Abstract
Purpose
Recent observations suggest that partial nephrectomy for small renal tumors may be associated with improved survival compared with radical nephrectomy. We evaluated survival in patients with renal tumors 4-7cm using a bi-institutional collaboration.
Methods
Combining institutional databases from Mayo Clinic and Memorial Sloan-Kettering, we identified 1,159 patients with sporadic, unilateral, solitary and localized renal masses 4.1–7.0 cm who underwent radical or partial nephrectomy between 1989 and 2006. Patient outcome was compared using Cox proportional hazards regression models.
Results
Among the 1,159 patients, 873 (75%) and 286 (25%) were treated with radical and partial nephrectomy, respectively. Patients treated with partial (vs radical) nephrectomy were significantly more likely to have a solitary kidney (10% vs 0.2%, p<0.001) and chronic kidney disease (15% vs 7%, p<0.001). Median duration of follow-up for survivors was 4.8 years (range 0-19). There was not a significant difference in overall survival when comparing patients treated with radical and partial nephrectomy (p=0.8). Interestingly, in a subset of 943 patients with RCC, those treated with radical nephrectomy were significantly more likely to die from RCC compared with those treated with partial nephrectomy (hazard ratio 2.16; 95% CI 1.04–4.50; p=0.039) although this association only approached statistical significance in a multivariable analysis (hazard ratio 1.97; 95% CI 0.92–4.20; p=0.079).
Conclusions
Our results suggest that overall and cancer-specific survival is not compromised when partial nephrectomy is utilized for patients with 4-7cm renal cortical tumors. With the benefit of preserving renal function, our results support the use of partial nephrectomy whenever technically feasible for renal tumors up to 7cm.
Keywords: Kidney neoplasms, Nephrectomy, Carcinoma, renal cell, Survival, Treatment outcome
INTRODUCTION
Nearly half a century ago, radical nephrectomy became standard of care for renal cortical tumors while partial nephrectomy was reserved for imperative situations such as a solitary kidney. With increased experience and technical advances, coupled with an upsurge in the discovery of small and incidental renal masses, partial nephrectomy has been accepted as a safe and preferred alternative to radical nephrectomy for most small renal tumors, even in the setting of a normal contralateral kidney.1-5 However, the utilization of partial nephrectomy has yet to become widespread as national databases, including the Surveillance, Epidemiology and End Results (SEER) Registry, indicate that at least as recent as 2001, most patients with a small renal mass are treated with radical nephrectomy.6, 7
More recent observations suggest that radical nephrectomy for renal tumors fl4 cm significantly increases the risk of de novo chronic renal failure compared with partial nephrectomy,1 which is particularly concerning given that chronic kidney disease is associated with an elevated risk of cardiovascular morbidity, hospitalization, and death.8 Consistent with this, we recently reported that patients with renal tumors fl4cm treated with radical nephrectomy were significantly more likely to die from any cause compared with those treated with partial nephrectomy.4, 9 In the current report, we combine data from the Mayo Clinic and Memorial Sloan-Kettering Cancer Center (MSKCC) and evaluate survival for patients with renal masses 4 – 7cm treated with radical vs. partial nephrectomy.
MATERIALS AND METHODS
Patient Selection
After obtaining Institutional Review Board approval at both the Mayo Clinic and MSKCC, each respective nephrectomy registry/database was queried. Combined, we identified 1,159 patients who were treated with radical or partial nephrectomy between 1989 and 2006 at Mayo Clinic (N=602) or MSKCC (N=557) for a sporadic, solitary, unilateral, NX/N0, M0, solid renal mass between 4.1 and 7.0 cm in maximum diameter. Most patients (91.4%) were treated with open surgery while 99 (8.5%) were treated laparoscopically. Patients with peripheral perinephric or renal sinus fat invasion (pT3a) were eligible for study, although patients with pT3b, pT3c, or pT4 disease were excluded.
Clinicopathologic Features
The clinicopathologic features studied included age at surgery, gender, symptoms at presentation (local or systemic), Charlson comorbidity index, diabetes, presence of a solitary kidney, preoperative serum creatinine, estimated glomerular filtration rate (GFR),10 chronic kidney disease, the 2002 primary tumor classification, tumor size (defined pathologically), and histologic subtype. Men with a preoperative serum creatinine >1.6 or women with a preoperative serum creatinine >1.4 were considered to have chronic kidney disease.
Statistical Methods
Clinicopathologic features were compared between institutions and between patients treated with radical and partial nephrectomy using Wilcoxon rank sum, chi-square, and Fisher's exact tests. Overall and cancer-specific survival were estimated using the Kaplan-Meier method. Associations of clinicopathologic features and type of surgery with death from any cause and death from RCC were evaluated using Cox proportional hazards regression models and summarized with hazard ratios and 95% confidence intervals (CI). The duration of follow-up was calculated from the date of surgery to the date of death or last follow-up. Statistical analyses were performed using the SAS software package (SAS Institute; Cary, North Carolina). All tests were two-sided and p-values <0.05 were considered statistically significant.
RESULTS
All Patients
Clinicopathologic features for the 1,159 patients under study are summarized in Table 1. There were 873 (75%) patients treated with radical and 286 (25%) patients treated with partial nephrectomy. Median age at surgery was 65 (range 22 – 95); median Charlson comorbidity index was 1 (range 0 – 11); median preoperative serum creatinine was 1.1 (range 0.5 – 11.2); median preoperative GFR was 65.4 (range 4.7 - 155.6); and median tumor size was 5.5 cm (range 4.1 – 7.0).
Table 1.
Summary of Clinicopathologic Features for 1,159 Patients Treated with Radical or Partial Nephrectomy for a 4.1 – 7.0cm Renal Mass
| Feature | N (%) |
|---|---|
| Age at Surgery (years) | |
| <65 | 586 (50) |
| ≥65 | 573 (50) |
| Gender | |
| Female | 425 (37) |
| Male | 734 (63) |
| Symptoms at Presentation (N=1,142) | 438 (38) |
| Systemic Symptoms at Presentation (N=1,142) | 92 (8) |
| Charlson Comorbidity Index (N=1,052) | |
| 0 | 454 (43) |
| >0 | 598 (57) |
| Diabetes (N=1,133) | 107 (9) |
| Solitary Kidney | 31 (3) |
| Chronic Kidney Disease (N=1,146) | 105 (9) |
| Type of Surgery | |
| Open RN | 785 (68) |
| Open NSS | 275 (24) |
| Laparoscopic RN | 88 (8) |
| Laparoscopic NSS | 11 (1) |
| 2002 Primary Tumor Classification | |
| pT1b | 1092 (94) |
| pT3a | 67 (56) |
| Maximal Tumor Size (cm) | |
| 4.1 – 5.0 | 505 (44) |
| 5.1 – 6.0 | 355 (31) |
| 6.1 – 7.0 | 299 (26) |
| Histologic Subtype | |
| Clear Cell RCC | 784 (68) |
| Papillary RCC | 160 (14) |
| Chromophobe RCC | 82 (7) |
| Collecting Duct RCC | 2 (0.2) |
| RCC, Not Otherwise Specified | 8 (0.7) |
| Benign | 123 (11) |
| Histologic Subtype | |
| RCC | 1036 (89) |
| Benign | 123 (11) |
A comparison of clinicopathologic features between patients treated with radical and partial nephrectomy is shown in Table 2. Patients treated with radical nephrectomy tended to be older and female and were more likely to have larger tumors with perinephric or renal sinus fat invasion compared with patients treated with partial nephrectomy. Median tumor size for patients treated with radical nephrectomy was 5.5 cm (range 4.1 – 7.0) compared with 5.0 cm (range 4.1 – 7.0) for patients treated with partial nephrectomy (p<0.001). As expected, patients with a solitary kidney or chronic kidney disease were more likely to be treated with partial nephrectomy. Median preoperative GFR for patients treated with partial nephrectomy was 63 (range 8 - 148) compared with 66 (range 5 - 156) for patients treated with radical nephrectomy (p=0.022).
Table 2.
Comparison of Clinicopathologic Features by Type of Surgery for 1,159 Patients
| Radical N=873 | Partial N=286 | ||
|---|---|---|---|
| Feature | N (%) | P-value | |
| Age at Surgery (years) | |||
| <65 | 422 (48) | 164 (57) | 0.008 |
| ≥65 | 451 (52) | 122 (43) | |
| Gender | |||
| Female | 335 (38) | 90 (32) | 0.036 |
| Male | 538 (62) | 196 (68) | |
| Symptoms at Presentation (N=1,142) | 343 (40) | 95 (34) | 0.089 |
| Systemic Symptoms at Presentation (N=1,142) | 70 (8) | 22 (8) | 0.904 |
| Charlson Comorbidity Index (N=1,052) | |||
| 0 | 341 (43) | 113 (45) | 0.536 |
| >0 | 459 (57) | 139 (55) | |
| Diabetes (N=1,133) | 73 (9) | 34 (12) | 0.088 |
| Solitary Kidney | 2 (0.2) | 29 (10) | <0.001 |
| Chronic Kidney Disease (N=1,146) | 63 (7) | 42 (15) | <0.001 |
| Type of Surgery | |||
| Open | 785 (90) | 275 (96) | 0.001 |
| Laparoscopic | 88 (10) | 11 (4) | |
| 2002 Primary Tumor Classification | |||
| pT1b | 815 (93) | 277 (97) | 0.028 |
| pT3a | 58 (7) | 9 (3) | |
| Maximal Tumor Size (cm) | |||
| 4.1 – 5.0 | 330 (38) | 175 (61) | <0.001 |
| 5.1 – 6.0 | 289 (33) | 66 (23) | |
| 6.1 – 7.0 | 254 (29) | 45 (16) | |
| Histologic Subtype | |||
| Clear Cell RCC | 629 (72) | 155 (54) | <0.001 |
| Papillary RCC | 100 (12) | 60 (21) | |
| Chromophobe RCC | 50 (6) | 32 (11) | |
| Collecting Duct RCC | 2 (0.2) | 0 | |
| RCC, Not Otherwise Specified | 7 (0.8) | 1 (0.4) | |
| Benign | 85 (10) | 38 (13) | |
| Histologic Subtype | |||
| RCC | 788 (90) | 248 (87) | 0.091 |
| Benign | 85 (10) | 38 (13) | |
At last follow-up 345 patients had died at a median of 4.7 years following surgery (range 0 – 18.2). Among the 814 patients who were still alive at last follow-up, the median duration of follow-up was 4.8 years (range 0 – 19.1); 92 (11%) patients had less than one year of follow-up. Estimated overall survival rates (95% CI, number still at risk) at 1, 3, 5, 7, and 10 years following surgery were 96% (95 – 97, 1,021), 89% (87 – 91, 770), 79% (77 – 82, 543), 70% (67 – 74, 349), and 57% (53 – 62, 185), respectively. Among the 873 patients treated with radical nephrectomy, at last follow-up 290 died at a median of 4.7 years following surgery (range 0 – 18.2). Among the 583 radical nephrectomy patients still alive at last follow-up, the median duration of follow-up was 5.3 years (range 0 – 19.1). Fifty-five of the 286 patients treated with partial nephrectomy died at a median of 4.1 years following surgery (range 0 – 15). Among the 231 partial nephrectomy patients still alive at last follow-up, the median duration of follow-up was 3.4 years (range 0 – 17).
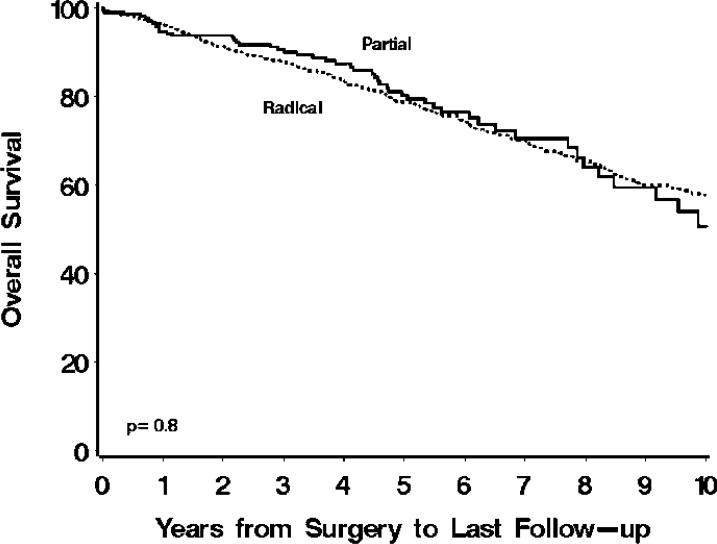
Univariately, patients treated with radical nephrectomy were 5% more likely to die from any cause compared with patients treated with partial nephrectomy, although this difference was not statistically significant (hazard ratio 1.05; 95% CI 0.78 – 1.40; p=0.8; Figure 1.) Adjustment for each of the clinicopathologic features, one feature at a time, did not appreciably change the risk of death from any cause for patients treated with radical versus partial nephrectomy. In addition, there was no evidence of statistically significant interactions between the clinicopathologic features and type of surgery, indicating that the risk of death from any cause for patients did not differ significantly when stratified by levels of the clinicopathologic features studied. Lastly, a multivariable analysis indicated that older age at surgery, higher Charlson comorbidity index, chronic kidney disease, pT3a disease, larger tumor size, and malignant histology were jointly statistically significantly associated with death from any cause (Table 3). After adjusting for these features, there was not a statistically significant association between type of surgery and death from any cause (p=0.7).
Figure 1.
Overall survival for 873 patients treated with radical and 286 patients treated with partial nephrectomy. Estimated overall survival rates (95% CI, number still at risk) at 1, 3, 5, 7, and 10 years following surgery were 96% (94 – 98, 789), 88% (86 – 90, 607), 79% (76 – 82, 448), 70% (67 – 74, 310), and 58% (54 – 63, 170), respectively, for patients treated with radical compared with 95% (92 – 97, 232), 91% (87 – 95, 163), 80% (74 – 87, 95), 71% (63 – 80, 39), and 51% (39 – 66, 15), respectively, for patients treated with partial nephrectomy.
Table 3.
Multivariable models looking at death from any cause and cancer-specific death
| Death from Any Cause | ||
|---|---|---|
| Feature | HR (95% CI) | P-value |
| Age at Surgery (years) | 1.05 (1.04 – 1.06)* | <0.001 |
| Charlson Comorbidity Index | 1.23 (1.17 – 1.30)* | <0.001 |
| Chronic Kidney Disease | ||
| Absent | 1.0 (reference) | |
| Present | 1.76 (1.29 – 2.41) | <0.001 |
| 2002 Primary Tumor Classification | ||
| pT1b | 1.0 (reference) | |
| pT3a | 1.96 (1.34 – 2.86) | <0.001 |
| Maximal Tumor Size (cm) | 1.16 (1.03 – 1.31)* | 0.015 |
| Histologic Subtype | ||
| Benign | 1.0 (reference) | |
| RCC | 2.18 (1.12 – 4.24) | 0.022 |
| Type of Surgery | ||
| Partial | 1.0 (reference) | |
| Radical | 0.94 (0.69 – 1.27) | 0.665 |
| Death from RCC | ||
|---|---|---|
| Feature | HR (95% CI) | P-value |
| Age at Surgery (years) | 1.02 (1.00 – 1.04)* | 0.019 |
| Chronic Kidney Disease | ||
| Absent | 1.0 (reference) | |
| Present | 2.91 (1.55 – 5.47) | <0.001 |
| 2002 Primary Tumor Classification | ||
| pT1b | 1.0 (reference) | |
| pT3a | 4.35 (2.50 – 7.56) | <0.001 |
| Maximal Tumor Size (cm) | 1.66 (1.29 – 2.16)* | <0.001 |
| Type of Surgery | ||
| Partial | 1.0 (reference) | |
| Radical | 1.97 (0.92 – 4.20) | 0.079 |
HRs represent a 1-unit increase in the feature listed.
Cancer-specific Survival
Among the 1,036 patients with RCC, there were 943 who were either still alive at last follow-up or whose cause of death could be determined, including 704 (75%) patients treated with radical and 239 (25%) patients treated with partial nephrectomy. Eighty-two patients died from RCC at a median of 3.3 years following surgery (range 0 – 16.2). Estimated cancer-specific survival rates (95% CI , number still at risk) at 1, 3, 5, 7, and 10 years following surgery were 99% (98 – 99, 835), 95% (94 – 97, 623), 92% (90 – 94, 435), 90% (87 – 92, 281), and 86% (83 – 90, 150), respectively.
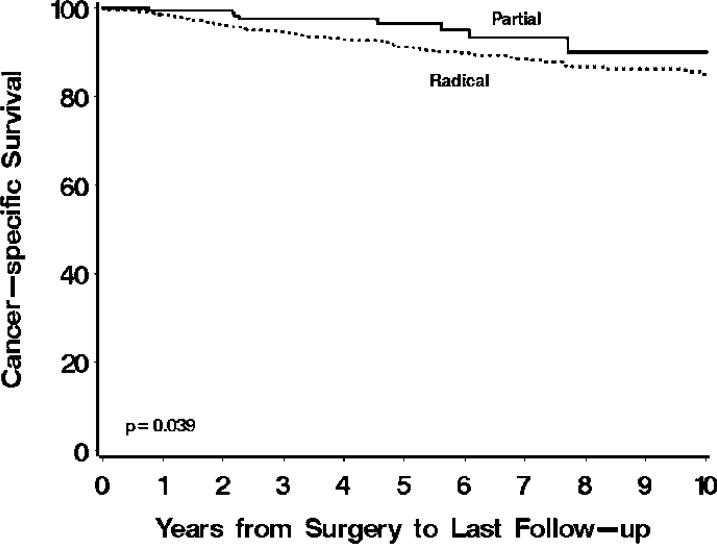
At last follow-up, 74 of the 704 patients treated with radical had died from RCC at a median of 3.3 years following surgery (range 0 – 16.2) while only 8 of the 239 patients treated with partial nephrectomy died from RCC at a median of 3.4 years following surgery (range 0.8 – 7.7). Univariately, patients treated with radical were over twice as likely to die from RCC compared with patients treated with partial nephrectomy (hazard ratio 2.16; 95% CI 1.04 – 4.50; p=0.039; Figure 2). A multivariable analysis indicated that older age at surgery, chronic kidney disease, pT3a disease, and larger tumor size were jointly significantly associated with death from RCC (Table 3). After adjusting for these features, patients treated with radical were still nearly twice as likely to die from RCC compared with patients treated with partial nephrectomy, although this difference only approached statistical significance (hazard ratio 1.97; 95% CI 0.92 – 4.20; p=0.079). Similar results were obtained if the definition of chronic kidney disease was changed to GFR <45 (data not shown).
Figure 2.
Cancer-specific survival for 704 patients treated with radical and 239 patients treated with partial nephrectomy. Estimated cancer-specific survival rates (95% CI, number still at risk) at 1, 3, 5, 7, and 10 years following surgery were 98% (97 – 99, 640), 95% (93 – 96, 483), 91% (89 – 93, 354), 89% (86 – 91, 248), and 85% (81 – 89, 135), respectively, for patients treated with radical compared with 99.5% (99 – 100, 195), 98% (95 – 100, 140), 97% (93 – 99.7, 81), 93% (88 – 99, 33), and 90% (82 – 99, 15), respectively, for patients treated with partial nephrectomy.
DISCUSSION
In this bi-institution collaboration, we combined data from two tertiary-care centers that have long advocated for the use of partial nephrectomy. We have previously reported that oncologic outcome following partial nephrectomy is not compromised for select patients with renal masses 4-7cm in size.5, 11 We have also suggested that overall survival may be diminished if radical nephrectomy is utilized in lieu of partial nephrectomy for renal tumors fl4cm in size.4, 9 In this study, we observed that overall survival is similar between partial and radical nephrectomy for T1b-appearing renal masses, despite the fact that those receiving partial nephrectomy were more likely to be diabetic and have chronic kidney disease or a solitary kidney. These results support the use of partial nephrectomy for select patients with renal masses up to 7cm, a practice that as of 2001 was utilized for <6% of patients with 4-7cm renal masses according to SEER registry data.6
Partial nephrectomy remains underutilized in the surgical management of renal tumors. The most contemporary SEER registry data (2000-2001) suggest that partial nephrectomy is performed in less than 1/2 of patients with tumors <2cm, approximately 1/5 of patients with tumors 2-4cm, and in only a small fraction (<6%) of patients with tumors 4-7cm.6 With increasing experience, complications from partial nephrectomy have been minimized.2, 3, 12, 13 Furthermore, laparoscopic partial nephrectomy has now been investigated with excellent oncologic and functional outcomes.14 While we expect updated SEER data to demonstrate that partial nephrectomy use has increased, we suspect that radical nephrectomy is currently utilized for the vast majority of patients with tumors 4-7cm.
Previous observations from our institutions demonstrate a significantly increased risk of chronic kidney disease among patients treated with radical compared with partial nephrectomy for renal tumors in elective situations.1, 15 In fact, among patients with a preoperative GFR >60, radical nephrectomy was significantly associated with a 12-fold increased risk of new onset GFR <45 compared with partial nephrectomy.1 It is important to note that chronic kidney disease, in a graded fashion, is associated with significant cardiovascular morbidity, hospitalization, and mortality.8 Consistent with this, radical nephrectomy may increase the risk of death from any cause compared with partial nephrectomy for small renal masses, even in the absence of dialysis.4, 9 Furthermore, renal insufficiency is associated with an increased risk of hip fracture even for patients who have not progressed to dialysis.16 Thus, the previous dogma that dialysis is uncommon after radical nephrectomy is inappropriate; death and disability from chronic kidney disease exists even in the absence of dialysis.
In this report, interestingly, we observed that cancer-specific survival is improved for patients treated with partial compared with radical nephrectomy. While this association did not remain statistically significant on multivariable analysis, the hazard ratio remained similar suggesting that radical nephrectomy was associated with nearly a 2-fold increased risk of cancer-specific death. Intuitively, this does not make sense and we do not propose that partial nephrectomy improves cancer-specific survival for patients with T1b-appearing renal masses. Those treated with radical nephrectomy were more likely to have larger tumors, clear cell histology, and perinephric or renal sinus fat invasion, all features reported to increase the risk of cancer-specific death.17-19 Furthermore, we also suggest that patients treated with partial nephrectomy in the current study were more likely to have exophytic tumors, which may be biologically less aggressive than more central or hilar renal masses,20 perhaps due to the close proximity of the renal sinus with its associated rich network of veins and lymphatics.21 Additionally, surgeon selection bias may have influenced the results in that more aggressive appearing tumors may have been selected for radical nephrectomy. However, these results provide assurance regarding the oncologic efficacy of partial nephrectomy for renal tumors up to 7cm.
This study is not without limitations. Our analysis represents a retrospective investigation and is subject to the biases inherent with this approach. Another important limitation is the inherent selection bias when comparing patients with renal tumors who undergo different surgical procedures, namely partial vs radical nephrectomy. Patients with multiple comorbid conditions may have been more likely treated with radical nephrectomy which would have impacted our overall survival results. However, our results demonstrate that patients treated with partial nephrectomy were more often in imperative situations (i.e. solitary kidney, chronic kidney disease, and diabetes), and coupled with a nearly identical Charlson co-morbidity Index between the two groups, we believe that our results support that partial nephrectomy is appropriate for select patients with T1b-appearing renal masses.
In elective situations, health related quality of life is improved with partial compared with radical nephrectomy.22 Hospital costs and length of stay are similar for partial and radical nephrectomy.23, 24 Partial nephrectomy reduces the risk of chronic kidney disease and complications thereof including hip fractures and cardiovascular morbidity and mortality. Our results suggest that partial nephrectomy does not compromise overall or cancer-specific mortality compared with radical nephrectomy for tumors up to 7cm. Appropriate patients with T1b-appearing renal tumors should be offered partial nephrectomy or referred to a center that performs the procedure as removal of the entire kidney, even if performed laparoscopically, may have long-term adverse consequences.
CONCLUSION
Our results suggest that overall survival is similar for T1b renal mass patients treated with radical or partial nephrectomy. While likely due to patient selection and other unforeseen factors, we also observed an improvement in cancer-specific survival for patients treated with partial vs radical nephrectomy, merely suggesting that partial nephrectomy does not compromise oncologic outcome. Collectively, our results support the use of partial nephrectomy whenever technically feasible patients with tumors up to 7cm in size.
ACKNOWLEDGEMENT
We thank Tom Manion for his assistance with data abstraction.
Abbreviations
- MSKCC
Memorial Sloan-Kettering Cancer Center
- CI
Confidence Interval
- SEER
Surveillance, Epidemiology and End Results
- GFR
Glomerular Filtration Rate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 3.Thompson RH, Leibovich BC, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol. 2005;174:855. doi: 10.1097/01.ju.0000169453.29706.42. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 5.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 6.Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 7.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112:511. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.Huang WC, Elkin EB, Levey AS, Jang TL, Russo P. Partial nephrectomy versus radical nephrectomy in patients with small renal tumors--is there a difference in mortality and cardiovascular outcomes? J Urol. 2009;181:55. doi: 10.1016/j.juro.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 11.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4-7 cm. BJU Int. 2006;97:939. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RH, Frank I, Lohse CM, Saad IR, Fergany A, Zincke H, et al. The impact of ischemia time during open nephron sparing surgery on solitary kidneys: a multi-institutional study. J Urol. 2007;177:471. doi: 10.1016/j.juro.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha E, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. 2003;170:64. doi: 10.1097/01.ju.0000072272.02322.ff. [DOI] [PubMed] [Google Scholar]
- 14.Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70. doi: 10.1016/j.juro.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 15.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 16.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 17.Frank I, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 18.Thompson RH, Blute ML, Krambeck AE, Lohse CM, Magera JS, Leibovich BC, et al. Patients with pT1 renal cell carcinoma who die from disease after nephrectomy may have unrecognized renal sinus fat invasion. Am J Surg Pathol. 2007;31:1089. doi: 10.1097/PAS.0b013e31802fb4af. [DOI] [PubMed] [Google Scholar]
- 19.Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol. 2003;27:612. doi: 10.1097/00000478-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Schachter LR, Bach AM, Snyder ME, Kattan MW, Russo P. The impact of tumour location on the histological subtype of renal cortical tumours. BJU Int. 2006;98:63. doi: 10.1111/j.1464-410X.2006.06179.x. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Leibovich BC, Cheville JC, Webster WS, Lohse CM, Kwon ED, et al. Is renal sinus fat invasion the same as perinephric fat invasion for pT3a renal cell carcinoma? J Urol. 2005;174:1218. doi: 10.1097/01.ju.0000173942.19990.40. [DOI] [PubMed] [Google Scholar]
- 22.Lesage K, Joniau S, Fransis K, Van Poppel H. Comparison between open partial and radical nephrectomy for renal tumours: perioperative outcome and health-related quality of life. Eur Urol. 2007;51:614. doi: 10.1016/j.eururo.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Uzzo RG, Wei JT, Hafez K, Kay R, Novick AC. Comparison of direct hospital costs and length of stay for radical nephrectomy versus nephron-sparing surgery in the management of localized renal cell carcinoma. Urology. 1999;54:994. doi: 10.1016/s0090-4295(99)00348-9. [DOI] [PubMed] [Google Scholar]
- 24.McKiernan JM, Teschendorf B, Katz J, Herr HW, Russo P. A comparison of hospital-based charges following partial and radical nephrectomy. Urol Oncol. 2002;7:3. doi: 10.1016/s1078-1439(01)00135-1. [DOI] [PubMed] [Google Scholar]