Abstract
Mentha longifolia (wild mint) is a popular folk remedy. Some parts of this plant have been used in traditional medicine of Iran and other countries. Many studies have shown various pharmacological and therapeutic effects of the plant. Our aim in preparing this study was to review the traditional uses of M. longifolia together with the pharmacological and therapeutic effects of its entire extract and major compounds. Mentha longifolia is an herb with a wide range of pharmacological properties such as antimicrobial, gastrointestinal, and nervous system effects. Pulegone is the main compound of the plant responsible for most of its pharmacological effects followed by menthone, isomenthone, menthol, 1, 8-cineole, borneol, and piperitenone. Moreover, the plant may dose-dependently exert toxic effects in different systems of the body. Based on the review of various studies, it can be concluded that M. longifolia is a potential natural source for the development of new drugs. However, further studies are required to determine the precise quality and safety of the plant to be used by clinicians.
KEY WORDS: Mentha longifolia, menthol, pharmacological effects, traditional use
INTRODUCTION
The wild mint (Mentha longifolia L. family Lamiaceae) grows extensively in Mediterranean regions, Europe, Australia, and North Africa.[1,2,3] The plant is a variable perennial with a peppermint-scented aroma. It has a creeping rhizome with straight to creeping stems 40-120 cm in height. The leaves are oblong-elliptical to lanceolate, thinly to densely tomentose, green to greyish-green above and white below. The flowers are 3-5 mm long, lilac, purplish, or white, produced in dense clusters on tall, branched, and tapering spikes. M. longifolia is used in the pharmaceutical, tobacco and food industries and particularly in cosmetology. Different parts of the plant including its leaves, flower, stem, bark, and seeds have been also used widely in traditional folk medicine as antimicrobial, carminative, stimulant, antispasmodic and for the treatment of various diseases such as headaches and digestive disorders [Table 1].[4] In pharmacological research, there is enough indication for different biological effects of M. longifolia [Table 2] and the chemical compounds present in the essential oil of the plant.
Table 1.
Traditional uses of M. longifolia
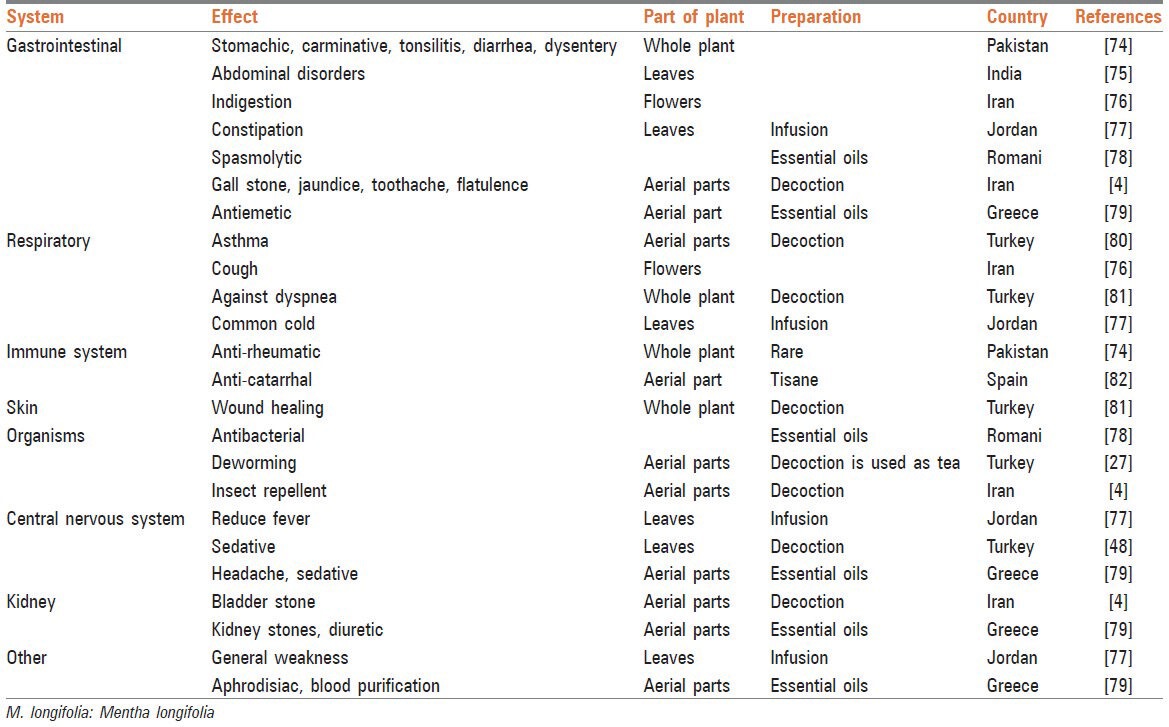
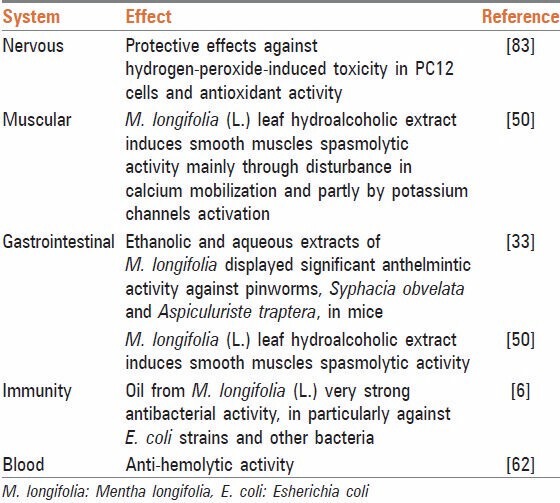
Table 2.
The pharmacological effects of M. longifolia (L.)
Studies carried out on the chemical composition of the plant have shown that the main chemical compounds present in M. longifolia essential oil are monoterpenes [Figure 1], particularly oxygenated ones such as pulegone, menthone, isomenthone, menthol, 1,8-cineole, borneol, and piperitenone oxide.[5] Among them, menthol is the most important component responsible for most of the pharmacological effects of the plant.[6,7] It is a waxy, crystalline substance, clear or white in color, which is solid at room temperature and melts at slightly high temperatures. Mentha is also found in the essential oils of other members of the mint family (Mentha spp.) such as peppermint and horse mint. Gas chromatography mass spectrometry analysis has shown that the main compounds within essential oil of M. longifolia are: Menthol (19.4-32.5%), menthone (20.7-28.8%), pulegone (7.8-17.8%), 1,8-cineole (5.6-10.8%), which have imperative roles in various effects of this plant.[8] This article reviews the pharmacological effects of the total extract [Table 2] and the most active ingredient [Table 3] of M. longifolia (menthol) and its applications in traditional folk medicine [Table 1].
Figure 1.
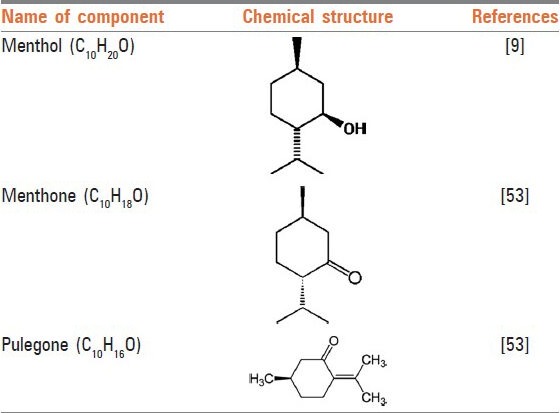
Structures of some active constituents of Mentha longifolia
Table 3.
The pharmacological effects of menthol
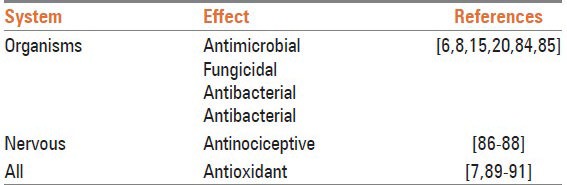
ANTIMICROBIAL ACTIVITY
Usage of M. longifolia in the treatment of throat irritation, mouth and sore throat is widespread.[9] Studies have shown that plants of the genus Mentha possess significant antimicrobial activities,[6] mainly due to the presence of oxygenated monoterpenes in their chemical composition.[10,11,12,13] The essential oil of M. longifolia has shown interesting antimicrobial activity against Escherichia coli, Salmonella typhimurium,[14] Listeria monocytogenes, Aspergillus flavus, Botrytis cinerea, Fusarium oxysporum, Pseudomonas aeruginosa, Aspergillus niger,[15] Trichophyton longifusus, Microsporm canis,[16] and Mucor ramamnianus.[17] The most sensitive micromycetes against the extract of this plant were shown to be Cladosporium fulvum, Penicillium ochrochloron, and Cladosporium cladosporioides with a lethal dose of 2.5 μL/mL.[6] A clinical study of methanolic extract and essential oil of M. longifolia showed that the essential oil has stronger and broader spectrum of antimicrobial activity compared with the methanolic extract. In another in vitro study, the anti-protozoal effect of ethanolic extract of M. longifolia against Entamoeba histolytica and Giardia duodenalis trophozoites was evaluated.[18] The essential oil of the plant showed fungistatic and fungicidal activity that was significantly higher than that of the costlier fungicide bifonazole.[6] Menthol has been shown to be an antimicrobial and antifungal agent against ringworm and other fungal infestations of different kinds.[11,12,13] Anticandidial effect of menthol against Candida albicans (zone of inhibition range: 7.1-18.5 mm; minimal inhibitory concentration (MIC): 125.0 μg/mL) is comparable to amphotericin B (zone of inhibition: 10.2 mm; MIC: 7.8 μg/mL). Menthol is also effective against dental bacteria.[9] It has commonly been reported that Gram-positive bacteria are more vulnerable to essential oils of the plant than Gram-negative bacteria.[11,13,19,20] However, alkaloids isolated from M. longifolia have pronounced effects against growth of Gram-negative bacteria such as E. coli.[21] One study on five flavonoids separated from M. longifolia extract showed that the quercetin-3-O-glucoside had the maximum antibacterial activity among the flavonoids tested.[22] Apigenin is a common dietary flavonoid that is found in Mentha spp. and has many biological effects including antimicrobial activity.[23,24,25] Other studies have shown the antimicrobial activity of M. longifolia against the two yeasts C. albicans and Saccharomyces cerevisiae (diameter of the inhibition zones in 25 and 28 mm respectively).[26,27,28] In vitro anti-Vibrio effects of the essential oils obtained from M. longifolia have been also shown against Vibrio spp. This effect has been seen in administration of M. longifolia in cases of gastrointestinal and extra-intestinal troubles related to the consumption of insufficiently cooked seafood or contact with contaminated sea water with Vibrio alginolyticus, Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio fluvialis strains.[29] There is a report that piperitone from M. longifolia reduces the nitrofurantoin resistance of strains of Enterobacteriaceae and increases the value of the antimicrobial activity of nitrofurantoin, which is used for the treatment of urinary tract infections.[30] Pulegone is considered as the main composition of M. longifolia against molds and against Klebsiella pneumoniae.[5,31] Combination of nisin and the essential oil of M. longifolia showed significant inhibitory effect on the growth of the vegetative forms of Bacillus subtilis at 25°C. Nevertheless, the sole essential oil of the plant did not expressively inhibit bacterial growth at 25°C.[32] Ethanolic and aqueous extracts from M. longifolia showed significant anthelmintic activity against pinworms, Syphacia obvelata and Aspiculuris tetraptera, in mice.[33] In one study, M. longifolia was found highly effective (>88%) in spore germination tests against some fungi.[34] Many studies have also reported the insecticidal activity of M. Longifolia. Feeding on this plant was found to cause death in Chrysolina herbacea. Piperitenoneoxide is the main integral that is attributed to the insecticidal activity of the plant (LC50, 9.95 mg/L).[35] It is similarly shown that M. longifolia essential oil has 100% repellence against Sitophilus zeamais (10, 15, 20 days old),[36,37] and Tribolium castaneum (25 days old).[38] Two studies have reported the high efficacy of the ethanolic extract of M. longifolia against third- and fourth-instar larvae of house mosquito Culex pipiens (LC50-26.8 ppm),[39] and against Sitophilus oryzae (24.2% repellency).[40]
EFFECTS ON NERVOUS SYSTEM
It was shown in a study that the aqueous leaf extract of M. longifolia has antinociceptive and antipyretic properties. Comparatively high LD (50) values obtained for oral and intraperitoneal administration of the plant extract confirmed that the plant extract is nontoxic to mice.[41] In another study, methanolic extracts of M. longifolia were tested for their possible protective effects against hydrogen-peroxide-induced toxicity in PC12 cells, antioxidant activity (by 2, 2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) [ABTS] and xanthine/xanthine oxidase methods) and neurochemical properties (monoamine oxidase A (MAO-A) inhibition, acetylcholinesterase inhibition and affinity to the gamma-aminobutyric acid (A) receptors). This plant exhibited antioxidant and MAO-A inhibitory activities.[42] M. longifolia essential oil exhibited a stronger central nervous system (CNS) depressant effect.[43] The effect of M. longifolia crude ethanol extract, as well as fractions rich in apigenin glycosides, luteolin glycosides and phenolic acids have been also examined on CNS function. Phenolic acids are found to possess noteworthy spasmodic, choleretic and CNS simulative effects.[44]
EFFECTS ON GASTROINTESTINAL SYSTEM
Mentha longifolia leaves are used in herbal recipes, which are used for gastrointestinal disorders. Here, the leaves of M. longifolia are locally boiled in water along with cardamom seed or leaves powder is given along with green tea to children. It is used as antiemetic, particularly in chronic diarrhea. M. longifolia is used as a carminative in gas trouble and eaten in the form of chutney, especially in summer along with butter to stop diarrhea.[45,46,47] It is similarly used for the treatment of abdominal pain.[48] The leaf extract of M. longifolia, exerts relaxant effects on intestinal smooth muscle, consistent with the traditional use of the plant to treat gastrointestinal disorders such as diarrhea and colic. This plant exhibits spasmolytic activity mainly through varying calcium mobilization and partly by potassium channels activation due to the presence of calcium channel blocking constituents.[49,50] In a castor oil induced diarrheal model, the crude extract of M. longifolia at doses of 100-1000 mg/kg, provided 31-80% protection, similar to loperamide. The calcium channel blocking activity was further confirmed when pretreatment of the tissue with M. longifolia crude extract caused a rightward shift in the Ca++ concentration-response curves, similar to verapamil. Activity-directed fractionation revealed that the petroleum spirit fraction was more potent than the parent crude extract and aqueous fraction.[49] In isolated ruminal and abomasal preparations, essence of M. longofolia (0.1-100 g/mL) exhibited a weak spasmogenic effect followed by relaxation and complete (P < 0.05) abolition of the spontaneous contraction at the highest dose (1000 g/mL). In contrast, rat ileum only showed a dose-dependent relaxation effect, and tissues preincubated with essence reduced the acetylcholine (ACh)-induced contraction. Essence (1000 g/mL) meaningfully repressed the effect of ACh suggesting that the effect may be mediated via cholinergic receptors on smooth muscle. The essence profoundly changed gastrointestinal smooth muscle contraction in a dose-dependent and tissue-specific manner.[51] Another study examined the effects of piperitenone oxide, an important chemical constituent of the essential oil of many Mentha spp. such as M. longifolia, M. spicata, M. rotundifolia, M. suaveolens and M. villosa on the guinea pig ileum. Piperitenone oxide (30-740 μg/ml) relaxed basal tonus without significant alteration in the resting membrane potential.[52]
ANTIOXIDANT EFFECT
Gulluce et al.[7] designed a study to evaluate the antioxidant activities of the essential oil and methanol extract of M. Longifolia. The extract showed much better activity than the essential oil in antioxidant activity assays employed, e.g. in the inhibition of free radical 2, 2-diphenyl-1-picrylhydrazyl (DPPH) and β carotene/linoleic acid systems. Other studies presented the phenolic compounds as the main cause of better antioxidant effect of methanol extract than the essential oil.[15] There is a positive connection between antioxidant activity potential and amount of phenolic compounds.[28] The antioxidant activity by ABTS assay showed IC50 values of 476.3 ± 11.7 for M. longifolia.[5] In some studies, apigenin derivatives are determined as antioxidative molecules.[54,55,56] In another study, the free radical-scavenging potential (1, 1-diphenyl-2-picrylhydrazyl scavenging activity) of nine Mentha spp. was investigated to evaluate and explore new potential sources for natural antioxidants. The activity was performed after different time intervals with the incubation period of 30 min. The methanolic extracts of M. longifolia showed significant antioxidant activity (79%).[57] Berselli et al.[58] have shown the efficacy of a 1 h preincubation with the highest nontoxic dose of a characterized M. longifolia extract (80 μg/mL) in protecting human keratinocytes (NCTC2544) from chemically tempted oxidative stress (500 μM H2O2 for 2, 16, and 24 h). Cellular viability was meaningfully protected by mint, which limited protein and DNA damage, reduced lipid peroxidation, and conserved glutathione and superoxide dismutase activity in the shorter phases of oxidative stress induction. Weigh level, a mixture of extract of four plants (Alchemilla vulgaris, Olea europaea, M. longifolia and Cuminum cyminum) used in traditional Arabic and Islamic medicine as well as in European herbal medicine, showed noteworthy antioxidant properties at so low absorptions (10 μg/mL) as measured by the lipid peroxidation technique.[59] In another study, monoterpene ketones (menthone and isomenthone) were shown as the most powerful scavenging complexes present in the essential oils of M. longifolia.[6] The antioxidant effect of the contents of the plant varies when different methods of extraction are used. For instance, in one study the highest antioxidant activity determined by the ferric decreasing antioxidant property (FRAP) and DPPH assays was attributed to extracts taken from naturally dried herbs (2.76 ± 0.15 m/mol Fe2+/Mg of the dry extract and EC50 = 0.022 ± 0.001 mg/ml) due to the highest general content of phenols (113.8 ± 2.0 mg of gallic acid/g of the dry extract) and flavonoids. On the other hand, the lowest antioxidant activity was elicited from the extracts of herbs dried in the laboratory oven (1.13 ± 0.11 m/mol Fe2+/mg of the dry extract and EC50 = 0.033 ± 0.001 mg/mL).[60,61] This shows the importance of the methods by which the plant is dried off before preparation of the extracts. Hydro-alcoholic extract of this plant showed good antioxidant activities in several in vitro assay systems including DPPH radical-scavenging system, nitric oxide-scavenging system, Fe2+ chelating ability, linoleic acid model, and scavenging hydrogen-peroxide.[62] In another study, the antioxidant activity of M. longifolia extract was investigated with four different methods such as DPPH, ABTS radical, reducing power assay and FRAP. M. longifolia exhibited the strongest activity as ABTS scavenger compared to DPPH and FRAP.[63] It was shown that high antioxidant activity by tissues of many M. longifolia variants native to Israel resulted in part, from antioxidant activity of rosmarinic acid (RA).[64]
IN VITRO TOXICOLOGICAL EVALUATION
The effects of the essential oil from the leaves of M. longifolia on a few biochemical parameters of Wistar rats were investigated in one study. The oil at 125, 250, 375, and 500 μL/kg of body weight reduced (P < 0.05) the red blood cells and lymphocytes with no definite pattern on the white blood cells and mean cell volume. The optimal doses meaningfully increased the population of neutrophils, monocytes and large unstained cells. In addition, liver-body weight ratio and serum concentrations of cholesterol, triglyceride, high-density lipoprotein-cholesterol, and inorganic phosphate were increased; however, no change was observed in the heart body weight ratio and the levels of serum low-density lipoprotein-cholesterol, Na(+), Ca(2+), Cl(−), K(+), creatinine, and uric acid. The oil at 500 μL/kg of body weight also increased the kidney-body weight ratio. In contrast, the oil reduced the serum urea and atherogenic index. The total and conjugated bilirubin, together with the entire protein and albumin, in the serum increased only with oil at 125 μL/kg of body weight. The serum alkaline phosphatase activity also increased with no significant change in those of gamma-glutamyltransferase and alanine and aspartate aminotransferase. The results specify dose- and parameter-specific effect of the essential oil. Although the essential oil from M. longifolia leaves may not predispose to atherosclerosis, it may raise the practical activity of the rat liver at the lowest dose investigated. Therefore, the essential oil from M. longifolia may not be completely “safe” at the doses investigated.[65] In vitro cytotoxicity test was also performed by brine shrimp lethality bioassay. At 40 μg/mL, which was the least attentiveness used, the oil was not toxic; all the brine shrimps survived. While maximum mortalities happened at 200 μg/mL and the least mortalities were observed at 40 μg/mL.[66] Due to the major decrease of the potentially harmful pulegone and menthone by oven-drying, it is recommended that this herb should be oven-dried or cooked before consumption in order to reduce toxicity. Eating of the raw plant should be discouraged, particularly in patients with a history of liver disease or those taking cytochrome P450-inducing drugs.[67] Acute Toxicity effect of flavonoids extracted from M. longifolia was measured, and the quercetin-3-O-glucoside had the lowest values with an LD of 5 mg/kg. The apigenin and luteolin-7-O-glucoside, luteolin-7, 3’-O-diglucoside had the same effect with an LD of 4 mg/kg. The kaempferol-3-O-glucoside showed an LD of 3 mg/kg and was thus considered as the most toxic compound among the tested flavonoids.[22]
CYTOTOXIC ACTIVITY
Three flavonoids, apigenin-7-O-glucoside, apigenin- 7-O-rutinoside, and apigenin-7-O-glucuronide, were isolated from M. longifolia by using E. coli WP2 genotoxicity assay directed fractionation procedures. The mutagenic and anti-mutagenic properties of each flavonoid were evaluated using the same genotoxicity assay. Apigenin-7-O-glucoside, apigenin-7-O-rutinoside, and apigenin-7-O-glucuronide were shown to possess significant anti-mutagenic activity against 2-AF and N-methyl-N-nitro-N′nitrosoguanidine induced mutagenicity. The response was dose-dependent, and the inhibition rates were between 25.3% (apigenin-7-O-glucoside with S9-2.0 μM/plate) and 59.0% (apigenin-7-O-rutinoside without S9-2.0 μM/plate). Apigenin derivatives can be thought as genetically safe at tested concentrations since they did not show a mutagenic activity.[56] The cytotoxicity of M. longifolia essential oil with seasonal variation was tested on breast cancer (MCF-7) and prostate cancer (LNCaP) cell lines using the MTT assay. The cytotoxicity of the essential oils against MCF-7 showed an IC50 of 45.2 and 50.6 μg/mL for summer and winter samples, correspondingly and against LNCaP the IC50 values were 43.5 and 52.0 μg/mL, respectively.[10] In another study that was designed to assess the mutagenic and antimutagenic activities of luteolin derivatives (luteolin 7-O-glucoside, luteolin 7-O-rutinoside and luteolin 7-O-glucuronide) isolated from M. longifolia by using Ames Salmonella test (TA 1535 and TA1537 strains), The highest inhibition rates for luteolin 7-O-glucoside, luteolin 7-O-rutinoside and luteolin 7-O-glucuronide on TA1537 strain were 84.03%, 87.63% and 67.77%, respectively. Similarly, in the antimutagenicity assays performed with the TA1535 strain, the inhibition rates for luteolin 7-O-glucoside and luteolin 7-O-rutinoside was 23.86% and 23.76% respectively.[68] The possible antimutagenic potential of apigenin 7-O-rutinoside (A7R) was examined against mutagens ethyl methane sulfonate (EMS) and acridine (AC) in an eukaryotic cell system S. cerevisiae RS112. The results showed that A7R has dissimilar inhibition rates against EMS and AC-induced mutagenicity. Therefore, the properties of A7R are of excessive pharmacological importance and might be useful for decreasing the risk of reactive oxygen species-related diseases.[69] In another similar study on the mutagenic and anti-mutagenic activities of RA, a phenolic compound isolated from M. longifolia the possible anti-mutagenic potential of RA was examined against EMS and AC in S. cerevisiae RS112. Only one concentration of RA showed a mutagenic effect in the highest concentrations used. The lower concentrations of RA meaningfully subdued EMS and AC-induced mutations. The highest inhibition rates in yeast DEL assay ranged from 10% (4 μM/mL concentration in EMS-induced DEL events) to 63.3% (2 μM/mL concentration in AC-induced DEL events).[70]
NUTRITIONAL USAGE
The Van Herby Cheese is a traditional milk product, which includes local herb species in eastern Turkey. M. longifolia had the highest lead concentration of 1.69 mg/kg among the herbs present in the cheese.[71] The effects of M. longifolia essential oil in concentrations of 0, 50, 150 and 300 ppm and Lactobacillus casei (108-109 CFU/mL) on the growth of Staphylococcus aureus and L. monocytogenes during the manufacturing, ripening and storage of Iranian white-brined cheese was investigated in one study. The development of the two pathogens was significantly reduced (P < 0.01) by both ethylene oxide (EO) concentrations ≥50 ppm and the probiotic and their combination in the standard manufacturing and storage process conditions of the cheese. In addition, the treatment containing 150 ppm of this EO combined with probiotic had a favorable inhibitory effect on the growth of two pathogenic micro-organisms and also was the most appropriate treatment in sensory assessment.[72] M. longifolia essential oil has been also shown to have a relation with viability and cellular ultrastructure of L. casei during ripening and storage of probiotic Feta cheese. The essential oil in the concentrations ranging from 0.0% to 0.03% was trialed: the 0.03% treatment resulted in the highest viability of L. casei and the lowest pH value compared with other treatments (P < 0.05). Electron microscopy has shown that essential oil caused no harm to L. casei. These data show favorable effects of M. longifolia on best maintenance of L. casei at the end of cheese storage period.[73]
CONCLUSION
Mentha longifolia demonstrates a wide range of antibiotic activity against various bacteria, yeasts, insects, etc. The essential oil is a more potent antimicrobial than the hydroalcoholic extract. It is also a potential antinociceptive and carminative. Although high LD50 values have been calculated for oral and intraperitoneal administration of the extract, it has been shown that the plant is not thoroughly safe. M. longifolia may raise the liver activity even at the safest doses administered in rat. Pulegone and menthone are the major harmful compounds present in the plant. Since oven-drying decreases the amount of these compounds in the plant, it is recommended that this plant be oven-dried or cooked before consumption in order to make it safer. Moreover, care should be taken when this plant is consumed along with drugs which induce P450 enzymes. This review reveals the potential of this plant to be used as a novel source for the development of new drugs; however, more studies are required to determine its precise quality and quantity to be used in humans as a therapeutic source.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Harley RM, Brighton CA. Chromosome number in the genus Mentha L. Bot J Linn Soc. 1977;74:71–96. [Google Scholar]
- 2.Chambers H. Mentha: Genetic resources and the collection at USDA-ARSNCGR-Corvallis. Lam Newsl. 1992;1:3–4. [Google Scholar]
- 3.Stamenkovic V. Revised and Expanded. 2nd ed. Leskovac: NIGP Trend; 2005. Our Non-Harming Medicinal Herbs. [Google Scholar]
- 4.Naghibi F, Mosaddegh M, Motamed SM, Ghorbani A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. Iran J Pharm Res. 2005;4:63–79. [Google Scholar]
- 5.Mkaddem M, Bouajila J, Ennajar M, Lebrihi A, Mathieu F, Romdhane M. Chemical composition and antimicrobial and antioxidant activities of Mentha (longifolia L. and viridis) essential oils. J Food Sci. 2009;74:M358–63. doi: 10.1111/j.1750-3841.2009.01272.x. [DOI] [PubMed] [Google Scholar]
- 6.Mimica-Dukic N, Bozin B, Sokovic M, Mihajlovic B, Matavulj M. Antimicrobial and antioxidant activities of three Mentha species essential oils. Planta Med. 2003;69:413–9. doi: 10.1055/s-2003-39704. [DOI] [PubMed] [Google Scholar]
- 7.Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, et al. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103:1449–56. [Google Scholar]
- 8.Hajlaoui H, Snoussi M, Ben Jannet H, Mighri Z, Bakhrouf A. Comparison of chemical composition and antimicrobial activities of Mentha longifolia L. ssp. longifolia essential oil from two Tunisian localities (Gabes and Sidi Bouzid) Ann Microbiol. 2008;58:513–20. [Google Scholar]
- 9.Al-Bayati FA. Isolation and identification of antimicrobial compound from Mentha longifolia L. leaves grown wild in Iraq. Ann Clin Microbiol Antimicrob. 2009;8:20. doi: 10.1186/1476-0711-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain AI, Anwar F, Nigam PS, Ashraf M, Gilani AH. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J Sci Food Agric. 2010;90:1827–36. doi: 10.1002/jsfa.4021. [DOI] [PubMed] [Google Scholar]
- 11.Karaman I, Sahin F, Güllüce M, Ogütçü H, Sengül M, Adigüzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol. 2003;85:231–5. doi: 10.1016/s0378-8741(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Kitic D, Jovanovic T, Ristic M, Palic R, Stojanovic G. Chemical composition and antimicrobial activity of the essential oil of Calamintha nepeta (L.) Savi ssp. glandulosa (Req.) P.W. Ball from Montenegro. J Essent Oil Res. 2002;14:150–2. [Google Scholar]
- 13.Sahin F, Karaman I, Güllüce M, Ogütçü H, Sengül M, Adigüzel A, et al. Evaluation of antimicrobial activities of Satureja hortensis L. J Ethnopharmacol. 2003;87:61–5. doi: 10.1016/s0378-8741(03)00110-7. [DOI] [PubMed] [Google Scholar]
- 14.Hafedh H, Fethi BA, Mejdi S, Emira N, Amina B. Effect of Mentha longifolia L. ssp longifolia essential oil on the morphology of four pathogenic bacteria visualized by atomic force microscopy. Afr J Microbiol Res. 2010;4:1122–7. [Google Scholar]
- 15.Hajlaoui H, Trabelsi N, Noumi E, Snoussi M, Fallah H, Ksouri R, et al. Biological activities of the essential oils and methanol extract of tow cultivated mint species (Mentha longifolia and Mentha pulegium) used in the Tunisian folkloric medicine. World J Microbiol Biotechnol. 2009;25:2227–38. [Google Scholar]
- 16.Khattak S, Rehman SU, Khan T, Shah HU, Shad AA, Ahmad M. In vitro screening for biological pharmacological effects of indigenous medicinal plants, Mentha longifolia and Aloe vera. J Chem Soc Pak. 2004;26:248–51. [Google Scholar]
- 17.Gibriel YA, Hamza AS, Gibriel AY, Mohsen SM. In vivo effect of mint (Mentha viridis) essential oil on growth and aflatoxin production by Aspergillus flavus isolated from stored corn. J Food Saf. 2011;31:445–51. [Google Scholar]
- 18.El-Badry AA, Al-Ali KH, El-Badry YA. Activity of Mentha longifolia and Ocimum basilicum against Entamoeba histolytica and Giardia duodenalis. Sci Parasitol. 2010;11:109–17. [Google Scholar]
- 19.Mann CM, Cox SD, Markham JL. The outer membrane of Pseudomonas aeruginosa NCTC 6749 contributes to its tolerance to the essential oil of Melaleuca alternifolia (tea tree oil) Lett Appl Microbiol. 2000;30:294–7. doi: 10.1046/j.1472-765x.2000.00712.x. [DOI] [PubMed] [Google Scholar]
- 20.Cosentino S, Tuberoso CI, Pisano B, Satta M, Mascia V, Arzedi E, et al. In-vitro antimicrobial activity and chemical composition of sardinian thymus essential oils. Lett Appl Microbiol. 1999;29:130–5. doi: 10.1046/j.1472-765x.1999.00605.x. [DOI] [PubMed] [Google Scholar]
- 21.Al-Younis NK, Argushy ZM. Antibacterial evaluation of some medicinal plants from Kurdistan region. J Duhok Univ. 2009;12:256–61. [Google Scholar]
- 22.Akroum S, Bendjeddou D, Dand S, Lalaoui K. Antibacterial activity and acute toxicity effect of flavonoids extracted from Mentha longifolia. Am Eurasian J Sci Res. 2009;4:93–6. [Google Scholar]
- 23.Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg Med Chem. 2003;11:1995–2000. doi: 10.1016/s0968-0896(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa M, Hisama M. Antimutagenic activity of flavonoids from Chrysanthemum morifolium. Biosci Biotechnol Biochem. 2003;67:2091–9. doi: 10.1271/bbb.67.2091. [DOI] [PubMed] [Google Scholar]
- 25.Xiao M, Shao YD, Yan WD, Zhang ZZ. Measurement and correlation of solubilities of apigenin and apigenin7-O-rhamnosylglucoside in seven solvents at different temperatures. J Chem Thermodyn. 2011;43:240–3. [Google Scholar]
- 26.Sarac N, Ugur A. Antimicrobial activities and usage in folkloric medicine of some Lamiaceae species growing in Mugla, Turkey. Eurasian J Bio Sci. 2007;4:28–37. [Google Scholar]
- 27.Yigit D, Yigit N, Ozgen U. An investigation on the anticandidal activity of some traditional medicinal plants in Turkey. Mycoses. 2009;52:135–40. doi: 10.1111/j.1439-0507.2008.01552.x. [DOI] [PubMed] [Google Scholar]
- 28.Gursoy N, Sihoglu-Tepe A, Tepe B. Determination of in vitro antioxidative and antimicrobial properties and total phenolic contents of Ziziphora clinopodioides, Cyclotrichium niveum, and Mentha longifolia ssp. typhoides var. typhoides. J Med Food. 2009;12:684–9. doi: 10.1089/jmf.2008.0102. [DOI] [PubMed] [Google Scholar]
- 29.Snoussi M, Hajlaoui H, Noumi E, Usai D, Sechi LA, Zanetti S, Bakhrouf A. In vitro anti-Vibrio spp. activity and chemical composition of some Tunisian aromatic plants. World J Microbiol Biotechnol. 2008;24:3071–6. [Google Scholar]
- 30.Shahverdi AR, Rafii F, Tavassoli F, Bagheri M, Attar F, Ghahraman A. Piperitone from Mentha longifolia var. chorodictya Rech F. reduces the nitrofurantoin resistance of strains of enterobacteriaceae. Phytother Res. 2004;18:911–4. doi: 10.1002/ptr.1566. [DOI] [PubMed] [Google Scholar]
- 31.Lang G, Buchbauer G. A review on recent research results (2008-2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr J. 2011;27:13–39. [Google Scholar]
- 32.Pajohi MR, Tajik H, Farshid AA, Basti AA, Hadian M. Effects of Mentha longifolia L. essential oil and nisin alone and in combination on Bacillus cereus and Bacillus subtilis in a food model and bacterial ultrastructural changes. Foodborne Pathog Dis. 2011;8:283–90. doi: 10.1089/fpd.2010.0675. [DOI] [PubMed] [Google Scholar]
- 33.Kozan E, Küpeli E, Yesilada E. Evaluation of some plants used in Turkish folk medicine against parasitic infections for their in vivo anthelmintic activity. J Ethnopharmacol. 2006;108:211–6. doi: 10.1016/j.jep.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Abou-Jawdah Y, Sobh H, Salameh A. Antimycotic activities of selected plant flora, growing wild in Lebanon, against phytopathogenic fungi. J Agric Food Chem. 2002;50:3208–13. doi: 10.1021/jf0115490. [DOI] [PubMed] [Google Scholar]
- 35.Cordero C, Zebelo SA, Gnavi G, Griglione A, Bicchi C, Maffei ME, et al. HS-SPME-GC×GC-qMS volatile metabolite profiling of Chrysolina herbacea frass and Mentha spp. leaves. Anal Bioanal Chem. 2012;402:1941–52. doi: 10.1007/s00216-011-5600-4. [DOI] [PubMed] [Google Scholar]
- 36.Odeyemi OO, Masika P, Afolayan AJ. Insecticidal activities of essential oil from the leaves of Mentha longifolia L. subsp. capensis against Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae) Afr Entomol. 2008;16:220–5. [Google Scholar]
- 37.Kumar A, Shukla R, Singh P, Singh AK, Dubey NK. Use of essential oil from Mentha arvensis L. to control storage moulds and insects in stored chickpea. J Sci Food Agric. 2009;89:2643–9. [Google Scholar]
- 38.Pascual-Villalobos MJ, Robledo A. Screening for anti-insect activity in Mediterranean plants. Ind Crops Prod. 1998;8:183–94. [Google Scholar]
- 39.Cetin H, Cinbilgel I, Yanikoglu A, Gokceoglu M. Larvicidal activity of some Labiatae (Lamiaceae) plant extracts from Turkey. Phytother Res. 2006;20:1088–90. doi: 10.1002/ptr.2004. [DOI] [PubMed] [Google Scholar]
- 40.Saljoqi AU, Afridi MK, Khan SA, Rehman S. Effects of six plant extracts on rice weevil Sitophilus oryzae L. in the stored wheat grains. J Agric Biol Sci. 2006;1:1–5. [Google Scholar]
- 41.Amabeoku GJ, Erasmus SJ, Ojewole JA, Mukinda JT. Antipyretic and antinociceptive properties of Mentha longifolia Huds. (Lamiaceae) leaf aqueous extract in rats and mice. Methods Find Exp Clin Pharmacol. 2009;31:645–9. doi: 10.1358/mf.2009.31.10.1441861. [DOI] [PubMed] [Google Scholar]
- 42.López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. Neuroprotective and neurochemical properties of mint extracts. Phytother Res. 2010;24:869–74. doi: 10.1002/ptr.3037. [DOI] [PubMed] [Google Scholar]
- 43.Pérez Raya MD, Utrilla MP, Navarro MC, Jiménez J. CNS activity of Mentha rotundifolia and Mentha longifolia essential oil in mice and rats. Phytother Res. 2006;4:232–4. [Google Scholar]
- 44.Mimica-Dukiç N, Jakovljeviç V, Mira P, Gasiç O, Zabo A. Pharmacological study of Mentha longifolia phenolic extracts. Pharm Biol. 1996;34:359–64. [Google Scholar]
- 45.Jan S, Khan MA, Uddin S, Murad W, Hussain M, Ghani A. Herbal recipes used for gastrointestinal disorders in Kaghan valley, nwfp, Pakistan. Pak J Weed Sci Res. 2008;14:169–200. [Google Scholar]
- 46.Khan SW, Khatoon S. Ethnobotanical studies on some useful herbs of Haramosh and Bugrote valleys in Gilbit, Northern areas of Pakistan. Pak J Bot. 2008;40:43–58. [Google Scholar]
- 47.Hussain K, Shahazad A, Zia-ul-Hussnain S. An ethnobotanical survey of important wild medicinal plants of Hattar District Haripur, Pakistan. Ethnobotanical Lealf. 2008;12:29–35. [Google Scholar]
- 48.Cakilcioglu U, Khatun S, Turkoglu I, Hayta S. Ethnopharmacological survey of medicinal plants in Maden (Elazig-Turkey) J Ethnopharmacol. 2011;137:469–86. doi: 10.1016/j.jep.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 49.Shah AJ, Bhulani NN, Khan SH, Ur Rehman N, Gilani AH. Calcium channel blocking activity of Mentha longifolia L. explains its medicinal use in diarrhoea and gut spasm. Phytother Res. 2010;24:1392–7. doi: 10.1002/ptr.3263. [DOI] [PubMed] [Google Scholar]
- 50.Naseri MK, Naseri ZG, Mohammadian M, Birgani MO. Ileal relaxation induced by Mentha longifolia (L.) leaf extract in rat. Pak J Biol Sci. 2008;11:1594–9. doi: 10.3923/pjbs.2008.1594.1599. [DOI] [PubMed] [Google Scholar]
- 51.Jalilzadeh AG, Maham M, Dalir-Naghadeh B, Kheiri F. Effects of Mentha longifolia essential oil on ruminal and abomasal longitudinal smooth muscle in sheep. J Essent Oil Res. 2012;24:61–9. [Google Scholar]
- 52.Sousa PJ, Magalhães PJ, Lima CC, Oliveira VS, Leal-Cardoso JH. Effects of piperitenone oxide on the intestinal smooth muscle of the guinea pig. Braz J Med Biol Res. 1997;30:787–91. doi: 10.1590/s0100-879x1997000600014. [DOI] [PubMed] [Google Scholar]
- 53.Stanfill SB, Calafat AM, Brown CR, Polzin GM, Chiang JM, Watson CH, et al. Concentrations of nine alkenylbenzenes, coumarin, piperonal and pulegone in Indian bidi cigarette tobacco. Food Chem Toxicol. 2003;41:303–17. doi: 10.1016/s0278-6915(02)00230-2. [DOI] [PubMed] [Google Scholar]
- 54.Nagy M, Krizková L, Mucaji P, Kontseková Z, Sersen F, Krajcovic J. Antimutagenic activity and radical scavenging activity of water infusions and phenolics from ligustrum plants leaves. Molecules. 2009;14:509–18. doi: 10.3390/molecules14010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romanová D, Vachálková A, Cipák L, Ovesná Z, Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48:104–7. [PubMed] [Google Scholar]
- 56.Baris O, Karadayi M, Yanmis D, Guvenalp Z, Bal T, Gulluce M. Isolation of 3 flavonoids from Mentha longifolia (L.) Hudson subsp. longifolia and determination of their genotoxic potentials by using the E. coli WP2 test system. J Food Sci. 2011;76:T212–7. doi: 10.1111/j.1750-3841.2011.02405.x. [DOI] [PubMed] [Google Scholar]
- 57.Ahmad N, Fazal H, Ahmad I, Abbasi BH. Free radical scavenging (DPPH) potential in nine Mentha species. Toxicol Ind Health. 2012;28:83–9. doi: 10.1177/0748233711407238. [DOI] [PubMed] [Google Scholar]
- 58.Berselli PV, Zava S, Montorfano G, Corsetto PA, Krzyzanowska J, Oleszek W, et al. A mint purified extract protects human keratinocytes from short-term, chemically induced oxidative stress. J Agric Food Chem. 2010;58:11428–34. doi: 10.1021/jf1020285. [DOI] [PubMed] [Google Scholar]
- 59.Said O, Saad B, Fulder S, Khalil K, Kassis E. Weight loss in animals and humans treated with “weighlevel”, a combination of four medicinal plants used in traditional arabic and islamic medicine. Evid Based Complement Alternat Med 2011. 2011:874538. doi: 10.1093/ecam/nen067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stanisavljevic DM, Stojicevic SS, Ðorcevic SM, Zlatkovic BP, Veliĉkovic DT, Karabegovic IT, et al. Antioxidant activity, the content of total phenols and flavonoids in the ethanol extracts of Mentha longifolia (l.) Hudson dried by the use of different techniques. Chem Ind Chem Eng Q. 2012;18:411–20. [Google Scholar]
- 61.Khan FA, Khan A, Hussain J, Khattak MR, Shah SM, Hassan M. Assessment of antioxidant and antibacterial activities of Mentha longifolia. J Pharm Res. 2011;4:2338–9. [Google Scholar]
- 62.Ebrahimzadeh MA, Nabavi SM, Nabavi SF. Antioxidant and antihemolytic activities of Mentha longifolia. Pharmacologyonline. 2010;2:464–71. [Google Scholar]
- 63.Raj JX, Bajpjpai PK, Kumar PG, Murugan PM, Kumar J, Chaurasia OP, et al. Determination of total phenols, free radical scavenging and antibacterial activities of Mentha longifolia Linn. Hudson from the cold desert, Ladakh, India. Pharmacogn J. 2010;2:470–5. [Google Scholar]
- 64.Dudai N, Segey D, Haykin-Frenkel D, Eshel A. Genetic variation of phenolic compounds content essential oil composition and antioxidative activity in Israel-grown Mentha longifolia L. ISHS Acta Hortic. 2006;709:69–78. [Google Scholar]
- 65.Odeyemi OO, Yakubu MT, Masika PJ, Afolayan AJ. Toxicological evaluation of the essential oil from Mentha longifolia L. subsp. capensis leaves in rats. J Med Food. 2009;12:669–74. doi: 10.1089/jmf.2008.0136. [DOI] [PubMed] [Google Scholar]
- 66.Okoh OO, Afolayan AJ. The effects of hydrodistillation and solvent free microwave extraction methods on the chemical composition and toxicity of essential oils from the leaves of Mentha longifolia L. subsp. capensis. Afr J Pharm Pharmacol. 2011;5:2474–8. [Google Scholar]
- 67.Asekun OT, Grierson DS, Afolayan AJ. Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. Capensis. Food Chem. 2007;101:995–8. [Google Scholar]
- 68.Orhan F, Baris Ö, Yanmis D, Bal T, Güvenalp Z, Güllüce M. Isolation of some luteolin derivatives from Mentha longifolia (L.) Hudson subsp. longifolia and determination of their genotoxic potencies. Food Chem. 2012;135:764–9. doi: 10.1016/j.foodchem.2012.04.137. [DOI] [PubMed] [Google Scholar]
- 69.Gulluce M, Orhan F, Adiguzel A, Bal T, Guvenalp Z, Dermirezer LO. Determination of antimutagenic properties of apigenin-7-O-rutinoside, a flavonoid isolated from Mentha longifolia (L.) Huds. ssp. longifolia with yeast DEL assay. Toxicol Ind Health. 2013;29:534–40. doi: 10.1177/0748233712442732. [DOI] [PubMed] [Google Scholar]
- 70.Gulluce M, Yanmis D, Orhan F, Bal T, Karadayi M, ªahin F. Determination of antimutagenic properties of rosmarinic acid, a phenolic compound isolated from Mentha longifolia ssp. longifolia with yeast DEL assay. Microbes Appl Res. 2011;107:526–30. [Google Scholar]
- 71.Tuncturk M, Tuncturk R, Sekeroglu N, Ertus MM, Ozgokce F. Lead concentrations of herbs used in Van Herby cheese. Nat Prod Commun. 2011;6:1473–4. [PubMed] [Google Scholar]
- 72.Ehsani A, Mahmoudi R. Effects of Mentha longifolia L. essential oil and Lactobacillus casei on the organoleptic properties and on the growth of Staphylococcus aureus and Listeria monocytogenes during manufacturing, ripening and storage of Iranian white-brined cheese. Int J Dairy Technol. 2012;66:70–6. [Google Scholar]
- 73.Mahmoudi R, Tajik H, Ehsani A, Farshid AA, Zare P, Hadian M. Effects of Mentha longifolia L. essential oil on viability and cellular ultrastructure of Lactobacillus casei during ripening of probiotic Feta cheese. Int J Dairy Technol. 2012;66:77–82. [Google Scholar]
- 74.Hamayun M, Khan SA, Sohn EY, Lee IJ. Folk medicinal knowledge and conservation status of some economically valued medicinal plants of District Swat, Pakistan. Lyonia. 2006;11:101–13. [Google Scholar]
- 75.Dwivedi S, Dwivedi A, Dwivedi SN. Folk lore uses of some plants by the tribes of Madhya Pradesh with special reference to their conservation. Ethnobotanical Lealf. 2008;12:763–71. [Google Scholar]
- 76.Pirbalouti AG, Malekpoor F, Enteshari S, Yousefi M, Momtaz H, Hamedi B. Antibacterial activity of some folklore medicinal plants used by Bakhtiari tribal in Southwest Iran. Int J Biol. 2010;2:55–63. [Google Scholar]
- 77.Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10:9. doi: 10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spiridon I, Bodirlau R, Teaca CA. Total phenolic content and antioxidant activity of plants used in traditional Romanian herbal medicine. Cent Eur J Biol. 2011;6:388–96. [Google Scholar]
- 79.Karousou R, Balta M, Hanlidou E, Kokkini S. “Mints”, smells and traditional uses in Thessaloniki (Greece) and other Mediterranean countries. J Ethnopharmacol. 2007;109:248–57. doi: 10.1016/j.jep.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 80.Tuzlacı E, Doğan A. Turkish folk medicinal plants, IX: Ovacık (Tunceli) Marmara Pharm J. 2010;14:136–43. [Google Scholar]
- 81.Ozgen U, Kaya Y, Houghton P. Folk medicines in the villages of Ilıca District (Erzurum, Turkey) Turk J Biol. 2012;36:93–106. [Google Scholar]
- 82.Agelet A, Vallès J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or very rare uses of previously known medicinal plants. J Ethnopharmacol. 2003;84:211–27. doi: 10.1016/s0378-8741(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 83.López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI. Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res. 2009;34:1955–61. doi: 10.1007/s11064-009-9981-0. [DOI] [PubMed] [Google Scholar]
- 84.Ghoulami S, Il Idrissi A, Fkih-Tetouani S. Phytochemical study of Mentha longifolia of Morocco. Fitoterapia. 2001;72:596–8. doi: 10.1016/s0367-326x(01)00279-9. [DOI] [PubMed] [Google Scholar]
- 85.Rasooli I, Rezaei MB. Bioactivity and chemical properties of essential oils from Zataria multiflora Boiss and Mentha longifolia (L.) Huds. J Essent Oil Res. 2002;14:141–6. [Google Scholar]
- 86.Santos FA, Rao VS. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 2000;14:240–4. doi: 10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 87.Rice KC, Wilson RS. (-)-3-Isothujone, a small nonnitrogenous molecule with antinociceptive activity in mice. J Med Chem. 1976;19:1054–7. doi: 10.1021/jm00230a015. [DOI] [PubMed] [Google Scholar]
- 88.Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: A natural analgesic compound. Neurosci Lett. 2002;322:145–8. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- 89.Oyedeji OA, Afolayan AJ. Chemical composition and antibacterial activity of the essential oil isolated from South African Mentha longifolia L. subsp. capensis (Thunb.) Briq. J Essent Oil Res. 2006;18:57–9. [Google Scholar]
- 90.Kaur C, Kapoor HP. Anti-oxidant activity and total phenolic content of some Asian vegetables. Int J Food Sci Technol. 2002;37:153–63. [Google Scholar]
- 91.Daferera DJ, Ziogas BN, Pollissiou MG. The effectiveness of plant essential oils on the growth of botrycinera, Fusarium sp. And Clavibacter michiganensis ssp. Michiganensis. Crop Prot. 2003;22:39–44. [Google Scholar]


