Abstract
Background
The highly selective α2-adrenoreceptor agonist, dexmedetomidine, exerts neuroprotective, analgesic, anti-inflammatory and sympatholytic properties that may be beneficial for perinatal asphyxia. The optimal safe dose for pre-clinical newborn neuroprotection studies is unknown.
Methods
Following cerebral hypoxia-ischaemia, dexmedetomidine was administered to nine newborn piglets in a de-escalation dose study in combination with hypothermia (whole body cooling to 33.5°C). Dexmedetomidine was administered with a loading dose of 1 μg/kg and maintenance infusion at doses from 10 to 0.6 μg/kg/h. One additional piglet was not subjected to hypoxia-ischaemia. Blood for pharmacokinetic analysis was sampled pre-insult and frequently post-insult. A one-compartment linear disposition model was used to fit data. Population parameter estimates were obtained using non-linear mixed effects modelling.
Results
All dexmedetomidine infusion regimens led to plasma concentrations above those associated with sedation in neonates and children (0.4–0.8 μg/l). Seven out of the nine piglets with hypoxia-ischaemia experienced periods of bradycardia, hypotension, hypertension and cardiac arrest; all haemodynamic adverse events occurred in piglets with plasma concentrations greater than 1 μg/l. Dexmedetomidine clearance was 0.126 l/kg/h [coefficient of variation (CV) 46.6.%] and volume of distribution was 3.37 l/kg (CV 191%). Dexmedetomidine clearance was reduced by 32.7% at a temperature of 33.5°C. Dexmedetomidine clearance was reduced by 55.8% following hypoxia-ischaemia.
Conclusions
Dexmedetomidine clearance was reduced almost tenfold compared with adult values in the newborn piglet following hypoxic-ischaemic brain injury and subsequent therapeutic hypothermia. Reduced clearance was related to cumulative effects of both hypothermia and exposure to hypoxia. High plasma levels of dexmedetomidine were associated with major cardiovascular complications.
Neonatal encephalopathy consequent on perinatal hypoxia-ischaemia occurs in 1–3/1000 term births in the developed world and frequently leads to serious and tragic consequences that devastate lives and families, with huge financial burdens for society.1 Although the recent introduction of cooling represents a significant advance, despite treatment around 40% survive with adverse neurodevelopmental function.2 There is an unmet need for novel, safe and effective therapies to optimise brain protection following brain injury around birth. Pre-clinical3 and clinical studies4 have emphasised the importance of sedation to realise the full benefit of therapeutic hypothermia. There is also increasing evidence that infection/inflammation plays a part in the pathogenesis of neonatal encephalopathy in both high- and low-income socioeconomic groups.5 A sedative that enhances macrophage phagocytosis and bacterial clearance, minimising inflammation-induced brain injury would be particularly useful in these patients.
Dexmedetomidine is a highly selective α2-adrenoreceptor agonist that confers sedative, anti-inflammatory, analgesic, sympatholytic and organ-protective properties.6 Many of these properties, including sedation, are transduced via α2-adrenoreceptor signalling,7 although imidazoline receptor signalling may also contribute to the cardiovascular and organ-protective properties.8 Dexmedetomidine has extensive experimental support for its neuroprotective effects via both α2- and non-α2-adrenoceptor-mediated mechanisms of action9–12 and has shown neuroprotection in neonatal models of hypoxic-ischemic brain injury13 and anaesthetic brain injury in rodents.14–16 Dexmedetomidine has superior anti-inflammatory effects compared with other sedative drugs and may protect against sepsis-induced brain and other organ injury.17,18
During therapeutic hypothermia (core body cooling to 33.5°C for 72 h within 6 h of birth) in ventilated infants with moderate to severe neonatal encephalopathy, the current practice in most neonatal intensive care units includes sedation with a morphine infusion to minimise discomfort.19 In rodent studies, however, opioids have been seen to augment hypoxic-ischaemic neuronal damage20 in contrast to dexmedetomidine, which was neuroprotective.9,13 Dexmedetomidine use has been described in premature neonates21,22 term neonates23,24 and infants.24,25 Dexmedetomidine may be associated with dose-dependent cardiovascular effects in children; such effects may be opposing and depend on central or peripheral actions.25–27 Specifically, bradycardia, hypotension and hypertension may occur to varying degrees depending on the plasma concentration.28 Therefore, close monitoring of circulatory dynamics and careful dose titration of dexmedetomidine has been recommended; this is particularly important under hypothermic conditions where there is potential for altered drug pharmacokinetics and pharmacodynamics.29 Specific studies in the newborn are also vital because dexmedetomidine clearance has been reported to be one third that described in adults, rapidly increasing to 85% of the adult value by 1 year of age.23
Pharmacokinetic (PK) studies are therefore needed prior to pre-clinical neonatal studies of dexmedetomidine neuroprotection with hypothermia. The aim of this study was to investigate the influence of hypothermia and hypoxia-ischaemia on dexmedetomidine pharmacokinetics in a piglet perinatal asphyxia model.
Methods
Anaesthesia and surgical preparation
All animal experiments were performed under UK Home Office Guidelines [Animals (Scientific procedures) Act, 1986]. Ten male piglets aged less than 24 h, with a weight range of 1.6–2.0 kg were anaesthetised and surgically prepared as described previously.30 Briefly, piglets were sedated with intramuscular midazolam (0.2 mg/kg), and arterial oxygen saturation (SpO2) was monitored (Nonin Medical, Plymouth, MN, USA). Isoflurane anaesthesia (4% v/v; Abbott Laboratories, Maidenhead, Berkshire, UK) was initially given through a facemask to facilitate tracheostomy and intubation and was used for maintenance anaesthesia (3% during surgery and 2% otherwise). Piglets were mechanically ventilated; ventilator settings were adjusted to maintain partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) at 8–13 kPa and 4.5–6.5 kPa, respectively, allowing for temperature and fraction of inspired oxygen (FiO2) correction of the arterial blood sample.
A double-lumen umbilical venous catheter (Vigon, Ecouen, France) was inserted for infusion of maintenance fluids (10% dextrose, 60 ml/kg/day before insult and 40 ml/kg/day after resuscitation), fentanyl (3–6 μg/kg/h; Mercury Farmer, Berlin, Germany), and antibiotics [benzylpenicillin (Genus Pharmaceutical, Newbury, Berkshire, UK) 50 mg kg, every 12 h and gentamicin (Patheon, Swindon, Wiltshire, UK) 4 mg/kg, once a day] through one lumen and the other lumen was kept for dexmedetomidine infusion only. An umbilical arterial catheter (Vigon) was inserted for continuous monitoring of heart rate and arterial blood pressure, and intermittent blood sampling was used to measure plasma dexmedetomidine concentrations, PaO2, PaCO2, pH, electrolytes, glucose and lactate (data not shown, Abbot Laboratories). Bolus infusions of 0.9% saline (Baxter, Thetford, Norfolk, UK; 10 ml/kg), dopamine and dobutamine (5–20 μg/kg/min), adrenaline (0.1–1.5 μg/kg/min) and noradrenaline (0.02–1 μg/kg/min) maintained mean arterial blood pressure above 40 mmHg. All animals received continuous physiological monitoring (SA Instruments Inc, Stony Brook, NY, USA) and intensive life support throughout the study. Arterial lines were maintained by infusing 0.9% saline solution (Baxter; 0.3 ml/h); heparin sodium was added at a concentration of 0.5 IU/ml to prevent line blockage.
Both common carotid arteries were surgically isolated at the level of the fourth cervical vertebra and encircled by remotely controlled vascular occluders (OC2A, In Vivo Metric, Healdsburg, CA, USA). After surgery, piglets were positioned prone in a plastic pod, and the head was immobilised securely below the magnetic resonance spectroscopy (MRS) surface coil on each side.
Cerebral hypoxia-ischaemia
In the MRS system, transient hypoxia-ischaemia was induced by remote occlusion of both common carotid arteries, using inflatable vascular occluders and also reducing FiO2 to 0.09. During transient hypoxia-ischaemia, cerebral energetic changes were monitored every 2 min by phosphorus-31 (31P) MRS and the β-nucleotide triphosphate (NTP; mainly ATP) peak height was automatically quantified. Once the β-NTP peak height had fallen to 40% of baseline, titration began by adjusting FiO2 to maintain the β-NTP peak height at 35% (30–40%) of baseline value for 12.5 min. At the end of this 12.5-min period, the animal was resuscitated by deflating the occluders and increasing FiO2 to 0.21. 31P magnetic resonance spectra were acquired for a further 1 h to follow recovery after resuscitation. Acute energy depletion (AED) was calculated by the time integral of the change in β-NTP peak area relative to the exchangeable phosphate pool [EPP; EPP = inorganic phosphate (Pi) + phosphocreatine (PCr) + (2γ + β) − nucleotide triphosphate (NTP)] during hypoxia-ischaemia and the first 60 min after resuscitation as described previously.30
Experimental groups
Before transient hypoxia-ischaemia, baseline data were acquired after stabilisation of the animal in the MRS system. After resuscitation, piglets were administered dexmedetomidine in combination with hypothermia. Fentanyl infusion was stopped following dexmedetomidine bolus and was substituted by dexmedetomidine infusion. Whole body cooling was achieved in less than 90 min using a water mattress and maintained for a total of 18–24 h at target rectal temperature of 33.5°C. Dexmedetomidine was administered with a loading dose of 1 μg/kg over 20 min and then infused for 46–48 h at the following doses: i) 10 μg/kg/h started at 4 h after hypoxic ischemic insult (HI), n = 1; ii) 2–10 μg/kg/h variable dose started with 2 μg/kg/h at 4 h with increments of 2 μg/kg/h 4 hourly, n = 1; iii) 1.5 μg/kg/h started at 4 h after hypoxia-ichaemia, n = 1; iv) 1.5 μg/kg/h started at 30 min post hypoxia-ischaemia, n = 3; v) 0.8 μg/kg/h started at 30 min post hypoxia-ischaemia, n = 1; vi) 0.6 μg/kg/h started at 30 min post hypoxia-ischaemia, n = 2; vii) 1.5 μg/kg/h as control with no hypoxia-ischaemia, n = 1. Piglets were cooled: group i, ii and iii had cooling from 2–26 h post HI while group iv, v and vi had cooling from 4–22 h post hypoxia-ischaemia (Table 1). Group vii was not cooled. A solution of 4 μg/ml dexmedetomidine was made by adding 200 μg (2 ml) dexmedetomidine to 48 ml 10% glucose and used for infusion.
Table 1.
Summary of the dexmedetomidine maintenance dose, start time and cooling periods in relation to the hypoxic-ischaemic insult
| Dexmedetomidine maintenance dose (μg/kg/h) | Start time of dexmedetomidine dose | Cooling period | Number (n) |
|---|---|---|---|
| (h after hypoxia-ischaemia) | (h after hypoxia-ischaemia) | ||
| 10 | 4 | 2–26 | 1 |
| 2–10 | 4 | 2–26 | 1 |
| 1.5 | 4 | 2–26 | 1 |
| 1.5 | 0.5 | 4–22 | 3 |
| 0.8 | 0.5 | 4–22 | 1 |
| 0.6 | 0.5 | 4–22 | 2 |
| 1.5 | – | No cooling | 1 |
At the end of hypothermic treatment, piglets were re-warmed at rate of 0.5°C/h to normothermia (38.5°C for a piglet). Blood for dexmedetomidine PK assay was sampled at baseline (pre-insult), 0.5, 1, 2, 6, 9, 12, 24 and 48 h post-insult. Blood for chemistry and blood gas analysis was taken at baseline and then every 6 h post hypoxic ischaemic insult (data not shown).
PK analysis
Population parameter estimations
A one-compartment linear disposition model was used to fit data to the PK model. Population parameter estimates were obtained using non-linear mixed effects modelling (NONMEM VII, Globomax LLC, Hanover, MD, USA).31 This model accounts for population parameter variability (between subjects) and residual variability (random effects) as well as parameter differences predicted by covariates (fixed effects). The population parameter variability in model parameters was modelled by a proportional variance model. An additive error (ERRADD) and a proportional term error term (ERRPROP) were used to characterise the residual unknown variability. The variance of the residual unidentified variability, ηRUV,i, was estimated.32 The population mean parameters, between subject variance and residual variance were estimated using the first-order conditional interaction estimate method using ADVAN1 TRANS2. Convergence criterion was three significant digits. A Compaq Digital Fortran Version 6.6A compiler with Intel Celeron 333 MHz CPU (Intel Corp., Santa Clara, CA, USA) under MS Windows XP (Microsoft Corp., Seattle, WA, USA) was used to compile NONMEM. The population parameter variability is modelled in terms of random-effect (η) variables. Each of these variables is assumed to have mean 0 and a variance denoted by ω,2 which is estimated. The covariance between two elements of η [e.g., clearance (CL) and volume of distribution (V)] is a measure of statistical association between these two variables. Their covariance is related to their correlation (R), i.e.,
The covariance of clearance and distribution volume variability was incorporated into the model.
Covariate analysis
The parameter values were standardised for a body weight of 70 kg using an allometric model33,34
where Pi is the parameter in the ith individual, Wi is the weight in the ith individual and Pstd is the parameter in a pig with a weight Wstd of 70 kg. This standardisation allows comparison of piglet parameter estimates with those reported for adults. The PWR exponent was 0.75 for clearance, 0.25 for half times and 1 for distribution volumes.34
Covariate analysis included a model investigating the impact of temperature on clearance:
where CLstd is the population estimates for CL, standardised to a 70 kg pig using allometric models, and Ftemp is a constant. Piglets also suffered an episode of AED, where they sustained ischemic brain injury similar to perinatal asphyxia. An additional scaling factor [hypoxia-ischaemia severity or acute energy depletion factor (FAED)] was applied to clearance at that time
Quality of fit
The quality of fit of the PK model to the data was sought by NONMEM's objective function and by visual examination of plots of observed vs. predicted concentrations. Models were nested and an improvement in the objective function was referred to the chi-square distribution to assess significance, e.g., an objective function change (ΔOBJ) of 3.84 is significant at α = 0.05.
Bootstrap methods, incorporated within the Wings for NONMEM program, provided a means to evaluate parameter uncertainty.35 A total of 1000 replications were used to estimate parameter confidence intervals. A visual predictive check (VPC),36 a modelling tool that estimates the concentration prediction intervals and graphically superimposes these intervals on observed concentrations after a standardised dose, was used to evaluate how well the model predicted the distribution of observed dexmedetomidine concentrations. Simulation was performed using 1000 subjects with characteristics taken from 10 studied piglets; these simulations were generated by randomly using values from the estimated parameters and their variability. This is an advanced internal method of evaluation37,38 and is considered better than the commonly used plots of observed vs. predicted values.39 For data such as these where covariates such as dose, weight and height are different for each piglet, we used a prediction corrected VPC (PC-VPC).40 Observations and simulations are multiplied by the population baseline value divided by the individual-estimated baseline.
Results
Piglets had a mean age of 22.9 h [standard deviation (SD) 1.2 h] and weight 1.76 kg (SD 0.23 kg). The PK analysis included data from 10 piglets that showed substantial variation in plasma concentrations over the 48 h study period (Fig. 1). Plasma dexmedetomidine concentrations were above the safe sedative level in human neonates (0.4–0.8 1 μg/ml) in all but one piglet. Analysis suggested that a two-compartment model was not superior to a one-compartment model (ΔOBJ 5.560 despite two additional parameters required for the two-compartment model). Population parameter estimates (between-subject variability) were CL 3.52 l/h 70 kg [coefficient of variation (CV) 46.6%] and V 235 l 70/kg (109.1%). The correlation of between-subject variability for CL and V was −0.756. Temperature reduced clearance (ΔOBJ 3.86); CL was reduced 32.7% at a temperature of 33.5°C. The hypoxic-ischaemic insult had further impact (ΔOBJ 11.15), reducing CL by 55.8%. These parameter estimates are shown in Table 2. The satisfactory PC-VPC plot for these PK data is shown in Fig. 2. From these analyses, we propose that dexmedetomidine 2 μg/kg followed by an infusion of 0.028 μg/kg/h when combined with hypothermia following hypoxic-ischaemic brain injury will provide a dexmedetomidine concentration of 0.5–0.6 μg/l.
Fig. 1.
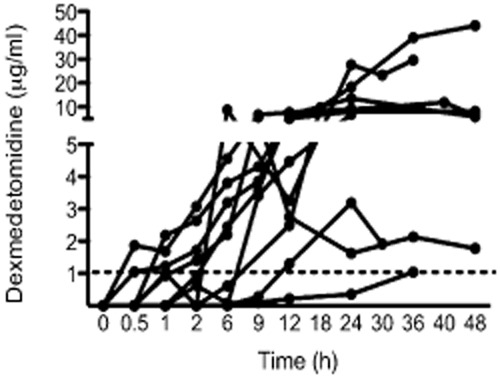
Dexmedetomidine plasma concentrations from nine piglets at varying infusion rates following hypoxic-ischaemic brain injury and co-administration with hypothermia. One additional piglet did not incur brain injury and did not undergo hypothermia. The safe sedative concentration in human neonates is 0.4–0.8 μg/ml; the dotted line is at 1 μg/ml. All piglets achieved dexmedetomidine concentrations above 1 μg/ml.
Table 2.
Standardised population pharmacokinetic parameter estimates
| Parameter | Estimate | %BSV | 95%CI |
|---|---|---|---|
| CLstd (l/h/70 kg) | 3.52 | 46.6 | 1.35, 9.01 |
| Vstd (l/70 kg) | 236 | 191 | 85.8, 497 |
| Ftemp | 0.0934 | – | 0.0127, 0.244 |
| FEAD | 0.558 | 2.6 | 0.329, 1.21 |
| ErrADD (mcg/l) | 0.171 | ηRUV 0.643 | 0.0356, 0.657 |
| ErrPROP (%) | 26 | 4.5, 48.9 |
BSV is the between-subject variability, CI is the confidence interval of the structural estimate; CLstd is the standardised clearance; Vstd is the standardised volume; additive and proportional error term respectively are ErrADD and ErrPROP.
FAED - acute energy depletion factor.
Fig. 2.

Visual predictive check for the dexmedetomidine pharmacokinetic (PK) model. All plots show median and 90% intervals (solid and dashed lines). Left hand plot shows all prediction corrected observed concentrations. Right hand plot shows prediction corrected percentiles (10%, 50%, 90%) for observations (lines with symbols) and predictions (lines) with 95% confidence intervals for prediction percentiles (gray shaded areas).
Haemodynamic instability was noted in piglets over study period with episodes of bradycardia, hypotension and hypertension (Table 3). Seven out of the nine piglets who received a hypoxic-ischaemic insult experienced cardiac arrest. All haemodynamic adverse events occurred in piglets with plasma concentrations greater than 1 μg/l.
Table 3.
Dexmedetomidine infusion rates and plasma concentrations for each piglet. The timing of the hypothermia (cooling to 33.5°C; HT), normothermia (NT) and rewarming (RW) phases are shown. The timing of the cardiac arrest and resuscitation are shown for those subjects with these events. The oxygen saturation (O2 sats), heart rate (HR) and mean arterial blood pressure (MABP) for time points corresponding to when dexmedetomidine plasma levels were taken are shown. (BL: baseline)
| Piglet | DEX dose μg/kg/h | Time | DEX plasma level (μg/ml) | Temp | Arrests? | O2 Sats % | HR Beat/min | MABP |
|---|---|---|---|---|---|---|---|---|
| Study 1 | ||||||||
| 1-1 | 0 | BL | – | NT | 98 | 136 | 37 | |
| 1-2 | 0 | 0.5 h | – | NT | 98 | 140 | 55 | |
| 1-3 | 0 | 1 h | – | NT | 97 | 147 | 56 | |
| 1-4 | 1.5 | 6 h | – | HT | 98 | 98 | 54 | |
| 1-5 | 1.5 | 9 h | 3.412 | HT | 98 | 90 | 46 | |
| 1-6 | 1.5 | 12 h | 4.473 | HT | 98 | 98 | 47 | |
| 1-7 | 1.5 | 24 h | 6.916 | RW | Fatal arrest | 100 | 94 | 37 |
| Study 2 | ||||||||
| 2-1 | 0 | BL | – | NT | 98 | 133 | 45 | |
| 2-2 | 1.5 | 0.5 h | 1.047 | NT | 98 | 132 | 51 | |
| 2-3 | 1.5 | 1 h | 1.226 | NT | 97 | 136 | 49 | |
| 2-4 | 1.5 | 2 h | 1.713 | NT | 97 | 135 | 46 | |
| 2-5 | 1.5 | 6 h | 3.191 | NT | 95 | 137 | 52 | |
| 2-6 | 1.5 | 9 h | 3.855 | NT | 98 | 145 | 50 | |
| 2-7 | 1.5 | 12 h | 5.141 | NT | 99 | 147 | 51 | |
| 2-8 | 1.5 | 24 h | 8.052 | NT | 98 | 149 | 49 | |
| 2-9 | 1.5 | 48 h | 8.115 | NT | 97 | 135 | 56 | |
| Study 3 | ||||||||
| 3-1 | 0 | BL | – | NT | 99 | 154 | 42 | |
| 3-2 | 1.5 | 0.5 h | – | NT | 96 | 166 | 53 | |
| 3-3 | 1.5 | 1 h | 2.179 | NT | 99 | 130 | 46 | |
| 3-4 | 1.5 | 2 h | 2.636 | NT | 99 | 131 | 48 | |
| 3-5 | 1.5 | 6 h | 3.809 | HT | 99 | 95 | 47 | |
| 3-6 | 1.5 | 9 h | 4.307 | HT | 98 | 92 | 41 | |
| 3-7 | 1.5 | 12 h | 6.660 | HT | 99 | 88 | 33 | |
| 3-8 | 1.5 | 24 h | 13.511 | RW | 99 | 164 | 43 | |
| 3-9 | 1.5 | 48 h | 5.877 | NT | 99 | 189 | 44 | |
| Study 4 | ||||||||
| 4-1 | 0 | BL | – | NT | 98 | 154 | 41 | |
| 4-2 | 1.5 | 0.5 h | 1.869 | NT | 98 | 141 | 46 | |
| 4-3 | 1.5 | 1 h | 1.685 | NT | 98 | 140 | 45 | |
| 4-4 | 1.5 | 2 h | 3.076 | NT | 98 | 134 | 46 | |
| 4-5 | 1.5 | 6 h | 4.563 | HT | 99 | 93 | 45 | |
| 4-6 | 1.5 | 9 h | 6.656 | HT | 99 | 84 | 46 | |
| 4-7 | 1.5 | 12 h | 7.763 | HT | 100 | 95 | 45 | |
| 4-8 | 1.5 | 24 h | 9.067 | RW | 97 | 131 | 35 | |
| 4-9 | 1.5 | 48 h | 7.470 | NT | 98 | 127 | 49 | |
| Study 5 | ||||||||
| 5-1 | 0 | BL | – | NT | 99 | 175 | 51 | |
| 5-2 | 1.5 | 0.5 h | – | NT | 99 | 187 | 50 | |
| 5-3 | 1.5 | 2 h | – | NT | 94 | 169 | 51 | |
| 5-4 | 1.5 | 6 h | 0.929 | HT | 100 | 111 | 48 | |
| 5-5 | 1.5 | 9 h | 2.449 | HT | 100 | 115 | 50 | |
| 5-6 | 1.5 | 12 h | 3.579 | HT | 100 | 153 | 45 | |
| 5-7 | 1.5 | 18 h | 7.065 | HT | 98 | 117 | 48 | |
| 5-8 | 1.5 | 24 h | 8.954 | RW | 98 | 105 | 33 | |
| 5-9 | 1.5 | 30 h | – | NT | 99 | 143 | 48 | |
| 5-10 | 1.5 | 40 h | 11.886 | NT | 80 | 146 | 34 | |
| 5-11 | 1.5 | 48 h | 6.506 | NT | Fatal arrest | 80 | 146 | 34 |
| Study 6 | ||||||||
| 6-1 | 0 | BL | – | NT | 94 | 125 | 44 | |
| 6-2 | 0 | 0.5 h | – | NT | 91 | 111 | 37 | |
| 6-3 | 0.8 | 1 h | 0.930 | NT | 94 | 100 | 58 | |
| 6-4 | 0.8 | 6 h | 1.400 | HT | 97 | 135 | 60 | |
| 6-5 | 0.8 | 12 h | 2.201 | HT | 96 | 103 | 50 | |
| 6-6 | 0.8 | 16 h | 5.767 | HT | 98 | 173 | 28 | |
| 6-7 | 0.8 | 24 h | 27.519 | RW | Arrest-resuscitated | 98 | 181 | 34 |
| 6-8 | 0.8 | 30 h | 1.620 | NT | 96 | 178 | 55 | |
| 6-9 | 0.8 | 36 h | 2.131 | NT | 97 | 160 | 78 | |
| 6-10 | 0.8 | 48 h | 1.772 | NT | 97 | 107 | 54 | |
| Study 7 | ||||||||
| 7-1 | 0 | BL | – | NT | 94 | 155 | 35 | |
| 7-2 | 0.6 | 0.5 h | – | NT | 93 | 231 | 54 | |
| 7-3 | 0.6 | 1 h | – | NT | 93 | 220 | 43 | |
| 7-4 | 0.6 | 2 h | 0.622 | NT | 95 | 190 | 44 | |
| 7-5 | 0.6 | 6 h | 0.082 | HT | 98 | 154 | 53 | |
| 7-6 | 0.6 | 9 h | 0.096 | HT | 99 | 145 | 72 | |
| 7-7 | 0.6 | 12 h | 0.221 | HT | 98 | 161 | 64 | |
| 7-8 | 0.6 | 18 h | 0.353 | HT | 98 | 125 | 59 | |
| 7-9 | 0.6 | 24 h | 1.041 | RW | Fatal arrest | 99 | 169 | 65 |
| Study 8 | ||||||||
| 8-1 | 0 | BL | – | NT | 88 | 235 | 45 | |
| 8-2 | 0.6 | 0.5 h | – | NT | 90 | 202 | 43 | |
| 8-3 | 0.6 | 1 h | – | NT | 94 | 189 | 35 | |
| 8-4 | 0.6 | 2 h | – | NT | 95 | 185 | 36 | |
| 8-5 | 0.6 | 6 h | – | HT | 97 | 178 | 51 | |
| 8-6 | 0.6 | 9 h | 0.276 | HT | 98 | 158 | 43 | |
| 8-7 | 0.6 | 12 h | 1.301 | HT | 98 | 151 | 44 | |
| 8-8 | 0.6 | 24 h | 3.190 | RW | Fatal arrest | 50 | 15 | |
| Study 9 | ||||||||
| 9-1 | 0 | BL | – | NT | 100 | 160 | 58 | |
| 9-2 | 0 | 0.5 h | – | NT | 99 | 144 | 61 | |
| 9-3 | 10 | 6 h | 8.736 | HT | 98 | 181 | 49 | |
| 9-4 | 10 | 12 h | 32.551 | HT | 99 | 141 | 41 | |
| 9-5 | 10 | 26 h | 27.614 | RW | 175 | 33 | ||
| 9-6 | 10 | 30 h | 23.353 | NT | 95 | 173 | 35 | |
| 9-7 | 10 | 36 h | 29.608 | NT | Fatal arrest | 98 | 170 | 35 |
| Study 10 | ||||||||
| 10-1 | 0 | BL | – | NT | 92 | 160 | 61 | |
| 10-2 | 0 | 0.5 h | – | NT | 98 | 167 | 51 | |
| 10-3 | 0 | 2 h | – | NT | 100 | 158 | 46 | |
| 10-4 | 2 | 6 h | 0.614 | HT | 99 | 92 | 38 | |
| 10-5 | 6 | 12 h | 2.489 | HT | 100 | 104 | 54 | |
| 10-6 | 8 | 18 h | 9.649 | HT | 99 | 124 | 40 | |
| 10-7 | 10 | 24 h | 18.369 | HT | Arrest-resuscitated | 99 | 126 | 58 |
| 10-8 | 10 | 36 h | 39.020 | NT | 95 | 185 | 42 | |
| 10-9 | 10 | 48 h | 44.115 | NT | 96 | 93 | 56 | |
Discussion
PK modelling in this piglet perinatal asphyxia model demonstrated that dexmedetomidine plasma concentrations are influenced by both hypothermia and a preceding hypoxic-ischaemic insult. All dexmedetomidine infusion regimens led to plasma concentrations above those associated with sedation in neonates and children (0.4–0.8 μg/l). Adverse cardiovascular events occurred in seven out of nine piglets receiving a dexmedetomidine infusion following hypoxia-ischaemia and therapeutic hypothermia. All haemodynamic adverse events occurred in piglets with plasma concentrations greater than 1 μg/l. These adverse cardiovascular events consisted of episodes of hypotension, bradycardia, hypertension and cardiac arrest; the events occurred at high drug concentrations due to much lower dexmedetomidine clearance than initially hypothesised. Further studies are needed in neonatal animal models to provide information on the safe dexmedetomidine dose for future neuroprotection studies.
Our initial dose was based on a previous study of adult pigs in which an infusion of 20 mcg/kg/h was administered with no adverse haemodynamic effects.41 As we knew that the clearance estimates of dexmedetomidine in neonates are 50% lower than adult values,24 we started with a dexmedetomidine infusion rate that was 50% lower. However, our data suggest that clearance estimates in neonatal piglets following hypoxia-ischaemia and during therapeutic hypothermia are approximately 10% those reported for clearance in adult humans (CL) 42.1 l/h 70/kg (CV 30.9%).24
In children in intensive care, a plasma concentration of 0.4–0.8 μg/l was associated with safe sedation (using simulation of published infusion rates).24 This is similar to the suggested effective sedative adult plasma concentration range of dexmedetomidine of 0.6–1.25 μg/l. All piglet dexmedetomidine concentrations were higher than 1.0 μg/l. Drug accumulation, attributable to both a poor initial clearance estimate in piglets, and further reduction due to the effects of cooling and preceding hypoxia-ischaemia over the 48 h study period, was associated with cardiovascular problems including bradycardia, hypotension and hypertension, culminating in cardiac arrest in seven piglets (Table 3). Bradycardia and hypotension are likely to have resulted from central α2A-adrenergic receptors causing sympatholysis.6 Bradycardia may be a particular problem in the neonate due to the heart's relatively fixed stroke volume. In contrast, stimulation of peripheral post-synaptic α2B-adrenergic receptors, and α1-adrenergic receptors, may be responsible for the pressor effect and hypertension.6 While dexmedetomidine shows 1200-fold selectivity for α2- over α1-adrenergic receptors, the high concentrations of dexmedetomidine achieved in our study suggest that α1- or α2B-adrenergic receptors may have contributed to the cardiovascular changes observed.
The findings that both hypoxia and hypothermia reduce dexmedetomidine clearance have important implications to the use of dexmedetomidine in babies with neonatal encephalopathy undergoing cooling. In two children with traumatic head injury undergoing therapeutic hypothermia, clinically significant bradycardia developed with dexmedetomidine; however, plasma concentrations were not measured.42 In adults, the majority (85%) of dexmedetomidine is metabolised in the liver by direct glucuronidation [5-diphosphoglucuronosyl transferase (UGT1A4 and UGT2B10)] and 15% by the cytochrome P450 enzymes (CYP450).28 Following perinatal hypoxia-ischaemia, multi-organ failure occurs in some infants, which may include liver dysfunction and decreased cardiac output;43 both factors can reduce dexmedetomidine clearance. CYP450 enzymes are known to be affected by hypothermia; drugs used in neonatal intensive care such as midazolam, fentanyl, propofol, vecuronium, phenobaribitol and propranolol all have reduced clearance.29,44 Hypothermia is thought to change the binding pocket confirmation of the CYP450, reduce the substrate affinity for the CYP450 binding sites and slow the rate of redox reactions performed by CYP450 enzymes.44 The uridine 5-diphosphoglucuronosyl transferase (UGT) activity has also been seen to be reduced with hypothermia in brain-injured adult patients.45
There are limitations to this study. Our sample size was limited because of the need to minimise the number of animals used for ethical reasons. The data show large variability of parameter estimates; however, future studies are planned with larger numbers and more intense sampling with and without hypothermia or hypoxia-ischaemia. The hepatic clearance is likely to be even more impaired in severe asphyxia than in our piglet hypoxia-ischaemia model (our model induces systemic hypoxia and localised cerebral ischaemia). Therefore, the hepatic hypoxia experienced in our model may be less compared with infants with severe multi-organ failure associated with neonatal encephalopathy.
Our data highlight the importance of understanding the pharmacokinetics of dexmedetomidine for future pre-clinical neuroprotection trials. Sato et al. saw no augmentation of hypothermic neuroprotection with dexmedetomidine in an adult incomplete cerebral ischaemia rodent model; however, plasma concentrations of dexmedetomidine were not measured.46 A biphasic neuroprotective response to low- and high-dose clonidine (another α2-adrenergic receptor agonist) was observed in a pre-term fetal sheep study: low-dose clonidine demonstrated a neuroprotective effect whereas high-dose clonidine was associated with hyperglycaemia, more frequent delayed seizures and loss of neuroprotection.47 It is vital that further studies relate plasma dexmedetomidine levels with brain protection to ascertain whether sedative or sub sedative doses are protective. Our PK analysis in a piglet perinatal asphyxia model with therapeutic hypothermia suggests that dexmedetomidine clearance in the piglet is affected by both temperature and prior hypoxic-ischaemic brain injury. Dexmedetomidine clearance may be reduced almost tenfold in the newborn following hypoxic-ischaemic brain injury with subsequent therapeutic hypothermia. From our one-compartment model, we estimate that a bolus of 2 μg/kg dexmedetomidine over 20 min followed by an infusion of 0.028 μg/kg/h dexmedetomidine during therapeutic hypothermia will achieve a plasma concentration of 0.5–0.6 μg/l (concentration associated with sedation in human newborns); this may be a suitable dose to use in pre-clinical studies assessing possible neuroprotective effects of dexmedetomidine following hypoxia-ischaemia. Further PK studies are vital in pre-clinical and clinical neuroprotection studies of dexmedetomidine.
In summary, this PK study in a piglet perinatal asphyxia model demonstrated adverse cardiovascular effects of dexmedetomidine; these cardiovascular events were associated with unexpectedly high dexmedetomiidne plasma levels secondary to reduced clearance due to cumulative effects of both hypothermia and exposure to hypoxia. Dexmedetomidine clearance may be further reduced in severe asphyxia with multi-organ failure. Dexmedetomidine clearance was reduced almost ten-fold in this neonatal asphyxia model compared with adult values, emphasising the critical importance of careful PK studies in the newborn.
Acknowledgments
The work was undertaken at UCH/UCL, which received a proportion of funding from the UK Department of Health's NIHR Biomedical Research Centres funding scheme.
Conflict of interest: R. D. S. has received speaker fees from Orion Pharma, Turku, Finland and Hospira, Chicago, USA. R. D. S. is the recipient of an unrestricted research grant from Orion Pharma, Turku, Finland.
Funding: This project is funded by a generous grant from the Garfield Weston Foundation and Action Medical Research.
Author contribution
Experimental studies and acquisition of data (G. K., I. F., J. R., M. E., K. B.).
Study design, data analysis and writing first draft (N. R., M. E., R. S.).
Data analysis and interpretation (M. M., J. S., B. A., B. F., P. G.).
Funding, study design, revising manuscript (N. R., R. S., P. G. J. H.).
Final approval of manuscript for publication (all authors).
References
- 1.Kurinczuk J, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86:329–338. doi: 10.1016/j.earlhumdev.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Edwards A, Brocklehurst P, Gunn A, Halliday H, Juszczak E, Levene M, Strohm B, Thoresen M, Whitelaw A, Azzoaprdi D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ. 2010;340:C363. doi: 10.1136/bmj.c363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thoresen M, Satas S, Løberg E, Whitelaw A, Acolet D, Lindgren C, Penrice J, Robertson N, Haug E, Steen PA. Twenty-four hours of mild hypothermia in unsedated newborn pigs starting after a severe global hypoxic-ischemic insult is not neuroprotective. Pediatr Res. 2001;50:405–411. doi: 10.1203/00006450-200109000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Simbruner G, Mittal RA, Rohlmann F, Much R neo.nEURO.network Trial Participants. Systemic hypothermia after neonatal encephalopathy: outcomes of neo.nEURO.network RC. Pediatrics. 2010;126:e771–778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- 5.Kasdorf E, Perlman J. Hyperthermia, inflammation, and perinatal brain injury. Pediatr Neurol. 2013;49:8–14. doi: 10.1016/j.pediatrneurol.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 6.Sanders R, Maze M. Alpha2-adrenoceptor agonists. Curr Opin Investig Drugs. 2007;8:25–33. [PubMed] [Google Scholar]
- 7.Nelson L, Lu J, Guo T, Saper C, Franks N, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Dahmani S, Paris A, Jannier V, Hein L, Rouelle D, Scholz J, Gressens P, Mantz J. Dexmedetomidine increases hippocampal phosphorylated extracellular signal-regulated protein kinase 1 and 2 content by an alpha 2-adrenoceptor-independent mechanism: evidence for the involvement of imidazoline I1 receptors. Anesthesiology. 2008;108:457–466. doi: 10.1097/ALN.0b013e318164ca81. [DOI] [PubMed] [Google Scholar]
- 9.Paris A, Mantz J, Tonner PH, Hein L, Brede M, Gressens P. The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A-adrenoceptor subtype. Anesth Analg. 2006;102:456–461. doi: 10.1213/01.ane.0000194301.79118.e9. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha 2-adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–332. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 11.Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P. Effects of alpha(2)-adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology. 2002;96:134–141. doi: 10.1097/00000542-200201000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 13.Ma D, Hossain M, Rajakumaraswamy N, Arshad M, Sanders RD, Franks NP, Maze M. Dexmedetomidine produces its neuroprotective effect via the alpha 2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502:87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Sanders RD, Xu J, Shu Y, Januszewski A, Halder S, Fidalgo A, Sun P, Hossain M, Ma D, Maze M. Dexmedetomidine attenuates isoflurane-induced neurocognitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 15.Sanders R, Sun P, Patel S, Li M, Maze M, Ma D. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2009;54:710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 16.Sanders R, Hassell J, Davidson A, Robertson NJ, Ma D. Impact of anaesthetics and surgery on neurodevelopment: an update. Br J Anaesth. 2013;110:i53–72. doi: 10.1093/bja/aet054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2008;32:1322–1326. doi: 10.1097/01.ccm.0000128579.84228.2a. [DOI] [PubMed] [Google Scholar]
- 18.Pandharipande P, Sanders R, Girard T, McGrane S, Thompson J, Shintani A, Herr DL, Maze M, Ely EW MENDS Investigators. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38. doi: 10.1186/cc8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azzopardi D, Strohm B, Edwards A, Dyet L, Halliday H, Juszczak E, Kapellou O, Levene M, Marlow N, Porter E, Thoresen M, Whitelaw A, Brocklehurst P TOBY Study Group. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 20.Kofke W, Garman R, Stiller R, Rose M, Garman R. Opioid neurotoxicity: fentanyl dose-response effects in rats. Anesth Analg. 1996;83:1298–1306. doi: 10.1097/00000539-199612000-00029. [DOI] [PubMed] [Google Scholar]
- 21.O'Mara K, Gal P, Ransommd J, Wimmermd JJ, Carlosmd R, Dimaguilamd M, Davonzomd C, Smith M. Successful use of dexmedetomidine for sedation in a 24-week gestational age neonate. Ann Pharmacother. 2009;43:1707–1713. doi: 10.1345/aph.1M245. [DOI] [PubMed] [Google Scholar]
- 22.O'Mara K, Gal P, Wimmer J, Ransom J, Carlos R, Dimaguila M, Davanzo CC, Smith M. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharmacol Ther. 2012;17:252–262. doi: 10.5863/1551-6776-17.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Potts A, Warman G, Anderson B. Dexmedetomidine disposition in children: a population analysis. Paediatr Anaesth. 2008;18:722–730. doi: 10.1111/j.1460-9592.2008.02653.x. [DOI] [PubMed] [Google Scholar]
- 24.Potts A, Anderson B, Warman G, Lerman J, Diaz S, Vilo S. Dexmedetomidine pharmacokinetics in pediatric intensive care-a pooled analysis. Paediatr Anaesth. 2009;19:1119–1129. doi: 10.1111/j.1460-9592.2009.03133.x. [DOI] [PubMed] [Google Scholar]
- 25.Potts A, Anderson B, Holford N, Vu T, Warman G. Dexmedetomidine hemodynamics in children after cardiac surgery. Pediatr Anaesth. 2010;20:425–433. doi: 10.1111/j.1460-9592.2010.03285.x. [DOI] [PubMed] [Google Scholar]
- 26.Wong J, Steil G, Curtis M, Papas A, Zurakowski D, Mason K. Cardiovascular effects of dexmedetomidine sedation in children. Anesth Analg. 2012;114:193–199. doi: 10.1213/ANE.0b013e3182326d5a. [DOI] [PubMed] [Google Scholar]
- 27.Su F, Hammer G. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10:55–66. doi: 10.1517/14740338.2010.512609. [DOI] [PubMed] [Google Scholar]
- 28.Mason K, Lerman J. Dexmedetomidine in children: current knowledge and future applications. Anesth Analg. 2011;113:1129–1142. doi: 10.1213/ANE.0b013e31822b8629. [DOI] [PubMed] [Google Scholar]
- 29.Zanelli S, Buck M, Fairchild K. Physiologic and pharmacologic considerations for hypothermia therapy in neonates. J Perinatol. 2011;31:377–386. doi: 10.1038/jp.2010.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorek A, Takei Y, Cady E, Wyatt J, Penrice J, Edwards A, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, Reynolds EOR. Delayed (‘secondary’) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1994;36:699–706. doi: 10.1203/00006450-199412000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Beal S, Sheiner L, Boeckmann A. NONMEM user's guide. San Francisco, CA: Division of Pharmacology, University of California; 1999. [Google Scholar]
- 32.Karlsson M, Jonsson E, Wiltse C, Wade J. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J Pharmacokinet Biopharm. 1998;26:207–246. doi: 10.1023/a:1020561807903. [DOI] [PubMed] [Google Scholar]
- 33.Holford N. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30:329–332. doi: 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 34.Anderson B, Meakin G. Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth. 2002;12:205–219. doi: 10.1046/j.1460-9592.2002.00616.x. [DOI] [PubMed] [Google Scholar]
- 35.Efron B, Tibshirani R. Bootstrap methods for standard error, confidence intervals and other methods of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 36.Post T, Freijer J, Ploeger B, Danhof M. Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn. 2008;35:185–202. doi: 10.1007/s10928-007-9081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tod M, Jullien V, Pons G. Facilitation of drug evaluation in children by population methods and modelling. Clin Pharmacokinet. 2008;47:231–243. doi: 10.2165/00003088-200847040-00002. [DOI] [PubMed] [Google Scholar]
- 38.Brendel K, Dartois C, Comets E, Lemenuel-Diot A, Laveille C, Tranchand B, Girard P, Laffont CM, Mentre F. Are population pharmacokinetic and/or pharmacodynamic models adequately evaluated? A survey of the literature from 2002 to 2004. Clin Pharmacokinet. 2007;46:221–234. doi: 10.2165/00003088-200746030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson M, Savic R. Diagnosing model diagnostics. Clin Pharmacol Ther. 2007;82:17–20. doi: 10.1038/sj.clpt.6100241. [DOI] [PubMed] [Google Scholar]
- 40.Bergstrand M, Hooker A, Wallin J, Karlsson M. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nunes S, Berg L, Raittinen L, Ahonen H, Laranne J, Lindgren L, Parviainen I, Ruokonen E, Tenhunen J. Deep sedation with dexmedetomidine in a porcine model does not compromise the viability of free microvascular flap as depicted by microdialysis and tissue oxygen tension. Anesth Analg. 2007;105:666–672. doi: 10.1213/01.ane.0000277488.47328.f5. [DOI] [PubMed] [Google Scholar]
- 42.Tobias J. Bradycardia during dexmedetomidine and therapeutic hypothermia. J Intensive Care Med. 2008;23:403–408. doi: 10.1177/0885066608324389. [DOI] [PubMed] [Google Scholar]
- 43.Shah P, Riphagen S, Beyene J, Perlman M. Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2004;89:F152–155. doi: 10.1136/adc.2002.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tortorici M, Kochanek P, Poloyac S. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35:2196–2204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 45.Fukuoka N, Aibiki M, Tsukamoto T, Seki K, Morita S. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60:225–230. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 46.Sato K, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand. 2010;54:377–382. doi: 10.1111/j.1399-6576.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 47.Dean JM, George S, Naylor AS, Mallard C, Gunn AJ, Bennet L. Partial neuroprotection with low-dose infusion of the alpha2-adrenergic receptor agonist clonidine after severe hypoxia in preterm fetal sheep. Neuropharmacology. 2008;55:166–174. doi: 10.1016/j.neuropharm.2008.05.009. [DOI] [PubMed] [Google Scholar]


