Abstract
A series of 1,4-dihydropyrimidine derivatives 3(a-t) were prepared from Biginelli reactions by using ethyl acetoacetate, substituted benzaldehyde and thiourea in the presence of piperidine and ethanol. The compounds 3(a-t) were reacted with dimethylsulphate, diethylsulphate, butyl bromide and benzyl chloride to give the new series of compounds 4(a-t). The structures of the newly synthesized compounds 4(a-t) were established by IR, 1H NMR, Mass spectra and elemental analysis. The synthesized compounds were evaluated for their in vitro anticancer activity by using Sulforhodamine B assay method against the growth of four humans cancer cell lines, antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli and for antifungal activity against Candida albicans and Aspergillus niger. The compounds exhibited good anticancer activity and moderate antibacterial and antifungal activities. Compounds 4b, 4c, 4d, 4g, 4i, 4n, 4o, 4q and 4s showed significant anticancer activity when compared with the doxorubicin as a standard reference drug.
Keywords: Anticancer, antimicrobial, Biginelli reaction, cell line, dihydropyrimidine, SRB assay method
Anticancer drug discovery and development is one of the most essential and rapidly changing avenues for medicinal chemist. The requirement for new chemotherapeutics in cancer is evident due to the limited capacity of drugs to cure or significantly prolong the survival of patients with disseminated tumours or certain leukemias. Combination chemotherapy of existing anticancer agents with diverse mechanism of action is one strategy employed to treat this disease[1]. Despite large number of antibiotics and chemotherapy available for medicinal use, the treatment of infectious diseases still remains an important and challenging problem. This is because of a combination of factors including emergence of resistance to current antimicrobial therapy and rapid increase of primary and opportunistic fungal infections in immunocompromised patients like those suffering from immunodeficiency syndrome (AIDS) or undergoing anticancer therapy and organ transplantation[2,3,4,5].
Pyrimidine derivatives have played a crucial role in the history of heterocyclic chemistry and have been used extensively as important pharmacophores and synthons in the field of organic chemistry and drug designing owning to their versatile chemotherapeutic importance. A significant amount of research effort has been focused on this nucleus. Pyrimidines are found to possess biomimetic and reactive pharmacophores due to their diverse medicinal properties such as antiviral[6], anticancer[7], antibacterial[8], antihypertensive[9], tyrosine kinase inhibitory[10], COX-2 inhibitory[11] and calcium channel blockade[12]. The presence of pyrimidine bases i.e. thymine, cytosine and uracil, the essential building blocks of nucleic acids, is one of the possible reasons for their activities. Pyrimidine ring is found in vitamins like riboflavin, thiamine and folic acid. Pyrimidine nucleus is also present in barbituric acid and its several derivatives, which are used as hypnotics. In light of these interesting biological activites, it was our interest to synthesize some new dihydropyrimidine derivatives and evaluate them for in vitro anticancer, antibacterial and antifungal activities.
MATERIALS AND METHODS
All chemicals were of laboratory grade and obtained from Merck, Mumbai. Melting points were determined on a Veego VMP-1 capillary melting point apparatus in open capillaries and are uncorrected. The purity of the compounds was ascertained by thin layer chromatography on silica gel G in various solvent systems using iodine vapors as detecting agents. IR spectra were recorded on a Jasco FT/IR-410 spectrometer in potassium bromide pellets and are expressed in cm-1. 1H NMR spectra was recorded on Brucker 400 MHz spectrophotometers using tetramethylsilane as internal standard. Chemical shifts are expressed in δ (ppm). Mass spectra were recorded on Jeol 5×102/DA-6000. Elemental analysis was carried out using Carlo Erba 1106 CHN analyzer.
General procedure for the preparation of compounds:
Ethyl-6-methyl-4-(substituted phenyl)-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate 3(a-t)[13,14] was prepared from acetoacetic ester (0.01 mol, 1.9 g), thiourea (0.01 mol, 0.9 g) and substituted aromatic aldehydes (0.01 mol). Piperidine was added (2 ml) as catalyst in the reaction mixture. It was stirred for 4 h and kept for 24-36 h to afford the product 3(a-t).
3a: Yield 43%; m.p. 215-216°; IR (KBr, ν, cm-1) 3200 (N-H), 3070 (Ar-H), 1680 (C=O), 1485 (C=C), 1120 (C=S), 1180 (C=N), 1080 (C-O); 1H NMR (DMSO-d6, δ, ppm) 1.2 (t, 3H, -OCH2-CH3); 2.4 (s, 3H, 6-CH3); 4.1 (q, 2H, -OCH2CH3); 5.4 (s, 1H, 4-CH); 7.5-8.2 (m, 4H, Ar-H); 9.5 (s, 1H, NH); 10.1 (s, 1H, NH);EI-MS m/z(% base): 321 (60.6), 304 (37.2), 292 (34.6), 248 (31.6), 199 (100), 171 (35.2); Anal. Calc. for C14H15N3O4S: C, 52.33%; H, 4.70%; N, 13.08%. found: C, 52.68%; H, 5.01%; N, 12.78%.
General procedure for synthesis of ethyl-6-methyl-2-(methylsulfanyl)-4-substituted phenyl-1,4-dihydropyrimidine-5-carboxylate 4(a-g)[15,16] was as follows. To tetrahydropyrimidine (0.004 mol) 3 dissolved in methanol NaOH solution which was prepared by dissolving NaOH (0.160 g) in water (2 ml) was added and the mixture was cooled. To this mixture dimethyl sulphate (0.004 mol, 0.4 ml) was added dropwise and stirred continuously. Then the mixture was refluxed for 3 h. The mixture was cooled and poured over ice. The product was filtered under reduced pressure, dried and recrystallised using methanol to give 4a-g.
General procedure for synthesis of ethyl-6-methyl-2-(ethylsulfanyl)-4-substituted phenyl-1, 4-dihydropyrimidine-5-carboxylate 4h-l[15,16]. To tetrahydropyrimidine (0.004 mol) 3 dissolved in methanol NaOH solution which was prepared by dissolving NaOH (0.160 g) in water (2 ml) was added and the mixture was cooled. To this mixture diethyl sulphate (0.004 mol, 0.6 ml) was added dropwise and stirred continuously. Then the mixture was refluxed for 3 h. The mixture was cooled and poured over ice. The product was filtered under reduced pressure, dried and recrystallised using methanol to give 4h-l.
General procedure for synthesis of ethyl-6-methyl-2-(butylsulfanyl)-4-substituted phenyl-1,4-dihydropyrimidine-5-carboxylate (4m-q)[17]. A mixture of powdered tetrahydropyrimidine 3 (0.004 mol), butylbromide (0.004 mol, 0.8 ml) and absolute alcohol (5 ml) were refluxed for 5 h. Then the product was allowed to separate at room temperature. The product was filtered under reduced pressure and crystallizedusing ethanol to give 4m-q.
General procedure for synthesis of ethyl-6-methyl-2-(benzylsulfanyl)-4-substitutedphenyl-1,4-dihydropyrimidin-5-carboxylate (4r-t)[18]. To tetrahydropyrimidine 3, (0.004 mol) dissolved in alcohol (2.5 ml) benzyl chloride (0.004 mol, 0.8 ml) was added and the mixture was refluxed for 4 h. The mixture was cooled at room temperature. The solid separated was filtered and recrystallised using ethanol to give 4r-t.
Ethyl-6-methyl-2-(methylsulfanyl)-4-(2-nitrophenyl)-1,4-dihydropyrimidine-5-carboxylate (4a). Yield 33%; m.p. 205-206°; IR (KBr, ν, cm-1) 3200 (N-H), 3070 (Ar-H), 1680 (C=O), 1485 (C=C), 1349 (N=O), 1180 (C=N), 1080 (C-O), 687 (C-S); 1H NMR (DMSO-d6, δ, ppm)1.0 (t, 3H, -OCH2-CH3); 2.4 (s, 3H, 6-CH3); 2.9 (s, 3H, S-CH3); 4.1 (q, 2H, -OCH2CH3); 5.8 (s, 1H, 4-CH); 7.5-7.9 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 335 (40.6), 305 (20.7), 298 (100), 283 (40.0), 269 (31.6), 225 (44.6), 176 (35.6); Anal. Calc. for C15H17N3O4S: C, 53.72%; H, 5.11%; N, 12.53%. found: C, 53.52%; H, 5.34%; N, 12.21%.
Ethyl-6-methyl-2-(methylsulfanyl)-4-(3-nitrophenyl)-1,4-dihydropyrimidine-5-carboxylate (4b). Yield 59%; m.p. 220-221°; IR (KBr, ν, cm-1) 3211 (N-H), 3125 (Ar-H), 1655 (C=O), 1485 (C=C), 1365 (N=O), 1215 (C=N), 1075 (C-O), 681 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.0 (t, 3H, -OCH2-CH3); 2.4 (s, 3H, 6-CH3); 2.9 (s, 3H, S-CH3); 4.1 (q, 2H, -OCH2CH3); 5.4 (s, 1H, 4-CH); 7.6-8.4 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 335 (38.5), 305 (22.5), 298 (100), 283 (42.1), 269 (34.7), 225 (49.6), 176 (31.7); Anal. Calc. for C15H17N3O4S: C, 53.72%; H, 5.11%; N, 12.53%. found: C, 53.69%; H, 5.08%; N, 12.98%.
Ethyl-6-methyl-2-(methylsulfanyl)-4-(4-methoxyphenyl)-1,4-dihydropyrimidine-5-carboxylate (4c). Yield 35%; m.p. 135-136°; IR (KBr, ν, cm-1)3175 (N-H), 3095 (Ar-H), 1660 (C=O), 1460 (C=C), 1250 (C-O-C), 1195 (C=N), 1098 (C-O), 688 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.2 (t, 3H, -OCH2-CH3); 2.4 (s, 3H, 6-CH3); 2.9 (s, 3H, S-CH3); 3.8 (s, 3H, OCH3); 4.1 (q, 2H, -OCH2CH3); 5.4 (s, 1H, 4-CH); 6.8-7.3(m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 320 (29.7), 305 (15.0), 291 (100), 247 (49.5), 213 (82.0), 185 (37.9); Anal. Calc. for C16H20N2O3S: C, 59.98%; H, 6.29%; N, 8.74%. found: C, 59.12%; H, 5.98%; N, 8.98%.
Ethyl-6-methyl-2-(methylsulfanyl)-4-(3,4-dimethoxyphenyl)-1,4-dihydropyrimidine-5-carboxylate (4d). Yield 80%; m.p. 110-111°; IR (KBr, ν, cm-1)3256 (N-H), 3095 (Ar-H), 1661 (C=O), 1555 (C=C), 1235 (C-O-C), 1180 (C=N), 1115 (C-O), 677 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 2.3 (s, 3H, 6-CH3); 2.4 (s, 3H, S-CH3); 3.8 (s, 6H, -OCH3); 4.1 (q, 2H, -OCH2CH3); 5.4 (s, 1H, 4-CH); 6.7-7.1 (m, 3H, Ar-H); 8.8 (s, 1H, NH); EI-MS m/z (% base): 351 (35.6), 335 (20.0), 305 (18.3), 291 (100), 213 (70.1), 185 (32.5); Anal. Calc. for C17H22N2O4S: C, 58.27%; H, 6.33%; N, 7.99%. found: C, 57.23%; H, 6.01%; N, 7.23%.
Ethyl-6-methyl-2-(methylsulfanyl)-4-phenyl-1,4-dihydropyrimidine-5-carboxylate (4e). Yield 70%; m.p. 165-166°; IR (KBr, ν, cm-1)3198 (N-H), 3115 (Ar-H), 1680 (C=O), 1575 (C=C), 1298 (C=N), 1120 (C-O), 671 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.2 (t, 3H, -OCH2-CH3); 2.3 (s, 3H, 6-CH3); 2.4 (s, 3H, S-CH3); 4.1 (q, 2H, -OCH2CH3); 5.8 (s, 1H, 4-CH); 7.2-7.6 (m, 5H, Ar-H); 9.2(s, 1H, NH); EI-MS m/z (% base): 291 (30.5), 276 (18.6), 244 (32.6), 218 (100), 214 (60.6); Anal. Calc. for C15H18N2O2S: C, 62.04%; H, 6.25%; N, 9.65%. found: C, 61.45%; H, 6.58%; N, 9.65%.
Ethyl-6-methyl-2-(methylsulfanyl)-4-(4-chlorophenyl)-1,4-dihydropyrimidine-5-carboxylate (4f). Yield 72%; m.p. 170-171°; IR (KBr, ν, cm-1) 3211 (N-H), 3085 (Ar-H), 1671 (C=O), 1598 (C=C), 1295 (C=N), 1088 (C-O), 751 (C-Cl), 660 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.2 (t, 3H, -OCH2-CH3); 2.3 (s, 3H, 6-CH3); 2.4 (s, 3H, S-CH3); 4.1 (q, 2H, -OCH2CH3); 5.6 (s, 1H, 4-CH); 7.4-7.7 (m, 4H, Ar-H); 10.1 (s, 1H, NH); EI-MS m/z (% base): 325 (49.5), 310 (33.6), 275 (100), 252 (80.0), 141 (48.2); Anal. Calc. for C15H17ClN2O2S: C, 55.47%; H, 5.27%; N, 10.91%. found: C, 54.25%; H, 5.98%; N, 11.85%.
Ethyl-6-methyl-2-(methylsulfanyl)-4-(4-methylphenyl)-1,4-dihydropyrimidine-5-carboxylate (4g). Yield 78%; m.p. 175-176°; IR (KBr, ν, cm-1) 3345 (N-H), 3205 (Ar-H), 1680 (C=O), 1575 (C=C), 1325 (C=N), 1210 (C-O), 688 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 2.1 (s, 3H, S-CH3); 2.3 (s, 3H, Ar-CH3); 2.4 (s, 3H, 6-CH3); 4.1 (q, 2H, -OCH2CH3); 5.3 (s, 1H, 4-CH); 7.0-7.2 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 304 (37.5), 289 (31.3), 275 (40.1), 231 (100), 216 (28.1); Anal. Calc. for C16H20N2O2S: C, 63.13%; H, 6.62%; N, 9.20%. found: C, 64.32%; H, 6.68%; N, 9.56%.
Ethyl-2-(ethylsulfanyl)-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyrimidine-5-carboxylate (4h). Yield 35%; m.p. 108-109°; IR (KBr, ν, cm-1) 3285 (N-H), 3145 (Ar-H), 1688 (C=O), 1578 (C=C), 1385 (N=O), 1278 (C=N), 1138 (C-O), 714 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 1.3 (t, 3H, -S-CH2-CH3); 2.3 (s, 3H, 6-CH3); 2.9 (m, 1H, S-CH2); 3.1 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.6 (s, 1H, 4-CH); 7.1-7.3 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 350 (15.9), 321 (35.7), 275 (30.0), 246 (20.6), 202 (40.2), 187 (100), 153 (25.6); Anal. Calc. for C16H19N3O4S: C, 55.00%; H, 5.48%; N, 12.03%. found: C, 54.12%; H, 5.34%; N, 12.35%.
Ethyl-2-(ethylsulfanyl)-6-methyl-4-(4-methylphenyl)-1,4-dihydropyrimidine-5-carboxylate (4i). Yield 52%; m.p. 120-121°; IR (KBr, ν, cm-1) 3266 (N-H), 3145 (Ar-H), 1658 (C=O), 1538 (C=C), 1298 (C=N), 1165 (C-O), 711 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 1.3 (t, 3H, -S-CH2-CH3); 2.3 (s, 3H, 6-CH3); 2.4 (s, 3H, Ar-CH3); 3.1 (m, 1H, S-CH2); 3.3 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.5 (s, 1H, 4-CH); 7.4-8.2 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 320 (19.7), 291 (20.7), 246 (30.0), 230 (25.6), 215 (40.6), 156 (100), 121 (20.6); Anal. Calc. for C17H22N2O2S: C, 64.12%; H, 6.96%; N, 8.80%. found: C, 64.48%; H, 6.23%; N, 9.11%.
Ethyl-2-(ethylsulfanyl)-6-methyl-4-(3,4-dimethoxyphenyl)-1,4-dihydropyrimidine-5-carboxylate (4j). Yield 60%; m.p. 182-183°; IR (KBr, ν, cm-1) 3225 (N-H), 3158 (Ar-H), 1668 (C=O), 1585 (C=C), 1260 (C-O-C), 1188 (C=N), 1147 (C-O), 688 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 1.2 (t, 3H, -S-CH2-CH3); 2.4 (s, 3H, 6-CH3); 2.9 (m, 1H, S-CH2); 3.1 (m, 1H, S-CH2); 3.70 (s, 6H, Ar-3,4(OCH3)2); 4.1 (q, 2H, -OCH2CH3); 5.5 (s, 1H, 4-CH); 6.8-7.3 (m, 3H, Ar-H); 9.2 (s, 1H, NH); EI-MS m/z (% base): 366 (20.70, 337 (30.1), 308 (40.1), 292 (35.0), 230 (40.7), 215 (80.0), 165 (100), 121 (18.8); Anal. Calc. for C18H22N2O4S: C, 59.32%; H, 6.64%; N, 7.69%. found: C, 59.98%; H, 6.69%; N, 8.12%.
Ethyl-2-(ethylsulfanyl)-6-methyl-4-phenyl-1,4-dihydropyrimidine-5-carboxylate (4k). Yield 72%; m.p. 175-176°; IR (KBr, ν, cm-1) 3225 (N-H), 3147 (Ar-H), 1680 (C=O), 1575 (C=C), 1225 (C=N), 1110 (C-O), 688 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 1.2 (t, 3H, -S-CH2-CH3); 2.3 (s, 3H, 6-CH3); 2.9 (m, 1H, S-CH2); 3.1 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.5 (s, 1H, 4-CH); 6.8-7.3(m, 5H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 304 (7.8), 275 (40.5), 255 (38.8), 226 (70.3), 198 (23.6), 169 (100), 128 (25.6), 111 (30.4), 83 (30.0), 71 (60.8); Anal. Calc. for C16H20N2O2S: C, 63.13%; H, 6.62%; N, 9.20%. found: C, 63.68%; H, 6.12%; N, 8.98%.
Ethyl-2-(ethylsulfanyl)-6-methyl-4-(4-chlorophenyl)-1,4-dihydropyrimidine-5-carboxylate (4l). Yield 40%; m.p. 115-116°; IR (KBr, ν, cm-1) 3278 (N-H), 3245 (Ar-H), 1660 (C=O), 1545 (C=C), 1325 (C=N), 1238 (C-O), 780 (C-Cl), 688 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 1.3 (t, 3H, -S-CH2-CH3); 2.3 (s, 3H, 6-CH3); 2.9 (m, 1H, S-CH2); 3.1 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.5 (s, 1H, 4-CH); 7.4-7.7 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 339 (20.6), 310 (30.7), 281 (20.6), 246 (40.7), 214 (20.6), 170 (100); Anal. Calc. for C16H19ClN2O2S: C, 56.71%; H, 5.65%; N, 10.46%. found: C, 56.22%; H, 5.11%; N, 10.11%.
Ethyl-2-(butylsufanyl)-6-methyl-4-(3-nitrophenyl)-1,4-dihydropyrimidine-5-carboxylate (4m). Yield 77%; m.p. 185-186°; IR (KBr, ν, cm-1) 3267 (N-H), 3149 (Ar-H), 1665 (C=O), 1548 (C=C), 1349 (N=O), 1288 (C=N), 1235 (C-O), 681 (C-S); 1H NMR (DMSO-d6, δ, ppm) 0.8 (t, 3H, CH3 of S-butyl); 1.1 (t, 3H, -OCH2-CH3); 1.3 (m, 2H, -CH2-CH3 of S-butyl); 1.5 (m, 2H, -CH2-CH2-CH3 of S-butyl); 2.6 (s, 3H, 6-CH3); 3.2 (m, 1H, S-CH2); 3.6 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.8 (s, 1H, 4-CH); 6.8-7.4 (m, 4H, Ar-H); 9.1 (s, 1H, NH); EI-MS m/z (% base): 337 (10.9), 348 (30.6), 291 (30), 275 (28.6), 229 (100), 143 (30.1), 82 (30.5); Anal. Calc. for C18H23N3O4S: C, 57.28%; H, 6.14%; N, 11.13%. found: C, 57.11%; H, 5.88%; N, 10.88%.
Ethyl-2-(butylsufanyl)-6-methyl-4-(4-methoxyphenyl)-1,4-dihydropyrimidine-5-carboxylate (4n). Yield 42%; m.p. 117-118°; IR (KBr, ν, cm-1) 3345 (N-H), 3205 (Ar-H), 1680 (C=O), 1575 (C=C), 1325 (C=N), 1280 (C-O-C), 1210 (C-O), 681 (C-S); 1H NMR (DMSO-d6, δ, ppm) 0.8 (t, 3H, CH3 of S-butyl); 1.1 (t, 3H, -OCH2-CH3); 1.3 (m, 2H, -CH2-CH3 of S-butyl); 1.5 (m, 2H, -CH2-CH2-CH3 of S-butyl); 2.6 (s, 3H, 6-CH3); 3.2 (m, 1H, S-CH2); 3.6 (m, 1H, S-CH2); 3.7 (s, 3H, Ar-OCH3); 4.1 (q, 2H, -OCH2CH3); 5.4 (s, 1H, 4-CH); 7.0-7.6 (m, 4H, Ar-H); 8.6 (s, 1H, NH); EI-MS m/z (% base): 363 (8.1), 362 (6.6), 333 (100), 305 (25.7), 289 (33.4), 277 (48), 255 (89.8), 199 (40), 171 (20.6), 82 (36.8); Anal. Calc. for C19H26N2O3S: C, 62.96%; H, 7.23%; N, 7.73%. found: C, 63.65%; H, 7.54%; N, 8.01%.
Ethyl-2-(butylsufanyl)-6-methyl-4-(3,4-dimethoxyphenyl)-1,4-dihydropyrimidine-5-carboxylate(4o). Yield 72%; m.p. 140-141°; IR (KBr, ν, cm-1)3322 (N-H), 3258 (Ar-H), 1685 (C=O), 1575 (C=C), 1345 (C=N), 1278 (C-O-C), 1215 (C-O), 681 (C-S); 1H NMR (DMSO-d6, δ, ppm) 0.8 (t, 3H, CH3 of S-butyl); 1.1 (t, 3H, -OCH2-CH3); 1.3 (m, 2H, -CH2-CH3 of S-butyl); 1.5 (m, 2H, -CH2-CH2-CH3 of S-butyl); 2.6 (s, 3H, 6-CH3); 3.2 (m, 1H, S-CH2); 3.6 (m, 1H, S-CH2); 3.7 (s, 6H, Ar-(OCH3)2); 4.1 (q, 2H, -OCH2CH3); 5.8 (s, 1H, 4-CH); 6.8-7.3 (m, 3H, Ar-H); 8.6 (s, 1H, NH); EI-MS m/z (% base): 394 (12.6), 365 (20.7), 337 (100), 277 (48), 255 (79.3), 199 (40.8), 171 (20.6); Anal. Calc. for C20H28N2O4S: C, 61.20%; H, 7.19%; N, 7.14%. found: C, 60.88%; H, 7.66%; N, 7.88%.
Ethyl-2-(butylsufanyl)-6-methyl-4-phenyl-1,4-dihydropyrimidine-5-carboxylate (4p). Yield 70%; m.p. 142-143°; IR (KBr, ν, cm-1) 3325 (N-H), 3205 (Ar-H), 1680 (C=O), 1575 (C=C), 1322 (C=N), 1198 (C-O), 681 (C-S); 1H NMR (DMSO-d6, δ, ppm) 0.8 (t, 3H, CH3 of S-butyl); 1.1 (t, 3H, -OCH2-CH3); 1.3 (m, 2H, -CH2-CH3 of S-butyl); 1.5 (m, 2H, -CH2-CH2-CH3 of S-butyl); 2.6 (s, 3H, 6-CH3); 3.2 (m, 1H, S-CH2); 3.6 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.8 (s, 1H, 4-CH); 6.8-7.2 (m, 5H, Ar-H); 8.6 (s, 1H, NH); EI-MS m/z (% base): 332 (30.6), 255 (24.3), 198 (18.3), 169 (100), 128 (15.8), 111 (30.6), 71 (89); Anal. Calc. for C18H24N2O2S: C, 65.03%; H, 7.28%; N, 8.43%. found: C, 65.78%; H, 7.48%; N, 8.21%.
Ethyl-2-(butylsufanyl)-6-methyl-4-(4-chlorophenyl)-1,4-dihydropyrimidine-5-carboxylate (4q). Yield 62%; m.p. 138-139°; IR (KBr, ν, cm-1) 3298 (N-H), 3147 (Ar-H), 1688 (C=O), 1575 (C=C), 1325 (C=N), 1155 (C-O), 721 (C-Cl), 681 (C-S); 1H NMR (DMSO-d6, δ, ppm) 0.8 (t, 3H, CH3 of S-butyl); 1.1 (t, 3H, -OCH2-CH3); 1.3 (m, 2H, -CH2-CH3 of S-butyl); 1.5 (m, 2H, -CH2-CH2-CH3 of S-butyl); 2.6 (s, 3H, 6-CH3); 3.2 (m, 1H, S-CH2); 3.6 (m, 1H, S-CH2); 4.1 (q, 2H, -OCH2CH3); 5.8 (s, 1H, 4-CH); 7.1-7.7 (m, 4H, Ar-H); 8.6 (s, 1H, NH); EI-MS m/z (% base): 367 (10.9), 338 (22.1), 322 (100), 310 (17.9), 277 (40), 255 (38.7), 111 (25.7); Anal. Calc. for C18H23ClN2O2S: C, 58.92%; H, 6.32%; N, 9.66%. found: C, 58.11%; H, 6.56%; N, 9.87%.
Ethyl-2-(benzylsufanyl)-6-methyl-4-(3,4-dimethoxyphenyl)-1,4-dihydropyrimidine-5-carboxylate(4r). Yield 40%; m.p. 153-154°; IR (KBr, ν, cm-1)3266 (N-H), 3128 (Ar-H), 1668 (C=O), 1575 (C=C), 1325 (C=N), 1278 (C-O-C), 658 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 2.5 (s, 3H, 6-CH3); 3.9 (d, 6H, 3,4-(OCH3)2); 4.1 (q, 2H, -OCH2CH3); 4.2 (d, 1H, S-CH2); 4.9 (d, 1H, S-CH2); 5.8 (s, 1H, 4-CH); 7.2-7.9 (m, 8H, Ar-H); 12.0 (s, 1H, NH); EI-MS m/z (% base): 427 (16.3), 398 (7.8), 353 (5.2), 335 (16.8), 289 (18.2), 91 (100), 65.0 (100), 58 (15.2); Anal. Calc. for C23H26N2O4S: C, 64.77%; H, 6.14%; N, 6.57%. found: C, 64.53%; H, 6.24%; N, 6.65%.
Ethyl-2-(benzylsufanyl)-6-methyl-4-phenyl-1,4-dihydropyrimidine-5-carboxylate (4s). Yield 76%; m.p. 170-171°; IR (KBr, ν, cm-1) 3325 (N-H), 3225 (Ar-H), 1680 (C=O), 1575 (C=C), 1325 (C=N), 678 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 2.5 (s, 3H, 6-CH3); 4.1 (q, 2H, -OCH2CH3); 4.5 (d, 1H, S-CH2); 5.0 (d, 1H, S-CH2); 5.8 (s, 1H, 4-CH); 7.2-7.9 (m, 10H, Ar-H); 12.0 (s, 1H, NH); EI-MS m/z (% base): 367 (17.9), 338 (4.6), 289 (19.3), 276 (5.2), 144 (2.3), 91 (100), 77 (6.0), 65 (9.5), 58 (14.4); Anal. Calc. for C21H22N2O2S: C, 68.83%; H, 6.05%; N, 7.64%. found: C, 68.70%; H, 6.15%; N, 7.44%.
Ethyl-2-(benzylsufanyl)-6-methyl-4-(4-chlorophenyl)-1,4-dihydropyrimidine-5-carboxylate (4t). Yield 65%; m.p. 192-193°; IR (KBr, ν, cm-1)3315 (N-H), 3243 (Ar-H), 1665 (C=O), 1578 (C=C), 1338 (C=N), 718 (C-Cl), 678 (C-S); 1H NMR (DMSO-d6, δ, ppm) 1.1 (t, 3H, -OCH2-CH3); 2.5 (s, 3H, 6-CH3); 4.1 (q, 2H, -OCH2CH3); 4.3 (d, 1H, S-CH2); 4.9 (d, 1H, S-CH2); 5.8 (s, 1H, 4-CH); 7.2-7.9 (m, 9H, Ar-H); 10.0 (s, 1H, NH); EI-MS m/z (% base): 402 (21.6), 373 (8.9), 329 (15.2), 311 (12.6), 290 (75), 91 (100), 65 (12.6); Anal. Calc. for C21H21ClN2O2S: C, 62.91%; H, 5.28%; N, 6.99%. found: C, 63.25%; H, 5.48%; N, 7.11%.
Evaluation of in vitro anticancer activity:
The anticancer activities of the newly synthesized compounds in four concentrations were studied at Advanced Center for Treatment, Research and Education in Cancer (ACTREC), Mumbai by Sulforhodamine B (SRB) assay using four cancer cell lines (Human Colon SW620, Human Breast MCF7, Human Cervix HeLa and Human Hepatoma HEPG2) that were maintained in ideal laboratory conditions[19]. The cell lines were grown in RPMI 1640 medium containing 10% fetal bovine serum and 2 mM L-glutamine. For present screening experiment, cells were inoculated into 96 well microtiter plates 90 μl/well at appropriate plating densities, depending on the doubling time of individual cell lines. After cell inoculation, the micro titer plates were incubated at 37°, in 5% CO2, 95% air and 100% relative humidity for 24 h prior to addition of experimental drugs.
After 24 h, cells from one plate of each cell line were fixed in situ with trichloroacetic acid (TCA), to represent a measurement of the cell population for each cell line at the time of drug addition (Tz). Experimental extracts were solubilized in appropriate solvent at 400-fold, the desired final maximum test concentration and were frozen prior to use. At the time of drug addition, an aliquot of frozen concentrate was thawed and diluted 10 times the desired final maximum test concentration with complete medium containing test article at a concentration of 100, 200, 400 and 800 μg/ml. Aliquots of 10 μl of these different dilutions were added to the appropriate micro-titer wells already containing 90 μl of cell suspension, resulting in the required final drug concentrations of 10, 20, 40 and 80 μg/ml. For each of the experiments a known anticancer drug doxorubicin at concentrations of 10, 20, 40 and 80 μg/ml was used as a positive control.
Endpoint Measurement:
After compound addition, plates were incubated at standard conditions for 48 h and assay was terminated by the addition of cold TCA. Cells were fixed in situ by the gentle addition of 50 μl of cold 30 % (w/v) TCA (final concentration, 10 % TCA) and incubated for 60 min at 4°. The supernatant was discarded; the plates were washed five times with tap water and air-dried. SRB solution (50 μl) at 0.4 % (w/v) in 1 % acetic acid was added to each of the wells, and plates were incubated for 20 min at room temperature. After staining, unbound dye was recovered and the residual dye was removed by washing five times with 1% acetic acid. The plates were air-dried. Bound stain was subsequently eluted with 10 mM Trizma base, and the absorbance was read on an ELISA Plate Reader at a wavelength of 540 nm with 690 nm reference wavelength. Percent growth was calculated on a plate-by-plate basis for test wells relative to control wells. Percent Growth was expressed as the ratio of average absorbance of the test well to the average absorbance of the control wells×100.
Using the six absorbance measurements [time zero (Tz), control growth (C), and test growth in the presence of drug at the four concentration levels (Ti)]; the percentage growth was calculated at each of the drug concentration levels. Percent growth inhibition was calculated as, [(Ti-Tz)/(C-Tz)]×100 for concentrations for which Ti>/=Tz (Ti-Tz) positive or zero [(Ti-Tz)/Tz]×100 for concentrations for which Ti
The dose response parameters were calculated for each test article. Growth inhibition of 50 % (GI50) was calculated from [(Ti-Tz)/(C-Tz)]×100=50, which is the drug concentration resulting in a 50% reduction in the net protein increase (as measured by SRB staining) in control cells during the drug incubation. The drug concentration resulting in total growth inhibition (TGI) was calculated from Ti=Tz. The LC50 (concentration of drug resulting in a 50% reduction in the measured protein at the end of the drug treatment as compared to that at the beginning) indicating a net loss of cells following treatment is calculated from [(Ti-Tz)/Tz]×100=-50.
Values were calculated for each of these three parameters if the level of activity was reached; however, if the effect was not reached or was exceeded, the values for that parameter were expressed as greater or less than the maximum or minimum concentration tested. GI50≤10 μg/ml is considered as active for pure compounds.
Evaluation of in vitro antimicrobial activity:
All the clinically isolated bacterial strains namely Staphylococcus aureus (MTCC 7443), Bacillus subtilis (MTCC 1133), Pseudomonas aeruginosa (MTCC 2036), Escherichia coli (MTCC 2118) and fungal strains namely Candida albicans (MTCC 1637) and Aspergillus niger (MTCC 7369) were obtained from Institute of Microbial Technology, Chandigarh, India.
The in vitro activities of the compounds were tested in Sabouraud dextrose broth (SDB) (Hi-media, Mumbai) for fungi and nutrient broth (NB) (Hi-media, Mumbai) for bacteria by two-fold serial dilution method.[20] The respective test compounds 4a-t were dissolved in dimethylsulfoxide to obtain 1mg/ml stock solution. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37±1° while fungal spores from 1 to 7 days old Sabouraud agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104-105 cfu/ml. The final inoculum size was 105 cfu/ml for antibacterial assay and 1.1-1.5×102 cfu/ml for antifungal assay. Testing was performed at pH 7.4±0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 ml solution of test compound was added to 1.6 ml of seeded broth to form the first dilution. It was further serially diluted two folds till six dilutions. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in biological oxygen demand (BOD) incubators at 37±1° for bacteria and 25° for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observation after 24 h (for bacteria) and 72-96 h (for fungi) of incubation. Ciprofloxacin was used as standard for antibacterial studies and fluconazole was used as standard for antifungal studies.
RESULTS AND DISCUSSION
In this study, twenty new compounds 4a-t have been synthesized with scaffold 1,4-dihydropyrimidine and their in vitro anticancer, antibacterial and antifungal activities were evaluated. The compounds 3a-t were prepared from Biginelli reactions by using ethyl acetoacetate, substituted benzaldehyde and thiourea in presence of piperidine and ethanol. The compounds 3a-t were reacted with dimethyl sulpahte, diethyl sulphate, butyl bromide and benzyl chloride to give the new series of compounds 4a-t. The synthetic procedure for preparation of title compounds is given in Scheme 1. The structures of the compounds were confirmed by spectral methods IR, 1H NMR, MS and elemental analysis.
Scheme 1.
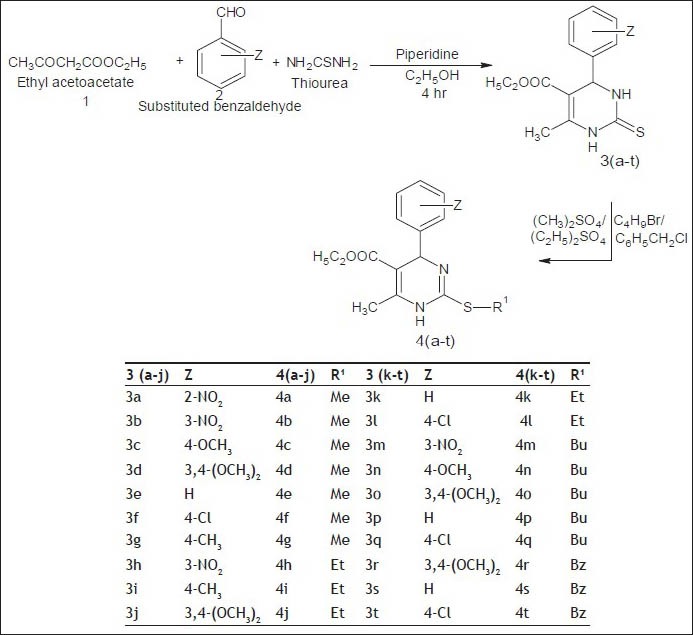
Synthesis of title compounds.
Z= 2-NO2, 3-NO2, 4-OCH3, 4-Cl, 2-CH3, 4-CH3, H; R1 = CH3, C2H5, C4H9, CH2C6H5. Me: Methyl; Et: ethyl; Bu: butyl; Bz: benzyl.
In IR spectra, some significant stretching bands due to N-H, C=O and C=N were observed at 3345-3198, 1688-1665 and 1345-1222 cm-1 respectively. In 1H NMR spectra of 4a-t, the signal due three hydrogen of −OCH2CH3 protons appeared at 1.0-1.2 ppm as triplet and two hydrogen of −OCH2CH3 appeared at 4.1 ppm as quartet. The 1,4-dihydropyrimidine N-H, 6-CH3, Ar-OCH3 and Ar-CH3 protons were observed at 8.6-10.1, 2.3-2.9, 3.7-3.8 and 2.3-2.4 ppm respectively. All the aromatic protons were observed in the expected regions. Mass spectra of compounds showed (M+1)+ and (M+2)+ peaks in agreement with their molecular formula.
The 1,4-dihydropyrimidine derivatives were evaluated for in vitro anticancer activity against the human cancer cell lines compared with the doxorubicin (ADR) as a reference drug at ACTREC, Mumbai by SRB assay using four cancer cell lines (Human Colon SW620, Human Breast MCF7, Human Cervix HeLa and Human Hepatoma HEPG2). The LC50, TGI and GI50 obtained with selected cell lines are summarized in Table 1.
TABLE 1.
IN VITRO ANTICANCER ACTIVITY OF THE SYNTHESIZED COMPOUNDS
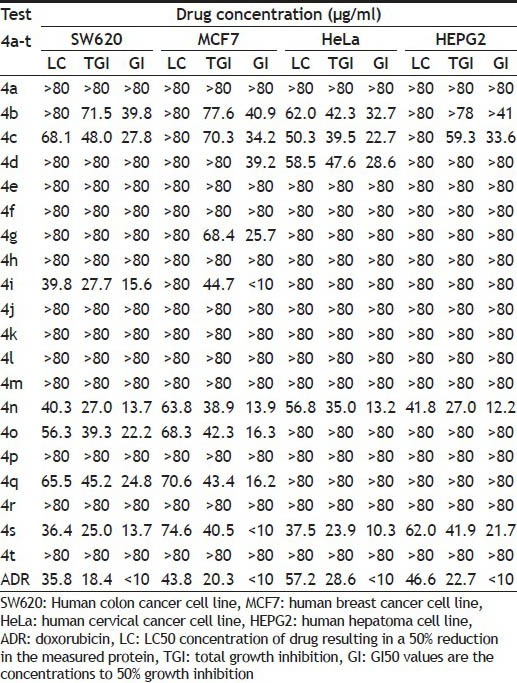
The compounds 4b, 4c, 4n and 4s showed anticancer activity against all four cell lines used in the study with GI50 in range of 32.7-41.8, 22.7-34.2, 12.2-13.9 and <10.0-21.7, respectively. Compounds 4i, 4o and 4q also showed anticancer activity against two cell lines (Human colon cancer SW620 and Human colon cancer MCF7) with GI50 in range of <10.0-15.6, 16.3-22.2 and 16.2-24.8 respectively. Moreover, 4i and 4s showed most prominent activity (GI50=<10.0) as compared with doxorubicin standard drug (GI50=<10) against Human breast cancer cell line MCF7.
Compound 4d exhibited anticancer activity against cell lines (Human breast cancer MCF7 and human cervix cancer HeLa) with GI50 in range of 28.6-39.2 whereas 4g showed GI50 value 25.7 against Human breast cancer MCF7. The compounds 4d and 4g showed lesser activity as compared with doxorubicin standard drug (GI50=<10). All the other synthesized compounds did not show significant anticancer activity.
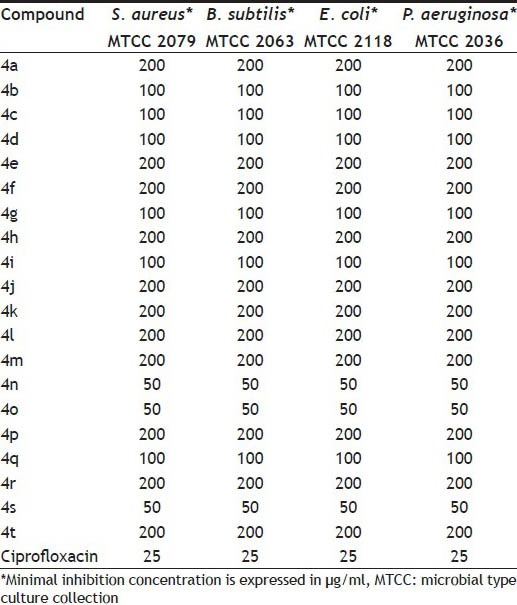
All the newly synthesized compounds 4(a-t) were tested in vitro for their antibacterial activity against two Gram-positive strains S.aureus, B.subtilis and two Gram-negative strains E.coli, P.aeruginosa. Minimum inhibitory concentration (MIC) in mg/ml is represented in Table 2. Compounds 4n, 4o and 4s exerted moderate activities with the MIC value of 50 mg/ml against tested Gram positive and Gram-negative strains. Remaining compounds did not show significant activity against tested microorganisms.
TABLE 2.
IN VITRO ANTIBACTERIAL ACTIVITY DATA OF THE SYNTHESIZED COMPOUNDS
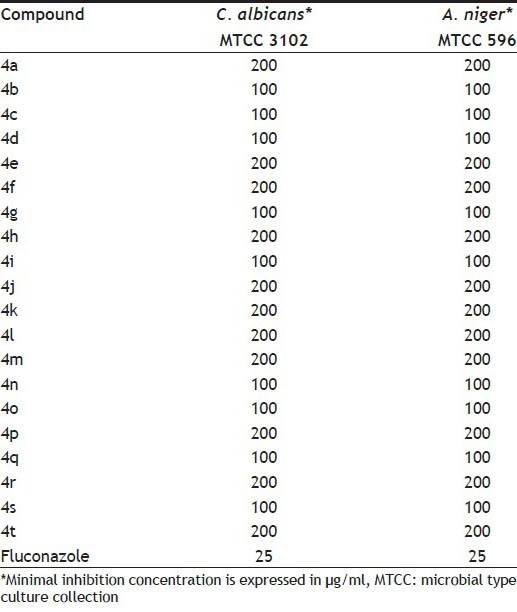
All the title compounds were screened for their antifungal potential and minimum inhibitory concentration (MIC) in mg/ml, which have been reported in Table 3. All the compounds did not show significant activity against C.albicans and A.niger.
TABLE 3.
IN VITRO ANTIFUNGAL ACTIVITY DATA OF THE SYNTHESIZED COMPOUNDS
In conclusion, the objective of this study was to design and synthesize 1,4-dihydropyrimidine derivatives. All the compounds were evaluated for their in vitro anticancer, antibacterial and antifungal activities. The results of anticancer activity evaluation demonstrated that the in vitro anticancer effect of some of the synthesized compounds are significant, however still there is a need for further exploration of these for other synthetic and biological possibilities so that this skeleton can be used as a novel anticancer scaffold for further modification and design of novel potent compounds.
Footnotes
Rana, et al.: Synthesis of Novel Substituted Dihydropyrimidines
REFERENCES
- 1.Baviskar BA, Khadabadia SS, Deorea SL, Shiradkar MR. Synthesis of clubbed triazolyl indeno[1,2-C] isoquinolines as an novel anticancer agent. Der Pharmacia Sinica. 2012;3:24. [Google Scholar]
- 2.Lamb DC, Kelly DE, Baldwin BC, Kelly SL. Differential inhibition of human CYP3A4 and Candida albicans CYP51 with azole antifungal agents. Chem Biol Interact. 2000;125:165–75. doi: 10.1016/s0009-2797(99)00169-6. [DOI] [PubMed] [Google Scholar]
- 3.John HB, John MB. 11th ed. New York: Lippincott Williams and Wilkins; 2004. Wilson and Gisvold's Textbook of Organic Medicinal and Pharmaceutical Chemistry; p. 217. [Google Scholar]
- 4.Sun Q, Xu J, Cao Y, Zhang W, Wu Q, Zhang D, et al. Synthesis of novel triazole derivatives as inhibitors of cytochrome P450 14α-demethylase (CYP51) Eur J Med Chem. 2007;42:1226–33. doi: 10.1016/j.ejmech.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Dirlam JP, Presslitz JE, Williams BJ. Synthesis and antibacterial activity of some 3 [(alkylthio)methyl]quinoxaline-1-oxide derivatives. J Med Chem. 1983;26:1122–6. doi: 10.1021/jm00362a007. [DOI] [PubMed] [Google Scholar]
- 6.Nasr MN, Gineinah MM. Pyrido[2, 3-d]pyrimidines and pyrimido[5’,4’:5,6]pyrido[2, 3-d]pyrimidines as new antiviral agents: Synthesis and biological activity. Arch Pharm (Weinheim) 2002;335:289–95. doi: 10.1002/1521-4184(200208)335:6<289::AID-ARDP289>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 7.Sondhi SM, Johar M, Rajvanshi S, Dastidar SG, Shukla R, Raghubir R, et al. Anticancer, anti-Inflammatory and analgesic activity evaluation of heterocyclic compounds synthesized by the reaction of 4-isothiocyanato-4-methylpentan-2-one with Substituted o-phenylenediamines, o-diaminopyridine and (Un) substituted. Aust J Chem. 2001;54:69–74. [Google Scholar]
- 8.Lanjewar KR, Rahatgaonkar AM, Chorghade MS, Saraf BD. Synthesis and antimicrobial activity of 5-(2-aminothiazol-4-yl)-3,4-dihydro-4-phenyl pyrimidin-2(1H)-one. Indian J Chem. 2009;48B:1732–7. [Google Scholar]
- 9.Rana K, Kaur B, Kumar B. Synthesis and antihypertensive activity of some dihydropyrimidines. Indian J Chem. 2004;43B:1553–7. [Google Scholar]
- 10.Dai Y, Guo Y, Frey RR, Ji Z, Curtin ML, Ahmed AA, et al. Thienopyrimidine ureas as novel and potent multi targeted receptor tyrosine kinase inhibitors. J Med Chem. 2005;48:6066–83. doi: 10.1021/jm050458h. [DOI] [PubMed] [Google Scholar]
- 11.Reddy GJ, Sailaja SS, Rao KS. Synthesis of 2-aryl-7-(3-oxo-2H-[1,4]-benzoxazin-6-yl)pyrazolo[1,5-a]pyrimidines as potential COX-2 inhibitors. Indian J Chem. 2004;44:204–6. [Google Scholar]
- 12.Atwal KS, Rovnyak GC, Kimball SD, Floyd DM, Moreland S, Swanson BN, et al. Dihydropyrimidine calcium channel blockers. 2. 3-substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J Med Chem. 1990;33:2629–35. doi: 10.1021/jm00171a044. [DOI] [PubMed] [Google Scholar]
- 13.Folkers K, Harwood HJ, Johnson TB. Synthesis of 2-keto-1,2,3,4-tetrahydropyrimidines. J Am Chem Soc. 1932;54:3751–8. [Google Scholar]
- 14.Gupta R, Gupta AK, Paul S, Kachroo PL. Improved synthesis of some ethyl 4-aryl-6-methyl-1,2,3,4-tetrahydropyrimidin-2-one/thione-5-carboxylates by microwave irridation. Indian J Chem. 1995;34B:151–2. [Google Scholar]
- 15.O’Reilly BC, Atwal KS. Synthesis of substituted 1,2,3,4-tetrahydro-6-methyl-2-oxo-5-pyrimidinecarboxylic acid esters: The Biginelli condensation revisited. Heterocycles. 1987;26:1185–8. [Google Scholar]
- 16.Atwal KS, O’Reilly BC, Gougoutas JZ, Malley MF. Synthesis of substituted 1,2,3,4-tetrahydro-6-methyl-2-thioxo-5-pyrimidinecarboxylic acid esters. Heterocycles. 1987;26:1189–92. [Google Scholar]
- 17.Atwal KS, Rovnyak GC, O’Reilly BC, Schwartz J. Substituted 1,4-dihydropyrimidines. 3. synthesis of selectively functionalized 2-hetero-1,4-dihydropyrimidines. J Org Chem. 1989;54:5898–907. [Google Scholar]
- 18.Biginelli P. Aldehyde-urea derivatives of aceto- and oxaloacetic acids. Gazz Chim Ital. 1893;23:360–416. [Google Scholar]
- 19.Skehn P, Storeng R, Scudiero A, Monks J, McMohan D, Vistica D, et al. New colorimetric cytotoxicity assay for anticancer drug screening. J Natl Cancer Inst. 1990;82:1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 20.Gopalakrishnan M, Thanusu J, Kanagarajan V, Govindaraju R. Design, syntheis and in vitro microbiological evaluation of 6,6-dimethyl-7,9-diaryl-1,2,4,5-tetraazaspiro[4,5]decan-3-thiones- A new series of tailor made compounds. J Enzyme Inhib Med Chem. 2009;24:406–12. doi: 10.1080/14756360802188099. [DOI] [PubMed] [Google Scholar]


