Abstract
Introduction:
Helicobacter pylori (H. pylori) infection has been associated with increased levels of inflammatory cytokines and subsequent insulin resistance and epidemiologically linked to type 2 diabetes.
Objectives:
To study the prevalence rate of H. pylori infection in type 2 diabetes and its relation with HbA1C levels.
Materials and Methods:
In this cross-sectional case-control study, 80 patients (≥18 years) who met the Americans with Disabilities Act (ADA) criteria for diabetes were recruited. Similarly, 60 age, sex, and education matched healthy controls were taken. They were tested for H. pylori infection by rapid urease test, histological examination of antral endoscopic biopsy specimens and serology. The relationship between H. pylori infection and levels of plasma glucose and HbA1C was assessed.
Results:
Out of the 80 patients of type 2 diabetes, H. pylori infection was found in 62 (77.5%) while it was present in only 35 (58.3%) of 60 controls, which was found to be significant (Chi-square test: 5.919, df = 1, P value = 0.015). Mean HbA1C among diabetics with H. pylori infection was 8.19 ± 1.16% and without H. pylori infection was 6.9 ± 0.84% (t = 4.3872, P value = 0.0001).
Conclusions:
Prevalence of H. pylori infection was significantly higher in diabetes as compared to controls. Presence of H. pylori infection significantly correlated with the level of HbA1C.
Keywords: HbA1C, H. pylori, insulin resistance, type 2 diabetes
INTRODUCTION
H. pylori infection may have an impact on cardiovascular conditions, insulin resistance, and metabolic syndrome potentially mediated by elevations in inflammatory markers such as C-reactive protein (CRP) and Interlukin-6 (IL-6).[1] Elevated levels of inflammatory cytokines may lead to phosphorylation of serine residues on the insulin receptor substrate, which prevents its interaction with insulin receptors, inhibiting insulin action.[2] Mammalian stomach produces leptin and ghrelin, two hormones involved in energy homeostasis and whose interactions affect obesity, insulin sensitivity, and glucose homeostasis. Increasing evidence indicates that H. pylori is involved in the regulation of these hormones.[3] The study was designed to study association of H. pylori infection in type 2 diabetes.
MATERIALS AND METHODS
This single point cross-sectional case control study was conducted at a tertiary care hospital from April 2012 to August 2013. In this study, 80 patients (aged ≥18 years) who were diagnosed to have diabetes as per Americans with Disabilities Act (ADA; 2012) criteria were studied. Patients with type 1 diabetes, history of intake of antibiotics, proton pump inhibitors, H2 receptor blockers, or antacids in last 4 weeks and with past and present evidence of active gastrointestinal bleeding, jaundice, or post gastric surgery were excluded from the study. Microvascular complications viz neuropathy, retinopathy, and nephropathy and macrovascular complications viz coronary artery disease (CAD) and cerebrovascular accident (CVA) were assessed. Diabetic neuropathy was assessed using subjectively with monofilament test and vibration sense and objectively using nerve conduction velocity. Retinopathy was assessed for with indirect ophthalmoscopy and nephropathy with urine protein and micral tests. Macrovascular complications were assessed with history of CAD and CVA, electrocardiography (ECG) and head computrd tomography (CT). Written informed consent was taken and the study was approved by the Ethics Committee. The control group comprised of age, sex, socioeconomic status, and education matched normal healthy volunteers. The exclusion criteria were the same as those for cases. Controls were screened for the absence of type 2 diabetes with fasting plasma glucose, oral glucose tolerance test (OGTT), and glycated hemoglobin (HBA1C). Diabetics and healthy volunteers were tested for H. pylori infection by rapid urease test, histopathological examination of antral endoscopic biopsy specimens, and serology. Data was analyzed with the appropriate statistical methods. Chi-square test and Two Sample Proportion Tests were used to calculate the P value using Decision Support System (DSS) calculator. Tests were considered significant if P values were less than 0.05.
RESULTS
In the present study, out of the 80 patients of type 2 diabetes, H. pylori infection was found in 62 (77.5%) while it was present in only 35 (58.3%) of 60 controls, which was found to be significant (x2 = 5.9, df = 1, P = 0.02, power of the study was 81.5%). Mean HbA1C among diabetics with H. pylori infection was significantly greater than H. pylori-negative diabetics (8.2 ± 1.2% and 6.9 ± 0.8%, respectively, t value = 4.39 P = 0.0001) [Figure 1]. H. pylori positivity was maximum in groups that had higher HbA1C level as demonstrated in Table 1. The patients with H. pylori seropositivity had significantly high post prandial blood sugar (PPBS), serum triglycerides and serum low-density lipoprotein (LDL) levels than those with negative H. pylori as depicted in Table 2.
Figure 1.
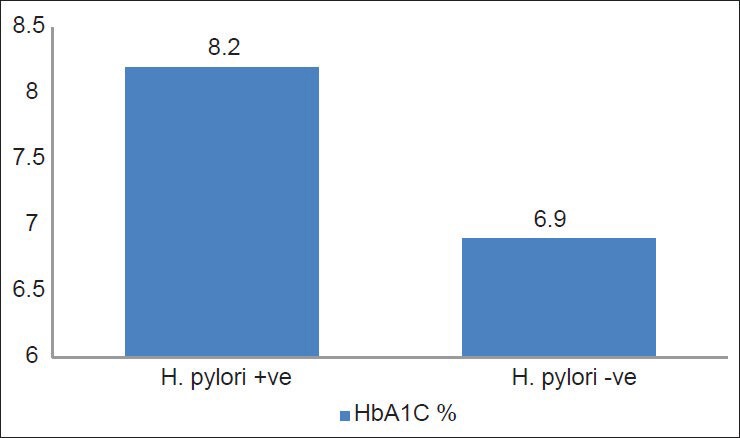
Mean HbA1C levels in H. pylori-positive and -negative cases
Table 1.
HbA1C levels among H. pylori-positive and -negative cases

Table 2.
H. pylori infection among type 2 diabetic patients in relation to some laboratory data
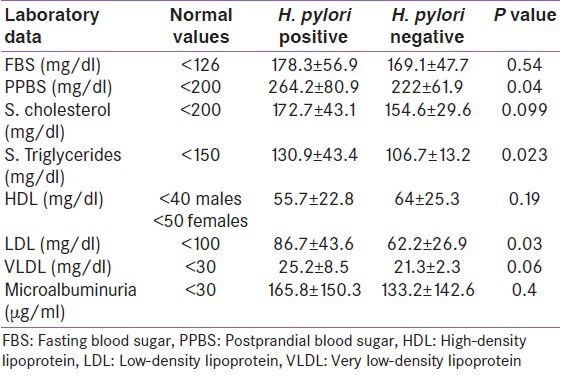
H. pylori positivity is maximum in lower and upper lower classes of socioeconomic status as compared from the upper class of both diabetic and control subjects. It was 92.8% in lower class and 87.5% in upper lower class for diabetic group [Table 3]. The difference was found to be significant (x2 = 10.2, df = 4, P = 0.04). Similarly, the difference was significant in controls as well (88.8% in lower class and 76.9% upper lower class for controls, x2 = 10.3, df = 4, P = 0.03). Mean duration of type 2 diabetes in H. pylori-positive group was 4.2 ± 4 years and in H. pylori-negative group was 2.8 ± 2.7 years which was found to be insignificant (t value = 1.4, df = 78, P = 0.16) [Figure 2].
Table 3.
Socioeconomic status wise distribution of H. pylori infection among diabetic and non-diabetic subjects
Figure 2.
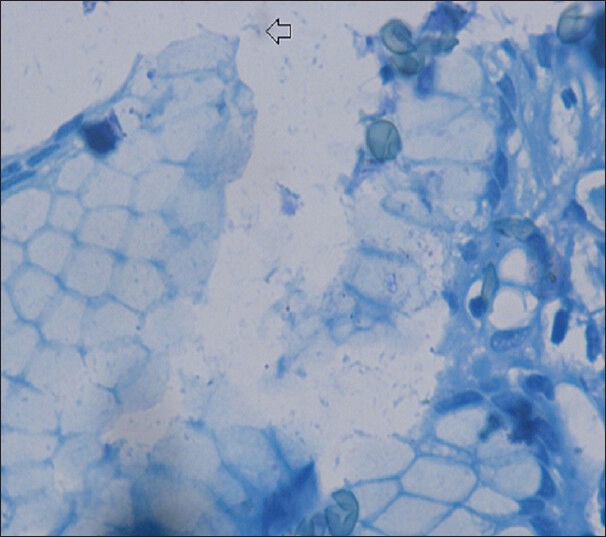
Antral biopsy showing H. pylori (Loeffler's methylene blue, ×1000)
H. pylori positivity was maximum in diabetic retinopathy (DR) group (non-proliferative diabetic retinopathy [NPDR] and proliferative diabetic retinopathy [PDR]) as compared to patients without retinopathy, which was insignificant (P = 0.31). It was 86.9% in NPDR and 85.7% in PDR group. H. pylori positivity was insignificantly higher in diabetic nephropathy group (P = 0.29). Diabetic patients with DN had 85.7% H. pylori positivity while it was 74.5% in diabetic patients without DN.
H. pylori positivity was more among diabetic patients with macrovascular complication (Coronary artery disease [CAD] and Stroke) group which was insignificant (P = 0.31). Among diabetics, macrovascular complication group had 90% H. pylori positivity while it was 75.5% in patients without macrovascular complication.
In the present study maximum patients were in the age group 51-60 years (36.3%) and 41-50 years (23.8%) while this fraction was 33.3% and 23.3%, respectively for the control population which was not found to be significant (P = 0.99) and showed approximately equal age-wise distribution between cases and controls. Similarly, the mean ages in case and control groups were 55.6 ± 10.7 and 54.7 ± 11.1 years, respectively which was insignificant (P = 0.59). The gender distribution in the cases were male 65% and female 35% with M: F ratio 1.9 and in controls males 63.3% and female 36.7% with M: F ratio 1.7, which was also found to be insignificant (P = 0.84). The socioeconomic status was also balanced between case and control group with insignificant differences (P = 0.99) [Table 3].
The difference in H. pylori seropositivity in relation to type of therapy was statistically non-significant (P = 0.6).
DISCUSSION
The present study found a higher prevalence of H. pylori infection in diabetics as compared to non-diabetics. H. pylori infection has been positively related to the prevalence of type 2 diabetes in most studies[4,5,6,7,8,9], while some studies found no such association.[10,11,12] In a recent prospective study done by Jeon et al., it was found that those who were seropositive for H. pylori at enrolment were 2.7 times more likely at any given time to develop type 2 diabetes than seronegative individuals (hazard ratio 2.69 [95% CI 1.10-6.60]).[13] However, it is in contrast to a comparable prevalence of H. pylori infection in diabetics and non-diabetics reported by other authors: 33% vs. 32%[10], 28.1% vs. 29.25%[14], 37.3% vs. 35.2%[15], and 50.8% vs. 56.4%[16], respectively.
There are also significant limitations of earlier studies. Foremost, pathogen infection was determined based on seropositivity to IgG antibodies. IgG antibodies reflect prior infection, but are not sensitive indicators of current infection or the chronicity of prior infections. Though their results were insignificant, it is possible that active pathogen infection, or chronic active infection, is associated with systemic inflammation and greater risk of type 2 diabetes. Notably, IgG antibodies were used to define infection in most studies, while some studies did not use IgG antibodies for assessing the relation between type 2 diabetes and H. pylori infection.[5,6,8] Regardless of the means by which pathogen infection was assessed, inferences from cross sectional data exploring the relation between pathogens and type 2 diabetes are tenuous, as the temporal direction of the relationship is unclear. Cani et al., proposed that pathogen infection may lead to inflammation and type 2 diabetes.[17] An alternate theory by Butler et al., suggests that hyperglycaemia may impair host defences and predispose to infection.[18] Another hypothesis is that gastroduodenal conditions resulting from H. pylori infection could delay gastric emptying, which has been postulated to cause poor glucose control in insulin-dependent children with longstanding diabetes. Possible predisposing conditions (e.g., gastroparesisdiabeticorum) and many other factors related to H. pylori colonization of gastric mucosa in type 2 diabetes remain unknown.[15]
Among the three different methods namely biopsy of the mucosa, the rapid urease test and the presence of serum H pylori antibodies, different studies have used either one or any two of these methods for studying H. pylori infection. However, in the present study, H pylori infection was investigated by all three methods, so the incidence of H pylori infection in type 2 diabetes patients in the present study may be more accurate.
In the present study, increased levels of HbA1C were associated with H. pylori infection among type 2 diabetes group. As the glycemic control improved, the prevalence of H. pylori decreased. Mean HbA1C among diabetics with H. pylori infection was significantly greater than H. pylori-negative diabetics. It may be possible that a good glycemic control could hinder H. pylori colonization. H. pylori seropositivity, especially H. pylori cag A positivity, was positively associated (P < 0.01, NHANES III; P = 0.02, NHANES 1999-2000) with HbA1C levels after excluding individuals with history of type 2 diabetes and controlling for potential confounders.[19] In contrast tothese findings Gillum et al., found that H. pylori infection status was not significantly associated with HbA1C in men aged 40-70 years with or without history of type 2 diabetes.[20] Candelli and co-workers also found that H. pylori eradication in type 1 diabetes does not affect glycemic control in such patients as evidenced by HbA1C level and daily insulin requirement.[14]
Although the present study is a cross-sectional study, reverse causation is not likely H. pylori is acquired almost exclusively in childhood[2], but there is no clear mechanism explaining how glucose intolerance which presents in later life would increase risk of H. pylori colonization. The most plausible hypothesis is that H. pylori directly or indirectly increase levels of HbA1C in adulthood. Infection with H. pylori was found to correlate with elevated levels of CRP, IL-6, and tumour necrosis factor-α, which are markers of inflammation implicated in insulin resistance and development of type 2 diabetes.[2] The inflammation hypothesis, however, was not substantiated in present study, since we did not measure IL-6 and CRP levels in H. pylori seropositive individuals or in those who had type 2 diabetes.
The present study provides evidences that H. pylori seropositivity was associated with atherogenic lipid profile among patients with type 2 diabetes. H. Pyloriin fection was associated with significant increase in triglyceride and LDL level and had insignificant effect on levels of both high-density lipoprotein (HDL) and total cholesterol. Sung and his co-workers found that H. Pylori infection in healthy Korean adults was associated with atherogenic lipid profile (increase in total cholesterol, triglyceride, LDL cholesterol, and decrease in HDL cholesterol).[21] According to Kanbay et al., H. pylori infection affected lipid metabolism in a way that increased the risk of atherosclerosis and has been regarded as an independent risk factor for coronary artery disease.[22] Dursen et al., found insignificant differences in lipid profile between H. pylori infected and non-infected diabetics, thus affirming the metabolic neutrality of H. pylori infection in terms of serum lipids.[23] However, Moghimi et al., found that H. pylori eradication has been reported to modify some parameters of lipids and homeostasis.[24]
Study done by Sung and co-workers had many limitations (subjects included were healthy, young, self-selected, and of relatively high socioeconomic status).[21] While in the present study, patients were aged ≥18 years and of relatively lower socioeconomic status. Association of H. Pylori infection with atherogenic lipid profile may be due to lipopolysaccharides present in this gram-negative bacteria which stimulate the production of many cytokines including TNF- α, which inhibit lipoprotein lipase activity leading to mobilization of lipids from the tissues and elevated serum triglycerides and lower HDL cholesterol levels.[21]
In the present study, an inverse relationship between the prevalence of H. pylori infection and socioeconomic class was observed. Prevalence was higher in lower socioeconomic status. These findings are in concordance with Begue et al., who had reported higher prevalence of H. pylori infection among low socioeconomic class.[25] This could well reflect the standard of living among such subjects including hygiene, overcrowding, and water supply. Various studies have reported that poor hygiene, overcrowding, and insalubrity increase the isolation rates of H. pylori.
The present study found insignificant relations between H. Pylori seropositivity and microvascular complications (degree of retinopathy and nephropathy as indicated by level of micro albuminuria) or prevalence of macrovascular complications (CAD and Stroke).
Tasi and Huang found insignificant association between H. Pylori infection and angiographically documented CAD.[26] Bielanski performed an epidemiological study in which no clear correlation between H. pylori infection and CAD could be found.[27] Biagi et al. found no positive association between seroprevalence of H. pylori infection and CAD.[28] On the other hand, Mbenza et al. found that H. pylori infection might be one of the risk factors for atherosclerosis thorough inflammation (fibrinogen) and modulation of glucose and lipid profiles, which may be prevented by low antibiotics.[7] Gillum et al. revealed no consistent associations of H. pylori infection with diabetes prevalence but it was associated with CAD prevalence.[20] Hadidy et al. found H. pylori seropositivity had no relation to the degree of diabetic retinopathy and also no significant difference in prevalence of H. Pylori infection among diabetic patients with no evidence of macrovascular complications and those with macrovascular complications.[29]
The differences between previous studies and the present study may be due to predominant effects of cardiovascular risk factors in diabetic patients then direct effects of H. pylori infection. Another explanation is the role of toxigenic strains of H. pylori infection (Cag A and Vac A strains) which were proved to be highly related to CAD and atherosclerotic plaque instability.[30] However, these toxigenic strains were not examined in the present study.
One of major strength of this study is its matched case control design which is controlled for multiple factors, including age, sex, socioeconomic status, and education by design and analysis, thereby minimizing bias due to confounding. Cases were recruited on basis of diabetic status measured by objective laboratory values which avoided selection biases. There were few limitations too. The cross-sectional design of this study limits the ability to assess causality. Due to the constraints of a time bound study and because of the stringent selection criteria, the sample size was small and hence the results are subjected to Type II error and they cannot be generalized. Also only a small percentage of the population was seronegative for H. pylori which limited the power of the study and increased the width of our confidence intervals.
Prevalence of H. pylori infection was significantly higher in type 2 diabetes as compared to controls. Diabetics with poor glycemic control had significantly increased prevalence of H. pylori infection as denoted by increased HbA1C level in H. pylori-positive diabetic group. Also H pylori infection was more common among people from lower socioeconomic strata. S. triglyceride and LDL levels were significantly higher in H. pylori-positive as compared to H. pylori-negative diabetics. We also concluded that H. Pylori infection is not related to duration of diabetes and type of therapy for diabetes and has no direct relation to microvascular (diabetic retinopathy and nephropathy) or macrovascular (CAD and Stroke) complications among diabetic patients. Larger prospective studies investigating the impact of H. pylori infection on type 2 diabetes and corresponding mediating factors are warranted. If the relationship between the two is established preventive measures may be implemented for this treatable disorder especially in high risk communities.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Nabipour I, Vahdat K, Jafari SM, Pazoki R, Sanjdideh Z. The association of metabolic syndrome and Chlamydia pneumoniae, Helicobacter pylori, cytomegalovirus, and herpes simplex virus type 1: The Persian Gulf Healthy Heart Study. Cardiovasc Diabetol. 2006;5:25. doi: 10.1186/1475-2840-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francois F, Roper J, Joseph N, Pei Z, Chhada A, Blaser MJ, et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol. 2011;11:37. doi: 10.1186/1471-230X-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon L, Tornóczky J, Tóth M, Jámbor M, Sudár Z. The significance of Campylobacter pylori infection in gastroenterologic and diabetic practice. Orv Hetil. 1989;130:1325–9. [PubMed] [Google Scholar]
- 5.Quadri R, Rossi C, Catalfamo E, Masoero G, Lombardo L, Rovera L, et al. Helicobacter pylori infection in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2000;10:263–6. [PubMed] [Google Scholar]
- 6.Gulcelik NE, Kaya E, Demirbas B, Culha C, Koc G, Ozkaya M, et al. Helicobacter pylori prevalence in diabetic patients and its relationship with dyspepsia and autonomic neuropathy. J Endocrinol Invest. 2005;28:214–7. doi: 10.1007/BF03345375. [DOI] [PubMed] [Google Scholar]
- 7.Longo-Mbenza B, Nkondi Nsenga J, Vangu Ngoma D. Prevention of the metabolic syndrome insulin resistance and the atherosclerotic diseases in Africans infected by Helicobacter pylori infection and treated by antibiotics. Int J Cardiol. 2007;121:229–38. doi: 10.1016/j.ijcard.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Quatrini M, Boarino V, Ghidoni A, Baldassarri AR, Bianchi PA, Bardella MT. Helicobacter pylori prevalence in patients with diabetes and its relationship to dyspeptic symptoms. J Clin Gastroenterol. 2001;32:215–7. doi: 10.1097/00004836-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 9.So WY, Tong PC, Ko GT, Ma RC, Ozaki R, Kong AP, et al. Low plasma adiponectin level, white blood cell count and Helicobacter pylori titre independently predict abnormal pancreatic beta-cell function. Diabetes Res Clin Pract. 2009;86:89–95. doi: 10.1016/j.diabres.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Xia HH, Talley NJ, Kam EP, Young LJ, Hammer J, Horowitz M. Helicobacter pylori infection is not associated with diabetes mellitus, nor with upper gastrointestinal symptoms in diabetes mellitus. Am J Gastroenterol. 2001;96:1039–46. doi: 10.1111/j.1572-0241.2001.03604.x. [DOI] [PubMed] [Google Scholar]
- 11.Gasbarrini A, Ojetti V, Pitocco D, De Luca A, Franceschi F, Candelli M, et al. Helicobacter pylori infection in patients affected by insulin-dependent diabetes mellitus. Eur J Gastroenterol Hepatol. 1998;10:469–72. doi: 10.1097/00042737-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Arslan D, Kendirci M, Kurtoglu S, Kula M. Helicobacter pylori infection in children with insulin dependent diabetes mellitus. J Pediatr Endocrinol Metab. 2000;13:553–6. doi: 10.1515/jpem.2000.13.5.553. [DOI] [PubMed] [Google Scholar]
- 13.Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520–5. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candelli M, Rigante D, Marietti G, Nista EC, Crea F, Bartolozzi F, et al. Helicobacter pylori, gastrointestinal symptoms, and metabolic control in young type 1 diabetes mellitus patients. Pediatrics. 2003;111:800–3. doi: 10.1542/peds.111.4.800. [DOI] [PubMed] [Google Scholar]
- 15.Anastasios R, Goritsas C, Papamihail C, Trigidou R, Garzonis P, Ferti A. Helicobacter pylori infection in diabetic patients: Prevalence and endoscopic findings. Eur J Intern Med. 2002;13:376. doi: 10.1016/s0953-6205(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 16.Ko GT, Chan FK, Chan WB, Sung JJ, Tsoi CL, To KF, et al. Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr Res. 2001;27:171–7. doi: 10.1081/erc-100107178. [DOI] [PubMed] [Google Scholar]
- 17.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 18.Butler SO, Btaiche IF, Alaniz C. Relationship between hyperglycemia and infection in critically ill patients. Pharmacotherapy. 2005;25:963–76. doi: 10.1592/phco.2005.25.7.963. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Blaser MJ. Association between gastric Helicobacter pylori colonization and glycated hemoglobin levels. J Infect Dis. 2012;205:1195–202. doi: 10.1093/infdis/jis106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillum RF. Infection with Helicobacter pylori, coronary heart disease, cardiovascular risk factors, and systemic inflammation: The Third National Health and Nutrition Examination Survey. J Natl Med Assoc. 2004;96:1470–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Sung KC, Rhee EJ, Ryu SH, Beck SH. Prevalence of Helicobacter pylori infection and its association with cardiovascular risk factors in Korean adults. Int J Cardiol. 2005;102:411–7. doi: 10.1016/j.ijcard.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 22.Kanbay M, Gür G, Yücel M, Yilmaz U, Boyaciolu S. Does eradication of Helicobacter pylori infection help normalize serum lipid and CRP levels? Dig Dis Sci. 2005;50:1228–31. doi: 10.1007/s10620-005-2764-9. [DOI] [PubMed] [Google Scholar]
- 23.Dursun M, Bahceci M, Tuzcu A, Yilmaz S, Canoruc F. Insulin sensitivity, cell function and serum lipid levels in Helicobacter pylori positive, non-obese, young adult males. Turk J Med Sci. 2004;34:103–7. [Google Scholar]
- 24.Moghimi J, Toussy J, Babaei M, Mousavi S, Tamadon M. Effect of H. pylori eradication on short term control of glycemia in patients with type 2 diabetes millitus. Saudi Med J. 2007;28:475–6. [PubMed] [Google Scholar]
- 25.Begue RE, Mirza A, Compton T, Gomez R, Vargas A. Helicobacter pylori infection and insulin requirement among children with type 1 diabetes mellitus. Pediatrics. 1999;103:e83. doi: 10.1542/peds.103.6.e83. [DOI] [PubMed] [Google Scholar]
- 26.Tasi CJ, Huang TY. Relation of Helicobacter pylori infection and angiographically documented coronary artery disease. Dig Dis Sci. 2000;45:1227–32. doi: 10.1023/a:1005522624004. [DOI] [PubMed] [Google Scholar]
- 27.Bielanski W. Epidemiological study on Helicobacter pylori infection and extragastroduodenal disorders in Polish population. J Physiol Pharmacol. 1999;50:723–33. [PubMed] [Google Scholar]
- 28.Biagi B, Fabbrini D, Bocchini S. Seroprevalence of Helicobacter pylori infection in a group of hospitalized geriatric patients. Panminerva Med. 2000;42:183–6. [PubMed] [Google Scholar]
- 29.El Hadidy M, Abdul-Aziz MY, Mokhtar AR, El Ata MM, El Gwad SS. Helicobacter pylori infection and vascular complications in patients with type 2 diabetes mellitus. J Taibah Univ Med Sci. 2009;4:62–72. [Google Scholar]
- 30.Gabrielli M, Santoliquido A, Cremonini F, Cicconi V, Candelli M, Serricchio M, et al. CagA-positive cytotoxic H. pylori strains as a link between plaque instability and -atherosclerotic stroke. Eur Heart J. 2004;25:64–8. doi: 10.1016/j.ehj.2003.10.004. [DOI] [PubMed] [Google Scholar]


