Abstract
Objective
Maternal-to-child-transmission of HIV-1 infection remains a significant cause of HIV-1 infection despite successful prevention strategies. Testing protective HIV-1 vaccines remains a critical priority. The immunogenicity of ALVAC-HIV vCP1521 (ALVAC) in infants born to HIV-1 infected women in Uganda was evaluated in the first pediatric HIV-1 vaccine study in Africa.
Design
HPTN 027 was a randomized, double blind, placebo-controlled phase I trial to evaluate the safety and immunogenicity of ALVAC in 60 infants born to HIV-1 infected mothers with CD4 counts > 500 cell/μL that were randomized to the ALVAC vaccine or placebo. ALVAC-HIV vCP1521 is an attenuated recombinant canarypox virus expressing HIV-1 clade E env, clade B gag and protease gene products.
Methods
Infants were vaccinated at birth, 4, 8 and 12 weeks of age with ALVAC or placebo. Cellular and humoral immune responses were evaluated using IFN-γ ELISpot, CFSE proliferation, intracellular cytokine staining, binding and neutralizing antibody assays. Fisher's exact test was used to compare positive responses between study arms.
Results
Low levels of antigen specific CD4 and CD8 T cell responses (intracellular cytokine assay) were detected at 24 months (CD4 – 6/36 vaccine vs. 1/9 placebo; CD8 – 5/36 vaccine vs. 0/9 placebo) of age. There was a non-significant trend toward higher cellular immune response rates in vaccine recipients compared to placebo. There were minimal binding antibody responses and no neutralizing antibodies detected.
Conclusions
HIV-1 exposed infants are capable of generating low levels of cellular immune responses to ALVAC vaccine, similar to responses seen in adults.
Keywords: HIV vaccine, infants, immunogenicity, Africa, HIV prevention
Introduction
Mother-to-child-transmission (MTCT) of HIV-1 infection remains a significant cause of HIV-1 infection worldwide, despite successful prevention strategies. In the absence of antiretrovirals (ARV), vertical transmission is estimated 21-45% with postnatal transmission through breast milk accounting for over one-third of all transmission.[1–5] Promotion of breastfeeding in most developing countries has been central to maternal and child health.[6] Discouraging breastfeeding in these countries to protect against HIV-1 infection places infants at greater risk of poor growth and increased morbidity and mortality.[7-10] Identifying a simple, safe and effective method of protecting infants from HIV while breastfeeding remains a priority.
Combined antenatal, perinatal, and postnatal infant ARV interventions offer effective protection from MTCT, but the difficult logistics and cost of reaching all pregnant women with HIV-1 prevention of MTCT (PMTCT) services and continuing ARVs in the infant or mother for the duration of breastfeeding has slowed the elimination of pediatric HIV-1 infection in resource limited settings. The global plan to reduce pediatric infections to less than 5% by the year 2015 requires strategies to improve PMTCT uptake to over 95% throughout pregnancy and breastfeeding, to safely reduce the duration of breastfeeding, and to support medication adherence, which is a challenge during both pregnancy and breastfeeding.[11-13] Identification of an effective HIV-1 vaccine that could be given to all infants after birth would significantly enhance these elimination efforts.[14]
The need for the successful development and testing of an adult HIV-1 vaccine applies equally to infants. However, pediatric vaccine studies have been hampered by unique concerns, including the ability of the immature neonatal immune system to respond, regulatory protections for vulnerable populations, constraints of small blood volumes that limit the breadth of safety and immunogenicity evaluations, simultaneous exposure to an HIV-1 vaccine and HIV-1 in breast milk, and the effect of an HIV-1 vaccine on the infant immune response to standard Uganda National Expanded Programme on Immunization (UNEPI) immunizations given in the same period. Despite these concerns, HIV vaccines trials have been successfully completed in infants and HIV-1 vaccines have been shown to be safe in the pediatric population.[15-17]
Limited data exist on the immunogenicity of preventive HIV-1 vaccines in children. In the AIDS Clinical Trials Group (ACTG) 230 trial, infants born to HIV infected women in the United States (US) were immunized with recombinant gp120 vaccines or adjuvant and HIV-specific lymphoproliferative responses were detected.[16,18] The ALVAC HIV-1 vCP205 expressing gp120 MN, linked to the LAI strain transmembrane domain of gp41 and its entire gag and pol genes also elicited lymphoproliferative responses in vaccine recipients, rare mucosal IgA but no measurable vaccine elicited plasma antibodies were detected.[17] The ALVAC-HIV vCP1452 vaccine with and without a subunit rgp120 envelope boost [19] was evaluated for safety and immunogenicity in infants born to HIV-1-infected women and showed that HIV-1 exposed infants were capable of responding to HIV-1 vaccines despite the presence of maternal antibody. The Thai RV144 HIV vaccine efficacy trial showed that a prime-boost HIV-1 vaccine regimen conferred 30% protection against HIV-1 acquisition, using the same ALVAC-HIV vCP1521 vaccine as this study as a prime followed by boosting with two recombinant envelope proteins from HIV-1 subtype B and E (AIDSVAX B/E).[20] The Thai trial provides the first evidence that a preventive vaccine for HIV may be feasible, with studies of possible correlates of protection underway.[21]
Our study was designed prior to the availability of efficacy and correlates of risk data from the above RV144. As such, the significance of the protein boost and a correlate of risk based on antibody response was not readily evident at the time of study initiation. While the prime boost regimen (e.g., ALVAC prime + gp120 boost) was felt to be the preferred regimen, it was felt necessary to document the performance of each component of a potential vaccination regimen separately before combining them. Thus the use of vCP1521 not only allowed for generation of safety data that could be attributed to ALVAC, it also had the potential to generate essential information on the capability of the neonatal immune system to respond to an HIV vaccine in the presence of maternally derived HIV antibodies. The larger safety experience with vCP1521, provided a favorable risk benefit profile to test in this vulnerable population.
The immunogenicity (cell-mediated and humoral responses) of ALVAC vCP1521 in infants born to HIV-1 infected Ugandan women with CD4 cell counts > 500 cells/μL was evaluated in the HPTN 027 trial, the first pediatric HIV-1 vaccine study in Africa.
Methods
Study population
HIV-1 infected pregnant women attending the antenatal clinics at the Mulago National Referral Hospital in Kampala, Uganda that were eligible during initial screening and consented were asked to return to the hospital for delivery. After birth, cord blood was obtained and infant eligibility for enrollment was assessed. Enrolled infants were randomized 4:1 to receive ALVAC or 0.9% saline placebo on or before Day 3 post birth, and at 4, 8, and 12 weeks. Infants were followed at the study clinic every two weeks through week 14 and at months 5, 6, 9, 12, 15, 18, 21 and 24 after birth. HIV-1 DNA PCR assays were performed on a pre-vaccination sample and at various time points to determine the HIV-1 status of the infants. Study vaccinations were discontinued in infants identified as HIV-1 infected after randomization. At age 18 and 24 months, rapid HIV-1 testing was also performed and if positive, confirmed by Western blot. Infants who received one or more doses of vaccine and were subsequently discontinued from study vaccination due to an adverse event, HIV-1 infection or any other reason remained in follow up through 24 months of age. Immunological evaluations were discontinued in any child that did not receive at least 2 doses of vaccine.
As part of routine care, study women and infants received the Mulago Hospital standard PMTCT regimens [single dose nevirapine +/- azidothymidine (AZT)] according to Uganda Ministry of Health (MOH) guidelines at the time of the study, which were consistent with World Health Organization (WHO) 2006 recommendations. In addition, they received counseling on the risks and benefits of breastfeeding according to the WHO and Uganda MOH recommendations.
This study was approved by the National HIV/AIDS Research Committee in Uganda, the Johns Hopkins Medicine Institutional Review Board, Uganda National Council of Science and Technology and the Makerere-Johns Hopkins Institutional Biosafety Committee. Import approval for the study products was provided by the Uganda National Drug Authority. Written informed consent for each infant in the study was provided by the infant's mother after demonstrating an understanding of the study purpose and procedures.
Vaccines
ALVAC HIV-1 vCP1521 is a recombinant canarypox vaccine developed by Virogenetics Corporation (Troy, NY) and manufactured by Sanofi Pasteur (Marcy-l'Étoille, France). The recombinant canarypox expresses gene products from the HIV-1 clade E env, clade B gag and protease. The genes are inserted into the C6 locus under the control of vaccine virus H6 and I3L promoters, respectively. The gp120 env sequence is derived from the subtype E strain TH023, but the anchoring part of the gp41 is derived from subtype B strain (LAI). The gag and protease sequences are also derived from subtype B. ALVAC-infected cells present Env and Gag proteins in a near-native conformation.[22] Sodium Chloride Injection USP, 0.9% was used as the placebo control.
Cell Mediated Immunity Assays
HIV specific cell mediated immunity was assessed using three assays: T cell cytokine production, quantified by enzyme-linked immunosorbent spot (ELISPOT) assays; T cell proliferation by carboxyfluorescein diaccetate succinimidyl (CFSE) dilution, using the CellTrace™ CFSE Cell Proliferation Kit; and lastly, flow-based intracellular cytokine staining.
Antigens
Peptides corresponding to the vaccine sequences of HIV-1 Env gp160 (consensus Group M, 212 peptides) and Gag (consensus group B, 49 peptides) were synthesized as 15 amino acids (aa) overlapping by 11 aa (NIH/NIAID repository, catalog numbers 6451 and 5189, respectively). The consensus M peptides were not matched to the vaccine antigens. The final concentration of individual peptides was 1μg/mL per peptide.
ELISPOTassay
Interferon gamma (IFN-γ) ELISPOT assay was performed using freshly isolated peripheral blood mononuclear cells (PBMC) as previously described at birth and 14 weeks of age (2 weeks after last vaccination).[23–25] Briefly, ninety-six well nitrocellulose plates (Millititer, Millipore Corp., Bedford, Massachusetts) were coated with monoclonal antibody (mAb) 1-D1K (Mabtech, Macka, Sweden) overnight at 4°C. PBMC were added in duplicate wells at 105 cells/well and stimulated with pools of overlapping peptides (20-40 peptides at final concentration 1μg/mL per peptide). Pooled peptides corresponding to the consensus group M HIV-1 Env gp160 (3 pools) and consensus clade B Gag (1 pool) were obtained from the NIAID repository and consisted of 15 aa overlapping by 11 aa. Negative and positive controls used were not stimulated (no peptide) as well as phytohemagglutinin (PHA) stimulated (2 μg/mL, Sigma, St Louis, MO) PBMC, respectively. Spots were counted using a CTL Analyzer and software version 2.8 (CTL Analyzers LLC, Cleveland, OH). A statistical distribution free resampling method was used to determine positivity similar to current HIV Vaccine Trials Network (HVTN) vaccine studies.[23]
CFSE proliferation assay
The CFSE flow-based proliferation assay in this study uses similar methodology to previously published clinical trials in adults [26] and this assay correlates to other functional assays performed in HIV positive and HIV negative subject studies.[27]. Cells were cultured in the presence of antigen for five days at 37°C and 5% CO2, then harvested and stained for surface markers with the following antibodies: CD3 APC, CD4 PE, CD8 PerCp-Cy5.5 (BD Biosciences, San Jose, CA). Samples were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). All flow analysis was performed using FlowJo software (TreeStar, Ashland, OR). Proliferation was measured by the extent of CFSE dilution. Staphylococcal Enterotoxin B (SEB) (Sigma-Aldrich, St. Louis, MO) stimulation was used as a positive control. Results with less than 1% background response, and greater than 5% SEB response were considered valid. Only data with a minimum of 5,000 acquired events of CD3+CD4+ or CD3+CD8+ were analyzed. Results greater than twice the background values and more than 0.1% after subtraction of background were considered positive. CFSE and intracellular cytokine assays were performed on specimens from birth, 10 weeks, and 24 months of age.
Flow-based intracellular cytokine staining
Detection of antigen-specific cytokine production was performed using PBMC incubated with Env, Gag, or cytomegalovirus (CMV) peptide pools for 6 hours at 37°C and 5% CO2 in the presence of brefeldin A (Sigma) and monensin (Golgi Stop, BD Biosciences, San Jose, CA).[28] SEB (1 μg/mL, Sigma) and media alone were used as positive and negative background controls, respectively. Cells were subsequently stained with anti-IFN-γ FITC, anti-CD4 PerCP-Cy5.5, anti-CD3 AmCyan, anti-CD8 APC-Cy7, anti- interleukin 2 (IL-2) APC (BD Biosciences) and anti-CD107a PE (BD Pharmigen). Dead cells were excluded from analysis using a violet excited viability dye (LIVE/DEAD Fixable Dead Cell Stain; Invitrogen).[29] A minimum of 30,000 CD3+ cells per sample were acquired using an 8-color flow cytometer (LSRII, BD Biosciences) Results were expressed as: Percent cytokine positive, CD4+ or CD8+ T cells (Percent positive = % antigen-specific - % negative control). Responses greater than 2 times the background were considered positive.
Humoral immune response assays
Humoral immune responses were measured using qualitative and quantitative antibody binding enzyme-linked immunosorbent assay (ELISA) and neutralizing antibodies as described below.
Binding Antibody ELISA Assays
The presence of plasma antibodies was assessed using a validated ELISA procedure.[19] Qualitative (single-point values) measurements of binding antibodies were determined from cryopreserved plasma at weeks 10 and 14 and months 6, 12, 18 and 24. Quantitative end-point titers at month 18 and 24 were determined for those with positive values in the qualitative assay. In the qualitative assay, one dilution of plasma at 1:50 dilution was tested in duplicate whereas in the quantitative assay, six serial dilutions of plasma beginning at 1:50 were tested in duplicate in microtiter plates coated with the following antigens: gp41 (clade B), gp120MN (clade B, VaxGen), p24 (subtype B) and DP31–a synthetic AVERY peptide not present in the vaccine (AnaSpec Incorporated, San Jose, California, USA). For each antigen in antibody assays, the background mean optical density (OD) of no antigen plate was subtracted from the mean with the vaccine or placebo to obtain the OD value analyzed. The OD value was set to zero if the above calculation yielded a negative value. An antigen-specific OD ≥ 0.2 was considered positive. A titre was considered as the highest dilution giving a positive OD value ≥0.2.[19,30]
Neutralization assays
Plasma samples that were antibody positive from the binding assays at months 18 and 24 were also tested for neutralizing activity against two tier 1 strains of HIV-1: SF162.LS (subtype B), MW965.26 (subtype C) and an MLV negative control virus using a standardized TZM-bl neutralization assay as previously described.[31] Neutralization titers were defined as the dilution at which relative luminescence units (RLU) were reduced by 50% compared to virus control wells after subtraction of background RLU. Neutralizing antibody titers of ≥ 10 were considered positive.
Statistical analysis
Data from all infants who received the full set of four ALVAC vaccine doses and all the placebo recipients were included in the descriptive analyses. Flow-based T cell responses were analyzed using a two- sided Fisher's exact test in comparing the proportion of infants with positive T-cell response at each visit between the vaccine and the placebo arms. Antibody responses were summarized by boxplots across study visits.
Results
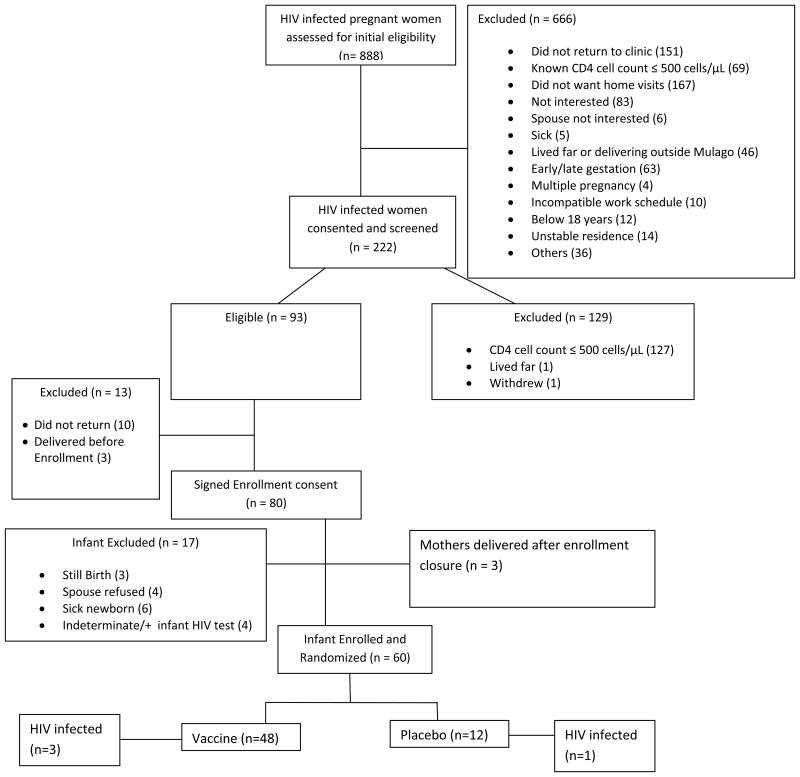
From October 2006 to May 2007, 888 HIV infected pregnant women were assessed for initial eligibility and of these 222 consented and were screened. Of the 93 subsequently eligible women, 60 infants born (Figure 1) were enrolled in the study and randomized (48 vaccine, 12 placebo).[15] All 60 infants received at least one study vaccination, however, only 47 infants (38 vaccine, 9 placebo) received all four study vaccinations. Thirteen infants were discontinued from receiving the full vaccine schedule. Four discontinuations were due to HIV-1 infection; three were diagnosed with HIV-1 infection at birth (2 vaccine, 1 placebo) and one infant in the vaccine arm with a negative PCR at birth had HIV infection detected at two weeks of age. No infants in the study became infected after 2 weeks of age during the 24 month follow-up.
Figure 1.
HPTN 027 CONSORT diagram.
Flow chart showing patterns of women and infants from assessment of HIV infected women for eligibility to infant enrollment showing reasons for exclusion.
Overall performance of the study
The multi- step recruitment procedures started with community sensitization. During these sensitization workshops, the commonly raised questions included: what is the composition of the vaccine?; how it might protect infants?; what is the duration of any possible protection?, what are the vaccine importation procedures; could the vaccine cause HIV infection?; would there be compensation in case of harm?; what is a placebo?; what is the role of the father/male partner?; and what is the duration of follow-up? These were addressed by study staff. Workshop participants identified perceived barriers to study participation including husband disapproval, lack of health research information among community leaders, the extended period from initiation of the trial to receipt of trial results, and rumors about the use of expired vaccines which were all addressed during participant recruitment.
The multi-step recruitment procedure further included; provision of written consent by a pregnant woman to be screened for eligibility during the antenatal period. Those eligible mothers then underwent a second written consent process to have their newborn screened and enrolled in the study and lastly when a child was born, a mother was required to give verbal consent to continue with randomization of an eligible infant.
After provision of the second consent usually obtained during the antenatal period, the woman was escorted home by staff health visitor to obtain locator information used for tracing the mother and child in case of a missed visit. In addition, mothers agreed to deliver at the hospital. These requirements were challenging and potentially eligible mother/infant pairs declined to participant. However, the trial had outstanding retention and visit adherence for those mothers who consented and whose infants were enrolled.
Cell Mediated Immune Responses
ELISPOT assay
No antigen-specific responses were detected at birth or two weeks after the last immunization by the ELISPOT assay (data not shown). Responses to PHA (positive control) were detected in all samples.
Flow-based T cell responses
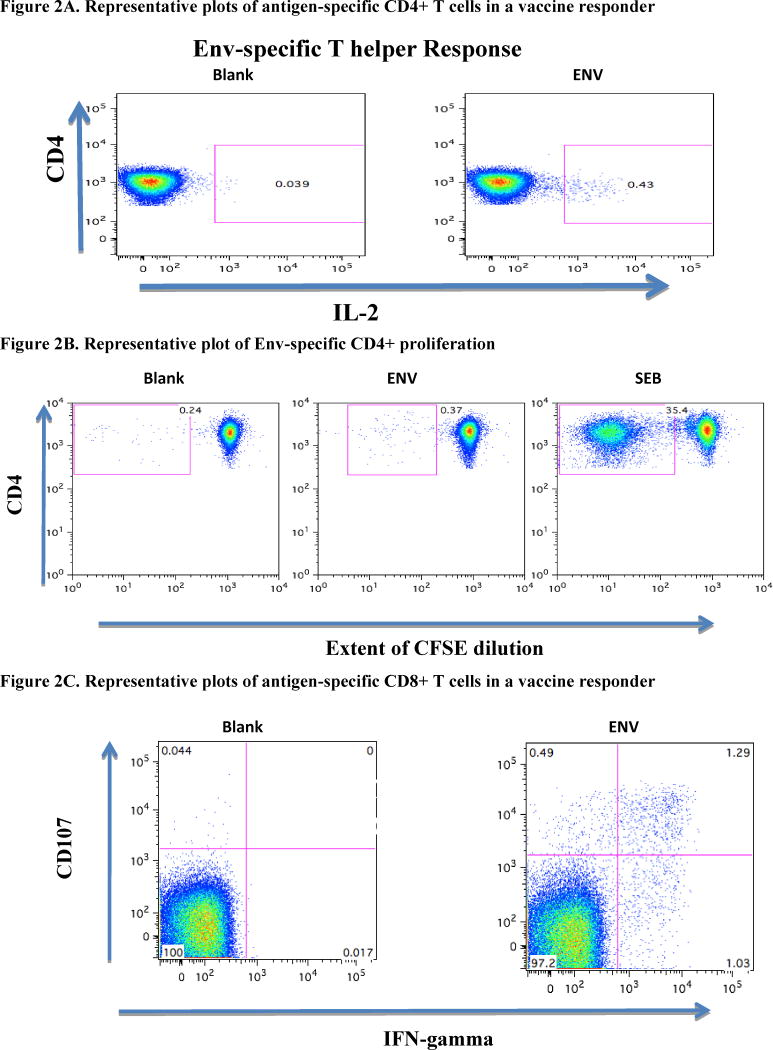
In the assessment of intracellular cytokine production, low levels of vaccine-specific IFN-γ were detected in vaccine responders (Table 1). A representative plot of a CD4-mediated, Env-specific IL-2 and CFSE response is shown for one individual (Figure 2A and 2B, respectively). Representative CD8-mediated T cell response is shown for one infant in Figure 2C. Vaccine recipients maintained a higher response rate two years after immunization, although statistical significance was not observed (Table 1). All infants with evaluable data demonstrated significant cytokine production and proliferation following SEB stimulation as a control.
Table 1. HIV-specific T cell immune responses in the 38 infants who received the full set of ALVAC vaccinations and all placebo recipients.
| Birth | Week 10 | Month 24 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (%) | ALVAC (%) | Pa | Placebo (%) | ALVAC (%) | Pa | Placebo (%) | ALVAC (%) | Pa | |
| CD4b ICS | 0/11 (0) | 0/33 (0) | -- | 2/9 (22.2) | 2/38 (5.3) | 0.16 | 1/9 (11.1) | 6/36 (16.7) | 1.00 |
| CD4c CFSE | 0/11 (0) | 2/34 (5.9) | 1.00 | 0/7 (0) | 9/31 (29.0) | 0.16 | 0/7 (0) | 0/30 (0) | -- |
| CD8b ICS | 0/11 (0) | 0/33 (0) | -- | 1/9 (11.1) | 1/38 (2.6) | 0.35 | 0/9 (0) | 5/36 (13.9) | 0.57 |
| CD8c CFSE | 0/11 (0) | 1/34 (2.9) | 1.00 | 0/7 (0.0) | 3/31 (9.7) | 1.00 | 0/7 (0) | 0/30 (0) | - |
P-values from two-sided Fisher's Exact test
Effector assay: Intracellular cytokine and/or CD107
CFSE proliferation
Figure 2.
A. Representative plots of antigen-specific CD4+ T cells in a vaccine responder. PBMC were stimulated with Env peptides then stained with anti-IL2 PE, 4 anti-CD4 PerCP-Cy5.5, anti-CD3 AmCyan, and analyzed by flow cytometry. Samples were first gated on the CD3+/CD4+ lymphocyte population then the percent of IL-2 positive CD4+ T cells were determined.
B. Representative Plot of Env-specific CD4 proliferation. CFSE-labeled PBMC were stimulated with ENV peptides for 5 days then assessed for proliferation by flow cytometry. Results are expressed as percent of proliferating CD8+ T-cells as measured by the extent of CFSE dilution. Positive proliferation is defined as >0·1% net and at least twice background.
C. Representative plots of antigen-specific CD8+ T cells in a vaccine responder. PBMC were stimulated with Env peptides then stained with anti-CD107a PE, anti-IFN-γ FITC, anti-CD4 PerCP-Cy5.5, anti-CD3 AmCyan and anti-CD8 APC-Cy7, and analyzed by flow cytometry. Samples were first gated on the CD3+/CD8+ lymphocyte p opulation then the percent of CD107 and IFN-γ positive CD8+ T cells were determined.
Humoral immune responses
Plasma binding antibody responses
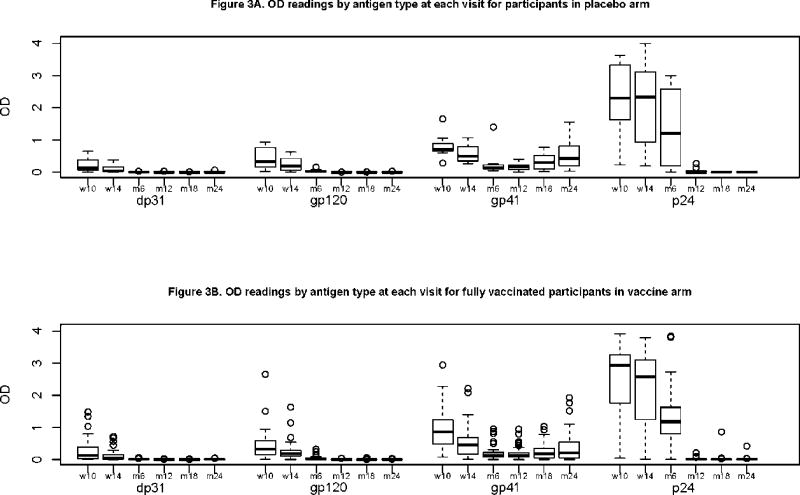
With the exception of p24 antigen, binding antibody responses to other antigens tested were generally low, with median OD of less than 1.0 (Figures 3A & 3B). Maternal antibodies, as measured by DP31 qualitative ELISA (an HIV-1 antigen not included in the vaccine) disappeared by month 6. Antibody responses generally disappeared by 12 months of age for gp120 and 18 months for p24. Antibodies to gp41 did not disappear in both the vaccine and placebo recipients. These non-vaccine specific antibodies showed a similar pattern of a decline in both the placebo and vaccine arms by month 6 with a non-significant rise by month 24 (Figures 3A & 3B).
Figures 3A & 3B.
Single Point ELISA Optical Density readings by antigen type at each visit for participants in placebo arm (a) and vaccine arm (b). Qualitative (single-point titers) measurements of binding antibodies were determined from cryopreserved serum at weeks week 10, 14, month 6, 12, 18, 24). One dilution of serum at 1:50 dilution were tested in duplicate in microtiter plates coated with the following antigen: gp41 (subtype B), gp120MN (Subtype B, VaxGen), p24 (subtype B) and DP31–a synthetic AVERY peptide not present in the vaccine (AnaSpec Incorporated, San Jose, California, USA). An assay was considered positive if the antigen-specific optical density (OD) was ≥ 0.2, i.e., after subtraction of the OD of the no antigen plate
Antibody responses at 18 and 24 months were quantified in specimens from individuals who had a positive single point ELISA. Ten individuals had titres of 1/300 to gp41; four in the placebo arm. One ALVAC vaccine recipient had a p24 antibody titre of 1/300. This child also had persistent p24 and gp160 antibodies at 18 and 24 months by western blot, despite persistently HIV-1 negative DNA and RNA PCR results.
Neutralizing antibodies
No vaccine induced neutralizing antibodies were detected in the vaccine arm at month 18 or month 24 (data not shown).
Summary of individual immune responses
Overall, 19 out of the 38 infants in the vaccine arm that received all four vaccinations had no cellular immunes responses detected at any time point. In the other 19, there were 10 infants with a positive response on one assay, 8 infants with responses on two assays, and 1 infant with responses on three assays (Table 2). The one HIV-uninfected vaccine recipient with persistent p24 and gp160 antibody positivity at 18 and 24 months, also had a positive CD8 T cell response on the intracellular cytokine staining assay at 24 months.
Table 2. Individual vaccine recipients with any positive cellular immunogenicity assay.
| Birth | 10 Weeks | 24 Months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4 ICS | CD8 ICS | CD4 CFSE | CD8 CFSE | CD4 ICS | CD8 ICS | CD4 CFSE | CD8 CFSE | CD4 ICS | CD8 ICS | CD4 CFSE | CD8 CFSE |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | POS | ND | ND | ND | ND |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | POS | POS | NEG | NEG | NEG | NEG | ND | ND |
| NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG | POS | POS | ND | ND |
| NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | POS | POS | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | ND | ND | ND | ND |
| NEG | NEG | NEG | NEG | POS | POS | ND | ND | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | ND | ND |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | POS | NEG | NEG | NEG | NEG |
| NEG | NEG | POS | POS | NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG |
| NEG1 | NEG | NEG | NEG | NEG | NEG | ND | ND | NEG | POS | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | POS | NEG | NEG | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | NEG | NEG | POS | POS | NEG | NEG |
| NEG | NEG | NEG | NEG | NEG | NEG | POS | NEG | NEG | NEG | NEG | NEG |
This infant also had persistent humoral antibody responses on binding antibody assays, HIV rapid testing, and western blot despite being persistently HIV DNA and RNA negative
Discussion
The HPTN 027 trial represents the first study of an HIV-1 preventive vaccine in children born to HIV-1 infected mothers in Africa. HIV vaccination is an attractive approach to HIV prevention for children because an HIV-1 immunogen could potentially be combined with other routine childhood vaccinations and be given to all infants. In light of the encouraging results from the phase III vaccine trial in Thailand [20,21], where moderate protection was observed, it is important to expand our understanding of vaccine responses as they relate to protective HIV immune responses in children at risk for HIV acquisition.
Responses to HIV-1 vaccines may differ between adults and infants because of the presence of maternal antibodies and maturational differences in the neonatal immune system.[30,31] In this study, the presence of maternal antibodies was detected in the samples collected at early visits, with decreasing levels over later visits. This vaccine was able to induce very low binding antibodies sustained up to 24 months in only one individual (2.5%). Previous studies using canarypox vaccine alone in adults [34] and in children [19] have reported similar low levels of antibody responses. This is attributed to the poor immunogenicity of the vaccine and the lack of a protein boost.[19] It is unlikely that the use of subtype B antigens in our binding assays contributed to the low responses detected given the similar results found in the other studies mentioned above in which the vaccine and the ELISA antigens were matched. The presence of low level gp41 antibodies in both the vaccine and placebo arms may represent either persistent maternal antibodies or an assay problem, although our control samples were consistent.
This assay was used in another trial [19] among infants born to HIV-1 infected mothers in the U.S. using the vCP1452 vaccine that had gp120, DP31, gp41, gag and parts of pol. However, in this trial the gp41 antibodies were not measured and for that reason we have no information regarding gp41 antibodies in other international settings.
In this study, an unboosted clade E envelope vaccine failed to elicit neutralizing antibodies. Unlike binding antibodies, both clade specific and cross clade neutralizing antibodies have been reported.[35,36] We have previously reported low level cross neutralization between subtype E and A,[37] but subtype specific envelope vaccines may be more relevant for this population. The presence of binding antibodies in the absence of neutralization was observed in the RV144 trial, but only after receipt of the boost. A subset of the IgG antibodies that mapped to the V1/V2 region correlated inversely with rate of HIV-1 infection.[20,21]. Similarly, in infants who received ALVAC vCP1452 with an rgp120 subunit boost demonstrated HIV specific antibody responses (binding and neutralizing) in contrast to those receiving ALVAC vCP 1452 alone. [19]
Though the CFSE flow-based proliferation assay in this study uses similar methodology to previously published clinical trials in adults, [26], the responses were very low. Extensive data flow-based proliferation assay in neonates and infants are lacking. Nevertheless, T cell proliferation has previously been shown to be significantly decreased in the absence of recombinant protein booster in ALVAC clinical trial of vCP1452 in infants born to HIV infected mother, consistent with our data. [17]
The ideal HIV-1 vaccine would be capable of generating a protective immune response against all HIV-1 subtypes, affording widespread applicability. Only by comparing vaccine efficacy in subtype-matched populations to partially or completely unmatched ones can the impact of HIV-1 diversity ultimately be understood. The predominant HIV-1 strains isolated from Uganda are clade A and D viruses and their various unique recombinant forms with a small contribution from clades C and B virus.[38,39] The Ugandan clade D HIV-1 virus is genetically similar to the US clade B virus. Portions of the gag gene are conserved among virus subtypes and gag-specific CTL elicited by ALVAC vaccines may cross-react CTL on non-clade B primary viruses.[40-46] Furthermore, a previous prime-boost trial (vCP205 alone or boosted with Chiron SF2 gp120/MF59) showed that CD8+ CTL from some vaccine recipients recognized target cells infected with non-B viruses, including subtype E.[40] T cell responses were elicited in this study although at low levels so future vaccine design in infants may also require a prime-boost approach to optimize the cellular immune response.
This study demonstrates the ability of the neonatal immune system to respond to a subtype E/B HIV-1 vaccine, albeit at low levels. However, the critical correlates of immunity for an HIV-1 vaccine in pediatric populations remain unknown. Development of novel targeted assays that permit assessment of innate and adaptive immune responses with limited amounts of blood will significantly improve the ability to detect potential responses. More pediatric studies of candidate HIV vaccines are warranted, including Phase III clinical trials, to identify effective HIV-1 vaccines to prevent infection in HIV-1 exposed infants. Ideally, identification of a vaccine regimen that could be given to all infants that would protect not only against infection during breastfeeding, but with a sustained effect through adolescence and adult would contribute to a generation free of HIV infection and AIDS.
Acknowledgments
The HPTN 027 team would like to thank Melissa Allen for her longstanding dedication to this study. We acknowledge Mr. Michael Muganga, Dr. Jennifer Serwanga, and Mr. Kharmis Tomusange at the MRC/UVRI Unit, Entebbe, who contributed to the performance of the humoral immune assays and Drs. Peter Mugyenyi and Cissy Kityo for their leadership and support for this study at the Joint Clinical Research Center. This study would not have been possible without the willingness and commitment of Ugandan women and their families to contribute to the search for an HIV vaccine. In addition, appreciation is given to the MUJHU Research Collaboration HPTN 027 study team for their dedication to the care and follow-up of study participants, the staff at MUJHU and the Joint Clinical Research Center, Sanofi-Pasteur, SCHARP, and FHI 360. Special thanks to Drs. Samuel Adeniyi-Jones, Jean Louis Excler, Jack Moye and Clemensia Nakabiito for their support. We dedicate this study to the memory of Dr. Mary Lou Clements and Prof. Francis Mmiro, the initial protocol immunologist when the study was first conceptualized and the Uganda site principal investigator, respectively.
Source of Support: The HIV Prevention Trials Network (HPTN) 027 study was funded by the US National Institutes of Health (NIH), initially through the HPTN and later through the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group. The HPTN (U01AI46749) has been funded by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Drug Abuse (NIDA), and National Institute of Mental Health (NIMH). The IMPAACT Group (U01AI068632) has been funded by NIAID, NICHD, and NIMH. The study product was provided for free by Sanofi-Pastuer
Footnotes
Potential conflict of interest: None declared
This study is registered with ClinicalTrials.gov, identifier NCT00098163.
Contributor Information
Pontiano Kaleebu, Email: pontiano.kaleebu@mrcuganda.org.
Harr Freeya Njai, Email: harrnjai@gmail.com.
Lei Wang, Email: lwang@scharp.org.
Norman Jones, Email: Norman.Jones@cdph.ca.gov.
Isaac Ssewanyana, Email: sewyisaac@yahoo.co.uk.
Paul Richardson, Email: pricha18@jhmi.edu.
Kenneth Kintu, Email: kkintu@mujhu.org.
Lynda Emel, Email: lemel@scharp.org.
Philippa Musoke, Email: pmusoke@mujhu.org.
Mary Glenn Fowler, Email: mgfowler@mujhu.org.
San-San Ou, Email: sou@scharp.org.
Laura Guay, Email: lguat@pedaids.org.
Philip Andrew, Email: pandrew@fhi360.org.
Lynn Baglyos, Email: Lynn.Baglyos@sanofipasteur.com.
Huyen Cao team, Email: caohuyen@gmail.com.
References
- 1.Bertolli J, Louis ME, Simonds RJ, et al. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J Infect Dis. 1996;174:722–6. doi: 10.1093/infdis/174.4.722. [DOI] [PubMed] [Google Scholar]
- 2.Ekpini ER, Wiktor SZ, Satten GA, et al. Late postnatal mother-to-child transmission of HIV-1 in Abidjan, Cote d'Ivoire. Lancet. 1997;349:1054–9. doi: 10.1016/s0140-6736(96)06444-6. [DOI] [PubMed] [Google Scholar]
- 3.Miotti PG, Taha TE, Kumwenda NI, et al. HIV transmission through breastfeeding: a study in Malawi. JAMA. 1999;282:744–9. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 4.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–74. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 5.Leroy V, Karon JM, Alioum A, et al. Postnatal transmission of HIV-1 after a maternal short-course zidovudine peripartum regimen in West Africa. AIDS. 2003;17:1493–501. doi: 10.1097/00002030-200307040-00010. [DOI] [PubMed] [Google Scholar]
- 6.WHO Collaborative Study Team. Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–5. [PubMed] [Google Scholar]
- 7.Fowler MG, Bertolli J, Nieburg P. When is breastfeeding not best? The dilemma facing HIV-infected women in resource-poor settings. JAMA. 1999;282:781–3. doi: 10.1001/jama.282.8.781. [DOI] [PubMed] [Google Scholar]
- 8.Humphrey J, Iliff P. Is breast not best? Feeding babies born to HIV-positive mothers: bringing balance to a complex issue. Nutr Rev. 2001;59:119–27. doi: 10.1111/j.1753-4887.2001.tb06999.x. [DOI] [PubMed] [Google Scholar]
- 9.Kafulafula G, Hoover DR, Taha TE, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1- uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 10.Onyango-Makumbi C, Bagenda D, Mwatha A, et al. Early weaning of HIV-exposed uninfected infants and risk of serious gastroenteritis: findings from two perinatal HIV prevention trials in Kampala, Uganda. J Acquir Immune Defic Syndr. 2010;53:20–27. doi: 10.1097/QAI.0b013e3181bdf68e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UNAIDS. Countdown to Zero: Global Plan Towards the Elimination of New HIV Infections Among Children by 2015 and Keeping Their Mothers Alive. UNAIDS. 2011 [Google Scholar]
- 12.Nachega J, Uthman O, Anderson J, Peltzer K, Wampold S, Cotton M, et al. Adherence to ART during and after pregnancy in low-income, middle-income, and high-income countries: a systematic review and meta-analysis. AIDS. 2012 doi: 10.1097/QAD.0b013e328359590f. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myer L, Cornell M, Fox M, Garone D, Wood R, Prozesky H, Ndirangu J, Keiser O, Boulle A, IeDEA-Southern Africa Collaboration Loss to follow-up and mortality among pregnant and non-pregnant women initiating antiretroviral therapy across South Africa. 19th Conference on Retroviruses and Opportunistic Infections; Seattle Washington. March 5-8, 2012; Abstract #22. [Google Scholar]
- 14.Mofenson LM. Prevention of mother-to-child HIV-1 transmission--why we still need a preventive HIV immunization strategy. J Acquir Immune Defic Syndr. 2011;58:359–362. doi: 10.1097/QAI.0b013e318235517e. [DOI] [PubMed] [Google Scholar]
- 15.Kintu K, Andrew P, Musoke P, et al. Feasilbility and Safety of ALVAC-HIV vCP1521 Vaccine in HIV-exposed Infants in Uganda: Results form the First Vaccine Trial in Africa. JAIDS. 2013 doi: 10.1097/QAI.0b013e31827f1c2d. published ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham CK, Wara DW, Kang M, et al. Safety of 2 recombinant human immunodeficiency virus type 1 (HIV-1) envelope vaccines in neonates born to HIV-1-infected women. Clin Infect Dis. 2001;32:801–7. doi: 10.1086/319215. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DC, McFarland EJ, Muresan P, et al. Safety and immunogenicity of an HIV-1 recombinant canarypox vaccine in newborns and infants of HIV-1-infected women. J Infect Dis. 2005;192:2129–2133. doi: 10.1086/498163. [DOI] [PubMed] [Google Scholar]
- 18.Borkowsky W, Wara D, Fenton T, et al. Lymphoproliferative responses to recombinant HIV-1 envelope antigens in neonates and infants receiving gp120 vaccines. AIDS Clinical Trial Group 230 Collaborators. J Infect Dis. 2000;181:890–896. doi: 10.1086/315298. [DOI] [PubMed] [Google Scholar]
- 19.McFarland EJ, Johnson DC, Muresan P, et al. HIV-1 vaccine induced immune responses in newborns of HIV-1 infected mothers. AIDS. 2006;20:1481–1489. doi: 10.1097/01.aids.0000237363.33994.45. [DOI] [PubMed] [Google Scholar]
- 20.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 21.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang ZY, Kuli-Zade I, Spearman P. Efficient human immunodeficiency virus (HIV)-1 Gag-Env pseudovirion formation elicited from mammalian cells by a canarypox HIV vaccine candidate. J Infect Dis. 1999;180:1122–32. doi: 10.1086/315028. [DOI] [PubMed] [Google Scholar]
- 23.Moodie Z, Huang Y, Gu L, Hural J, Self SG. Statistical positivity criteria for the analysis of ELISPOT assay data in HIV-1 vaccine trials. J Immunol Methods. 2006;315:121–32. doi: 10.1016/j.jim.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Peiperl L, Morgan C, Moodie Z, et al. Safety and immunogenicity of a replication-defective adenovirus type 5 HIV vaccine in Ad5-seronegative persons: a randomized clinical trial (HVTN 054) PLoS One. 2010;5:e13579. doi: 10.1371/journal.pone.0013579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507–15. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Souza MS, Ratto-Kim S, Chuenarom W, et al. The Thai phase III trial (RV144) vaccine regimen induces T cell responses that preferentially target epitopes within the V2 region of HIV-1 envelope. J Immunol. 2012;188:5166–5176. doi: 10.4049/jimmunol.1102756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abate G, Eslick J, Newman FK, et al. Flow-cytometric detection of vaccinia-induced memory effector CD4(+), CD8(+), and gamma delta TCR(+) T cells capable of antigen-specific expansion and effector functions. J Infect Dis. 2005;192:1362–1371. doi: 10.1086/444423. [DOI] [PubMed] [Google Scholar]
- 28.Robinson HL. HIV/AIDS vaccines: 2007. Clin Pharm Ther. 2007;82:686–93. doi: 10.1038/sj.clpt.6100408. [DOI] [PubMed] [Google Scholar]
- 29.McMichael AJ. HIV vaccines. Annu Rev Immunol. 2006;24:227–55. doi: 10.1146/annurev.immunol.24.021605.090605. [DOI] [PubMed] [Google Scholar]
- 30.Goepfert PA, Horton H, McElrath MJ, et al. High-dose recombinant Canarypox vaccine expressing HIV-1 protein, in seronegative human subjects. J Infect Dis. 2005;192:1249–1259. doi: 10.1086/432915. [DOI] [PubMed] [Google Scholar]
- 31.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005;12:1–12. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 32.Siegrist CA. Neonatal and early life vaccinology. Vaccine. 2001;19:3331–3346. doi: 10.1016/s0264-410x(01)00028-7. [DOI] [PubMed] [Google Scholar]
- 33.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. doi: 10.1038/nri1394. [DOI] [PubMed] [Google Scholar]
- 34.Cao H, Kaleebu P, Hom D, Flores J, Agrawal D, Jones N, Serwanga J, Okello M, Walker C, Sheppard H, El-Habib R, Klein M, Mbidde E, Mugyenyi P, Walker B, Ellner J, Mugerwa R. Immunogenicity of a recombinant human immunodeficiency virus (HIV)-canarypox vaccine in HIV-seronegative Ugandan volunteers: results of the HIV Network for Prevention Trials 007 vaccine study. J Infect Dis. 2003;187:887. doi: 10.1086/368020. [DOI] [PubMed] [Google Scholar]
- 35.Verrier F, Burda S, Belshe R, et al. A human immunodeficiency virus prime-boost immunization regimen in humans induces antibodies that show interclade cross-reactivity and neutralize several X4-, R5-, and dualtropic clade B and C primary isolates. J Virol. 2000;74:10025–10033. doi: 10.1128/jvi.74.21.10025-10033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Binley JM, Wrin T, Korber B, et al. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol. 2004;78:13232–13252. doi: 10.1128/JVI.78.23.13232-13252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber J, Fenyö EM, Beddows S, Kaleebu P, Björndal A. Neutralization serotypes of human immunodeficiency virus type 1 field isolates are not predicted by genetic subtype. J Virol. 1996;70:7827–7832. doi: 10.1128/jvi.70.11.7827-7832.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ssemwanga D, Ndembi N, Lyagoba F, et al. HIV type 1 subtype distribution, multiple infections, sexual networks, and partnership histories in female sex workers in Kampala, Uganda. AIDS Res Hum Retroviruses. 2012;28:357–65. doi: 10.1089/aid.2011.0024. [DOI] [PubMed] [Google Scholar]
- 39.Yirrell DL, Kaleebu P, Morgan D, et al. Inter- and intra-genic intersubtype HIV-1 recombination in rural and semi-urban Uganda. AIDS. 2002;16:279–286. doi: 10.1097/00002030-200201250-00018. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari G, Humphrey W, McElrath MJ, et al. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc Natl Acad Sci USA. 1997;94:1396–401. doi: 10.1073/pnas.94.4.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buseyne F, Chaix ML, Fleury B, et al. Cross-clade-specific cytotoxic T lymphocytes in HIV-1-infected children. Virology. 1998;250:316–24. doi: 10.1006/viro.1998.9373. [DOI] [PubMed] [Google Scholar]
- 42.Lynch JA, deSouza M, Robb MD, et al. Cross-clade cytotoxic T cell response to human immunodeficiency virus type 1 proteins among HLA disparate North Americans and Thais. J Infect Dis. 1998;178:1040–6. doi: 10.1086/515652. [DOI] [PubMed] [Google Scholar]
- 43.Fukada K, Tomiyama H, Wasi C, et al. Cytotoxic T-cell recognition of HIV-1 cross-clade and clade-specific epitopes in HIV-1-infected Thai and Japanese patients. AIDS. 2002;16:701–11. doi: 10.1097/00002030-200203290-00005. [DOI] [PubMed] [Google Scholar]
- 44.Thomson MM, Pérez-Alvarez L, Nájera R. Molecular epidemiology of HIV-1 genetic forms and its significance for vaccine development and therapy. Lancet Infect Dis. 2002;2:461–471. doi: 10.1016/s1473-3099(02)00343-2. [DOI] [PubMed] [Google Scholar]
- 45.Yusim K, Kesmir C, Gaschen B, et al. Clustering patterns of cytotoxic T-lymphocyte epitopes in human immunodeficiency virus type 1 (HIV-1) proteins reveal imprints of immune evasion on HIV-1 global variation. J Virol. 2002;76:8757–68. doi: 10.1128/JVI.76.17.8757-8768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Groen G, Nyambi PN, Beirnaert E, et al. Genetic variation of HIV type 1: relevance of interclade variation to vaccine development. AIDS Res Hum Retroviruses. 1998;14:S211–21. [PubMed] [Google Scholar]