Abstract
Background
Omega-3 (n-3) fatty acids have previously been shown to reduce the risk of cardiac events, cardiac death, and all-cause mortality in randomized controlled trials. However, recent data have challenged the benefits of n-3 fatty acids in the current era of optimal medical therapy.
Methods
We performed a literature review indicating important limitations that must be considered when interpreting the recent negative n-3 fatty acids trials.
Results
Our review found relative strengths and weaknesses of both the older and more recent studies, along with many possible explanations for the disparate results. The principal difference between the older and the more recent n-3 studies was a greater use of background optimal medical therapy that may have reduced the benefit from n-3s. Additionally, some of the more recent n-3 trials used relatively low doses or tested n-3 supplementation on top of a relatively high baseline intake of n-3s.
Conclusion
Despite the recent negative data about n-3 fatty acids, the overall evidence still supports the American Heart Association recommendation of 1 gram of eicosapentaenoic acid/docosahexaenoic acid per day for patients with coronary heart disease.
Keywords: Fatty acids–omega-3, fish oils, myocardial infarction
INTRODUCTION
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (omega-3 polyunsaturated fatty acids [n-3-PUFAs]) are mainly present in marine fish oil. Ever since the low cardiovascular (CV) mortality rate in Greenland Eskimos, known for their high fish intake, came to light 28 years ago,1 several studies have investigated the possible role of n-3-PUFAs in treating and preventing cardiovascular disease (CVD). Evidence from observational studies and randomized controlled trials (RCTs) regarding the role of fish oil in primary and secondary prevention of CVD has been promising. However, not all studies have shown consistent results. Many studies and metaanalyses report conflicting results that show positive and negative effects of n-3-PUFAs for prevention of CV events, CV death, and mortality.2-4 Despite the conflicting data, current guidelines recommend 2 servings of fatty fish per week for the general population and 1 g/d of n-3-PUFAs for patients with coronary heart disease (CHD).3
Controversy regarding the efficacy of n-3-PUFAs in the primary and secondary prevention of CVD has led to recent debate. On a closer analysis of the current body of evidence, many of the discrepancies in the results of various trials can be explained on the basis of faulty study design (short follow-up, low baseline CV risk, lack of power, high baseline n-3-PUFA intake), inadequate dose of n-3-PUFAs used, and differences in study populations.4
Demographics and the baseline intake of fish in the study population are important points to consider. Many of the studies that have failed to demonstrate positive effects of n-3-PUFAs on CV outcomes have been conducted in populations with a high background level of fish intake.4 In such populations, maintaining a contrast between the n-3-PUFA intake levels of the intervention and the control groups is difficult. This hypothesis is supported by the low level of CV events in both arms of the studies showing a lack of benefit of n-3-PUFAs. One could argue that the maximum benefit of n-3-PUFAs was already present in both arms of the studies because of the high fish intake in the populations.
Several hypotheses have been presented regarding the role of n-3-PUFAs in preventing CVDs (Table 1).5–88 The relative importance of these mechanisms for the reduced CV risk associated with replete n-3 status remains unclear.
Table 1.
Proposed Mechanisms of Action of Omega-3 Polyunsaturated Fatty Acids

In the following sections, we present observational studies, randomized trials, and recent metaanalyses.
OBSERVATIONAL STUDIES
Kromhout et al Study
Because Greenland Eskimos' consumption of a high amount of fish and marine mammals is generally regarded as being responsible for the low incidence of fatal CHD in this population, a cross-sectional study was conducted on 852 middle-aged men in the Netherlands.1 The study recorded the fish consumption of these patients and followed them for 20 years. Mortality from CHD showed an inverse relation with the amount of fish consumption, with people consuming at least 30 g of fish every day showing a 50% lower mortality rate from CHD. Noteworthy is the fact that the amount of fish being consumed every day in this population is very large—possibly several times larger than the average fish intake in the US population.
Zutphen Study
The Zutphen Study was a cohort study that assessed the relationship between n-3-PUFA consumption and sudden cardiac death (SCD) in 1,373 males.89 The risk of CHD (hazard ratio [HR] 0.32, 95% confidence interval [CI] 0.13-0.80 at age 50; HR 1.34, 95% CI 0.58-3.12 at age 80) and SCD (HR 0.46, 95% CI 0.27-0.78) was lower in men with increased fish consumption. The statistical significance of the decrease in the risk of CHD with fatty fish consumption decreased with increasing age. Additionally, no clear dose response of fish intake on the reduction of risk was apparent. Fish consumption, especially in people younger than 65 years, may significantly lower the risk for CHD and SCD. The lower incidence of SCD in these patients could be related to the antiarrhythmic effects of n-3-PUFAs.
Cardiovascular Health Study
A case-control study was conducted within the Cardiovascular Health Study, a cohort study, designed to assess the effects of n-3-PUFAs in older patients with CHD.90 Patients with fatal myocardial infarction (MI) and other CHD and patients with nonfatal MI were matched to randomly selected controls. The plasma phospholipid n-3 concentration, a biomarker of n-3-PUFA intake, was measured in the blood samples obtained from the patients almost 2 years before the events. Patients with a higher plasma phospholipid concentration were at a lower risk of fatal CHD (odds ratio [OR] 0.32, 95% CI 0.13-0.78, P=0.01). Conversely, the plasma phospholipid concentration had no relation to nonfatal MI. The association of n-3-PUFAs with a lower risk of fatal CHD but not with nonfatal MI suggests a possible reduction in SCD with increased intake.
RANDOMIZED TRIALS
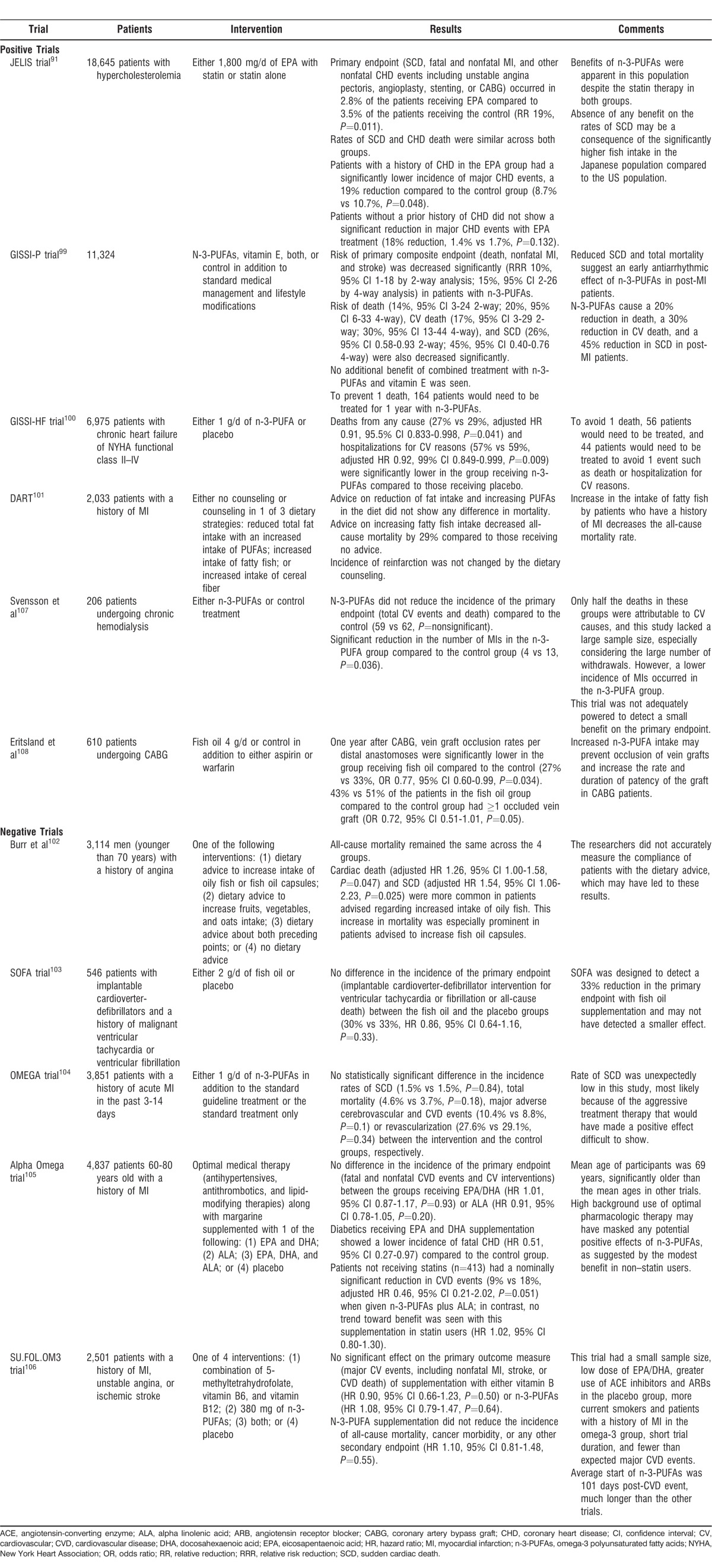
JELIS Trial
In the Japan EPA Lipid Intervention Study (JELIS), 18,645 patients with hypercholesterolemia were randomized to receive either 1,800 mg/d of EPA with statin or statin alone.91 The primary endpoint of the trial was any major CHD event, defined as SCD; fatal and nonfatal MI; and nonfatal CHD events including unstable angina pectoris, angioplasty, stenting, or coronary artery bypass graft (CABG). The primary endpoint occurred in 2.8% of the patients receiving EPA compared to 3.5% of the patients receiving the control (relative reduction [RR] 19%, P=0.011). The rates of SCD and CHD death were similar in both groups. Patients with a history of CHD in the EPA group had a significantly lower incidence of major CHD events, a 19% reduction compared to the control group (8.7% vs 10.7%, P=0.048). Conversely, patients without a prior history of CHD did not show a significant reduction in major CHD events with EPA treatment (18% reduction, 1.4% vs 1.7%, P=0.132). The results of this study show that in patients with hypercholesterolemia, EPA treatment reduces the incidence of major CHD events. This finding is especially prominent in hypercholesterolemic patients with a history of CHD. Additionally, the benefits of n-3-PUFAs were apparent in this population despite the statin therapy in both the intervention and control groups.
The absence of any benefit on the rates of SCD may be a consequence of the significantly higher fish intake in the Japanese population compared to the US population.92 N-3-PUFAs and fish oil have a nonlinear relation with the primary prevention of SCD and CHD death, presumed to reach maximum benefit around 250 mg/d.84 One to 2 servings of fish per week is associated with a reduced risk of CV death in western countries,93 but higher amounts do not seem to result in a greater reduction in SCD.84,93 However, Japanese men consume more than 3 times this amount from childhood; thus the entire JELIS population might already have been ingesting levels of n-3-PUFAs sufficient to achieve optimal prevention of SCD.94 This can be seen by the gross difference in the rates of SCD between the JELIS trial and other trials testing n-3-PUFAs.
A significant inverse association has been shown between levels of marine-derived n-3-PUFAs and carotid intima-media thickness in Japanese individuals, independent of traditional CV risk factors.95 Japanese patients have been shown to have a 2-fold higher serum level of n-3-PUFAs than white patients and Japanese American patients in the United States.95 Thus, the higher levels of n-3-PUFAs in Japanese patients may provide antiatherosclerotic benefits. Moreover, the incidence of CHD in Japan is less than half that of the United States,96 and the percentage of surface involvement of raised lesions in coronary arteries in men aged 30-34 years is less than one-third for Japanese vs US white patients.97
GISSI-P Trial
The GISSI (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico)-Prevenzione trial was an RCT designed to assess the effect of n-3-PUFA supplementation on mortality in patients with a history of recent MI.98,99 A total of 11,324 patients were randomized to receive n-3-PUFAs, vitamin E, both n-3-PUFAs and vitamin E, or to serve as controls in addition to receiving the standard medical management and lifestyle modifications. The study had a primary composite endpoint of death, nonfatal MI, and stroke. In patients treated solely with n-3-PUFAs, the risk of occurrence of the primary endpoint was decreased significantly (relative risk reduction [RRR] 10% by 2-way analysis; 15% by 4-way analysis). The risks of death (14% by 2-way analysis; 20% by 4-way analysis), CV death (17% by 2-way analysis; 30% by 4-way analysis), and SCD (26% by 2-way analysis; 45% by 4-way analysis) were also decreased significantly. However, no additional benefit of combined treatment with n-3-PUFAs and vitamin E was seen. In terms of absolute numbers, 164 patients would need to be treated for 1 year with n-3-PUFAs to prevent 1 death. Worthy of note is that n-3-PUFAs are inexpensive and free from any major adverse effects, making this reduction in all-cause mortality rather impressive and difficult to improve even when looking at trials testing statins. The reduction in SCD and total mortality points toward an antiarrhythmic effect of n-3-PUFAs in patients early post-MI. This beneficial effect of n-3-PUFAs is in addition to their well-known antiatherosclerotic and antithrombotic effects. In summary, GISSI-P showed that n-3 fatty acids cause a 20% reduction in death, a 30% reduction in CV death, and a 45% reduction in SCD in patients who have recently experienced an MI.
GISSI-HF Trial
The GISSI-HF trial (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico – Heart Failure), an RCT, enrolled 6,975 patients with chronic heart failure of New York Heart Association functional class II–IV and randomized them to receive either 1 g/d of n-3-PUFA or placebo.100 Deaths from any cause (27% vs 29%, adjusted HR 0.91, 95.5% CI 0.833-0.998, P=0.041) and hospitalizations for CV reasons (57% vs 59%, adjusted HR 0.92, 99% CI 0.849-0.999, P=0.009) were significantly lower in the group receiving n-3-PUFAs compared to those receiving placebo. Fifty-six patients would need to be treated with n-3-PUFAs to avoid 1 death, and 44 patients would need to be treated to avoid 1 event such as death or hospitalization because of CV reasons.
DART
The Diet and Reinfarction Trial (DART) was conducted to determine whether n-3-PUFAs have a role in the secondary prevention of MI in patients with a history of MI.101 In this trial, 2,033 patients with a history of MI were randomized to receive either no counseling or dietary counseling according to 1 of 3 dietary strategies: (1) reduced total fat intake with an increased intake of PUFAs, (2) increased intake of fatty fish, or (3) increased intake of cereal fiber. Patients in the group who received advice on reducing fat intake and increasing PUFAs did not show any difference in mortality compared to the other groups. Conversely, those who received advice on increasing fatty fish intake decreased all-cause mortality by 29% compared to those receiving no advice. The dietary counseling did not change the incidence of reinfarction. This study shows that an increase in the intake of fatty fish by patients who have a history of MI decreases the all-cause mortality rate.
Burr et al
In a similar study by the DART investigators, 3,114 men younger than 70 years with a history of angina received 1 of the following interventions: (1) dietary advice to increase oily fish intake or fish oil capsules; (2) dietary advice to increase fruits, vegetables, and oats intake; (3) dietary advice about both preceding points; or (4) no dietary advice.102 Although all-cause mortality remained the same across the 4 groups, cardiac death (adjusted HR 1.26, 95% CI 1.00-1.58, P=0.047) and SCD (adjusted HR 1.54, 95% CI 1.06-2.23, P=0.025) were more common in patients advised to increase their intake of oily fish. The increase in mortality was especially prominent in patients advised to increase their intake of fish oil capsules. Inferences from the results of this trial are limited because the researchers did not accurately measure the compliance of patients with the dietary advice.
SOFA Trial
The Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) trial was conducted to assess the antiarrhythmic effects of n-3-PUFAs.103 A total of 546 patients with implantable cardioverter-defibrillators and a history of malignant ventricular tachycardia or ventricular fibrillation were randomized to receive either 2 g/d of fish oil or placebo. The primary endpoint for the trial was implantable cardioverter-defibrillator intervention for ventricular tachycardia, fibrillation, or all-cause death. No significant difference was seen in the incidence of the primary endpoint between the fish oil and the placebo groups (30% vs 33%, HR 0.86, 95% CI 0.64-1.16, P=0.33). The results of this study did not support the idea that fish oil has an antiarrhythmic effect. However, SOFA was designed to detect a 33% reduction in the primary endpoint with fish oil supplementation. Fish oil may have had a benefit smaller than 33%; therefore, the antiarrhythmic effect of n-3-PUFAs cannot be ruled out from this trial alone.
OMEGA Trial
In the OMEGA trial, 3,851 patients with a history of acute MI in the past 3 to 14 days were randomized to receive either 1 g/d of n-3-PUFAs in addition to the standard guideline treatment or the standard treatment only.104 The incidence of SCD, total mortality rates, and nonfatal CV events was recorded. No statistically significant difference was seen in the incidence rates of SCD (1.5% vs 1.5%, P=0.84), total mortality (4.6% vs 3.7%, P=0.18), major adverse cerebrovascular and CVD events (10.4% vs 8.8%, P=0.1), or revascularization (27.6% vs 29.1%, P=0.34) between the intervention and the control groups, respectively. The results of this study implied that the addition of n-3-PUFAs to the best available treatment therapy for patients with a recent history of MI does not lead to any significant benefit. The rate of SCD was unexpectedly low in this study, most likely attributable to the aggressive treatment therapy used in these patients. In cases in which aggressive therapy has been used, the benefit of any new intervention becomes difficult to prove.
Alpha Omega Trial
The Alpha Omega trial was an RCT in which 4,837 patients between the ages of 60 and 80 years with a history of MI were randomized to receive margarine supplemented with one of the following: (1) EPA and DHA; (2) alpha linolenic acid (ALA); (3) EPA, DHA, and ALA; or (4) placebo.105 All patients were already receiving optimal medical therapy, including antihypertensives, antithrombotics, and lipid-modifying therapies. Major CVD events, including fatal and nonfatal CVD events and CV interventions, were the primary outcome. No difference was seen in the incidence of the primary endpoint between the groups receiving EPA/DHA (HR 1.01, 95% CI 0.87-1.17, P=0.93) and solely ALA (HR 0.91, 95% CI 0.78-1.05, P=0.20). In this trial, supplementation with low doses of either EPA and DHA or ALA did not reduce the incidence of major CVD events in patients with a history of MI who were already receiving optimal pharmacologic therapy. However, patients with diabetes who received EPA and DHA supplementation showed a lower incidence of fatal CHD (HR 0.51, 95% CI 0.27-0.97) compared to the control group. This trial enrolled participants with a mean age of 69 years, which is significantly older than the mean ages in other trials. Additionally, patients not receiving statins (n=413) had a nominally significant reduction in CVD events (9% vs 18%, adjusted HR 0.46, 95% CI 0.21-2.02, P=0.051) when given n-3-PUFAs plus ALA; in contrast, no trend toward benefit was seen with this supplementation in statin users (HR 1.02, 95% CI 0.80-1.30). Alpha Omega did not show a benefit of n-3-PUFAs; however, the high background use of optimal pharmacologic therapy may have masked any potential positive effects.
SU.FOL.OM3 Trial
The SU.FOL.OM3 (Supplémentation en Folates et Omega-3) trial investigated the effects of n-3-PUFAs and vitamin B on the incidence of major CVD events in patients with a history of CHD or stroke.106 A total of 2,501 patients with a history of MI, unstable angina, or ischemic stroke were randomized to receive 1 of 4 interventions: (1) a combination of 5-methyltetrahydrofolate, vitamin B6, and vitamin B12; (2) 380 mg of n-3-PUFAs; (3) both preceding therapies; or (4) placebo. The primary outcome measure was major CV events, including nonfatal MI, stroke, or CVD death. No significant effect on the primary outcome measure was seen with supplementation of either vitamin B (HR 0.90, 95% CI 0.66-1.23, P=0.50) or n-3-PUFAs (HR 1.08, 95% CI 0.79-1.47, P=0.64). Additionally, n-3-PUFA supplementation did not reduce the incidence of all-cause mortality, cancer morbidity, or any other secondary endpoint (HR 1.10, 95% CI 0.81-1.48, P=0.55). Several factors could have influenced the lack of apparent benefit of n-3-PUFA supplementation in the patients enrolled in this trial: small sample size; low dose of EPA/DHA (380 mg); greater use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in the placebo group; more current smokers and patients with a history of MI in the omega-3 group; short trial duration; and fewer than expected major CVD events, leading to a 15% lower than expected power to test the benefits of n-3-PUFAs in this trial (ie, 20% power to detect a 25% benefit of n-3s). Additionally, the average start of n-3-PUFAs was 101 days post-CVD event in this trial, which is much longer than the 16 days post-MI in the GISSI-P trial.
Hemodialysis Patients
Svensson et al tested benefits of n-3-PUFAs for the secondary prevention of CV events in patients undergoing chronic hemodialysis in an RCT that assigned 206 patients to receive either n-3-PUFAs or control treatment.107 A composite primary outcome of total CV events and death was seen in 59% of the patients. Although n-3-PUFAs did not reduce the incidence of the primary endpoint compared to the control (59 endpoints vs 62 endpoints, P=nonsignificant), the number of MIs in the n-3-PUFA group was significantly reduced compared to the control group (4 vs 13, P=0.036). Only half of the deaths in these patients were attributable to CV causes, and this study lacked a large sample size, especially considering the large number of withdrawals. Moreover, this trial was not adequately powered to detect a small benefit on the primary endpoint. The lower incidence of MI with n-3-PUFA treatment in secondary prevention patients undergoing chronic hemodialysis is clinically relevant as these patients are at a high risk of recurrent CV events.
Patients with CABG
Eritsland et al showed that n-3-PUFAs provide CV benefit in patients undergoing CABG; 610 patients undergoing CABG were randomized to receive either 4 g/d of fish oil or control therapy in addition to either aspirin or warfarin.108 One year after the CABG, vein graft occlusion rates per distal anastomoses were significantly lower in the group receiving fish oil compared to the control group (27% vs 33%, OR 0.77, 95% CI 0.60-0.99, P=0.034). Similarly, 43% vs 51% of the patients in the fish oil group compared to the control group had ≥1 occluded vein graft (OR 0.72, 95% CI 0.51-1.01, P=0.05). Therefore, increased n-3-PUFA intake may be able to prevent occlusion of vein grafts and increase the rate and duration of patency of the graft in CABG patients.
RECENT METAANALYSES
Rizos et al
A metaanalysis conducted on 20 RCTs including 68,680 patients evaluated the effects of n-3-PUFAs on all-cause mortality, CV death, SCD, MI, and stroke.109 The results did not show n-3-PUFA supplementation to be associated with a reduced risk of all-cause mortality (RR 0.96, 95% CI 0.91-1.02; absolute risk reduction [RD] −0.004, 95% CI −0.01-0.02), CV death (RR 0.91, 95% CI 0.85-0.98; RD −0.01, 95% CI −0.02-0.00), SCD (RR 0.87, 95% CI 0.75-1.01; RD −0.003, 95% CI −0.012-0.006), MI (RR 0.89, 95% CI 0.76-1.04; RD −0.002, 95% CI −0.007-0.002), or stroke (RR 1.05, 95% CI 0.93-1.18; RD 0.001, 95% CI −0.002-0.004). Lewis and colleagues explained the lack of apparent benefit of n-3-PUFAs in this metaanalysis by pointing out that the mean dose of n-3-PUFAs given to patients in studies included in this review was 1.51 g/d (perhaps not high enough), some of the studies had small sample sizes, and diverse sources of n-3-PUFAs may affect their efficacy.110 In addition, the authors chose <0.0063 as the P value for significance instead of the usual P value of 0.05. This measure was unnecessary and may have led to misinterpretation of the results.111
Delgado-Lista et al
In a metaanalysis of 21 studies conducted on the CV effects of n-3-PUFAs, the risk of a CV event of any kind was significantly decreased by 10% (OR 0.90, 95% CI 0.85-0.96, P=0.001).2 Similarly, CV death was decreased by 9% (OR 0.91, 95% CI 0.83-0.99, P=0.03), and fatal and nonfatal CHD events were decreased by 18% (OR 0.82, 95% CI 0.75-0.90, P<1×10−4). Total mortality was also reduced for the patients receiving n-3-PUFAs (5% reduction of risk, OR 0.95, 95% CI 0.89-1.02, P=0.15).
Kwak et al
These beneficial results were not shown for n-3-PUFAs in the metaanalysis by Kwak et al.112 Inclusion of small trials, lack of inclusion of GISSI-P and DART, and inclusion of trials testing low doses of EPA/DHA (SU.FOL.OM3) could have contributed to the lack of demonstrated CV benefit of n-3-PUFAs.113 However, in a subanalysis limited to the 5 included studies in which lipid-lowering agents were not commonly used, a trend toward reduction in CVD events was noted in the omega-3 group (RR=0.74, 95% CI 0.54-1.03). This trend very likely would have been statistically significant if the large GISSI-P study had not been excluded for lack of double-blinding.
DISCUSSION
In light of the evidence that statin therapy exerts a prominent antiarrhythmic effect114 and the protection observed with n-3-PUFA supplementation among non–statin users (but not among statin users) in the Alpha Omega trial and the Kwak metaanalysis, concurrent statin therapy can be reasonably suspected to render superfluous the antiarrhythmic benefit of modest intakes of n-3-PUFAs, hence accounting for the null findings of several recent n-3-PUFA supplementation studies. Because SCD is the first symptom of CHD in a significant proportion of cases and most asymptomatic people developing CHD do not take statins, modest supplemental intakes of n-3-PUFAs may have important potential for prevention of SCD in non–statin users. Many patients cannot tolerate statins. Moreover, the JELIS study suggests that high intakes of n-3-PUFAs may be protective even in the context of statin usage, likely by evoking protective mechanisms independent of arrhythmia prevention. Hence, even among statin users, the possible benefits of high intakes of n-3-PUFAs merit further exploration in controlled trials.
Compared to populations without high intakes of n-3-PUFAs, those with very high lifelong intakes of n-3-PUFAs are notable for low CV risk and less arterial atherosclerosis. This trend may reflect the interaction of a large number of protective mechanisms, as demonstrated in rodent and clinical studies, as well as concurrent low consumption of red meat. Although high intakes of n-3-PUFAs may be required to evoke many of these mechanisms, epidemiology and some clinical trials suggest that intakes of n-3-PUFAs as low as 250 mg/d can provide protection from SCD and cardiac arrhythmias, especially in non–statin users. The failure of modest doses of n-3-PUFAs to confer protection in some recent trials may reflect the competing antiantiarrhythmic benefits of concurrent therapy with statins and possibly other CV drugs. Increased baseline intakes of n-3-PUFAs in recent study populations, owing to increased public awareness of the protection afforded by n-3-PUFAs, may also have obscured the impact of modest supplemental intakes of n-3-PUFAs in these studies (Table 2). Hence, supplemental n-3-PUFAs are most likely to be protective in non–statin users who rarely consume fatty fish. The quite low CV risk enjoyed by populations with very high dietary intakes of n-3-PUFAs and the favorable impact of 1.8 g of EPA daily among the statin users of the JELIS trial suggest that prolonged high intakes of n-3-PUFAs may confer benefit even in patients receiving modern statin-based pharmacotherapy; hence, high-dose n-3-PUFA supplementation merits further exploration in the secondary prevention of CV events. The antiatherogenic, antithrombotic, and antiinflammatory benefits of n-3-PUFAs appear to require considerably higher intakes than those adequate for prevention of SCD.
Table 2.
Summary of Important Positive and Negative Omega-3 Polyunsaturated Fatty Acids Trials
CONCLUSION
Despite the recent negative data about n-3 fatty acids, the overall evidence still supports the American Heart Association recommendation of n-3-PUFAs (1 g/d) for secondary prevention of CHD.
Footnotes
Financial Disclosure: Dr DiNicolantonio works for a company that sells omega-3 products, but he does not personally profit from the sales. Drs O>'Keefe and Lavie have both served as speakers and consultants to GlaxoSmithKline. Dr Lavie is also a speaker and consultant for Amarin. Mark McCarty is owner and science director of a small nutraceutical company that sells, among other products, a fish oil supplement. Dr O'Keefe is the founder and has major ownership interest in CardioTabs, a company that markets omega-3s. Dr Meier and Asfandyar Niazi disclose no conflicts of interest.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Kromhout D, Bosschieter EB. de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985 May 9;312(19):1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- 2.Delgado-Lista J, Perez-Martinez P, Lopez-Miranda J, Perez-Jimenez F. Long chain omega-3 fatty acids and cardiovascular disease: a systematic review. Br J Nutr. 2012 Jun;107((suppl 2)):S201–S213. doi: 10.1017/S0007114512001596. [DOI] [PubMed] [Google Scholar]
- 3.Stone NJ. Fish consumption, fish oil, lipids, and coronary heart disease. Circulation. 1996 Nov 1;94(9):2337–2340. doi: 10.1161/01.cir.94.9.2337. [DOI] [PubMed] [Google Scholar]
- 4.DiNicolantonio JJ, Niazi AK, O'Keefe JH, Lavie CJ. Explaining the recent fish oil trial “failures.”. J Glycomics Lipidomics. 2013 Dec;3(1): Epub 2012 Nov 15. [Google Scholar]
- 5.Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006 Aug;17(4):387–393. doi: 10.1097/01.mol.0000236363.63840.16. [DOI] [PubMed] [Google Scholar]
- 6.Harris WS, Muzio F. Fish oil reduces postprandial triglyceride concentrations without accelerating lipid-emulsion removal rates. Am J Clin Nutr. 1993 Jul;58(1):68–74. doi: 10.1093/ajcn/58.1.68. [DOI] [PubMed] [Google Scholar]
- 7.Harris WS, Connor WE, Alam N, Illingworth DR. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J Lipid Res. 1988 Nov;29(11):1451–1460. [PubMed] [Google Scholar]
- 8.Schirmer SH, Werner CM, Binder SB, et al. Effects of omega-3 fatty acids on postprandial triglycerides and monocyte activation. Atherosclerosis. 2012 Nov;225(1):166–172. doi: 10.1016/j.atherosclerosis.2012.09.002. Epub 2012 Sep 13. [DOI] [PubMed] [Google Scholar]
- 9.Volek JS, Gómez AL, Kraemer WJ. Fasting lipoprotein and postprandial triacylglycerol responses to a low-carbohydrate diet supplemented with n-3 fatty acids. J Am Coll Nutr. 2000 Jun;19(3):383–391. doi: 10.1080/07315724.2000.10718935. [DOI] [PubMed] [Google Scholar]
- 10.Harris WS, Hustvedt BE, Hagen E, Green MH, Lu G, Drevon CA. N-3 fatty acids and chylomicron metabolism in the rat. J Lipid Res. 1997 Mar;38(3):503–515. [PubMed] [Google Scholar]
- 11.Roche HM, Gibney MJ. Long-chain n-3 polyunsaturated fatty acids and triacylglycerol metabolism in the postprandial state. Lipids. 1999 Jan;34((1)(suppl)):S259–S265. doi: 10.1007/BF02562313. [DOI] [PubMed] [Google Scholar]
- 12.Qi K, Fan C, Jiang J, et al. Omega-3 fatty acid containing diets decrease plasma triglyceride concentrations in mice by reducing endogenous triglyceride synthesis and enhancing the blood clearance of triglyceride-rich particles. Clin Nutr. 2008 Jun;27(3):424–430. doi: 10.1016/j.clnu.2008.02.001. Epub 2008 Mar 24. [DOI] [PubMed] [Google Scholar]
- 13.Sanders TA, Oakley FR, Miller GJ, Mitropoulos KA, Crook D, Oliver MF. Influence of n-6 versus n-3 polyunsaturated fatty acids in diets low in saturated fatty acids on plasma lipoproteins and hemostatic factors. Arterioscler Thromb Vasc Biol. 1997 Dec;17(12):3449–3460. doi: 10.1161/01.atv.17.12.3449. [DOI] [PubMed] [Google Scholar]
- 14.Smith BK, Sun GY, Donahue OM, Thomas TR. Exercise plus n-3 fatty acids: additive effect on postprandial lipemia. Metabolism. 2004 Oct;53(10):1365–1371. doi: 10.1016/j.metabol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011 Nov 8;58(20):2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 16.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002 Aug;20(8):1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005 Sep 27;112(13):1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. Epub 2005 Sep 19. [DOI] [PubMed] [Google Scholar]
- 18.O'Keefe JH, Jr, Abuissa H, Sastre A, Steinhaus DM, Harris WS. Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol. 2006 Apr 15;97(8):1127–1130. doi: 10.1016/j.amjcard.2005.11.025. Epub 2006 Mar 3. [DOI] [PubMed] [Google Scholar]
- 19.Djoussé L, Akinkuolie AO, Wu JH, Ding EL, Gaziano JM. Fish consumption, omega-3 fatty acids and risk of heart failure: a meta-analysis. Clin Nutr. 2012 Dec;31(6):846–853. doi: 10.1016/j.clnu.2012.05.010. Epub 2012 Jun 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charnock JS, McLennan PL, Abeywardena MY. Dietary modulation of lipid metabolism and mechanical performance of the heart. Mol Cell Biochem. 1992 Oct 21;116((1-2)):19–25. doi: 10.1007/BF01270564. [DOI] [PubMed] [Google Scholar]
- 21.Ghio S, Scelsi L, Latini R, et al. Effects of n-3 polyunsaturated fatty acids and of rosuvastatin on left ventricular function in chronic heart failure: a substudy of GISSI-HF trial. Eur J Heart Fail. 2010 Dec;12(12):1345–1353. doi: 10.1093/eurjhf/hfq172. Erratum in: Eur J Heart Fail. 2011 Sep;13(9):1042. [DOI] [PubMed] [Google Scholar]
- 22.Grimsgaard S, Bønaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998 Jul;68(1):52–59. doi: 10.1093/ajcn/68.1.52. [DOI] [PubMed] [Google Scholar]
- 23.McLennan PL, Barnden LR, Bridle TM, Abeywardena MY, Charnock JS. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc Res. 1992 Sep;26(9):871–877. doi: 10.1093/cvr/26.9.871. [DOI] [PubMed] [Google Scholar]
- 24.Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006 Jan 15;97(2):216–222. doi: 10.1016/j.amjcard.2005.08.025. Epub 2005 Nov 21. [DOI] [PubMed] [Google Scholar]
- 25.Peoples GE, McLennan PL, Howe PR, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol. 2008 Dec;52(6):540–547. doi: 10.1097/FJC.0b013e3181911913. [DOI] [PubMed] [Google Scholar]
- 26.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002 May 14;105(19):2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 27.Xin W, Wei W, Li X. Effects of fish oil supplementation on cardiac function in chronic heart failure: a meta-analysis of randomised controlled trials. Heart. 2012 Nov;98(22):1620–1625. doi: 10.1136/heartjnl-2012-302119. Epub 2012 Jul 3. [DOI] [PubMed] [Google Scholar]
- 28.Nodari S, Triggiani M, Campia U, et al. Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2011 Feb 15;57(7):870–879. doi: 10.1016/j.jacc.2010.11.017. Epub 2011 Jan 6. [DOI] [PubMed] [Google Scholar]
- 29.Hein GJ, Bernasconi AM, Montanaro MA, et al. Nuclear receptors and hepatic lipidogenic enzyme response to a dyslipidemic sucrose-rich diet and its reversal by fish oil n-3 polyunsaturated fatty acids. Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E429–E439. doi: 10.1152/ajpendo.00513.2009. Epub 2009 Dec 1. [DOI] [PubMed] [Google Scholar]
- 30.Sato A, Kawano H, Notsu T, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010 Oct;59(10):2495–2504. doi: 10.2337/db09-1554. Epub 2010 Aug 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka N, Zhang X, Sugiyama E, et al. Eicosapentaenoic acid improves hepatic steatosis independent of PPARalpha activation through inhibition of SREBP-1 maturation in mice. Biochem Pharmacol. 2010 Nov 15;80(10):1601–1612. doi: 10.1016/j.bcp.2010.07.031. Epub 2010 Aug 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wada S, Yamazaki T, Kawano Y, Miura S, Ezaki O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J Hepatol. 2008 Sep;49(3):441–450. doi: 10.1016/j.jhep.2008.04.026. Epub 2008 Jun 11. [DOI] [PubMed] [Google Scholar]
- 33.González-Périz A, Horrillo R, Ferré N, et al. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009 Jun;23(6):1946–1957. doi: 10.1096/fj.08-125674. Epub 2009 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomised controlled clinical trial. Arch Dis Child. 2011 Apr;96(4):350–353. doi: 10.1136/adc.2010.192401. Epub 2011 Jan 12. [DOI] [PubMed] [Google Scholar]
- 35.Masterton GS, Plevris JN, Hayes PC. Review article: omega-3 fatty acids - a promising novel therapy for non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2010 Apr;31(7):679–692. doi: 10.1111/j.1365-2036.2010.04230.x. [DOI] [PubMed] [Google Scholar]
- 36.Kenny D, Warltier DC, Pleuss JA, Hoffmann RG, Goodfriend TL, Egan BM. Effect of omega-3 fatty acids on the vascular response to angiotensin in normotensive men. Am J Cardiol. 1992 Nov 15;70(15):1347–1352. doi: 10.1016/0002-9149(92)90773-r. [DOI] [PubMed] [Google Scholar]
- 37.Chin JP, Gust AP, Nestel PJ, Dart AM. Marine oils dose-dependently inhibit vasoconstriction of forearm resistance vessels in humans. Hypertension. 1993 Jan;21(1):22–28. doi: 10.1161/01.hyp.21.1.22. [DOI] [PubMed] [Google Scholar]
- 38.Harris WS, Rambjør GS, Windsor SL, Diederich D. N-3 fatty acids and urinary excretion of nitric oxide metabolites in humans. Am J Clin Nutr. 1997 Feb;65(2):459–464. doi: 10.1093/ajcn/65.2.459. [DOI] [PubMed] [Google Scholar]
- 39.Stirban A, Nandrean S, Götting C, et al. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr. 2010 Mar;91(3):808–813. doi: 10.3945/ajcn.2009.28374. Epub 2010 Jan 13. [DOI] [PubMed] [Google Scholar]
- 40.Leeson CP, Mann A, Kattenhorn M, Deanfield JE, Lucas A, Muller DP. Relationship between circulating n-3 fatty acid concentrations and endothelial function in early adulthood. Eur Heart J. 2002 Feb;23(23):216–222. doi: 10.1053/euhj.2001.2728. [DOI] [PubMed] [Google Scholar]
- 41.Dangardt F, Osika W, Chen Y, et al. Omega-3 fatty acid supplementation improves vascular function and reduces inflammation in obese adolescents. Atherosclerosis. 2010 Oct;212(2):580–585. doi: 10.1016/j.atherosclerosis.2010.06.046. Epub 2010 Jul 21. [DOI] [PubMed] [Google Scholar]
- 42.Rizza S, Tesauro M, Cardillo C, et al. Fish oil supplementation improves endothelial function in normoglycemic offspring of patients with type 2 diabetes. Atherosclerosis. 2009 Oct;206(2):569–574. doi: 10.1016/j.atherosclerosis.2009.03.006. Epub 2009 Mar 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodfellow J, Bellamy MF, Ramsey MW, Jones CJ, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. J Am Coll Cardiol. 2000 Feb;35(2):265–270. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 44.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000 Sep 12;102(11):1264–1269. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 45.Wright SA, O'Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008 Jun;67(6):841–848. doi: 10.1136/ard.2007.077156. Epub 2007 Sep 17. [DOI] [PubMed] [Google Scholar]
- 46.Nestel P, Shige H, Pomeroy S, Cehun M, Abbey M, Raederstorff D. The n-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid increase systemic arterial compliance in humans. Am J Clin Nutr. 2002 Aug;76(2):326–330. doi: 10.1093/ajcn/76.2.326. [DOI] [PubMed] [Google Scholar]
- 47.McVeigh GE, Brennan GM, Cohn JN, Finkelstein SM, Hayes RJ, Johnston GD. Fish oil improves arterial compliance in non-insulin-dependent diabetes mellitus. Arterioscler Thromb. 1994 Sep;14(9):1425–1429. doi: 10.1161/01.atv.14.9.1425. [DOI] [PubMed] [Google Scholar]
- 48.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA. Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study. Atherosclerosis. 2009 Aug;205(2):538–543. doi: 10.1016/j.atherosclerosis.2008.12.013. Epub 2008 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He K, Liu K, Daviglus ML, et al. Associations of dietary long-chain n-3 polyunsaturated fatty acids and fish with biomarkers of inflammation and endothelial activation (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009 May 1;103(9):1238–1243. doi: 10.1016/j.amjcard.2009.01.016. Epub 2009 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Micallef MA, Munro IA, Garg ML. An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr. 2009 Sep;63(9):1154–1156. doi: 10.1038/ejcn.2009.20. Epub 2009 Apr 8. [DOI] [PubMed] [Google Scholar]
- 51.van Bussel BC, Henry RM, Schalkwijk CG, et al. Fish consumption in healthy adults is associated with decreased circulating biomarkers of endothelial dysfunction and inflammation during a 6-year follow-up. J Nutr. 2011 Sep;141(9):1719–1725. doi: 10.3945/jn.111.139733. Epub 2011 Jul 13. Erratum in: J Nutr. 2011 Dec;141(12):2258. [DOI] [PubMed] [Google Scholar]
- 52.Zampelas A, Panagiotakos DB, Pitsavos C, et al. Fish consumption among healthy adults is associated with decreased levels of inflammatory markers related to cardiovascular disease: the ATTICA study. J Am Coll Cardiol. 2005 Jul 5;46(1):120–124. doi: 10.1016/j.jacc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 53.Murakami K, Sasaki S, Takahashi Y, et al. Total n-3 polyunsaturated fatty acid intake is inversely associated with serum C-reactive protein in young Japanese women. Nutr Res. 2008 May;28(5):309–314. doi: 10.1016/j.nutres.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006 Feb;91(2):439–446. doi: 10.1210/jc.2005-1303. Epub 2005 Oct 18. [DOI] [PubMed] [Google Scholar]
- 55.Klein-Platat C, Drai J, Oujaa M, Schlienger JL, Simon C. Plasma fatty acid composition is associated with the metabolic syndrome and low-grade inflammation in overweight adolescents. Am J Clin Nutr. 2005 Dec;82(6):1178–1184. doi: 10.1093/ajcn/82.6.1178. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-Garcia E, Schulze MB, Manson JE, et al. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr. 2004 Jul;134(7):1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 57.Kelley DS, Siegel D, Fedor DM, Adkins Y, Mackey BE. DHA supplementation decreases serum C-reactive protein and other markers of inflammation in hypertriglyceridemic men. J Nutr. 2009 Mar;139(3):495–501. doi: 10.3945/jn.108.100354. Epub 2009 Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang TM, Hsieh SC, Chen JW, Chiang AN. Docosahexaenoic acid and eicosapentaenoic acid reduce C-reactive protein expression and STAT3 activation in IL-6-treated HepG2 cells. Mol Cell Biochem. 2013 May;377((1-2)):97–106. doi: 10.1007/s11010-013-1574-1. Epub 2013 January 30. [DOI] [PubMed] [Google Scholar]
- 59.Satoh N, Shimatsu A, Kotani K, et al. Highly purified eicosapentaenoic acid reduces cardio-ankle vascular index in association with decreased serum amyloid A-LDL in metabolic syndrome. Hypertens Res. 2009 Nov;32(11):1004–1008. doi: 10.1038/hr.2009.145. Epub 2009 Sep 18. [DOI] [PubMed] [Google Scholar]
- 60.McCarty MF. Strategies for controlling serum amyloid A, a key mediator of the impact of systemic inflammation on cardiovascular disease. 2014 http://catalyticlongevity.org/strategies-for-controlling-serum-amyloid-a-a-key-mediator-of-the-impact-of-systemic-inflammation-on-cardiovascular-disease/. Accessed June 25. [Google Scholar]
- 61.Serhan CN. Novel lipid mediators and resolution mechanisms in acute inflammation: to resolve or not? Am J Pathol. 2010 Oct;177(4):1576–1591. doi: 10.2353/ajpath.2010.100322. Epub 2010 Sep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nowak JZ. Anti-inflammatory pro-resolving derivatives of omega-3 and omega-6 polyunsaturated fatty acids [in Polish] Postepy Hig Med Dosw (Online) 2010 Mar 17;64:115–132. [PubMed] [Google Scholar]
- 63.Serhan CN. Novel omega-3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005 Jan;105(1):7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Serhan CN, Gotlinger K, Hong S, Arita M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins Other Lipid Mediat. 2004 Apr;73((3-4)):155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 65.Serhan CN, Arita M, Hong S, Gotlinger K. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their endogenous aspirin-triggered epimers. Lipids. 2004 Nov;39(11):1125–1132. doi: 10.1007/s11745-004-1339-7. [DOI] [PubMed] [Google Scholar]
- 66.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005 Mar;8(2):115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 67.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008 Mar;153((1)(suppl)):S200–S215. doi: 10.1038/sj.bjp.0707489. Epub 2007 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Serhan CN. Systems approach with inflammatory exudates uncovers novel anti-inflammatory and pro-resolving mediators. Prostaglandins Leukot Essent Fatty Acids. 2008 Sep-Nov;79((3-5)):157–163. doi: 10.1016/j.plefa.2008.09.012. Epub 2008 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Calviello G, Su HM, Weylandt KH, Fasano E, Serini S, Cittadini A. Experimental evidence of ω-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: their potential role in inflammatory, neurodegenerative, and neoplastic diseases. Biomed Res Int. 2013;2013: doi: 10.1155/2013/743171. Epub 2013 Apr 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jones ML, Mark PJ, Keelan JA, et al. Maternal dietary omega-3 fatty acid intake increases resolvin and protectin levels in the rat placenta. J Lipid Res. 2013 Aug;54(8):2247–2254. doi: 10.1194/jlr.M039842. Epub 2013 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caughey GE, Mantzioris E, Gibson RA, Cleland LG, James MJ. The effect on human tumor necrosis factor alpha and interleukin 1 beta production of diets enriched in n-3 fatty acids from vegetable oil or fish oil. Am J Clin Nutr. 1996 Jan;63(1):116–122. doi: 10.1093/ajcn/63.1.116. [DOI] [PubMed] [Google Scholar]
- 72.Kelley DS, Taylor PC, Nelson GJ, et al. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999 Apr;34(4):317–324. doi: 10.1007/s11745-999-0369-5. [DOI] [PubMed] [Google Scholar]
- 73.Trebble TM, Wootton SA, Miles EA, et al. Prostaglandin E2 production and T cell function after fish-oil supplementation: response to antioxidant cosupplementation. Am J Clin Nutr. 2003 Sep;78(3):376–382. doi: 10.1093/ajcn/78.3.376. [DOI] [PubMed] [Google Scholar]
- 74.Rees D, Miles EA, Banerjee T, et al. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. 2006 Feb;83(2):331–342. doi: 10.1093/ajcn/83.2.331. [DOI] [PubMed] [Google Scholar]
- 75.Vedin I, Cederholm T, Freund-Levi Y, et al. Reduced prostaglandin F2 alpha release from blood mononuclear leukocytes after oral supplementation of omega3 fatty acids: the OmegAD study. J Lipid Res. 2010 May;51(5):1179–1185. doi: 10.1194/jlr.M002667. Epub 2009 Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR. Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest. 1993 Feb;91(2):651–660. doi: 10.1172/JCI116245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.An WS, Kim HJ, Cho KH, Vaziri ND. Omega-3 fatty acid supplementation attenuates oxidative stress, inflammation, and tubulointerstitial fibrosis in the remnant kidney. Am J Physiol Renal Physiol. 2009 Oct 1;297(4):F895–F903. doi: 10.1152/ajprenal.00217.2009. Epub 2009 Aug 5. [DOI] [PubMed] [Google Scholar]
- 78.von Schacky C, Kiefl R, Jendraschak E, Kaminski WE. N-3 fatty acids and cysteinyl-leukotriene formation in humans in vitro, ex vivo, and in vivo. J Lab Clin Med. 1993 Feb;121(2):302–309. [PubMed] [Google Scholar]
- 79.Shearer GC, Harris WS, Pedersen TL, Newman JW. Detection of omega-3 oxylipins in human plasma and response to treatment with omega-3 acid ethyl esters. J Lipid Res. 2010 Aug;51(8):2074–2081. doi: 10.1194/jlr.M900193-JLR200. Epub 2009 Aug 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siegel G, Ermilov E. Omega-3 fatty acids: benefits for cardio-cerebro-vascular diseases. Atherosclerosis. 2012 Dec;225(2):291–295. doi: 10.1016/j.atherosclerosis.2012.09.006. Epub 2012 Sep 18. [DOI] [PubMed] [Google Scholar]
- 81.Rix TA, Christensen JH, Schmidt EB. Omega-3 fatty acids and cardiac arrhythmias. Curr Opin Clin Nutr Metab Care. 2013 Mar;16(2):168–173. doi: 10.1097/MCO.0b013e32835bf39b. [DOI] [PubMed] [Google Scholar]
- 82.Costanzo S, di Niro V, Di Castelnuovo A, et al. Prevention of postoperative atrial fibrillation in open heart surgery patients by preoperative supplementation of n-3 polyunsaturated fatty acids: an updated meta-analysis. J Thorac Cardiovasc Surg. 2013 Oct;146(4):906–911. doi: 10.1016/j.jtcvs.2013.03.015. Epub 2013 Apr 12. [DOI] [PubMed] [Google Scholar]
- 83.Rix TA, Joensen AM, Lundbye-Christensen S, Riahi S, Schmidt EB, Overvad K. Moderate consumption of marine n-3 fatty acids is associated with a lower risk of atrial fibrillation - a cohort study [poster abstract] Europace. 2013 Jun;15((suppl 2)): [Google Scholar]
- 84.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006 Oct 18;296(15):1885–1899. doi: 10.1001/jama.296.15.1885. Erratum in: JAMA. 2007 Feb 14;297(6):590. [DOI] [PubMed] [Google Scholar]
- 85.von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999 Apr 6;130(7):554–562. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 86.Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007 Mar;191(1):162–167. doi: 10.1016/j.atherosclerosis.2006.03.005. Epub 2006 Apr 17. [DOI] [PubMed] [Google Scholar]
- 87.Landmark K, Abdelnoor M, Kilhovd B, Dørum HP. Eating fish may reduce infarct size and the occurrence of Q wave infarcts. Eur J Clin Nutr. 1998 Jan;52(1):40–44. doi: 10.1038/sj.ejcn.1600510. [DOI] [PubMed] [Google Scholar]
- 88.Landmark K, Abdelnoor M, Urdal P, et al. Use of fish oils appears to reduce infarct size as estimated from peak creatine kinase and lactate dehydrogenase activities. Cardiology. 1998 Jan;89(2):94–102. doi: 10.1159/000006763. [DOI] [PubMed] [Google Scholar]
- 89.Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: the Zutphen study. Eur Heart J. 2008 Aug;29(16):2024–2030. doi: 10.1093/eurheartj/ehn294. Epub 2008 Jul 18. [DOI] [PubMed] [Google Scholar]
- 90.Lemaitre RN, King IB, Mozaffarian D, Kuller LH, Tracy RP, Siscovick DS. N-3 polyunsaturated fatty acids, fatal ischemic heart disease, and nonfatal myocardial infarction in older adults: the Cardiovascular Health Study. Am J Clin Nutr. 2003 Feb;77(2):319–325. doi: 10.1093/ajcn/77.2.319. [DOI] [PubMed] [Google Scholar]
- 91.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007 Mar 31;369(9567):1090–1098. doi: 10.1016/S0140-6736(07)60527-3. Erratum in: Lancet. 2007 Jul 21;370(9583):220. [DOI] [PubMed] [Google Scholar]
- 92.Barringer TA, Harris WS. Omega-3 fatty acid deficiency and cardiovascular disease. In: McNamara RK, editor. The Omega-3 Fatty Acid Deficiency Syndrome: Opportunities for Disease Prevention. New York, NY: Nova Science Publishers;; 2013. pp. 213–232. In. ed. [Google Scholar]
- 93.Kris-Etherton PM, Harris WS, Appel LJ. American Heart Association. Nutrition Committee. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002 Nov;106(21):2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. Erratum in: Circulation. 2003 Jan 28;107(3):512. [DOI] [PubMed] [Google Scholar]
- 94.Ministry of Health and Welfare. Annual report of the National Nutrition Survey in 1998 [in Japanese] Tokyo, Japan: Daiichi Publishing; 2000. [Google Scholar]
- 95.Sekikawa A, Curb JD, Ueshima H, et al. Marine-derived n-3 fatty acids and atherosclerosis in Japanese, Japanese-American, and white men: a cross-sectional study. J Am Coll Cardiol. 2008 Aug 5;52(6):417–424. doi: 10.1016/j.jacc.2008.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sekikawa A, Kuller LH. Coronary heart disease mortality in the United States among black and white men 35–44 years old by state. CVD Prev. 1999;2:212–221. [Google Scholar]
- 97.Takei H, Strong JP, Yutani C, Malcom GT. Comparison of coronary and aortic atherosclerosis in youth from Japan and the USA. Atherosclerosis. 2005 May;180(1):171–179. doi: 10.1016/j.atherosclerosis.2004.11.014. Epub 2005 Jan 26. [DOI] [PubMed] [Google Scholar]
- 98.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002 Apr 23;105(16):1897–1903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 99.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999 Aug 7;354(9177):447–455. Erratum in: Lancet. 2001 Feb 24;357(9256):642. Lancet. 2007 Jan 13;369(9556):106. [PubMed] [Google Scholar]
- 100.Gissi-HF Investigators. Tavazzi L, Maggioni AP, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Oct 4;372(9645):1223–1230. doi: 10.1016/S0140-6736(08)61239-8. Epub 2008 Aug 29. [DOI] [PubMed] [Google Scholar]
- 101.Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989 Sep 30;2(8666):757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 102.Burr ML, Ashfield-Watt PA, Dunstan FD, et al. Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr. 2003 Feb;57(2):193–200. doi: 10.1038/sj.ejcn.1601539. [DOI] [PubMed] [Google Scholar]
- 103.Brouwer IA, Zock PL, Camm AJ, et al. Effect of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators: the Study on Omega-3 Fatty Acids and Ventricular Arrhythmia (SOFA) randomized trial. JAMA. 2006 Jun 14;295(22):2613–2619. doi: 10.1001/jama.295.22.2613. [DOI] [PubMed] [Google Scholar]
- 104.Rauch B, Schiele R, Schneider S, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010 Nov 23;122(21):2152–2159. doi: 10.1161/CIRCULATIONAHA.110.948562. Epub 2010 Nov 8. [DOI] [PubMed] [Google Scholar]
- 105.Kromhout D, Giltay EJ, Geleijnse JM. Alpha Omega Trial Group. N–3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010 Nov 18;363(21):2015–2026. doi: 10.1056/NEJMoa1003603. Epub 2010 Aug 28. [DOI] [PubMed] [Google Scholar]
- 106.Galan P, Kesse-Guyot E, Czernichow S, et al. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010 Nov 29;341: doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Svensson M, Schmidt EB, Jørgensen KA, Christensen JH. OPACH Study Group. N-3 fatty acids as secondary prevention against cardiovascular events in patients who undergo chronic hemodialysis: a randomized, placebo-controlled intervention trial. Clin J Am Soc Nephrol. 2006 Jul;1(4):780–786. doi: 10.2215/CJN.00630206. Epub 2006 Jun 14. [DOI] [PubMed] [Google Scholar]
- 108.Eritsland J, Arnesen H, Grønseth K, Fjeld NB, Abdelnoor M. Effect of dietary supplementation with n-3 fatty acids on coronary artery bypass graft patency. Am J Cardiol. 1996 Jan 1;77(1):31–36. doi: 10.1016/s0002-9149(97)89130-8. [DOI] [PubMed] [Google Scholar]
- 109.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012 Sep 12;308(10):1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 110.Lewis EJH. Omega-3 fatty acid supplementation and cardiovascular disease events [letter] JAMA. 2013 Jan 2;309(1):27. doi: 10.1001/jama.2012.116654. [DOI] [PubMed] [Google Scholar]
- 111.Vlachopoulos C, Richter D, Stefanadis C. Omega-3 fatty acid supplementation and cardiovascular disease events. JAMA. 2013 Jan 2;309(1):28. doi: 10.1001/jama.2012.116651. [DOI] [PubMed] [Google Scholar]
- 112.Kwak SM, Myung SK, Lee YJ, Seo HG. Korean Meta-analysis Study Group. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta-analysis of randomized, double-blind, placebo-controlled trials. Arch Intern Med. 2012 May 14;172(9):686–694. doi: 10.1001/archinternmed.2012.262. [DOI] [PubMed] [Google Scholar]
- 113.DiNicolantonio JJ, O'Keefe JH, Lavie CJ. The big ones that got away: omega-3 meta-analysis flawed by excluding the biggest fish oil trials. Arch Intern Med. 2012 Oct 8;172(18):1427. doi: 10.1001/archinternmed.2012.3755. [DOI] [PubMed] [Google Scholar]
- 114.Abuissa H, O'Keefe JH, Bybee KA. Statins as anti-arrhythmics: a systematic review part II: effects on risk of ventricular arrhythmias. Clin Cardiol. 2009 Oct;32(10):549–552. doi: 10.1002/clc.20435. [DOI] [PMC free article] [PubMed] [Google Scholar]


