INTRODUCTION
Despite considerable advances in pediatric HIV care and treatment,[1] the global burden of pediatric HIV infection remains great, particularly in sub-Saharan Africa. With infrastructure to support timely diagnosis and antiretroviral (ARV) medications for pregnant HIV-infected mothers and infants, mother-to-child HIV transmission rates may be reduced from greater than 25% to less than 5%.[2, 3] In 2010 however, an estimated 390,000 new pediatric infections occurred, with 98% of these occurring in low and middle income countries (LMIC).[1] In 2011, The Joint United Nations Programme on HIV/AIDS (UNAIDS) recently framed an initiative to reduce new pediatric HIV infections globally by 90%, provide ARV medications for all HIV-infected children, and reduce AIDS-related infant deaths by >50% by 2015.[4] Optimized medication regimens for pregnant women and HIV-exposed or infected infants, access to maternal[5] and early infant[6–9] diagnostic testing, retention in services,[10–12] and ARV adherence [13–15] are vital for improving prevention of mother-to-child HIV transmission (PMTCT) and HIV treatment effectiveness. Additionally, adult caregivers must also be able to accurately follow ARV-based prophylaxis or treatment plans in order to prevent and/or treat new pediatric HIV infections.
Caregiver errors in administering liquid medications to children are frequent in the United States (US),[16–19] with as many as 30–70% of caregivers measuring or administering liquid over-the-counter medications incorrectly.[17–20] While factors that contribute to dosing errors are incompletely understood, medication-specific factors, such as concentration, the health-care provider’s communication skills, and caregiver characteristics, such as health literacy, defined as “the degree to which individuals have the capacity to obtain, process and understand basic health information and services needed to make appropriate health decisions,”[21] have been found to play a role.[22] Exposure to a range of dosing instruments, such as cups, syringes, and spoons, may also contribute to confusion on the part of the caregiver,[20, 23, 24] particularly in the setting of complex instructions.
Little is known about caregiver dosing accuracy for liquid medications in LMICs. Pediatric ARV medications such as zidovudine, commonly used in prophylactic[2] or treatment regimens for pediatric HIV infection,[25] may have serious adverse consequences from either overdosing or underdosing. In sub-Saharan Africa, characteristics that contribute to dosing errors, such as low health literacy, are common among caregivers.[26] We hypothesized that in a population of adults in sub-Saharan Africa who were receiving cART for their own HIV infections, lower HIV health literacy would be associated with dosing errors for liquid zidovudine. Our objectives were to assess the ability of our study population to accurately dose liquid zidovudine using two commonly available dosing instruments and to evaluate the impact of HIV health literacy on dosing accuracy for liquid zidovudine in Mozambique.
METHODS
Study Design
Between August and November 2012, we enrolled a convenience sample of adults receiving combination antiretroviral therapy (cART) for HIV infection in Maputo Province, Mozambique to participate in a primary, validation study designed to validate a novel measure of HIV health literacy (the HIV Literacy Test or HIV-LT). The study took place at two public clinic sites: Polana Caniço, an urban facility located near the Maputo city center, and Marracuene, a rural facility located approximately 40 km from the center of the city of Maputo. Our cross-sectional assessment of dosing ability was nested within this primary study.
Study Setting
Both clinic facilities are staffed by Clinical Officers assigned by the Ministry of Health and offer adult and pediatric HIV care and treatment services, as well as a broad range of other services including antenatal care and enrollment in PMTCT and tuberculosis care and treatment. . During the time period of the study, the Mozambican national guidelines[27] recommended “opt-out” PMTCT services based on universal offering of testing and treatment as indicated during pregnancy as well zidovudine/nevirapine dual therapy (or occasionally cART-based approaches) for the exposed infant. Caregivers of HIV-exposed/infected infants and children receive written prescriptions from the health care provider for ARV medications for the child. Typically, full bottles of liquid ARV formulations are dispensed by the on-site pharmacy. Although dosing cups or syringes are dispensed when available, no single standardized dosing instrument is routinely offered. Caregivers may receive brief dosing instructions at the pharmacy; however, no formal counseling is provided systematically.
Study Participants
HIV-infected Portuguese-speaking adults aged 18–49 years presenting to either health center for HIV-related care and treatment were included. To be eligible, participants must also have been receiving cART for at least three months. Subjects were excluded if they were unable to communicate in basic Portuguese or did not pass vision screening.
Measures
Data included interview and observational measures. All study-related communications were conducted in Portuguese, the official language of Mozambique and the language most commonly used in the health centers.[26] The primary outcome variable was dosing accuracy. Primary predictor variables were dosing instrument type and HIV health literacy.
Literacy measurement
Each participant was administered the HIV-LT, a novel 16-item scale designed to measure literacy and numeracy skill applied to HIV self-management. The HIV-LT was designed to assess common literacy- and numeracy-related tasks that adults with HIV infection must perform in order to participate in care. These tasks include the ability to dose oral medications, determine the timing of follow-up appointments, and understand the risk of HIV transmission and treatment side effects. Scores were calculated as the number of items answered correctly and ranged from 0–16 with higher scores indicating greater literacy. The HIV-LT was validated as part of the primary study,[28] and the internal reliability was excellent (Kuder-Richardson 20 coefficient = 0.87).
Dosing instruments
A standard dosing cup (10 mL) and a standard dosing syringe (10 mL) were used that were obtained from Mozambican clinics. The plastic cup had clear etched calibration markings at every 2.5 mL interval. The syringe had black calibration markings printed numerically at each mL interval, with hash marks every intervening 0.2 mL.
Participants were shown a prescription card and were read a scripted prompt and instructed to measure a hypothetical pediatric dose of zidovudine suspension [2.5 mL (25 mg)] using both the cup and syringe. The measured dose was weighed using an electronic digital prescription scale accurate to 0.05g (Zieis; Apple Valley, MN; USA). For both the cup and syringe, the magnitude of error was calculated by comparing the weight of the measured dose (weight of the instrument containing the measured dose minus the pre-assessment instrument weight) to a reference weight.[23] The average weight of 2.5 mL of zidovudine as measured by five pediatricians was recorded to establish the reference weights for both the cup and the syringe. No zidovudine was administered to study participants or their children.
Dosing accuracy was recorded as a categorical variable. Dosing errors were defined as either no error (within 20% of the reference weight), any error (any dosing error ≥20% deviation from the reference weight), or major error (≥40% deviation from the reference weight).[23]
Socioeconomic data and health status
Sociodemographic data were collected by standardized interview and included age, sex, educational status attained, years of education, primary language spoken, employment status and type, household income, and number and ages of children in the household. Caregivers were also categorized by the presence of children <5, as well as <18 years, in the household.
Statistical analysis
Sample size
Our subjects were enrolled to participate in a primary study designed to validate the HIV-LT, and validation of this scale served as the basis for sample size calculations for our substudy. For scale validation, generally approximately 10–20 subjects are needed per item;[29] therefore, we aimed to enroll 320 participants for the 16-item HIV-LT.
Data were analyzed using Stata® version 12.0 statistical software (StataCorp LP, College Station, Texas, USA). Continuous variables were compared between sites using the Student’s t-test; categorical variables were compared between groups using the Chi-square test. The frequency of dosing errors was reported by calculating the proportion of subjects who committed any dosing error [≥20% deviation from reference dose] and major dosing errors (≥40% deviation) for each dosing instrument in the total population; comparisons of dosing error frequency were made according to literacy level, sex, and the presence of a child <5 years of age in the household. The proportions of errors for each dosing instrument were compared in the total population, as well as in participants with children <5 years in the household and between the groups with below-average versus above-average HIV-LT scores using the Wilcoxon signed-rank test. Multivariable logistic regression analysis was conducted to analyze the relationships between HIV-LT score and the occurrence of errors for each dosing instrument. For the multivariable model, a number of covariates were specified a priori, including the adult’s age, sex, clinic site, employment, income, years of education, duration of ARV therapy, language spoken at home, and number of children <18 years of age in the household.
All study procedures were approved by the Institutional Review Boards at Vanderbilt University Medical Center (Nashville, TN; USA) and Universidade Eduardo Mondlane (Maputo, Mozambique). All participants provided informed consent prior to study enrollment.
RESULTS
Our recruitment and enrollment process is outlined in Figure 1. We approached 578 potential subjects; 102 (17.6%) refused or could not communicate in basic Portuguese. Refusals were primarily due to concerns about delaying appointments. Of 476 individuals who provided informed consent, 316 individuals (66.4%) were eligible and completed all study measures. Demographic characteristics are described in Table 1. The mean age of subjects was 34.8 years (SD: 6.7); 76% were women. Nearly 90% of subjects had at least one child <18 years or under in the household, and nearly half of all subjects had at least one child <5 years of age in the household. Levels of education, income, and employment were significantly lower in subjects enrolled in the rural Marracuene clinic than in the urban Polana Caniço clinic. The mean HIV-LT score in our sample was 6.7 (SD 4.1). HIV-LT scores were strongly correlated with general literacy (ρ = 0.8), general numeracy (ρ = 0.7) and education (ρ = 0.7) (p<0.001). Participants enrolled at the rural Marracuene clinic scored significantly lower on the HIV-LT than those at Polana Caniço [mean (SD) HIV-LT scores 5.3 (4.0) and 7.7 (3.9), respectively (p <0.0001)]. Sociodemographic characteristics were compared between participants with and without children <5 years of age in the household (data not shown). The group with children <5 were slightly younger [33.8 (6.7) vs 35.7 (6.6); p=0.02] and had more children in the household [3.5 (1.8) vs 1.8 (1.4); p<0.0001] than the group without children <5 years in the household.
Figure 1.
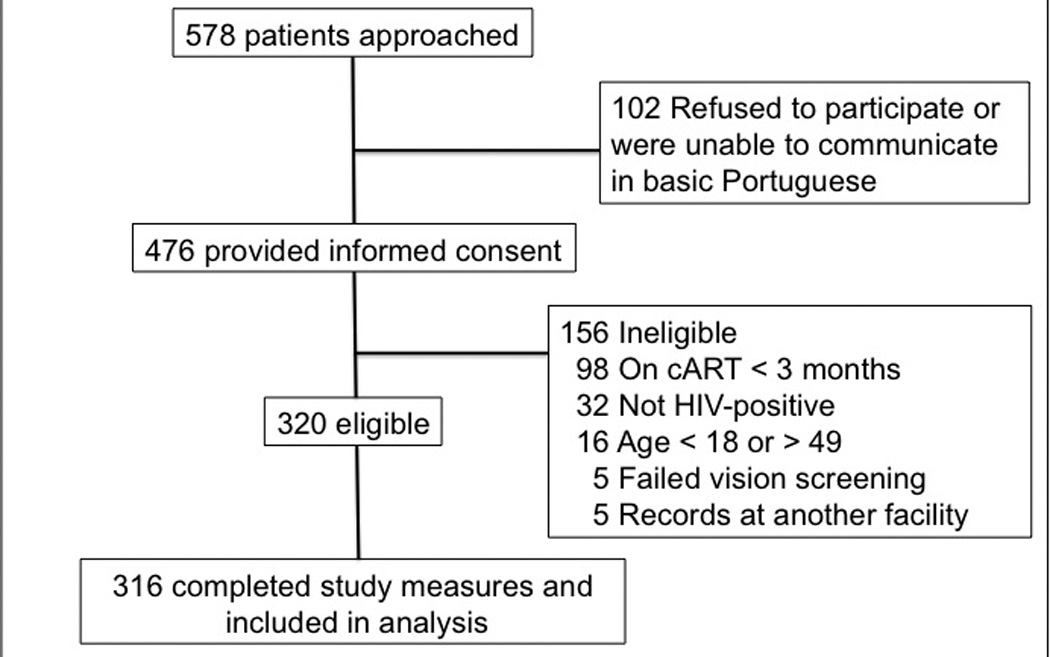
Recruitment process for 316 HIV-infected Mozambican adults enrolled in the pediatric dosing study for antiretroviral medications
Table 1.
Sociodemographic Characteristics of 316 HIV-infected Mozambican adults enrolled in the pediatric dosing study for antiretroviral medications
| Characteristic | All Subjects (n=316) |
Polana Caniço (n=178) |
Marracuene (n=138) |
p-value |
|---|---|---|---|---|
| Age, mean (SD), y | 34.8 (6.7) | 34.6 (6.8) | 35.0 (6.5) | 0.61 |
| Sex, female; No. (%) | 240 (76.0) | 136 (76.4) | 104 (75.4) | 0.83 |
| Occupation,+ No. (%) | <0.001 | |||
| Work outside the home | 153 (49.0) | 102 (57.6) | 51 (37.8) | |
| Unemployed or other | 163 (51.0) | 76 (42.4) | 87 (62.2) | |
| Monthly income; No. (%) | 0.001 | |||
| 0–5999 MZN* | 230 (72.8) | 117 (65.7) | 113 (81.9) | |
| 6000 MZN or more | 86 (27.2) | 61 (34.3) | 25 (18.1) | |
| Education, mean (SD), y | 6.1 (3.6) | 7.1 (3.5) | 4.8 (3.3) | <0.0001 |
| Language at home, No. (%) | <0.001 | |||
| Portuguese | 108 (34.2) | 82 (46.1) | 26 (18.9) | |
| Time on ARVs,# mean (SD), y | 2.8 (2.4) | 3.0 (2.5) | 2.4 (2.1) | 0.02 |
| Presence of child < 18 y, No. (%) | 281 (88.9) | 157 (88.2) | 124 (89.9) | 0.64 |
| Presence of child < 5 y, No. (%) | 146 (46.2) | 81 (45.5) | 65 (47.1) | 0.78 |
| Children in household, mean (SD) | 2.6 (1.8) | 2.6 (2.0) | 2.6 (1.6) | 0.95 |
| HIV-LT score, mean (SD) | 6.7 (4.1) | 7.7 (3.9) | 5.3 (4.0) | <0.0001 |
n=312
n=310
MZN=Mozambican Meticais (6000 MZN≈202 USD)
NOTE: y=years; SD=standard deviation; No.=number; HIV-LT= HIV Literacy Test
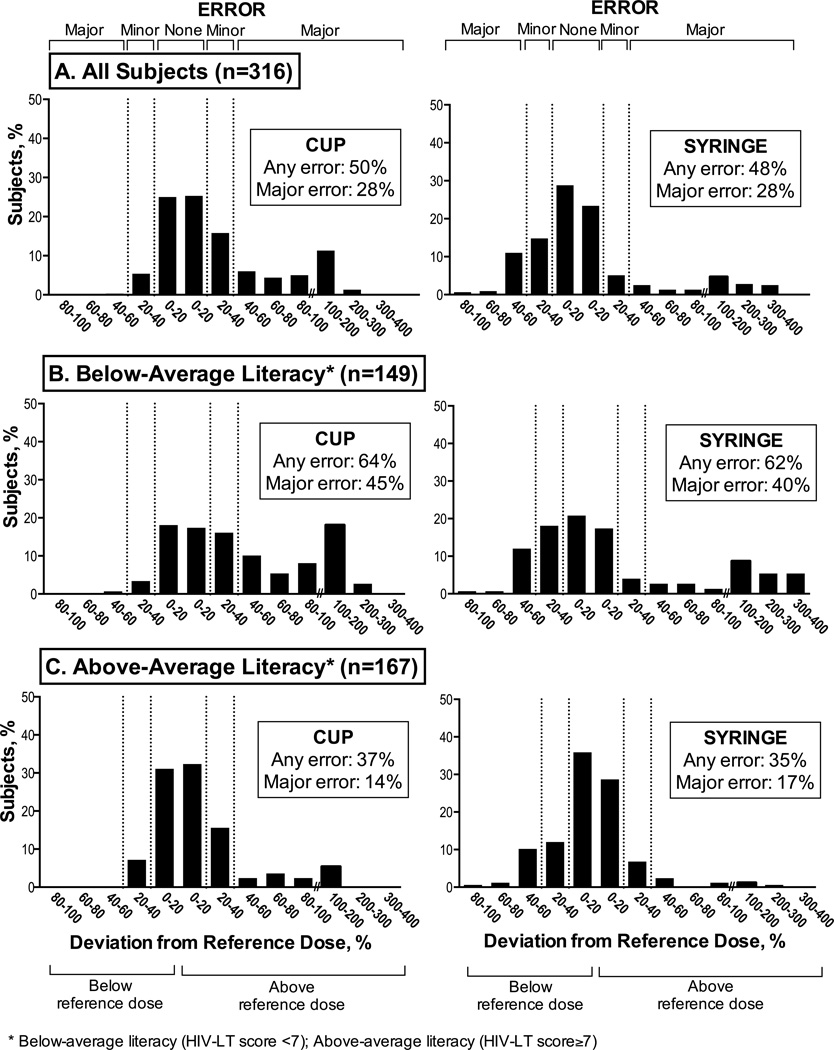
The proportions of dosing errors using both the cup and syringe are reported in Figure 2. Using the cup, 157 subjects (49.7%) made any error, including 90 subjects (28.4%) who committed a major dosing error. Using the syringe, dosing errors were also common, with 151 (47.8%) of subjects committing any error, and 88 of these (27.9%) committing major errors. Nearly 90% of errors made using the cup were overdosing errors, while the majority of errors using the syringe were underdosed (58%). Comparing the occurrence of dosing errors by dosing instrument, there were no significant differences in the proportion of dosing errors using the dosing cup compared with the dosing syringe (Figure 2A). No significant differences existed in the proportion of dosing errors between subjects with children under the age of five years in the household compared to those without. Similarly, there were no differences in proportion of dosing errors by sex. <
Figure 2. Proportion of dosing errors according to dosing instrument and by literacy level (n=316).
There was no significant difference in the proportion of dosing errors using the dosing cup compared with the dosing syringe (Figure 2A). Using cups, participants with below-average literacy (2B, left figure) were more likely than participants with above-average literacy (2C, left figure) to make any dosing error (p<0.001) and major dosing errors (p<0.001). With the syringe, participants with below-average literacy (2B, right figure) were more likely than participants with above-average literacy (2C, right figure) to make any dosing error (p<0.001) and major dosing errors (p<0.001).
Subjects with below-average literacy were significantly more likely to commit dosing errors than those with above-average literacy using both the cup and syringe (Figure 2B and 2C). After adjustment for covariates, higher HIV-LT score remained significantly associated with reduced odds of committing any error and major errors using either dosing instrument (Table 2), in both the total population and the subpopulation with at least one child less than five years of age in the household. Subjects with above-average literacy had significantly reduced odds of committing any error using both the dosing cup [AOR 0.52 (95% CI 0.29–0.92), p=0.03] and syringe [AOR 0.39 (95% CI 0.19–0.80), p=0.01]. Aside from HIV-LT score, in the adjusted model, only education was significantly associated with reduced major errors using both the cup (p=0.021) and the syringe (p=0.038).
Table 2.
Association of HIV-LT Score with Dosing Errors by Dosing Instrument
| Any Dosing Error (≥20%) |
Major Dosing Error (≥40%) |
|||||
|---|---|---|---|---|---|---|
| HIV-LT Score | AOR* | 95% CI | p-value | AOR* | 95% CI | p-value |
| All Subjects (n=316) | ||||||
| Per Item Correct (Cup) | 0.91 | (0.84–0.99) | 0.03 | 0.84 | (0.75–0.92) | 0.001 |
| Per Item Correct (Syringe) | 0.82 | (0.75–0.90) | <0.001 | 0.88 | (0.80–0.97) | 0.01 |
| Subjects with Child <5 in Household | ||||||
| Per Item Correct (Cup) | 0.84 | (0.73–0.97) | 0.02 | 0.79 | (0.64–0.98) | 0.03 |
| Per Item Correct (Syringe) | 0.87 | (0.75–0.99) | 0.04 | 0.86 | (0.72–1.02) | 0.09 |
Adjusted for age, sex, clinic site, years of education, income, years on antiretroviral therapy, employment, and number of children ≤ 18 years of age
DISCUSSION
To our knowledge, this study is the first to evaluate dosing accuracy for a pediatric ARV medication, particularly in a low-income country[30] with high HIV prevalence. Dosing errors were exceedingly common, despite the fact that all subjects were receiving cART for their own HIV infections. Alongside efforts toward developing improved pediatric ARV formulations and enhancing global access to diagnostic technologies and interventions for HIV infection, our study highlights serious concern as to a caregiver’s ability to accurately dose infants for PMTCT or child cART. Our findings suggest that targeted interventions to improve health care communication and instructions about pediatric liquid ARV dosing are likely a critical component of efforts to improve pediatric ARV adherence, particularly in settings in which tablet and/or dissolvable formulations are unavailable in appropriate dosages for infants and small children.
As early as 1975, the American Academy of Pediatrics recommended the use of dosing syringes for highest accuracy for pediatric liquid medication dosing.[24, 31, 32] To date, no such standard of care has been adopted in Mozambique. In 2008, Yin et al[23] found that the use of the dosing syringe enhanced dosing accuracy for liquid acetaminophen compared to the cup in an urban US population. In contrast to these findings and others,[23, 24, 33] syringe error rates in our population were comparatively high, possibly reflecting less familiarity with syringe dosing. Given that the advantage of the dosing syringe in reducing the proportion of dosing errors was not clearly established in our study population, additional studies are needed to determine if recommendations for the use of dosing syringes in the US also apply to other settings, particularly resource-limited settings, where other factors that may contribute to dosing inaccuracy may be more prevalent.
Given the potentially narrow therapeutic window of zidovudine, our definitions for errors were liberal relative to the US Pharmacopeia’s standard acceptable volumetric error for calibrated devices.[38], Thus, our findings can be considered a minimum estimate of the problem. Zidovudine is the ARV medication most frequently associated with severe toxicity, usually anemia and leukopenia, in pediatric HIV patients in Africa.[39] These toxicities may occur at concentrations near those associated with the optimal antiviral effect[40, 41] and more advanced HIV infection at the time of ARV initiation, other comorbidities,,[42, 43] or concomitant medications[44][45] may further exacerbate ARV toxicities. However, the consequences of underdosing zidovudine are also critical, including risk of mother-to-child transmission, virologic failure, and resistance. However in Mozambique, overdosing ARV medications exposes the child not only to toxicity from supratherapeutic doses of medication initially, but also to the risks associated with underdosing when the medication supply is completed before refills are obtained,. Despite the fact that the syringe was not superior to the cup in our population with regards to the proportion of errors, our finding that overdosing errors were less common with the syringe, as previously demonstrated in a study from the U.S.,[24] lends support for the syringe as the preferred dosing instrument in this setting.
Strengths of our study include use of standard measuring approaches, precise calibration of errors, and a novel measure of HIV health literacy. Limitations include using a hypothetical dosing scenario that may not truly reflect the ability of caregivers to administer zidovudine doses to children that are relevant to PMTCT and cART of pediatric HIV infection in real-world settings. Because we designed the study to validate the HIV-LT in a general population of adults on treatment for HIV, participants were not necessarily caring for children receiving ARV medications, although nearly half of our study population had children under five in the home, and there were no differences in the dosing accuracy performance of this subpopulation. Additionally, subjects were provided 10 mL dosing instruments, allowing more opportunity to overdose than underdose. Since no biologic outcomes were measured, it remains unclear if the proportion and magnitude of dosing errors seen in our study population translate into clinically relevant outcomes. Finally, the individuals captured in our cross-sectional study, HIV-infected individuals with experience in adult ARV dosing, likely represent those with the most consistent access to care and the greatest dosing abilities, and may underestimate the burden of liquid medication dosing errors in more remote areas in Mozambique, where lower literacy and numeracy abilities may be more prevalent.[26]
In summary, dosing errors for a liquid pediatric antiretroviral medication were very common among HIV-infected adults in Maputo, Mozambique and were significantly associated with lower HIV health literacy. The HIV-LT could be used in clinic settings to identify caregivers of HIV-exposed/infected children who may benefit from additional dosing instruction or counseling strategies. In addition to the many complex social and financial barriers to ARV adherence, our findings suggest that addressing caregiver dosing accuracy is a critical component of enhancing adherence to pediatric ARV medications throughout southern Africa and should encourage programs to review their standard plans for giving medicines to young infants. Given the serious implications for successful PMTCT and pediatric HIV treatment outcomes, we urge development of targeted approaches to improving health care instructions and dosing skills for caregivers at the pediatric point of care.
ACKNOWLEDGEMENTS
Author responsibilities: LMH, JAT, and PJC initiated the project, designed the study, developed the protocol and designed the tools for collecting data. LMH, JAT, and SG participated in data collection. LMH, JAT, and PJC cleaned and analyzed the data. LMH drafted and made revisions to the manuscript. JAT, SG, MS, RLR, SHV, and PJC contributed to interpretation of the analysis and revision of the manuscript. LMH and PJC are guarantors for the study. All authors had full access to all of the data, including statistical reports and tables, and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest and Source of Funding: All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare that (1) LMH, JAT, SG, MS, RLR, SHV, and PJC have no support from any company for the submitted work; (2) LMH, JAT, SG, MS, RLR, SHV, and PJC have no relationships to companies that might have an interested in the submitted work in the previous 3 years; (3) their spouses, partners, or children have no financial relationships that may be relevant to the submitted work; and (4) LMH, JAT, SG, MS, RLR, SHV, and PJC have no non-financial interests that may be relevant to the submitted work. Dr. Howard was funded by the Childhood Infections Research Program Training Grant #IT32AI095202-01. Dr. Tique was funded by AIDS International Training and Research Program NIH grant #D43TW001035. Researchers were independent from funders, and funders had no role in study design, data collection, analysis, interpretation, decision to publish or preparation of the article. The content is solely the responsibility of the authors.
Footnotes
All authors declare that the research was conducted in accordance with the Declaration of Helskinki. Experiments were conducted with the understanding and the consent of each participant, and the responsible ethical committees have approved the experiments.
All authors declare that they have read and approved the paper, that they meet the criteria for authorship as established by the International Committee of Medical Journal Editors, that they believe that the paper represents honest work, and that they are able to verify the validity of the results reported.
Contributors: Dr. Kathryn Edwards critically reviewed the manuscript.
Contributor Information
Leigh M. Howard, Instructor, Pediatric Infectious Diseases, Vanderbilt University School of Medicine, 1161 21Avenue South; CCC-5319 MCN, Nashville, TN 37212; USA, leigh.howard@vanderbilt.edu.
José A. Tique, Clinical Advisor, Friends in Global Health, LLC, Avenida da Maguiguana, N° 32, CP 604, Maputo, Moçambique.
Sandra Gaveta, Fellow, Departamento de Saúde da Comunidade, Faculdade de Medicina da Universidade Eduardo Mondlane, Avenida Salvador Allende, N°702, CP: 257, Maputo, Moçambique.
Mohsin Sidat, Professor, Departamento de Saúde da Comunidade, Faculdade de Medicina da Universidade Eduardo Mondlane, Avenida Salvador Allende, N°702, CP: 257, Maputo, Moçambique.
Russell L. Rothman, Associate Professor, Internal Medicine and Pediatrics, Vanderbilt University School of Medicine, 2525 West End Ave., Suite 600, Nashville, Tennessee 37203-1738.
Sten H. Vermund, Professor of Pediatrics, Internal Medicine, and Obstetrics and Gynecology, Vanderbilt University School of Medicine, 2525 West End Avenue, Suite 750, Nashville, TN 37203-1738.
Philip J. Ciampa, Instructor, Internal Medicine and Pediatrics, Vanderbilt University School of Medicine, 2525 West End Avenue, Suite 725, Nashville, TN 37203-1738.
REFERENCES
- 1.Organization WH. Global HIV/AIDS Response: Epidemic update and health sector progress towards Universal Access - Progress Report. 2011 [Google Scholar]
- 2.Study EC. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–465. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 3.Chetty T, Knight S, Giddy J, Crankshaw TL, Butler LM, Newell ML. A retrospective study of Human Immunodeficiency Virus transmission, mortality and loss to follow-up among infants in the first 18 months of life in a prevention of mother-to-child transmission programme in an urban hospital in KwaZulu-Natal, South Africa. BMC Pediatr. 2012;12:146. doi: 10.1186/1471-2431-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.HIV/AIDS JUNPo. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. 2011:2011–2015. [Google Scholar]
- 5.Spensley A, Sripipatana T, Turner AN, Hoblitzelle C, Robinson J, Wilfert C. Preventing mother-to-child transmission of HIV in resource-limited settings: the Elizabeth Glaser Pediatric AIDS Foundation experience. Am J Public Health. 2009;99:631–637. doi: 10.2105/AJPH.2007.114421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke GS, Little KE, Bland RM, Thulare H, Newell ML. Need for timely paediatric HIV treatment within primary health care in rural South Africa. PLoS One. 2009;4:e7101. doi: 10.1371/journal.pone.0007101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejiokem MC, Faye A, Penda IC, Guemkam G, Ateba Ndongo F, Chewa G, et al. Feasibility of early infant diagnosis of HIV in resource-limited settings: the ANRS 12140-PEDIACAM study in Cameroon. PLoS One. 2011;6:e21840. doi: 10.1371/journal.pone.0021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook RE, Ciampa PJ, Sidat M, Blevins M, Burlison J, Davidson MA, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambezia, Mozambique. J Acquir Immune Defic Syndr. 2011;56:e104–e109. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcos Y, Phelps BR, Bachman G. Community strategies that improve care and retention along the prevention of mother-to-child transmission of HIV cascade: a review. J Int AIDS Soc. 2012;15(Suppl 2):17394. doi: 10.7448/IAS.15.4.17394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciampa PJ, Burlison JR, Blevins M, Sidat M, Moon TD, Rothman RL, et al. Improving retention in the early infant diagnosis of HIV program in rural Mozambique by better service integration. J Acquir Immune Defic Syndr. 2011;58:115–119. doi: 10.1097/QAI.0b013e31822149bf. [DOI] [PubMed] [Google Scholar]
- 12.Ciampa PJ, Tique JA, Juma N, Sidat M, Moon TD, Rothman RL, et al. Addressing poor retention of infants exposed to HIV: a quality improvement study in rural Mozambique. J Acquir Immune Defic Syndr. 2012;60:e46–e52. doi: 10.1097/QAI.0b013e31824c0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boateng D, Kwapong GD, Agyei-Baffour P. Knowledge, perception about antiretroviral therapy (ART) and prevention of mother-to-child-transmission (PMTCT) and adherence to ART among HIV positive women in the Ashanti Region, Ghana: a cross-sectional study. BMC Womens Health. 2013;13:2. doi: 10.1186/1472-6874-13-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mepham S, Zondi Z, Mbuyazi A, Mkhwanazi N, Newell ML. Challenges in PMTCT antiretroviral adherence in northern KwaZulu-Natal, South Africa. AIDS Care. 2011;23:741–747. doi: 10.1080/09540121.2010.516341. [DOI] [PubMed] [Google Scholar]
- 15.Kirsten I, Sewangi J, Kunz A, Dugange F, Ziske J, Jordan-Harder B, et al. Adherence to combination prophylaxis for prevention of mother-to-child-transmission of HIV in Tanzania. PLoS One. 2011;6:e21020. doi: 10.1371/journal.pone.0021020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zandieh SO, Goldmann DA, Keohane CA, Yoon C, Bates DW, Kaushal R. Risk factors in preventable adverse drug events in pediatric outpatients. J Pediatr. 2008;152:225–231. doi: 10.1016/j.jpeds.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 17.Frush KS, Luo X, Hutchinson P, Higgins JN. Evaluation of a method to reduce over-the-counter medication dosing error. Arch Pediatr Adolesc Med. 2004;158:620–624. doi: 10.1001/archpedi.158.7.620. [DOI] [PubMed] [Google Scholar]
- 18.Li SF, Lacher B, Crain EF. Acetaminophen and ibuprofen dosing by parents. Pediatr Emerg Care. 2000;16:394–397. doi: 10.1097/00006565-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gribetz B, Cronley SA. Underdosing of acetaminophen by parents. Pediatrics. 1987;80:630–633. [PubMed] [Google Scholar]
- 20.Simon HK, Weinkle DA. Over-the-counter medications. Do parents give what they intend to give? Arch Pediatr Adolesc Med. 1997;151:654–656. doi: 10.1001/archpedi.1997.02170440016003. [DOI] [PubMed] [Google Scholar]
- 21.Medicine Io. Health Literacy: A Prescription to End Confusion. Washington, D.C: National Academies Press; 2004. [PubMed] [Google Scholar]
- 22.Yin HS, Dreyer BP, van Schaick L, Foltin GL, Dinglas C, Mendelsohn AL. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch Pediatr Adolesc Med. 2008;162:814–822. doi: 10.1001/archpedi.162.9.814. [DOI] [PubMed] [Google Scholar]
- 23.Yin HS, Mendelsohn AL, Wolf MS, Parker RM, Fierman A, van Schaick L, et al. Parents' medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164:181–186. doi: 10.1001/archpediatrics.2009.269. [DOI] [PubMed] [Google Scholar]
- 24.Sobhani P, Christopherson J, Ambrose PJ, Corelli RL. Accuracy of oral liquid measuring devices: comparison of dosing cup and oral dosing syringe. Ann Pharmacother. 2008;42:46–52. doi: 10.1345/aph.1K420. [DOI] [PubMed] [Google Scholar]
- 25.Organization WH. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. 2010 [PubMed] [Google Scholar]
- 26.Ciampa PJ, Vaz LM, Blevins M, Sidat M, Rothman RL, Vermund SH, et al. The association among literacy, numeracy, HIV knowledge and health-seeking behavior: a population-based survey of women in rural Mozambique. PLoS One. 2012;7:e39391. doi: 10.1371/journal.pone.0039391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaz PME, Santos P, Mboa C. In: Manual de Tratamento da Criança com Infecção pelo HIV/SIDA em Moçambique. Saú Md., editor. 2011. [Google Scholar]
- 28.Tique JAHL, Gaveta S, Sidat M, Rothman RL, Vermund SH, Ciampa PJ. Measuring Health Literacy in Patients with HIV Infection in Mozambique: Development and Validation of the HIV Literacy Test. Aids and Behavior. 2013 doi: 10.1007/s10461-016-1348-3. (In review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorsuch RL. Factor Analysis. Hillsdale, NJ: Lawrence Erlbaum Associates; 1983. [Google Scholar]
- 30.Group TWB. Country and Lending Groups. 2013 [Google Scholar]
- 31.Yaffe SJ, Bierman CW, Cann HM, Cohen SN, Freeman J, Segal S, et al. Inaccuracies in administering liquid medication. Pediatrics. 1975;56:327–328. [PubMed] [Google Scholar]
- 32.Paul I, Yin HS. Out with teaspoons, in with metric units. AAP News. 2012:10–11. [Google Scholar]
- 33.Madlon-Kay DJ, Mosch FS. Liquid medication dosing errors. J Fam Pract. 2000;49:741–744. [PubMed] [Google Scholar]
- 34.Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124(Suppl 3):S289–S298. doi: 10.1542/peds.2009-1162E. [DOI] [PubMed] [Google Scholar]
- 35.Janisse HC, Naar-King S, Ellis D. Brief report: Parent's health literacy among high-risk adolescents with insulin dependent diabetes. J Pediatr Psychol. 2010;35:436–440. doi: 10.1093/jpepsy/jsp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedman RB, Jones SK, Lin A, Robin AL, Muir KW. Influence of parental health literacy and dosing responsibility on pediatric glaucoma medication adherence. Arch Ophthalmol. 2012;130:306–311. doi: 10.1001/archopthalmol.2011.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeWalt DA, Hink A. Health literacy and child health outcomes: a systematic review of the literature. Pediatrics. 2009;124(Suppl 3):S265–S274. doi: 10.1542/peds.2009-1162B. [DOI] [PubMed] [Google Scholar]
- 38.Pharmacopeia U. Teaspoon. In. [Google Scholar]
- 39.Sauvageot D, Schaefer M, Olson D, Pujades-Rodriguez M, O'Brien DP. Antiretroviral therapy outcomes in resource-limited settings for HIV-infected children <5 years of age. Pediatrics. 2010;125:e1039–e1047. doi: 10.1542/peds.2009-1062. [DOI] [PubMed] [Google Scholar]
- 40.Pizzo PA, Eddy J, Falloon J, Balis FM, Murphy RF, Moss H, et al. Effect of continuous intravenous infusion of zidovudine (AZT) in children with symptomatic HIV infection. N Engl J Med. 1988;319:889–896. doi: 10.1056/NEJM198810063191401. [DOI] [PubMed] [Google Scholar]
- 41.Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC, et al. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985;82:7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prendergast A, Bwakura-Dangarembizi MF, Cook AD, Bakeera-Kitaka S, Natukunda E, Nahirya Ntege P, et al. Hospitalization for severe malnutrition among HIV-infected children starting antiretroviral therapy. AIDS. 2011;25:951–956. doi: 10.1097/QAD.0b013e328345e56b. [DOI] [PubMed] [Google Scholar]
- 43.Koethe JR, Heimburger DC. Nutritional aspects of HIV-associated wasting in sub-Saharan Africa. Am J Clin Nutr. 2010;91:1138S–1142S. doi: 10.3945/ajcn.2010.28608D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brentlinger PE, Behrens CB, Micek MA. Challenges in the concurrent management of malaria and HIV in pregnancy in sub-Saharan Africa. Lancet Infect Dis. 2006;6:100–111. doi: 10.1016/S1473-3099(06)70383-8. [DOI] [PubMed] [Google Scholar]
- 45.Harries AD, Chimzizi R, Zachariah R. Safety, effectiveness, and outcomes of concomitant use of highly active antiretroviral therapy with drugs for tuberculosis in resource-poor settings. Lancet. 2006;367:944–945. doi: 10.1016/S0140-6736(06)68387-6. [DOI] [PubMed] [Google Scholar]