Abstract
Background and Aims:
The excessive and repeated use of antibiotics in medicine has led to the development of antibiotic-resistant microbial strains, including Staphylococcus aureus whose emergence of antibiotic-resistant strains has reduced the number of antibiotics available to treat clinical infections caused by this bacterium. In this study, antioxidant and antimicrobial activities of methanolic extract of Xanthium strumarium L. leaves were evaluated on methicillin-susceptible and methicillin-resistant Staphylococcus aureus (MRSA) spp.
Materials and Methods:
Antiradical and antioxidant activities X. strumarium L. leaf extract were evaluated based on its ability to scavenge the synthetic 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical and by the paired diene method, respectively, whereas the antimicrobial activity was assayed by the disc diffusion method.
Statistical Analysis:
Data were subjected to analysis of variance following an entirely random design to determine the least significant difference at P < 0.05 using SPSS v. 11.5.
Results and Conclusions:
The IC50 values of the extract were 0.02 mg/mL and 0.09 mg/mL for the antioxidant and DPPH-scavenging capacity, respectively. X. strumarium extract affected both methicillin-sensitive Staphylococcus aureus and MRSA, though antibacterial activity was more effective on methicillin-susceptible S. aureus spp. The antibacterial and antioxidant activities exhibited by the methanol extract may justify the traditional use of this plant as a folk remedy worldwide.
KEY WORDS: 1,1-diphenyl-2-picrylhydrazyl; antioxidant activity; methicillin-resistant Staphylococcus aureus; methicillin-sensitive Staphylococcus aureus; Staphylococcus aureus; Xanthium strumarium L
INTRODUCTION
Many medicinal plants are considered as important natural remedies for the treatment of various diseases. The excessive and repeated use of some synthetic drugs in modern medicine has led to the development of antibiotic-resistant microbial strains, including Staphylococcus aureus.[1] The emergence of antibiotic-resistant strains of this bacterium reduces the number of antibiotics available to treat clinical infections caused by this pathogen.[2] S. aureus is a highly variable pathogen with considerable impact on human health. It is responsible for a wide range of hospital and community-acquired infections globally, from skin infections and food poisoning to life-threatening conditions such as toxic-shock syndrome, endocarditis, pneumonia, bacteremia, and osteomyelitis.[3,4]
Xanthium strumarium L. is an annual plant belonging to the family Asteraceae. In Iran, X. strumarium is available between August and September. In many countries, different plant parts, especially fruit and root, are used as remedies. Various parts of this plant species were found to possess useful medicinal properties such as antitrypanosomal,[5] diuretic,[6] hypoglycemic,[7] anthelmintic,[8] antifungal,[9] antileishmanial,[9] antiulcerogenic,[10] and anti-inflammatory[11,12] activities, it is also known to inhibit proliferation of human cancer cells in vitro[13] and to exert a neuroprotective activity on the central nervous system.[14]
The chemical constituents of X. strumarium include phenolic compounds such as ferulic acids, chlorogenic acids and thiazolidinediones;[15] caffeic acid, 1,3,5-tri-O-caffeoyl quinic acid and 1,5-di-O-caffeoyl quinic acid;[16] isoprenoids as β-sitosterol and strumasterol;[17] monoterpene and sesquiterpene hydrocarbons;[17] xanthanolide sesquiterpene lactones[18] and triterpenoid saponins.[19] In addition, Srinivas et al.[20] reported, high levels of alkaloids, phenolic acids, and diterpenes and significant concentrations of saponins, glycosides, fixed oils, and phytosterols in X. strumarium.[20] The main aim of the present study was to carry out in vitro tests on X. strumarium from Iran by to assess the antioxidant and antimicrobial activities of methanolic leaf extracts.
MATERIALS AND METHODS
Plant material and extract
Leaves of X. strumarium were collected between August and September 2012 from the area of Hamun Lake of Zabol (31° 1’ 43” N, 61° 30’ 4” E), Sistan and Baluchestan Province, Iran. The plant was taxonomically identified by a botanist at the herbarium of Department of Botany, Shahid Beheshti University, Iran. 20 g of dried leaves were and extracted in 200 mL 85% methanol using a shaker water bath for 24 h at 25°C. After filtration with Whatman No. 1 filter paper, filtrate was concentrated by a rotary evaporator at 50°C for 30 min., to remove solvent from the extract. Solid extract was dissolved in 20 mL of distilled water. This working solution was used for all tests in this study.
Antioxidant activity
Antioxidant activity was determined by the paired diene method.[21] The antioxidant activity measured represents the capacity of the plant extract to inhibit the peroxidation of linoleic acid, in which the double bond is changed to a paired diene. Each extract sample (0.01-30 mg/mL) in methanol (100 μL) was blended with 3 mL of 10 mM linoleic acid (Sigma Chemical Co., St. Louis, MO, USA) to form an emulsion in 0.2 M sodium phosphate buffer (pH 6.6) in test tubes, and then placed in the dark at 37°C to stimulate oxidation. After incubation for 17 h, 7 mL of 70% methanol in deionized water was added, and the absorbance of the mixture was measured at 234 nm against a blank in a Hitachi U-2001 spectrophotometer (Tokyo, Japan). Antioxidant activity was measured as follows:
Antioxidant activity (%) = [(∆A234 of control − ∆A234 of sample)/∆A234 of control] ×100.
IC50 value (mg/mL) is the efficient concentration at which the antioxidant capacity was inhibited by 50%, and was gained by interpolation from linear regression analysis Analyses were repeated 3 times (technical replicates). α-tocopherol, butylated hydroxyanisole (BHA) and ascorbic acid (Sigma-Aldrich, USA) were used as standard controls.
Scavenging ability on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radicals
The scavenging ability on the synthetic (DPPH, Sigma) free radical, determined according to Shimada et al.,[22] is the capability of the extract to respond rapidly with DPPH radicals and to scavenge most DPPH radical molecules. The test was repeated 3 times. α-tocopherol, BHA and ascorbic acid (Sigma-Aldrich, USA) were used as standards. A volume of 5 mL of the methanolic extract (0.5-40 mg/mL) was mixed with 1 mL of methanolic solution containing DPPH radicals, resulting in a final concentration of 0.2 mM DPPH. The mixture was shaken vigorously, left to stand for 40 min., in the dark, and the absorbance was read at 517 nm against a blank. The scavenging ability was determined as follows:
Scavenging ability (%) = [(∆A517 of control − ∆A517 of sample)/∆A517 of control] ×100.
IC50 value (mg/mL) is the efficient concentration at which the antioxidant activity was inhibited by 50% and DPPH radicals were scavenged by 50%, and was gained by interpolation from linear regression analysis.
Bacterial isolates
The S. aureus strains used in this study were clinical isolates from patients with S. aureus infections, obtained from the Microbiological Laboratory of the Central Hospital in Zabol, Iran. Isolated were identified by biochemical (catalase, coagulase and DNase) and molecular tests. Isolated methicillin-resistant Staphylococcus aureus (MRSA) were identified by screening tests on Mueller-Hinton agar (MHA, Torlak, Berlin, Germany) complemented with 5% NaCl and 1 mg/mL oxacillin-impregnated disc.[23] The two strains used in this study were ATTC 25923 (MRSA) and PTCC 1341 (MSSA).
Disc-diffusion assay
Antimicrobial tests were carried out by the disc diffusion method using 100 μL of bacteria suspension (containing 2.0 × 108 CFU/mL of bacteria) dispersed on MHA in sterilized Petri dishes (60 mm in diameter). To the discs (6 mm in diameter, HI Media Laboratories Pvt. Ltd., Mumbai, India) placed on the inoculated agar, 50, 100, 200, and 300 μL of leaf extracts were added. The inoculated plates were maintained at 4°C for 2 h and later incubated at 37°C for 24 h. Antimicrobial activity was determined by measuring the zone of inhibition (mm) against the test bacterial (MRSA and MSSA) strains.
Statistical analysis
The extract was prepared in triplicate for antioxidant and antibacterial tests. Data were subjected to analysis of variance following an entirely random design to determine the least significant difference at P < 0.05, using statistical software package (SPSS, version 11.5, IBM Corporation, NY, USA). All results are expressed as mean ± standard deviation.
RESULTS
The results on antioxidant and antiradical activities of the tested extract are summarized in Table 1. The levels of both antioxidant and DPPH radical scavenging capacities are inversely correlated with their IC50 values. The IC50 values of antioxidant activity were 0.04, 0.06, 3.12, and 0.02 mg/mL for α-tocopherol, BHA, ascorbic acid and X. strumarium leaf extract, respectively. For the radical scavenging capacity, IC50 values were 1.01, 0.27, 4.01, and 0.09 mg/mL for α-tocopherol, BHA, ascorbic acid and X. strumarium extract, respectively. In both assays, activity of X. strumarium methanol extract was significantly higher than that of the three tested reference compounds (P < 0.05) [Table 1]. The results of antibacterial activity of the leaf extract are shown in Figures 1 and 2. Our result showed that inhibition zones for MSSA (PTCC 1341) bacteria were 12.11 ± 0.12 (f), 16.08 ± 0.14 (d), 23.06 ± 0.04 (b), and 26.00 ± 0.00 (a) mm at concentrations of 50, 10, 200, and 300 μL of plant extract, respectively (P < 0.05) [Figure 1]. The inhibition zones for MSSA isolates were 11.01 ± 0.03 (g), 14.03 ± 0.00 (e), 18.06 ± 0.14 (c), and 26.0.1 ± 0.02 (a) mm, at concentrations of 50, 10, 200, and 300 μL of plant extract, respectively (P < 0.05) [Figure 1]. Inhibition zones relative to MRSA (ATTC 25923) strain were 8.11 ± 0.00 (e), 12.11 ± 0.11 (c), 15.01 ± 0.00 (b), and 18.4 ± 0.07 (a) mm at concentrations of 50, 10, 200, and 300 μL of plant extracts, respectively (P < 0.05) [Figure 2]. Inhibition areas obtained for MRSA isolates were 6.4 ± 0.2 (f), 8.12 ± 0.01 (d), 12.2 ± 0.5 (c), and 17.8 ± 0.9 (b) mm, at concentrations of 50, 10, 200, and 300 μL of plant extracts, respectively (P < 0.05) [Figure 2]. Our results showed a dose-response correlation between the plant extract concentration and the inhibition of bacterial growth.
Table 1.
IC50 values (mg/mL) of the X. strumarium leaf extract in two tests: Paired diene method and DPPH radical scavenging assay
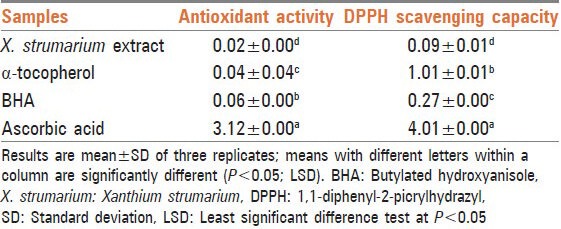
Figure 1.
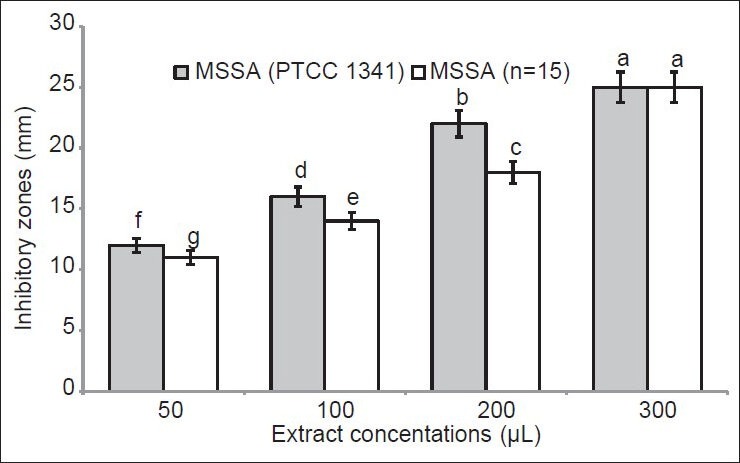
Antibacterial activity of Xanthium strumarium leaf extract against methicillin-sensitive Staphylococcus aureus (MSSA) standard (PTCC 1341) and the clinical isolate MSSA (n = 15) measured as diameter of the zone of inhibition (mm). Different letters indicate significant differences according to the least significant difference test at P < 0.05. All results are expressed as mean ± standard deviation
Figure 2.
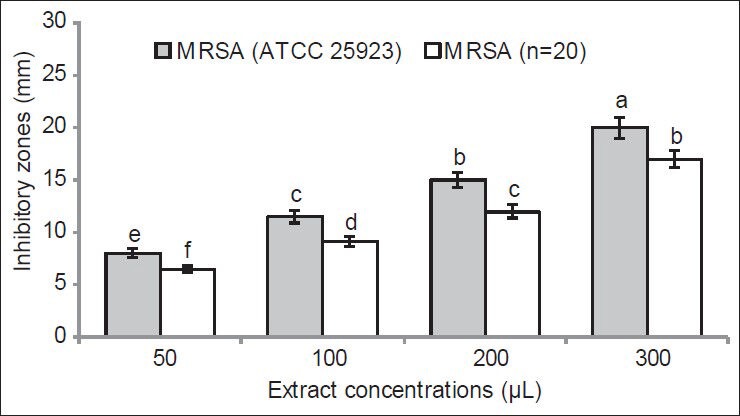
Antibacterial activity of Xanthium strumarium leaf extracts against methicillin-resistant Staphylococcus aureus (MRSA) standard (ATTC 25923) and the clinical isolate MRSA (n = 20) mea-sured as diameter of the zone of inhibition (mm). Different letters indi-cate significant differences according to the least significant difference test at P < 0.05. All results are expressed as mean ± standard deviation
DISCUSSION
According to the European Antimicrobial Surveillance System, MRSA represents currently a huge burden for many healthcare institutions and it is by far the most significant antibiotic-resistant acquired pathogen worldwide.
In previous studies, ethanol extracts from leafs of Eremophila alternifolia (Myoporaceae), Eremophila duttonii R.Br. (Myoporaceae), Amyema quandong (Lindl.) Tiegh. (Loranthaceae) and from the stem base of Lepidosperma viscidum R.Br. (Cyperaceae), traditional Australian medicinal plants, showed antibacterial activity against MRSA.[24] Essential oils of Thymus vulgaris L. (Lamiaceae), Eucalyptus globulus Labill. (Myrtaceae)[25] and Sinapis arvensis L. (Brassicaceae)[26] were also effective against clinical isolates of MRSA in disc diffusion assay. More recently, antimicrobial activity of essential oil of Daucus crinitus was reported with the same method, even if resistance or sensitivity to methicillin of S. aureus strains used were not specified by authors.[27] As regards X. strumarium, a methanol leaf extract exhibited a significant inhibitory activity on the growth of S. aureus,[28] and similar results were reported on chloroform and ethanol fractions from X. strumarium leaves.[29] Again, in both studies, the sensitivity to methicillin of the isolates was not specified.[28,29] Interestingly, chlorhexidine gluconate (1% and 4%) exerted a high biocide activity on both MSSA and MRSA.[30]
Our results showed that the maximum concentration of the extract (300 μL) was inhibitory both against MSSA and MRSA strains, with the highest inhibition zones of 25 mm and 20 mm, respectively. The inhibitory activity of the plant extract against MSSA was higher than against MRSA, i.e. in other words, X. strumarium exerted a higher antimicrobial effect on MSSA than on MRSA. Antibacterial activity of the methanol extract of X. strumarium leaves was previously reported by Srinivas et al.[20] and ascribed to the main components, alkaloids, phenolic acids, and saponins, phytochemicals with well-known antimicrobial properties.[30]
1,1-diphenyl-2-picrylhydrazyl assay is a sensitive method widely used to assess the free radical scavenging activity of plant extracts or isolated phytochemicals. DPPH is a stable free radical which accepts an electron or hydrogen radical to turn into a stable diamagnetic molecule.[31,32] This test possesses many advantages compared with other methods, such as good stability, sensitivity, feasibility, and handiness.[33] Antioxidant activity was expressed as the IC50 (mg/mL), which is the effective concentration at which the antioxidant activity was inhibited by 50%, gained by interpolation from linear regression analysis. Very recently, Kamboj et al.[34] have reported that, among all different organs of X. strumarium, leaf ethanol extract, with high levels of phenolics and the highest amount of flavonoids, showed the highest antioxidant activity. Finally, the relevant antioxidant and antiradical capacities of X. strumarium suggest a potential use for the prevention and treatment of diseases correlated with oxidative stress.
CONCLUSION
The antibacterial activity may be possibly attributed to the presence of phenolic acids, flavonoids, tannins and triterpinoids in the methanol extract, as reported in literature. The antibacterial and antioxidant activities exhibited by the methanol extract may justify the traditional use of this plant as folk remedy worldwide. X. strumarium has emerged as a relevant medicinal plant by virtue of its documented biological properties and possible applications.
ACKNOWLEDGMENT
The authors are very grateful to Department of Range and Watershed Management, Faculty of Natural Resources, University of Zabol for financial support.
Footnotes
Source of Support: The authors are very grateful to Department of Range and Watershed Management, Faculty of Natural Resources, University of Zabol for financial support.
Conflict of Interest: None declared.
REFERENCES
- 1.Miri A, Rad JS, Alfatemi SMH, Rad MS. A study of Antibacterial potentiality of some plants extracts against multi-drug resistant human pathogens. Ann Biol Res. 2013;4:35–41. [Google Scholar]
- 2.Holman L. Methicillin-resistant Staphylococcus aureus. Radiol Technol. 2013;84:307–10. [PubMed] [Google Scholar]
- 3.Alfatemi SMH, Rad JS, Rad MS, Mohsenzadeh S, da Silva JAT. Chemical composition, antioxidant activity and in vitro antibacterial activity of Achillea wilhelmsii C. Koch essential oil on methicillin-susceptible and methicillin-resistant Staphylococcus aureus spp. 3. Biotech. 2014:1–6. doi: 10.1007/s13205-014-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akineden O, Hassan AA, Schneider E, Usleber E. Enterotoxigenic properties of Staphylococcus aureus isolated from goats’ milk cheese. Int J Food Microbiol. 2008;124:211–6. doi: 10.1016/j.ijfoodmicro.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Talakal TS, Dwivedi SK, Sharma SR. In vitro and in vivo antitrypanosomal activity of Xanthium strumarium leaves. J Ethnopharmacol. 1995;49:141–5. doi: 10.1016/0378-8741(95)01313-x. [DOI] [PubMed] [Google Scholar]
- 6.Nieves JL, Padilla L, Del Carmen M, Rodríguez HR, Simón GG, Freixas C, et al. Efecto diurético del Xanthium strumarium L.(Guizazo de Caballo) Rev Cuba Plant Med. 1999;1:22–5. [Google Scholar]
- 7.Hsu FL, Chen YC, Cheng JT. Caffeic acid as active principle from the fruit of Xanthium strumarium to lower plasma glucose in diabetic rats. Planta Med. 2000;66:228–30. doi: 10.1055/s-2000-8561. [DOI] [PubMed] [Google Scholar]
- 8.Sharma SR, Singh D, Khan FA, Swarankar CP, Bhagwan PS. Anthelmintic activity of Xanthium strumarium against Haemonchus contortus infection in sheep. Indian J Anim Sci. 2003;73:342–4. [Google Scholar]
- 9.Lavault M, Landreau A, Larcher G, Bouchara JP, Pagniez F, Le Pape P, et al. Antileishmanial and antifungal activities of xanthanolides isolated from Xanthium macrocarpum. Fitoterapia. 2005;76:363–6. doi: 10.1016/j.fitote.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Favier LS, María AO, Wendel GH, Borkowski EJ, Giordano OS, Pelzer L, et al. Anti-ulcerogenic activity of xanthanolide sesquiterpenes from Xanthium cavanillesii in rats. J Ethnopharmacol. 2005;100:260–7. doi: 10.1016/j.jep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 11.Kim IT, Park YM, Won JH, Jung HJ, Park HJ, Choi JW, et al. Methanol extract of Xanthium strumarium L. possesses anti-inflammatory and anti-nociceptive activities. Biol Pharm Bull. 2005;28:94–100. doi: 10.1248/bpb.28.94. [DOI] [PubMed] [Google Scholar]
- 12.Anjoo K, Kumar SA. Phytopharmacological review of Xanthium strumarium L. (Cocklebur) J Pharm Pract Res. 2010;4:129–39. [Google Scholar]
- 13.Kim YS, Kim JS, Park SH, Choi SU, Lee CO, Kim SK, et al. Two cytotoxic sesquiterpene lactones from the leaves of Xanthium strumarium and their in vitro inhibitory activity on farnesyltransferase. Planta Med. 2003;69:375–7. doi: 10.1055/s-2003-38879. [DOI] [PubMed] [Google Scholar]
- 14.Mandala SC, Dharab AK, Kumara CK, Maitic BC. Neuropharmacological activity of Xanthium strumarium Linn. extract. J Herbs Spices Med Plants. 2001;8:69–77. [Google Scholar]
- 15.Qin L, Han T, Li H, Zhang Q, Zheng H. A new thiazinedione from Xanthium strumarium. Fitoterapia. 2006;77:245–6. doi: 10.1016/j.fitote.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Bisht NPS, Singh R. Chemical investigation of the leaves of Xanthium strumarium L. J Indian Chem Soc. 1978;55:707–8. [Google Scholar]
- 17.Taher HA, Ubiergo GO, Talenti ECJ. Constituents of the essential oil of Xanthium strumarium. J Nat Prod. 1985;48:857–7. doi: 10.1021/np50035a036. [DOI] [PubMed] [Google Scholar]
- 18.Sheu S, Hsu F, Tai H, Sheu M, Huang M. Determination of xanthii constituents by high-performance liquid chromatography and capillary electrophoresis. J Food Drug Anal. 2003;11:67–71. [Google Scholar]
- 19.Yadava RN, Jharbade J. Novel biologically active triterpenoid saponin from the leaves of Xanthium strumarium Linn. Asian J Chem. 2007;19:1224–30. [Google Scholar]
- 20.Srinivas PV, Rao RU, Venkateshwarulu EL, Kumar AC. Phytochemical screening and in vitro antimicrobial investigation of the methanolic extract of Xanthium strumarium leaf. Int J Drug Dev Res. 2011;3:245–51. [Google Scholar]
- 21.Lingnert H, Vallentin K, Eriksson CE. Measurement of antioxidative effect in model system. J Food Process Preserv. 1979;3:87–103. [Google Scholar]
- 22.Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 23.Li W, Roberts DP, Dery PD, Meyer SL, Lohrke S, Lumsden RD, et al. Broad spectrum anti-biotic activity and disease suppression by the potential biocontrol agent Burkholderia ambifaria BC-F. Crop Prot. 2002;21:129–35. [Google Scholar]
- 24.Palombo EA, Semple SJ. Antibacterial activity of Australian plant extracts against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) J Basic Microbiol. 2002;42:444–8. doi: 10.1002/1521-4028(200212)42:6<444::AID-JOBM444>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Tohidpour A, Sattari M, Omidbaigi R, Yadegar A, Nazemi J. Antibacterial effect of essential oils from two medicinal plants against methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2010;17:142–5. doi: 10.1016/j.phymed.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Rad JS, Alfatemi MH, Rad MS, Sen DJ. Phytochemical and Antimicrobial Evaluation of the Essential Oils and Antioxidant Activity of Aqueous Extracts from Flower and Stem of Sinapis arvensis L. Am J Advan Drug Deliv. 2013;1:1–10. [Google Scholar]
- 27.Bendiabdellah ME, Amine Dib ME, Meliani N, Muselli A, Nassim D, Tabti B, et al. Antibacterial activity of Daucus crinitus essential oils along the vegetative life of the plant. J Chem. 2013;1:7–12. [Google Scholar]
- 28.Mahida Y, Mohan JS. Screening of plants for their potential antibacterial activity against Staphylococcus and Salmonella spp. Nat Prod Rad. 2007;6:301–5. [Google Scholar]
- 29.Khuda F, Iqbal Z, Khan A, Nasir ZF, Khan MS. Validation of some of the ethnopharmacological uses of Xanthium strumarium and Duchesnea indica. Pak J Bot. 2012;44:1199–201. [Google Scholar]
- 30.Bruneton J. France: Lavoisiler Publishing Co; 1995. Pharmacognosy, Phytochemistry, Medicinal Plants; pp. 265–380. [Google Scholar]
- 31.Suresh PK, Sucheta S, Sudarshana VD, Selvamani P, Latha S. Antioxidant activity in some selected Indian medicinal plants. Afr J Biotechnol. 2008;7:1826–8. [Google Scholar]
- 32.Rad JS, Alfatemi SMH, Rad MS, Iriti M. Free Radical Scavenging and Antioxidant Activities of Different Parts of Nitraria schoberi L. TBAP; 2014;4:44–51. [Google Scholar]
- 33.Sarla S, Prakash MA, Apeksha R, Subhash C. Free radical scavenging (DPPH) and ferric reducing ability (FRAP) of Aphanamixis polystachya (Wall) Parker. Int J Drug Dev Res. 2011;3:271–4. [Google Scholar]
- 34.Kamboj A, Atri P, Saluja AK. Phytochemical screening, in vitro evaluation of antioxidant and free radical scavenging activity of leaves, stems and roots of Xanthium strumarium L., (Compositeae) Br J Pharm Res. 2014;4:1–22. [Google Scholar]


