Abstract
Background
Although the incidence of gastric cancer is declining during the last half century, this cancer still is the second morbid cancer in the world after lung cancer. The incidence of gastric cancer is 26 per 100,000 in Iran. This study evaluated the effect of Alpinia galangal on AGS cells (human gastric adenocarcinoma epithelial cell line) and L929 cells (as a standard cell line originated from mouse fibroblast cells).
Methods
After culturing the cells in Roswell Park Memorial Institute (RPMI) medium, the cells were incubated with different doses of Alpinia galangal (0 (control), 125, 250, 500, 750 and 1000 µg/ml) in 24, 48 and 72 hour periods and then, cells viability were assessed using MTT based cell proliferation assay.
Results
After 24 hours, the percentage of living AGS cells compared to the control group showed no significant decrease at the concentrations of 125 and 250µg/ml. But in the rest concentrations were significant (p<0.05). Only, the percentage of surviving L929 cells at concentration of 125µg/ml of the extract was not significant, but these percentages in the other concentrations were significant. After 48 and 72h incubation, in the last three extract concentrations, the percentage of living AGS and L929 cells significantly decreased compared to control cells (p<0.05).
Conclusion
We have demonstrated, using cell culture model, anti-proliferative effect of aqueous extract of Alpinia galangal on human gastric tumor (AGS) and L929 cell lines. This effect was prominent in high concentrations.
Keywords: Alpinia galangal, AGS, L929, MTT
Introduction
Cancer is the anomalous growth of cells in our bodies that can lead to death. Cancer cells usually attack and destroy normal cells. These cells are born due to imbalance in the body and by correcting this unevenness; the cancer may be treated [1]. Every year, millions of people are diagnosed with cancer, leading to death. Consistent with the American Cancer Society, deaths arising from cancer constitute 2-3% of the annual deaths recorded worldwide [2].
Cancers affecting the digestive tract are among the most common of all the cancers associated with aging. About one out of every 14 men and women in America is diagnosed with gastrointestinal cancer at some time in his/her life [3].
Alpinia galangal is widely cultivated in China, India, and Southeast Asian countries such as Thailand, Indonesia, and Philippines. The rhizomes of this plant are used in traditional medicine for several purposes, such as stomachicin China, or for carminative, anti-flatulent, antifungal, and anti-itching in Thailand. In chemical studies of A. galangal, was reported to possess various biological activities, such as antitumor [4, 5], anti-inflammatory, pungency, antifungal, antioxidative, and xanthine oxidase inhibitory activity [5-9].
There is no report available on the effects of aqueous extract of Alpinial galangal on gastric adenocarcinoma. The present study was designed to examine the effect of this extract on the in vitro proliferation of AGS cells, originated from gastric carcinoma. In addition, we chose L929 cell lines as non-tumor cell, for comparison of the effect of extract on both cell lines.
Materials and Methods
Aqueous extract preparation
Fresh whole plants of Alpinia galangal were purchased from a local market in Mashhad, Iran. The fresh plants were sliced and dried in a tray-dryer oven at 50°C for 24h, after which they were ground in a blender (National, MXT2GN, Taiwan) to make powder. Then, the different concentrations of Alpinia galangal (125, 250, 500, 750 and 1000 µg/ml) were prepared [10].
Morphologic examinations
AGS and L929 cells were obtained from National Cell Bank of Iran (NCBI). Cells were cultivated in a 1:1 mixture of Dulbeccos Modified Eagles Medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution. The cultured were humidified at atmosphere and fed 2-3 times a week until they approached confluence. Then, cell aggregates were treated with 0.25% trypsin-EDTA solutions. EDTA solutions mechanically were dispersed using a 10ml pipette. Trypsin activity was inhibited by adding growth medium and cells were centrifuged at 1000 rpm for 5min. Supernatant was removed and cell pellet was seeded into seven 50cm2- flasks. After 24h, both cells were incubated with different concentrations of the extract into the flasks. Cells were observed under light inverted microscope for shape, granulation and suspension from 24 to 72h.
Cell Viability Assay
The effect of aqueous extract of Alpinia galangal on AGS and L929 cells proliferation was determined. All five Alpinia galangal extracts, at the concentration of 0-1000 µg/mL, dissolved in dimethyl sulfoxide (DMSO) (Sigma-Aldrich), were added to the wells, 24h after seeding of 5 ×103 cells per well of 96-well plate. DMSO was used as a solvent control. After 24 and 48h of incubation, 20μL of 5mg/mL MTT (Sigma Chemical Company) solution was added to each well, and the plates were wrapped with aluminum foil and incubated for 4h at 37°C. The purple formazan product was dissolved by addition of 100μL of 100% DMSO to each well. The absorbance was monitored at 570nm (measurement) and 630nm (reference) using a 96-well plate reader (Bio-Rad, Hercules, Calif, USA). Data were collected for each four replicates and used to calculate the means and the standard deviations. Optical density of AGS and L929 cells incubated in the presence and absence of the different concentrations of Alpinia galangal was compared together and the percentage of surviving cells was determined using the following formula:
Surviving cells (%) in compared to the controls =
Formula 1
Statistical analysis
Data was analyzed by SPSS software version 11.5. All data were presented as the mean ± standard deviation (SD). One way ANOVA followed by post hoc Tukey's multiple comparison tests were used for statistical evaluation. Statistical significance was determined at p<0.05.
Results
Morphologic changes
After 72h, AGS and also L929 cells with low concentrations (125 and 250 µg/ml) of extract in sense of morphology were very similar to the control. Morphological effects of the extract on both cells started at concentration of 500µg/ml. A complete response from the extract was seen on both cells at concentrations 750 and 1000 µg/ml, so that cells appeared as round and suspended with increase granulation of cytoplasm when compared to the control. The granulation of cytoplasm also was seen on times 24 and 48 h after addition of extract. However, this effect was lower than 72h (data not shown).
MTT assay results
a) After 24 hours
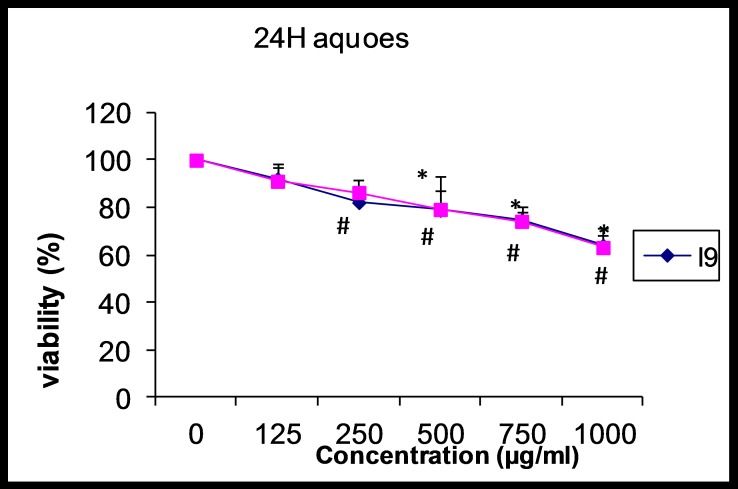
After 24 hours, the percentage of living AGS cells compared to control showed no significant decrease at concentrations of 125 and 250 µg/ml. But in the rest concentrations were significant (p<0.05). The percentage of surviving L929 cells at the extract concentration of 125 µg/ml just not be significant, but in the other concentrations were significant. Also, there was a direct correlation between increasing in the extract concentration and decreasing of surviving AGS and L929 cells. The maximum decrease in the percentage of surviving cells in the both cells was observed at the concentration of 1000 µg/ml (Figure 1).
Figure 1.
The effect of aqueous extract of Alpinia galangal (concentrations of 0 (control), 125, 250, 500, 750 and 1000 μg/ml) on viability of AGS and L929 cells after 24 hours. Each point represents the Mean±SD.
b) After 48 hours
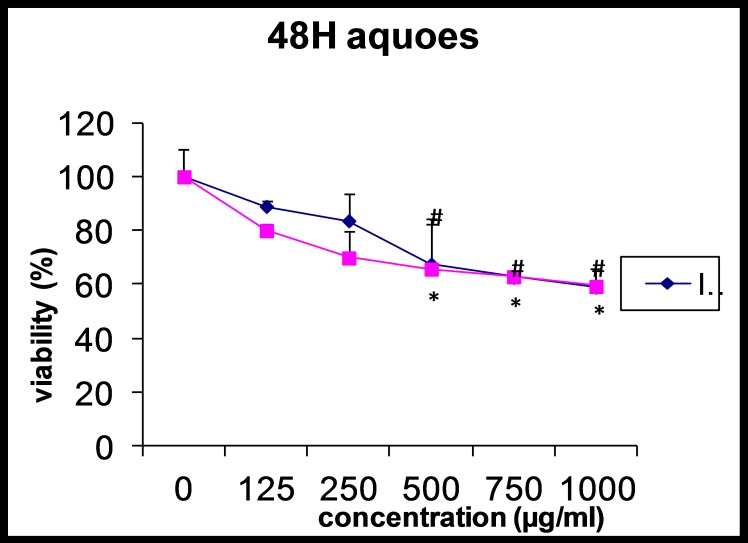
As shown in figure 2, the percentage of living AGS and L929 cells were significantly decreased at the last three concentrations of the extract compared with control cells (p<0.05). Also, there was a correlation between increase in the extract concentration and surviving AGS and L929 cells. The maximum decrease of surviving AGS cells was observed at 250 µg/ml while in the L929 cells was at 500 µg/ml.
Figure 2.
Effect of aqueous extract of Alpinia galangal (concentrations of 0 (control), 125, 250, 500, 750 and 1000 μg/ml) on viability of AGS and L929 cells after 48 hours. Each point represents the Mean±SD.
c) After 72 hours
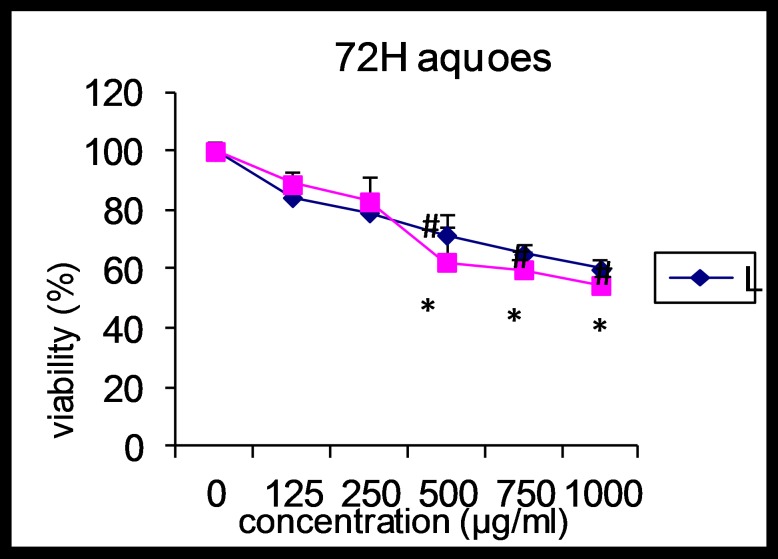
These changes became more prominent in third day, so that percentage of surviving AGS and L929 cells at concentrations of 500, 750 and 1000 µg/ml of extract showed significant decrease compared with control cells (p<0.05). The maximum decrease of surviving AGS cells was at 500µg/ml while in the L929 cells were in 1000µg/ml. Also, there was a correlation between increase in the extract concentration and decrease of surviving AGS and L929 cells. Effect of the Alpinia galangal extract on percent of surviving AGS and L929 cells after 72h is shown in figure 3.
Figure 3.
Effect of aqueous extract of Alpinia galangal (concentrations of 0 (control), 125, 250, 500, 750 and 1000 μg/ml) on viability of AGS and L929 cells after 72 hours. Each point represents the Mean±SD.
Discussion
No previous literature was found to show the effectiveness of aqueous extract of Alpinia galangal on AGS and L929 cells. Our data demonstrated that this extract influences on AGS and L929 cells proliferation. Effect of the extract was started from 24h and continued for 48h and 72h, so that maximum anti proliferative effect appeared after 72h. In MTT assay, statistical analysis indicated that the extract significantly inhibited the proliferation of both cells in concentrations higher than 500µg/ml. These results demonstrated that aqueous extract of Alpinia galangal can inhibit growth of human gastric tumor cell lines in vitro. But this inhibition can also occur in non-tumor L929 cells. These properties may arise from several mechanisms including inhibition of gene mutation, suppressing the formation of DNA- adducts, change of enzyme activity or induction of apoptosis [11].
Various in vitro and in vivo studies in diverse cancer cell lines have showed obviously the potential of Alpinia species as anticancerous plant in animal models. For instance, pinostrobin chalcone, as a new compound, has been isolated from A. mutica which displays remarkable cytotoxic potential to different human carcinoma cell lines such as MCF7 and CaSki cells with significant IC50 values [12].
Nam et al. identified [13] that A. galangal rhizome extract has two compounds named, viz. 10-(S)-10-acetoxychavicolacetate and p-coumaryl alcoholc-O-methyl ether. The first compound, showed significant cytotoxic activity against human cancer cell lines like A549, SNU638, HT1080, HL60 and HCT116, while, the next compound revealed specific activity against SNU638. On the other hand, cytotoxic activity has been revealed with four different compounds separated from A. officinalrum and only 7-(3,4-dihydroxyphenyl)-1-(4-hydroxy-3-methoxyphenyl)-4-en-3-heptanone has notable cytotoxic activity against HepG2, MCF-7 and SF-268 in some other cancer cell lines [14]. In the work of Lu et al. it was shown that A. officinarum has the whitening effects on melanin biosynthesis in B16 (mouse melanoma cell line). So that, the flavonoid mixture and galangin exhibited a broad absorption band at 270-290 nm related to the UV-B area supporting that galangin could be a whitening agent and a capable candidate for prevention of skin cancer [15].
Additionally, Acetoxychavicol acetate (ACA), a compound isolated from an n-pentane/diethyl ether-soluble extract of dried rhizomes, act as an antiulcer and antitumor agents as well as an inhibitor of chemically induced carcinogenesis is event [16]. It was also shown that extract from the dried rhizomes of A. galangal showed strong protective effects against gastric lesions induced with ethanol, which it is stronger than clinically used medicines such as omeprazole, cimetidine, and cetraxate hydrochloride [17, 18].
Conclusion
In this study, using cell culture model, anti-proliferative effect of aqueous extract of Alpinia galangal on human gastric tumor (AGS) and L929 cell lines was demonstrated. This effect was more dominant in high concentrations.
Acknowledgments
The authors are thankful for contributions of those who helped them to carry out this study.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this study.
Authors' Contribution
Zakieh Keshavarzi designed and wrote this article, Mosa-Al- Reza Hadjzadeh, Habib Ghanbari and Jalil Tavakol Afshari collected and analyzed the data. All authors read and approved the final manuscript.
REFERENCES
- 1.Visvader J. Cells of origin in cancer. Nature. 2011;469:314–22. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun M. Cancer Statistics. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Moffatt J, Hashimoto M, Kojima A, Kennedy DO, Murakami A, Koshimizu K, Ohigashi H, Matsui-Yuasa I. Apoptosis induced by 1V-acetoxychavicol acetate in Ehrlich as cites tumor cells is associated with modulation of polyamine metabolism and caspase-3 activation. Carcinogenesis. 2000;21(12):2151–7. doi: 10.1093/carcin/21.12.2151. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Q, Hirose Y, Yoshimi N, Murakami A, Koshimizu K, Ohigashi H, Sakata K, Matsumoto Y, Sayama Y, Mori H. Further investigation of the modifying effect of various chemo preventive agents on apoptosis and cell proliferation in human colon cancer cells. J Cancer Res Clin Oncol. 2002;128(10):539–46. doi: 10.1007/s00432-002-0373-y. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Murakami A, Ohto Y, Torikai K, Tanaka T, Ohigashi H. Suppression of tumor promoter-induced oxidative stress and inflammatory responses in mouse skin by a superoxide generation inhibitor 1V-acetoxychavicol acetate. Cancer Res. 1998;58(21):4832–9. [PubMed] [Google Scholar]
- 6.Yang X, Eilerman RG. Pungent principle of Alpinia galanga (L.) Swartz and its applications. J Agric Food Chem. 1999;47(4):1657–62. doi: 10.1021/jf9808224. [DOI] [PubMed] [Google Scholar]
- 7.Janssen AM, Scheffer JJ. Acetoxychavicol acetate, an antifungal component of Alpinia galanga. Planta Med. 1985;51(6):507–11. [PubMed] [Google Scholar]
- 8.Kubota K, Ueda Y, Yasuda M, Masuda A. Occurrence and antioxidative activity of 1V-acetoxychavicol acetate and its related compounds in the rhizomes of Alpinia galanga during cooking. Chem. 2001;274:601–7. [Google Scholar]
- 9.Noro T, Sekiya T, Katoh M, Oda Y, Miyase T, Kuroyanagi M, Ueno A, Fukushima S. Inhibitors of xanthine oxidase from Alpinia galanga. Chem Pharm Bull. 1988;36(1):244–48. [Google Scholar]
- 10.Hadjzadeh M. R, Tavakol Afshari J, Barati M. The inhibitory effects of ethanolic extract Alpinia Galangal on colonic cancer cells (HT-29) and L929 cells In Vitro. Urmia Med J. 2009;3(69):572–82. [Google Scholar]
- 11.Bianchini F, Vainio H. Allium Vegetables and Organosulfur Compounds: Do They Help Prevent Cancer? Environ Health Perspect. 2001;109(9):893–902. doi: 10.1289/ehp.01109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malek SN, Phang CW, Ibrahim H, Norhanom AW, Sim KS. Phytochemical and cytotoxic investigations of Alpinia mutica rhizomes. Molecules. 2011;16(1):583–9. doi: 10.3390/molecules16010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nam JW, Kim SJ, Han AR, Lee SK, Seo EK. Cytotoxic phenylpropanoids from rhizomes of Alpinia galanga. J Appl Pharmacol. 2005;13:263–6. [Google Scholar]
- 14.An N, Zou Z, Tian Z, Luo XZ, Yang S, Xu LZ. Diaryl heptanoids from the rhizomes of Alpinia officinarum and their anticancer activity. Fitoterapia. 2008;79(1):27–31. doi: 10.1016/j.fitote.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lu YH, Lin-Tao Wang ZT, Wei DZ, Xiang HB. Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J Enzyme Inhib Med Chem. 2007;22(4):433–8. doi: 10.1080/14756360601141562. [DOI] [PubMed] [Google Scholar]
- 16.Murakami A, Toyota K, Ohura S, Koshimizu K, Ohigashi H. Structure-activity relationships of (10S)-10-Acetoxychavicol acetate, a major constituent of a southeast Asian condiment plant Languas galangal, on the inhibition of tumor-promoter-induced Epstein-Barr virus activation. J Agric Food Chem. 2000;48:1518–23. doi: 10.1021/jf990528r. [DOI] [PubMed] [Google Scholar]
- 17.Matsuda H, Morikawa T, Managi H, Yoshikawa M. Antiallergic principles from Alpinia galanga: structural requirements of phenylpropanoids for inhibition of degranulation and release of TNF-alpha and IL-4 in RBL-2H3 cells. Bioorg Med Chem Lett. 2003;13(19):3197–202. doi: 10.1016/s0960-894x(03)00710-8. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda H, Pongpiriyadacha Y, Morikawa T, Ochi M, Yoshikawa M. Gastroprotective effects of phenylpropanoids from the rhizomes of Alpinia galanga in rats: structural requirements and mode of action. Eur J Pharmacol. 2003;471(1):59–67. doi: 10.1016/s0014-2999(03)01785-0. [DOI] [PubMed] [Google Scholar]