Abstract
Background
Propofol is commonly used and well tolerated for induction of general anesthesia and is also used as a sedative in the intensive care unit. However, in rare cases, the agent may cause a fatal condition known as propofol infusion syndrome (PRIS).
Case Report
We present a case of PRIS that could have been fatal in a previously healthy male patient with multiple gunshot wounds.
Conclusion
Because patients typically exhibit other potentially fatal comorbidities, PRIS is always a diagnosis of exclusion. The true incidence of PRIS remains unknown, and more objective criteria for its diagnosis need to be established.
Keywords: Acidosis, acute kidney injury, hypnotics and sedatives, intensive care units, propofol, rhabdomyolysis, wounds–gunshot
INTRODUCTION
Propofol, a popular sedative hypnotic, is commonly used for induction of general anesthesia and sedation in the intensive care unit (ICU). It is preferred over other agents for its rapid onset of action, rapid emergence from sedation, and reduced likelihood of nausea and vomiting. Despite these advantages, propofol in rare cases can cause a fatal condition known as propofol infusion syndrome (PRIS). The diagnosis of PRIS is usually made by exclusion because patients often exhibit other potentially fatal comorbidities, including metabolic acidosis, acute renal failure, and rhabdomyolysis. We report a case of PRIS that could have been fatal in a previously healthy male patient with multiple gunshot wounds.
CASE REPORT
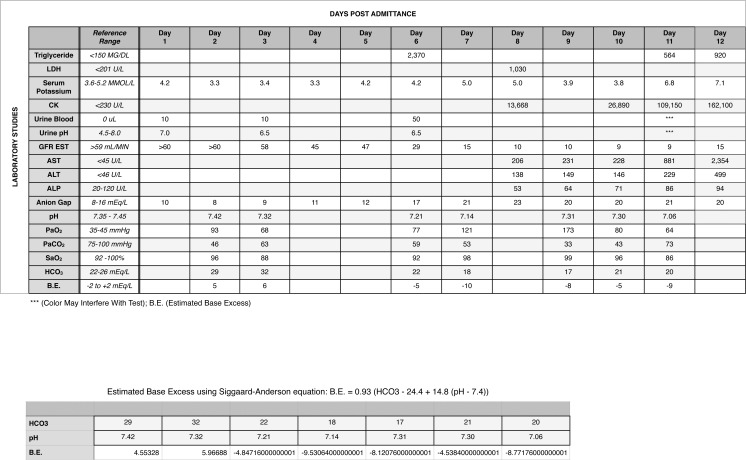
A 32-year-old, 168 kg African American male victim of gunshot wounds presented to the emergency room and underwent an exploratory laparotomy with resection of the terminal ileum and primary reanastomosis. Other injuries included a pelvic hematoma with myonecrosis and transection of the membranous urethra. No evidence of compartment syndrome was seen in any of the extremities. Postoperatively, the patient developed adult respiratory distress syndrome (ARDS) that required continuous propofol sedation at a dose of 50-125 mcg/kg/min for mechanical ventilation (day 3). Propofol was discontinued on the eighth postoperative day in response to the development of metabolic acidosis, rhabdomyolysis, and acute renal failure; hemodialysis was instituted (day 9). Continuous norepinephrine infusion was required to maintain blood pressure during hemodialysis. By the tenth postoperative day, the patient required multiple vasopressors in addition to norepinephrine to maintain cardiac output. The patient suffered a cardiac arrest the following day and could not be successfully resuscitated. The Figure shows the patient's laboratory values over time until his death on the eleventh postoperative day (day 12). In addition to the arterial blood gases and widening anion gap consistent with ARDS and progressive renal insufficiency, respectively, his most significant laboratory values included a high spike in serum triglyceride (2,370 mg/dL) on day 6; spiking serum potassium levels starting on day 7 (range: 3.8-7.1 mmol/L); increasing creatine kinase levels starting on day 8 (range: 13,688-162,100 U/L); and elevated hepatic transaminases beginning on day 8. Of these abnormal laboratory values, the early spike in serum triglyceride and the progressively rising creatine kinase levels with a widening base deficit (range: −5 to −10) would have been consistent with a clinical diagnosis of PRIS.
Figure.
Patient laboratory values over time. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; B.E., estimated base excess; CK, creatine kinase; GFR EST, estimated glomerular filtration rate; HCO3, sodium bicarbonate; LDH, lactate dehydrogenase; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; SaO2, saturation level of oxygen.
Gross autopsy findings included the following gunshot wounds: (1) a completely penetrating wound of the left upper arm with soft tissue injuries, (2) a completely penetrating wound of the left thigh with soft tissue injuries, (3) a completely penetrating wound of the anterior right thigh with soft tissue injuries, and (4) a reentry wound in the right groin with bullet impact and fracture of the L5 vertebral body. A jacketed bullet fragment was recovered from the retroperitoneum. Extensive hemorrhage into the pelvic musculature had occurred. Partial small bowel resection of the terminal ileum was present with an intact reanastomosis. Two hundred milliliters of bloody peritoneal fluid were collected on the lateral aspect of the right hepatic lobe. The lungs demonstrated acute bilateral pneumonia with pulmonary congestion and edema. Cardiomegaly (770 g) was present with concentric left ventricular hypertrophy (1.6 cm). The liver demonstrated evidence of chronic passive congestion without hepatomegaly or evidence of fatty liver. The serum was not lipemic.
Although the patient demonstrated metabolic acidosis, acute renal failure, and rhabdomyolysis, his other comorbidities included morbid obesity, cardiomegaly, and extensive muscle trauma from gunshot wounds, all of which could have resulted in metabolic acidosis, renal failure, and cardiac arrest. Because PRIS is a clinical diagnosis, the coroner's pathological diagnoses in this case included bilateral acute pneumonia, acute hepatic congestion, and cardiomegaly with fatal cardiac arrhythmia.
DISCUSSION
Despite more than 20 years of intensive research, the complete pathophysiological mechanisms responsible for PRIS have not been identified. Potential risk factors for PRIS include high-dose infusions of propofol for lengthy periods. This risk factor was first described in a series of 5 cases reported in 1992 in relatively healthy children with acute epiglottitis or tracheobronchitis who died after being sedated with propofol in the ICU. In these cases, the pediatric patients developed metabolic acidosis, lipemic serum, and refractory bradycardia progressing to asystole.1
The name PRIS was coined in 1998 when Bray summarized 13 additional propofol-related deaths of children, all of whom exhibited a similar constellation of symptoms, including metabolic acidosis, lipemic serum, and refractory bradycardia progressing to asystole.2 In 1996, Marinella was the first author to suggest that a propofol reaction should be included in the differential diagnosis of metabolic acidosis developing in adult patients during long-term sedation with propofol.3 In 1998, the first case of PRIS in an adult was reported. In this case, nearly all of the earlier presenting signs of PRIS in pediatric patients were described, including hypoxia, metabolic acidosis, rhabdomyolysis, renal failure, and cardiac dysfunction.4
The true incidence of PRIS is unknown. In 2009, Roberts et al evaluated 1,017 critically ill patients receiving propofol infusions for longer than 24 hours and reported the incidence of PRIS to be 1.1%.5 Fong et al analyzed 1,139 suspected cases of PRIS from the United States Food and Drug Administration's MedWatch system and estimated an incidence of approximately 30%.6 In addition to the confusion regarding the true incidence of PRIS, no consensus exists on the management of PRIS other than early recognition and discontinuation of propofol.
The clinical manifestations that define PRIS include the development of metabolic acidosis, rhabdomyolysis (skeletal > cardiac), cardiac arrhythmias (including right bundle branch block, Brugada-like syndrome, atrial fibrillation, supraventricular tachycardia, ventricular tachycardia, ventricular fibrillation, and electromechanical dissociation), acute renal failure, lipemic serum, hepatomegaly, and fatal cardiac arrest.2 The onset of PRIS might be related to inhibition of intracellular energy production by mitochondria, possibly by 2 mechanisms: (1) inhibition of transportation of long-chain fatty acids into cells during the nutritionally deficient states of critical illnesses and/or (2) inhibitory effects on the intracellular mitochondrial respiratory chain.7 The metabolic derangements in PRIS appear to be triggered by (1) metabolic stress and high energy demand during critical illness in susceptible patients; (2) low carbohydrate supplies, especially in children; and (3) high availability of fats, as in propofol's emulsion of soybean oil and egg whites.8,9 However, anything that inhibits effective cellular aerobic respiration may result in lactic acidosis that, if left untreated, can result in rhabdomyolysis, hyperkalemia, and, ultimately, acute renal failure. Other risk factors for PRIS include respiratory infection, severe head injury, propofol sedation for more than 48 hours at doses >4 mg/kg/h, and increased catecholamine and glucocorticoid serum levels.8 The metaanalysis by Fong et al found that death from PRIS was more likely if the patient was younger than 18 years old, received a vasopressor, or developed any of the following symptoms: cardiac arrhythmias, rhabdomyolysis, impairment in renal function, metabolic acidosis, or dyslipidemia.6
In our case, the patient displayed many of the diagnostic criteria for PRIS, including the development of metabolic acidosis, rhabdomyolysis, acute renal failure requiring hemodialysis, and, later, unanticipated cardiac arrest. The most important preexisting risk factor for PRIS in our case was the patient's sedation with propofol for more than 48 hours. Although the autopsy showed no evidence of hepatomegaly and the serum was not lipemic, the chronic passive congestion of the liver could have resulted from congestive heart failure in an obese patient with cardiomegaly. The only gross anatomical findings at autopsy that may have supported a diagnosis of PRIS included extensive muscle damage in the pelvic musculature and 2 extremities, which were more likely caused by wound ballistics and independently associated with rhabdomyolysis.
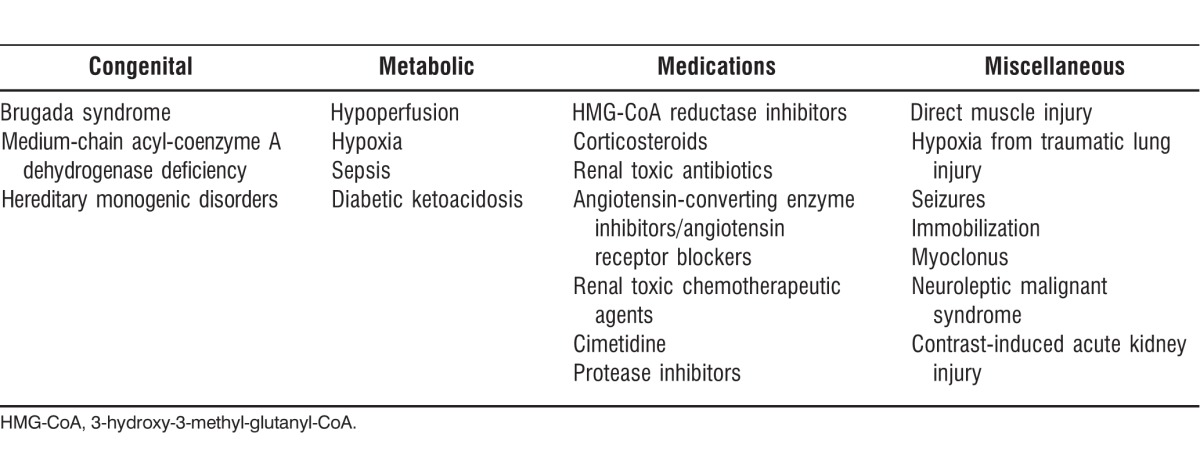
A differential diagnosis of PRIS is presented in the Table. Congenital cardiac conduction disorders such as Brugada syndrome, an autosomal dominant disorder in right ventricular conduction, can result in sudden death. Genetic polymorphisms that affect lipid metabolism, such as medium-chain acyl-coenzyme A dehydrogenase deficiency, may also be risk factors for PRIS and should be included in the differential diagnosis. Medications can also be a cause of laboratory derangements resembling PRIS. The medications that should be considered when ruling out PRIS either have the capability to cause muscle injury or have been shown to cause renal damage. Finally, miscellaneous conditions that need to be considered in the differential diagnosis of PRIS include the use of typical antipsychotics in bipolar patients and the presence of ongoing seizures in epileptic patients, both of which can cause rhabdomyolysis.
Table.
Differential Diagnosis of Propofol Infusion Syndrome
CONCLUSION
A presumptive diagnosis of PRIS includes rhabdomyolysis, hyperkalemia, hyperlipidemia, and acute renal failure in adults receiving high-dose propofol infusions (>4 mg/kg/h) for prolonged (>48 hours) periods. Although Roberts et al5 reported a 1.1% incidence rate of PRIS in a multihospital study, level III trauma center hospitals may have higher incidence rates of PRIS cases for several reasons including the following: (1) more trauma patients with soft tissue injuries, (2) more trauma patients with head injuries requiring prolonged sedation for mechanical ventilation, and (3) greater use of propofol over other sedative-hypnotics for sedation in the ICU.
The true incidence of PRIS is unknown, and more objective criteria for its diagnosis need to be established. Future large prospective, randomized, controlled trials comparing outcomes of several sedation protocols in ICU patients are needed to determine the true incidence of PRIS, to identify genetically susceptible patients, and to develop clinical guidelines for propofol sedation without increasing the risks of PRIS. If possible, propofol should not be used for sedation longer than 3 days. During propofol infusions, clinicians should monitor arterial blood gases, serum triglycerides, creatine kinase, all electrolytes (particularly potassium), serum lactate levels, and creatinine.
Footnotes
The authors have no financial or proprietary interest in the subject matter of this article.
This article meets the Accreditation Council for Graduate Medical Education and the American Board of Medical Specialties Maintenance of Certification competencies for Patient Care and Medical Knowledge.
REFERENCES
- 1.Parke TJ, Stevens JE, Rice AS, et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992 Sep 12;305(6854):613–616. doi: 10.1136/bmj.305.6854.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray RJ. Propofol infusion syndrome in children. Paediatr Anaesth. 1998;8(6):491–499. doi: 10.1046/j.1460-9592.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 3.Marinella MA. Lactic acidosis associated with propofol. Chest. 1996 Jan;109(1):292. doi: 10.1378/chest.109.1.292. [DOI] [PubMed] [Google Scholar]
- 4.Hanna JP, Ramundo ML. Rhabdomyolysis and hypoxia associated with prolonged propofol infusion in children. Neurology. 1998 Jan;50(1):301–303. doi: 10.1212/wnl.50.1.301. [DOI] [PubMed] [Google Scholar]
- 5.Roberts RJ, Barletta JF, Fong JJ, et al. Incidence of propofol-related infusion syndrome in critically ill adults: a prospective, multicenter study. Crit Care. 2009;13(5):R169. doi: 10.1186/cc8145. Epub 2009 Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong JJ, Sylvia L, Ruthazer R, Schumaker G, Kcomt M, Devlin JW. Predictors of mortality in patients with suspected propofol infusion syndrome. Crit Care Med. 2008 Aug;36(8):2281–2287. doi: 10.1097/CCM.0b013e318180c1eb. [DOI] [PubMed] [Google Scholar]
- 7.Diedrich DA, Brown DR. Analytic reviews: propofol infusion syndrome in the ICU. J Intensive Care Med. 2011 Mar-Apr;26(2):59–72. doi: 10.1177/0885066610384195. [DOI] [PubMed] [Google Scholar]
- 8.Motsch J, Roggenbach J. Propofol infusion syndrome [in German] Anaesthesist. 2004 Oct;53(10):1009–1022. 1023–1024. doi: 10.1007/s00101-004-0756-3. quiz. [DOI] [PubMed] [Google Scholar]
- 9.Otterspoor LC, Kalkman CJ, Cremer OL. Update on the propofol infusion syndrome in ICU management of patients with head injury. Curr Opin Anaesthesiol. 2008 Oct;21(5):544–551. doi: 10.1097/ACO.0b013e32830f44fb. [DOI] [PubMed] [Google Scholar]