Abstract
Aims:
Percutaneous aspiration and ethanol injection (PEI) is effective in managing predominantly cystic (>50% cystic) thyroid nodules with efficacy ranging from 38-85%. We aimed to evaluate efficacy, safety, and factors determining outcomes of PEI in managing simple cystic (purely cystic) vs. complex cystic (having both cystic and solid components) thyroid nodules.
Materials and Methods:
Predominantly cystic thyroid nodules, without any ultrasonography and/or fine needle aspiration, evidence of malignancy were aspirated ultrasonography guided. Sterile 100% ethanol (50-100% volume aspirated) was injected and reviewed monthly for 3 months and thereafter 3 monthly. Responders were defined as ≥ 50% reduction in nodule volume.
Results:
Sixty-five patients out of 152 considered underwent PEI. Sixty patients [simple cystic (42) and complex cystic (18)] with mean follow-up of 12.3 ± 2.88 months were analyzed. Response rate of PEI was 78.33% [simple cystic (92.86%) and complex cystic (44.44%) nodules; P < 0.001]. Also, 31.67% patients achieved remission at 1st month. And, 46.67% patients achieved remission between 1-6-months follow-up. Kaplan Meier analysis showed significantly improved outcomes in patients with simple cystic nodules (P < 0.001). Cox-regression revealed type of nodule (simple cystic vs. complex cystic) to be predictive of outcome (P = 0.034). Complex cystic nodules were 67.6% less likely to go into remission, compared to simple cystic nodules. Baseline nodule size, aspirate, or volume of ethanol injected did not predict outcome.
Conclusions:
PEI is safe and should be treatment of choice for simple cystic thyroid nodules. PEI for complex cystic thyroid nodules are associated with lower response, increased recurrence, and need for repeated PEI.
Keywords: Complex cystic nodule, percutaneous ethanol ablation, simple cystic nodule thyroid cyst
INTRODUCTION
Thyroid nodules are common discreet lesions in the parenchyma of thyroid gland which can either be palpated or made out during imaging like ultrasonography (USG).[1] Clinically palpable thyroid nodules have a prevalence of 4-7% in the general population.[2] However, the prevalence increases to 20-76% when USG is used for detection.[3] Prevalence of clinical palpable goiter is higher in eastern India ranging from 9-33% from different studies done in the past decade[4,5,6] as compared to 9-15% in rest of the country.[7,8] The combination of high prevalence of goiter along with normal urine iodine excretion observed in eastern India[4,5,6] is an indication of transition from iodine deficient to an iodine-sufficient state, a result of more than 2 decades of universal salt iodinization. Goiter and the associated thyroid nodules result in anxiety, cosmetic disfigurement, and rarely compressive symptoms necessitating surgical removal, a procedure inherently associated with risks and complications.
Percutaneous sclerotherapy has been suggested to be an effective alternative, especially in patients with cystic nodules. Simple cystic (purely cystic) and complex cystic (having both cystic and solid components) constitutes 6-28% of all thyroid nodules, are usually benign, filled with cellular debris or blood, and are a result of degeneration or hemorrhage into a hyperplastic nodule or adenoma.[9] Among the various compounds (sodium tetradocyl sulfate, hydroxypolyethoxydodecan, tetracycline, and ethanol) tried for sclerosis of cystic thyroid nodules, outcomes are best and most studied with ethanol.[10] However, data on outcomes of percutaneous aspiration and ethanol injection (PEI) in resolution of thyroid nodules is highly variable in different studies (success rate: 38-85%), which may be due to different populations studied and the heterogeneous nature of thyroid nodules evaluated, ranging from purely cystic to purely solid nodules.[11,12,13,14,15]
Data of PEI in managing cystic thyroid nodules is not available from eastern India. Hence, the aim of this study was to evaluate the efficacy and safety of PEI in managing predominantly cystic benign thyroid nodules in euthyroid individuals, with special reference to simple cystic nodules as compared to complex cystic nodules (having both cystic and solid components). Study outcomes were assessed in terms of responders and non-responders. Responders and non-responders were defined as ≥50% and < 50% reduction in nodule volume, respectively, on USG during follow-up. We also planned to evaluate whether the final outcomes are affected by the baseline nodule size, amount of fluid aspirated, nature of fluid aspirated (serous/turbid), and the volume of ethanol injected. This would help us in delineating the cohort of patients who are most likely to respond to PEI, thus reducing failure rates and need for recurrent sittings of PEI.
MATERIALS AND METHODS
The study was conducted as collaboration between the department of radiodiagnosis and department of endocrinology and metabolism. The study period was from August 2011 till February 2014. Consecutive patients who had presented to the endocrinology clinic with complaints of goiter were considered. Thyroid function was evaluated, and assessment of goiter for thyroid nodules was done using USG. Nodules having one or more high risk features of malignancy on USG (irregular margins, hypoechoic nodules, nodules taller than broader, loss of peripheral halo, microcalcifications, increased central vascularity)[16] underwent USG-guided fine needle aspiration (FNA) and cytology evaluation. Clinically and biochemically euthyroid patients who on clinical examination and neck USG had thyroid nodules which were predominantly cystic (≥50% cystic) with at least 2 ml cystic component on USG were included in the study. Patients were excluded if they had 2 or more high risk feature of malignancy on thyroid USG[16] or FNA suggestive of malignancy, suspicious, or indeterminate cytology. The study was approved by the institutional ethics committee. The study protocol was explained to all the patients and only those who gave informed written consent were included. The study protocol has been elaborated in Figure 1.
Figure 1.
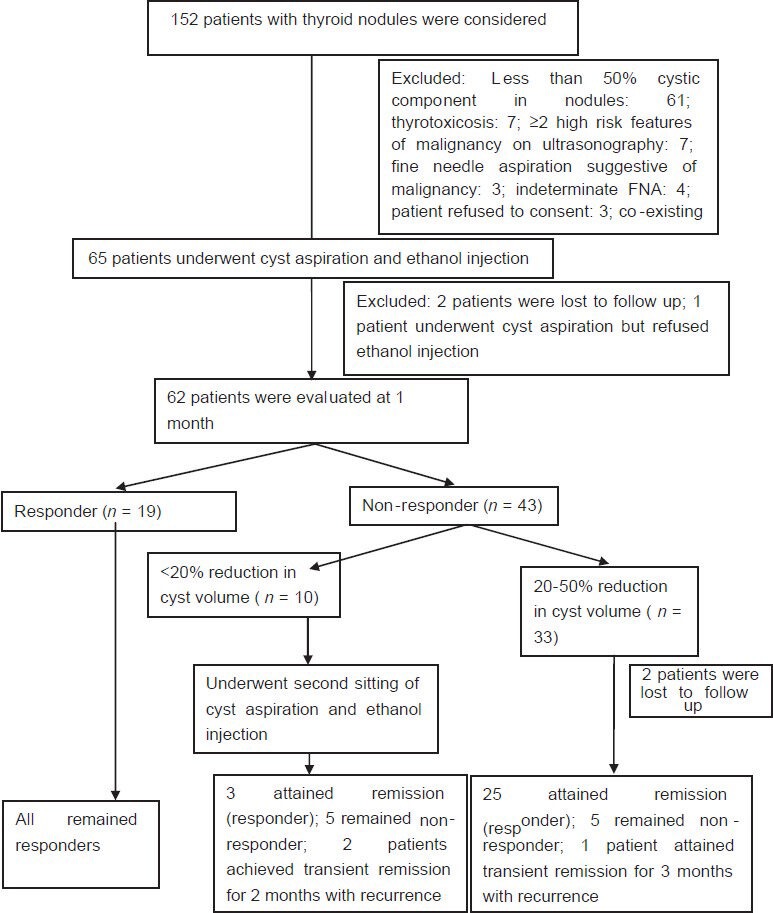
Study protocol
USG neck and thyroid was performed using Toshiba Xario-XG machine (Model number: SSA-680A, Japan) with high frequency transducer of 7.5-12 MHz. Thyroid nodules were classified as simple cystic nodules which were purely cystic without any solid component, or complex cystic nodules, which had both solid and cystic components [Supplementary Figures 1 and 2]. The volume of the simple cystic nodule or the cystic component of the complex cystic nodule was calculated using the formula 0.52 × length × breadth × height.[17] The cysts were aspirated USG guided under aseptic conditions using a 22 G (25 mm) needle with a 20 ml syringe in supine position with neck extended. Aspiration of maximal amount of fluid possible was attempted in each patient. In patients with thick fluid not draining with the 22 G needle, a second re-aspiration was attempted using a 20 G or 18 G needle. Sterile 100% ethanol specially manufactured by Bengal Chemicals (Calcutta, India) of around 50-100% volume of fluid aspirated was injected into the cyst by changing the syringe with the needle in situ [Supplementary Figures 3 and 4]. The alcohol was left inside the cyst without re-aspiration. The patients were reviewed clinically and ultrasonographically monthly for the first 3 months and thereafter 3 monthly. Patients with reduction of nodule volume by ≥50% of baseline were defined as responders, and the rest were defined as non-responders. Among non-responders at 1 month, those with < 20% reduction in cyst volume underwent a second aspiration followed by ethanol injection using the same procedure as mentioned above.
Supplementary Figure 1.
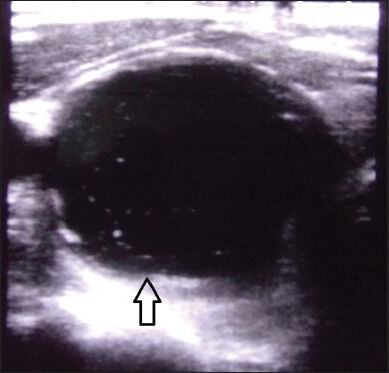
Ultrasonography neck showing simple cystic thyroid nodule
Supplementary Figure 2.
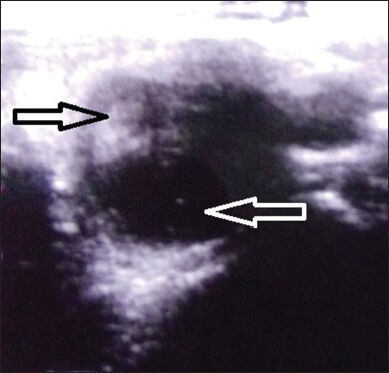
Ultrasonography neck showing complex cystic thyroid nodule (solid component: Black arrow; cystic component: White arrow)
Supplementary Figure 3.
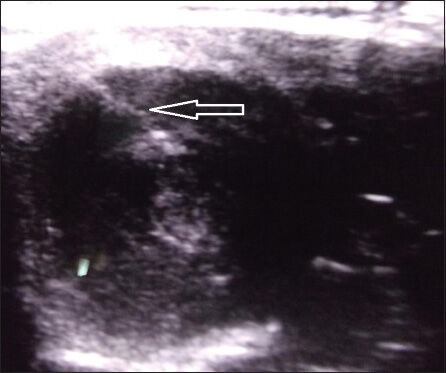
Ultrasonography showing needle inside a thyroid nodule (white arrow)
Supplementary Figure 4.
Ultrasonography showing ethanol injected into thyroid nodule
Statistical analysis
All data were elaborated as mean ± standard deviation. Continuous variables were analyzed using t-tests and discrete variables were analyzed using Chi-square test. P < 0.05 was considered statistically significant. Follow-up outcomes (responders) of aspiration and ethanol injection were plotted using Kaplan-Meir analysis. Cox-regression was done with all baseline parameters to evaluate their role in predicting responders. Statistical Package for the Social Sciences (SPSS) version 16 was used for statistical analysis.
RESULTS
Sixty-five out of the initially considered 152 patients who fulfilled all inclusion and exclusion criteria underwent PEI [Figure 1]. Nineteen patients were responders and 43 non-responders at 1-month follow-up. Ten patients with < 20% reduction in nodule volume underwent second PEI [Figure 1]. Twenty-five of the remaining 33 non-responders attained remission by 9 months of follow-up [Figure 1]. All the 19 responders at 1 month were simple cystic nodules, remained in remission at the end of study, and had >80% reduction in nodule volume from baseline [Figure 1]. Response rate of PEI at the end of study was78.33% (47/60 patients) with a mean follow-up duration of 12.3 ± 2.88 months. Among non-responders, at the end of the study, the majority of them (12/13) had 20-50% reduction in nodule volume from baseline. Only one patient had < 20% reduction in nodule volume as compared to baseline.
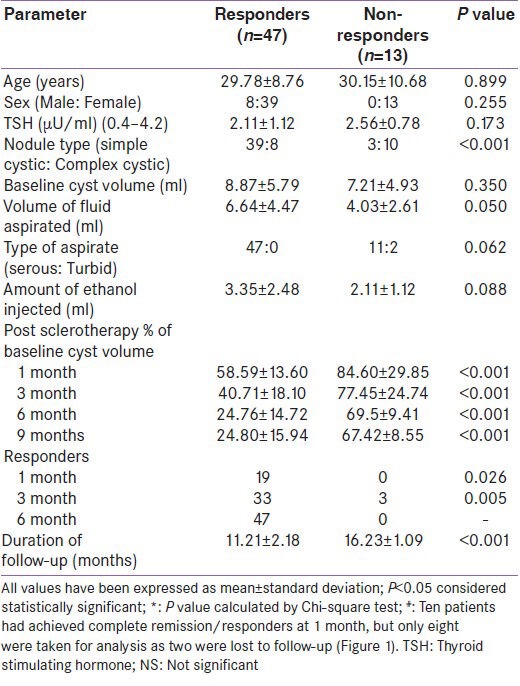
Baseline nodule volume was comparable in responders as compared to non-responders [Table 1]. Volume of fluid aspirated and amount of ethanol injected was higher in responders as compared to non-responders, which approached statistical significance (P = 0.05 and 0.06, respectively). Responders were more likely to have simple cystic nodules [P < 0.001; Table 1]. Residual nodule volumes were significantly lower in responders as compared to non-responders during follow-up [Table 1].
Table 1.
Clinical and thyroid cyst characteristics of responders as compared to non-responders to percutanous aspiration and ethanol sclerotherapy at 9 months
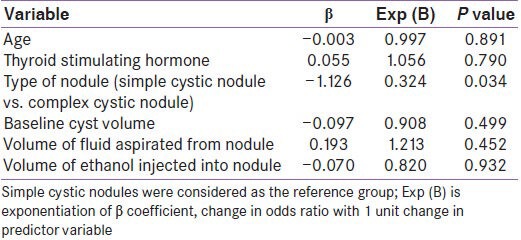
Comparing simple cystic nodules (n = 42) to complex cystic nodules (n = 18), there was no difference in the baseline nodule volume and nature of fluid aspirated. However, the volume of fluid aspirated and the amount of ethanol injected was significantly higher in simple cystic nodules as compared to complex cystic nodules [Table 2]. Post-sclerotherapy residual volumes were significantly lower in simple cystic nodules during follow-up [Table 2]. Simple cystic nodules had significantly higher response rates at 1, 3, 6, and 9 months follow-up [Table 2]. Sclerotherapy response rate in simple cystic nodules at the end of study was 92.86% (39/42 patients) as compared to 44.44% (8/18 patients) for complex cystic nodules [P < 0.001) [Table 2]. Kaplan-Meir analysis showed significantly improved PEI outcomes in patients with simple cystic nodules [P < 0.001; Figure 2]. Cox-regression revealed that among all the baseline parameters, only the type of nodule (simple cystic vs. complex cystic) was predictive of response to PEI [P = 0.034; Table 3]. Patients with complex cystic nodules were 67.6% less likely to go into remission as compared to simple cystic nodule following PEI [Table 3].
Table 2.
Percutanous aspiration and ethanol injection outcomes of simple cystic vs. complex cystic thyroid nodules
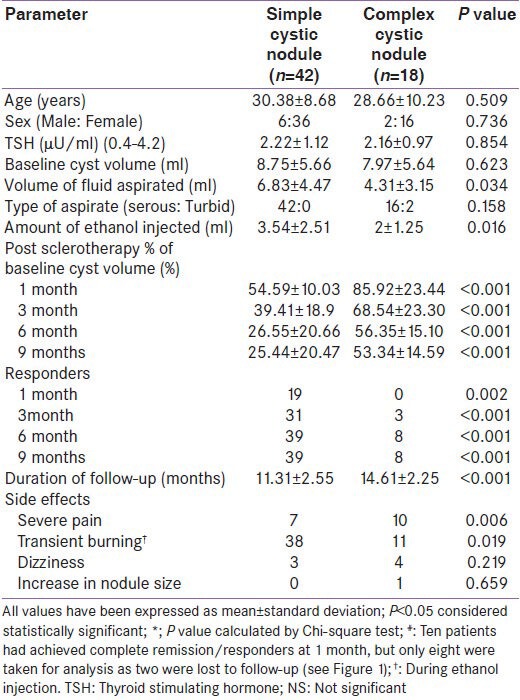
Figure 2.
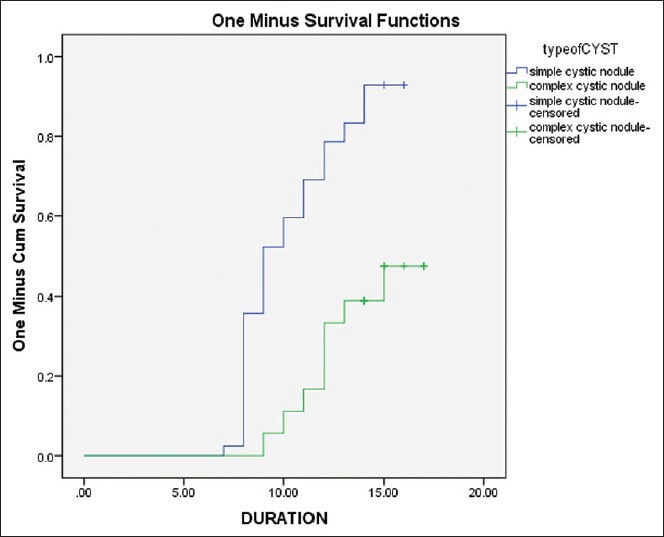
Kaplan Meir analysis of complete response outcomes of simple cystic nodules (Group-1) vs. complex cystic nodules (Group-2) which underwent percutanous aspiration and ethanol sclerotherapy Outcome was defined as complete response, i.e., ≥ 50% reduction in cyst volume from baseline. Y-axis indicates percentage of patients in each group attaining complete response. X-axis indicates follow-up duration of follow-up in months; P < 0.001 [Log Rank test (Mantel-Cox)]
Table 3.
Cox-regression analysis of baseline parameters of cystic thyroid nodules which can predict response rate
Most common side effect noted was transient burning during PEI followed by transient dizziness which was comparable in both simple cystic and complex cystic [Table 2]. Major complications like intra-cyst hemorrhage, peri-thyroidal ethanol leakage, local compressive effects, fever/infection was not observed. Severe pain during PEI was more common in patients with complex cystic nodules [P = 0.006; Table 2]. Need for double puncture during PEI was comparable in both the groups [Table 2]. Increase in cyst size after PEI was noted in only one patient of complex cystic nodule which spontaneously reduced at 1-month follow-up.
DISCUSSION
Use of simple aspiration in the management of cystic thyroid nodules is limited by a high recurrence rate of up to 80%, which lead to the development of sclerotherapy of thyroid nodules.[18] The use of USG-guided PEI as an alternative to surgery in managing thyroid nodules was first proposed by Livraghi et al., for autonomously functioning thyroid nodules, which were later extended to management of benign cystic thyroid nodules.[18] PEI has been reported to have success rate of 82-85% (volume reduction >85% from baseline) after an average of two session in the management of thyroid cysts.[11,12] Ethanol causes reduction in volume of cystic thyroid nodules by causing cellular dehydration, protein denaturation, coagulation necrosis, small vessel thrombosis, leading to reactive fibrosis.[18] Efficacy of PEI in the management of predominantly cystic hyperfunctioning nodules is lower with a success rate of 64-95% (volume reduction ≈ 66%), a higher recurrence rate, and need for more sittings of PEI (≈4 per patient).[13,14] Outcomes have been reported to be worst in solid thyroid nodules (success rate ≈ 38%) believed to be due to poor diffusion of ethanol in the solid tissue along with early wash out of ethanol due to the increased vascularity of a solid nodule as compared to a thyroid cyst.[15]
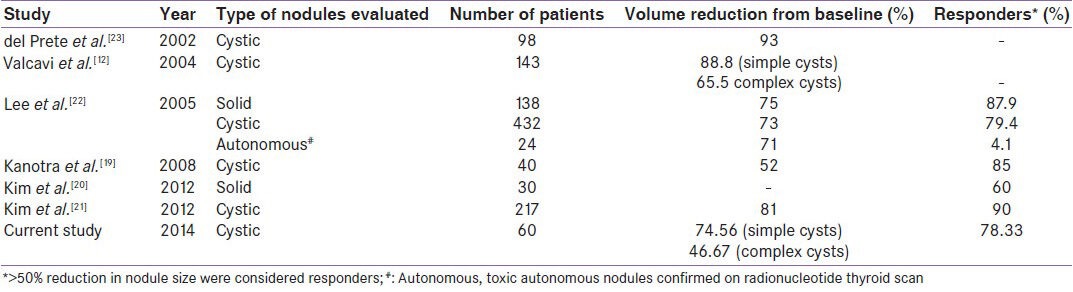
The overall response rate of PEI in our study was 78.33% which is comparable to previous reported data from across the globe [Table 4],[12,19,20,21,22,23] and India. PEI resulted in >50% reduction in 13 out of 15 patients (86.67%) with predominantly cystic thyroid nodules (>2/3rd cystic component) in a study from Tamil Nadu.[24] The largest study till date reported an impressive response rate of 87.9% for solid nodules and 79.4% of cystic nodules.[22] In that study, 41%, 23%, 20% patients with solid nodules underwent PEI 2, 3, and 4 times, respectively. Four patients underwent six episodes of PEI.[22] Similarly in patients with cystic nodules 47%, 25%, and 8% patients underwent PEI 2, 3, and 4 times, respectively.[22] PEI 3 and 2 patients underwent PEI 6 and 7 times, respectively.[22] Hence, multiple episodes of PEI over a long period of 3 years may explain the excellent outcomes.
Table 4.
Outcomes of percutaneous aspiration and ethanol injection for managing thyroid nodules in literature
Our study showed that baseline cyst volume, amount of fluid aspirated, nature of fluid aspirated (serous/turbid), or volume of ethanol injected did not predict the final outcome [Table 1]. This is similar to most of the studies which have not observed any correlation between the amounts of ethanol injected to the final response rates.[20,21,22] However, some studies have observed better response rates in patients with larger baseline cysts.[22] Alcohol was not re-aspirated from the nodules as studies have shown that outcomes and complication rates were not improved in patients in whom alcohol was re-aspirated, but the entire procedure took double the normal time.[20] The single most important factor which predicted the final outcome was the type of nodule aspirated, viz. simple cystic nodule vs. complex cystic nodule, which is consistent with previous reports.[12,20,21,22,23]
An important observation from our study was that only 19/60 patients (31.67%) achieved remission at the 1st month. A significant number of patients (28/60; 46.67%) achieved remission between 1 and 6 months of follow up. Hence, it may be worthwhile to wait beyond 1 month to allow for spontaneous remission following PEI. Another important observation in our study was that there was no further change in response rate beyond 6 months of follow-up. Ten patients in our study with < 20% reduction in cyst volume at 1 month had a second sitting of PEI. Additional sitting of PEI in extremely poor responders resulted in only a 30% response rate (3/10). Hence, repeated PEI in extremely poor responders may not improve the outcomes greatly.
PEI was found to be safe in our study without any major complications. This is similar to previous reports which have also suggested PEI to be safe with the common adverse effects being local pain, dizziness, flushing, dysphonia, and very rarely recurrent laryngeal nerve damage.[9,12] Severe pain was more common in patients with complex cystic nodules. Local pain is believed to be due to leakage of ethanol into the subcutaneous tissue.[19]
Our study demonstrated that PEI is ideal for managing patients with goiter due to simple cystic nodules as response rates are excellent with low rates of recurrence. Presence of solid tissue significantly worsens the outcomes. Limitations of this study include the relatively small number of patients evaluated and the duration of follow-up.
In spite of these limitations, our study clearly demonstrated that PEI should be the treatment of choice in patients with simple cystic thyroid nodules. PEI in patients with complex cystic thyroid nodules are associated with a lower rate of remission, increased recurrence, and need for repeated PEI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Singer PA, Cooper DS, Daniels GH, Ladenson PW, Greenspan FS, Levy EG, et al. Treatment guidelines for patients with thyroid nodules and well-differentiated thyroid cancer. American Thyroid Association. Arch Intern Med. 1996;156:2165–72. [PubMed] [Google Scholar]
- 3.Tan GH, Gharib H. Thyroid incidentalomas: Management approaches to non- palpable nodules discovered incidentally on thyroid imaging. Ann Intern Med. 1997;126:226–31. doi: 10.7326/0003-4819-126-3-199702010-00009. [DOI] [PubMed] [Google Scholar]
- 4.Biswas AB, Das DK, Chakraborty I, Biswas AK, Sharma PK, Biswas R. Goiter prevalence, urinary iodine, and salt iodization level in sub-Himalayan Darjeeling district of West Bengal, India. Indian J Public Health. 2014;58:129–33. doi: 10.4103/0019-557X.132291. [DOI] [PubMed] [Google Scholar]
- 5.Biswas AB, Chakraborty I, Das DK, Chakraborty A, Ray D, Mitra K. Elimination of iodine deficiency disorders--current status in Purba Medinipur district of West Bengal, India. Indian J Public Health. 2008;52:130–5. [PubMed] [Google Scholar]
- 6.Chandra AK, Tripathy S, Ghosh D, Debnath A, Mukhopadhyay S. Goitre prevalence and the state of iodine nutrition in the sundarban delta of north 24-parganas in West Bengal. Asia Pac J Clin Nutr. 2006;15:357–61. [PubMed] [Google Scholar]
- 7.Marwaha RK, Tandon N, Ganie MA, Kanwar R, Sastry A, Garg MK, et al. Status of thyroid function in Indian adults: Two decades after universal salt iodization. J Assoc Physicians India. 2012;60:32–6. [PubMed] [Google Scholar]
- 8.Marwaha RK, Tandon N, Garg MK, Desai A, Kanwar R, Sastry A, et al. Thyroid status two decades after salt iodization: Country-wide data in school children from India. Clin Endocrinol (Oxf) 2012;76:905–10. doi: 10.1111/j.1365-2265.2011.04307.x. [DOI] [PubMed] [Google Scholar]
- 9.Senchenkov A, Staren ED. Ultrasound in head and neck surgery: Thyroid, parathyroid, and cervical lymph nodes. Surg Clin North Am. 2004;84:973–1000. doi: 10.1016/j.suc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Porenta M, Fettich JJ. Treatment of thyroid cysts by sclerosation. Radiobiol Radiother (Berl) 1985;26:249–54. [PubMed] [Google Scholar]
- 11.Bennedbaek FN, Hegedus L. Treatment of recurrent thyroid cysts with ethanol: A randomized double-blind controlled trial. J Clin Endocrinol Metab. 2003;88:5773–7. doi: 10.1210/jc.2003-031000. [DOI] [PubMed] [Google Scholar]
- 12.Valcavi R, Frasoldati A. Ultrasound-guided percutaneous ethanol injection therapy in thyroid cystic nodules. Endocr Pract. 2004;10:269–75. doi: 10.4158/EP.10.3.269. [DOI] [PubMed] [Google Scholar]
- 13.Tarantino L, Francica G, Sordelli I, Sperlongano P, Parmeggiani D, Ripa C, et al. Percutaneous ethanol injection of hyperfunctioning thyroid nodules: Long-term follow-up in 125 patients. AJR Am J Roentgenol. 2008;190:800–8. doi: 10.2214/AJR.07.2668. [DOI] [PubMed] [Google Scholar]
- 14.Papini E, Panunzi C, Pacella CM, Bizzarri G, Fabbrini R, Petrucci L, et al. Percutaneous ultrasound-guided ethanol injection: A new treatment of toxic autonomously functioning thyroid nodules? J Clin Endocrinol Metab. 1993;76:411–6. doi: 10.1210/jcem.76.2.8432784. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Lee HK, Lee JH, Ahn IM, Choi CG. Efficacy of sonographically guided percutaneous ethanol injection for treatment of thyroid cysts versus solid thyroid nodules. AJR Am J Roentgenol. 2003;180:1723–6. doi: 10.2214/ajr.180.6.1801723. [DOI] [PubMed] [Google Scholar]
- 16.Hong YJ, Son EJ, Kim EK, Kwak JY, Hong SW, Chang HS. Positive predictive values of sonographic features of solid thyroid nodule. Clin Imaging. 2010;34:127–33. doi: 10.1016/j.clinimag.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 17.Bruneton JS. Berlin, Germany: Springer-Verlag; 1987. Ultrasonography of the Neck; p. 6. [Google Scholar]
- 18.Livraghi T, Paracchi A, Ferrari C, Bergonzi M, Garavaglia G, Raineri P, et al. Treatment of autonomous thyroid nodules with percutaneous ethanol injection. Radiology. 1990;175:827–9. doi: 10.1148/radiology.175.3.2188302. [DOI] [PubMed] [Google Scholar]
- 19.Kanotra VS, Lateef M, Kirmani O. Non-surgical management of benign thyroid cysts: Use of ultrasound-guided ethanol ablation. Postgrad Med J. 2008;84:639–43. doi: 10.1136/pgmj.2008.072777. [DOI] [PubMed] [Google Scholar]
- 20.Kim DW, Rho MH, Park HJ, Kwag HJ. Ultrasonography- guided ethanol ablation of pre dominantly solid thyroid nodules: A preliminary study for factors that predict the outcome. Br J Radiol. 2012;85:930–6. doi: 10.1259/bjr/81849588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YJ, Baek JH, Ha EJ, Lim HK, Lee JH, Sung JY, et al. Cystic versus predominantly cystic thyroid nodules: Efficacy of ethanol ablation and analysis of related factors. Eur Radiol. 2012;22:1573–8. doi: 10.1007/s00330-012-2406-5. [DOI] [PubMed] [Google Scholar]
- 22.Lee SJ, Ahn IM. Effectiveness of percutaneous ethanol injection therapy in benign nodular and cystic thyroid diseases: Long term follow up experience. Endocr J. 2005;54:455–62. doi: 10.1507/endocrj.52.455. [DOI] [PubMed] [Google Scholar]
- 23.Del Prete S, Caraglia M, Russo D, Vitale G, Giuberti G, Marra M, et al. Percutaneous ethanol injection efficacy in the treatment of large symptomatic thyroid cystic nodules: Ten-year follow-up of a large series. Thyroid. 2002;12:815–21. doi: 10.1089/105072502760339398. [DOI] [PubMed] [Google Scholar]
- 24.Jayesh SR, Mehta P, Cherian MP, Ilayaraja V, Gupta P, Venkatesh K. Efficacy and safety of USG-guided ethanol sclerotherapy in cystic thyroid nodules. Indian J Radiol Imaging. 2009;19:199–202. doi: 10.4103/0971-3026.54879. [DOI] [PMC free article] [PubMed] [Google Scholar]


