the American Dietetic Association. 2010;110:1477–1484. doi: 10.1016/j.jada.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman SS, Graham BG, Sanly-Miller EA, Peterson-Dancy M. Elevated and sustained desire for sweet taste in African-Americans. A potential factor in the development of obesity. Nutrition (Burbank, Los Angeles County, Calif) 2000;16:886–893. doi: 10.1016/s0899-9007(00)00403-2. [DOI] [PubMed] [Google Scholar]
- Shafaie Y, Koelliker Y, Hoffman DJ, Tepper BJ. Energy intake and diet selection during buffet consumption in women classified by the 6-n-propylthiouracil bitter taste phenotype. The American Journal of Clinical Nutrition. 2013;98:1583–1591. doi: 10.3945/ajcn.113.058818. [DOI] [PubMed] [Google Scholar]
- Sharafi M, Hayes JE, Duffy VB. Masking vegetable bitterness to improve palatability depends on vegetable type and taste phenotype. Chemosens Percept. 2013;6:8–19. doi: 10.1007/s12078-012-9137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RSJ. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. The British Journal of Nutrition. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Seimon RV, Otto B, Keast RSJ, Clifton PM, Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. The American Journal of Clinical Nutrition. 2011;93:703–711. doi: 10.3945/ajcn.110.007583. [DOI] [PubMed] [Google Scholar]
- Sullivan SA, Birch LL. Pass the sugar, pass the salt. Experience dictates preference. Developmental Psychology. 1990;26:546–551. [Google Scholar]
- Tepper BJ. Does genetic taste sensitivity to PROP influence food preferences and body weight? Appetite. 1999;32:422. doi: 10.1006/appe.1999.0240. [DOI] [PubMed] [Google Scholar]
- Tepper BJ. Nutritional implications of genetic taste variation. The role of PROP sensitivity and other taste phenotypes. Annual Review of Nutrition. 2008;28:1–22. doi: 10.1146/annurev.nutr.28.061807.155458. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Koelliker Y, Zhao L, Ullrich NV, Lanzara C, d’Adamo P, et al. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity (Silver Spring, Md) 2008;16:2289–2295. doi: 10.1038/oby.2008.357. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Neilland M, Ullrich NV, Koelliker Y, Belzer LM. Greater energy intake from a buffet meal in lean, young women is associated with the 6-n-propylthiouracil (PROP) non-taster phenotype. Appetite. 2011;56:104–110. doi: 10.1016/j.appet.2010.11.144. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Nurse RJ. Fat perception is related to PROP taster status. Physiology & Behavior. 1997;61:949–954. doi: 10.1016/s0031-9384(96)00608-7. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Nurse RJ. PROP taster status is related to fat perception and preference. Annals of the New York Academy of Sciences. 1998;30:802–804. doi: 10.1111/j.1749-6632.1998.tb10662.x. [DOI] [PubMed] [Google Scholar]
- Tepper BJ, Ullrich NV. Influence of genetic taste sensitivity to 6-n-propylthiouracil (PROP), dietary restraint and disinhibition on body mass index in middle-aged women. Physiology & Behavior. 2002;75:305–312. doi: 10.1016/s0031-9384(01)00664-3. [DOI] [PubMed] [Google Scholar]
- Welsh JA, Sharma A, Cunningham SA, Vos MB. Consumption of added sugars and indicators of cardiovascular disease risk among US adolescents. Circulation. 2011;123:249–257. doi: 10.1161/CIRCULATIONAHA.110.972166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandstra EH, de Graaf C. Sensory perception and pleasantness of orange beverages from childhood to old age. Food Quality and Preference. 1998;9:5–12. [Google Scholar]