Abstract
The often fatal outcome of ovarian cancer (OC) is related to inadequate detection methods, which may be overcome by development of nuclear imaging agents. Cancer targeting peptides have been identified using in vivo bacteriophage (phage) display technology; however, the majority of these ligands target tumor vasculature. To overcome this problem, a two-tier phage display method was employed to select an ovarian cancer targeting peptide with good pharmacokinetic and imaging properties. A fUSE5 15-amino acid peptide library was screened against xenografted human OC SKOV-3 tumors in mice, which was followed by selection against enriched SKOV-3 cells. The selected peptide RSLWSDFYASASRGP (J18) was synthesized with a GSG-spacer and a 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA) chelator and radiolabeled with 111In. SKOV-3 xenografted mice were used to evaluate the biodistribution and single photon emission computed tomography (SPECT) imaging capabilities of the radiolabeled peptide. Competitive binding experiments using 111In-DOTA-GSG-J18 indicated that the peptide displayed a half maximal inhibitory concentration (IC50) value of 10.5 ± 1.1 μM. Biodistribution studies revealed that tumor uptake was 1.63 ± 0.68, 0.60 ± 0.32, 0.31 ± 0.12 and 0.10 ± 0.02% injected dose/g at 30 min, 1 h, 2 h and 4 h post-injection of 111In-DOTA-GSG-J18, respectively. SPECT/CT imaging demonstrated good tumor uptake and minimal background binding. This study demonstrated successful utilization of a two-tier phage display selection process to identify an ovarian cancer avid peptide with excellent SPECT/CT imaging capabilities.
Keywords: Ovarian cancer, peptide, phage display, SPECT imaging
Introduction
Ovarian cancer (OC) is the fifth leading cause of cancer deaths in women, and has been termed the silent killer due to a predominantly asymptomatic disease development and quick dissemination of aggressive metastatic cells. The late manifestations of OC cause ~70% of patients to be diagnosed at advanced stage disease, at which point five-year survival rates are merely 30-45%. In contrast, the minority of women diagnosed at the early stages of OC exhibit good five-year survival rates of > 90%, emphasizing the importance of diagnosis at early onset [1,2]. Currently, standard detection methods involve evaluation of CA-125 (Mucin 16) serum levels and ultrasonography, which are both mostly successful for late-stage OC. In fact, only 50% of patients with stage I disease test positive for elevated CA-125 serum levels, which highlight the need for identification and development of efficacious detection methods of OC [3].
Various cancers have been successfully imaged using radionuclide-coupled peptides [4-6], and while these ligands generally display lower binding affinities compared to antibodies, they offer advantages in regard to tumor targeting by exhibiting low immunogenicity, rapid blood-clearance and excretion through the urine [7]. High-throughput strategies such as bacteriophage (phage) display are utilized in the discovery of such peptide ligands [8,9]. Phage display is a well-established technique that allows selection of ligands from large phage libraries, in which random peptide sequences are displayed on coat proteins [10]. Cancer targeting peptides can be identified by in vivo phage display selections against tumor targets in live animals, and have resulted in peptides with great stability and biodistribution. However, most of these peptides have been found to bind the tumor vasculature rather than actual cancer cells [11,12]. Therefore, careful design of the phage display selection enables identification of peptides that are able to extravasate the vasculature, bind specific cells of interest and possess desirable pharmacokinetics.
To overcome this problem, our laboratory developed a two-tier phage display technique that involves in vivo selection rounds in tumor-bearing mice followed by screening against cultured tumor cells in vitro. In the current study, we hypothesized that this rigid selection process against xenografted human OC (SKOV-3) tumors in mice and enriched cultured SKOV-3 cells would identify peptide ligands that could be utilized in single-photon emission computed tomography (SPECT) imaging. This study resulted in identification of an OC targeting peptide RSLWSDFYASASRGP (J18), which was synthesized with a GSG-spacer and conjugated to a 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetraacetic acid (DOTA)-chelator. The conjugated peptide was radiolabeled with 111In and evaluated in regard to its stability and binding affinity for SKOV-3 cells. The pharmacokinetic properties and SPECT imaging capabilities of 111In -DOTA-GSG-J18 were determined in SKOV-3 xenografted mice, and the results showed that the peptide exhibited good tumor targeting and retention and was able to successfully localize ovarian tumors.
Materials and methods
Chemicals and reagents
111InCl3 was purchased from Mallinckrodt Chemicals (St. Louis, MO). Unless otherwise stated, chemicals were obtained from Thermo Fisher Scientific (Waltham, MA).
Cell lines and cell culture
The human ovarian adenocarcinoma cell line SKOV-3 and human normal ovarian cells (HS-832) were purchased from American Type Tissue Culture. Both cell lines were cultivated in RPMI 1640 (custom) with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1.7 μM insulin and 48 mg/ml gentamicin, and maintained at 37°C in 5% CO2.
Animals and handling
Solid tumors (1 cm) were established in 4-6-week-old female nude (nu/nu) mice (Harlan, Indianapolis, IN) over a period of 8 weeks. The animals were inoculated subcutaneously in the shoulder under gas anesthesia (3.5% isoflurane, Baxter Healthcare Corp. Deerfield, IL) with 1x107 SKOV-3 cells. All animal studies were conducted according to NIH Guidelines for Care and Use of Laboratory Animals and the Policy and Procedures for Animal Research of the Harry S. Truman Veterans Memorial Hospital.
Phage display selections of tumor binding phage clones
For selection of tumor targeting phage clones, a linear 15-mer fUSE5 phage display library (a gift from Dr. George Smith) was used [10]. The library was pre-cleared from vasculature and non-tumor target binding phage according to previous methods [13]. The pre-cleared phage library (1012 pfu) was injected into xenografted SKOV-3 tumor-bearing nude mice and allowed to circulate for 1 h. The animals were sacrificed, tumors were collected and minced, and bound phage were eluted and amplified as previously described [13]. The selection was repeated a total of four times. Tumor-derived SKOV-3 cells were enriched using MACS® MicroBeads (Milteniy Biotech, Auburn, CA) technology [14], and used in a final round of selection. Selected phage were analyzed for binding to SKOV-3 and HS-832 cells in a micropanning assay per previous methods [13].
Peptides
Solid-phase Fmoc chemistry was used to synthesize peptides in a 396 multiple peptide synthesizer (Advanced Chem Tech, Louisville, KY). Peptides were synthesized with a DOTA-chelator linked to a GSG-spacer at the NH2-terminus. Purification was performed using reverse-phase high-pressure liquid chromatography (RP-HPLC; Beckmann Coulter System Gold HPLC, Beckmann Coulter, Fullerton, CA) on a C18 column (Novapack Reverse Phase, Waters, Milford, MA) and lyophilized and stored at -20°C. The peptides were identified by electrospray ionization mass spectrometry (Mass Consortium Corp, San Diego, CA).
Peptide radiolabeling and stability
The selected peptide was radiolabeled with 111In (111In-DOTA-GSG-J18) by incubating 100 μg peptide with 18.5 MBq 111In-Cl3 in 0.5 M NH4-acetate buffer, pH 5.0, at 85°C for 1 h. For blocking experiments, 1 mg peptide was incubated with 10-4 M non-radioactive indium under similar conditions. The non-radioactive and radiolabeled peptides were purified using RP-HPLC (LC-20A Prominence, Shimadzu, Columbia, MD) on a C18 column (Higgins Analytical Inc., Mountain View, CA; 25-35% acetonitrile/0.1% trifuoroacetic acid) over 20 min. Acetonitrile was removed by further purification on an Empore® Extraction Disk C18 cartridge (Phenomenex, Torrance, CA), and the peptide was eluted with 70% ethanol and the pH adjusted to neutral by 0.5 M 2-[4-(2-hydroxyethyl) piperazin-1-yl]ethanesulfonic acid (HEPES; final concentration) buffer. For animal experiments, the ethanol was evaporated under N2 and the peptide was resuspended in 0.1 M HEPES buffer, pH 7. Peptide stability was analyzed in mouse serum over time (0 h, 1 h, 2 h, 4 h, 6 h and 24 h) at 37°C, and degradation was monitored by RP-HPLC on a C18 column. In short, at the end of each incubation mouse serum protein was precipitated by addition of acetonitrile, and removed by centrifugation and filtration using a 0.22 μm filter. The filtrate was then loadedonto the HPLC.
Cell binding studies
Ovarian cancer specificity of 111In-DOTA-GSG-J18 was evaluated in a cell binding assay as previously described [15], except SKOV-3 or HS-832 cells (1x106) were incubated with 2x105 cpm of 111In-DOTA-GSG-J18 in DMEM, 1% bovine serum albumin at 37°C for different periods of time (0 min, 0.5, 1, 2 and 4 h). Bound radioactivity was measured using a Genesys™ Genii™ Multi-Well Gamma Counter (Laboratory Technologies, Inc, Maple Park, IL).
To determine the half maximal inhibitory concentration (IC50) of the peptide ligand to OC cells (SKOV-3), competition experiments were performed with the radiolabeled (111In-DOTA-GSG-J18) and the non-radioactive counterpart (In-DOTA-GSG-J18) according to previous studies [15]. In brief, cells (1x106) were incubated with 2x105 cpm of 111In-DOTA-GSG-J18 and va-rying concentrations of In-DOTA-GSG-J18 (10-13 to 10-4 M) for 1 h at 37°C. Cell-bound radioactivity was measured, and the IC50-value was calculated using graphPad prism software.
Biodistribution of peptide 111In-DOTA-GSG-J18 in SKOV-3 tumor-bearing nude mice
Nude mice (n=4) received tail-vein injections of approximately 0.17 Mbq of 111In-GSG-DOTA-J18, and were subsequently housed separately. The animals were sacrificed by cervical dislocation at 30 min, 1 h, 2 h and 4 h post-injection, and organs were removed, weighed and counts were determined. Radioactive uptake in the tumor and in normal organs was calculated and expressed as percentage of injected dose per gram of tissue (%ID/g). For blocking experiments, tumor-bearing mice (n=4) were pre-injected with 100 μg nonradioactive In-DOTA-GSG-J18 peptide. After 5 min, the mice were injected with 0.17 MBq of 111In-DOTA-GSG-J18, and the animals were sacrificed after 1 h and the blocking efficiency was evaluated.
Small animal SPECT/CT studies
Nude mice bearing xenografted SKOV-3 tumors were injected through the tail-vein with 13.0 MBq of 111In-DOTA-GSG-J18 and imaged 1 h post-injection using a small-animal MicroCAT II SPECT/CT scanner (Siemens Medical Solutions, Malvern, PA) with a high-resolution 2 mm pinhole collimator at the Harry S. Truman Veterans Memorial Hospital Biomolecular Imaging Center. For blocking experiments, mice were pre-injected with 170 μg of nonradioactive In-labeled DOTA-GSG-J18 peptide. After 5 min, mice were injected with 13.0 MBq of 111In-DOTA-GSG-J18 and imaged after a total of 1 h using a SPECT/CT scanner as described above.
Statistical analysis
For statistical analysis an unpaired Student’s t-test was performed to determine significance using Prism Graphpad Software. A P-value of 0.05 or less was considered significant.
Results
Phage display selection
In order to select peptides with good in vivo pharmacokinetic and tumor targeting properties, a two-tier phage display selection protocol was performed. In the last round of selection, 31 individual phage clones were identified, and were used in micropanning experiments to identify clones with high SKOV-3-to-HS-832 binding ratios. Results showed that phage clones pJ18 exhibited the highest SKOV-3-to-HS832 binding ratios of 6.57, indicating preferable binding to the human OC cell line (Table 1). Based on these results pJ18 was chosen for further analysis.
Table 1.
Micropanning assay to determine binding of selected phage clones to SKOV-3 and HS-832 cells
| Phage No. | Peptide sequence | SKOV-3 to HS-832 ratio |
|---|---|---|
| pJ1 | RTEVPVLSFTSPLTG | 1.76 |
| pJ2 | GDVWLFKTSTSHFAR | 1.91 |
| pJ3 | AREYGTRFSLIGGYR | 0.21 |
| pJ4 | HAAFEPRGDVRHTLL | 2.00 |
| pJ5 | LGRAGQSYPSFARGL | 0.52 |
| pJ6 | PIFPVVSSSGSSSSP | 1.58 |
| pJ7 | PLSHGSVVYPRSSLG | 1.36 |
| pJ8 | RRDTVPRSLSAPLSW | 0.59 |
| pJ9 | PAVASTSSLIIDGPF | 2.29 |
| pJ10 | HPPLASVWHVSVPL | 0.83 |
| pJ11 | LHDFRSPIYASLLGF | 1.53 |
| pJ12 | AGDGGLGRVAAGARV | 1.15 |
| pJ13 | RVFHLWPHPTSTLSA | 0.25 |
| pJ14 | APLSYNFASMPFMSG | 0.73 |
| pJ15 | HPGWFDSAWFRAVSR | 1.24 |
| pJ16 | ARDSRCGGFLGCGVT | 1.36 |
| pJ17 | AMVRGFSFGMSRGSD | 1.94 |
| pJ18 | RSLWSDFYASASRGP | 6.57 |
| pJ19 | SYSVVNSPWCDGTCD | 1.25 |
| pJ20 | SRDGLHSFCYVGCPP | 0.40 |
| pJ21 | GVGDADGFIPVISAV | 0.59 |
| pJ22 | PVFFRLSPVTEGGGV | 1.40 |
| pJ23 | FPSYPFIAYSLQTPV | 1.23 |
| pJ24 | RRLPHLMPFEGSVFL | 3.55 |
| pJ25 | GPHFDYRTGLGWRFG | 1.35 |
| pJ26 | LGKGLTGSALSLSAL | 2.29 |
| pJ27 | YGVTPSPRSPWATAH | 3.43 |
| pJ28 | VFVDGARYSTASDSL | 2.83 |
| pJ29 | GAGIFGPWGVFAAVP | 1.21 |
| pJ30 | GYRSAFVPFVARGGH | 3.65 |
| pJ31 | RYRVGFTPGTIAAVL | 1.13 |
SKOV-3 or HS-832 cells were incubated with individual phage clones and eluted using 2.5% CHAPS. Phage binding was determined by titer and the SKOV-3 to HS-832 ratio was calculated.
Peptide synthesis, radiolabeling and stability
Peptide J18 was synthesized with a GSG-spacer between the DOTA-group and the NH2-terminus to avoid potential steric hindrance. The peptide (DOTA-GSG-J18) was successfully labeled with 111In or non-radioactive In and purified by RP-HPLC. The radiolabeled peptide and its non-radioactive counterpart eluted with retention times of 24.6 min and 24.5 min, respectively. High labeling efficiency of 98% and radiochemical purity of 98.6 ± 1.6% (mean ± SD), for 111In-DOTA-GSG-J18 were observed. The yield of radiolabeled peptide was ~20% after HPLC and C-18 cartridge purification.
The stability of 111In-DOTA-GSG-J18 was evaluated in mouse serum at 37°C for different time intervals and analyzed by RP-HPLC. Results showed that the radiolabeled peptide exhibited a half-life of 5.2 ± 0.43 h (mean ± SD) in mouse serum (Figure 1).
Figure 1.
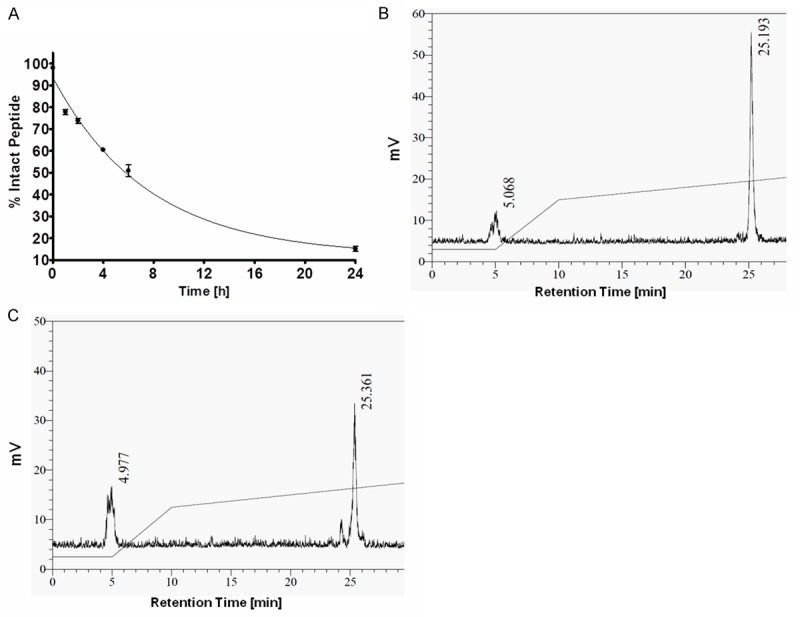
Stability of 111In-DOTA-GSG-J18 under physiological conditions. (A) Time course of intact radiolabeled peptide in mouse serum at 37°C. (B, C) HPLC chromatograms of collected radiolabeled peptide after incubations of 1 h (B) and 4 h (C) in mouse serum.
Cell binding
The specificity of 111In-DOTA-GSG-J18 for OC cells was determined in a cell-binding assay using SKOV-3 and HS-832 cells. For this, 111In-DOTA-GSG-J18 was incubated with 1x106 cells for different periods of time, and the bound radioactivity was measured and calculated as percentage of total counts per minute (% total cpm). Binding of the radiolabeled peptide to both cell lines increased from 0 min to 1 h, after which the binding gradually decreased. Nevertheless, in comparison to HS-832 cells, 111In-DOTA-GSG-J18 exhibited significantly higher binding to SKOV-3 cells after 2 h, demonstrating specificity for this cell line (Figure 2).
Figure 2.

Binding properties of 111In-DOTA-GSG-J18 to OC SKOV-3 (■) and normal ovarian HS-832 (▲) cells. Cells were incubated with radiolabeled peptide for different periods of time. Although, binding of 111In-DOTA-GSG-J18 to SKOV-3 cells was significantly higher (p < 0.05) at 2 h, binding to HS-832 cells was also observed.
Further validation of specific binding of radiolabeled peptide to SKOV-3 cells was determined by a competition assay in the presence of different concentrations of the correlating non-radioactive peptide. Results showed that the amount of bound radioactivity decreased in a dose-dependent manner with increasing concentrations of non-radiolabeled peptide, indicating that binding of 111In-DOTA-GSG-J18 was out-competed by In-DOTA-GSG-J18 (Figure 3). Based on these data, an IC50 value of 10.5 ± 1.1 μM (mean ± SE) for 111In-DOTA-GSG-J18 for SKOV-3 cells was calculated.
Figure 3.
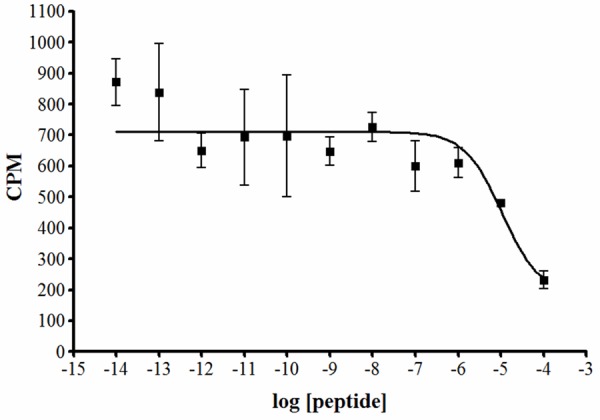
Cell binding competition study of 111In-DOTA-GSG-J18 and its non-radioactive counterpart. SKOV-3 cells were incubated with 111In-DOTA-GSG-J18 and various concentrations (10-14 to 10-4 M) of the correlating non-radiolabeled peptide In-DOTA-GSG-J18 for 1 h at 37°C. Bound radioactivity was measured and the IC50 value was 10.5 ± 1.1 μM (mean ± SE).
Pharmacokinetics of 111In-GSG-DOTA-J18 peptide in SKOV-3 tumor-bearing nude mice
Indium-111 labeled DOTA-GSG-J18 peptide was evaluated in regard to its pharmacokinetic properties in nude mice bearing xenografted SKOV-3 tumors. Radioactive uptake in the tumor was calculated to be 1.63 ± 0.68, 0.60 ± 0.32, 0.31 ± 0.12 and 0.10 ± 0.02% ID/g at 30 min, 1 h, 2 h and 4 h, respectively (Table 2). The radioligand cleared from the blood and exhibited levels of 2.37 ± 0.94, 0.40 ± 0.24, 0.03 ± 0.03 and 0.19 ± 0.02% ID/g at 30 min, 1 h, 2 h and 4 h, respectively. The tumor-to-blood ratio increased from 0.69 at 30 min to 1.50 and 6.00 at 1 h and 2 h, respectively, indicating rapid blood clearance and kinetically favorable tumor uptake and retention. Equally high tumor-to-muscle ratios were observed as early as 1 h post-injection. Radioactive uptake in normal organs was, as expected, the highest in the kidneys due to renal clearance being the major route of excretion for most peptide ligands [16]. The kidney uptake was highest after 30 min at 6.71 ± 3.08 and decreased to 2.70 ± 0.95, 1.67 ± 0.43 and 1.75 ± 0.17% ID/g at 1 h, 2h and 4 h, respectively. Of all other organs, only the lungs, liver and spleen demonstrated noticeable radioligand uptake, which decreased rapidly after 1 h. Very little radioactivity was detected in the remaining organs and bone. In order to determine the specificity of tumor uptake, the radiolabeled peptide was subjected to an in vivo competition study with its non-radioactive counterpart. The results indicated that the non-radioactive peptide significantly blocked uptake of the radioactive counterpart by approximately 48%. The non-radioactive ligand did not significantly affect the uptake of 111In-labeled peptide in normal organs.
Table 2.
Biodistribution of 111In-DOTA-GSG-J18 in SKOV-3 tumor-bearingnude mice
| Pharmacokinetics of 111In-DOTA-GSG-J18 | |||||
|---|---|---|---|---|---|
| %ID/g | |||||
| Tissues | 30 min | 1 h† | 1 h-block | 2 h‡ | 4 h |
| Tumor | 1.63 ± 0.68 | 0.60 ± 0.32 | 0.31 ± 0.12* | 0.18 ± 0.03 | 0.10 ± 0.02 |
| Blood | 2.37 ± 0.94 | 0.40 ± 0.24 | 0.23 ± 0.11 | 0.03 ± 0.03 | 0.19 ± 0.02 |
| Heart | 0.67 ± 0.27 | 0.13 ± 0.08 | 0.07 ± 0.03 | 0.02 ± 0.01 | 0.05 ± 0.01 |
| Lung | 2.42 ± 0.48 | 1.06 ± 0.82 | 0.83 ± 0.19 | 0.49 ± 0.36 | 0.62 ± 0.32 |
| Liver | 1.42 ± 0.37 | 0.51 ± 0.48 | 0.37 ± 0.02 | 0.23 ± 0.11 | 0.84 ± 0.13 |
| Spleen | 1.77 ± 0.46 | 0.68 ± 1.01 | 0.16 ± 0.03 | 0.19 ± 0.24 | 1.58 ± 0.36 |
| Stomach | 0.36 ± 0.14 | 0.11 ± 0.04 | 0.08 ± 0.03 | 0.04 ± 0.02 | 0.04 ± 0.00 |
| Large intestine | 0.34 ± 0.17 | 0.15 ± 0.05 | 0.07 ± 0.02 | 0.27 ± 0.06 | 0.28 ± 0.19 |
| Small intestine | 0.63 ± 0.29 | 0.32 ± 0.11 | 0.15 ± 0.04 | 0.15 ± 0.08 | 0.06 ± 0.01 |
| Intestines | 0.49 ± 0.22 | 0.25 ± 0.08 | 0.12 ± 0.03 | 0.20 ± 0.07 | 0.15 ± 0.08 |
| Kidneys | 6.71 ± 3.08 | 2.70 ± 0.95 | 1.98 ± 0.29 | 1.67 ± 0.43 | 1.75 ± 0.17 |
| Brain | 0.09 ± 0.03 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Muscle | 0.32 ± 0.17 | 0.06 ± 0.03 | 0.04 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Pancreas | 0.65 ± 0.18 | 0.11 ± 0.05 | 0.08 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 |
| Bone | 0.31 ± 0.13 | 0.06 ± 0.05 | 0.05 ± 0.03 | 0.02 ± 0.01 | 0.08 ± 0.02 |
| Skin | 1.28 ± 0.68 | 0.22 ± 0.11 | 0.22 ± 0.08 | 0.09 ± 0.03 | 0.10 ± 0.01 |
| Uptake ratio | |||||
| Tumor to blood | 0.69 | 1.50 | 1.35 | 6.00 | 0.53 |
| Tumor to muscle | 5.10 | 10.00 | 7.75 | 18.00 | 10.00 |
Data are represented as %ID/g (mean ± SD), or as uptake ratio of tumor to normal tissue for female nude mice (n=4) bearing SKOV-3 xenografted tumors. Mice were sacrificed at different times post-injection of 111In-DOTA-GSG-J18.
n=7;
n=5;
p=0.03; significant difference between tumor uptake of radiolabeled peptide 1 h post-injection with and without the presence of its non-radioactive counterpart.
SPECT/CT tumor imaging
The SPECT/CT imaging ability of 111In-DOTA-GSG-J18 was evaluated in SKOV-3 xenografted nude mice (Figure 4A). The tumors were clearly visualized with high tumor-to background contrast, and the kidney uptake was concurrent with the pharmacokinetic data. Excretion of the radioligand was visible in the bladder, while little to no radioligand uptake was detected in other normal organs. Peptide tumor specificity was determined by blocking 111In-DOTA-GSG-J18 tumor uptake in the presence of non-radioactive peptide. The image showed that radioactive tumor uptake was successfully blocked (Figure 4B), while radioactive uptake in the kidneys and other organs was unchanged, indicating that the radioligand exhibits specificity for SKOV-3 tumors.
Figure 4.
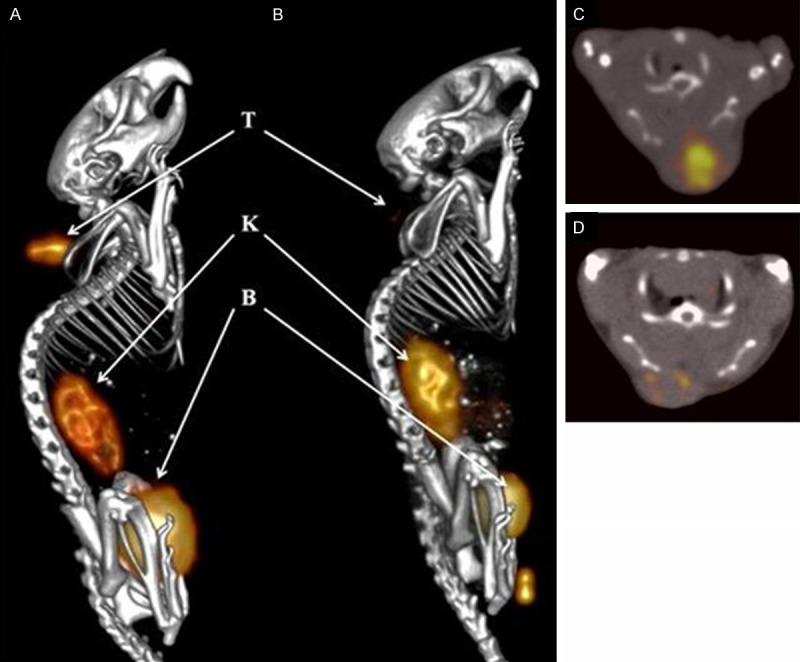
SPECT/CT study with nude mice carrying xenografted SKOV-3 tumors. (A) 111In-DOTA-GSG-J18 (13.0 MBq) or (B) In-DOTA-GSG-J18 followed by 111In-DOTA-GSG-J18 (13.0 MBq) were injected into the tail vein of SKOV-3 tumor-bearing nude mice. (C, D) Axial SPECT/CT images of tumor uptake of (C) 111In-DOTA-GSG-J18 and (D) In-DOTA-GSG-J18 followed by 111In-DOTA-GSG-J18. The live image was acquired 1 h post-injection under isoflurane anesthesia in a Siemens small-animal SPECT/CT scanner. The pictures show left lateral fused SPECT/CT images. T=tumor, K=kidneys and B=bladder. Created by 3D OSEM Fan beam (Feldkamp). Image processing was performed with Inveon Research Workplace processing software.
Discussion
The poor prognosis of OC is a direct result of asymptomatic disease development and a lack of adequate diagnostic techniques [1]. Many OC detection methods are under investigation; however, most show high specificity while exhibiting low sensitivity or vice versa [17,18]. The need for new diagnostic tools combined with the fatality of OC, emphasizes the great need for development of efficacious detection methods.
Peptide-based radionuclide imaging agents are rapidly evolving due to implementation of combinatorial strategies including phage display technology [8,9,19]. While several peptides have been identified by in vitro phage display selections [20,21], it is generally accepted that in vivo selections offer advantages in obtaining optimal targeting and pharmacokinetic properties [11,12,22-24]. A limited group of peptides have been identified in this manner, of which the most well-known example is identification of a RGD-containing peptide that binds vasculature expressed αvβ3-integrin, and has been employed in both positron emission tomography (PET) and SPECT imaging of the human tumor vasculature [22,25,26]. Other examples of peptides selected via in vivo phage display include the rhabdomyosarcoma targeting sequence CQQSNRGDRKRC [23], and several peptides that bind tumor vasculature receptors [11,12,24]. In contrast, our selection protocol initially selects for a sub-population of the library with desirable pharmacokinetics and extravasation abilities, which is followed by a subsequent in vitro selection that ensures tumor cell binding rather than vasculature targeting.
While many of these peptides show great promise; the majority of peptides target the tumor vasculature rather than tumor cells. Instead, our laboratory has developed a two-tier phage display screening method designed to select tumor-cell targeting peptides with high in vivo stability and good pharmacokinetic properties. The rigid screening process resulted in identification of peptide J18, which was synthesized with a DOTA-chelator and GSG-spacer and labeled with 111In. The radiolabeled peptide demonstrated excellent stability under physiological conditions, indicating good potential in vivo stability [27]. A comparison of the binding patterns of the radiolabeled peptide for OC and normal ovarian cells were investigated, and although 111In-DOTA-GSG-J18 showed minimal binding to HS-832 cells, the peptide exhibited significantly higher binding to SKOV-3 cells after 2 h. These results may indicate that the radiolabeled peptide binds to an antigen that is overexpressed by SKOV-3 cells, but is present at low levels on normal ovarian cells. Several cell receptors are known to exhibit such expression patterns, including the folate receptor-α and the ErbB2/Her2/neu receptor, among others [28,29]. Competition studies demonstrated that binding of 111In-DOTA-GSG-J18 to OC cells was specific, in that it was outcompeted by increasing concentrations of the correlating non-radioactive peptide. Based on this data, an IC50-value of 10.5 ± 1.1 μM (mean ± SE) for the radiolabeled peptide was calculated, which is in correlation with what has previously been reported for phage display peptides selected against live cells [30].
A competition biodistribution study with 111In-DOTA-GSG-J18 in the presence of non-radiolabeled peptide demonstrated specific binding to the tumor, while radioactive uptake in other organs was non-specific. Additionally, low levels of radioactivity in the blood as well as rapid clearance indicated good stability of the radionuclide-chelator complex as free 111In would bind to metal conjugating serum proteins resulting in prolonged circulation. Both the small molecular size and the positive charge of the peptide at physiological pH indicate that the majority should be cleared through the kidneys. This is evident from the biodistribution results, and correlates with the fact that renal clearance is the preferred route of excretion for most radiolabeled peptides [16]. Renal retention of radiolabeled compounds may lead to nephrotoxicity [31]. However, kidney uptake of 111In-DOTA-GSG-J18 rapidly decreased after only 1 h, thereby minimizing potential kidney exposure. Low non-specific retention of radioactivity in the liver and spleen may be due to hydrophobicity of the peptide and the fact that increased lipophilicity tends to direct a compoundtowards hepatic excretion [32]. Finally, the lungs showed some non-specific retention of radioactivity, which may be due to high perfusion and increased exposure to the blood circulation.
In vivo SPECT/CT imaging of SKOV-3 xenografted mice using 111In-DOTA-GSG-J18 revealed high tumor-to-background ratios, and successfully localized the tumor. In correlation with the pharmacokinetic studies, the majority of the radioactivity was found in the kidneys, and renal excretion of the peptide was clearly evident from high levels in the bladder. Furthermore, tumor uptake of the radioactive peptide was successfully blocked by injection of the non-radioactive counterpart showing specificity of the peptide for the SKOV-3 tumor.
To date, very few in vivo OC imaging agents have been reported. Of the few, a tetrameric vascular cell adhesion molecule 1 (VCAM-1) targeting peptide is among the most successful. Although the VCAM-1 targeting peptide exhibited excellent tumor homing, its use is limited to advanced stages of OC. In fact, VCAM-1 was not detectable on stage I tumors [9]. The peptide 18-DOTA was employed in successful SPECT/CT imaging of SKOV-3 metastases in mice and showed prolonged tumor retention and minimal radioactive uptake in normal organs. In order to further improve binding affinity, tumor uptake and retention, affinity maturation, alanine scanning, or display of the peptide as multiple copies on a scaffold may be performed in future studies. Additionally, identification of the peptide antigen will aid in the optimization of the targeting agent, and potentially shed light upon the pathology of ovarian cancer. Currently, the antigen remains unknown; however, future studies including proteomic analysis will be performed.
Here, we demonstrated that 111In-DOTA-GSG-J18 images xenografted human ovarian SKOV-3 tumors in live nude mice using SPECT/CT, and exhibits rapid renal clearance. This peptide may be useful in advancing the diagnosis of OC and allows detection of early stages of the malignancy.
Conclusion
The SKOV-3 specific peptide J18 was selected by a two-tier phage display screening process and evaluated for its binding, pharmacokinetic and SPECT/CT imaging properties. The peptide demonstrated good tumor uptake and retention and successfully imaged SKOV-3 tumors in xenografted nude mice. In conclusion, the J18 peptide may be an effective imaging agent of OC.
Acknowledgements
We acknowledge the contributions of Lisa Watkinson, Terry Carmack and Marie Dickerson. This work was supported by a Veterans Affairs (VA) Merit Review Award BX000964 and the National Institutes of Health (NIH) 1R21CA134960.
Disclosure of conflict of interest
None.
References
- 1.Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current Management Strategies for Ovarian Cancer. Mayo Clin Proc. 2007;82:751–770. doi: 10.4065/82.6.751. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Neesham D. Ovarian cancer screening. Aust Fam Physician. 2007;36:126–128. [PubMed] [Google Scholar]
- 4.Bakker WH, Krenning EP, Reubi JC, Breeman WA, Setyono-Han B, de Jong M, Kooij PP, Bruns C, van Hagen PM, Marbach P, et al. In vivo application of [111In-DTPA-D-Phe1] -octreotide for detection of somatostatin receptor-positive tumors in rats. Life Sci. 1991;49:1593–1601. doi: 10.1016/0024-3205(91)90053-e. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Cheng Z, Miao Y, Jurisson SS, Quinn TP. Alpha-melanocyte-stimulating hormone peptide analogs labeled with technetium-99m and indium-111 for malignant melanoma targeting. Cancer. 2002;94:1196–1201. doi: 10.1002/cncr.10284. [DOI] [PubMed] [Google Scholar]
- 6.Deutscher SL, Figueroa SD, Kumar SR. In-labeled KCCYSL peptide as an imaging probe for ErbB-2-expressing ovarian carcinomas. J Labelled Comp Radiopharm. 2009;52:583–590. doi: 10.1002/jlcr.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behr TM, Gotthardt M, Barth A, Behe M. Imaging tumors with peptide-based radioligands. Q J Nucl Med. 2001;45:189–200. [PubMed] [Google Scholar]
- 8.Karasseva NG, Glinsky VV, Chen NX, Komatireddy R, Quinn TP. Identification and characterization of peptides that bind human ErbB-2 selected from a bacteriophage display library. J Protein Chem. 2002;21:287–296. doi: 10.1023/a:1019749504418. [DOI] [PubMed] [Google Scholar]
- 9.Scalici JM, Thomas S, Harrer C, Raines TA, Curran J, Atkins KA, Conaway MR, Duska L, Kelly KA, Slack-Davis JK. Imaging VCAM-1 as an Indicator of Treatment Efficacy in Metastatic Ovarian Cancer. J Nucl Med. 2013;54:1883–9. doi: 10.2967/jnumed.112.117796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 11.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 12.Yao VJ, Ozawa MG, Trepel M, Arap W, McDonald DM, Pasqualini R. Targeting pancreatic islets with phage display assisted by laser pressure catapult microdissection. Am J Pathol. 2005;166:625–636. doi: 10.1016/S0002-9440(10)62283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton JR, Kelly KA, Mahmood U, Weissleder R, Deutscher SL. In vivo selection of phage for the optical imaging of PC-3 human prostate carcinoma in mice. Neoplasia. 2006;8:772–780. doi: 10.1593/neo.06331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke C, Titley J, Davies S, O’Hare MJ. An immunomagnetic separation method using superparamagnetic (MACS) beads for large-scale purification of human mammary luminal and myoepithelial cells. Epithelial Cell Biol. 1994;3:38–46. [PubMed] [Google Scholar]
- 15.Kumar SR, Deutscher SL. 111In-labeled galectin-3-targeting peptide as a SPECT agent for imaging breast tumors. J Nucl Med. 2008;49:796–803. doi: 10.2967/jnumed.107.048751. [DOI] [PubMed] [Google Scholar]
- 16.Vegt E, de Jong M, Wetzels JFM, Masereeuw R, Melis M, Oyen WJG, Gotthardt M, Boerman OC. Renal Toxicity of Radiolabeled Peptides and Antibody Fragments: Mechanisms, Impact on Radionuclide Therapy, and Strategies for Prevention. J Nucl Med. 2010;51:1049–1058. doi: 10.2967/jnumed.110.075101. [DOI] [PubMed] [Google Scholar]
- 17.Leung F, Diamandis EP, Kulasingam V. From bench to bedside: discovery of ovarian cancer biomarkers using high-throughput technologies in the past decade. Biomark Med. 2012;6:613–625. doi: 10.2217/bmm.12.70. [DOI] [PubMed] [Google Scholar]
- 18.Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013;415:341–345. doi: 10.1016/j.cca.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 19.Peletskaya EN, Glinsky G, Deutscher SL, Quinn TP. Identification of peptide sequences that bind the Thomsen-Friedenreich cancer-associated glycoantigen from bacteriophage peptide display libraries. Molec Divers. 1996;2:13–18. doi: 10.1007/BF01718695. [DOI] [PubMed] [Google Scholar]
- 20.Leinonen J, Wu P, Koivunen E, Narvanen A, Stenman UH. Development of novel peptide ligands modulating the enzyme activity of prostate-specific antigen. Scand J Clin Lab Invest Suppl. 2000;233:59–64. [PubMed] [Google Scholar]
- 21.Koistinen H, Narvanen A, Pakkala M, Hekim C, Mattsson JM, Zhu L, Laakkonen P, Stenman UH. Development of peptides specifically modulating the activity of KLK2 and KLK3. Biol Chem. 2008;389:633–642. doi: 10.1515/BC.2008.076. [DOI] [PubMed] [Google Scholar]
- 22.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 23.Witt H, Hajdin K, Iljin K, Greiner O, Niggli FK, Schafer BW, Bernasconi M. Identification of a rhabdomyosarcoma targeting peptide by phage display with sequence similarities to the tumour lymphatic-homing peptide LyP-1. Int J Cancer. 2009;124:2026–2032. doi: 10.1002/ijc.24170. [DOI] [PubMed] [Google Scholar]
- 24.Essler M, Ruoslahti E. Molecular specialization of breast vasculature: a breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc Natl Acad Sci. 2002;99:2252–2257. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. MicroPET and autoradiographic imaging of breast cancer alpha v-integrin expression using 18F- and 64Cu-labeled RGD peptide. Bioconjug Chem. 2004;15:41–49. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Hsieh WY, Jiang Y, Kim YS, Sreerama SG, Chen X, Jia B, Wang F. Evaluation of a (99m)Tc-labeled cyclic RGD tetramer for noninvasive imaging integrin alpha(v)beta3-positive breast cancer. Bioconjug Chem. 2007;18:438–446. doi: 10.1021/bc0603081. [DOI] [PubMed] [Google Scholar]
- 27.Jain RK. Delivery of molecular and cellular medicine to solid tumors. J Control Release. 1998;53:49–67. doi: 10.1016/s0168-3659(97)00237-x. [DOI] [PubMed] [Google Scholar]
- 28.Ross JF, Chaudhuri PK, Ratnam M. Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines. Physiologic and clinical implications. Cancer. 1994;73:2432–2443. doi: 10.1002/1097-0142(19940501)73:9<2432::aid-cncr2820730929>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 29.Hung MC, Zhang X, Yan DH, Zhang HZ, He GP, Zhang TQ, Shi DR. Aberrant expression of the c-erbB-2/neu protooncogene in ovarian cancer. Cancer Lett. 1992;61:95–103. doi: 10.1016/0304-3835(92)90166-s. [DOI] [PubMed] [Google Scholar]
- 30.Ma C, Yin G, Yan D, He X, Zhang L, Wei Y, Huang Z. A novel peptide specifically targeting ovarian cancer identified by in vivo phage display. J Pept Sci. 2013;19:730–736. doi: 10.1002/psc.2555. [DOI] [PubMed] [Google Scholar]
- 31.Wessels BW, Konijnenberg MW, Dale RG, Breitz HB, Cremonesi M, Meredith RF, Green AJ, Bouchet LG, Brill AB, Bolch WE, Sgouros G, Thomas SR. MIRD pamphlet No. 20: the effect of model assumptions on kidney dosimetry and response--implications for radionuclide therapy. J Nucl Med. 2008;49:1884–1899. doi: 10.2967/jnumed.108.053173. [DOI] [PubMed] [Google Scholar]
- 32.Hosseinimehr SJ, Tolmachev V, Orlova A. Liver uptake of radiolabeled targeting proteins and peptides: considerations for targeting peptide conjugate design. Drug Discov Today. 2012;17:1224–1232. doi: 10.1016/j.drudis.2012.07.002. [DOI] [PubMed] [Google Scholar]


