Abstract
Uterine leiomyomas (ULMs) are benign oestrogen-dependent tumours of the myometrium. They are the most common tumours of the female genital tract, affecting around 77% of the female population. ULMs are more common in Black women than White women. These tumours tend to develop earlier and be more numerous, larger in size and more symptomatic in Black women than other ethnic groups. The molecular mechanism underlying this ethnic disparity is not fully understood. Polymorphism of genes involved in oestrogen synthesis and/or metabolism (COMT, CYP17), variation in the expression levels or function of oestrogen and progesterone receptors or retinoic acid nuclear receptors (retinoid acid receptor-α, retinoid X receptor-α), or aberrant expression of micro-RNAs are some of the molecular mechanisms that may be involved.
Keywords: molecular genetics, race, leiomyoma
Uterine leiomyomas (ULMs) are benign, monoclonal tumours of the smooth muscle cells of the myometrium. They are composed of large amounts of extracellular matrix containing collagen, fibronectin and proteoglycan.1 ULMs may cause significant morbidity through their presence in the uterus and pelvic cavity. These benign tumours are a significant cause of pelvic pain, abnormal uterine bleeding, infertility and pregnancy complications.2
ULMs are the most common tumours of the female genital tract. Serial sectioning at 2-mm intervals of 100 consecutive total hysterectomy specimens revealed the presence of leiomyomas in 77% of cases.3 The rate of hospitalization of women for ULMs is 3.0 per 1000 women-years in the USA. Around 200 000 hysterectomies and 30 000 myomectomies are performed each year to treat women with ULMs,4,5 and the overall costs for inpatient surgeries for ULMs are US$ 2.1 billion per year.1
OESTROGEN DEPENDENCY OF ULMS
There is plenty of clinical and research evidence that ULMs are oestrogen-dependent tumours. The risk of developing ULMs increases with age during the premenopausal years; however, tumours typically regress and/or become asymptomatic with the onset of menopause.6 Obesity, age at menarche and unopposed oestrogen exposure have been linked to an increased risk of ULMs, and cigarette smoking, use of oral contraceptives and parity have been identified as protective factors.7 Moreover, using gonadotrophin-releasing hormone agonists leads to shrinkage of ULMs through the suppression of ovarian oestrogen production to postmenopausal levels.8 At the molecular level, primary ULM cells express oestrogen receptors (OR) and progesterone receptors (PR). Rodent leiomyoma cells derived from the Eker rat model for this disease proliferate in response to oestrogen in culture, and this response can be inhibited by oestrogen antagonists such as ICI 182780, tamoxifen and raloxifene.9 In addition, an elevated transcriptional response to oestrogen in leiomyoma cells suggests that these tumours may have increased responsiveness or be hypersensitive to oestrogen stimulation.10
EPIDEMIOLOGICAL STUDIES OF RACIAL DIFFERENCES IN THE INCIDENCE OF ULMS AND OTHER OESTROGEN-RELATED DISORDERS
ULMs do not affect all races equally. Increased prevalence of ULMs in African-American women was observed more than 100 years ago. A report published in 1894 on the prevalence of fibrosis processes in the dark-skinned races identified leiomyomas as one of the diseases considered as peculiar to the dark-skinned races. The author attributed this to ‘something in the blood of the dark-skinned people that predisposes to the development of fibrous growth’.11
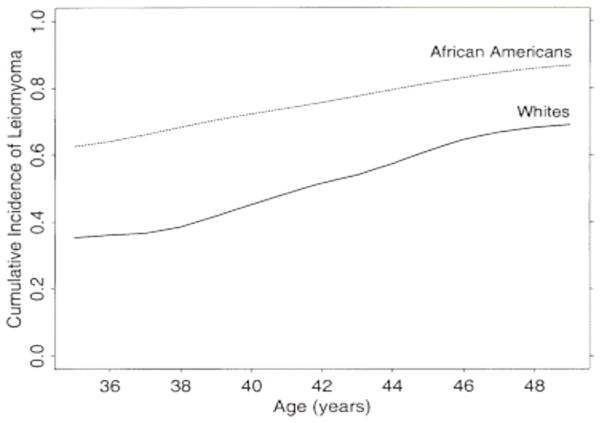
Recent data have confirmed the racial differences in ULMs. Marshall et al prospectively studied a large cohort of premenopausal women with intact uteri and no history of ULMs for 4 years. They found that the age-standardized rates of ultrasound- or hysterectomy-confirmed leiomyoma were significantly higher in Black women compared with White women. Similarly, Baird et al12 demonstrated that the incidence of myomas was 60% among African-American women by 35 years of age, and the incidence increased to over 80% by 50 years of age. Caucasian women had an incidence of 40% by 35 years of age and almost 70% by 50 years of age (Figure 1).
Figure 1.
Estimated age-specific cumulative incidence of uterine leiomyomas for Black and White women aged 35–49 years.12
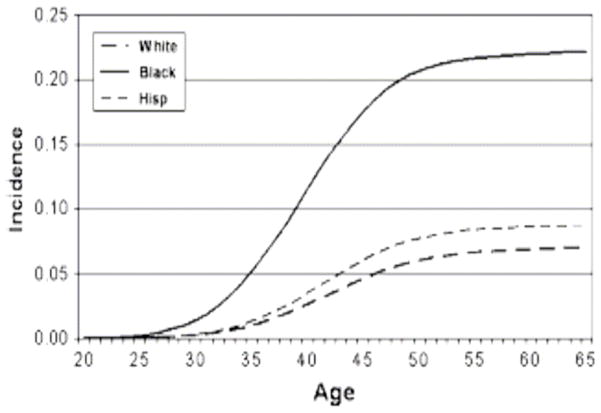
The ethnic differences in the incidence of ULMs were reflected in the hysterectomy rates in different ethnic groups. Kjerulff et al13 found that the annual age-adjusted hysterectomy rate was significantly higher in Black women compared with White women. ULMs were the primary diagnosis for 65.4% of hysterectomies in Black women, compared with only 28.5% in White women. Also, Myers et al14 reported a three-fold increase in hysterectomy rates in Black women compared with White or Hispanic women. Black women had a lifetime risk of hysterectomy approaching 22% (Figure 2).
Figure 2.
Cumulative incidence of hysterectomy by race.14
Kjerulff et al13 found that the average ages at ULM diagnosis and hysterectomy were younger for Black women than White women. The average uterine weight was heavier for Black women compared with White women. Black women were more likely to have seven or more ULMs than White women, and a higher proportion of Black women reported severe symptoms in the form of anaemia or severe pelvic pain. The molecular background behind the ethnic disparity in ULMs is not fully understood.
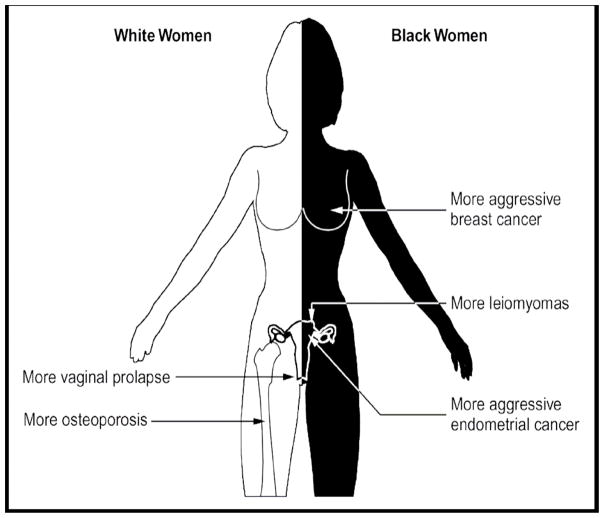
Epidemiological studies have shown that, in addition to ULMs, several other oestrogen-dependent disorders show ethnic/racial disparity in their incidence and biological behaviours. White women have higher incidence rates of endometrial and breast cancers than African-American women.15 In contrast, African-Americans have higher mortality rates from breast and endometrial cancer than White women.16 This is related to a greater frequency of adverse clinicopathological features in African-American women, including advanced tumours, high-grade tumours and more aggressive histology, compared with White women.17–19
Moreover, Black women have a lower risk for osteoporosis compared with White women. Age-adjusted estimates of osteoporosis of the hip are significantly higher for postmenopausal White women than Black women.20 In addition, Black race appears to be protective against the development of pelvic floor disorders such as urinary stress incontinence and pelvic organ prolapse, which are more common in White women.21 Figure 3 summarizes the ethnic disparities in the incidence of oestrogen-dependent diseases.
Figure 3.
Ethnic disparities in the incidence and biological behaviours of oestrogen-dependent diseases.
The racial/ethnic disparity in the incidence and biological behaviours of ULMs in addition to other oestrogen-dependent diseases suggests that different races may exhibit differences in oestrogen biosynthesis and/or metabolism. This concept appears more plausible as racial differences in the exposure to risk factors associated with ULMs could not explain the differences in their incidence between races. In the work by Marshall et al, even after adjustment for factors such as marital status, body mass index, age at first birth, years since last birth, history of infertility, age at first oral contraceptive use and current alcohol consumption, ULM incidence rates in Black women were still significantly higher than those in White women.
POLYMORPHISM OF GENES INVOLVED IN OESTROGEN SYNTHESIS AND/OR METABOLISM (CYP17 AND COMT)
CYP17
Polymorphism of genes coding for different enzymes involved in oestrogen biosynthesis and/or metabolism have been investigated in different studies as a possible mechanism. One of these genes is CYP17 which codes for the cytochrome P450C17α enzyme. This enzyme mediates both steroid 17α-hydroxlyase and 17, 20-lyase activities, and functions at key branch points in human steroidogenesis.22 The 5′ untranslated region of CYP17 contains a single base pair polymorphism, a T (designated as A1) to a C (designated as A2), 34 base pairs upstream from the initiation of translation and 27 base pairs downstream from the transcription start site.23 Among premenopausal women, there appears to be a steady increase in serum oestradiol and progesterone concentrations depending on the number of A2 alleles that a woman carries, with the A2/A2 genotype corresponding to the highest concentrations.24
Amant et al25 studied 89 Black South African and 56 Caucasian women who underwent hysterectomy. Blood samples were withdrawn from these women, and the hysterectomy specimens were examined pathologically for the presence (study group) or absence (control group) of ULMs. DNA was isolated from the blood cells and was used in a polymerase chain reaction to determine the CYP17 genotype of the patient. To determine the impact on the incidence of ULMs, CYP17 genotype in addition to other risk factors associated with ULMs (age, parity, age at last birth, weight, body mass index, menopausal status, cigarette smoking and oral contraceptive use) were put in a statistical model. Age, race and parity appeared to affect the incidence of ULMs in that model which included Caucasian and Black South African women. Logistic regression analysis in Caucasian women showed that oral contraceptives were protective against the development of ULMs regardless of CYP17 genotype. Logistic regression applied in Black South African women showed that age and CYP17 polymorphism were correlated positively with the presence of ULMs. Using categorical data analysis, the risk for ULM development among Black South African women with the CYP17 A2/A2 genotype was shown to be increased, whereas the risk in Black South African women with the CYP17 A1/A1 and A1/A2 genotypes was shown to be lower (Table 1). Also, ULMs were larger in size in women with the CYP17 A2/A2 genotype compared with women with the A1/A2 or A1/A1 genotypes; however, the difference was not statistically significant. The authors hypothesized that higher levels of oestrogen in African women homozygous for CYP17 A2 allele expose the myometrium to a stronger stimulatory effect, which may, in the long term, result in spontaneous mutations and uncontrolled growth; an important feature of ULMs.
Table 1.
Risk of Black South African women developing uterine leiomyomas based on CYP17 genotype.25
| CYP17 genotype | Odds ratio | 95% confidence interval |
|---|---|---|
| A1A1 | 3.80 | 0.98–14.67 |
| A1A2 | 3.83 | 1.56–9.41 |
| A2A2 | Infinite | 1.18–infinite |
COMT
Catechol-O-methyltransferase (COMT) is a ubiquitous enzyme that catalyses methyl conjugation of the hydroxyl groups of catechol oestrogens. Specifically, it catalyses the conversion of 2,4 hydroxy oestradiol to 2,4 methoxy oestradiol. Therefore, regulation of COMT activity may indirectly modulate the biological effects of oestrogen and play an aetiological role in leiomyoma formation.26 A common genetic polymorphism, G to A transition at codon 158, resulting in a valine-to-methionine substitution, is associated with thermal instability and a four-fold decrease in enzymatic activity. The genotypes designated in relation to the predicated enzymatic activity of the protein are high (Val/Val), intermediate (Val/Met) and low (Met/Met) activity.27
In recent work by the authors’ group, COMT gene polymorphism was studied in 186 women with ULMs and 142 women without ULMs. All subjects had a hysterectomy, and the presence (study group) or absence (control group) of ULMs was documented at histological level. Genotyping was performed using DNA isolated from normal myometrium, and was confirmed with DNA isolated from peripheral blood cells. The Val/Val (high activity) genotype was highly represented in ULM patients (39%) compared with the controls (21%) from all ethnic groups. However, the homozygous Met/Met (low activity) genotype was less represented in ULM patients (12%) compared with the controls (27%). The heterozygous Val/Met genotype did not differ significantly between cases (49%) and controls (52%). Within each ethnic group, the Val/Val genotype was significantly more common in ULM cases than controls (Table 2).
Table 2.
Distribution of COMT genotypes in women with and without uterine leiomyomas by ethnic group.49
| African–American | White | Hispanic | ||||
|---|---|---|---|---|---|---|
| Genotype | With leiomyoma (%) | Without leiomyoma (%) | With leiomyoma (%) | Without leiomyoma (%) | With leiomyoma (%) | Without leiomyoma (%) |
| Val/Val | 42 (52) | 6 (27) | 15 (25) | 14 (15) | 16 (35) | 6 (21) |
| Val/Met | 36 (44) | 14 (64) | 29 (50) | 44 (48) | 26 (56) | 18 (64) |
| Met/Met | 3 (4) | 2 (9) | 15 (25) | 34 (37) | 4 (9) | 4 (15) |
Using multiple logistic models, White women had the lowest occurrence of leiomyomas. African-American and Hispanic women were 5.3 and 2.1 times more likely to have ULMs than White women, respectively. Overall, women with the Val/Val genotype were 2.5 times more likely to have ULMs compared with women with the Met/Met genotype (controlling for ethnicity). Conversely, COMT Val/Met and Met/Met did not mediate significantly different associations with ULMs.
The natural distribution of COMT genotypes in different racial groups was also addressed in the authors’ study. African-American women had a high frequency of the Val/Val genotype (47%) and a low frequency of the Met/Met genotype (5%); heterozygous Val/Met was 49%. In sharp contrast, White women had a low frequency of the Val/Val genotype (19%) and a higher frequency of the Met/Met genotype (33%); heterozygous Val/Met was 48%.
Overall, these data show that the high-activity COMT (Val/Val) genotype is associated with ULMs in all ethnic groups. This genotype is more common in African-Americans than other races, and this may be associated with the higher incidence of ULMs in that ethnic group.
The exact relationship between COMT gene polymorphism and leiomyoma is not yet clear. COMT converts 2-hydroxy oestradiol to 2-methoxy oestradiol. 2-hydroxy oestradiol has been found to work as as anti-oestrogen in many tissue systems.28,29 On the other hand, 2-methoxy oestradiol has been demonstrated to possess a mitogenic effect on different cell types.30–32 Therefore, the high-activity COMT genotype (Val/Val) would derive rapid and efficient conversion of the anti-oestrogenic metabolite (2-hydroxy oestradiol) into the more mitogenic counterpart (2-methoxy oestradiol), thus creating a high oestrogenic cellular milieu. Conversely, the low-activity COMT genotype (Met/Met) would lead to the accumulation of 2-hydroxy oestradiol, creating a low oestrogenic environment. As ULMs are oestrogen dependent, a higher frequency of occurrence would be associated with the COMT Val/Val genotype than the low oestrogenic Met/Met genotype. In agreement with this hypothesis were the findings of Reddy et al,33 who demonstrated lower levels of 2-hydroxy oestradiol in leiomyomas compared with adjacent normal myometrium and implicated this in the tumourgenesis. In the same context, two reports in American34 and Finnish27 populations described a significantly decreased risk of breast cancer in premenopausal women with a COMT Met/Met genotype.
In-vitro data have confirmed the effects of the COMT genotype on the phenotype of myometrial and ULM cells.35 The Val/Val primary myometrial cells showed a significantly higher proliferation rate, greater transcriptional response to oestrogen (as evidenced by higher luciferase reporter transactivation) and a gene expression profile expressive of high oestrogenic milieu (increased expression of cyclo-oxygenase 2, cyclin D1, PR-A, PR-B and Bcl2, and decreased expression of BAX) compared with their Met/Met counterparts. This confirms the high oestrogenic drive of myometrial cells of the COMT Val/Val genotype.
Variations in steroid receptor expression were one of the molecular mechanisms evaluated by researchers to explain the racial differences in ULMs. Several recent reports have attempted to expose leiomyomas to gene arrays, and suggested no significant difference in OR expression levels in leiomyomas compared with adjacent normal myometrium.36 In addition, two studies failed to show significant differences in the expression of ORs and PRs in the myometrium between Black and White women.37,38
DYSREGULATION OF RETINOIC ACID NUCLEAR RECEPTORS
More recently, Wei et al39 applied immunohistochemistry with high-density tissue micro-array to identify the ethnic differences in the expression of selected gene products between Black, Asian, Hispanic and White women with ULMs. Relative protein expression was determined by normalizing the absolute immunoscores in ULMs to that of the adjacent normal myometrium. The absolute expression value of OR-α in both normal myometrium and ULMs was higher in Black women compared with other ethnic groups; however, when the relative OR-α expression was calculated, ULMs of Black women did not differ significantly from those of other ethnic groups. In ULMs of Black women, the relative expression of PR-A (upregulated in relation to normal myometrium), retinoid acid receptor-α (RAR-α; downregulated) and retinoid X receptor-α (RXR-α; no change from adjacent myometrium) differed significantly from other ethnic groups (Table 3). About one-third of ULMs from Black women subclustered together in association with a group of upregulated gene products. Many other gene products, including local growth factors, insulin-like growth factor signalling proteins and cell proliferation markers, were dysregulated in ULMs, but showed non-significant differences between the ethnic groups (Table 3).
Table 3.
Results of immunoscores by ethnicity status, median and range of difference between uterine leiomyomas and matched myometrium.39
| Biomarkers | Black | Asian | Hispanic | White | Pa | Pb |
|---|---|---|---|---|---|---|
| ORα | 0 (−3,5) | 0.25 (−2, 5.5) | 0 (−3.5, 6.5) | 0 (−3, 3) | NS | |
| PR-A | 2.8 (−7, 10) | 0 (−5, 6) | 0 (−5, 5.5) | 1.75 (−6, 6) | 0.02 | < 0.02c |
| RAR-α | −0.5 (−10, 5) | 1.25 (−4, 7.5) | −1 (−6, 9) | 2 (−8, 10) | <0.02 | 0.01d |
| RXR-α | 0 (−6, 2.5) | 1.25 (−4, 5.5) | 0.5 (−4, 10) | 0.75 (−3, 5.5) | NS | 0.03e |
| GCR | −1 (−4,1) | −1 (−4.5, 0.5) | −1 (−2, 1) | −1.5 (−5, 1) | NS | 0.07f |
| AIB1 | 2 (−4, 9) | 2 (−4, 10) | 1 (−8.5, 8) | 1.25 (−4, 6) | NS | 0.08g |
| SRC1 | 0.5 (−1, 2) | 0.5 (−0.5, 2.5) | 0 (−2, 1.5) | 0.75 (−1, 2) | 0.03 | 0.01h |
| IGF2 | 2 (−2, 7.5) | 2.5 (0, 6) | 2 (−11, 6) | 3 (−0.5, 6) | NS | < 0.04i |
| IGF1Rβ | 0 (−0.5, 1.5) | 0 (−1, 1.5) | 0.5 (−1, 1) | 0 (−1, 1.5) | NS | |
| Tuberin | −2 (−6, 3) | −2 (−5, 1.5) | −1 (−5, 4) | −1 (−6, 3) | NS | |
| Hamartin | 0 (−1, 1) | 0.5 (−1,1) | −0.3 (−1, 2) | 0.5 (−1, 1.5) | NS | |
| BCL2 | 0 (−3.5, 3) | 0.5 (−3, 6.5) | 0.5 (−2, 4.5) | 0.5 (−2.5, 3.5) | NS | < 0.07e |
| MIB1 | 1 (−2, 26.5) | 1 (−28, 30) | 2.5 (−9.5, 24 | 1.8 (−1, 18.5) | NS | |
| HMGA2 | 0.5 (−1, 2) | 1 (0,2) | 1 (−1, 3) | 0.5 (−0.5, 3) | NS | |
| CD24 | 0.5 (0, 1) | 0.5 (−1, 1.5) | 1 (−1, 2) | 0 (0, 1.5) | NS | < 0.02i |
| PDGF | 0 (−1, 4) | 0 (0, 6) | 0 (−2, 4) | 0.(0, 8) | NS | |
| EGFR | 0 (−1.5,2) | 0 (−1.5, 1.5) | 0 (−2, 1.5) | 0 (−1.5, 2) | NS | |
| Factor VIII | −5 (−20, 0) | −5 (−20, 5) | −5 (−20, 5) | −10 (−25, 10) | NS |
For overall differences between ethnic groups using Kruskal-Wallis test
For pair-wise differences suing Wilcoxon rank sum test
Blacks versus Hispanics
Blacks versus Asians
Blacks versus all other groups
Whites versus all other groups
Whites and Hispanics versus blacks and Asians
Whites versus Hispanics
Hispanics versus all other groups
NS, not statistically significant.
OR, oestrogen receptor; PR, progesterone receptor; RAR-α, retinoid acid receptor-α; RXR-α, retinoid X receptor-α.
As ULMs are hormone dependent, the differential expression of steroid hormone receptors (OR and PR) among different races would be of crucial importance to explain the ethnic differences in the incidence of these benign tumours. The downregulation of retinoic acid receptors (RAR-α and RXR-α) in ULMs of Black women in comparison with their upregulation in other ethnic groups indicates dysregulation of retinoic acid metabolism in ULMs of Black women. Other studies have shown abnormal expression of genes coding for enzymes involved in retinoic acid metabolism in ULMs.40,41 However, the exact role of retinoic acid and its nuclear receptors in the ethnical disparity of ULMs still needs to be elucidated.
POLYMORPHISM OF OESTROGEN RECEPTOR GENES
The authors investigated whether racial differences in the incidence of ULMs may be related to variation in the function of the steroid receptors, rather than the expression level. The distribution of two common OR gene polymorphisms was assessed between Black, Hispanic and White women with or without ULMs. The polymorphisms tested were in the first intron of the OR gene and included a T/C polymorphism that is recognized by the restriction endonuclease PvuII, and an A/G polymorphism recognized by XbaI restriction enzyme. The T and C alleles correspond to the presence (p allele) or absence (P allele), respectively, of the restriction site. Similarly, the A and G alleles correspond to the presence (x allele) or absence (X allele), respectively, of the restriction site. Genotypes for PvuII and XbaI polymorphisms were termed PP, Pp and pp, and XX, Xx and xx, respectively.
According to the authors’ results, the PP genotype was associated with significantly greater risk of ULMs among Black and White women, but not among Hispanic women (Table 4). Using the logistic model, White women had the lowest incidence of leiomyomas. Black and Hispanic women were 9.7 and 2.4 times more likely, respectively, to have ULMs than White women. Overall, women with the PP genotype were 6.4 times more likely to have ULMs compared with women with the pp genotype. Furthermore, the PP genotype was significantly more common in cases with severe disease (uterine weight > 400 g) and was associated with younger age at hysterectomy compared with the pp genotype.
Table 4.
Distribution of PvuII genotypes of the oestrogen receptor-α gene among women with or without uterine leiomyomas in different ethnic groups.
| Genotype or allele | Black patients with leiomyoma (%) | Black control patients without leiomyoma (%) | P-value | White patients with leiomyoma (%) | White control patients without leiomyoma (%) | P-value | Hispanic patients with leiomyoma (%) | Hispanic control patients without leiomyoma (%) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| PP | 36 (39) | 3 (14) | 0.004 | 17 (28) | 1 (2) | 0.001 | 8 (18) | 6 (12) | NS |
| Pp | 34 (37) | 9 (43) | NS | 23 (38) | 99 (62) | NS | 23 (51) | 18 (35) | NS |
| pp | 22 (24) | 9 (43) | NS | 21 (34) | 57 (36) | NS | 14 (31) | 27 (53) | NS |
NS, not statistically significant.
The authors also addressed the distribution of different OR genotypes in various ethnic groups. Black women had a significantly high frequency of the PP genotype (35%) compared with White women (13%) and Hispanic women (16%). In contrast, White and Hispanic women had a higher frequency of the pp genotype (38% and 40%, respectively) compared with Black women (27%). There was no significant difference in the Pp heterozygous genotype among the three ethnic groups
The strong association between ULMs and the PP genotype of ORs, and the in-vitro data of higher cellular proliferation in myometrial cells harbouring the same genotype detected in the authors’ study, together with the results of other studies that detected more ULM-related hysterectomies and higher bone mineral densities in women with the PP genotype,42,43 indicate the higher prevalence of the P allele in more potent local oestrogenic environments.
It is not fully understood how the polymorphism at the Pvu II locus, which is located in the first intron of the OR gene, alters the oestrogenic response. There are a number of possibilities; the first intron may contain a regulatory site (like an enhancer) to control the gene function, this polymorphism may lead to differential mRNA splicing with different functional proteins, or this polymorphism may serve as a marker in linkage with other, as yet unidentified, regulatory regions.
ABERRANT EXPRESSION OF MICRO-RNAS
Micro-RNAs (miRNAs) are a class of small, non-coding RNAs which are transcribed by RNA polymerase II. miRNAs regulate cell proliferation, differentiation and cell death during development.44 They are expressed aberrantly in certain types of tumour.45,46 Many genes are dysregulated in ULMs, and some of this dysregulation may be due to abnormal expression of miRNAs.47
Wang et al48 collected 55 ULMs and matched myometrium from 41 patients of different ethnic groups for micro-array-based global miRNA expression analysis. They demonstrated that ULMs from Black women showed more than two-fold overexpression in certain miRNAs, including miR-23a/b, let-7s, miR-145, miR-197, miR-411 and miR-412 (Table 2), compared with tumours from White women. The miRNA expression profile from other racial groups (Asian and Hispanic) appears to be in between that of Black and White women.
One of the predicted target genes of miR-23b is TGIF (TGFB-induced factor). TGIF plays a role in inhibiting retinoic-acid-dependent RXR-α transcription. ULMs in Black women exhibit minimal change of RXR-α expression compared with ULMs in other racial groups, in which a higher level of overexpression of RXR-α is evident.49
In conclusion, the incidence of ULMs in Black women is much higher than in women of other ethnicities. The molecular background of this racial difference is not fully understood. Polymorphism of genes involved in oestrogen synthesis and/or metabolism (COMT, CYP17), variation in the expression levels or function of steroid receptors (OR, PR) or retinoic acid nuclear receptors (RAR-α, RXR-α), or aberrant expression of miRNAs may be some of the molecular mechanisms involved.
Table 5.
Differential expression of micro-RNAs associated with race in uterine leiomyomas.
| Average fold change | ||||
|---|---|---|---|---|
| Black | White | Other | ||
| Number of tumours | 20 | 19 | 9 | P-value |
| MiR21 | 5.0783 | 1.9764 | 2.2327 | 0.013 |
| miR23b | 5.0092 | 1.6317 | 1.6517 | 0.009 |
| miR27a | 2.2770 | 1.4710 | 1.4901 | 0.002 |
| miR16-1 | 2.1789 | 1.2272 | 1.4181 | 0.021 |
| let7e | 2.0704 | 1.2671 | 1.6273 | 0.016 |
| miR30a | 1.7230 | 1.3591 | 1.2947 | 0.026 |
| let7i | 1.6203 | 1.2933 | 1.3304 | 0.008 |
| let7g | 1.3641 | 1.0398 | 1.2809 | 0.010 |
| miR191 | 1.2981 | 0.9857 | 0.7997 | 0.003 |
| miR25 | 1.1375 | 1.3144 | 0.9150 | 0.022 |
| miR384 | 0.9919 | 1.2465 | 0.7923 | 0.006 |
| miR224 | 0.9263 | 1.1381 | 0.8883 | 0.019 |
| miR194-1 | 0.8659 | 0.8728 | 0.5680 | 0.022 |
| miR302b* | 0.7810 | 0.9738 | 0.5563 | 0.011 |
| miR19a | 0.7802 | 1.1314 | 0.7924 | 0.010 |
| miR217 | 0.7507 | 0.9787 | 0.6299 | 0.023 |
| miR323 | 0.7326 | 0.9496 | 0.6332 | 0.013 |
| miR330 | 0.7205 | 1.0471 | 0.7552 | 0.008 |
| miR141 | 0.6961 | 0.9702 | 0.4284 | 0.000 |
| miR200a | 0.6804 | 0.9977 | 0.5808 | 0.022 |
| miR142 | 0.6216 | 0.9424 | 0.4765 | 0.011 |
| miR207 | 0.6011 | 0.8764 | 0.4662 | 0.013 |
| miR18 | 0.5881 | 0.8948 | 0.6798 | 0.004 |
| miR345 | 0.5143 | 0.7016 | 0.7956 | 0.013 |
| miR184 | 0.5128 | 0.6297 | 0.3225 | 0.010 |
| miR376b | 0.4237 | 0.6708 | 0.7820 | 0.013 |
| miR324 | 0.4227 | 0.7202 | 0.2864 | 0.006 |
| miR203 | 0.3963 | 0.8328 | 0.5984 | 0.001 |
| miR412 | 0.3957 | 0.8787 | 0.4966 | 0.017 |
| miR144 | 0.3785 | 0.6279 | 0.3752 | 0.014 |
| miR212 | 0.3066 | 0.5654 | 0.7880 | 0.013 |
Research agenda.
the exact molecular explanation for the association between the A2/A2 genotype of CYP17, the Val/Val genotype of COMT and the PP genotype of ORs with higher incidence of ULMs
the exact role played by retinoic acid receptors in the tumourigenesis of ULMs and the implications on explaining racial disparity in the incidence of these benign tumours
the interaction between aberrantly expressed miRNAs and dysregulation of retinoic acid receptors in the pathogenesis of ULMs in different ethnic groups
Acknowledgments
This research was supported in part by the Clinical Research Center of Meharry Medical College, Grant #P20RR011792 from the National Institutes of Health and RCMI Clinical Research Infrastructure Initiative, R01 HD 046228, and G12 RR003032.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Essam El-Din R. Othman, Department of Obstetrics and Gynaecology, Assiut Faculty of Medicine, Assiut, Egypt.
Ayman Al-Hendy, Center for Women Health Research, Department of Obstetrics and Gynecology, Meharry Medical College, 1005 Dr. D.B. Todd Jr. Blvd. George Hubbard Hospital, 5th Floor, Room 5131C, Nashville, Tennessee 37208, USA.
References
- 1.Parker WH. Etiology, symptomatology, and diagnosis of uterine myomas. Fertil Steril. 2007;87:725–736. doi: 10.1016/j.fertnstert.2007.01.093. [DOI] [PubMed] [Google Scholar]
- 2.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 3.Cramer SF, Patel A. The frequency of uterine leiomyoma. Am J Clin Pathol. 1990;94:435–438. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549–555. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 6.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 7.Marshall LM, Spiegelman D, Goldman MB, et al. A prospective study of reproductive factors and oral contraceptive use in relation to the risk of uterine leiomyomata. Fertil Steril. 1998;70:432–439. doi: 10.1016/s0015-0282(98)00208-8. [DOI] [PubMed] [Google Scholar]
- 8.Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 2001;2:CD000547. doi: 10.1002/14651858.CD000547. [DOI] [PubMed] [Google Scholar]
- 9.Howe SR, Gottardis MM, Everitt JI, et al. Rodent model of reproductive tract leiomyomata. Establishment and characterization of tumor-derived cell lines. Am J Pathol. 1995;146:1568–1579. [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen J, DyReyes VM, Barbieri RL, Coachman DM, Miksicek RJ. Leiomyoma primary cultures have elevated transcriptional response to estrogen compared with autologous myometrial cultures. J Soc Gynecol Investig. 1995;2:542–551. doi: 10.1016/1071-5576(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 11.Balloch E. The relative frequency of fibroid processes in the dark skinned races. Med News. 1894 [Google Scholar]
- 12.Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;1881:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 13.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–490. [PubMed] [Google Scholar]
- 14.Myers E, Barber M, Couchman G, et al. Management of uterine fibroids. 1 AHRQ Evidence Reports, No. 24. AHRQ Publication No. 01-E052. 2001. Available at: http://www.ncbi.nlm.nih.gov/books/bv.fcgi?rid¼hstat1.chapter47755. [PMC free article] [PubMed] [Google Scholar]
- 15.SEER: Surveillance, Epidemiology, and End Results. Bethesda, MD: Surveillance Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute; 2006. Available at: http://seer.cancer.gov/ [Google Scholar]
- 16.Bach PB, Schrag D, Brawley OW, Galaznik A, Yakren S, Begg CB. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. doi: 10.1001/jama.287.16.2106. [DOI] [PubMed] [Google Scholar]
- 17.Madison T, Schottenfeld D, James SA, Schwartz AG, Gruber SB. Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–2111. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Setiawan VW, Pike MC, Kolonel LN, Nomura AM, Goodman MT, Henderson BE. Racial/ethnic differences in endometrial cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;165:262–270. doi: 10.1093/aje/kwk010. [DOI] [PubMed] [Google Scholar]
- 19.Blackman D, Masi C. Racial and ethnical disparity in breast cancer mortality. Are we doing enough to address the root causes? J Clin Oncol. 2006;24:2170–2178. doi: 10.1200/JCO.2005.05.4734. [DOI] [PubMed] [Google Scholar]
- 20.Pothiwala P, Evans E, Chapman-Novakofskik K. Ethnic variation in risk for osteoporosis among women: a review of biological and behavioral factors. J Women’s Health. 2006;15:709–719. doi: 10.1089/jwh.2006.15.709. [DOI] [PubMed] [Google Scholar]
- 21.Anger J, Rodriguez L, Wang Q, Chen E, Pachos C, Litwin M. Racial disparities in the surgical management of stress urinary incontinence among female Medicare beneficiaries. J Urol. 2007;177:1846–1850. doi: 10.1016/j.juro.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 22.Brentano ST, Picado-Leonard J, Mellon SH, Moore CCD, Miller WL. Tissue-specific cyclic adenosine 3′,5′-monophosphate-induced, and phorbol sterrepressed transcription from the human P450cl7 promoter in mouse cells. Mol Endocrinol. 1990;4:1972–1979. doi: 10.1210/mend-4-12-1972. [DOI] [PubMed] [Google Scholar]
- 23.Carey A, Waterworth D, Patel K, et al. Polycystic ovaries and premature male pattern baldness are associated with one allele of the steroid metabolism gene CYP17. Hum Mol Genet. 1994;3:1873–1876. doi: 10.1093/hmg/3.10.1873. [DOI] [PubMed] [Google Scholar]
- 24.Feigelson HS, Shames LS, Pike MC, Coetzee GA, Stanczyk FZ, Henderson BE. Cytochrome P450c17alpha gene (CYP17) polymorphism is associated with serum estrogen and progesterone concentrations. Cancer Res. 1998;58:585–587. [PubMed] [Google Scholar]
- 25.Amant F, Dorfling CM, de Brabanter J, et al. A possible role of the cytochrome P450c17alpha gene (CYP17) polymorphism in the pathobiology of uterine leiomyomas from black South African women: a pilot study. Acta Obstet Gynecol Scand. 2004;83:234–239. [PubMed] [Google Scholar]
- 26.Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Mitrunen K. Polymorphic catechol-O-methyltransferase gene and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:635–640. [PubMed] [Google Scholar]
- 28.Bradlow LH. 2-Hydroxyesterone: the “good” estrogen. J Endocrinol. 1996;150:S259–S265. [PubMed] [Google Scholar]
- 29.Vandewalle B. Opposite effects of estrogen and catecholestrogen on hormone-sensitive breast cancer cell growth and differentiation. Mol Cell Endocrinol. 1989;61:239–246. doi: 10.1016/0303-7207(89)90135-4. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z-J, Zhu BT. Concentration-dependent mitogenic and antiproliferative actions of 2-methoxyestradiol in estrogen receptor positive human breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:265–275. doi: 10.1016/j.jsbmb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee SN, Sengupta K, Banerjee S, Saxena NK, Banerjee SK. 2-Methoxyestradiol exhibits a biphasic effect on VEGF-A in tumor cells and upregulation is mediated through ER-alpha: a possible signaling pathway associated with the impact of 2-ME2 on proliferative cells. Neoplasia. 2003;5:417–426. doi: 10.1016/s1476-5586(03)80044-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lippert C, Seeger H, Mueck AO. The effect of endogenous estradiol metabolites on the proliferation of human breast cancer cells. Life Sci. 2003;72:877–883. doi: 10.1016/s0024-3205(02)02305-6. [DOI] [PubMed] [Google Scholar]
- 33.Reddy VV, Hanjani P, Rajan R. Synthesis of catechol estrogens by human uterus and leiomyoma. Steroids. 1981;37:195–203. doi: 10.1016/s0039-128x(81)80017-7. [DOI] [PubMed] [Google Scholar]
- 34.Lavigne JA, Helzlsouer KJ, Huang HY, et al. An association between the allele coding for a low activity variant of catechol-O-methyltransferase and the risk for breast cancer. Cancer Res. 1997;57:5493–5497. [PubMed] [Google Scholar]
- 35.Al-Hendy A, Salama SA. Catechol-O-methyltransferase polymorphism is associated with increased uterine leiomyoma risk in different ethnic groups. Soc Gynecol Investig. 2006;13:136–144. doi: 10.1016/j.jsgi.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Chegini N, Verala J, Luo X, Xu J, Williams RS. Gene expression profile of leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. J Soc Gynecol Investig. 2003;10:161–171. doi: 10.1016/s1071-5576(03)00004-2. [DOI] [PubMed] [Google Scholar]
- 37.Sadan O, van Iddekinge B, Savage N, Robinson M, Zakut H. Ethnic variation in estrogen and progesterone receptor concentration in leiomyoma and normal myometrium. Gynecol Endocrinol. 1998;2:275–282. doi: 10.3109/09513598809107651. [DOI] [PubMed] [Google Scholar]
- 38.Amant F, Huys E, Geurts-Moespot A, et al. Ethnic variations in uterine leiomyoma biology are not caused by differences in myometrial estrogen receptor alpha levels. J Soc Gynecol Invest. 2003;102:105–109. doi: 10.1016/s1071-5576(02)00253-8. [DOI] [PubMed] [Google Scholar]
- 39.Wei J-J, Chiriboga L, Arslan A, Melamed J, Yee H, Mittal K. Ethnic differences in expression of the dysregulated proteins in uterine leiomyoma. Hum Reprod. 2006;21:57–67. doi: 10.1093/humrep/dei309. [DOI] [PubMed] [Google Scholar]
- 40.Quade BJ, Wang TY, Sornberger K, Cin PD, Mutter GL, Morton CC. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosomes Cancer. 2004;40:97–108. doi: 10.1002/gcc.20018. [DOI] [PubMed] [Google Scholar]
- 41.Arslan AA, Gold LI, Mittal K, et al. Gene expression studies provide clues to the pathogenesis of uterine leiomyoma: new evidence and a systematic review. Hum Reprod. 2005;204:852–863. doi: 10.1093/humrep/deh698. [DOI] [PubMed] [Google Scholar]
- 42.Weel AE, Uitterlinden AG, Westendorp IC, et al. Estrogen receptor polymorphism predicts the onset of natural and surgical menopause. J Clin Endocrinol Metab. 1999;84:3146–3150. doi: 10.1210/jcem.84.9.5981. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi S. Association of bone mineral density with polymorphism of the estrogen receptor gene. J Bone Miner Res. 1996;11:306–311. doi: 10.1002/jbmr.5650110304. [DOI] [PubMed] [Google Scholar]
- 44.Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development. 2005;132:4653–4662. doi: 10.1242/dev.02073. [DOI] [PubMed] [Google Scholar]
- 45.He H, Jazdzewski K, Li W, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci USA. 2005;102:19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Pregnancy after uterine artery embolization for leiomyomata: the Ontario Multicenter Trial. Obstet Gynecol. 2005;105:67–76. doi: 10.1097/01.AOG.0000149156.07061.1f. [DOI] [PubMed] [Google Scholar]
- 47.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Zhang X, Obijuru L, et al. A micro DNA signature associated with race tumor size, and target gene activity in human uterine leiomyoma. Genes Chromosomes Cancer. 2007;46:336–347. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 49.Al-Hendy A, Salama SA. Ethnic differences of estrogen receptor-α polymorphism is associated with higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86:686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]