Abstract
Context:
Accumulating evidence suggests that vitamin D deficiency is associated with increased risk of stroke. Contributing mechanisms have been linked to the association of vitamin D deficiency with the presence of hypertension, diabetes mellitus, and atherosclerosis, however, the evidence is conflicting.
Aims:
This study sought to determine the association of vitamin D deficiency with ischemic stroke and its risk factors.
Settings and Design:
Cross-sectional case control study.
Subjects and Methods:
We measured serum 25-hydroxyvitamin D [25(OH) D] and intact parathyroid hormone (iPTH) levels in 73 patients of ischemic stroke, presenting within 7 days of onset of stroke and compared with 70 age and gender matched controls.
Statistical Analysis Used:
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 17.0 for Windows).
Results:
The mean age of patients and controls was 59.9 ± 11.2 years and 57.9 ± 9.7 years, respectively (P = 0.26). Of 67.1% patients were men as compared to 65.7% controls (P = 0.86). There was no significant difference in the prevalence of vitamin D deficiency/insufficiency (P = 0.25), mean 25(OH) D levels (P = 0.75), and iPTH levels (P = 0.10) between cases and controls. No association of vitamin D deficiency/insufficiency was found with the prevalent risk factors in cases of ischemic stroke.
Conclusions:
Vitamin D deficiency does not bear an association with ischemic stroke or its risk factors.
Keywords: Asian, ischemic, Indian, risk, stroke, Vitamin D
INTRODUCTION
A poor vitamin D status is now recognized as a public health problem affecting almost every second person worldwide.[1] Evidence from many prospective population based studies has indicated that poor vitamin D status is predictive for future strokes.[2,3,4,5,6,7] A meta-analysis on 25-hydroxyvitamin D [25(OH) D] and symptomatic ischemic stroke has found stepwise increasing risk of symptomatic ischemic stroke with decreasing concentration of plasma 25(OH) D.[2] Data from patient based cross-sectional studies also supports this association, but the evidence is limited.[8,9,10]
Studies from India have uniformly pointed to low 25(OH) D levels in Indian population in all age groups and in all regions, despite plenty of sunshine.[11] Indian epidemiological data also reveals high age-standardized prevalence and annual incidence rates of first-ever stroke in Indians as compared to non-Asian populations.[12,13] Hence, it was interesting to explore the association between the two. This study aimed at determining the association of vitamin D deficiency/insufficiency with ischemic stroke and its risk factors.
SUBJECTS AND METHODS
Study design and participants
In this cross-sectional, hospital based (latitude 30.74°N) case control study, 73 patients were included who were diagnosed with ischemic stroke. The inclusion criteria were: i) Presentation within 7 days of onset of ischemic stroke, ii) Prestroke Modified Rankin Score (MRS) < 2, iii) Age ≥ 35 years. Patients on vitamin D and calcium supplementation, those with renal and hepatic impairment, and those who underwent thrombolysis were excluded. All patients had a CT or MRI brain scan at baseline. A total of 70 age- and gender-matched healthy controls were included. The study was approved by the Institute ethics committee and all subjects gave a written informed consent.
Data collection
A questionnaire-based direct interview was used to collect information on disease profile, demographic variables, risk factors, and medications used. Patients underwent measurements like serum 25(OH) D, intact parathyroid hormone (iPTH), serum total calcium, inorganic phosphorous, lipid profile, HbA1c, fasting plasma glucose (FPG), renal and liver function tests.
Serum iPTH and 25(OH) D were measured by chemiluminescence assay using commercially available kits (Elecsys 2010 system, Roche diagnostic, Germany). Individuals whose serum 25(OH) D value was less than 50 nmol/L (20 ng/mL) were classified as deficient, 50 to 74 nmol/L (20 - 29 ng/ml) as insufficient and ≥75 nmol/L (30 ng/ml) were identified as sufficient.[14] HbA1c was estimated by Bio-Rad 10 system (Bio-Rad, Hercules, CA) functioning on HPLC-based ion exchange chromatography. Serum albumin, calcium, phosphorus, lipid profile, FPG, liver function and renal function tests were done by auto-analyser (Modular P500 Roche Diagnostic, Germany). Blood pressure measurements were carried out by mercury sphygmomanometer and hypertension was defined as systolic blood pressure (SBP) ≥140 mm Hg or diastolic blood pressure (DBP) ≥90 mm Hg, or use of antihypertensive therapy.[15] Criteria for diabetes mellitus were a FPG ≥ 126 mg% (7 mmol/L) or HbA1c ≥ 6.5% or use of insulin or oral hypoglycaemic agents.[16] Dyslipidemia was defined as either of serum triglycerides (TG) ≥1.7 mmol/L (150 mg/dl), or high-density lipoprotein cholesterol (HDL-C) < 1.03 mmol/L (40 mg/dl) in men and 1.3 mmol/L (50 mg/dl) in women or low density lipoprotein cholesterol (LDL-C) >2.6 mmol/L (100 mg/dl).[17] Waist circumference was measured in a horizontal plane above the iliac crest. A value of >90 cm in men and >80 cm in women was taken as abnormal as per the ethnic specific cut points proposed by the International Diabetes Federation.[18]
A total of 70 healthy age-and gender-matched subjects were evaluated as controls in the study. The vitamin D levels in stroke patients were compared to that of healthy controls. In subgroup analysis, we compared the association between 25(OH) D levels in stroke patients with the prevalence of diabetes, hypertension, obesity, and dyslipidemia.
Statistical analysis
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 17.0 for Windows). Using the Power/Sample size calculator, our sample size came was 65 (taking a confidence interval of 95%, power of study as 90%, prevalence of vitamin D insufficiency as 50% in healthy adults[19] and 77% in patients with acute stroke).[10] All quantitative variables like age, blood pressure, waist circumference, FPG, HbA1c, 25(OH) D levels, iPTH levels, calcium profile, and lipid profile were estimated using measures of central location, i.e. mean, median and measures of dispersion, i.e. standard deviation. Normality of data was checked by Kolmogorov Smirnov tests of normality. For normally distributed data means were compared using t-test for two groups. For skewed data, Mann Whitney test was applied for comparison of two groups. For more than two groups Kruskal Wallis test was applied. Qualitative or categorical variables, e.g. obesity, hypertension, diabetes mellitus, etc., were presented as percentages. Proportions were compared using Chi-square or Fisher's exact test whichever was applicable. All statistical tests were two-sided and performed at a significance level of α =0.05.
RESULTS
Demographic data for the study population (73 cases and 70 controls) are summarized in Table 1. The mean ± SD age of cases and controls was 59.9 ± 11.2 and 57.9 ± 9.7 years, respectively (P = 0.26). The proportion of men in cases and controls were 67.1% and 65.7%, respectively (P = 0.86). The proportion of smokers among cases (24.7%) was significantly higher than that in controls (10%) (P = 0.02). Significantly higher values of waist circumference, SBP, DBP, TG, FPG, and HbA1c were found in cases than in controls as shown in Table 1. LDL-C and HDL-C were significantly higher in controls as compared to cases.
Table 1.
Baseline characteristics of cases and controls

Only 27.4% of cases and 34.3% of controls were vitamin D sufficient. There was no significant difference among cases and controls in vitamin D status and iPTH levels as shown in Table 1. To see the effect of seasonal difference, the vitamin D levels of the cases in summers (April-September) and winters (October–March) were compared. The mean vitamin D levels were 62 ± 40.5 nmol/L (24.8 ± 16.2 ng/ml) and 67 ± 36.5 nmol/L (26.8 ± 14.6 ng/ml), respectively. The difference between the two groups was not statistically significant (P = 0.61). Also on comparing the mean vitamin D levels between cases [67 ± 36.5 nmol/L (26.8 ± 14.6 ng/ml)] and controls [65.5 ± 36.3 nmol/L (26.2 ± 14.5 ng/ml)] in similar season (October-March), no significant difference was found (P = 0.86).
There was high prevalence of traditional risk factors of stroke among cases. Hypertension, diabetes mellitus, dyslipidemia, and obesity were present in 87.7%, 54.8%, 87.7%, and 56.2% of cases, respectively. After study, 24.7% were smoker and 6.8% of cases had non-valvular atrial fibrillation.
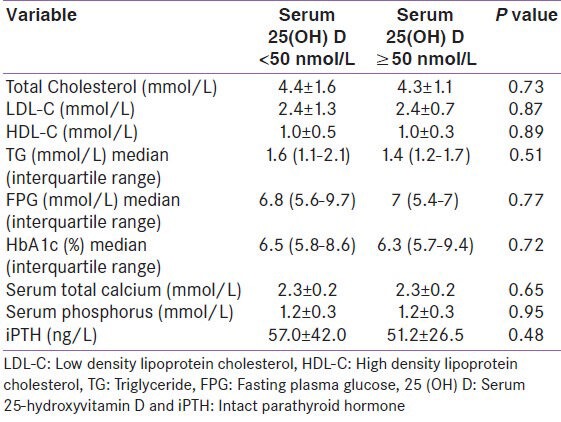
We analyzed the risk factor prevalence among cases as per their vitamin D status. The comparison was made between individuals who were vitamin D sufficient and those who were either vitamin D insufficient or vitamin D deficient (i.e. between cases with vitamin D levels ≥75 nmol/L and < 75 nmol/L, as shown in Table 2 and ≥50 nmol/L and < 50 nmol/L, as shown in Table 3). No statistical difference was observed either in the prevalence of hypertension, diabetes mellitus, obesity, dyslipidemia, ischemic heart disease, non-valvular atrial fibrillation, and smoking [Tables 2 and 3] or in the biochemical parameters (TC, LDL, HDL, TG, FPG, HbA1c, serum total calcium, serum inorganic phosphorous, and iPTH) as per vitamin D status among cases [Tables 4 and 5].
Table 2.
Characteristics of cases as per vitamin D status, (25 (OH) D <75 nmol/L vs. ≥75 nmol/L)

Table 3.
Characteristics of cases as per vitamin D status, (25 (OH) D <50 nmol/L vs. ≥50 nmol/L)
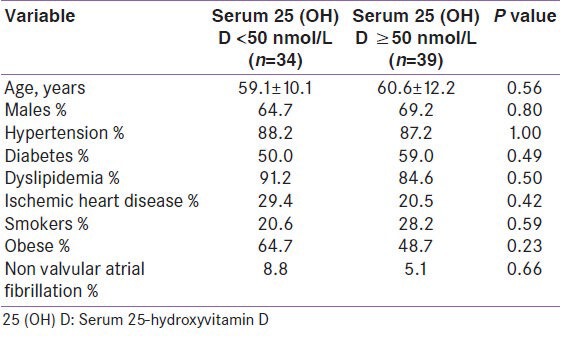
Table 4.
Biochemical parameters of cases as per vitamin D status, (25 (OH) D <75 nmol/L vs ≥75 nmol/L)
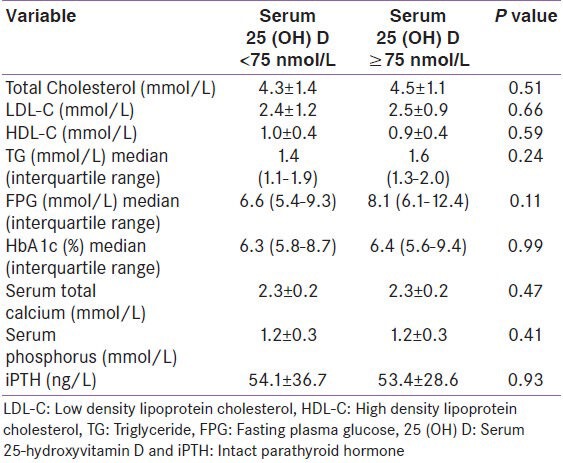
Table 5.
Biochemical parameters of cases as per vitamin D status, (25 (OH) D <50 nmol vs ≥50 nmol/L)
DISCUSSION
We found no association of low vitamin D status with ischemic stroke or its prevalent risk factors. Although, prospective population-based studies have shown that low 25(OH) D levels are predictive of future stroke,[2,4] our study being a cross-sectional hospital-based study did not demonstrate this association. We have restricted our comparison on this association to other cross-sectional studies.
Poole et al., found 34 of 44 patients (77%) with stroke with low serum 25(OH) D levels (< 50 nmol/L) which were substantially lower than healthy elderly subjects.[8] The inclusion criterion in the study were however hemiplegia involving lower limb, inability to walk independently 1 week after stroke. The sample for 25(OH) D level was collected up to 30 days of a first-ever stroke, whereas we evaluated 25(OH) D levels within 7 days of stroke and our stroke population was not restricted to lower limb plegics. Prolonged immobilization contributes to lowered vitamin D levels. Moreover, they included both ischemic and hemorrhagic strokes, which make the study population heterogenous.
In a study of elders, receiving home services, 25(OH) D levels less than 50 nmol/L was associated with stroke with or without dementia in fully adjusted logistic regression models.(OR = 2.3, 95% CI 1.1-4.8). However, when subjects with stroke were stratified by the presence or absence of dementia, 25(OH) D status did not emerge to be a significant factor.[9]
Another cross-sectional analysis data from NHANES III reported strong and independent relationship of 25(OH) D deficiency with prevalent cardiovascular disease (CVD). However, CVD outcome was based on self report. The data did not provide information about the date of onset CVD or any subsequent life style changes, both of which could have occurred long before the patients’ participation in the study.[10]
In terms of strengths, the inclusion criteria of our study avoided false associations between the prevalence of vitamin D deficiency and stroke, as patients were recruited within 7 days of onset of stroke, did not have a past history of stroke (Vitamin D deficiency is common in post-stroke immobilised patients), and were ambulatory prior to stroke as in some previous studies.
This study has some limitations. Being a cross-sectional study, it has a limited ability to determine a temporal association between vitamin D deficiency and ischemic stroke. The participants in this study were recruited from patients attending tertiary level health services and results cannot be generalized to a community dwelling population.
In conclusion, vitamin D deficiency/insufficiency does not bear an association with ischemic stroke or its prevalent risk factors. It might be a marker of greater comorbidity rather than being causally related to stroke.
ACKNOWLEDGMENT
We acknowledge the help of Mrs. Kusum Chopra for statistical calculation of the manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pilz S, Tomaschitz A, Drechsler C, Zittermann A, Dekker JM, März W. Vitamin D supplementation: A promising approach for the prevention and treatment of strokes. Curr Drug Targets. 2011;12:88–96. doi: 10.2174/138945011793591563. [DOI] [PubMed] [Google Scholar]
- 2.Brøndum-Jacobsen P, Nordestgaard BG, Schnohr P, Benn M. 25-Hydroxyvitamin D and symptomatic ischemic stroke: An original study and meta-analysis. Ann Neurol. 2013;73:38–47. doi: 10.1002/ana.23738. [DOI] [PubMed] [Google Scholar]
- 3.Kojima G, Bell C, Abbott RD, Launer L, Chen R, Motonaga H, et al. Low dietary vitamin D predicts 34-year incident stroke: The Honolulu Heart Program. Stroke. 2012;43:2163–7. doi: 10.1161/STROKEAHA.112.651752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: A prospective study and meta-analysis. Stroke. 2012;43:1470–7. doi: 10.1161/STROKEAHA.111.636910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, et al. Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J. 2010;31:2253–61. doi: 10.1093/eurheartj/ehq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JL, May HT, Horne BD, Bair TL, Hall NL, Carlquist JF, et al. Intermountain Heart Collaborative (IHC) Study Group. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol. 2010;106:963–8. doi: 10.1016/j.amjcard.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Kilkkinen A, Knekt P, Aro A, Rissanen H, Marniemi J, Heliövaara M, et al. Vitamin D status and the risk of cardiovascular disease death. Am J Epidemiol. 2009;170:1032–9. doi: 10.1093/aje/kwp227. [DOI] [PubMed] [Google Scholar]
- 8.Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–5. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 9.Buell JS, Dawson-Hughes B, Scott TM, Weiner DE, Dallal GE, Qui WQ, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74:18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kendrick J, Targher G, Smits G, Chonchol M. 25-Hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–60. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Harinarayan CV, Joshi SR. Vitamin D status in India–its implications and remedial measures. J Assoc Physicians India. 2009;57:40–8. [PubMed] [Google Scholar]
- 12.Das SK, Banerjee TK. Stroke: Indian scenario. Circulation. 2008;118:2719–24. doi: 10.1161/CIRCULATIONAHA.107.743237. [DOI] [PubMed] [Google Scholar]
- 13.Gunarathne A, Patel JV, Gammon B, Gill PS, Hughes EA, Lip GY. Ischemic stroke in South Asians: A review of the epidemiology, pathophysiology, and ethnicity-related clinical features. Stroke. 2009;40:e415–23. doi: 10.1161/STROKEAHA.108.535724. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 15.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee: Seventh report of the joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and Classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on Detection, Evaluation and Treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Zimmet P, Shaw J IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: A new worldwide definition. Lancet. 2005;366:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 19.Ramakrishnan S, Bhansali A, Bhadada SK, Sharma R, Walia R, Ravikiran M, et al. Vitamin D status and its seasonal variability in healthy young adults in an Asian Indian urban population. Endocr Pract. 2011;17:185–91. doi: 10.4158/EP10155.OR. [DOI] [PubMed] [Google Scholar]


