Abstract
Objective
Cardiovascular disease (CVD) is the leading cause of death in severe psychiatric disorders (depression, schizophrenia). Here, we provide evidence of how the effects of oxidative stress on fatty acid (FA) and one-carbon (1-C) cycle metabolism, which may initially represent adaptive responses, might underlie comorbidity between CVD and psychiatric disorders.
Method
We conducted a literature search and integrated data in a narrative review.
Results
Oxidative stress, mainly generated in mitochondria, is implicated in both psychiatric and cardiovascular pathophysiology. Oxidative stress affects the intrinsically linked FA and 1-C cycle metabolism: FAs decrease in chain length and unsaturation (particularly omega-3 polyunsaturated FAs), and lipid peroxidation products increase; the 1-C cycle shifts from the methylation to transsulfuration pathway (lower folate and higher homocysteine and antioxidant glutathione). Interestingly, corresponding alterations were reported in psychiatric disorders and CVD. Potential mechanisms through which FA and 1-C cycle metabolism may be involved in brain (neurocognition, mood regulation) and cardiovascular system functioning (inflammation, thrombosis) include membrane peroxidizability and fluidity, eicosanoid synthesis, neuroprotection and epigenetics.
Conclusion
While oxidative-stress-induced alterations in FA and 1-C metabolism may initially enhance oxidative stress resistance, persisting chronically, they may cause damage possibly underlying (co-occurrence of) psychiatric disorders and CVD. This might have implications for research into diagnosis and (preventive) treatment of (CVD in) psychiatric patients.
Keywords: cardiovascular disease, fatty acids, homocysteine, oxidative stress, psychiatry
Summations
Oxidative stress and its effects on fatty acid and 1-carbon cycle metabolism may underlie co-occurrence of cardiovascular and psychiatric disorders.
During oxidative stress, fatty acids shorten in chain length and decrease in unsaturation and peroxidation, while the 1-carbon cycle shifts from the methylation to the transsulfuration pathway.
While these changes initially may enhance oxidative stress resistance, persisting chronically, they may cause damage.
Considerations
Interpreting fatty acid and 1-carbon metabolism changes as an adaptive response may partly explain disappointing results of supplementation of fatty acid and/or 1-carbon cycle components, for example B-vitamins, folate and antioxidants.
The oxidative-stress-induced pattern of fatty acid and 1-carbon metabolism changes does not seem to be specific to cardiovascular or psychiatric disorders, but is also found in other oxidative-stress-related diseases and during ageing.
A central role of oxidative stress in the link between psychiatric and cardiovascular disease stresses the need for monitoring of signs of oxidative stress (e.g. waist circumference) in psychiatric patients and treatment aimed at preventing oxidative stress development (e.g. diet, exercise, cognitive therapy).
Introduction
Relevance
Cardiovascular disease (CVD), including coronary heart disease, stroke and peripheral arterial disease, is the most frequent cause of excess mortality in patients with severe psychiatric disorders, such as schizophrenia, bipolar disorder and major depressive disorder (MDD) (1, 2). These patients have a doubled risk of dying from CVD, especially at an earlier age (1). Traditionally, the focus has been on schizophrenia, but CVD is of equal concern for patients with bipolar disorder or MDD (1–8). For example, MDD raises CVD risk 2.4 times (41% of MDD patients are at increased risk) (9), also prospectively (10).
On top of great personal suffering, CVD comorbidity in psychiatry causes substantial excess societal costs. Patients with high CVD risk have a more complex presentation of their psychiatric disorders, greater burden of disease, less favourable response to treatment and an adverse course and outcome (1, 2). Moreover, for example in bipolar disorder, CVD treatment accounts for 70% of total treatment costs (11). Therefore, improved understanding of CVD pathogenesis in psychiatric disorders is warranted.
However, pathophysiological mechanisms underlying the mutual association between psychiatric disorders and CVD are complex and still largely unknown. A better understanding of these mechanisms could i) provide directions for researchers investigating treatment and prevention options and ii) increase awareness among healthcare professionals for CVD risk in psychiatric patients, particularly general practitioners and psychiatrists, thereby iii) ensure early diagnosis and treatment.
Hypotheses and outline
In this review, we provide data indicating that oxidative stress underlies both psychiatric disorders and CVD (Part iii). In Part iv, we review the evidence that fatty acid (FA) metabolism mediates the manifestations of oxidative stress. Subsequently, we summarize FA metabolism alterations in psychiatric disorders and CVD. In Part v, we discuss how oxidative stress induces interrelated alterations in the methionine or 1-C cycle and FA metabolism and thereby may play an integrative role in oxidative-stress-associated pathophysiology of psychiatric disorders and CVD. In Part vi, we interpret studies that simultaneously assessed these biological alterations (oxidative stress, FA metabolism and 1-C cycle). By integrating these pathophysiological mechanisms (oxidative stress, FA and 1-C metabolism), a common pattern of specific biological alterations seems to emerge. Finally, we argue that this pattern may initially represent an adaptive process (Part vii).
Aims of the study
Hereby, we aim at shedding critical new light on the role of oxidative stress in i) the comorbidity of psychiatric disorders and CVD, ii) biochemical alterations observed in psychiatric patients vs. healthy controls and iii) results of intervention studies (e.g. supplementation, nutritional, psychological and physical exercise therapy) in this population.
Material and methods
We conducted a literature search in medline, embase and psycinfo databases, which we complemented with review articles and cross-references. We used search terms around oxidative stress, FA metabolism and the 1-C cycle, in combination with terms covering psychiatric disorders and CVD. It is beyond the scope of this review to address all relevant clinical studies addressing the role of oxidative stress, FAs and the 1-C cycle in CVD and psychiatry. Instead, we focussed on i) large-scale studies, reviews and/or meta-analyses addressing oxidative stress, FA metabolism or the 1-C cycle in CVD and/or psychiatric disorders; ii) specific studies combining clinical psychiatric and CVD characteristics with more detailed measurement of oxidative stress, FA metabolism or the 1-C cycle; and iii) studies simultaneously assessing oxidative stress, FA metabolism and/or the 1-C cycle in cardiovascular or psychiatric patients.
Both clinical concepts (CVD and psychiatric disorders) in our hypotheses and consequently search strategy cover wide areas. CVD includes, for example, stroke, coronary heart disease, type 2 diabetes mellitus and hypertension, while psychiatric disorders include MDD, schizophrenia and bipolar disorder. Because our hypotheses concern general and broad relations between psychiatric disorders and CVD risk, which do not seem to be specific to a particular psychiatric disorder or CVD entity, we chose not to limit our search strategy. However, as a result, many different forms of psychiatric disorders and CVD (risk factors) may be covered in this review. For CVD, examples of risk factors are insulin resistance, (visceral) obesity, dyslipidaemia, hypertension, subclinical inflammation and thrombosis, as encompassed by the debated concept ‘metabolic syndrome’ (12–14). To improve clarity and readability, we collectively addressed all these separate risk factors as CVD risk factors, where possible. In addition, we combined evidence regarding several psychiatric disorders. When a subdivision should be made, we addressed this in the text, for example, in the section Specificity.
We limited our search to articles published before May 2013 (without early date constraints). We focussed on recent articles, although we included older publications where warranted. We first excluded articles based on title and abstract (when available). Subsequently, at least two of us independently evaluated selected manuscripts. Finally, we integrated relevant data in a narrative review.
Results
Oxidative stress as shared underlying mechanism
Oxidative stress: the concept
Oxidative stress affects metabolism, signalling and functioning of cell types particularly relevant to pathogenesis of CVD and psychiatric disorders, such as neurons, endothelial cells, immune cells and platelets (15, 16). So, what is oxidative stress, and how does it originate?
Living with oxygen (O2), that is, breathing, causes lifelong stress. Mitochondria use oxygen for energy production, thereby generating reactive oxygen species (ROS) as potentially toxic byproducts, also called free radicals. This makes mitochondria the major source of ROS (17). At low levels, ROS are essential for adequate functioning of multiple physiological systems, including intracellular messaging, apoptosis and immunity. However, at high levels, ROS may cause cellular impairment by altering DNA, proteins and lipids (18, 19).
Therefore, to handle ROS levels inherent in living in an oxygen-rich environment, organisms have multiple layers of antioxidant defence at their disposal, consisting of i) damage removal, repair or replacement systems; ii) antioxidant enzymes such as superoxide dismutases, catalases and glutathione peroxidases; and iii) dietary (e.g. vitamins A, C and E and polyphenols) and endogenous antioxidants (e.g. glutathione), which all inactivate ROS (18, 20). Despite this efficient defence, some oxidative damage is inherent in aerobic life. This is believed to underlie ageing and affect human lifespan.
In conclusion, organisms must continuously confront and control both ROS and antioxidants. This balance – often referred to as redox potential – is tightly regulated and specific to each biological site. Interference with this balance may be deleterious. So, oxidative stress can be defined as a ‘disturbance in the pro-oxidant/antioxidant balance in favour of the former, leading to potential damage’ (18, 20).
Causes of oxidative stress
Several studies indicate that changes in oxidative stress are largely attributable to dietary factors and physical activity levels. Various modern lifestyle factors greatly increase mitochondrial ROS production, for example i) high saturated FA and sugar intake and ii) physical inactivity. In addition, iii) smoking, alcohol and lack of fresh fruits and vegetables (antioxidants) also intensify oxidative stress (13). Seemingly contradictory, on the short term, physical activity increases oxidative stress. However, in the long term, exercise-induced oxidative stress promotes biogenesis, maintenance and clearance of dysfunctional mitochondria, thereby diminishing ROS production (21, 22). In addition, this exercise-induced oxidative stress improves endogenous antioxidant defence capacity. Thereby, regular physical activity results in a net decrease in oxidative stress (23). Importantly, because mitochondria are the main source of ROS production, inherited and/or acquired mitochondrial dysfunction will strongly enhance oxidative stress (17). Being the source, mitochondrial membranes are subject to ROS exposure themselves; oxidative damage to these membranes may even further intensify ROS production, potentially creating a vicious cycle. Of note, brain cells also produce ROS during metabolism of important neurotransmitters in mood and psychosis (e.g. serotonin, noradrenalin and dopamine) by monoaminooxidase A and B, located on the mitochondrial membrane (16, 24).
Last but not least, psychological stress – severe life stress in particular – induces a variety of morphological and neurochemical modifications; among them oxidative stress is invariably observed (16).
Oxidative stress in CVD and psychiatric disorders
Oxidative stress in CVD
Considerable data indicate that ROS and oxidative stress are important features of CVD (25). A meta-analysis supported an inverse association between antioxidant enzymes (superoxide dismutase, glutathione peroxidase and catalase) and CVD (26). In addition, several studies implicate oxidative stress in CVD pathogenesis, including development of atherosclerosis and type 2 diabetes mellitus (27, 28). Increased oxidative stress was also associated with CVD risk factors in subjects that did not develop CVD yet (27), suggesting that oxidative stress may be an early causative factor in CVD pathology rather than a late consequence.
Indeed, elevated oxidative stress precedes insulin resistance, the first manifestation of CVD risk. Increased oxidative stress is probably the causal pathway that links, for example, excess caloric intake to insulin resistance (28). Furthermore, increased mitochondrial ROS production is the common feature of many different models of insulin resistance, including chronic treatment with insulin, corticosteroids, proinflammatory cytokines or lipids (29). This may suggest that insulin resistance – as a response to oxidative stress – could have an adaptive function: by decreasing glucose uptake, insulin resistance limits excess energy supply and thereby diminishes mitochondrial ROS production (28–30).
Oxidative stress in psychiatric disorders
Although it accounts for only 2% of total body mass, the brain consumes 20% of body energy (16). Besides high oxygen utilization, two more reasons make the brain most vulnerable to oxidative damage: first, its modest antioxidant defences and second, its highly oxidizable substrate, that is, lipids comprise 60–65% of brain dry weight (see ‘Discussion’). This may explain the basic role of oxidative stress in psychiatric disorders and how it may function as a common pathogenetic mechanism. Evidence, although still inconsistent at some points (31), is available for increased ROS concentrations and depletion of antioxidant defences (e.g. glutathione) in MDD, schizophrenia and bipolar depression (32–37). Notably, reductions in plasma antioxidant capacity are seen in patients with chronic disease as well as early in the course of schizophrenia. In addition, evidence for genetic/acquired impaired mitochondrial function in psychiatric disorders is also growing (24, 24, 38, 39).
Two sides of the same coin
In sum, increased oxidative stress may be intrinsically involved in the shared disposition for both psychiatric disorders and CVD.
This excessive ROS production is caused by cumulative effects of not only i) genetic factors, for example a mitochondrial dysfunction, ii) severe psychological stress and iii) environmental factors, that is, an intensified form of the aforementioned modern lifestyle (excessive food intake, physical inactivity, smoking), but also, for example, malnutrition, alcohol consumption and infectious diseases. This may suggest that psychiatric disorders and CVD represent two sides of the same coin: increased oxidative stress. Then, how does oxidative stress affect the brain and cardiovascular system resulting in psychiatric disorders and CVD? In the next paragraphs, we propose that FA peroxidation may provide the explanation of how oxidative stress effects are mediated in the brain and cardiovascular system.
Fatty acids and their oxidation products as potential mediators
Fatty acids: general aspects
FAs are main components of the phospholipid bilayer, which forms the membrane of all cells and subcellular organelles (e.g. mitochondria). The 2 FA residues bound to the glycerol backbone in phospholipids determine important membrane characteristics (40) (Fig. 1). First, we provide an overview of characteristics of different FA subclasses. Then we show how these FAs influence membrane characteristics important in pathophysiology of psychiatric disorders and CVD, particularly membrane susceptibility to oxidative stress.
Fig. 1.
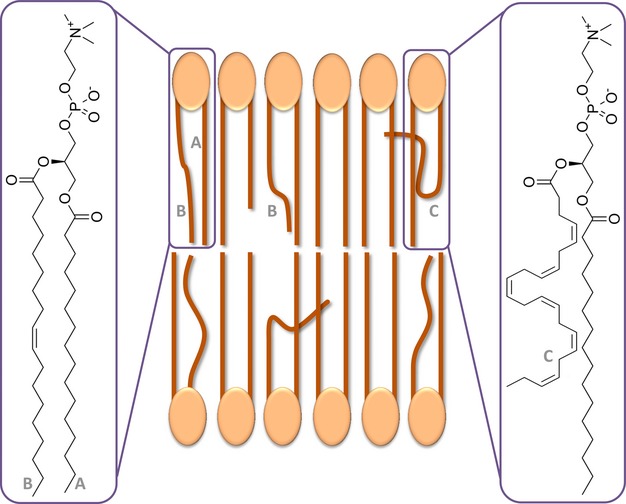
Fatty acids in membrane phospholipid bilayer. (A) Saturated fatty acid; (B) monounsaturated fatty acid; (C) polyunsaturated fatty acid.
Fatty acids: nomenclature, synthesis and metabolism
FAs consist of hydrocarbon (CHx) chains of varying length, containing no saturated FAs (SFAs), one monounsaturated FAs (MUFAs) or multiple double bonds [polyunsaturated FAs (PUFAs)].
The major PUFAs belong to the omega (ω or n) ω-3, -6 and -9 series. Omega-3 and ω-6 FAs are called essential FAs, because man is incapable of de novo synthesis. Dietary precursors alpha-linolenic acid (ALA; C18:3ω-3) and linolenic acid (LA; C18:2ω-6) can be enzymatically converted into long-chain PUFAs by elongases and desaturases (Fig. 2; (40–43)). However, because of limited conversion capacity, direct consumption of longer-chain ω-3 and ω-6 FAs, for example C20:4ω-6, arachidonic acid (AA), and C20:5ω-3, eicosapentaenoic acid (EPA), and C22:6ω-3, docosahexaenoic acid (DHA), from fatty fish, remains important. There is competition between elongases and desaturases for ω-3 and ω-6 FAs, so dietary ω-3/ω-6 balance influences synthesis. A ratio of ω-3/ω-6 of ∼1 : 4 is thought to be evolutionarily optimal, but has risen to at least 1 : 15 because of the above-mentioned modern lifestyle (44).
Fig. 2.
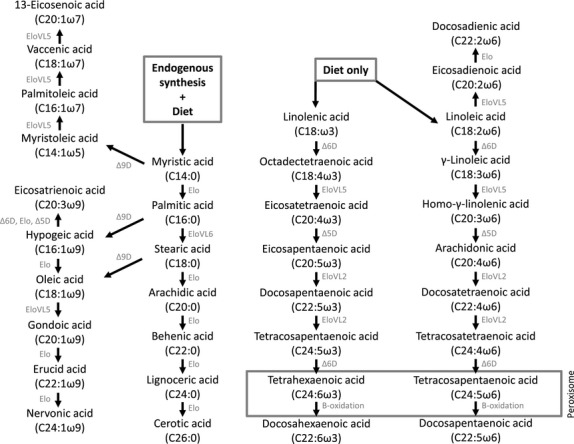
Pathways of fatty acid metabolism.
Plasma FA concentrations are thought to reflect dietary intake, while longer-term impact is better reflected in erythrocyte (membrane), liver and adipose tissue. However, it becomes increasingly clear that FA profiles are also substantially regulated by endogenous FA metabolism (44). The relationship between intake and incorporation into peripheral tissues is nonlinear and influenced by genetic factors, age, gender and oxidative stress generated by lifestyle (stress, smoking, alcohol, physical inactivity; (44)).
Fatty acids: structural role
FA length and saturation influence phospholipid membrane permeability, rigidity and fluidity. Double bonds cause curvatures in FAs, resulting in less compact arrangement in the membrane. DHA with its six double bonds therefore mainly determines increased membrane fluidity. SFAs on the contrary, by their compact arrangement, lead to ‘stiffer’, less fluid membranes (Fig. 1). This is important because cell membrane fluidity influences membrane-bound receptor functioning, thereby signal transduction, ion transport, membrane potential and receptor sensitivity (45). This way, the principal PUFAs in the brain – DHA, EPA and AA – are thought to be involved in regulation of cognitive processes, mood and affect (46). Thus far, clinical research mainly focussed on ω-3 and ω-6 PUFAs. However, SFAs and MUFAs have their own distinct roles. For example, nervonic acid (C24:1ω-9) is a major constituent of nerve's myelin sheaths. Finally, FAs are also essential components of sphingolipids and ceramides, important structural membrane components (47).
Fatty acids: functional roles
Regarding their functional role, associations of FAs with various pathophysiological processes involved in psychiatric disorders and/or CVD have been reported. For example, FAs regulate sympathetic activity (48, 49) and are associated with endocannabinoid signalling (50, 51) and hypothalamic–pituitary–adrenal (HPA) axis activity (52). In addition, DHA increases brain-derived neurotrophic factor (BDNF; (53)), which could explain ω-3 PUFA's reported neuroprotective effects (54–56). Besides these pathways, in the cardiovascular system, FAs have additional effects on triglyceride production, heart rate, myocardial efficiency, blood pressure, vascular resistance, endothelial dysfunction and thrombosis (57). Finally, FAs, as components of the aforementioned sphingolipids and ceramides, mediate responses of these signalling molecules to, for example, oxidative stress (58).
Fatty acids: (non-)enzymatic oxidation
Importantly, FAs' effects may drastically change under influence of oxidation. Phospholipid membrane FAs form a major target of enzymatic and non-enzymatic oxidation, resulting in production of lipid peroxidation products (LPOs). Their double bonds make PUFAs particularly susceptible to oxidation (59).
Regarding enzymatic lipid peroxidation, enzymes (e.g. cyclooxygenases, lipoxygenases) produce eicosanoids such as prostaglandins, leukotrienes and thromboxanes. These eicosanoids regulate inflammation and coagulation: in general, those derived from ω-6 PUFAs (e.g. AA) enhance, whereas ω-3 PUFA-derived (e.g. EPA) eicosanoids suppress these processes. In addition, DHA-derived oxidation products such as docosanoids (e.g. resolvins and neuroprotectins) have neuroprotective effects ((60, 61); Fig. 3).
Fig. 3.
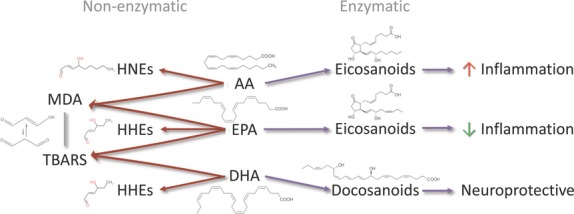
(Non-)enzymatic lipid peroxidation products of AA, EPA and DHA.
Non-enzymatic oxidation is caused by ROS attack of PUFAs and produces manifold potentially harmful LPOs, such as malondialdehyde (general LPO measure), 8-isoprostane (LPO generated by AA peroxidation), and hydroxynonenals (ω-6 PUFA-derived LPOs) and hydroxyhexenals (ω-3 PUFA-derived LPOs). Types of LPOs produced by ROS depend on the FAs in the phospholipid bilayer, with each FA precursing a specific LPO. These different LPOs regulate immune response, antioxidant compounds and enzymes (60–62).
Taken together, data indicate that depending on their composition and concentration, FAs give rise to specific peroxidation products, which acquire novel biological activities not possessed by their unoxidized precursors, potentially important for (patho)physiology of psychiatric disorders and CVD.
FA alterations in CVD and psychiatric disorders: clinical studies
Here, we aim at providing evidence for a specific pattern of FA alterations shared between psychiatric and CVD patients, by focussing on i) reviews and/or meta-analyses addressing FA alterations in CVD and/or psychiatric disorders, ii) studies combining CVD criteria with measurement of a wider FA spectrum, that is, SFA, MUFA, ω-3, ω-6 and ω-9 FAs, with or without activity estimates of desaturases and elongases, and iii) case–control studies including CVD criteria and LPO measurement.
FA alterations in CVD
FA: prospective studies in CVD
A 20-year follow-up study demonstrated that high Δ9-, Δ6- and low Δ5-desaturase activity predicted CVD risk, as well as CVD mortality (63). In 379 men (30–49 years old), SFAs were positively associated with 10-year CVD risk, even after adjustment for lifestyle (64). A prospective 7-year follow-up study involving 2724 subjects yielded comparable results. Erythrocyte 16:1ω-7, 18:3ω-3 and Δ9- and Δ6-desaturase activities were directly related to CVD risk, whereas Δ5-desaturase was inversely associated. Dietary FAs showed only modest to low correlations with erythrocyte FAs and were not significantly associated with CVD risk (65). In a recent prospective study (N = 2424), SFAs with an even chain length were found to be positively and ω-6 PUFA inversely related to subsequent CVD risk (66).
FA: cross-sectional studies in CVD
Cross-sectional analyses (N = 2980) showed associations between CVD risk and erythrocyte PUFAs, particularly LA and higher ω-6 unsaturated FAs (C18:3ω-6 and C20:3ω-6) (67). In a study of 210 men, increased SFAs, Δ9- and Δ6-desaturase- and decreased Δ5-desaturase activities were all associated with CVD risk (68). Likewise, in another study (N = 929), total PUFAs, ω-3 PUFAs and ω-3/ω-6 ratio were significantly lower in subjects with increased CVD risk. Plasma ω-3 PUFAs were inversely associated with CVD risk. In addition, high plasma total FAs increased CVD risk three times (69).
In Tunisian subjects (N = 1975) with increased CVD risk, SFAs, MUFAs and Δ9-desaturase activity were increased and positively associated with CVD risk factors, but main PUFAs (LA, DHA, AA) and Δ5-desaturase activity were decreased. In addition, CVD risk was inversely associated with PUFA concentrations (70).
LPO-CVD
With regard to LPOs, 528 obese individuals from the Framingham Offspring Study demonstrated that CVD risk was associated with increasing concentrations of the LPO 8-isoprostane, suggesting that lipid peroxidation is indeed associated with CVD risk (71). In another study, 8-isoprostane increased as CVD risk increased, with the correlation with visceral fat being stronger than with any other variable (72).
Studies in psychiatric disorders
MDD/BP-FA
Importantly, comparable FA alterations are seen in patients with psychiatric disorders. In a 14-study meta-analysis, EPA, DHA and total ω-3 PUFAs were lower in MDD patients than in controls (73). In a cross-sectional analysis of 40 medication-free patients with MDD (N = 20) and bipolar disorder (N = 20), erythrocyte DHA was i) significantly lower relative to controls, ii) inversely correlated with indices of Δ9-desaturase activity and iii) associated with elevations in 18:1ω-9 and Δ6-desaturase activity (74).
In a case–control study of 137 patients with recurrent MDD, concentrations of most SFAs and MUFAs and additionally erythrocyte PUFAs, all with >20C chain length, were significantly lower than in controls. In contrast, most shorter-chain (≤18C) SFAs and MUFAs were significantly higher in patients. Estimated activities of several elongases in patients' plasma were significantly altered, whereas Δ9-desaturase activity for C14:0 and C18:0 was significantly higher (75).
In an 8-year follow-up population study, no consistent prospective association of depression risk with any serum FA was found, in particular not with EPA, DPA and DHA (76). Unfortunately, results of long-term prospective studies including FA spectrum, LPOs and CVD risk factors in MDD patients are currently lacking.
MDD/BP-LPO
Increased lipid peroxidation was shown in MDD patients as expressed by a significant increase in the LPO-marker MDA. Moreover, a very significant increase in LPOs was observed in recurrent MDD patients as compared to the first-episode group (35). In both bipolar and MDD patients, the LPO MDA was significantly increased compared with controls (32). In 54 MDD patients, serum LPO was significantly higher compared with healthy controls (34). Furthermore, elevated serum LPOs were found in different phases of bipolar disorder and schizophrenia compared with controls (33).
Interestingly, recently, FAs, LPOs and the prime CVD risk factor insulin resistance were combined in 47 MDD patients and controls. In patients, increased concentrations of palmitoleic acid (C16:1ω-7) and total MUFAs, together with a decreased ω-6 PUFAs, were found, with increases in SFAs. Moreover, Δ6-desaturase activity was significantly increased. Concomitantly, MDD patients had higher plasma triglycerides, LPO and insulin resistance. Importantly, FA composition of MDD patients revealed changes similar to those usually observed in patients with insulin resistance without comorbid depression (77). Moreover, recently, a relationship was found between white matter integrity and lipid peroxidation in bipolar disorder (36).
Schizophrenia FA
Recently, a systematic review and meta-analysis were performed for docosapentaenoic acid (DPA, C22:5ω-3), DHA, LA and AA in patients with schizophrenia (78). Combining 642 patients (169 antipsychotic-naïve) and 574 controls provided substantial evidence that decreased DPA, DHA and AA are associated with the schizophrenia syndrome, apart from possible influences of antipsychotic medication. Given result heterogeneity, conclusions should be interpreted cautiously.
Schizophrenia LPO
For reports regarding lipid peroxidation in schizophrenia, we first refer to excellent recent reviews (37, 79, 80). Findings include increased LPOs (including MDA, 8-isoprostane and hydroxynonenals). In a case–control study, plasma MDA was higher and red blood cell SOD and catalase significantly lower in schizophrenic patients and their unaffected siblings (81). In addition, an inverse relationship was found between LPOs and erythrocyte DHA and AA in antipsychotic-naïve patients (82).
Comparable pattern of FA alterations in CVD and psychiatric disorders
After reviewing the above literature, an oxidative-stress-associated pattern of alterations in FA and LPOs seems to emerge, characterized by i) increased SFAs and MUFAs, decreased long-chain PUFAs and ω-3/ω-6 ratios, increased Δ6- and Δ9-desaturase activities and decreased Δ5-desaturase activity, together with ii) increases in LPOs, in patients with CVD or psychiatric disorders (MDD, schizophrenia, bipolar disorder). Therefore, also in view of the above-described oxidative-stress-induced structural and functional effects on FA metabolism, FAs together with their (non-)enzymatic peroxidation products may underlie (part of) the clinical overlap between CVD risk factors and psychiatric disorders. However, what mechanisms can explain these effects of oxidative stress on FA metabolism? In the subsequent part of this review, we propose that the methionine–homocysteine or 1-C(arbon) cycle may play an integrating role in translating the effects of oxidative stress on FA metabolism.
The 1-C cycle as integrator
General aspects
Here, we will review studies indicating that the 1-C cycle acts as an integrator, because it regulates both oxidative stress and methylation. In the 1-C cycle, the amino acid homocysteine is a key intermediate (83). First, in the transsulfuration pathway, homocysteine can be catabolized to the most important intracellular antioxidant glutathione, with vitamin B6 as cofactor (84). Second, in the transmethylation pathway, homocysteine can be transformed to S-adenosylmethionine, with vitamin B12 and folate as cofactors. S-adenosylmethionine is a universal donor of methyl groups, which are used for FA and phospholipid production, but also in epigenetic regulation of DNA transcription ((83, 85); Fig. 4).
Fig. 4.
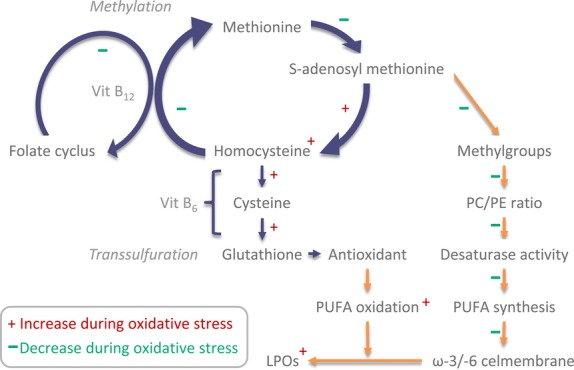
Interaction between the 1-C cycle and FA metabolism and alterations during oxidative stress.
The 1-C cycle and oxidative stress
Oxidative stress interfaces with the 1-C cycle. Accumulating evidence shows that oxidative stress increases the key 1-C cycle intermediate homocysteine, while decreasing folate (86). This may be because folate has been implicated as direct ROS scavenger and can act as antioxidant in vivo, being degraded/depleted in the process. Thereby, folate appears to be a major determinant of homocysteine increase (86, 87).
The 1-C cycle and FA metabolism
Besides this link of the 1-C cycle with oxidative stress, it is also tightly connected to FA metabolism (Fig. 4). First, methyl groups donated by S-adenosylmethionine are used for methylation of phospholipids, which are responsible for PUFA transport from liver to brain. Second, methyl group donation also regulates activity of desaturases and elongases responsible for ω-3 and ω-6 PUFA synthesis (88, 89). Third, methyl groups are also utilized in FA elongation. Importantly, finally, via DNA methylation, activity of all enzymes involved in the 1-C-cycle, FA and oxidative metabolism may be influenced (epigenetic regulation).
Changes in 1-C cycle in CVD and psychiatric disorders
1-C cycle in CVD
A 26-article meta-analysis concluded that each 5 μm homocysteine increase independently enhanced CHD risk by approximately 20% (90). In addition, meta-analysis of prospective studies showed that folate is inversely associated with CVD risk (91). Furthermore, a population study (N = 1108) observed an association between CVD risk (insulin resistance) and serum homocysteine (92).
1-C cycle in psychiatry
Involvement of the 1-C cycle in psychiatric disorders is supported by substantial evidence. For example, novel epigenetic findings demonstrate how the 1-C-derived methyl donor S-adenosylmethionine influences expression of key genes in the brain affecting memory, learning, cognition and behaviour, whose expression was found to be reduced in psychiatric patients (93–95).
In the largest sample examined to date concerning psychiatric symptomatology, a cross-sectional study of 11 757 participants, a significant positive relationship was found between elevated homocysteine and actual depressive symptoms (96). Another recent unique study in medication-naïve first-episode psychotic patients found significantly lower blood vitamin B12 and folate compared with matched controls. These reductions paralleled significant increases in plasma homocysteine and cortisol (97).
Similar pattern of 1-C cycle alterations in CVD and psychiatric disorders
In both psychiatric disorders and CVD, a pattern of alterations in key 1-C cycle components (homocysteine, vitamin B12, folate) seems to emerge. This pattern is characterized by increased homocysteine and glutathione, together with decreased concentrations of folate, vitamin B12 and S-adenosylmethionine (83, 87, 88). These alterations may be interpreted as a switch from the transmethylation pathway to the transsulfuration pathway. Being linked to oxidative stress on the one hand, and FA metabolism on the other, the 1-C metabolism is well positioned to integrate the effects of oxidative stress on FA metabolism. However, is this integrating role of the 1-C cycle supported by studies simultaneously assessing these three factors?
Fatty acids and the 1-C cycle: the integrated picture
Overview
We proposed a model in which oxidative-stress-associated alterations in FA metabolism, translated by integrative changes in the 1-C cycle, explain comorbidity of psychiatric disorders and CVD. The oxidative-stress-induced shift in the 1-C cycle from the methylation to the transsulfuration pathway may limit bioavailability of methyl groups, thereby possibly leading to decreases in FA chain length and unsaturation. Following this model, it can be hypothesized that studies that simultaneously assessed these factors will observe increases in oxidative stress accompanied by associated corresponding alterations in FA metabolism (reductions in long-chain PUFAs and increases in SFAs, MUFAs and LPOs) and the 1-C cycle (increased glutathione and homocysteine and decreased folate) (Fig. 4). In this subsequent Part (vi), we will review studies that applied such a combined approach.
Oxidative stress, the 1-C cycle and FAs: integrated clinical studies
Thus far, clinical studies combining CVD risk factors, parameters of oxidative stress, the 1-C cycle, FA metabolism and (non-)enzymatic LPOs are still scarce. In healthy men, homocysteine was inversely related to plasma AA and DHA, total ω-3 PUFAs and ω-3/ω-6 PUFA ratio (98). Severus et al. (99) were the first to draw attention to the interaction between ω-3 FAs, homocysteine and the increased mortality in MDD patients. Furthermore, in an uncontrolled study in 44 MDD patients, normal plasma homocysteine coincided with a decrease in erythrocyte membrane ω-3 FA and a significant positive association between the sum of ω-6 FAs and homocysteine was found (100).
Essential FA and B-vitamin status were assessed in 61 schizophrenic patients. Patients had high erythrocyte SFAs, MUFAs and low ω-3 and ω-6 series PUFAs, together with low vitamin B12 and high homocysteine. Importantly, homocysteine variance proved to be best explained by folate (101). Kale et al. (97) found that reductions in folate and vitamin B12 and increases in homocysteine were accompanied by significantly reduced membrane DHA.
Therefore, this handful of clinical studies on MDD integrating oxidative stress markers, FAs and the 1-C cycle indeed suggest a close interaction between FA metabolism and the 1-C cycle in handling oxidative stress.
Discussion
Summary
So far, we provided evidence that CVD and psychiatric disorders often co-occur (Part i), which may be explained by the underlying role of oxidative stress (Part iii). In Part iv, we provided evidence that FA metabolism may be an important mediator of the effects of oxidative stress on the brain and cardiovascular system. We supported this view by showing a specific corresponding pattern of oxidative-stress-associated comparable FA alterations in psychiatric disorders and CVD, consisting of shorter, less unsaturated FAs, and increases in LPOs. In Part v, we proposed that the 1-C cycle may translate the effect of oxidative stress on FA metabolism: indeed, psychiatric disorders and CVD are both associated with oxidative-stress-associated increases in homocysteine and reductions in folate. This is corroborated by studies observing associations of FA metabolism with 1-C cycle parameters in response to oxidative stress (Part vi).
While thus far these alterations are mainly considered to be harmful, here, we review data indicating that this pattern may well (partly) represent an (initially) adaptive response to increased oxidative stress.
Evidence for an adaptive potential
Apparent discrepancy between observed alterations and supplementation studies
Above reviewed alterations in FA metabolism and the 1-C cycle gave rise to clinical trials aiming at ‘normalizing’ these concentrations to treat and/or prevent psychiatric disorders and CVD. Increases in homocysteine are being supplemented with folate and B-vitamins, and decreases in long-chain PUFAs are being supplemented with EPA and DHA. Indeed, homocysteine falls and FA concentrations rise following supplementation. However, oxidative stress parameters do not always improve after FA supplementation (102). In addition, thus far, no clear clinical benefits could be demonstrated for ω-3 FA and/or folate and other B-vitamin supplementation in CVD or psychiatric disorders. Meta-analysis of randomized, double-blind, placebo-controlled trials in patients with a history of CVD showed insufficient evidence for a secondary preventive effect of ω-3 FA supplements against overall cardiovascular events (103–105). In addition, a recent meta-analysis of trials on ω-3 FA treatment of MDD involving 731 depressed patients suggests a small, but non-significant benefit of ω-3 FA for MDD, nearly entirely attributable to publication bias (106). A third updated review of PUFA supplementation for schizophrenia revealed persistent inconclusive results (107). Likewise, data from recent large randomized controlled trials have shown that there is no clear benefit of lowering homocysteine concentrations with folate or B-vitamins (108, 109). This lack of clinical effect of interventions aimed at lowering homocysteine supports the view that homocysteine is not an instigator, but rather an indicator of oxidative stress in CVD and psychiatric disorders (86, 87). These negative results of supplementation trials seem puzzling and in contrast to the distinct alterations in FA metabolism and the 1-C cycle in CVD and psychiatric patients. In addition, meta-analyses suggest that if any effect of FA supplementation can be noted, it is particularly for EPA (110), while it is mainly DHA, which differs between depressed patients and controls (111).
This apparent discrepancy between observational studies reporting clear alterations and supplementation studies showing inconsistent effects led us to the hypothesis that the observed alterations in FA metabolism and the 1-C cycle may (partly) consist of adaptive responses to increased oxidative stress.
Return to normal after combating oxidative stress
If these alterations represent adaptive responses to increased oxidative stress, one would expect that by combating oxidative stress (e.g. by weight reduction and/or physical exercise), a return to ‘normal’ values may be seen, that is, i) insulin resistance decreases, ii) in the 1-C cycle, homocysteine decreases and folate rises and iii) in FA metabolism, SFAs and MUFAs decrease and PUFAs increase, in parallel with a decrease in LPO. Although evidence remains scarce thus far, some indications exist (21, 112–115). If this pattern is further corroborated in well-designed randomized controlled trials, this may strengthen the view that interrelated alterations in FAs and the 1-C cycle induced by oxidative stress at least initially may represent an adaptive response.
Biochemical explanation for a potentially adaptive effect
In addition, interpreting the observed FA alterations – that is, shorter chains and less unsaturation – as an adaptive response, may make sense from a biochemical perspective. The FA alterations – the decrease in PUFAs in particular – make cell membranes less peroxidizable. This decreased peroxidizability may make cells more resilient to oxidative stress. This could also explain why mammal species with a more peroxidation-resistant membrane live longer (59), and offspring of human nonagenarians have more peroxidation-resistant erythrocyte membranes than controls (116). In addition, rat skeletal muscle mitochondrial membranes, highly exposed to oxidative stress, have more MUFAs and less PUFAs compared with whole muscle membranes (117). This decreased membrane unsaturation may reflect selective pressure towards membranes that are more resistant to oxidative damage by ROS produced in their vicinity. The negative effect of low polyunsaturation on membrane fluidity may be counterbalanced by the higher percentage of MUFA and the known low cholesterol content of mitochondrial membranes (117).
In addition, in the 1-C cycle, oxidative stress invokes a shift towards transsulfuration and rise in glutathione production. Glutathione as the major cellular antioxidant may thereby partly adaptively combat the oxidative stress that caused its formation. Moreover, although LPOs were considered to be harmful thus far, increasing data support an adaptive/protective role of LPOs. For example, LPOs were shown to acquire novel biological activities, including stimulation of antioxidant defences and the ability to regulate immune responses (15, 16, 21, 49, 60, 61), possibly counteracting the oxidative stress that generated them (118).
In sum, results indicate that, for example, lower ω-3 PUFA and higher homocysteine concentrations do not necessarily stand for harmful deficiencies/excesses, but may initially reflect adaptive alterations in FA and 1-C cycle metabolism to optimally handle oxidative stress.
Limitations and challenges
Role of Medication
Major psychotropic drugs are associated with increased CVD risk, especially weight gain. However, importantly, evidence for the bilateral association between CVD and psychiatric disorders predates psychotropic agents. So, altered glucose metabolism and dyslipidaemia seem to be integral to psychiatric disorders. Interestingly, therapeutic response to some antipsychotics seems associated with weight gain during treatment (119). Psychotropic drugs may work through intercalation in membrane phospholipids. Fluidity of membranes rich in essential FAs, influenced by diet, could be a contributing factor to the action of psychotropics (120). In addition, some antipsychotics and antidepressants have antioxidative effects possibly owing to effects on mitochondrial respiratory chain enzymes (121).
Specificity
Noteworthily, thus far, the discussed pattern of FA alterations (LPOs included) seems not specific to CVD or any psychiatric disorder, but is also found in other oxidative-stress-related diseases such as Alzheimer's and Parkinson's disease, as well as in normal ageing (122, 123). Therefore, one could consider oxidative stress as a relatively non-specific factor having nothing to do with underlying (patho)physiology of any (psychiatric) disease. However, we propose that oxidative stress and its influence on FA and 1-C cycle metabolism lies at the basis of a wide range of (patho)physiology. This aspecificity causes problems but may also hold promises.
For instance, how could it be that the clinical picture may greatly vary, despite this proposed common underlying (patho)physiology? This may be due to a multitude of different levels of modifying factors, varying from (epi)genetic variations regulating genes of, for example, the mitochondrial respiratory chain, FA metabolism and the 1-C cycle, LPO production and their different locations (brain, cardiovascular system), together with exogenous factors such as psychological stress (39, 124–127). For instance, the stress hormone cortisol translocates to mitochondria to regulate mitochondrial gene expression (24). Moreover, disease-specific neuroanatomical patterns in mitochondrial complex 1 alterations were found in schizophrenia, bipolar disorder and major depression (38). Nevertheless, further research is needed to develop more reliable diagnostic and prognostic markers, for example, to distinguish between diseases. Giustarini et al. (18) provide directions on how to more reliably measure oxidative stress, for example at tissue level, to detect more clear and specific relations between oxidative stress and various diseases. On the other hand, this generalizability of the discussed pattern suggests that interventions aimed at reducing oxidative stress may provide opportunities to improve health outcomes in general, including mental and cardiovascular health.
Reverse causality and confounding
While the above-proposed theoretical model may provide an interesting perspective to explain CVD and psychiatric comorbidity, evidence is far from conclusive. Most evidence is correlational, therefore limiting conclusions regarding causality. Although some prospective and/or intervention studies have been performed, and studies usually attempted to control for confounding, part of the described biological alterations may be explained by known confounders, including smoking, reduced dietary quality and lack of physical activity, which are all more common in psychiatric populations compared with the general public. On the other hand, these risk factors are all known to induce oxidative stress, which may suggest that instead of confounders, these factors represent mediating and/or moderating factors, with (e.g. bidirectional) effects on oxidative stress on their causal pathway. Hereafter, we will describe specific study designs to further test the above-proposed biological framework of oxidative-stress-induced alterations in FA and 1-C cycle metabolism.
Research implications
Oxidative stress decreasing interventions
Following the proposed framework, a successful lowering of oxidative stress would be expected to normalize FA and 1-C cycle alterations and consequently improve CVD risk and clinical outcomes. However, unfortunately, effectively lowering oxidative stress levels is not that easy, particularly in the brain (128).
Current antioxidant supplementation does not seem to be effective (18), coined as the antioxidant paradox. This can be explained because supplemented antioxidants i) do not enter the brain; ii) distort endogenous antioxidant responses and physiological oxidative stress (as noted above); and iii) not always act antioxidant in vivo (128–130). Alternatively, it may be more effective to prevent oxidative stress from arising in the first place. Future randomized controlled trials mutually combining add-on lifestyle interventions (e.g. diet, physical exercise) and investigation of (adjuvant) novel oxidative-stress-relieving treatments are therefore urgently needed. For example, effects on oxidative stress of N-acetylcysteine through the 1-C cycle, but also psychotherapy (21, 131–135), may be interesting topics of future investigation. In addition, it might be worthwhile to look for ways to prevent disrupted mitochondrial oxidative stress formation, that is, mitochondrial therapy (24, 136). Importantly, these studies should combine clinical outcomes with biochemical parameters, for example oxidative stress, (non-)enzymatic LPOs and the 1-C cycle, to understand underlying biological mechanisms.
Subgroups, windows of opportunity and personalized medicine
Another factor that may be of special interest to future trials may be definition of subgroups. For example, effects of folate and vitamin B12 on negative symptoms in schizophrenia depended on genetic variation in folate absorption (137). In genetically vulnerable groups, supplementation may have an effect, while in patients with decreased folate secondary to oxidative stress, supplementation may have no or even opposite effects. A similar idea can be noted for FA supplementation, where patients with low long-chain ω-3 PUFA concentrations resulting from genetically reduced enzymatic conversion of ALA into EPA and DHA may benefit from supplementation, while in case of adaptive low concentrations, supplementation will not be effective (138–140). An additional indication that such subgroups exist may be the observed bimodal distribution of FA concentrations in MDD and schizophrenia (141, 142).
Besides this cross-sectional classification, subgroups may also be defined longitudinally in time (staging), that is, supplementation effectiveness depends on timing. For instance, disease stage may influence supplementation effectiveness; for example, long-chain ω-3 PUFA was shown to reduce the rate of progression to first-episode psychotic disorder specifically in adolescents and young adults aged 13–25 years with subthreshold psychosis (143). This might be explained because supplementation took place early in the disease – during adolescent neurodevelopment. This period may provide a window of opportunity where it might be possible to interfere in the proposed pathophysiological cascade before the point of no return – that is, the stage in which the production of ROS damaged products starts to overwhelm ROS defence – consequently reducing the risk of potentially toxic LPO formation.
Future research aimed at disentangling these subgroups may help to find those subgroups of patients who may actually benefit from supplementation and at what time point, resulting in clearer effects in treatment trials. This will pave the way to develop personalized medicine interventions, for example specialized nutritional therapy (144).
Relation between inflammation and oxidative stress
Over the last decade, studies consistently reported increased levels of a variety of peripheral inflammatory biomarkers in psychiatric disorders, for example MDD (145, 146). Interestingly, as briefly indicated earlier, oxidative stress and inflammation are inextricably tied processes (147). Importantly, evidence indicates that this relation may be bidirectional: oxidative stress elicits an immune response, while immune activation may also result in oxidative stress. This chicken-or-egg question could be the topic of another review, but some points of clarification may better place this review in context. The main pathophysiological pathway proposed here is that oxidative stress induces (non) enzymatic initially adaptive alterations in, for example, membrane lipids (LPOs). Because of the above-discussed immunoregulatory effects of FAs and their peroxidation products, these alterations subsequently prime the immune system to adequately handle oxidative stress and restore the redox balance as much as possible. This would imply that MDD, as well as other psychiatric disorders and CVD, is primarily an oxidative-stress-based disorder with secondary inflammatory consequences.
However, in some cases, inflammation may be the causative factor. For example, (randomized) treatment with supraphysiological concentrations of TNFα and inflammatory disease are associated with development of psychiatric disorders. This may fit with our theoretical model, because both treatment with TNFα and inflammatory disease are known to result in elevated levels of oxidative stress, thereby making it one of the possible driving forces behind the described oxidative-stress-associated changes in FA metabolism and the 1-C cycle. However, this route appears to be only present in specific subtypes of patients (145, 148). Interestingly, in physiological concentrations, many cytokines have antioxidant properties, thereby potentially being involved in adaptive oxidative stress regulation (146, 149). In sum, disentanglement of these bidirectional relationships may be an interesting topic for future investigation.
Clinical implications
Supplementation risks
The above-provided evidence that decreases in, for example, FA concentrations do not necessarily represent shortages, and increases excesses, has important consequences for clinical treatment. True deficits should be supplemented, whereas decreases as adaptive responses could potentially be hindered or even be made harmful by supplementation, the more so, because potentially dangerous effects of FA supplementation have not been systematically studied thus far. Moreover, FAs in capsules may be prone to oxidation in and ex vivo, leading to production of biologically active, possibly harmful LPOs (150). Recent examples may be effects of DHA administration during pregnancy to prevent post-natal depression (151, 152). Another example of unintended negative effects was prevention of health-promoting effects of exercise by presumed antioxidants vitamin C and vitamin D (23).
As long as a just interpretation (adaptation vs. deficit) of FA alterations is not known, reluctance in supplementing is warranted. This contrasts the large number of people currently using diverse forms of supplementation, unsupported by solid scientific evidence (153).
As a more effective alternative, lowering oxidative stress by physical exercise, a healthy diet, reducing psychological stress (e.g. cognitive therapy and/or antidepressants) and weight loss have been proven beneficial for psychiatric symptomatology and CVD risk (3, 21, 133–135, 154–156). Therefore, these interventions should be routinely implemented in clinical care for these patients.
Monitoring CVD risk in psychiatric patients
Finally, in spite of consensus recommendations and guidelines, appropriate surveillance of anthropometric and metabolic parameters has not yet been rigorously implemented in psychiatric care (157). Obstacles to implementation need to be overcome by making CVD risk monitoring mandatory (158). The concept ‘metabolic syndrome’ (MetS) encompasses a cluster of CVD risk factors and may be a helpful tool for clinicians to assess CVD risk. Although there is continuing debate regarding the MetS criteria and concept, this clustering of risk factors is unequivocally linked to an increased risk for developing type 2 diabetes mellitus and CVD (12–14). Thereby, the concept MetS could guide clinicians which/when psychiatric patients should receive treatment for their increased CVD risk.
To conclude, oxidative stress elicits connected responses thought to be involved in the bilateral association between psychiatric disorder and CVD. In this review, we focussed on two main and interrelated factors that may be involved in handling of oxidative stress: FA metabolism and the 1-C cycle. An oxidative-stress-related pattern seems to emerge with FA-metabolism increases in SFAs and MUFAs and decreased PUFAs together with increased (non-)enzymatic LPOs. The 1-C cycle shifts away from the methylation pathway and production of methyl groups needed for PUFA production, neurotransmitters and DNA methylation, to the transsulfuration pathway resulting in synthesis of the major intracellular antioxidant glutathione.
We propose that these alterations primarily represent an adaptive response. This is corroborated by the fact that combating oxidative stress (particularly by physical exercise) may result in reversal of the alterations. However, although initially reversible and protective, the alterations may turn irreversible, irreparable and harmful. Combining clinical and biochemical criteria in randomized controlled trials aimed at combating oxidative stress in specifically selected patients may help in this distinction and consequently improve diagnosis of and (preventive) treatment for (CVD in) psychiatric disorders.
Acknowledgments
The authors would like to gratefully acknowledge the Academic Medical Center, University of Amsterdam and the Fatty Acids in Diabetes, Depression and Schizophrenia (FADDS) Study group for their support. Dr. H.G. Ruhé was supported by a NWO/ZonMW VENI-Grant #016.126.059.
Declaration of interest
All authors report no biomedical financial interests or potential conflicts of interest over the last 2 years, in general and also not specifically in relation to the present study. All funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Ohaeri JU, Akanji AO. Metabolic syndrome in severe mental disorders. Metab Syndr Relat Disord. 2011;9:91–98. doi: 10.1089/met.2010.0053. [DOI] [PubMed] [Google Scholar]
- 2.van Winkel R, van Os J, Celic I, et al. Psychiatric diagnosis as an independent risk factor for metabolic disturbances: results from a comprehensive, naturalistic screening program. J Clin Psychiatry. 2008;69:1319–1327. doi: 10.4088/jcp.v69n0817. [DOI] [PubMed] [Google Scholar]
- 3.Young AH, Grunze H. Physical health of patients with bipolar disorder. Acta Psychiatr Scand. 2013;127:3–10. doi: 10.1111/acps.12117. [DOI] [PubMed] [Google Scholar]
- 4.Von Hausswolff-Juhlin Y, Bjartveit M, Lindström E, Jones P. Schizophrenia and physical health problems. Acta Psychiatr Scand. 2009;119:15–21. doi: 10.1111/j.1600-0447.2008.01309.x. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre RS, Rasgon NL, Kemp DE, et al. Metabolic syndrome and major depressive disorder: co-occurrence and pathophysiologic overlap. Curr Diab Rep. 2009;9:51–59. doi: 10.1007/s11892-009-0010-0. [DOI] [PubMed] [Google Scholar]
- 6.McIntyre RS, Danilewitz M, Liauw SS, et al. Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord. 2010;126:366–387. doi: 10.1016/j.jad.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, de Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders- a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magalhães PV, Kapczinski F, Nierenberg AA, et al. Illness burden and medical comorbidity in the Systematic Treatment Enhancement Program for Bipolar Disorder. Acta Psychiatr Scand. 2012;125:303–308. doi: 10.1111/j.1600-0447.2011.01794.x. [DOI] [PubMed] [Google Scholar]
- 9.Kahl KG, Greggersen W, Schweiger U, et al. Prevalence of the metabolic syndrome in unipolar major depression. Eur Arch Psychiatry Clin Neurosci. 2012;262:313–320. doi: 10.1007/s00406-011-0277-4. [DOI] [PubMed] [Google Scholar]
- 10.Goldbacher EM, Bromberger J, Matthews KA. Lifetime history of major depression predicts the development of the metabolic syndrome in middle-aged women. Psychosom Med. 2009;71:266–272. doi: 10.1097/PSY.0b013e318197a4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo JJ, Keck PE, Li H, Patel NC. Treatment costs related to bipolar disorder and comorbid conditions among Medicaid patients with bipolar disorder. Psychiatr Serv. 2007;58:1073–1078. doi: 10.1176/ps.2007.58.8.1073. [DOI] [PubMed] [Google Scholar]
- 12.Oda E. Metabolic syndrome: its history, mechanisms, and limitations. Acta Diabetol. 2012;49:89–95. doi: 10.1007/s00592-011-0309-6. [DOI] [PubMed] [Google Scholar]
- 13.Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–712. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Fisman EZ, Tenenbaum A. The metabolic syndrome entanglement: cutting the Gordian knot. Cardiol J. 2014;21:1–5. doi: 10.5603/CJ.a2013.0054. [DOI] [PubMed] [Google Scholar]
- 15.Adibatla RM, Hatcher JF. Lipid oxidation and peroxidation in CNS health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2010;12:125–169. doi: 10.1089/ars.2009.2668. [DOI] [PubMed] [Google Scholar]
- 16.Schiavone S, Jacquet V, Trabace L, Krause KH. Severe life stress and oxidative stress in the brain: from animal models to human pathology. Antioxid Redox Signal. 2012;18:1475–1490. doi: 10.1089/ars.2012.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace DC. Colloquium paper: bioenergetics, the origins of complexity, and the ascent of man. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8947–8953. doi: 10.1073/pnas.0914635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: origin, link, measurement, mechanisms, and biomarkers. Crit Rev Clin Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- 19.Voss P, Siems W. Clinical oxidation parameters of aging. Free Radic Res. 2006;40:1339–1349. doi: 10.1080/10715760600953859. [DOI] [PubMed] [Google Scholar]
- 20.Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 2010;49:503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 21.Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. doi: 10.1155/2012/756132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yan Z, Lira VA, Greene NP. Exercise training-induced regulation of mitochondrial quality. Exerc Sport Sci Rev. 2012;40:159–164. doi: 10.1097/JES.0b013e3182575599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci USA. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manji H, Kato T, di Prospero NA, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 25.Sugamura K, Keaney JF. Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores-Mateo G, Carrillo-Santisteve P, Elosua R, et al. Antioxidant enzyme activity and coronary heart disease: meta-analyses of observational studies. Am J Epidemiol. 2009;170:135–147. doi: 10.1093/aje/kwp112. [DOI] [PubMed] [Google Scholar]
- 27.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 28.Urakawa H, Katsuki A, Sumida Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88:4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 29.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 30.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 32.Can M, Guven B, Atik L, Konuk N. Lipid peroxidation and serum antioxidant activity in patients with bipolar and major depressive disorders. Journal of Mood Disorders. 2011;1:14–18. [Google Scholar]
- 33.Kunz MC, Gama CS, Andreazza AC, et al. Elevated serum superoxide dismutase and thiobarbituric acid reactive substances in different phases of bipolar disorder and in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1677–1681. doi: 10.1016/j.pnpbp.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased plasma peroxides and serum oxidized low density lipoprotein antibodies in major depression: markers that further explain the higher incidence of neurodegeneration and coronary artery disease. J Affect Disord. 2010;125:287–294. doi: 10.1016/j.jad.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143:34–38. doi: 10.1016/j.jad.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Versace A, Andreazza AC, Young LT, et al. Elevated serum measures of lipid peroxidation and abnormal prefrontal white matter in euthymic bipolar adults: toward peripheral biomarkers of bipolar disorder. Mol Psychiatry. 2014;19:2000–2008. doi: 10.1038/mp.2012.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–409. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben-Sachar D, Karry R. Neuroanatomical pattern of mitochondrial complex I pathology varies between schizophrenia, bipolar disorder and major depression. PLoS ONE. 2008;3:e3676. doi: 10.1371/journal.pone.0003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner A, Boles RG. Beyond the serotonin hypothesis: mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 40.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobsson A, Westerberg R. Fatty acid elongases in 14 mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:215–249. doi: 10.1016/j.plipres.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 44.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Escriba PV, Gonzales-Ros JM, Goni FM, et al. Membranes: a meeting point for lipids, proteins and therapies. J Cell Mol Med. 2008;12:829–875. doi: 10.1111/j.1582-4934.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins Leukot Essent Fatty Acids. 2006;75:329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 47.Breslow DK, Weissman JS. Membranes in balance: mechanisms of sphingolipid homeostasis. Mol Cell. 2010;40:267–279. doi: 10.1016/j.molcel.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Delarue J, Matzinger O, Binnert C, Schneiter P, Chiolero R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes Metab. 2003;29:289–295. doi: 10.1016/s1262-3636(07)70039-3. [DOI] [PubMed] [Google Scholar]
- 49.Hamazaki K, Itomura M, Huan M, et al. Effect of omega-3 fatty acid-containing phospholipids on blood catecholamine concentrations in healthy volunteers: a randomized, placebo-controlled, double-blind trial. Nutrition. 2005;21:705–710. doi: 10.1016/j.nut.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 50.Lafourcade M, Larrieu T, Mato S, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- 51.Meijerink J, Balvers M, Witkamp R. N-acyl amines of docosahexaenoic acid and other n-3 polyunsatured fatty acids – From fishy endocannabinoids to potential leads. Br J Pharmacol. 2013;169:772–783. doi: 10.1111/bph.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mocking RJT, Ruhe HG, Assies J, et al. Relationship between the hypothalamic-pituitary-adrenal-axis and fatty acid metabolism in recurrent depression. Psychoneuroendocrinology. 2013;38:1607–1617. doi: 10.1016/j.psyneuen.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Rao JS, Ertley RN, Lee HJ, et al. N-3 polyunsaturated fatty acids deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Mol Psychiatry. 2006;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- 54.Beltz BS, Tlusty MF, Benton JL, Sandeman DC. Omega-3 fatty acids upregulate adult neurogenesis. Neurosci Lett. 2007;415:154–158. doi: 10.1016/j.neulet.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calderon F, Kim HY. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem. 2004;90:979–988. doi: 10.1111/j.1471-4159.2004.02520.x. [DOI] [PubMed] [Google Scholar]
- 56.Kawakita E, Hashimoto M, Shido O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience. 2006;139:991–997. doi: 10.1016/j.neuroscience.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 58.Quehenberger O, Armando AM, Brown AH, et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res. 2010;51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 60.Catala A. Lipid peroxidation of membrane phospholipids generates hydroxyl-alkenals and oxidized phospholipids in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47:469–484. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 62.Schonfeld P, Wojtczak L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med. 2008;45:231–241. doi: 10.1016/j.freeradbiomed.2008.04.029. [DOI] [PubMed] [Google Scholar]
- 63.Warensjo E, Riserus U, Vessby B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Am J Clin Nutr. 2006;84:442–444. doi: 10.1007/s00125-005-1897-x. [DOI] [PubMed] [Google Scholar]
- 64.Skidmore PML, Woodside JV, Mc Master C, et al. Plasma free fatty acid patterns and their relationship with CVD risk in a male middle-aged population. Eur J Clin Nutr. 2010;64:239–244. doi: 10.1038/ejcn.2009.144. [DOI] [PubMed] [Google Scholar]
- 65.Kröger J, Zieteman V, Enzenbach C, et al. Erythrocyte membrane fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer (EPIC)-Potsdam study. Am J Clin Nutr. 2011;93:127–142. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 66.Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS ONE. 2012;9:e1001255. doi: 10.1371/journal.pmed.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Enzenbach C, Kroger J, Zietemann V, et al. Erythrocyte membrane phospholipid polyunsaturated fatty acids are related to plasma C-reactive protein and adiponectin in middle-aged German women and men. Eur J Nutr. 2011;50:625–636. doi: 10.1007/s00394-011-0169-4. [DOI] [PubMed] [Google Scholar]
- 68.Zak A, Turzicka E, Vecka M, et al. Duffkova, and Severity of metabolic syndrome unfavourably influences oxidative stress and fatty acid metabolism in men. Tohoku J Exp Med. 2007;212:359–371. doi: 10.1620/tjem.212.359. [DOI] [PubMed] [Google Scholar]
- 69.Huang T, Bhulaidok S, Cai Z, et al. Plasma phospholipids n-3 polyunsaturated fatty acid is associated with metabolic syndrome. Mol Nutr Food Res. 2010;54:1628–1635. doi: 10.1002/mnfr.201000025. [DOI] [PubMed] [Google Scholar]
- 70.Sethom MM, Fares S, Feki M, et al. Plasma fatty acids profile and estimated elongase and desaturases activities in Tunisian patients with the metabolic syndrome. Prostaglandins Leukot Essent Fatty Acids. 2011;85:137–141. doi: 10.1016/j.plefa.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Meigs JB, Larson MG, Fox CS, Keaney JF, Vasan RS, Benjamin EJ. Association of oxidative stress, insulin resistance, and diabetes risk phenotypes: the Framingham Offspring Study. Diabetes Care. 2007;30:2529–2535. doi: 10.2337/dc07-0817. [DOI] [PubMed] [Google Scholar]
- 72.Fujita K, Nishizawa H, Funahashi T, Shimomura I, Shimabukuro M. Systemic oxidative stress is associated with visceral fat accumulation and the metabolic syndrome. Circ J. 2006;70:1437–1442. doi: 10.1253/circj.70.1437. [DOI] [PubMed] [Google Scholar]
- 73.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68:140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 74.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Assies J, Pouwer F, Lok A, et al. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PLoS ONE. 2010;5:e10635. doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Astorg P, Bertrais S, Alessandri JM, et al. Long-chain n-3 fatty acid levels in baseline serum phospholipids do not predict later occurrence of depressive episodes: a nested case-control study within a cohort of middle-aged French men and women. Prostaglandins Leukot Essent Fatty Acids. 2009;81:265–271. doi: 10.1016/j.plefa.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Vareka T, Vecka M, Jirak R, et al. Plasma fatty acid profile in depressive disorder resembles insulin resistance state. Neuro Endocrinol Lett. 2012;33(Suppl 2):83–86. [PubMed] [Google Scholar]
- 78.Hoen WP, Lijmer JG, Duran M, Wanders RJA, van Beveren NJM, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 79.Bitanirhirwe BKY, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal. 2011;15:2011–2035. doi: 10.1089/ars.2010.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ben Othmen L, Mechri A, Fendri C, et al. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:155–159. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 82.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophr Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 83.Stanger O, Fowler B, Piertzik K, et al. Homocysteine, folate and vitamin B12 in neuropsychiatric diseases: review and treatment recommendations. Expert Rev Neurother. 2009;9:1393–1412. doi: 10.1586/ern.09.75. [DOI] [PubMed] [Google Scholar]
- 84.Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGowan PO, Meany MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behaviour. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Widner B, Enzinger C, Laich A, Wirleitner B, Fuchs D. Hyperhomocysteinemia, pteridines and oxidative stress. Curr Drug Metab. 2002;3:225–232. doi: 10.2174/1389200024605091. [DOI] [PubMed] [Google Scholar]
- 87.Hoffman M. Hypothesis: hyperhomocysteinemia is an indicator of oxidant stress. Med Hypotheses. 2011;77:1088–1093. doi: 10.1016/j.mehy.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 88.Obeid R, Herrmann W. Homocysteine and lipids: S-adenosyl methionine as a key intermediate. FEBS Lett. 2009;583:1215–1225. doi: 10.1016/j.febslet.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 89.Selley ML. A metabolic link between S-adenosylhomocysteine and polyunsaturated fatty acid metabolism in Alzheimer's disease. Neurobiol Aging. 2007;28:1834–1839. doi: 10.1016/j.neurobiolaging.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 90.Humphrey LL, Fu R, Rogers K, Freeman M, Helfand M. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clin Proc. 2008;83:1203–1212. doi: 10.4065/83.11.1203. [DOI] [PubMed] [Google Scholar]
- 91.Wang ZM, Zhou B, Nie ZL, et al. Folate and risk of coronary heart disease: a meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2012;22:890–899. doi: 10.1016/j.numecd.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 92.Bjorck J, Hellgren M, Rastam L, Lindblad U. Associations between serum insulin and homocysteine in a Swedish population-a potential link between the metabolic syndrome and hyperhomocysteinemia: the Skaraborg project. Metabolism. 2006;55:1007–1013. doi: 10.1016/j.metabol.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 93.Folstein M, Liu T, Peter I, et al. The homocysteine hypothesis of depression. Am J Psychiatry. 2007;164:861–867. doi: 10.1176/ajp.2007.164.6.861. [DOI] [PubMed] [Google Scholar]
- 94.Krebs MO, Bellon A, Mainguy G, Jay TM, Frieling H. One-carbon metabolism and schizophrenia: current challenges and future directions. Trends Mol Med. 2009;15:562–570. doi: 10.1016/j.molmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 95.Sugden C. One-carbon metabolism in psychiatric illness. Nutr Res Rev. 2006;19:117–136. doi: 10.1079/NRR2006119. [DOI] [PubMed] [Google Scholar]
- 96.Gu P, Defina LF, Leonard D, John S, Weiner MF, Brown ES. Relationship between serum homocysteine levels and depressive symptoms: the Cooper Center Longitudinal Study. J Clin Psychiatry. 2012;73:691–695. doi: 10.4088/JCP.11m07223. [DOI] [PubMed] [Google Scholar]
- 97.Kale A, Naphade N, Sapkale S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and cortisol in never-medicated schizophrenic patients: implications for altered one-carbon-metabolism. Psychiatry Res. 2010;175:47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 98.Li D, Mann NJ, Sinclair AJ. A significant inverse relationship between concentrations of plasma homocysteine and phospholipid docosahexaenoic acid in healthy male subjects. Lipids. 2006;41:85–89. doi: 10.1007/s11745-006-5074-x. [DOI] [PubMed] [Google Scholar]
- 99.Severus WE, Littman AB, Stoll AL. Omega-3 fatty acids, homocysteine, and the increased risk of cardiovascular mortality in major depressive disorder. Harv Rev Psychiatry. 2001;9:280–293. doi: 10.1080/10673220127910. [DOI] [PubMed] [Google Scholar]
- 100.Assies J, Lok A, Bockting CL, et al. Fatty acids and homocysteine levels in patients with recurrent depression: an explorative pilot study. Prostaglandins Leukot Essent Fatty Acids. 2004;70:349–356. doi: 10.1016/j.plefa.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 101.Kemperman RFJ, Veurink M, van der Wal T, et al. Low essential fatty acid and B-vitamin status in a subgroup of patients with schizophrenia and its response to dietary supplementation. Prostaglandins Leukot Essent Fatty Acids. 2006;74:75–85. doi: 10.1016/j.plefa.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 102.Mocking RJT, Assies J, Bot M, Jansen EHJM, Schene AH, Pouwer F. Biological effects of add-on eicosapentaenoic acid supplementation in diabetes mellitus and co-morbid depression: a randomized controlled trial. PLoS ONE. 2012;7:e49431. doi: 10.1371/journal.pone.0049431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kwak SM, Myung SK, Lee YJ, Seo HG Korean Meta-Analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012;172:686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 104.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 105.Roncaglioni MC, Tombesi M, Avanzini F, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 106.Bloch MH, Hannestad J. Omega-3 fatty acids for the treatment of depression: systematic review and meta-analysis. Mol Psychiatry. 2012;17:1272–1282. doi: 10.1038/mp.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Joy CB, Mumby-Croft R, Joy LA. Polyunsaturated fatty acid supplementation for schizophrenia. Cochrane Database Syst Rev. 2006;19:CD001257. doi: 10.1002/14651858.CD001257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality: meta-analysis of 8 randomized trials involving 37 485 individuals. Arch Intern Med. 2010;170:1622–1631. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 109.Marti-Carvajal AJ, Sola I, Lathyris D, Karakitsu DE, Simancas-Racines D. Homocysteine-lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2013;1:CD006110. doi: 10.1002/14651858.CD006612.pub3. [DOI] [PubMed] [Google Scholar]
- 110.Martins JG, Bentsen H, Puri BK. Eicosapentaenoic acid appears to be the key omega-3 fatty acid component associated with efficacy in major depressive disorder: a critique of Bloch and Hannestad and updated meta-analysis. Mol Psychiatry. 2012;17:1144–1149. doi: 10.1038/mp.2012.25. [DOI] [PubMed] [Google Scholar]
- 111.McNamara RK, Hahn CG, Jandacek R, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biol Psychiatry. 2007;62:17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 112.Sartore G, Lapolla A, Reitano R, et al. Desaturase activities and metabolic control in type 2 diabetes. Prostaglandins Leukot Essent Fatty Acids. 2008;79:55–58. doi: 10.1016/j.plefa.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 113.Elizondo A, Araya J, Rodrigo R, et al. Effects of weight loss on liver and erythrocyte polyunsaturated fatty acid pattern and oxidative stress status in obese patients with non-alcoholic fatty liver disease. Biol Res. 2008;41:59–68. [PubMed] [Google Scholar]
- 114.Bell LN, Temm CJ, Saxena R, et al. Bariatric surgery-induced weight loss reduces hepatic lipid peroxidation levels and affects hepatic cytochrome P-450 protein content. Ann Surg. 2010;251:1041–1048. doi: 10.1097/SLA.0b013e3181dbb572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Haugaard SB, Madsbad S, Hoy CE, Vaag A. Dietary intervention increases n-3 long-chain polyunsaturated fatty acids in skeletal muscle membrane phospholipids of obese subjects. Implications for insulin sensitivity. Clin Endocrinol (Oxf) 2006;64:169–178. doi: 10.1111/j.1365-2265.2006.02444.x. [DOI] [PubMed] [Google Scholar]
- 116.Puca AA, Andrew P, Novelli V, et al. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008;11:63–72. doi: 10.1089/rej.2007.0566. [DOI] [PubMed] [Google Scholar]
- 117.Tsalouhidou S, Argyrou C, Theofilidis G, et al. Mitochondrial phospholipids of rat skeletal muscle are less polyunsaturated than whole tissue phospholipids: implications for protection against oxidative stress. J Anim Sci. 2006;84:2818–2825. doi: 10.2527/jas.2006-031. [DOI] [PubMed] [Google Scholar]
- 118.Higdon A, Diers AR, Oh JY, Landar A, Darley-Usmar VM. Cell signalling by reactive lipid species: new concepts and molecular mechanisms. Biochem J. 2012;442:453–464. doi: 10.1042/BJ20111752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Czobor P, Volavka J, Sheitman B, et al. Antipsychotic-induced weight gain and therapeutic response: a differential association. J Clin Psychopharmacol. 2002;22:244–251. doi: 10.1097/00004714-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 120.Oruch R, Lund A, Pryme IF, Holmsen H. An intercalation mechanism as a mode of action exerted by psychotropic drugs: results of altered phospholipid substrate availabilities in membranes? J Chem Biol. 2010;3:67–88. doi: 10.1007/s12154-009-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hroudova J, Fidar Z. In vitro inhibition of mitochondrial respiratory rate by antidepressants. Toxicol Lett. 2012;213:345–352. doi: 10.1016/j.toxlet.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 122.Bousquet M, Calon F, Cicchetti F. Impact of omega-3 fatty acids in Parkinson's disease. Ageing Res Rev. 2011;10:453–463. doi: 10.1016/j.arr.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 123.Lin PY, Chiu CC, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in dementia. J Clin Psychiatry. 2012;73:1245–1254. doi: 10.4088/JCP.11r07546. [DOI] [PubMed] [Google Scholar]
- 124.Sequeira A, Martin MV, Rollins B, et al. Mitochondrial mutations and polymorphisms in psychiatric disorders. Front Genet. 2012;3:103. doi: 10.3389/fgene.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rollins B, Martin MV, Sequeira PA, et al. Mitochondrial variants in schizophrenia, bipolar disorder, and major depressive disorder. PLoS ONE. 2009;4:e4913. doi: 10.1371/journal.pone.0004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rezin GT, Amboni G, Zugno AI, Quevedo J, Streck EL. Mitochondrial dysfunction and psychiatric disorders. Neurochem Res. 2009;34:1021–1029. doi: 10.1007/s11064-008-9865-8. [DOI] [PubMed] [Google Scholar]
- 127.Shao L, Martin MV, Watson SJ, et al. Mitochondrial involvement in psychiatric disorders. Ann Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 129.Halliwell B. The antioxidant paradox. Lancet. 2000;355:1179–1180. doi: 10.1016/S0140-6736(00)02075-4. [DOI] [PubMed] [Google Scholar]
- 130.Naviaux RK. Oxidative shielding or oxidative stress? J Pharmacol Exp Ther. 2012;342:608–618. doi: 10.1124/jpet.112.192120. [DOI] [PubMed] [Google Scholar]
- 131.Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–368. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 132.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends Pharmacol Sci. 2013;34:167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 133.Hsiao FH, Jow GM, Lai YM, et al. The long-term effects of psychotherapy added to pharmacotherapy on morning to evening diurnal cortisol patterns in outpatients with major depression. Psychother Psychosom. 2011;80:166–172. doi: 10.1159/000321558. [DOI] [PubMed] [Google Scholar]
- 134.Lok A, Mocking RJT, Ruhe HG, et al. Longitudinal hypothalamic-pituitary-adrenal axis trait and state effects in recurrent depression. Psychoneuroendocrinology. 2012;37:892–902. doi: 10.1016/j.psyneuen.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 135.Gulliksson M, Burell G, Vessby B, Lundin L, Toss H, Svardsudd K. Randomized controlled trial of cognitive behavioral therapy vs standard treatment to prevent recurrent cardiovascular events in patients with coronary heart disease: secondary Prevention in Uppsala Primary Health Care project (SUPRIM) Arch Intern Med. 2011;171:134–140. doi: 10.1001/archinternmed.2010.510. [DOI] [PubMed] [Google Scholar]
- 136.Swerdlow RH. Treating neurodegeneration by modifying mitochondria: potential solutions to a “complex” problem. Antioxid Redox Signal. 2007;9:1591–1603. doi: 10.1089/ars.2007.1676. [DOI] [PubMed] [Google Scholar]
- 137.Roffman JL, Lamberti JS, Achtyes E, et al. Randomized multicenter investigation of folate plus vitamin B12 supplementation in schizophrenia. JAMA Psychiatry. 2013;70:481–489. doi: 10.1001/jamapsychiatry.2013.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mocking RJ, Lok A, Assies J, et al. Ala54Thr fatty acid-binding protein 2 (FABP2) polymorphism in recurrent depression: associations with fatty acid concentrations and waist circumference. PLoS ONE. 2013;8:e82980. doi: 10.1371/journal.pone.0082980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lu Y, Vaarhorst A, Merry AH, et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS ONE. 2012;7:e41681. doi: 10.1371/journal.pone.0041681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hegarty BD, Parker GB. Marine omega-3 fatty acids and mood disorders – linking the sea and the soul. Acta Psychiatr Scand. 2011;124:42–51. doi: 10.1111/j.1600-0447.2011.01703.x. [DOI] [PubMed] [Google Scholar]
- 141.Bentsen H, Solberg DK, Refsum H, et al. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105. doi: 10.1016/j.biopsych.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 142.Mocking RJT, Assies J, Koeter MWJ, Ruhe HG, Lok A, Schene AH. Bimodal distribution of fatty acids in recurrent major depressive disorder. Biol Psychiatry. 2012;71:e3–e5. doi: 10.1016/j.biopsych.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 143.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–154. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 144.Horwitz RI, Cullen MR, Abell J, Christian JB. Medicine. (De)personalized medicine. Science. 2013;339:1155–1156. doi: 10.1126/science.1234106. [DOI] [PubMed] [Google Scholar]
- 145.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Raison CL, Miller AH. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D) Mol Psychiatry. 2013;18:15–37. doi: 10.1038/mp.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Terlecky SR, Terlecky LJ, Giordano CR. Peroxisomes, oxidative stress, and inflammation. World J Biol Chem. 2012;3:93–97. doi: 10.4331/wjbc.v3.i5.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Juel HB, Faber C, Svendsen SG, Vallejo AN, Nissen MH. Inflammatory cytokines protect retinal pigment epithelial cells from oxidative stress-induced death. PLoS ONE. 2013;8:e64619. doi: 10.1371/journal.pone.0064619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Serini S, Fasano E, Piccioni E, Cittadini ARM, Calviello G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem Res Toxicol. 2011;24:2093–2105. doi: 10.1021/tx200314p. [DOI] [PubMed] [Google Scholar]
- 151.Assies J, Mocking RJT, Pouwer F. Letter to the Editor. JAMA. 2011;305:360–361. doi: 10.1001/jama.2011.18. [DOI] [PubMed] [Google Scholar]
- 152.Makrides M, Gibson RA, McPhee AJ, Yelland L, Quinlivan J, Ryan P. Effect of DHA supplementation during pregnancy on maternal depression and neurodevelopment of young children: a randomized controlled trial. JAMA. 2010;304:1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 153.Guallar E, Stranges S, Mulrow C, Appel LJ, Miller IR., III Enough is enough: stop wasting money on vitamin and mineral supplements. Ann Intern Med. 2013;159:850–851. doi: 10.7326/0003-4819-159-12-201312170-00011. [DOI] [PubMed] [Google Scholar]
- 154.Daumit GL, Dickerson FB, Wang NY, et al. A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med. 2013;368:1594–1602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Scheewe TW, Backx FJG, Takken T, et al. Exercise therapy improves mental and physical health in schizophrenia: a randomised controlled trial. Acta Psychiatr Scand. 2013;127:464–473. doi: 10.1111/acps.12029. [DOI] [PubMed] [Google Scholar]
- 156.Sturm J, Plöderl M, Fartacek C, et al. Physical exercise through mountain hiking in high-risk suicide patients. A randomized crossover trial. Acta Psychiatr Scand. 2012;126:467–475. doi: 10.1111/j.1600-0447.2012.01860.x. [DOI] [PubMed] [Google Scholar]
- 157.Mitchell AJ, Hardy SA. Screening for metabolic risk among patients with severe mental illness and diabetes: a national comparison. Psychiatr Serv. 2013;64:1060–1063. doi: 10.1176/appi.ps.201200514. [DOI] [PubMed] [Google Scholar]
- 158.Rethink Mental Illness. 2013. Lethal Discrimination.


