Abstract
This study was carried out as a prerequisite to evaluate the therapeutic potential of Camellia varieties. The crude extracts of six different plants of green tea Camellia assamica and Camellia sinensis were tested against three Gram-positive and four Gram-negative bacteria using agar disk diffusion method at 50 mg/ml concentration. 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) and diphenyl-(2,4,6-trinitrophenyl)iminoazanium free radical scavenging methods were performed to evaluate the antioxidant potential. Phytochemical constituents and trace metals were detected through thin layer chromatography and Inductively Coupled Plasma Atomic Emission Spectrophotometer, respectively. The maximum inhibition of Staphylococcus aureus was recorded by dimethyl sulphoxide extracts of green tea varieties. The measured zone of inhibition of dimethyl sulphoxide extracts by Qimen was (10.00±0.0 mm), Japanese (10.00±0.0 mm), Turkish (10.00±0.0 mm), Indonesian (8.33±1.0 mm), P3 clone (10.00±0.0 mm) and Sri Lankan (10.00±0.0 mm). Maximum scavenging potential activity was found with ethanol, methanol and dimethyl sulphoxide extracts. Spot screening of TLC-developed plates indicated that the presence of active biological compounds such as flavonoids, proteins, phenols, alkaloids and glycosides also exhibited strong activity against tested bacterial strains. This study reveals the potential biological activities of Camellia assamica and Camellia sinensis having massive phytochemical constituents and trace elements.
Keywords: Antibacterial assay, antioxidant activity, phytochemical screening, Camellia sinensis, Camellia assanica
Plants are commonly used in treating or preventing specific ailments or diseases and are playing valuable role in health care. Probably, around 60% of world's population is relying on medicinal plants for their primary healthcare[1]. Reasons for the vast use of medicinal plants are high cost of allopathic drugs and their side effects[2]. Majority of the human beings died mostly due to infectious bacterial diseases in the developing countries[3]. The synthetic antibiotics that are being used also became ineffective because the pathogens got resistance against these antibiotics[4]. Various strategies have been developed during the past decade e.g., biological screening, isolation as well as clinical trials for a variety of plants to explore the secret of their therapeutic actions. Now a day, bioactive plant extracts are considered as a promising source of biological friendly antibacterial agents[5].
Camellia genus belongs to Theaceae family, found in southern and eastern Asia, from the Himalayas east to Japan and Indonesia. Green tea (Camellia) has received much attention as a beverage worldwide during last few decades due to its various beneficial effects on human health. Studies reveal that green tea possesses diverse pharmacological properties to lower incidence of oral cancer, stroke, cardiovascular diseases and fatness[6]. Green tea was also reported as useful against hepatitis and influenza viruses[7]. In the present study, we have investigated the bioactivity of following six different varieties of green tea, Camellia sinensis and Camellia assamica, cultivated at Shinkiari, Mansehra, Pakistan.
Fresh leaves of six different varieties of green tea such as Camellia sinensis (Qimen, P3 clone, Japanese and Turkish) and Camellia assamica (Indonesian and Sri Lankan) were collected and identified from National Tea Research Institute (NTRI) Shinkiari, Mansehra, Pakistan. Tea plants were selected based on ethano-medicinal importance. Leave samples were thoroughly rinsed with running tap water, dried under shade and were crushed in fine powder with the help of electric grinder. Approximately 20 g of leaf powder of each variety was separately soaked in 200 ml of both polar and non polar solvents viz., methanol, ethanol, chloroform, pet ether and dimethyl sulphoxide (DMSO) for 15-20 days. Each extract was filtered and stored at room temperature for further processing.
Trace metals such as sodium (Na), calcium (Ca), iron (Fe), manganese (Mn), copper (Cu), cobalt (Co), chromium (Cr), molybdenum (Mo), zinc (Zn), potassium (K) and magnesium (Mg) were detected in μg/g units by atomic absorption spectrophotometer and cross checked by inductively coupled plasma atomic emission spectrophotometer. Standard procedure was adopted for the acid digestion. Solid samples were digested in Aqua Regia (HCl:HNO3 (3:1)) for 3 days.
Gram-positive cocci (S. aureus, S. pyogene and S. epidermidis) and Gram-negative rod (P. aeruginosa, K. pneumonia, E. coli, and S. marcescens) clinical bacterial pathogens were isolated and identified in Microbial Biotechnology laboratory, University of Azad Jammu and Kashmir, Muzaffarabad, Pakistan[8].
Agar disc diffusion method was used to determine the antibacterial activity of various extracts of Camellia varieties as described by Alzoreky and Nakahara[9]. Nutrient agar and nutrient broth media (NAM; Oxide CMOO3 and NBM; CM1) were used for bacterial culture. The overnight culture was mixed with freshly prepared nutrient agar medium at 45° and the mixture was poured into the sterilized Petri dishes. All Petri dishes were kept at room temperature in laminar flow for solidification. The discs of 5 mm diameter were soaked with various prepared extracts and placed on agar surface. The Petri dishes were incubated at 37° for 48 h. Discs, soaked with chloroform, ethanol, methanol, dimethyl sulphoxide and pet ether were used as negative control. Microbial growth inhibition was assessed by measuring the zone of inhibited diameter (mm) after 48 h of incubation. Before each experiment, the optimal density (O.D.) of bacterial growth 107 colony forming units (cfu)/ml was measured through spectrophotometer at 600 nm[10]. Each experiment was performed in triplicate. The results of sensitivity tests were denoted as (0-<1 mm) for no sensitivity, (1-4 mm) for low sensitivity, (>4-8 mm) for moderate sensitivity and (>8-15 mm) for high sensitivity. Antibiogram analysis (ampicillin (10 μg/ml), penicillin G (10 μg/ml), tetracycline (30 μg/ml), amoxicillin (10 μg/ml), and oxytetracycline (30 μg/ml)) against all tested bacterial strains was assessed by agar disc diffusion method and used as positive control[11].
ABTS+ or 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) free radical scavenging activity was carried out to evaluate the antioxidant potential of Camellia extracts according to Re et al[12]. On the other hand DPPH [(diphenyl)-(2,4,6-trinitrophenyl)iminoazanium] free radical scavenging activity of Camellia extracts was also determined with slight modification[13]. Half milliliter of MeOH (0.1 mM) solution of DPPH was mixed with 20-150 μl of extracts, mixed with vortex vigorously and left for 30 min at 37° in an incubator. The volume (2 ml) was made up by adding methanol. Methanol was used as a baseline control. The absorbance was recorded at 517 nm. Water (20-150 μl) was used as a control and the percent scavenging activity was calculated by formula: %=[(Ao-Ai)/Ao]×100.
All extracts were analyzed for phytoconstituents such as glycosides, flavonoids, tannins, saponins, amino acids, terpenoides, alkaloids, and phenols[14,15]. The presence of major phytochemical constituents of Camellia extracts was further confirmed by thin layer chromatography (TLC) using precoated Silica gel 60F264 plates[16]. In order to get better resolution of the components, different screening systems were used (SS1:Chloroform/hexane/acetic acid (50:50:1), SS2:Chloroform/ethyl acetate/acetic acid (50:50:1), SS3:Methanol and chloroform (20:80), SS4:Petroleum ether and ethyl acetate (7:3) and SS5:Methanol and water (92:8)). The TLC plates were kept in the tank without touching the baseline and left for the development. The solvent front was marked, developed plates were dried and observed under normal and UV light (734 nm). The spots of various colors were also recorded. Rf value of each spot was calculated as Rf =distance travelled by the solute/distance travelled by the solvent.
After the development of chromatogram on TLC plates, various chemical sprays were used for the detection of phytochemicals[17]. For flavonoids, AlCl3 was used (yellow, green and blue fluorescence in longer wavelength UV light (360 nm) indicated positive results). Fast blue B reagent spray was used for the detection of phenols (red spots appear), 1% ninhydrin spray for amino acids (reddish spots appear), 37% formaldehyde in H2SO4 for alkaloids and urea in HCl for sugars (pink spots, ketoses in blue spots).
To measure the direct bioautography, agar overlay technique was used with minor modifications as demonstrated by Slusarenko et al[18]. All tested bacteria were used to screen bioautography. Extract (10 ml) was used to spot on the chromatograph silica gel plates. For separation, different solvent systems as mentioned above were used. The developed chromatogram was placed in sterilized Petri plates then the fresh overnight grown culture of tested bacteria was mixed with nutrient agar and poured over the chromatogram as a thin layer. The plates were left at room temperature for 5 min then left for incubation at 37° for overnight. The zone of growth inhibition was recorded around the active chromatogram spot.
TLC spot screening was performed using modified protocol of Joshi et al[19]. Spots on the preparative silica gel plates were scratched with the help of clean and dry spatula and collected in beaker containing 70% ethanol and left overnight. The content in the beaker was stirred and filtrated through Whatman No. 1 filter paper. The filtrate was collected in clean and dry beaker. The filtrate containing active compound was used for the determination of antimicrobial effect, assessed by the agar-well diffusion method[20].
Each experiment was repeated in triplicates. Mean±SD from absolute data was calculated using online calculator (http://easycalculation.com/statistics/standard-deviation.php). The comparison of antibacterial activity of solvent extracts with that of standard antibiotics was also determined by activity index (AI)[21] using formula, AI=(zone of inhibition of extract/zone of inhibition of antibiotic).
Trace metals such as sodium (Na), calcium (Ca), and magnesium (Mg) were detected in different varieties of Camellia through atomic absorption spectroscopy and cross confirmed by inductively coupled plasma atomic emission spectroscopy. It was observed that potassium was found in higher concentration. The quantity of other trace metals in P3 clone (Na: 32 μg/g, Ca: 7.8 μg/g, Mg: 6.4 μg/g), Sri Lankan (Na: 31 μg/g, Ca: 8.4 μg/g, Mg: 7.2 μg/g), Japanese (Na: 29 μg/g, Ca: 9.3 μg/g, Mg: 4.9 μg/g), Qimen (Na: 28 μg/g, Ca: 2.7 μg/g, Mg: <3 μg/g), Turkish (Na: 20.7 μg/g, Ca: 7.5 μg/g, Mg: 9.2 μg/g), and Indonesian (Na: 19.4 μg/g, Ca: 20.1 μg/g,Mg: 11.2 μg/g) was recorded. Cobalt, chromium and molybdenum were not detected whereas low quantity of iron, manganese, and zinc was found in dried leaf samples.
The results of antibacterial activity of Camellia sinensis (Qimen, P3 clone, Japanese, and Turkish) and Camellia assamica (Indonesian, and Sri Lankan) extracts through agar disc diffusion method are shown in Table 1. It was observed that methanol and ethanol showed the significant inhibition of all tested microorganisms, namely K. pneumonia, S. marcescens, S. pyogene, P. aeruginosa and S. epidermidis while dimethyl sulphoxide extracts exhibited the antibacterial activity of all tested bacteria. On the other hand pet ether (1-6 varieties) and chloroform (1-6) had minimum effect on all tested pathogens.
TABLE 1.
ZONE OF INHIBITION OF CAMELLIA EXTRACTS AGAINST PATHOGENIC BACTERIA
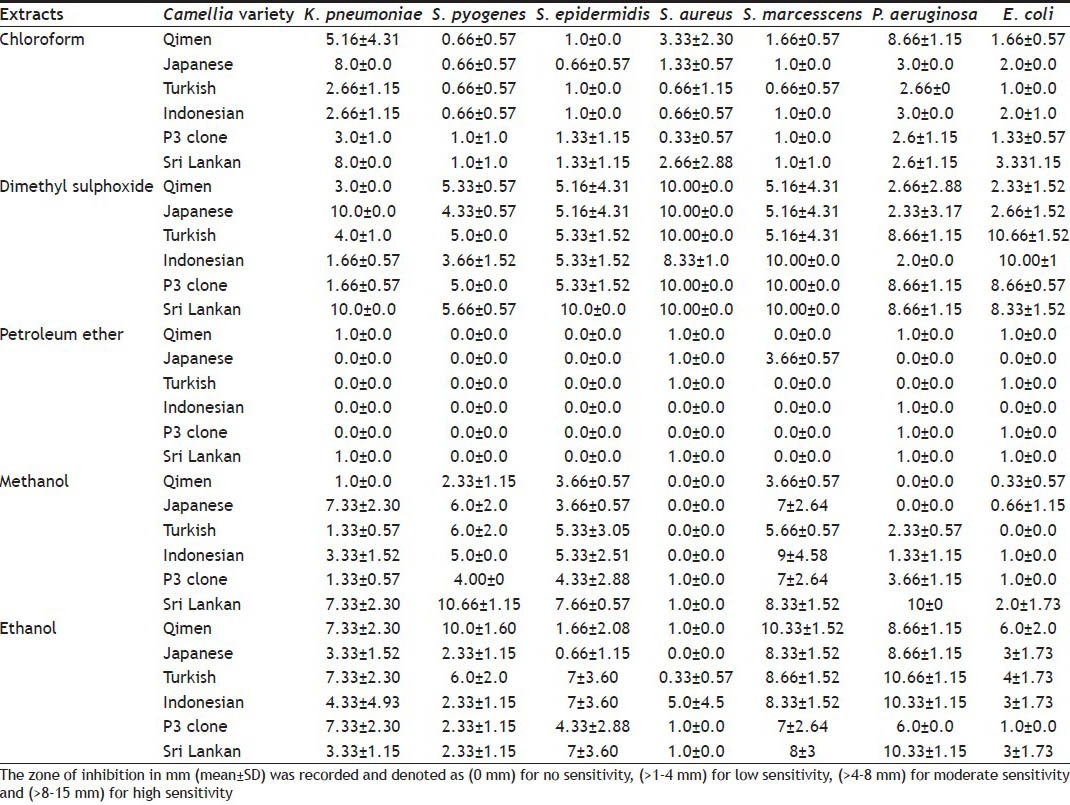
It is noteworthy that S. pyogene and P. aeruginosa were the most sensitive of the tested bacteria to the ethanol extract of Qimen, Japanese, Turkish, Indonesian and Sri Lankan varieties whereas S. marcescens, P. aeruginosa, and E. coli were susceptible to DMSO extracts of Indonesian, P3 clone and Sri Lankan at the concentration of 50 mg/ml (Table 1). Interestingly, the maximum inhibition of S. aureus was recorded when DMSO extracts of all varieties were used at same concentration. The measured zone of inhibition by Qimen was (10.00±0.0 mm), by Japanese (10.00±0.0 mm), Turkish (10.00±0.0 mm), by Indonesian (8.33±1.0 mm), by P3 clone (10.00±0.0 mm) and Sri Lankan (10.00±0.0 mm). Similarly, inhibition by Turkish (10.66±1.52 mm), Indonesian (10.00±0.0 mm), P3 clone (8.66±0.57 mm), and Sri Lankan (8.33±1.52 mm) was found against E. coli. On the other hand, moderate inhibition of S. epidermidis was indicated by all DMSO extracts of all varieties except extract of Sri Lankan variety (Table 1). Among the standard antibiotics, tetracycline significantly inhibited K. pneumonia (14.0±0.0 mm), followed by oxytertacycline (8.0±0.0 mm), ampicillin (6.0±0.0 mm) and amoxicillin (6.0±0.0 mm), respectively. On the other hand, all used antibiotics had no effect on the growth of S. pyogenes and S. aureus. In case of activity index analysis, higher or less activities were observed at 50 mg/ml concentration of all extracts than the standard drugs such as ampicillin, penicillin G, amoxicillin, tetracycline and oxytetracycline.
The preliminary phytochemical investigation revealed the presence of terpenoides, flavonoids, phenols, and alkaloids in all varieties of Camellia extracts whereas saponins and amino acids were not detected. On the other hand, glycosides and tannins were detected only in methanol, ethanol and DMSO extracts but tannins were also found in pet ether extracts. In TLC analysis, different absorbing bands were observed under UV light (254 nm and 336 nm). All varieties showed good separation of phytochemical constituents in the presence of SS1:Chloroform/hexane/acetic acid (50:50:1), SS2:Chloroform/ethyl acetate/acetic acid (50:50:1), SS4:Petroleum ether and ethyl acetate (7:3) and SS5:Methanol and water (92:8). Mostly, spots of yellow, green, brown, dark green and dark brown colors were observed on TLC plates which indicated the presence of flavonoids, phenols, glycosides and alkaloids, respectively. The presence of these phytochemical constituents were further reconfirmed on the TLC-developed plates by spraying the different chemicals such as AlCl3 spray indicated the presence of flavonoids, Fast blue B reagent spray for phenols, 37% formaldehyde in H2SO4 for alkaloids, 1% ninhydrin for amino acids and urea in HCl for sugars, respectively.
TLC bioautography of methanol, ethanol and DMSO extracts of Camellia varieties indicated the significant inhibition of S. aureus, S. epidermidis and S. marcescens, respectively. Similar results were recorded by spot screening of TLC–developed plates against all clinical bacterial pathogens. The zone of inhibition was recorded in range from 10.00±0.0 mm to 28.00±0.00 mm.
In order to determine the free radical scavenging power of Camellia varieties by DPPH and ABTS, five different solvents were used at 50 mg/ml concentration. The better results were found in ethanol, methanol and DMSO extracts as compared to chloroform and pet ether extracts. Both DPPH and ABTS showed similar results (fig. 1).
Fig. 1.
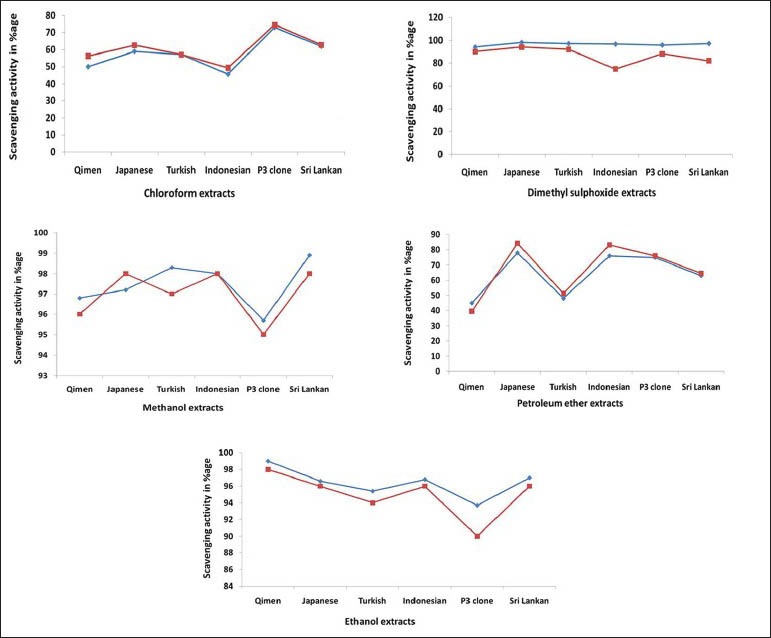
DPPH and ABTS.+ free radical scavenging activity of extracts of Camellia varieties.
 ABTS,
ABTS,  DPPH.
DPPH.
In current research two tea varieties such as China tea plant (Camellia sinensis) and Assam tea plant (Camellia assamica) were used. China tea plant is a big shrub with hard, thick and leathery leaves; and generally flowers white sometimes with pink pigmentation. Whereas Assam tea plant is a small tree with thin and glossy leaves; and white flowers, occasionally with pale yellow pigmentation. It has been shown that C. sinensis cultivated in China, Japan and Turkish whereas C. assamica cultivated in Indonechia and Sri lanka, respectively. P3 clone is the hybrid of Chinese tea varieties. Green tea is currently considered a source of dietary constituents capable with biological and pharmacological activities for human health. The extracts prepared from leaves to be used as medicine. The present study provides the information on the antibacterial and antioxidant importance of green tea varieties. Many therapeutic values of green tea has been explored including lowering body temperature, blood purification, aiding digestion, strengthening teeth and boons, boost immune system, suppress aging, lower the blood sugar levels and enhance the heart functions[22].
Essential and non essential trace metals in fruits and vegetables may decrease the chronic diseases such as cancer and cardiovascular diseases[23]. The higher concentrations of potassium, sodium, magnesium and calcium were found in the dry leaves of C. sinensis and C. assamica whereas iron, copper and zinc found in low concentration. These trace metals could also have great effect on the metabolic pathways of microbes[24]. Some toxic metals can stop the metabolic processes, can accumulate and interfere in the body of microbe. So, the interactions between the metals and microorganisms are diverse and lead to the death of nosocomial infections[25]. In view of above facts, it is expected that the green tea plants have high contents of important elements like Mg, Ca, K, and Fe, might play an important role in the maintenance of human health. Our results are consistent with the previous findings[26].
The plant extracts have been used for many years in pharmaceuticals, food preservation, natural therapies and alternative medicines[27]. In our study, the crude extracts exhibited varying degree of inhibitory activity, an effect that might be due to differences in the polysaccharide charges and cell wall structure of the bacteria. Gradisar et al.[28] demonstrated that phenolic compounds of green tea (catechins) inhibit the bacterial DNA gyrase and leading to bacterial cell death. Similarly, Ikigai et al.[29] also reported that catechins involved in the damaging of lipid bilayer of bacterial cell. Taylor et al.[30] also supported the antimicrobial properties of green tea.
Our in vitro studies showed that the screened phytochemical constituents supported the results of antibacterial assay. The leave extracts of Camellia varieties indicated the presence of active biological compounds such as flavonoids, proteins, phenols, alkaloids and glycosides which exhibited strong activity against selected bacterial strains. It has been shown that polar solvents having bioactive compounds involved in antimicrobial, anticancer and antioxidant activities[31]. Our findings are consistent with the previous report[32]. C. sinensis and C. assamica showed the moderate inhibition of both Gram-positive and Gram-negative bacteria. Antibacterial, antifungal and antioxidant activities of green tea have also been reported[31,32]. Ethanol, methanol and DMSO extracts of green tea varieties showed the inhibition of all tested bacteria. These extracts have chemical compounds which are responsible for the inhibition of S. aureus, S. epidermidis, K. pneumonia, S. marcescens, S. pyogene and P. aeruginosa. Turkmen et al.[32] reported the antibacterial, antioxidant and phytochemical screening of black tea.
Data presented in this study revealed potential in vitro antibacterial and antioxidant activities of Camellia varieties having both essential and non essential trace metals, and bioactive compounds. Further studies may require optimizing the extraction of bioactive compounds from green plants, chemical portrayal of such compounds and further testing of their effects in vivo.
ACKNOWLEDGMENTS
Authors are indebted to National Tea Research Institute (NTRI) for providing tea samples.
Footnotes
Bashir, et al.: Green Tea (Camellia) Plant Extracts as Antibacterial and Antioxidant Agents
REFERENCES
- 1.Bhat JA, Kumar M, Bussmann RW. Ecological status and traditional knowledge of medicinal plants in Kedarnath Wildlife Sanctuary of Garhwal Himalaya, India. J Ethnobiol Ethnomed. 2013;9:1. doi: 10.1186/1746-4269-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marwat SK, Khan MA, Ahmad M, Zafar M, Rehman F. Ethnomedicines for treatment of various diseases in D.I. Khan District. Sarhad J Agric. 2008;24:2. [Google Scholar]
- 3.Nathan C. Antibiotics at the crossroads. Nature. 2004;431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 4.Walsh FM, Amyes SG. Microbiology and drug resistance mechanisms of fully resistant pathogens. Curr Opin Microbiol. 2004;7:439–44. doi: 10.1016/j.mib.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–20. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 6.Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: A literature review. Chin Med. 2010;5:13. doi: 10.1186/1749-8546-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fassina G, Buffa A, Benelli R, Varnier OE, Noonan DM, Albini A. Polyphenolic antioxidant (-)-epigallocatechin-3-gallate from green tea as a candidate anti-HIV agent. AIDS. 2002;16:939–41. doi: 10.1097/00002030-200204120-00020. [DOI] [PubMed] [Google Scholar]
- 8.Awan UA, Andleeb S, Kiyani A, Zafar A, Shafique I, Riaz N, et al. Antimicrobial screening of traditional herbal plants and standard antibiotics against some human bacterial pathogens. Pak J Pharm Sci. 2013;26:1109–16. [PubMed] [Google Scholar]
- 9.Alzoreky NS, Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int J Food Microbiol. 2003;80:223–30. doi: 10.1016/s0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 10.Seeley HW, Vandemark PJ, Lee JJ. 4th ed. New York: W. H. Freeman and Company; 2001. Microbes in Action: A Laboratory Manual of Microbiology. [Google Scholar]
- 11.Kirby-Bauer A. Antimicrobial sensitivity testing by agar diffusion method. J Clin Pathol. 1996;44:493. [Google Scholar]
- 12.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans CA. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 13.You WC, Brown LM, Zhang L, Li JY, Jin ML, Chang YS, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 14.Trease GE, Evans WC. 15th ed. London: Saunders Publishers; 2002. Pharmacognosy; pp. 221–393. [Google Scholar]
- 15.Siddiqui AA, Ali M. 1st ed. New Delhi: CBS Publishers and distributors; 1997. Practical Pharmaceutical chemistry; pp. 126–31. [Google Scholar]
- 16.Wagner H, Bladt S. 2nd ed. New Delhi: Thompson Press Ltd; 2004. Plant drug analysis-A thin layer chromatography atlas. [Google Scholar]
- 17.Lacaille-Dubois MA. Bioactive Saponins from plants: Recent Development. In: Yaniv Z, Bacherach U, editors. Handbook of Medicinal plants. India: Harworth Press; 2007. [Google Scholar]
- 18.Slusarenko AJ, Longland AC, Whitehead IM. Convenient, sensitive and rapid assay for antibacterial activity of phytoalexins. Bot Helv. 1998;99:203–7. [Google Scholar]
- 19.Joshi B, Sah GP, Basnet BB, Bhatt MR, Sharma D, Subedi K, et al. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (Tulsi), Eugenia caryophyllata (Clove), Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem) J Microbiol Antimicrob. 2011;3:1–7. [Google Scholar]
- 20.Kingsbury DT, Wagner GE. Maryland: Williams and Wilkins; 1990. Microbiology; p. 36. [Google Scholar]
- 21.Shekhawat N, Vijayvergia R. Evaluation of antimicrobial potential of some medicinal plants against plant and human pathogens. J Pharm Res. 2010;3:700–2. [Google Scholar]
- 22.Sharangi AB. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.) – A review. Food Res Int. 2009;42:529–35. [Google Scholar]
- 23.Malin A, Qi D, Shu X, Gao Y, Friedmann J, Jin F, et al. Intake of fruits and vegetables and selected micronutrients in relation to the risk of breast cancer. Int J Cancer. 2003;105:413–8. doi: 10.1002/ijc.11088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehninger AL. The role of metal ions in enzyme systems. Physiol Rev. 1950;30:393–429. doi: 10.1152/physrev.1950.30.3.393. [DOI] [PubMed] [Google Scholar]
- 25.Pagmantidis V, Méplan C, van Schothorst EM, Keijer J, Hesketh JE. Suplementation of health volunteers with nutrionally relevant amounts of seleniun increases the expression of lymphoctyteprotien biosynthesis gene. Am J Clin Nutr. 2008;87:181–9. doi: 10.1093/ajcn/87.1.181. [DOI] [PubMed] [Google Scholar]
- 26.Schneider C, Segre T. Green tea potential health benefit. Am Fam Physician. 2009;79:591–4. [PubMed] [Google Scholar]
- 27.Jones FA. Herbs – useful plants. Their role in history and today. Eur J Gastroenterol Hepatol. 1996;8:1227–31. doi: 10.1097/00042737-199612000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Gradisar H, Pristovsek P, Plaper A, Jerala R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J Med Chem. 2007;50:264–71. doi: 10.1021/jm060817o. [DOI] [PubMed] [Google Scholar]
- 29.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–6. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 30.Taylor PW, Hamilton-Miller JM, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vijaya K, Ananthan S, Nalini R. Antibacterial effect of theaflavin, polyphenon 60 (Camellia sinensis) and Euphorbia hita on Shigella spp.-a cell culture study. J Ethanopharmacol. 1995;49:115–8. doi: 10.1016/0378-8741(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 32.Turkmen N, Velioglu YS, Sari F, Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–96. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]


