Abstract
The present study we investigated the hepatoprotective effects of Olea europaea fruit pulp extract against carbon tetrachloride-induced hepatic damage in experimental mice. Further we explored the antioxidant potential of the extract to substantiate the hepatoprotective properties. Biochemical parameters were analyzed in the serum of experimental mice using respective diagnostic kits. Antioxidant activities were measured following alkyl and hydroxyl radical scavenging assays. Compared with control groups, administration of the extract to carbon tetrachloride-treated mice significantly reduced the elevated serum levels of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase. The carbon tetrachloride-treated morphological changes in hepatocyte architecture were also reversed by extract pretreatment. Further, the carbon tetrachloride-treated increased serum cholesterol levels such as triglyceride and low density/very low-density lipoprotein in the liver were reversed in acute and chronic carbon tetrachloride-treated mice. The extract was also found to significantly increase the serum level of high-density lipoproteins in carbon tetrachloride-treated mice. Furthermore, the extract showed significant in vitro antioxidant actions by scavenging the alkyl and hydroxyl free radicals, substantiating its use in hepatoprotection. The concentration of the extract necessary for 50% inhibition of alkyl and hydroxyl radicals was 72.41 and 52.24 μg/ml, respectively. In conclusion, data from our study suggest that Olea europaea fruit pulp extract could prevent carbon tetrachloride-treated acute and chronic liver degeneration and attenuated the lipid levels elevated by carbon tetrachloride. The hepatoprotective activity exhibited by Olea europaea extract might possibly be through its antioxidant defense mechanisms.
Keywords: Olive fruit pulp, antioxidant, hepatotoxicity, carbon tetrachloride, free radicals
Hepatitis, food additives, alcohol, toxic chemicals, viral infection and pollutants are the major risk factors of liver toxicity, which is an important pathology worldwide. In experimental hepatopathy, carbon tetrachloride (CCl4) has been commonly used because it forms unstable trichloromethyl and peroxy radicals, which are capable of binding to proteins or lipids, initiating lipid peroxidation and liver damage[1]. It was well documented that experimentally induced cirrhotic response in animals by CCl4 has been shown to be similar to human liver cirrhosis[2,3,4].
One of the major features of metabolic syndrome is dyslipidemia, characterized by elevated levels of very low-density lipoprotein (VLDL), triglyceride (TG) and low density lipoprotein (LDL)[5]. Elevated plasma VLDL and TG levels are due, in large part, to an increase in hepatic overproduction of TG-enriched VLDL particles[6]. CCl4-induced toxicity, studied for its hepatotoxic properties, also in turn increase the accumulation of fat in the liver leading to hyperlipidemia.
Mounting evidence suggests that reactive oxygen species (ROS) play a pivotal role in liver-disease pathology and ROS have been proven to be associated with CCl4-induced hepatotoxicity[1,7,8,9]. Therefore, therapeutic approach using potential antioxidants might be an ideal approach to ameliorate liver damage and hyperlipidemic conditions. In recent decades, the potential use of natural products and their active constituents with immense antioxidant functions has attracted much interest from researchers in the treatment of various liver disorders[10,11].
Olea europaea Linn. (Oleaceae) commonly known as olive is among the oldest cultivated trees in the world with immense medicinal values. Olives and its associated products have been used widely as folk medicines in Spain, Italy, France, Greece, Israel, Morocco, Tunisia, Turkey and the Mediterranean islands for centuries[12]. Today, the olive plant is most well known for its fruit crop and oil. As a folk remedy, olives have been used to reduce the incidence of heart diseases[13]. Experimental studies on the fruits and leaf extracts from olives show that they possess antithrombotic, antihypertensive, anticancer, hypoglycemic, antiinflammatory, antimicrobial and antiatherogenic properties[14,15]. The major active components of olives are phenolic compounds including oleuropein, hydroxytyrosol, tyrosol, 4-hydroxyphenyl acetic acid, protocatechuic acid, caffeic acid and p-coumaric acid. Several other biologically active constituents are also present[14,15]. Although the hepatoprotective actions of olive leaf aqueous extract have been confirmed in rats[16], studies on the olive fruit pulp extracts and their beneficial effects on restoring the lipid profile against CCl4-induced hepatic damage have not been addressed. During the processing of olive extracts for its oil, the fruit pulp is treated as waste. However, many phenolic compounds remain in the by-products. Olive-mill waste water known as “alpechin” and olive oil cake known as “alperujo” contain a similar phenolic profile to that of the original fruit. Therefore, in the present investigation the hepatoprotective effects of Olea europaea fruit pulp extract (OFP-EA) and the changes in lipid profile was investigated against CCl4-induced acute and chronic hepatic damage in experimental mice. Further the antioxidant properties of OFP-EA were also evaluated to substantiate the hepatoprotective effects.
MATERIALS AND METHODS
Male ICR mice (15-16 g) were obtained from NARA Biotech (Seoul, Korea). The animals were kept under standard laboratory conditions. A 12 h light/dark cycle in a temperature and humidity controlled room with water and food ad libitum was maintained. All animal experiments were performed in accordance and approval with our Institutional Animal Care and Use Committee of Konkuk University and the International Guidelines for Handling of Laboratory Animals[17]. Mice were anaesthetized by ketamine (160 mg/kg)/xylazine (40 mg/kg) cocktail injections, intraperitoneally.
Preparation of OFP-EA extracts:
Green olive fruits collected at the end of September to about the middle of November were obtained from the local market, Seoul, South Korea. The collected fruit material was authenticated at Konkuk University, Korea and a voucher specimen (OL-KU2012) has been kept in our university herbarium for future reference. To obtain the olive fruit pulp extract, 500 g of the fruit were ground in a mixer and defatted three times with three volumes of 80% ethanol. The residue (fruit pulp) was extracted with absolute ethanol (EtOH) at 1:10 ratio (w/v) for 2 h in a heating mantle at 70-80°. The supernatant was filtered and concentrated in a rotatory evaporator at 50°. The ethanol extract of olive fruit pulp obtained (180 g) was resuspended in water: EtOH (9:1, v/v) and partitioned successively with n-hexane, ethyl acetate (EA) and n-butanol to obtain a final yield of 19.4, 52 and 27.27 %, respectively. Since EA fraction of olive fruit pulp (OFP-EA) extract showed potent antioxidant effect in our preliminary evaluation, further studies on hepatoprotective effects in CCl4-induced hepatic damage in mice was investigated using OFP-EA extract. The extract was dissolved in sterile distilled water and filtered on 0.22 μm filters before use. All reagents used in this study were of highest grade available commercially.
Carbon tetrachloride-induced hepatic injury:
The animals were randomly divided into four groups of six mice each. Group A served as the normal vehicle control and was administrated orally with 2 ml/kg corn oil daily for a period of 7 weeks. Group B orally administrated with 2 ml/kg body weight of CCl4 (20% CCl4 in corn oil) once a day for 7 weeks. The animals of Group C were pretreated with silymarin (200 mg/kg per day, dissolved into 0.1% DMSO) served as positive control and group D were pretreated with OFP-EA extract (200 mg/kg per day) orally for 7 weeks, respectively before CCl4 treatment. At the end of experiment, animals were sacrificed and the blood samples were collected into heparinized tubes for each group separately. Liver tissue was dissected out, washed immediately with ice-cold saline, weighted and then immediately stored at -70° until analysis. The same protocol was followed to achieve acute liver injury model in mice except that the time period was set for three days.
Biochemical assessment:
Liver damage was assessed by the estimation of serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) using commercially available test kits from BioVision (Milpitas, CA, USA) according to manufacturer's instructions. Total cholesterol, HDL-cholesterol, LDL/VLDL-cholesterol, triglyceride (TG) and alkaline phosphatase (ALP) were also estimated by using the respective diagnostic kits from BioVision.
Alkyl radical scavenging assay:
Alkyl radicals were generated by 2,2-azobis (2-amidinopropane)hydrochloride (AAPH, Sigma Chemical Co., St. Louis, MO, USA). Reaction mixtures containing 40 mM AAPH, 40 mM 4-POBN, and the indicated concentrations of tested samples (20, 40. 80 and 100 μg/ml of OFP-EA) diluted in PBS (pH 7.4) were incubated at 37 ° in a water bath for 30 min[18] and then transferred to 50 μl Teflon capillary tubes. The spin adduct was recorded using the ESR spectrometer. The following measurement conditions were used: Central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 10 mW, gain 6.3×105, and temperature 298 K. The radical-scavenging activities of the OPP-EA were calculated according to the following formula: Scavenging rate=(h0−hx)/h0×100%, where h0 and hx are the ESR signal intensities of samples with and without extract, respectively.
Hydroxyl radical scavenging assay:
Hydroxyl radicals were generated by the Fenton reaction and reacted rapidly with nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO, Sigma Chemical Co., St. Louis, MO, USA). The resulting DMPO-OH adduct was detected by ESR[19]. The ESR spectrum was recorded 2.5 min after mixing with phosphate buffer solution (pH 7.4) supplemented with 20 μl of 0.3 M DMPO, 20 μl of 10 mM FeSO4, and 20 μl of 10 mM H2O2 under the following conditions: Central field 3475 G, modulation frequency 100 kHz, modulation amplitude 2 G, microwave power 1 mW, gain 6.3×105, and temperature 298 K. The final concentration of each OPP-EA was 2 mg/ml. The calculation of scavenging activities were measured a mentioned in alkyl radical scavenging assay.
Statistical analysis:
All data are represented as the mean±SEM of at least three independent experiments. Statistical analyses were performed using SAS statistical software (SAS Institute, Cray, NC, USA) using Student's t-test and one-way analysis of variance, followed by Dunnett's multiple range tests. In all experiments P values less than 0.05 was considered statistically significant.
RESULTS
Effect of OFP-EA extract on CCl4-induced hepatotoxicity:
The serum biochemical data for the evaluation of CCl4-induced hepatotoxicity by measuring the ALT and AST as conventional indicator of the liver injury are shown in fig. 1a and b. There was a remarkable elevation of serum ALT and AST activities in the CCl4-treated group as compared to the vehicle control (ALT: 3.8 fold in acute injuries and 3.5 fold in chronic injuries; AST: 4 fold in acute and 4.2 fold in chronic), indicating CCl4-induced damage to the hepatic cells (P<0.01). However, administration of OFP-EA extract at a dosage of 200 mg/kg suppressed the elevated serum ALT and AST in a significant manner when compared to CCl4 alone treated group (P<0.05). The positive control silymarin treated group (200 mg/kg) also exhibited significant attenuation (P<0.05) when compared to CCl4 treated groups.
Fig. 1.
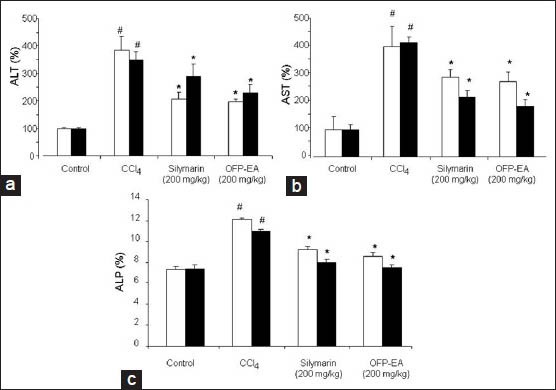
Effects of OFP-EA extract on serum ALT, AST and ALP in CCl4-treated mice.
Mice were administrated CCl4 dissolved in corn oil (2 ml/kg, 10% v/v) i.p. for 3 days for acute liver injury and orally for 7 weeks for chronic hepatic injury. Mice received silymarin (200 mg/kg) and OFP-EA extract (200 mg/kg each) before CCl4 intoxication and the levels of ALT (a), AST (b) and ALP (c) are measured using commercial kits, respective. Data are expressed as mean±SEM of six observations. #P<0.05, compared with control treated group and *P<0.05, compared with CCl4-treated group using one-way analysis of variance, followed by Dunnett's multiple range tests. OFP-EA: Olea europaea fruit pulp ethyl acetate extract, ALT: alanine aminotransferase, AST: aspartate aminotransferase, and ALP: alkaline phosphatase, □: acute liver injury,■: chronic liver injury.
The serum biochemical data for the evaluation of CCl4-induced hepatotoxicity by measuring the ALP as conventional indicator of the liver injury in both acute and chronic phase after CCl4 toxicity was shown in fig. 1c. There was an elevation of serum ALP activity in the CCl4-treated group as compared to the vehicle control (P<0.05). However, a significant decrease in the ALP activity was observed in CCl4-treated mouse by 200 mg/kg of OFP-EA extract (P<0.05). Silymarin (200 mg/kg) also exhibited significant attenuation of ALP serum levels, when compared with the CCl4-treated group (P<0.05). These results demonstrate that the levels of ALT, AST and ALP were significantly restored by treatment with OFP-EA extract and on par with standard drug silymarin.
Effects of OFP-EA on CCl4-induced chronic hepatic morphological changes:
The protective effect exerted by OFP-EA extract against CCl4-induced chronic hepatotoxicity was further confirmed by conventional histological assessment (fig. 2). As shown in fig. 2a, the histology of the liver sections of control group showed normal hepatic cells. The stained sections of CCl4 model group revealed extensive liver injuries characterized by moderate to severe hepatocellular hydropic degeneration and necrosis (fig. 2b). However, CCl4-intoxicated mice pretreated with 200 mg/kg silymarin and OFO-EA extract (200 mg/kg) markedly ameliorated the hepatic lesions (figs. 2c and d) when compared with CCl4-treated groups.
Fig. 2.
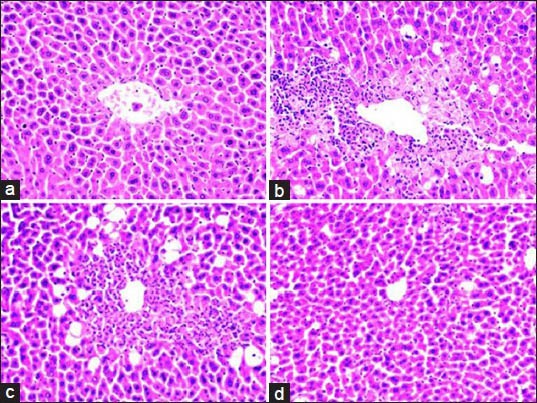
Effects of OFP-EA on hepatic morphological analysis.
Representative images at a magnification of 200 X of hematoxylin and eosin (H&E)-stained liver sections. (a) Control group with no noticeable histological changes, (b) CCl4-induced group showing severe histopathological changes like hydropic degeneration and necrosis, (c) silymarin (200 mg/kg) and CCl4 group, (d) OFP-EA (200 mg/kg) and CCl4 group (original magnification ×200). OFP-EA: Olea europaea fruit pulp ethyl acetate extract, CCl4: carbon tetrachloride.
Effects of OFP-EA extract on CCl4-treated elevated lipid levels:
The effect of OFP-EA extract on the cholesterol profile (total cholesterol, LDL/VLDL, TG and HDL) in both acute and chronic CCl4-indiced liver injury in mice is shown in figs. 3a and c. A significant increase in the total cholesterol, LDL/VLDL, TG and decreased HDL levels were observed in CCl4-intoxicated mice in both acute and chronic models, compared to the control group (P<0.05). However, pretreatment with OFP-EA extract (200 mg/kg) significantly attenuated the CCl4-induced increased serum levels of total cholesterol, TG, and LDL/VLDL in both acute and chronic mouse models (P<0.05) indicating that OFP-EA extract shows efficacy to inhibit the increased lipid levels inn CCl4-treated groups. Further the CCl4-treated decrease in HDL serum levels was reversed to near normal conditions when pretreated with OFP-EA extract (P<0.05). Silymarin (200 mg/kg) also attenuated the increased levels of total cholesterol, LDL/VLDL and TG and the decreased level of HDL in both acute and chronic models, which was consistent with the results from other researchers (P<0.05)[20].
Fig. 3.
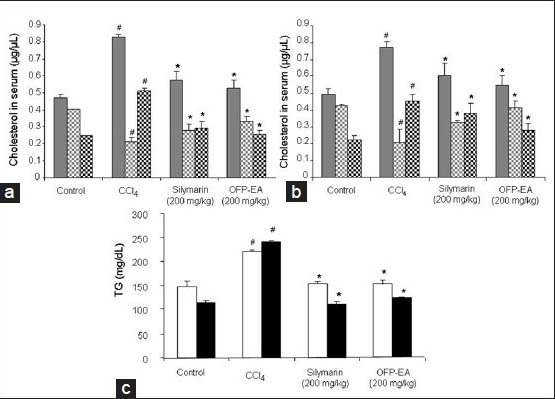
Effects of OFP-EA extract on serum cholesterol profile.
Mice were administrated CCl4 dissolved in corn oil i.p. for 3 days for acute liver injury and orally for 7 weeks for chronic hepatic injury. (a) Serum levels of total cholesterol, HDL and LDL/VLDL in acute liver injury. (b) Serum levels of total cholesterol, HDL and LDL/VLDL in chronic liver injury. (c) Serum levels of TG in acute and chronic liver injury. Mice received silymarin (200 mg/kg) and OFP-EA extract (200 mg/kg each) before CCl4 treatment and the serum levels were measured using commercial kits, respectively. Data are expressed as mean±SEM of six observations. #P<0.05, compared with control treated group and *P<0.05, compared with CCl4-treated group using one-way analysis of variance, followed by Dunnett's multiple range tests.  grey colored bar: total cholesterol,
grey colored bar: total cholesterol,  grey checks bar: HDL,
grey checks bar: HDL,  black checks bar: LDL/VLDL, □: acute liver injury, ■: chronic liver injury. OFP-EA: Olea europaea fruit pulp ethyl acetate extract, HDL: high-density lipoprotein, LDL/VLDL: low density/very lowdensity lipoprotein, TG: triglyceride.
black checks bar: LDL/VLDL, □: acute liver injury, ■: chronic liver injury. OFP-EA: Olea europaea fruit pulp ethyl acetate extract, HDL: high-density lipoprotein, LDL/VLDL: low density/very lowdensity lipoprotein, TG: triglyceride.
Alkyl free radical scavenging effects of OFP-EA:
The alkyl free radical scavenging activity of the OPP-EA extract was shown in fig. 4. The alkyl radical spin adduct of 4-POBN/free radicals was generated from AAPH at 37° after 30 min, and a decrease in the ESR signals was observed at increasing OPP-EA concentrations (20, 40, 80 and 100 μg/ml). The alkyl radical scavenging activity was nearly 80% at 100 μg/ml, and the IC50 value of the OPP-EA was 72.41 μg/ml (fig. 4a). The decrease in the ESR signals for alkyl radical scavenging effect of OFP-EA was shown in fig. 4b.
Fig. 4.
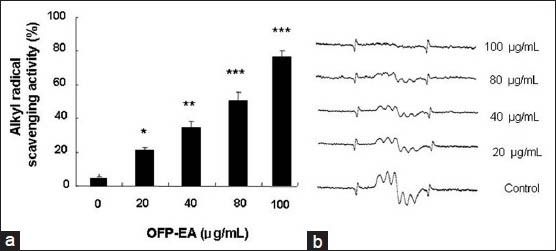
Effect of OFP-EA extract on alkyl radical scavenging activity.
(a) The capacities to scavenge the alkyl free radicals by different concentrations of OFP-EA extract. (b) ESR spectra. Data are expressed as mean±SEM of six observations. *P<0.05 compared with untreated control group by Student's t-test. OFP-EA: Olea europaea fruit pulp ethyl acetate extract.
Hydroxyl radical scavenging effects of OFP-EA:
The hydroxyl radical generated in the Fe2+/H2O2 system was trapped by DMPO, which formed the spin adduct detected by ESR spectroscopy, and the typical 1:2:2:1 ESR signal of the DMPO−OH adduct was observed. The height of the third peak in the spectrum represents the relative amounts of DMPO−OH adduct. The addition of the OPP-EA at indicated concentrations (25, 50, 100 and 200 μg/ml) resulted in a decrease in the peak corresponding to the DMPO−OH adduct. The maximum hydroxyl radical scavenging effect of OFP-EA was found to be 90.95% observed at 200 μg/ml concentrations (P<0.05, fig. 5a). The IC50 value of OPP-EA was determined to be 52.24 μg/ml. The decrease in the ESR signals for hydroxyl radical scavenging effect of OFP-EA was shown in fig. 5b.
Fig. 5.
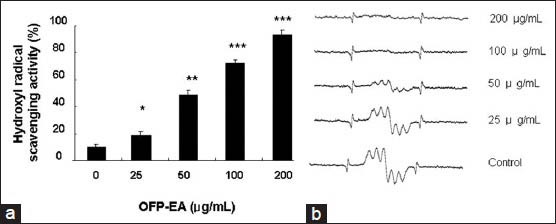
Effect of OFP-EA extract on hydroxyl radical scavenging activity.
(a) The capacities to scavenge the hydroxyl free radicals by different concentrations of OFP-EA extract. (b) ESR spectra. Data are expressed as mean±SEM of six observations. *P<0.05 compared with untreated control group by Student's t-test. OFP-EA: Olea europaea fruit pulp ethyl acetate extract.
DISCUSSION
Natural herbs rich in antioxidant bioactive compounds may exert beneficial effects towards human health including liver diseases. The model of acute and chronic intoxication with CCl4 has been used for decades to investigate the response of liver injury, because the elementary lesions caused by this hepatotoxin replicate those seen in most cases of human liver diseases[21,22]. In the present investigation we have identified the hepatoprotective effects of OFP-EA extract against CCl4-induced acute and chronic liver injury in mice. Estimation of serum enzymes such as ALT, AST and ALP is a useful quantitative marker of extent and type of hepatocellular injuries. Therefore, the elevated releases of ALT, AST and ALP in the circulation indicate severe damage to hepatic tissues during CCl4 toxicity[23]. In the present study, OFP-EA extract significantly inhibited the elevated levels of ALT, AST and ALP indicating a clear improvement of the functional status of the liver cells.
Histopathological examination of liver sections further substantiated with the inhibition of elevated serum enzymes in CCl4-induced hepatotoxicity. Liver sections of vehicle control group showed normal cellular architecture when compared with CCl4 intoxicated animals. The liver sections of mice treated with OFP-EA extract and silymarin followed by CCl4 intoxication showed a sign of protection as it was evident by absence of necrosis and vacuoles.
Liver plays an important role in the regulation of lipoprotein transport in serum and cholesterol metabolism. During lipoprotein transport, LDL and HDL appear to be especially pivotal. LDL, rich in cholesterol and cholesterol ester, is regarded as bad cholesterol, whereas HDL contains relatively little cholesterol and is regarded as good cholesterol. Reports suggested that high levels of serum cholesterol, LDL, and TG and low levels of HDL were induced with CCl4 in animal model systems[24,25,26]. Therefore, to evaluate whether OFP-EA extract has any influence in hyperlipidemia induced by CCl4, cholesterol catabolism was investigated. In the present study OFP-EA extract significantly prevented CCl4-induced liver damage as evidenced by reduced serum concentrations of cholesterol contents. Data form our study indicated that OFP-EA extract restored the CCl4-induced increased lipid levels to near normal levels.
Silymarin has been long used in the treatment for liver diseases as it restores liver enzyme activities due to its antioxidant properties, chelating metal ions, inhibiting lipid peroxidation, protecting the membrane permeability properties, and preventing liver glutathione depletion[27]. Therefore in the present study we used silymarin as standard agent to compare the effects of OFP-EA extract in CCl4-induced liver injury in mice. The extract showed similar or greater significant effect when compared with standard agent silymarin at the same dose tested in CCl4-induced acute and chronic liver damage in mice
Several reports have indicated that a pivotal mechanism in hepatoprotective effects may relate to the activities of antioxidants to scavenge ROS and/or alleviate CCl4-induced toxic effects by the prevention of toxic free radicals such as hydroxyl, alkyl and lipid peroxides[28,29,30]. Moreover, the initiation of oxidative stress in acute and chronic liver diseases is believed to be induced by increased free radicals[31]. Earlier studies revealed that olive oil phenols possessed strong antioxidant compounds[14,15,32,33]. In our present study, OFP-EA significantly scavenged the alkyl and hydroxyl free radicals significantly indicating that the fruit pulp extract of olive might also contain potential antioxidant agents. In conclusion, our findings showed that OFP-EA extract significantly prevented CCl4-induced liver damage as evidenced by decreased serum activities of AST, ALT and ALP. OFP-EA extract also modulated serum concentrations of cholesterol contents suggesting its beneficial effects in hepatoprotection. The hepatoprotective effects of OFP-EA extract might partly be due to its antioxidant properties. However, further work is necessary in exploring the exact molecular mechanisms and the compounds responsible in delivering such actions.
Footnotes
Kang and Koppula: Hepatoprotective Effects of Olive Fruit Pulp
REFERENCES
- 1.Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 2.Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit Rev Toxicol. 2003;33:105–36. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 3.Hsu YW, Tsai CF, Chuang WC, Chen WK, Ho YC, Lu FJ. Protective effects of silica hydride against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem Toxicol. 2010;48:1644–53. doi: 10.1016/j.fct.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Lee KJ, Choi JH, Jeong HG. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2007;45:2118–25. doi: 10.1016/j.fct.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Moller DE, Kaufman KD. Metabolic syndrome: A clinical and molecular perspective. Ann Rev Med. 2005;56:45–62. doi: 10.1146/annurev.med.56.082103.104751. [DOI] [PubMed] [Google Scholar]
- 6.Adiels M, Taskinen MR, Packard C, Caslake MJ, Soro-Paavonen A, Westerbacka J, et al. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologica. 2006;49:755–65. doi: 10.1007/s00125-005-0125-z. [DOI] [PubMed] [Google Scholar]
- 7.Vitagkione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr. 2004;44:575–86. doi: 10.1080/10408690490911701. [DOI] [PubMed] [Google Scholar]
- 8.Slater TF, Sawyer BC. The stimulatory effects of carbon tetrachloride on peroxidative reactions in rat liver fractions in vitro.Inhibitory effects of free radical scavengers and other agents. Biochem J. 1971;123:823–8. doi: 10.1042/bj1230823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicololgy. 2003;189:113–27. doi: 10.1016/s0300-483x(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 10.Vishnu Priya V, Niveda S, Pratiksha G, Gayathri R. A review of hepatoprotective natural products. Rec Res Sci Tech. 2010;2:49–52. [Google Scholar]
- 11.Adewusi EA, Afolayan AJ. A review of natural products with hepatoprotective activity. J Med Plant Res. 2010;4:1318–34. [Google Scholar]
- 12.Cook NC, Samman S. Flavonoids- chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem. 1996;7:66–76. [Google Scholar]
- 13.Covas MI. Bioactive effects of olive oil phenolic compounds in humans: Reduction of heart disease factors and oxidative damage. Inflammopharmacology. 2008;16:216–8. doi: 10.1007/s10787-008-8019-6. [DOI] [PubMed] [Google Scholar]
- 14.Rosignoli P, Fuccelli R, Fabiani R, Servili M, Morozzi G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J Nutr Biochem. 2013;24:1513–9. doi: 10.1016/j.jnutbio.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Caponio F, Alloggio V, Gomes T. Phenolic compounds of virgin olive oil: Influence of paste preparation techniques. Food Chem. 1999;64:203–9. [Google Scholar]
- 16.Enas A, Khalil M. Evaluation of the hepatoprotective activity of an aqueous extract of olive leaves in male albino rats. Egyp J Hosp Med. 2004;15:118–23. [Google Scholar]
- 17.Derrell C. Institute of Laboratory Animal Resources. Washington DC, USA: National Academy Press; 1996. Guide for the care and use of laboratory animals. [Google Scholar]
- 18.Hiramoto K, Johkoh H, Sako KI, Kikugawa K. DNA breaking activity of the carbon-centered radical generated from 2,2’-azobis (2-amidinopropane) hydrochloride (AAPH) Free Radic Res Commun. 1993;19:323–32. doi: 10.3109/10715769309056521. [DOI] [PubMed] [Google Scholar]
- 19.Rosen GM, Rauckman EJ. Spin trapping of superoxide and hydroxyl radicals. Methods Enzymol. 1984;105:198–209. doi: 10.1016/s0076-6879(84)05026-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen IS, Chen YC, Chou CH, Chuang RF, Sheen LY, Chiu CH. Hepatoprotection of silymarin against thioacetamide-induced chronic liver fibrosis. J Sci Food Agric. 2011;92:1441–7. doi: 10.1002/jsfa.4723. [DOI] [PubMed] [Google Scholar]
- 21.Morio LA, Chiu H, Sprowles KA, Zhou P, Heck DE, Gordon MK, et al. Distinct roles of tumor necrosis factor-alpha and nitric oxide in acute liver injury induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2001;172:44–51. doi: 10.1006/taap.2000.9133. [DOI] [PubMed] [Google Scholar]
- 22.Perez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3:112–20. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- 23.Khan RA, Khan MR, Sahreen S, Shah NA. Hepatoprotective activity of Sonchus asper against carbon tetrachloride-induced injuries in male rats: A randomized controlled trial. BMC Complement Altern Med. 2012;12:90. doi: 10.1186/1472-6882-12-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin HM, Tseng HC, Wang CJ, Lin JJ, Lo CW, Chou FP. Hepatoprotective effects of Solanum nigrum Linn extract against CCl 4 -induced oxidative damage in rats. Chem Biol Interact. 2008;171:283–93. doi: 10.1016/j.cbi.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Yang YS, Ahn TH, Lee JC, Moon CJ, Kim SH, Jun W, et al. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem Toxicol. 2008;46:380–7. doi: 10.1016/j.fct.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Kamalakkannan N, Rukkumani R, Aruna K, Varma PS, Viswanathan P, Menon VP. Protective effect of N-acetyl cysteine in carbon tetrachloride-induced hepatotoxicity in rats. Iranian J Pharmacol Therap. 2005;4:118–23. [Google Scholar]
- 27.Banaee M, Sureda A, Mirvaghefi AR, Rafei GR. Effects of long term silymarin oral supplementation on the blood biochemical profile of rainbow trout (Oncorhynchus mykiss) Fish Physiol Biochem. 2011;37:885–96. doi: 10.1007/s10695-011-9486-z. [DOI] [PubMed] [Google Scholar]
- 28.Naik SR, Panda VS. Antioxidant and hepatoprotective effects of Ginkgo biloba phytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007;27:393–9. doi: 10.1111/j.1478-3231.2007.01463.x. [DOI] [PubMed] [Google Scholar]
- 29.Hsu YW, Tsai CF, Chen WK, Lu FJ. Protective effects of seabuckthorn (Hippophae rhamnoids L) seed oil against carbon tetrachloride-induced hepatotoxicity in mice. Food Chem Toxicol. 2009;47:2281–8. doi: 10.1016/j.fct.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 30.Tsai CF, Hsu YW, Chen WK, Chang WH, Yen CC, Ho YC, et al. Hepatoprotective effect of electrolyzed reduced water against carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2009;47:2031–6. doi: 10.1016/j.fct.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B. Antioxidants and human disease: A general introduction. Nutr Rev. 1997;55:44–52. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 32.Raederstorff D. Antioxidant activity of olive polyphenols in humans: A review. Int J Vitam Nutr Res. 2009;79:152–65. doi: 10.1024/0300-9831.79.3.152. [DOI] [PubMed] [Google Scholar]
- 33.Kim MS, Koppula S, Jung SH, Kim JY, Lee HR, Lee SR, et al. Olea europaea Linn (Oleaceae) fruit pulp extract exhibits potent antioxidant activity and attenuates neuroinflammatory responses in lipopolysaccharide-stimulated microglial cells. Trop J Pharm Res. 2013;12:357–62. [Google Scholar]


