Abstract
Aim of the Study:
To evaluate bone mineral density (BMD) and levels of bone turnover markers in Egyptian children with classic congenital adrenal hyperplasia (CAH) caused by 21-hydroxylase deficiency and its relationship with disease-related variables.
Patients and Methods:
The study population consisted of 28 children from Upper Egypt with classic CAH, their mean age 8.3 ± 2.4 years and 28 age and sex matched healthy control. They were subjected to measurement of BMD of lumbar spines (L1-L4) and femoral neck using dual-energy-X-ray absorptiometry (DXA) and laboratory evaluation of bone turnover markers including Osteocalcin and serum receptor activator of nuclear factor κB-ligand (RANKL).
Result:
Children with CAH had significantly lower bone-mineral density (BMD) for both, vertebrae and femoral neck than controls. This difference is more obvious in children with poor control and in those receiving prednisone therapy. There was a significantly lower serum osteocalcin, and significantly higher serum RANKL levels in patients with CAH than the healthy controls. This differences is more obvious in children with poor control and in those receiving prednisone therapy. Total bone mineral content (BMC [gm]) have significant negative correlations to age (r = −0.81, P < 0.001), disease duration (r = −0.881, P < 0.001), 17 OH Progesterone level (r = −0.543, P < 0.05), RANKL level (r = −0.635, P < 0.05), and significant positive correlation with osteocalcin (r = 0.576, P < 0.001).
Conclusions:
Children from Upper Egypt with classic CAH may have reduced BMD and increase bone turnover compared with controls. This difference is more obvious in children with poor control and in those receiving prednisone therapy.
Recommendations:
Active monitoring of BMD in CAH children using Dual-energy X-ray absorptiometry (DEXA) scanning. Furthermore, effort should be done to bring hydrocortisone to Upper Egypt to replace prednisone in children with classic congenital adrenal hyperplasia.
Keywords: Bone mineral density, congenital adrenal hyperplasia, hydrocortisone, osteocalcin, receptor activator of nuclear factor κB-ligand
INTRODUCTION
Congenital adrenal hyperplasia (CAH) is an autosomal recessive disease due to defective biosynthesis of adrenal glucocorticoid with or without impairment of mineralocorticoid production. The commonest enzymatic defect is 21-hydroxylase deficiency. Patients with the classic form of CAH show androgen excess, with or without salt wasting. Treatment of the classical forms of CAH due to 21-hydroxylase deficiency involves lifelong glucocorticoid and mineralocorticoid hormonal replacement to prevent adrenal crises and normalize the elevated adrenocortical secretion of androgen steroid precursors.[1,2] However, oral glucocorticoid replacement cannot accomplish normal variations inserum cortisollevels. It is very well established that glucocorticoid excess inendogenous Cushing's syndrome and pharmacological glucocorticoid therapy can lead to osteoporosis via increasing bone resorption and decreasing bone formation. Serum receptor activator of nuclear factor κB-ligand (RANKL) and osteocalcin are biochemical markers of bone turnover have been developed to look at bone metabolism. Previous studies bone mineral density (BMD) and bone turnover markers in children and adults with classic CAH provided conflicting data.[3,4,5] The aim of the present study was to evaluate BMD and levels of bone turnover markers in Egyptian children with classic CAH and its relationship with disease-related variables.
PATIENTS AND METHODS
This cross-sectional controlled study was conducted during the period from March 2013 to November 2013. It included 28 children with classic salt loosing CAH (Group 1). The medical records of each patient were reviewed to confirm the diagnosis of congenital AH (salt loosing form) Criteria of salt loosing phenotype included history of salt-losing crisis with documented hyponatremia, hyperkalemia, and markedly elevated plasma renin activity. All participants were healthy, had normal vital signs, and were receiving no additional medications known to affect androgen secretion or bone metabolism. In addition, 28 apparently healthy age and sex matched children were studied as a control (Group 2). To be included in the study, the control subjects had to be without any acute disease and without clinical conditions involving the endocrine–metabolic system. Both patients and controls were recruited from Pediatric Endocrinology Outpatients Clinic in Assiut University Children Hospital, Assiut, Egypt. The study protocol was approved by the Ethical Committees of Assiut University Children Hospital, Egypt. Written informed consents were obtained from the parents of both patients and controls.
Inclusion criteria
Age > 4 years < 12 years, on steroid treatment > 3 years.
Exclusion criteria
Candidates were excluded if they had a history of chronic illness, other endocrinal problems, cardiac, hepatic, renal disease; they had one or more fractures of the vertebrae, wrist, or hip; or they had taken vitamin D preparation, calcium supplements, or Gonadotrophin releasing hormone analogue.
Prednisone dose was converted to hydrocortisone as 10 mg of hydrocortisone is the same of 2 mg of prednisone. Patients with serum 17-hydroxyprogesterone value of less than 10 nmol/l, were classified as well controlled (WC), and those with serum 17-hydroxyprogesterone value >10 nmol/l were classified as poorly controlled (PC).[6]
All children included in the study were subjected to:
Standard medical history
Anthropometric measurements: Height and weight were measured using a wall-mounted stadiometer and a calibrated weight scale, respectively with subjects wearing underwear only. Body mass index (BMI) of children and parents was calculated by using the formula; BMI = weight (kg)/height (m2). BMI standard deviation score (SDS) expressed according to the Egyptian data
Bone age (BA) was done by performing plain X-ray left hand and wrist according to the standards of Greulich and Pyle.
Bone mineral density measurement
Bone status was evaluated at the time of the study by using dual energy X-ray absorptiometry (DXA; NorlandEclips). Children were examined in supine antero-posterior position using the same scan mode. For all patients and controls, bone mineral content (BMC; g) and BMD (g/cm2) and bone area (cm2) of total body, the lumbar spine (L1-L4) and left proximal femur (femoral neck) were measured by DXA. Absolute values were converted into Z-scores (standard deviation from the mean of a healthy age and sex matched reference population).[7] Osteopenia was defined as Z scores between − 1 and − 2.5; whereas osteoporosis below − 2.5. So, Z-score > −1 SD was considered normal and low BMD was defined as a Z-score < -1 SD.
Laboratory investigations
Disease-related laboratory tests
Complete blood count (CBC), sodium, potassium, renin, testesterone 17 hydroxyprogesterone, and adrenocortcotrophic hormone (ACTH) were done.
Bone turnover-related laboratory tests
-
a)
Calcium (Ca), phosphorus (Ph) and total alkaline phosphatase (Menarini Diagnostics Kits, Italy; Technicon RA-1000 autoanalyzer)
-
b)
Serum osteocalcin was measured by a commercial radioimmunoassay (Incstar, Stillwater, MN, USA);[8] the intra- and inter-assay coefficients of variation (CVs) were 3.5% and 7%, respectively
-
c)
Serum RANKL levels was estimated by using ELISA kit, (Biomedica group, Biomedica Medicine product, GmbH and Co., Cat. No. BI-20422H). Intra- and interassay CV of the assay were < 10% and its detection limit was 0.08 pmol/l.
Statistical analysis
Data analysis was carried out using Statistical Package for Social Sciences (SPSS, version 16). Simple statistics such as frequency, arithmetic mean, and standard deviation (SD) were used. For comparison of the two groups, Student's t-test was used for parametric data and the Mann-Whitney U-test was used for nonparametric data. Linear correlations were performed by Spearman's or Pearson's test. For all analyses, P value of < 0.05 provided statistical significance.
RESULTS
The most relevant characteristics of patients are shown in Table 1.
Table 1.
Clinical and laboratory characteristics of congenital adrenal hyperplasia cases
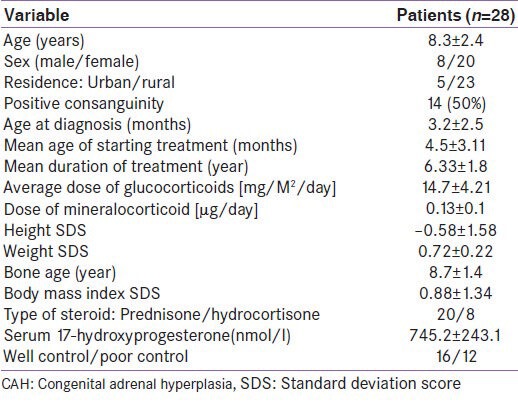
Differences between healthy controls and patients with classic CAH in the levels of the investigated bone metabolism, biochemical parameters and BMI are shown in Table 2. There was a significantly lower serum osteocalcin, and significantly higher serum RANKL levels in patients with CAH than the healthy controls.
Table 2.
Bone metabolism biochemical parameters in patients with classic congenital adrenal hyperplasia compared with healthy
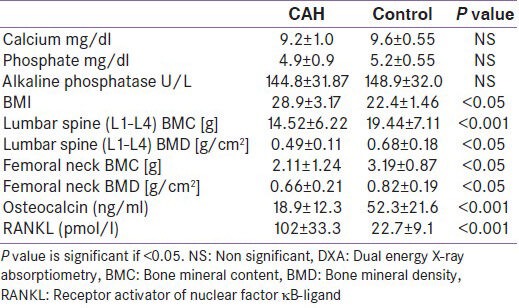
Differences between CAH cases with well control in the levels of the investigated biochemical parameters compared with those with poor control are shown in Table 3. There was a significantly lower serum osteocalcin, and significantly higher serum RANKL levels in children with poor control than the well control.
Table 3.
Bone metabolism biochemical parameters in poor controlled congenital adrenal hyperplasia children compared with well controlled
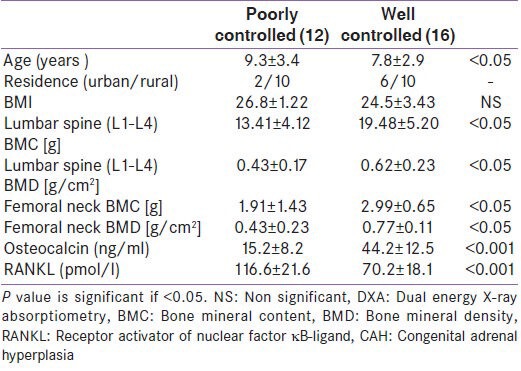
Differences between CAH cases on prednisone therapy in the levels of the investigated biochemical parameters compared with those on hydrocortisone are shown in Table 4. There was a significantly lower serum osteocalcin, and significantly higher serum RANKL levels in children prednisone therapy than those on hydrocortisone.
Table 4.
Bone metabolism biochemical parameters in congenital adrenal hyperplasia children on prednisone compared with those on hydrocortisone
Total BMC [gm] have significant negative correlations to age (r = −0.81, P < 0.001), duration of treatment (r = −0.887, P < 0.001), 17 OH Progesterone level (r = −0.543, P < 0.05), RANKL level (r = −0.635, P < 0.05), and significant positive correlation with osteocalcin (r = 0.576, P < 0.001) are shown in Table 5.
Table 5.
Correlation coefficient between total BMC [gm] and clinical and biochemical data of studied cases
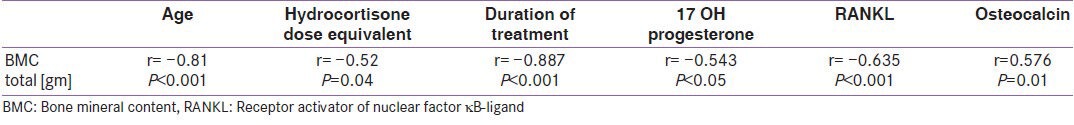
DISCUSSION
Children with congenital adrenal hyperplasia from Upper Egypt are usually attending pediatric Endocrinology Unit in Assuit Children University Hospital as it is the only specialized center for caring of these patients. Most of these children were treated with prednisolone rather hydrocortisone due to its high price of hydrocortisone and its unavailability. Furthermore, for children with poor controlled CAH are usually coming from rural areas in Upper Egypt, this may attributed to difficulty to attend the medical institution for follow up due to financial factors and long distance from their residency.
In the present study, the children with CAH had significant lower BMD values for both, vertebrae and proximal femur than controls. This result is in agreement with Cameron et al.[9] and de Almeida,[10] who confirmed the presence of inadequate mineralization in children with CAH. Reduced BMD is associated with increased fracture risk in children and teenagers as well. Children with CAH need chronic glucocorticoid therapy, both to replace congenital deficit in cortisol synthesis and to suppress the overproduction of androgens by adrenal cortex A possible reason for the high bone turnover rate found in CAH patients is represented by the interplay between exogenous glucocorticoids and endogenous production of androgens. Slightly higher doses of glucocorticoid taken chronically might affect bone metabolism and lead to alterations of bone mass in this condition. In particular, they could increase bone resorption rate.[11] Moreover, low androgen concentration is a known factor for reduced bone mass and altered bone metabolism. Suppressed androgen production with high dose of glucocorticoid may thus interfere with normal bone metabolism and lead to reduced bone mineral content. Careful hormonal balance must be maintained throughout childhood in order to maximize growth and limit a hyperandrogenic state.[12] In bone cells, at the molecular level, glucocorticoids regulate various functions including osteoblastogenesis, osteoclastogenesis, and the apoptosis of osteoblasts and osteocytes.[13] In contrary of our study, Fleischman et al.[14] in their study reported that BMD and BMC did not correlate with current glucocorticoid and androgen levels in this study. BMD correlates with cumulative, rather than current, steroid doses. Therefore, these and other data suggest that multiple factors, such as number of studied cases, age, duration, and dosage of therapy, contribute to bone status in children with CAH.[2]
In the present study, BMI was significantly higher in CAH patients compared with controls. The increased BMI could be the result of glucocorticoid use; other possible explanations for the increased fat mass could be adrenomedullary dysfunction with decreased catecholamine secretion, as recently described in CAH patients. Additionally, children with CAH had increased leptin levels and decreased insulin sensitivity, which has been ascribed to their adrenomedullary dysfunction.[15]
The serum levels of calcium, phosphorous, and total alkaline phosphatase were not significantly differed in children with CAH compared to controls. These results go in line with those of Hagenfeldt[4], who found that the serum level of calcium, phosphorus and alkaline phosphatase did not correlate with bone mineral density in CAH patients. This imply that biochemical tests of these levels are not useful indicators of osteopenia.
Biochemical markers of bone turnover have been developed to look at bone metabolism. In this study, we have shown that childrenwith CAH on steroid therapy have low serum osteocalcin and high serum RANKL levels, i.e. ourpatients have low bone formation markers and high bone resorption markers, this in agreement with Paganini[16] who report a similar result in their study. These biochemical markers of bone turnover provide potentially useful information but cannot be used alone to diagnose osteoporosis, to determine the severity of the disease, or to select a specific therapy.[17]
In the present study, children with elevated 17-hydroxyprogesterone levels (poor control) had significant reduction in bone density at all measured sites and a suppression of bone turnover compared with those with normal 17-hydroxyprogesterone (WC). The elevated 17-hydroxyprogesterone levels may have identified those individuals who were more resistant to therapy and, thus, were more likely to have required higher lifetime glucocorticoid exposures. However, the elevated levels may also represent non-compliant or intermittently compliant individuals and thus represent participants with less glucocorticoid exposure, which would be more difficult to explain physiologically. Therefore, these data suggest that multiple factors, such as body mass index, duration, and dosage of therapy contribute to bone status in children with CAH.[18,19,20]
In the present study, 20 of the studied CAH patient were on prednisone vs. 8 cases on hydrocortisone due to unavailability of hydrocortisone in Upper Egypt. In a comparison of CAH patients treated with prednisolone or hydrocortisone, we observed a significant reduction in bone density at all measured sites and a suppression of bone turnover markers in those patients taking prednisolone. This was despite the calculated equivalent dose of glucocorticoid being similar between the groups. Hassan et al. have previously reported that prednisolone treatment is not associated with a difference in measured quality of life.[13] As such, our data indicate that glucocorticoid replacement therapy with prednisolone is likely to have a selective adverse impact on bone relative to hydrocortisone therapy, and thus CAH patients currently taking prednisolone should have their therapy changed to hydrocortisone.
CONCLUSIONS
Children from Upper Egypt with classic CAH may have reduced BMD and increase bone turnover compared with controls. This differences is more obvious in children with poor control and in those receiving prednisone therapy.
Recommendations
Active monitoring of BMD in CAH children using DEXA scanning. Furthermore, effort should be done to bring hydrocortisone to Upper Egypt to replace prednisone in children with classic congenital adrenal hyperplasia.
Limitation of the study
Small sample size due to the frequency of the disease in question, one in 16,000, so, negative findings must therefore be interpreted with caution.
The heterogeneity of the adrenal status of the participants reflects the cross-sectional design, with measurements obtained at a single time point, and therefore may limit the ability to determine the lifelong effects of excess androgens on bone status.
Lean body mass, which is an important predictor of bone density, was not measured.
Footnotes
Source of Support: Nil
Conflict of Interest: No.
REFERENCES
- 1.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–88. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 2.Merke DP, Bornstein SR, Avila NA, Chrousos GP. NIH conference. Future directions in the study and management of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Ann Intern Med. 2002;36:320–34. doi: 10.7326/0003-4819-136-4-200202190-00012. [DOI] [PubMed] [Google Scholar]
- 3.Jaaskelainen J, Voutilainen R. Bone mineral density in relation to glucocorticoid substitution therapy in adult patients with 21-hydroxylase deficiency. Clin Endocrinol (Oxf) 1996;45:707–13. doi: 10.1046/j.1365-2265.1996.8620871.x. [DOI] [PubMed] [Google Scholar]
- 4.Hagenfeldt K, Martin Ritzen E, Ringertz H, Helleday J, Carlstrom K. Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur J Endocrinol. 2000;143:667–71. doi: 10.1530/eje.0.1430667. [DOI] [PubMed] [Google Scholar]
- 5.Girgis R, Winter JS. The effects of glucocorticoid replacement therapy on growth, bone mineral density, and bone turnover markers in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1997;82:3926–9. doi: 10.1210/jcem.82.12.4320. [DOI] [PubMed] [Google Scholar]
- 6.Bachelot A, Chakhtoura Z, Rouxel A, Dulon J, Touraine P. Hormonal treatment of congenital adrenal hyperplasiadue to 21-hydroxylase deficiency. Ann Endocrinol. 2007;68:274–80. doi: 10.1016/j.ando.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 7.Leonard MB, Propert KJ, Zemel BS, Stallings VA, Feldman HI. Discrepancies in pediatric bone mineral density reference data: Potential for misdiagnosis of osteopenia. J Pediatr. 1999;135:182–8. doi: 10.1016/s0022-3476(99)70020-x. [DOI] [PubMed] [Google Scholar]
- 8.Power MJ, Fottrel PF. Diagnostic methods and clinical applications. Crit Rev Lab Sci. 1991;28:287–335. doi: 10.3109/10408369109106867. [DOI] [PubMed] [Google Scholar]
- 9.Cameron FJ, Kaymakci B, Byrt EA, Ebeling PR, Warne GL, Wark JD. Bone mineral density and body composition in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1995;80:2238–43. doi: 10.1210/jcem.80.7.7608286. [DOI] [PubMed] [Google Scholar]
- 10.de Almeida Freire PO, de Lemos-Marini SH, Maciel-Guerra AT, Morcillo AM, Matias Baptista MT, de Mello MP, et al. Classical congenital adrenal hyperplasia due to 21-hydroxylasedeficiency: A cross-sectional study of factors involved in bone mineral density. J Bone Miner Metab. 2003;21:396–401. doi: 10.1007/s00774-003-0434-6. [DOI] [PubMed] [Google Scholar]
- 11.Mora S, Saggion F, Russo G, Weber G, Bellini A, Prinster C, et al. Bone density in young patients with congenital adrenal hyperplasia. Bone. 1996;18:337–40. doi: 10.1016/8756-3282(96)00003-8. [DOI] [PubMed] [Google Scholar]
- 12.Arisaka O, Hoshi M, Kanazawa S, Numata M, Nakajima D, Kanno S, et al. Effect of adrenal androgen and estrogen on bone maturation and bone mineral density. Metab Clin Exp. 2001;50:377–9. doi: 10.1053/meta.2001.21678. [DOI] [PubMed] [Google Scholar]
- 13.Rehman Q, Lane NE. Effect of glucocorticoids on bone density. Med Pediatr Oncol. 2003;41:212–6. doi: 10.1002/mpo.10339. [DOI] [PubMed] [Google Scholar]
- 14.Fleischman A, Ringelheim J, Feldman H, Gordon C. Bone mineral status in children with congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2007;20:227–35. doi: 10.1515/jpem.2007.20.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo CY, Weetman AP, Eastell R. Bone turnover and bone mineral density in patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 1996;45:535–41. doi: 10.1046/j.1365-2265.1996.00851.x. [DOI] [PubMed] [Google Scholar]
- 16.Paganini C, Radetti G, Livieri C, Braga V, Migliavacca D, Adami S. Height, bone mineral density and bone markers in congenital adrenal hyperplasia. Horm Res. 2000;54:164–8. doi: 10.1159/000053253. [DOI] [PubMed] [Google Scholar]
- 17.Rosen JF. The epidemiology and pathogenesis of osteoporosis. endotext.com. 2014. [Last accessed on 2014 Feb 20]. Available from: http://www.endotext.com .
- 18.Gussinye M, Carrascosa A, Potau N, Enrubia M, Vicens-Calvet E, Ibanez L, et al. Bone mineral density in prepubertal and in adolescent and young adult patients with the salt-wasting form of congenital adrenal hyperplasia. Pediatrics. 1997;100:671–4. doi: 10.1542/peds.100.4.671. [DOI] [PubMed] [Google Scholar]
- 19.Falhammar H, Filipsson Nyström H, Wedell A, Brismar K, Thorén M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168:331–41. doi: 10.1530/EJE-12-0865. [DOI] [PubMed] [Google Scholar]
- 20.Nermoen I, Bronstad I, Fougner KJ, Svartberg J, Oksnes M, Husebye, et al. Genetic, anthropometric and metabolic features of adult Norwegian patients with 21- hydroxylase deficiency. Eur J Endocrinol. 2012;167:507–16. doi: 10.1530/EJE-12-0196. [DOI] [PubMed] [Google Scholar]


