Abstract
Background
The opportunity to improve care by delivering decision support to clinicians at the point of care represents one of the main incentives for implementing sophisticated clinical information systems. Previous reviews of computer reminder and decision support systems have reported mixed effects, possibly because they did not distinguish point of care computer reminders from e‐mail alerts, computer‐generated paper reminders, and other modes of delivering ‘computer reminders’.
Objectives
To evaluate the effects on processes and outcomes of care attributable to on‐screen computer reminders delivered to clinicians at the point of care.
Search methods
We searched the Cochrane EPOC Group Trials register, MEDLINE, EMBASE and CINAHL and CENTRAL to July 2008, and scanned bibliographies from key articles.
Selection criteria
Studies of a reminder delivered via a computer system routinely used by clinicians, with a randomised or quasi‐randomised design and reporting at least one outcome involving a clinical endpoint or adherence to a recommended process of care.
Data collection and analysis
Two authors independently screened studies for eligibility and abstracted data. For each study, we calculated the median improvement in adherence to target processes of care and also identified the outcome with the largest such improvement. We then calculated the median absolute improvement in process adherence across all studies using both the median outcome from each study and the best outcome.
Main results
Twenty‐eight studies (reporting a total of thirty‐two comparisons) were included. Computer reminders achieved a median improvement in process adherence of 4.2% (interquartile range (IQR): 0.8% to 18.8%) across all reported process outcomes, 3.3% (IQR: 0.5% to 10.6%) for medication ordering, 3.8% (IQR: 0.5% to 6.6%) for vaccinations, and 3.8% (IQR: 0.4% to 16.3%) for test ordering. In a sensitivity analysis using the best outcome from each study, the median improvement was 5.6% (IQR: 2.0% to 19.2%) across all process measures and 6.2% (IQR: 3.0% to 28.0%) across measures of medication ordering.
In the eight comparisons that reported dichotomous clinical endpoints, intervention patients experienced a median absolute improvement of 2.5% (IQR: 1.3% to 4.2%). Blood pressure was the most commonly reported clinical endpoint, with intervention patients experiencing a median reduction in their systolic blood pressure of 1.0 mmHg (IQR: 2.3 mmHg reduction to 2.0 mmHg increase).
Authors' conclusions
Point of care computer reminders generally achieve small to modest improvements in provider behaviour. A minority of interventions showed larger effects, but no specific reminder or contextual features were significantly associated with effect magnitude. Further research must identify design features and contextual factors consistently associated with larger improvements in provider behaviour if computer reminders are to succeed on more than a trial and error basis.
Plain language summary
On screen point of care computer reminders to improve care and health
It is known that doctors do not always provide the care that is recommended or according to the latest research. Many strategies have been tried in an attempt to reduce this gap between what is recommended and what is done. A potentially low cost way to do this could be to use computer systems that remind physicians about important information while they make decisions. For example, a doctor could be ordering antibiotics for a child with an ear infection. At that point, the computer the doctor is working on displays a pop up window with a reminder about the evidence for the best dose and length of time the antibiotics should be prescribed.
This review found 28 studies that evaluated the effects of different on‐screen computer reminders. The studies tested reminders to prescribe specific medications, to warn about drug interactions, to provide vaccinations, or to order tests. The review found small to moderate benefits. The reminders improved physician practices by a median of 4%. In eight of the studies, patients' health improved by a median of 3%.
Although some studies showed larger benefits than these median effects, no specific reminders or features of how they worked were consistently associated with these larger benefits. More research is needed to identify what types of reminders work and when.
Summary of findings
Summary of findings 1. Summary of findings.
| Point‐of‐care computerized decision support systems with or without co‐intervention(s) compared with usual care or co‐intervention(s) | ||||||||
|
Patient or population: Physicians (any specialty) Settings: Point‐of‐care; the interventions were most commonly delivered in the outpatient setting, but were also delivered in the inpatient, long‐term care, and other clinical settings. The majority of interventions occurred in the United States, but interventions also occurred in several other countries Intervention: On‐screen tools designed to aid clinical decision‐making, with or without co‐intervention(s), that were delivered within routinely‐used clinical information systems (e.g. an electronic health record), accessible via physicians' usual workflow, and targeted the physician responsible for the clinical decision for which the on‐screen tool was providing support Comparison: Usual care or co‐intervention(s) | ||||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect: RR (95% CI) | Absolute effect: Median of median absolute improvements (IQR) | Absolute effect: Best of median absolute improvements (IQR) | No of Participants (Comparisons) | Quality of the evidence (GRADE) | Comments | |
| Assumed likelihood of outcome with comparison | Corresponding likelihood of outcome with intervention | |||||||
| All process outcomes | 1.29 (1.23 to 1.36) |
2.71% (0.52% to 9.5%) | 935 192 (114) |
Low1 | ||||
| Prescription of medications | 405 per 1000 | 470 per 1000 (454 to 486) |
1.16 (1.12 to 1.20) |
2.41% (‐0.08% to 6.76%) | 276 410 (64) |
Low2 | ||
| Prescription of recommended vaccines | 255 per 1000 | 386 per 1000 (329 to 451) |
1.51 (1.29 to 1.77) |
4.8% (1.56% to 7.65%) | 212 791 (30) |
Moderate3 | ||
| Test ordering | 412 per 1000 | 494 per 1000 (461 to 531) |
1.20 (1.12 to 1.29) |
1.96% (0.68% to 8.4%) |
539 528 (25) |
Low4 | ||
| Elements of recommended documentation | 275 per 1000 | 481 per 1000 (407 to 569) |
1.75 (1.48 to 2.07) |
6.08% (1.14% to 20.5%) |
66 725 (11) |
Low5 | ||
| Other process outcomes | 165 per 1000 | 269 per 1000 (243 to 299) |
1.63 (1.47 to 1.81) |
4.32% (1.03% to 10.4%) |
300 114 (32) |
Low1 | ||
|
RR: Risk Ratio; CI: Confidence interval; IQR: interquartile range *The basis for the assumedlikelihood of outcome with comparison was the median proportion of outcome recipients in the control group across studies, determined following application of the intervention to the intervention group.. The corresponding likelihood of outcome with intervention (and its 95% confidence interval) is based on the assumed risk in the comparison group and the RR of the intervention (and its 95% CI). | ||||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||||
1Quality of the evidence was downgraded by two levels. The evidence was downgraded by one level due to inconsistency; a notable minority of studies had anomalously large positive effect sizes, and quantitative measures of heterogeneity (I2 value and χ2 test) indicated the presence of inconsistency. The evidence was further downgraded by one level due to publication bias, as the funnel plot had substantial asymmetry in the direction of unduly favouring the intervention.
2Quality of the evidence was downgraded by two levels. Risk of bias downgraded the evidence by one level, as a substantial proportion of studies had a high risk of dissimilar baseline characteristics (24/64) and smaller but non‐negligible proportions of studies had high risks of other biases. Inconsistency also downgraded the evidence by one level, due to some studies reporting anomalously large positive effect sizes and quantitative measures of heterogeneity (I2 value and χ2 test) indicating the presence of inconsistency.
3Quality of the evidence was downgraded by one level due to inconsistency, which was indicated by quantitative measures of heterogeneity (I2 value and χ2 test).
4Quality of the evidence was downgraded by two levels. The evidence was downgraded by one level due to inconsistency, which was indicated by quantitative measures of heterogeneity (I2 value and χ2 test). The evidence was further downgraded by one level due publication bias. The funnel plot displayed substantial asymmetry in the direction of unduly favouring the intervention.
5Quality of the evidence was downgraded by two levels. The evidence was downgraded by one level due to inconsistency. Inconsistency was indicated by variation in study effect sizes, with multiple studies reporting anomalously large positive effect sizes and one study reporting an abnormally large negative effect size. There was also a borderline lack of confidence interval overlap between studies, and the presence of inconsistency was corroborated by quantitative measures of heterogeneity (I2 value and χ2 test). The evidence was downgraded by one additional level due to publication bias, as the funnel plot displayed substantial asymmetry in the direction of unduly favouring the intervention.
Background
Description of the condition
Gaps between recommended practice and routine care are widely known (McGlynn 2003; Quality of Health Care 2001; Schuster 1998). Interventions designed to close these gaps fall into a number of different categories: educational interventions (directed at clinicians or at patients), reminders (again, directed at clinicians or patients), audit and feedback of performance data, case management, and financial incentives to name a few (Shojania 2005). However, none of these categories of interventions confers large improvements in care, especially when evaluated rigorously. In fact, they often produce quite small benefits (Grimshaw 2004; Oxman 1995; Shojania 2006; Walsh 2006) and these benefits tend to involve process measures only, not patient outcomes.
Description of the intervention
Given the difficulty of changing the behaviour of healthcare providers and the resources required by many of the interventions that aim to do so, provider reminders offer a promising strategy, especially given their low marginal cost. Reminders delivered at the point of care prompt healthcare professionals to recall information that they may already know but could easily forget in the midst of performing other activities of care, or, in the case of decision support, provide information or guidance in an accessible format at a particularly relevant time. Paper‐based reminders have existed for many years and have ranged from simple notes attached to the fronts of charts (for example reminding providers of the need to administer an influenza vaccine) to more sophisticated pre‐printed order forms that include decision support (for example protocols for ordering and monitoring anti‐coagulants). Computer‐based reminders have the potential to address multiple topics and are automatic; therefore they represent a subset of reminders of great interest to those involved in quality improvement efforts.
How the intervention might work
A number of systematic reviews over the years have evaluated computerised reminders and decision support systems (Dexheimer 2008; Garg 2005; Hunt 1998; Kawamoto 2005). However, these reviews have tended to lump all forms of computerised reminders and decision support together, including, for instance, computer‐generated paper reminders and e‐mail alerts sent to providers, along with reminders generated at the point of care. It is this last category, computer reminders that prompt providers at the point of care, which represents the most promising form of computerised reminders. Such reminders, embedded into computerised provider order entry systems or electronic medical records, alert providers to important clinical information relevant to a targeted clinical task at the time the provider is engaged in performing the task.
Why it is important to do this review
While point of care computerised reminders have produced some well‐known successes (Dexter 2001; Kucher 2005; Overhage 1997), other trials have shown no improvements in care (Ansari 2003; Eccles 2002a; Montgomery 2000), including studies from institutions with well‐established computerised order entry systems (Dexter 2004; Sequist 2005; Tierney 2003). Therefore, we sought to quantify the expected magnitudes of improvements in processes and outcomes of care through the use of computerised reminders and decision support delivered at the point of care, and identify any features consistently associated with larger effects.
Objectives
In this review, we address the following questions:
Do on‐screen computer reminders effectively improve processes or outcomes of care?
Do any readily identifiable elements of on‐screen reminders influence their effectiveness (e.g. inclusion of patient‐specific information as opposed to generic reminders for a given condition, requiring a response from users).
Do any readily identifiable elements of the targeted activity (e.g. chart documentation, test ordering, medication prescribing) influence the effectiveness of on‐screen reminders?
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (with randomisation at the level of the patient or the provider) and quasi‐randomised trials, where allocation to intervention or control occurred on the basis of an arbitrary but not truly random process (for example even or odd patient identification numbers).
Types of participants
Any study in which the majority of participants (> 50%) consisted of physicians or physician trainees; we excluded studies that primarily targeted dentists, pharmacists, nurses, or other health professionals.
Types of interventions
The original protocol for this review defined 'on‐screen computer reminders' as follows:
Patient or encounter specific information that is provided via a computer console (either visually or audibly) and intended to prompt a healthcare professional to recall information usually encountered through their general medical education, in the medical records or through interaction with peers, and so remind them to perform or avoid some action to aid individual patient care (Gordon 1998).
This original definition served primarily to distinguish computer reminders that were literally presented to users on a computer screen (hence 'on‐screen reminders') from computer‐generated reminders that were simply printed out and placed in a paper chart. While this distinction remains germane (i.e. some studies still involve 'computer reminders' that are really paper‐based reminders that happen to have been generated by a computer), the use of computers in healthcare is now sufficiently widespread that the more important concept has become 'at the point of care', rather than merely 'on‐screen'. A reminder that is ‘on screen’ but not noticeable to clinicians during the target activities of interest is no more useful than a paper reminder placed in such a manner that clinicians must deviate from their usual charting activities in order to find it.
Thus, from an operational point of view, the focus of this review should be regarded as evaluating 'point‐of‐care computer reminders'. By 'point of care' we refer to delivery of the computer reminder to clinicians at the time they are engaged in the target activity of interest, such as prescribing medications, documenting clinical encounters in the medical record, and ordering investigations.
Operationally, we considered a reminder to qualify as delivered at the point of care if the following three criteria applied.
The reminder was delivered via the computer system routinely used by the providers targeted by the intervention ‐ typically an electronic medical record or computerised order entry program. For instance, a dedicated computer used solely for performing dose calculations for anticoagulants would not count as 'on‐screen/point of care', since it requires clinicians to depart from their usual workflow in order to avail themselves of the reminder or decision support provided by this separate system. We excluded such systems because they in effect require providers to remember to use the reminder system, thus undermining the fundamental purpose of a reminder.
The reminder was accessible from within the routinely used clinical information system (typically via a pop‐up screen or an icon that indicates the availability of the reminder or decision support feature). A decision support module that could only be accessed by remembering to call up a separate program or website would not count as a point of care reminder (again, because depending on clinicians’ remembering to call up the program without any prompting violates the notion of a ‘reminder’).
The reminder targeted the person responsible for the relevant clinical activity. For instance, if handwritten physician orders were entered by a clerk or pharmacist into a computer order entry system, any alert or decision support delivered via the computer system would not qualify as 'point of care' since, for the physician, it was the handwritten order that occurred at the point of care.
For settings without general computer order entry or electronic medical record systems, we allowed the possibility that some specific activities might still routinely occur using a computer system. For instance, an ambulatory clinic might have developed a computer‐based system for supporting preventive care activities, even if the rest of the ambulatory record remained paper‐based. Or, a hospital might have developed a computer program for ordering certain high‐risk drugs (for example chemotherapy or anticoagulants). If a study documented that over 90% of the target activity occurred using the computer system, we regarded such a system as delivering a de‐facto point of care computer reminder (since the documentation of > 90% use of the computer system for that activity implies that providers would generally not have to remember to use to the reminder).
Types of outcome measures
Eligible outcomes
In order to enhance the interpretability of the results, we categorised eligible outcomes as follows.
Dichotomous process adherence outcomes: the percentage of patients receiving a target process of care (e.g. prescription of a specific medication, documentation of performance of a specific task, such as referral to a consultant) or whose care was in compliance with an overall guideline.
Dichotomous clinical outcomes: true clinical endpoints (such as death or development of a pulmonary embolism), as well as surrogate or intermediate endpoints, such as achievement of a target blood pressure or serum cholesterol level.
Continuous clinical outcomes: various markers of disease or health status (e.g. mean blood pressure or cholesterol level).
Continuous process outcomes: any continuous measure of how providers delivered care (e.g. duration of antibiotic therapy, time to respond to a critical lab value).
We planned to include studies in the analysis only if they reported at least one clinical or process outcome (i.e. we excluded articles that reported only costs, lengths of stay, and other measures of resource use). As it turned out, meaningful analyses were possible only with the measures of process adherence. For these measures, in order to permit pooling across studies, we required that studies present data as the absolute percentage of patients who received the target process care in each study group (or in a manner that allowed us to calculate these percentages). For instance, we would not include a study that only reported the odds of patients receiving the process of care in the intervention group compared with the control. We made this decision partly because initial review revealed that the vast majority of studies reported their data as percentages of patients who received the process of interest, and partly because this format is most conducive to conveying the expected impacts of computer reminders, namely absolute improvements in adherence to a target process of care or clinical behaviour.
Primary outcomes
Although we planned to include any otherwise eligible study that reported the effect of computerised reminders on clinical outcomes, evaluating the impact of reminders on adherence to target processes of care represented the primary goal of our analysis. We recognise that improving patient outcomes represents the ultimate goal of any quality improvement activity. However, we focused on process improvements for this review because we wanted to capture the degree to which computer reminders achieve their main goal, namely changing provider behaviour (Mason 1999). The degree to which such behaviour changes ultimately improve patient outcomes will vary depending on the strength of the relationship between the targeted process of interest and patient level outcomes. In some cases, no such relationship may exist. For instance, the incentive to improve appropriate antibiotic use is usually the population level goal of reducing emergence of resistant microorganisms, not improving the outcomes of care for individual patients. In other cases, a presumed relationship between a given process of care and patient outcomes may be incorrect (for example we would no longer expect a reminder that encourages the use of hormone replacement therapy to improve cardiovascular outcomes in post‐menopausal women). Consequently, if we had focused on improvements in clinical endpoints and found that reminders achieved negligible improvements in such outcomes, we would not know if this reflected consistent failure of computer reminders to achieve their intended goal (changes in provider behaviour) or the fact that reminders had targeted processes with limited connections to patient outcomes.
Direction of improvements
Some studies target quality problems that involve ‘underuse,’ so that improvements in quality correspond to increases in the percentage of patients who receive a target process of care (for example increasing the percentage of patients who receive the influenza vaccine). However, other studies target ‘overuse’, so that improvements correspond to reductions in the percentage of patients receiving inappropriate or unnecessary processes of care (for example reducing the percentage of patients who receive antibiotics for viral upper respiratory tract infections). In order to standardise the direction of effects, all process outcomes were defined so that higher values represented an improvement. For example, data from a study aimed at reducing the percentage of patients receiving inappropriate medications would be captured as the complementary percentage of patients who did not receive inappropriate medications. Increasing this percentage of patients for whom providers did not prescribe the medications would thus represent an improvement.
Search methods for identification of studies
Electronic searches
We searched the MEDLINE database up to July 2008 using Medical Subject Headings for relevant forms of clinical information systems (for example Medical Order Entry Systems, Point‐of‐Care Systems, Ambulatory Care Information Systems) and combinations of text words such as ‘computer’ or ‘electronic’ with terms such as ‘reminder’, ‘prompt’, ‘alert’, ‘cue’, and ‘support’ (Appendix 1 to Appendix 2). We applied a methodological filter for any type of clinical trial. We also searched the EMBASE, CINAHL and CENTRAL databases using modified search strategies up to July 2008. In addition,we retrieved all articles related to computers and reminder systems or decision support from the Cochrane Effective Practice and Organisation of Care Group (EPOC) database (EPOC 2008) Finally, we scanned bibliographies from key articles. For non‐English language articles, we screened English translations of titles and abstracts and pursued full‐text translation where possible ( i.e. either to include or confirm exclusion).
Data collection and analysis
Study selection and data abstraction
Two investigators (from KS, AJ, AM) independently screened citations and abstracted included articles using a structured data entry form. In the initial screening, authors based their judgments about inclusion and exclusion solely on the titles and abstracts, but promoted articles to the next stage of the screening process whenever a decision could not be made with confidence. For the second stage of screening, we obtained full text for all references, with each article again judged independently by two authors.
Two authors independently abstracted the following information from articles that met all the inclusion criteria after the second stage of screening: clinical setting, participants, methodological details, characteristics of the reminders design and content, the presence of co‐interventions (for example educational materials or performance report cards distributed to clinicians in both study groups), and outcomes. The data abstraction form (available upon request) was based on the checklist developed by the Cochrane EPOC Group (EPOC 2008). The form was pilot tested and revised iteratively prior to its use for final data abstraction. We resolved discrepancies between authors during either the screening or abstraction stages by discussion between the two authors to achieve consensus. When a conflict could not be resolved, a third author was consulted to achieve consensus or generate a majority decision.
Quality assessment
As part of the data abstraction process, authors assessed the following quality criteria based on the Cochrane EPOC Group Data Collection Checklist: concealment of allocation, blinded assessment of primary outcomes, proportion of patients/providers followed up, baseline disparities in process adherence or outcomes in the study groups, protection against contamination, and unit of analysis errors (EPOC 2008).
Data analysis
We anticipated that the eligible studies would exhibit significant heterogeneity, due to variations in target clinical behaviours, patient and provider populations, methodological features, characteristics of the interventions, and the contexts in which they were delivered. One approach for addressing these sources of variation would involve meta‐regression. Given the number of potentially relevant covariates, however, meta‐regression would require many more studies than we anticipated finding. We also expected that many eligible studies would assign intervention status to the provider, rather than the patient, but would not take into account ‘cluster effects’ in the analysis (i.e. they would exhibit ‘unit of analysis errors’). Performing either a conventional meta‐analysis or meta‐regression using studies with unit of analysis errors would require us to make a number of assumptions about the magnitude of unreported parameters, such as the intra‐class correlation coefficients and the distributions of patients across clusters, in order to avoid spurious precision in 95% confidence intervals.
To preserve the goal of providing a quantitative assessment of the effects associated with computerised reminders, without resorting to numerous assumptions or conveying a misleading degree of confidence in the results, we chose to report the median improvement in process adherence (and inter‐quartile range) among studies that shared specific features of interest. This approach was first developed in a large review of strategies to foster the implementation of clinical practice guidelines (Grimshaw 2004) and subsequently applied to reviews of quality improvement strategies in a series of reports for the US Agency for Healthcare Research and Quality (Shojania 2004a; Shojania 2004b; Steinman 2006; Walsh 2006).
This method of reporting the median effect sizes across groups of studies involves two distinct uses of the term ‘median’. First, in order to handle multiple outcomes within individual studies, we calculated for each study the median improvement in process adherence across the various outcomes reported by that study. For example, if a study reported 10 process adherence outcomes, we would calculate the absolute difference between intervention and control values for each outcome in order to obtain the median improvement (and interquartile range) across all 10 such differences. This median would then contribute the single effect size for that study. We also captured whenever a study identified a primary outcome and separately analysed those studies. Further, we performed a sensitivity analysis in which, instead of the median outcome, we used the best outcome from each study. With each study then represented by a single, median outcome, we then calculated the median effect size and interquartile range across all included studies. It is this second use of the ‘median’ that is crucial to the method. Instead of providing a conventional meta‐analytic mean (an average weighted on the basis of the precision of the results from each study), we highlight the median effect achieved by included studies, along with an interquartile range for these effects.
The main potential drawback of this method of reporting the median effects of an intervention across a group of studies lies in the equal weight given to all studies (for example no weighting occurs on the basis of study precision). Note, however, that by using the median rather than the mean, the summary estimate is less likely to be driven by a handful of outlying results (such as large effects from small or methodologically poor studies). Moreover, we included an analysis of the impact of study size and various other methodological features on reported effect size. For instance, we compared the median effects across large and small studies (where large was defined as greater than or equal to the median sample size across all included studies). We performed the analysis of potential associations between study size and effect magnitude using various measures of sample size, including the numbers of patients (or episodes of care) without any adjustment for clustering, the effective sample size taking into account cluster effects (using values for intra‐class correlation coefficients available in the published literature (Campbell 2000)) and, finally, using the numbers of providers (or other cluster units) as the sample size.
We also compared the median effects across studies with and without various methodological markers of study quality, as well as certain features of the study context (for example ambulatory versus inpatient setting) and characteristics of the reminders (for example inclusion of patient‐specific information versus a generic alert, provision of an explanation for the reminder, requiring users to enter a response to the reminder before continuing with their work, requiring users to navigate through more than one reminder screen). We made all such comparisons using a non‐parametric rank‐sum test (Mann‐Whitney). We performed all statistical analyses using SAS version 9.1 (SAS Institute, Inc, Cary, NC).
Results
Description of studies
Results of the search
Our search identified 2036 citations, of which 1662 were excluded at the initial stage of screening and an additional 374 on full‐text review, yielding a total of 28 articles that met all inclusion criteria (Figure 4)(Bates 1999; Christakis 2001; Dexter 2001; Eccles 2002a; Filippi 2003; Flottorp 2002; Frank 2004; Hicks 2008; Judge 2006; Kenealy 2005; Kralj 2003 ‐ classified, excluded; Krall 2004; Kucher 2005; McCowan 2001; Meigs 2003; Overhage 1996; Overhage 1997; Peterson 2007; Rothschild 2007; Roumie 2006 ‐ classified, excluded; Safran 1995; Sequist 2005; Tamblyn 2003; Tape 1993; Tierney 2003; Tierney 2005; van Wyk 2008; Zanetti 2003). Four studies contained two comparisons (Eccles 2002a; Flottorp 2002; Kenealy 2005; van Wyk 2008), resulting in 32 included comparisons.
Of the 32 included comparisons, 19 came from US centers and 24 took place in outpatient settings (see 'Characteristics of included studies'). Most (26) trials used a true randomised design, with only six comparisons involving a quasi‐random design (typically allocating intervention status on the basis of even or odd provider identification numbers). Twenty‐six of the 32 included comparisons allocated intervention status at the level of providers or provider groups, rather than allocating patients (i.e. they were cluster trials).
Risk of bias in included studies
Allocation
Of the 32 comparisons in the review, concealed allocation definitely occurred in 14 comparisons (Christakis 2001; Dexter 2001; Flottorp 2002; Frank 2004; Kenealy 2005; McCowan 2001; Meigs 2003; Rothschild 2007; Roumie 2006 ‐ classified, excluded; Safran 1995; van Wyk 2008). The process of allocation concealment was unclear in 14 comparisons (Bates 1999; Eccles 2002a; Filippi 2003; Hicks 2008; Judge 2006; Krall 2004; Overhage 1996; Overhage 1997; Peterson 2007; Sequist 2005; Tierney 2003; Tierney 2005; Tamblyn 2003) and not done in four comparisons (Kralj 2003 ‐ classified, excluded; Kucher 2005; Tape 1993; Zanetti 2003).
Incomplete outcome data
The proportion of eligible practices or providers with complete follow up was reported in 14 comparisons (Christakis 2001; Flottorp 2002; Kenealy 2005; Krall 2004; McCowan 2001; Meigs 2003; Overhage 1997; Rothschild 2007; Roumie 2006 ‐ classified, excluded; Tamblyn 2003; van Wyk 2008). The proportion of eligible patients with complete follow up was reported in 12 comparisons (Filippi 2003; Hicks 2008; Kucher 2005; Meigs 2003; Overhage 1997; Rothschild 2007; Roumie 2006 ‐ classified, excluded; Safran 1995; Tamblyn 2003; Tierney 2003; Tierney 2005; Zanetti 2003). The number of subjects (professionals, practices or patients) lost to follow up was not clear in 11 comparisons (Bates 1999; Dexter 2001; Eccles 2002a; Frank 2004; Judge 2006; Kralj 2003 ‐ classified, excluded; Overhage 1996; Peterson 2007; Sequist 2005; Tape 1993).
Baseline disparities between study groups
Only seven comparisons reported data in a format that permitted calculation of baseline disparities between study groups. Across these studies, the median difference between adherence in the intervention and control groups was 0.00% (interquartile range (IQR): 2.0% greater adherence in the control to 0.0%).
Unit of analysis errors
Of the 26 comparisons with a clustered design, only 12 analysed their results in a manner that took clustering effects into account. Thus, the remaining 14 clustered comparisons exhibited unit of analysis errors.
Other quality criteria
Blinded assessment of study outcomes was generally not relevant, as data were typically derived from electronic systems that documented delivery of the target processes of care. Though not the focus of the review, many of the clinical outcomes were also objective ones, such as laboratory data, and so also did not require blinded assessment.
Effects of interventions
See: Table 1
Of the 32 comparisons that provided analysable results for improvements in process adherence, (Bates 1999; Christakis 2001; Dexter 2001; Eccles 2002a; Filippi 2003; Flottorp 2002; Frank 2004; Hicks 2008; Judge 2006; Kenealy 2005; Kralj 2003 ‐ classified, excluded; Krall 2004; Kucher 2005; McCowan 2001; Meigs 2003; Overhage 1996; Overhage 1997; Peterson 2007; Rothschild 2007; Roumie 2006 ‐ classified, excluded; Safran 1995; Sequist 2005; Tamblyn 2003; Tape 1993; Tierney 2003; Tierney 2005; van Wyk 2008; Zanetti 2003), 21 reported outcomes involving prescribing practices, six specifically targeted adherence to recommended vaccinations, 13 reported outcomes related to test ordering, three captured documentation, and seven reported adherence to miscellaneous other processes (for example composite compliance with a guideline).
Only nine comparisons reported pre‐intervention process adherence for intervention and control groups. For these comparisons, the marginal improvement in the intervention (i.e. the median improvement in the intervention group minus the improvement in the control group) was 3.8% (IQR): 0.4% to 7.9%).
Given the small number of studies that reported baseline adherence, improvements attributable to interventions were calculated as the absolute difference in post‐intervention adherence (i.e. the post‐intervention improvement in the target process of care observed in the intervention group minus that observed in the control group). Using this post‐intervention difference between study groups, the median improvements in process adherence associated with computer reminders were: 4.2 % (IQR: 0.8% to 18.8%) across all process outcomes, 3.3% (IQR: 0.5% to 10.6%) for improvements in prescribing behaviours, 3.8% (IQR: 0.5% to 6.6%) for improvements in vaccination, and 3.8% (IQR: 0.4% to 16.3%) for test ordering behaviours (Table 2). Table 2 also shows the results obtained when we used the outcome with the largest improvement from each study instead of the outcome with the median improvement.
1. Median improvements in process adherence across included studies.
| Dichotomous outcomes (number of intervention vs. control comparisons) | Median absolute improvement (Interquartile range) | |
| Using median outcome from each study | Using best outcome from each study | |
| All process outcomes (N = 32) |
4.2% (0.8% to 18.8%) |
5.6% (2.0% to 19.2%) |
| Prescription of medications (N = 21) |
3.30% (0.5% to 10.6%) |
6.2% (3.0% to 28.0%) |
| Prescription of recommended vaccines (N = 6) |
3.8% (0.5% to 6.6%) |
4.8% (0.5% to 7.8%) |
| Test ordering (N = 13) |
3.8% (0.4% to 16.30%) |
9.6% (0.6% to 24.0%) |
| Elements of recommended documentation (N = 3) |
0.0% (‐1.0% to 1.3%) |
2.0% (2.0% to 4.0%) |
| Other process outcomes (N = 7) |
1.0% (0.8% to 8.5%) |
4.0% (0.8% to 8.5%) |
The Table shows average improvements (expressed as the median and interquartile range) across included comparisons for different types of process outcomes. All process outcomes were defined so that higher values always represent an improvement. For example, data from a study aimed at reducing the percentage of patients receiving inappropriate medications would be captured as the complementary percentage of patients receiving appropriate medications, so that an increase in process adherence would represent an improvement.
Most studies reported multiple endpoints but did not specify a primary outcome. For the main analyses, we used the median improvement from each study (that is the median change in adherence to a target guideline or process of care across all such changes reported for the study) as the single representative outcome for that study. We then calculated the median improvements across all included studies for different types of process measures, as shown in the middle column of the table. The column to the far right presents the same results when we used the best improvement from each study as its representative outcome.
Eight comparisons reported dichotomous clinical endpoints; intervention patients experienced a median absolute improvement of 2.5% (IQR: 1.3% to 4.2%). These endpoints included intermediate endpoints, such as blood pressure and cholesterol targets, as well as clinical outcomes, such as development of pulmonary embolism and mortality. Blood pressure represented the most commonly reported outcome. Patients in intervention groups experienced a median reduction in their systolic blood pressure of 1.0 mmHg (IQR: 2.3 mmHg reduction to 2.0 mmHg increase). For diastolic blood pressure, the median reduction was 0.2 mmHg (IQR: 0.8 mm reduction to 1.0 mm increase).
Impacts of study features on effect sizes
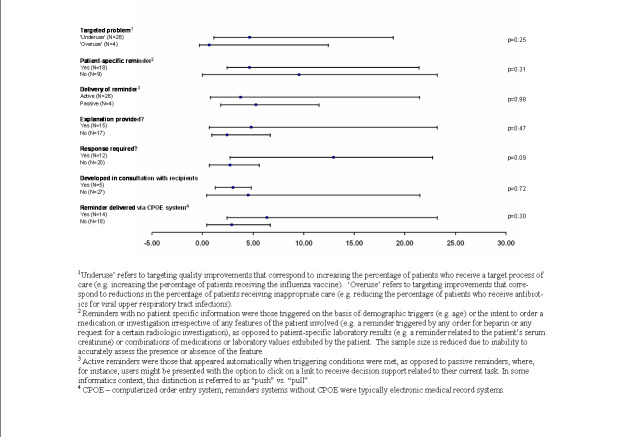
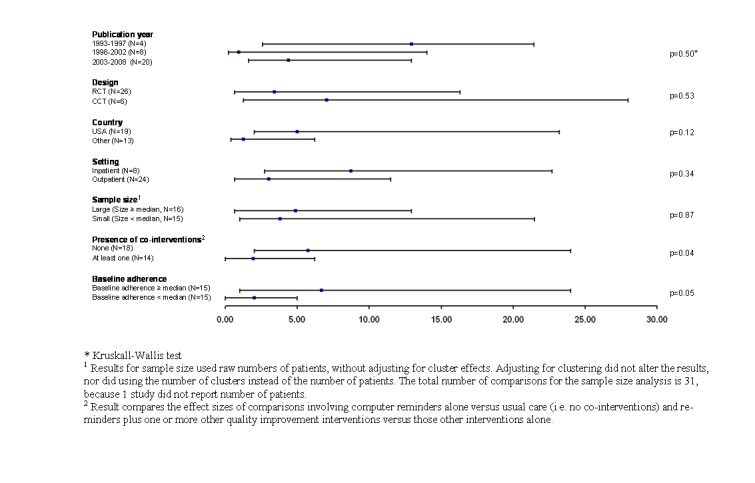
There were sufficient comparisons involving process adherence to permit various analyses of potential associations between various study features and the magnitude of effects (Figure 1). The six quasi‐randomised controlled trials reported larger improvements in process adherence than the 26 truly randomised comparisons (7.0%, IQR: 1.2% to 28.0% versus 3.4%: IQR 0.6% to 16.3%), but this difference was not statistically significant (P = 0.53). Sample size did not correlate with effect size, whether calculated on the basis of numbers of patients or providers (Figure 1).
1.
Median effects for process adherence by study feature
One might expect studies with low adherence in control groups to report larger improvements in care, but in fact studies with control adherence rates higher than the median across all studies had a non‐significant trend towards larger effect sizes (Figure 1). We analysed the potential impact of baseline adherence in several other ways (for example studies with baseline adherence in top quartile versus all others to look for a ‘ceiling effect’, and studies with baseline adherence in bottom quartile versus all others to look for a floor effect) but found no indication that baseline adherence significantly affected the magnitude of effect in the intervention group.
Interventions that targeted inpatient settings showed a trend towards larger improvements in processes of care than did those that occurred in outpatient settings: 8.7% (IQR: 2.7% to 22.7%) versus 3.0% (0.6% to 11.5%) for outpatient settings (P = 0.34). However, all interventions delivered in inpatient settings occurred at Brigham and Women’s Hospital in Boston or the Regenstreif Institute at the University of Indiana. Both of these institutions have mature ‘homegrown’ computerized provider order entry systems, and the recipients of computer reminders from these institutions consisted primarily of physician trainees, either of which factors may be more relevant than the fact of the inpatient setting.
Studies from the US reported slightly larger improvements in process adherence: 5.0% (IQR: 2.0% to 23.2%) versus 1.2% (IQR: 0.4% to 6.2%) for non‐US studies), but this difference was not significant (P = 0.12). Moreover, this trend at least partly reflected the results of studies from US institutions with long track records with clinical information systems (for example the Regenstreif Institute and Brigham and Women’s Hospital in Boston).
Grouping studies on the basis of track records in clinical informatics (for example analysing studies from Brigham and Women’s Hospital, the Regenstreif Institute and Vanderbilt University versus all others) did not result in significant differences, except in the case of Brigham and Women’s Hospital. The four studies from Brigham and Women’s Hospital by themselves reported significantly higher improvements in process adherence than all other studies: 16.8% (IQR: 8.7% to 26.0%) versus 3.0% (IQR: 0.5% to 11.5%; P = 0.04).
Lastly, the magnitude of effects attributable to computer reminders appeared to vary with the presence of co‐interventions (delivered to intervention and control groups). The 32 comparisons that reported process adherence outcomes included 18 that evaluated a computer reminder versus usual care and 14 that evaluated a computer reminder plus at least one other quality improvement intervention (for example educational materials) versus this same co‐intervention in the control group. Comparisons involving no co‐interventions (that is computer reminder alone versus usual care) showed a median improvement in process adherence of 5.7% (IQR: 2.0% to 24.0%), whereas studies of multifaceted interventions (that is computer reminders plus additional interventions versus those additional interventions alone) showed a median improvement in adherence of only 1.9% (IQR: 0.0% to 6.2%; P = 0.04 for this difference).
This apparent difference might reflect a ceiling effect, with co‐interventions delivered to the intervention and control groups leaving little room for computer reminders to demonstrate additional improvements. If this were the case, one would expect higher post‐intervention adherence rates in the control groups of studies that combined computer reminders with other interventions. However, the opposite proved true: post‐intervention values for process adherence (in both intervention and control groups) were in fact slightly higher in the studies involving comparisons of computer reminders by themselves, not in the studies involving additional interventions.
This relationship between comparison type and effect size at least partially reflected confounding by other studies features. For instance, dropping the four studies from Brigham and Women’s Hospital from the analysis substantially decreased the magnitude of the difference between studies with and without co‐interventions (median improvement of 0.9%, IQR: 0.0% to 5.0% versus 3.8%, IQR: 1.2% to 23.2%), and the difference was no longer statistically significant (P = 0.08). Also, of note, none of the P values reported in the analysis adjusted for multiple comparisons nor was stratification by the presence of co‐interventions a pre‐specified hypothesis for our analysis, further adding to the possibility that the observed difference reflects a chance association.
Features of computer reminders
We analysed a number of characteristics of the computer reminders (or the larger clinical information system) to look for associations with the magnitude of impact (Figure 2). The degree of improvement did not differ significantly between studies based on the type of quality problem targeted (underuse versus overuse of a given process of care), the conveyance of patient‐specific information versus a more generic alert, provision of an explanation for the alert, whether or not the reminder conveyed a specific recommendation, whether or not the authors of the study had developed the reminder, or the type of system used to deliver the reminder (CPOE versus electronic medical record).
2.
Median effects for process adherence by reminder feature
There was a trend towards larger effects with reminders that required users to enter a response of some kind (12.9%, IQR 2.7% to 22.7%) versus those that did not (2.7%, IQR: 0.6% to 5.6%; P = 0.09). However, this trend was confounded by the fact that all four comparisons from Brigham and Women's Hospital involved reminders that required responses from users. Dropping these four studies decreased the median effect of reminders that required user responses to 10.6% (IQR: 0.3% to 21.4%) and removed any appearance of statistical significance (P = 0.48). Of note, though, the magnitude of the difference remains substantial (10.6% versus 2.7%); it is possible that the lack of significance reflects lack of power.
We also analysed whether effect sizes differed between reminders that were 'pushed' onto users (that is users automatically received the reminder) versus reminders that required users to perform some action to receive it (that is users had to 'pull' the reminders). Only four comparisons involved 'pull' reminders and these showed comparable effects to 'push' reminders. Of note, however, one trial (van Wyk 2008) directly compared these two modes of reminder delivery. In this three‐armed cluster‐RCT of reminders for screening and treatment of hyperlipidemia, patients cared for at practices randomised to automatic alerts were more likely to undergo testing for hyperlipidemia and receive treatment than were patients seen at clinics where reminders were delivered to clinicians only ‘on‐demand.’
Sensitivity analysis
We reanalysed the potential predictors of effect size (study features and characteristics of the reminders) using a variety of alternate choices for the representative outcome from each study, including the outcome with the middle value (rather than a calculated median) and the best outcome (that is the outcome associated with the largest improvement in process adherence). None of these analyses substantially altered the main findings, including the lack of any significant association between study or reminder features and the magnitude of effects achieved by computer reminders. Of note, using the best outcome from each study rather than the median outcome, improvements attributable to reminders in studies at Brigham and Womens Hospital were no longer significantly larger than those achieved in studies from other centers (16.8%, IQR: 8.7% to 26.0% versus 4.6%, IQR: 2.0% to 13.4%; P = 0.09 for the comparison). However, the difference still appears large, so loss of significance may simply reflect the lack of power.
Discussion
Across 32 comparisons, computer reminders achieved small to modest improvements in care. The absolute improvement in process adherence was less than 4% for half of the included comparisons. Even when we included the best outcome from each comparison, the median improvement was only 5.6%. For improvements in prescribing, perhaps the behaviours of greatest general interest, improvements were even smaller.
With the upper quartile of reported improvements beginning at a 15% increase in process adherence, some studies clearly did show larger effects. However, we were unable to identify any study or reminder features that predicted larger effect sizes, except for a statistically significant (albeit unadjusted for multiple comparisons) difference in effects seen in studies involving the computer order entry system at Brigham and Women’s Hospital. A trend towards larger effects was seen for reminders that required users to enter a response in order to proceed, but this finding may have been confounded by the uneven distribution of studies from Brigham and Women’s Hospital. Thus, we do not know if the success of computer reminders at the Brigham partially reflects the design of reminders requiring user responses or if other features of the computer system or institutional culture of Brigham play the dominant role.
The finding that comparisons of computer reminders alone versus usual reported larger effect sizes than comparisons involving computer reminders and other co‐interventions represented an unexpected finding. Exploratory analyses did not reveal a plausible explanation for this result except that it may have reflected uneven distribution of confounders. One additional explanation might be that investigators chose to incorporate computer reminders in multifaceted interventions when attempting to change more complex (and therefore difficult to change) behaviours than those addressed by reminders alone. However, this unexpected finding may also constitute a chance association, especially as none of the P values reported in the analysis adjust for multiple comparisons.
A major potential limitation of our analysis was the heterogeneity of the interventions and the variable degree with which they were reported, including limited descriptions of key intervention features of the reminders and the systems through which they were delivered. We attempted to overcome this problem by abstracting basic attributes, such as whether user responses were required and whether or not the reminder contained patient‐specific information, but heterogeneity within even these apparently straightforward categories could mask important differences in effects. Also, other characteristics which we found difficult to operationalise for example the 'complexity' of the reminder), or which were inadequately reported, may also correlate with important differences in impact. This problem of limited descriptive detail of complex interventions and the resulting potential for substantial heterogeneity among included interventions in systematic reviews has been consistently encountered in the literature (Grimshaw 2003; Ranji 2008; Shojania 2005; Walsh 2006).
Our focus on the median effects across studies represents another potential limitation. However, as outlined in the 'Methods' section, we chose this approach precisely to avoid spurious precision due to heterogeneity and clustering effects that could not be taken into account in many studies. This approach is becoming increasingly common in Cochrane Reviews of interventions to change practice (Grimshaw 2004; Jamtvedt 2006; O'Brien 2007) and has also been used in other evidence syntheses (Grimshaw 2004; Shojania 2004b; Steinman 2006; Walsh 2006). This method conveys the range of effects associated with the intervention of interest and also allows for analysis of factors associated with effect size.
Additional studies continue to appear and we plan to assess eligible new studies formally for inclusion in six months. At that time we will also include a study that had previously been excluded as a time series, but which we have since decided merits inclusion as a controlled clinical trial (Durieux 2000 ‐ classified, excluded).
In summary, computer reminders delivered at the point of care have achieved variable improvements in target behaviours and processes of care. The small to modest median effects shown in our analysis may hide larger effects. However, the current literature does not suggest which features of the reminder systems, the systems with which they are delivered, or which target problems might consistently predict larger improvements.
Authors' conclusions
Implications for practice.
On‐screen computer reminders may become more prevalent as healthcare institutions advance in the use of computer technology. There appears to be a wide range of effects of the intervention, making it difficult to provide specific suggestions about how to maximize the benefits.
Implications for research.
Although some studies have clearly shown substantial improvements in care from point of care computer reminders it is concerning that the majority of studies have shown fairly small improvements across a range of process types. This finding of small to modest improvements is not unique to computer reminders. As had been said before, there are no 'magic bullets' when it comes to changing provider behavior and improving care (Shojania 2005; Oxman 1995). However, given that the opportunity to deliver computer reminders at the point of care represents one of the major incentives to implementing sophisticated clinical information systems, future research will need to identify key factors (related to the target quality problem or the design of the reminder) that reliably predict larger improvements in care from these expensive technologies.
What's new
| Date | Event | Description |
|---|---|---|
| 15 June 2021 | Review declared as stable | A related systematic review was published in September 2020 (https://doi.org/10.1136/bmj.m3216) and consequently there are no current plans to update this Cochrane Review. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 3, 2009
| Date | Event | Description |
|---|---|---|
| 7 December 2010 | Amended | Minor typo change to title |
| 11 November 2009 | Amended | Minor changes to figures |
Acknowledgements
We would like to acknowledge the assistance of Naghmeh Mojaverian and Jun Ji for their assistance with data extraction on this review. We would also like to acknowledge the assistance of Kathleen McGovern in preparing the review for publication. We would also like to acknowledge the contributions of Richard Gordon, Jeremy Wyatt and Rachel Rowe on the protocol for this review. Finally, we would like to thank Pierre Durieux, Richard Shiffman, Tomas Pantoja and Michelle Fiander for their helpful comments on earlier versions of this review.
Appendices
Appendix 1. MEDLINE search strategy
Strategy 1 (OVID)
1 "Forms and Records Control"/ 2 exp "Appointments and Schedules"/ 3 Medical Records Systems, Computerized/ 4 exp Decision Making, Computer‐Assisted/ 5 exp Artificial Intelligence/ 6 or/1‐5 7 Reminder Systems/ 8 (reminder$ or prompt$ or cue).tw. 9 or/7‐8 10 6 and 9 11 7 or 10 12 computer$.tw,hw. 13 11 and 12 14 (computer$ adj3 reminder$).tw. 15 or/13‐14 16 randomized controlled trial.pt. 17 controlled clinical trial.pt. 18 randomized controlled trials/ 19 random allocation/ 20 double blind method/ 21 single blind method/ 22 clinical trial.pt. 23 exp clinical trials/ 24 (clinical adj trial?).tw. 25 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 26 (random$ or placebo?).tw. 27 or/16‐26 28 animal/ 29 human/ 30 28 not (28 and 29) 31 27 not 30 32 15 and 31
Strategy 2 (PubMed)
#1 Search Ambulatory Care Information Systems [mh] OR Point‐of‐Care Systems [mh] OR Medical Order Entry Systems [mh] OR decision support systems, clinical [mh] OR drug therapy, computer‐assisted [mh] OR Medical Records Systems, Computerized [mh] OR Reminder Systems [mh] OR ((computer* [ti] OR electronic [ti]) AND (decision* [ti] OR support [ti] OR order* [ti] OR entry [ti] OR reminder* [ti] or prompt* [ti] or cue* [ti] OR alert* [ti])) #2 Search ((Randomised [ti] OR Randomized [ti] OR Controlled [ti] OR intervention [ti] OR evaluation [ti] OR Comparative [ti] OR effectiveness [ti] OR Evaluation [ti] OR Feasibility [ti]) AND (trial [ti] OR Studies [ti] OR study [ti] OR Program [ti] OR Design [ti])) OR Clinical Trial [pt] OR Randomized Controlled Trial [pt] #3 Search #1 and #2, Limits: English
Appendix 2. EPOC Register search strategy
[limit to RCT and CCT, 2005 ‐]
((reminder* or prompt* or cue*) and (computer* or on‐screen))
Appendix 3. CINAHL search strategy
1 exp Medical Records/ 2 ((form? or record?) adj (medical or control)).tw. 3 "Appointments and Schedules"/ 4 exp Patient Records Systems/ 5 exp Decision Making, Computer‐Assisted/ 6 exp Artificial Intelligence/ 7 artificial intelligence.tw. 8 natural language processing.tw. 9 or/1‐8 10 Reminder System/ 11 (reminder$ or prompt$ or cue).tw. 12 or/10‐11 13 9 and 12 14 10 or 13 15 computer$.tw,hw. 16 14 and 15 17 (computer$ adj3 reminder$).tw. 18 16 or 17 19 exp clinical trials/ 20 comparative studies/ 21 (clinical adj trial?).tw. 22 (random$ or placebo?).tw. 23 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 24 exp quasi‐experimental studies/ 25 or/19‐24 26 18 and 25
Appendix 4. EMBASE search strategy
1 Medical Record/ 2 ((form? or record?) adj (medical or control)).tw. 3 (patient? adj3 (schedul$ or appointment?)).tw. 4 (computer$ adj (medical or record?)).tw. 5 Computer Analysis/ 6 (decision? adj2 computer‐assisted).tw. 7 exp Artificial Intelligence/ 8 artificial intelligence.tw. 9 natural language processing.tw. 10 or/1‐9 11 Reminder System/ 12 (reminder$ or prompt$ or cue).tw. 13 or/11‐12 14 10 and 13 15 11 or 14 16 computer$.tw,hw. 17 15 and 16 18 (computer$ adj3 reminder$).tw. 19 17 or 18 20 Randomized Controlled Trial/ 21 (random$ or placebo?).tw. 22 clinical trial/ 23 (clinical adj trial?).tw. 24 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw. 25 or/20‐24 26 19 and 25
Data and analyses
Comparison 1. CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All | 114 | 935192 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.06, 0.09] |
| 1.2 Prescription | 64 | 276410 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [1.12, 1.20] |
| 1.3 Vaccination | 11 | 66725 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.29, 1.77] |
| 1.4 Testing | 30 | 212791 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.12, 1.29] |
| 1.5 Documentation | 25 | 539528 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.48, 2.07] |
| 1.7 Other | 32 | 300114 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.47, 1.81] |
1.1. Analysis.

Comparison 1: CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention), Outcome 1: All
1.2. Analysis.

Comparison 1: CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention), Outcome 2: Prescription
1.3. Analysis.

Comparison 1: CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention), Outcome 3: Vaccination
1.4. Analysis.

Comparison 1: CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention), Outcome 4: Testing
1.5. Analysis.

Comparison 1: CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention), Outcome 5: Documentation
1.7. Analysis.

Comparison 1: CDSS (+/‐ co‐intervention) vs. Usual care (+/‐ co‐intervention), Outcome 7: Other
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdel‐Kader 2011.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | University‐based outpatient general internal medicine practice, USA 248 patients, 30 providers |
|
| Interventions | Two reminders that were activated for patients with moderate to advanced chronic kidney disease (one suggested a referral to a nephrologist, a second suggested albumin quantification if not done within prior year) | |
| Outcomes | Process adherence (testing, documentation, other), clinical endpoint (laboratory test results, e.g. creatinine, hemoglobin) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in both control and intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Ansari 2003.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academically affiliated medical center, San Francisco, USA (San Francisco Veterans Affairs Medical Center) 115 patients, 49 providers of primary care for patients with congestive heart failure |
|
| Interventions | CDSS encouraging beta‐blocker use in eligible patients with heart failure | |
| Outcomes | Process adherence (prescribing), clinical endpoint (three outcomes related to hospitalization, mortality) | |
| Co‐Interventions | Educational: Distribution of educational materials and multiple (>1) educational sessions for providers in both control and interventions groups Beyond Clinician Education: Provision of list of patients eligible for beta‐blocker therapy, patient letter encouraging discussion of beta‐blocker therapy with provider in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Arts 2017.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practice clusters, the Netherlands 781 patients, 39 general practitioners across 18 practices |
|
| Interventions | CDSS determined recommended stroke prevention treatment based on patient risk status and informed the provider of discrepancies between current and recommended treatment | |
| Outcomes | Process adherence (other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Ambush, considered alert fatigue in design, conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, makes care recommendation, other concurrent CDSS, 'push' mode of delivery, targeted overuse and underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Awdishu 2016.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Inpatient and outpatient settings of academic medical center, San Diego, USA (University of California, San Diego) 1278 patients, 514 providers |
|
| Interventions | CDSS monitoring patient creatinine clearance and notifying physicians of necessity for renal dose adjustment or discontinuation of medications for patients with impaired renal function | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgment of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, makes care recommendation, possible to execute desired action, 'push' mode of delivery, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Baandrup 2010.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Two municipalities, Denmark 602 patients |
|
| Interventions | Reminder that popped up every time antipsychotic polypharmacy was about to be prescribed | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Baer 2013.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Primary care clinics affiliated with regional academic medical network, USA (Partners HealthCare System) 15 495 patients, 5 practices |
|
| Interventions | Patient self‐administered web‐based risk appraisal tool completed in waiting area that sends patient‐entered information on family history of cancer to electronic health record for clinicians to view. If accepted, populates coded fields and generates reminders about colon and breast cancer screening based on familial risk. | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Developed by study investigators, makes care recommendation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Bates 1999.
| Study characteristics | ||
| Methods | RCT | |
| Participants | All inpatients at academic medical center, Boston, USA (Brigham and Women’s Hospital) 939 episodes of care |
|
| Interventions | Reminder that was generated at the time a test that appeared to be redundant was ordered, prompting providers to consider cancelling the test | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Beeler 2014.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic medical center, Switzerland (University Hospital Zurich) 15 736 patients, 6 departments |
|
| Interventions | CDSS displayed for patients who did not receive a thromboprophylaxis order within the first 6h of admission or transfer. To improve specificity, he algorithm checked for thromboprophylaxis orders that were active within the 0–30h time frame after admission or transfer. | |
| Outcomes | Process adherence (prescribing), clinical endpoint (four outcomes pertaining to bleeding, heparin‐induced thrombocytopenia, venous thromboembolism) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, makes care recommendation, other concurrent CDSS, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Bell 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practice‐based research network, USA (Children’s Hospital of Philadelphia Pediatric Research Consortium) 19 450 patients, 12 practices |
|
| Interventions | Decision support for patients with asthma to improve adherence to national guidelines, including data‐entry tool, standardized documentation templates, order sets, and action/care plan for families | |
| Outcomes | Process adherence (prescribing, documentation, other) | |
| Co‐Interventions | Educational: Education session for providers in both intervention and control groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Appearance differed based on urgency, conveyed patient‐specific information, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Bennett 2018.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | N/A |
Bernstein 2017.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Internal medicine units, two academic medical centers, New Haven, USA (Yale New Haven Hospital and unnamed) 19 902 patients, 254 physicians |
|
| Interventions | Prompts physicians to refer smoking patients to a quitline, order tobacco cessation therapies, and document the patients’ smoking status | |
| Outcomes | Process adherence (prescribing, documentation, other) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: Audit and feedback in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgment of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, required provider input of clinical data, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | |
Beste 2015.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Eight VA facilities in the Pacific Northwest, USA 2884 patients |
|
| Interventions | CDSS intended to improve hepatocellular carcinoma surveillance by reminding clinicians to perform liver ultrasounds for patients with cirrhosis who had not received surveillance in the preceding 6 months | |
| Outcomes | Process adherence (testing, other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, makes care recommendation, other concurrent CDSS, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Boustani 2012.
| Study characteristics | ||
| Methods | RCT | |
| Participants | General medical ward, academic medical center, Indianapolis, USA (Wishard Memorial Hospital) 424 patients |
|
| Interventions | Reminder notifying physicians of presence of cognitive impairment, recommending early geriatric consultation, and suggesting discontinuation of urinary catheterization, physical restraints, and anticholinergic drugs | |
| Outcomes | Process adherence (prescribing, other), clinical endpoint (30‐day mortality, 30‐day readmission, hospital adverse event, mean length of hospital stay, home discharge) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, other concurrent CDSS, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Campbell 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Chak 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Primary care clinics affiliated with academic medical center, USA (University of California, Davis Health System) 2987 patients |
|
| Interventions | Reminder to screen foreign‐born Asian and Pacific Islander patients for chronic hepatitis B | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Ambush, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Chaturvedi 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Co 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Pediatric primary care practices, USA 412 patients, 79 providers, 12 practices |
|
| Interventions | Reminder to assess attention‐deficit/hyperactivity disorder (ADHD) symptoms every 3 to 6 months and ADHD note template with structured fields for symptoms, treatment effectiveness, and adverse effects | |
| Outcomes | Process adherence (documentation, other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, other concurrent CDSS, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Cote 2008a.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Cardiology telemetry and coronary care units in an academic medical center, Chicago, USA (Northwestern Memorial Hospital) 307 patients, 8 residents |
|
| Interventions | CDSS triggered when nonsteroidal anti‐inflammatory drugs were ordered suggesting gastrointestinal bleeding prophylaxis in high risk patients | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Cote 2008b.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Cardiology telemetry and coronary care units in an academic medical center, Chicago, USA (Northwestern Memorial Hospital) 320 patients, 8 residents |
|
| Interventions | CDSS triggered when nonsteroidal anti‐inflammatory drugs were ordered suggesting gastrointestinal bleeding prophylaxis in high risk patients | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Davis 2007.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Outpatient teaching pediatric clinic (Pediatric Care Center at the University of Washington) and rural/semi‐urban primary care pediatric clinic (Skagit Pedatrics), WA, USA 12 195 episodes of care, 88 providers |
|
| Interventions | CDSS presenting real‐time evidence to providers based on prescribing practices for acute otitis media, allergic rhinitis, sinusitis, constipation, pharyngitis, croup, urticaria, and bronchiolitis | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse and underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | |
Dean 2015.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Seven urban emergency departments, Utah, USA (Intermountain Healthcare) 4758 patients |
|
| Interventions | CDSS that calculated probability of pneumonia diagnosis and clinical severity using electronic clinical information, provided disposition and treatment recommendations | |
| Outcomes | Process adherence (other), Clinical endpoint (30‐day and inpatient mortality, hospitalization, 7‐day readmission, pleural effusion) | |
| Co‐Interventions | Educational: Single educational session for providers and academic detailing in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, developed in consultation with users, included supporting information on‐screen, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | N/A |
| Unit of analysis error | Unclear risk | N/A |
Dexter 2001.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General medicine inpatient service, urban teaching hospital, Indianapolis, USA (Wishard Memorial Hospital) 6371 patients, 8 provider teams |
|
| Interventions | Rule‐based CDSS generating prewritten orders for four preventive therapies (two vaccinations, prophylactic aspirin for cardiovascular disease, and venous thromboembolism prophylaxis) | |
| Outcomes | Process adherence (prescribing, vaccination) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Unclear risk | N/A |
Diaz 2018.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Pediatric emergency and urgent departments, two children’s hospitals, Delaware Valley and FL, USA (Nemours Children’s Health System) 50 patients, 28 physicians |
|
| Interventions | Prompts physicians to perform a neurovascular and musculoskeletal examination for patients with suspected elbow fracture | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: Single educational session for providers in both the control and intervention groups Beyond Clinician Education: Audit and feedback in both the control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | High risk | |
Diaz 2019.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care settings, Delaware Valley and FL, USA (Nemours Children’s Health System) 1051 patients, 13 physicians |
|
| Interventions | Guided physicians on how to screen for adolescent idiopathic scoliosis | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: Single educational session for providers in both control and intervention groups Beyond Clinician Education: Audit and feedback in both control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Decision support was complex, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | High risk | |
Downs 2006.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practices in Central Scotland and London, UK 236 patients, 18 practices |
|
| Interventions | CDSS producing prompts for the investigation and management of dementia | |
| Outcomes | Process adherence (other), clinical endpoint (diagnosis of dementia) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Dregan 2014.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Family practices within large research network, UK (Clinical Practice Research Datalink) 11 391 patients, 104 practices |
|
| Interventions | CDSS activated for patients on practice stroke register inviting physician to access prompts reminding them to adhere to guideline‐based secondary prevention (blood pressure control, recording strokes as hemorrhagic versus infarction, prescription of statins, and prescription of antiplatelet drugs) | |
| Outcomes | Process adherence (prescribing), clinical endpoint (blood pressure and cholesterol targets) | |
| Co‐Interventions | Education: Distribution of educational materials to providers in both control and intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, developed in consultation with users, included supporting information on‐screen, makes care recommendation, 'pull' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Eccles 2002a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Ambulatory general practices, UK 2335 patients, 60 practices |
|
| Interventions | Patient‐specific CDSS suggesting evidence‐based management for patients with angina (Control group received CDSS suggesting evidence‐based management for patients with asthma) |
|
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in both control and intervention groups; single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, makes care recommendation, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Eccles 2002b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Ambulatory general practices, UK 2363 patients, 60 practices |
|
| Interventions | Patient‐specific CDSS suggesting evidence‐based management for patients with asthma (Control group received CDSS suggesting evidence‐based management for patients with angina) |
|
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in both control and intervention groups; single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, makes care recommendation, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Feder 2011.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practices in two urban primary care trusts, UK (Bristol and Hackney) 143 868 patients, 48 practices |
|
| Interventions | Template in the electronic medical record linked to diagnoses for women experiencing domestic violence, such as depression, anxiety, irritable bowel syndrome, pelvic pain, and assault | |
| Outcomes | Process adherence (documentation, other) | |
| Co‐Interventions | Educational: Distribution of educational materials; multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: Audit and feedback; ad‐hoc telephone conversations and email exchanges with clinicians about referrals or advice; simplified referral pathway in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Field 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academically affiliated long‐term care facility, Canada 833 patients, 22 units |
|
| Interventions | CDSS providing patient‐specific recommendations in real‐time for adjusting dose and frequency of medications for residents with renal insufficiency | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, 'push' mode of delivery, targeted overuse and underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Fiks 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practice‐based research network, USA (Children’s Hospital of Philadelphia Pediatric Research Consortium) 23 418 episodes of care, 11 919 patients, 20 practices |
|
| Interventions | Reminder for influenza vaccine at office visits for children with asthma who were due for vaccine | |
| Outcomes | Process adherence (vaccination) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers and single educational session for providers in both control and intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Fiks 2013.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practice‐based research network, USA (Children’s Hospital of Philadelphia Pediatric Research Consortium) 11 245 patients, 22 practices |
|
| Interventions | Reminder for all routine adolescent vaccinations appearing prominently whenever patient encounter was opened within the electronic health record | |
| Outcomes | Process adherence (prescribing), clinical endpoint (outcomes pertaining to HPV vaccination status) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: Audit and feedback for providers in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Ambush, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Filippi 2003.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Ambulatory general practices, Italy 15 343 patients, 300 providers |
|
| Interventions | CDSS reminding providers to consider antiplatelet therapy in patients with diabetes | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in both control and intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Flottorp 2002a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Ambulatory general practices, Norway 9887 episodes of care, 120 practices |
|
| Interventions | Display of guidelines for appropriate use of antibiotics and laboratory testing in women with suspected urinary tract infection (control patients received identical interventions, but targeted to improve management of sore throat) | |
| Outcomes | Process adherence (prescribing, testing, other) | |
| Co‐Interventions | Educational: Educational materials for providers and patients, educational workshops for providers in intervention group Beyond Clinician Education: Financial incentives for providers in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Flottorp 2002b .
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Ambulatory general practices, Norway 16 939 episodes of care, 120 practices |
|
| Interventions | Display of guidelines for appropriate use of antibiotics and laboratory testing for patients with sore throat (control patients received identical interventions, but targeted to improve management of urinary tract infection in women) | |
| Outcomes | Process adherence (prescribing, testing, other) | |
| Co‐Interventions | Educational: Educational materials for providers and patients, educational workshops for providers in intervention group Beyond Clinician Education: Financial incentives for providers in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Frank 2004.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | Urban ambulatory practice, Australia 10507 patients, 10 providers |
|
| Interventions | CDSS for 12 preventive care activities (e.g. vaccinations; screening for cervical cancer, diabetes, and lipids; and documentation of allergies, weight, smoking, and blood pressure) | |
| Outcomes | Process adherence (testing, documentation, vaccination) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Gill 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care clinics in a national network of practices using same EHR, USA (Medical Quality Improvement Consortium) 64 150 patients, 105 providers, 25 offices |
|
| Interventions | Prompts during office visit regarding suboptimal screening, risk stratification, and management of dyslipidemia | |
| Outcomes | Process adherence (prescribing, testing), clinical endpoint (3 outcomes pertaining to lipid targets) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: Reporting tool to identify patients outside of office visits with suboptimal lipid care with standardized letter notifying these patients of their status in the intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, interruptive, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | N/A |
Gill 2011.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | National network of ambulatory practices using same EHR, USA (Centricity Healthcare User Research Network) 5234 patients, 119 clinicians, 27 offices |
|
| Interventions | Reminder suggesting adherence to guidelines for reducing gastrointestinal complications for patients on nonsteroidal anti‐inflammatory drugs | |
| Outcomes | Process adherence (other) | |
| Co‐Interventions | Educational: Distribution of educational materials and multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, developed in consultation with users, interruptive, 'push' mode of delivery, targeted overuse and underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Gonzales 2013.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices in integrated health care delivery system, PA, USA (Geisinger Health System) 8136 episodes of care, 22 practices |
|
| Interventions | CDSS providing structured template for documenting relevant history and physical examination elements in patients with acute respiratory tract infections. A clinical algorithm categorized the probability of having pneumonia, and triggered the most appropriate order set for a given patient with relevant testing and treatment options. | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Distribution of educational materials to patients, single educational session for providers in intervention group Beyond Clinician Education: Audit and feedback for providers in intervention group; clinical champions in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, possible to execute desired action, required provider input of clinical data, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Goud 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Cardiac rehabilitation clinics, Netherlands 2787 patients, 21 clinics |
|
| Interventions | Decision support that guided users through needs assessment procedure using structured dialogue and formulated patient‐specific rehabilitation programme on the basis of the needs assessment data (‘CARDSS’) | |
| Outcomes | Process adherence (other) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: Helpdesk services and financial incentives directed at providers in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, makes care recommendation, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Guiriguet 2016.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | 10 primary care centres, Barcelona, Spain 41 042 patients, 130 primary care physicians |
|
| Interventions | Prompted providers to promote patient participation in population‐based colorectal cancer screening program | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: Colorectal cancer screening program |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, makes care recommendation, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Gulliford 2014.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Family practices within large research network, UK (Clinical Practice Research Datalink) 603 409 patients, 100 practices |
|
| Interventions | CDSS encouraging either a no‐antibiotic or a delayed‐antibiotic approach during consultations with adults with acute respiratory tract infections | |
| Outcomes | Process adherence (prescribing,** other**), clinical endpoint (various outcomes pertaining to specialist consultation and antibiotic prescription) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, makes care recommendation, 'pull' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Unclear risk | N/A |
Gulliford 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Gupta 2014.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Academic medical center, CA, USA (VA Palo Alto Health Care System) 89 patients |
|
| Interventions | Reminder directing providers of patients who are candidates for implantable cardiac defibrillator to consider referral for consultation | |
| Outcomes | Process adherence (documentation, other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Gurwitz 2008.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic long‐term care facilities, Canada and USA 1118 patients, 29 units, 2 facilities |
|
| Interventions | CDSS linked with CPOE intended to prevent adverse drug events by flagging serious drug‐drug interactions and high‐risk prescriptions | |
| Outcomes | Clinical endpoint (preventable adverse drug events) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, interruptive, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Unclear risk | N/A |
Hicks 2008.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Community‐ and hospital‐based primary care clinics affiliated with large urban academic medical center, Boston, USA (Brigham and Women’s Hospital) 1834 patients, 12 clinics |
|
| Interventions | CDSS with guideline‐based reminders for management of patients with hypertension | |
| Outcomes | Process adherence (prescribing), clinical endpoint (uncontrolled blood pressure) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Holt 2010.
| Study characteristics | ||
| Methods | CCT | |
| Participants | General practices, West Midlands, UK 36 092 patient, 18 practices |
|
| Interventions | Reminder that encouraged cardiovascular risk stratification (‘e Nudge’) | |
| Outcomes | Process adherence (documentation), clinical endpoint (cardiovascular events) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, included supporting information on‐screen, interruptive, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
Holt 2017.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | 46 primary care practices, Central and South East England, UK 6429 patients |
|
| Interventions | Reminded physicians to prescribe oral anticoagulants for eligible patients with atrial fibrillation | |
| Outcomes | Process adherence (prescribing), Clinical endpoint (8 outcomes pertaining to stroke, transient ischemic attack, and haemorrhage) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, developed by study investigators, interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Unclear risk | N/A |
Hoye 2013.
| Study characteristics | ||
| Methods | CCT nested within cluster RCT | |
| Participants | Urban and rural practices in 11 counties participating in continuing medical education groups, Norway 16 188 dispensed prescriptions, 156 providers |
|
| Interventions | CDSS triggered when printing a prescription for antibiotics for respiratory tract infection requesting confirmation that the prescription was a delayed prescription | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in both control and intervention arms Beyond Clinician Education: Audit and feedback in both control and intervention arms |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, interruptive, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Judge 2006.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academically‐affiliated long‐term care facility, Canada 3843 episodes of care, 7 wards |
|
| Interventions | CDSS intended to improve medication safety at the time of order entry by flagging potential severe drug interactions, recent abnormal lab test results, requirement of special monitoring, dose reduction in elderly patients, or requirement of prophylactic measures | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, 'push' mode of delivery, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | High risk | |
Karlsson 2018.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | 42 primary care clinics, Östergötland, Sweden 14 800 patients |
|
| Interventions | Reminder to initiate anticoagulation therapy for eligible patients with atrial fibrillation or atrial flutter | |
| Outcomes | Process adherence (other), Clinical endpoint (various outcome pertaining to stroke, transient ischemic attack, bleeding) | |
| Co‐Interventions | Educational: Distribution of educational materials and for providers in both the control and intervention groups; single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Kenealy 2005.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Outpatient general practices, Auckland, New Zealand 2662 patients, 52 providers, 33 practices |
|
| Interventions | Icon suggesting diabetes screening for patients considered eligible for screening | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: Distribution of educational materials and single educational session for providers in both control and interventions groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, makes care recommendation, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Krall 2004.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Ambulatory family and internal medicine practices, regional managed care group, USA (Kaiser Permanente Northwest) 1076 patients, 100 providers |
|
| Interventions | Patient‐specific CDSS encouraging prescription of ASA for primary or secondary prevention in patient population at high risk of cardiovascular disease | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, considered alert fatigue in design, conveyed patient‐specific information, included supporting information on‐screen, interruptive, other concurrent CDSS, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Kucher 2005.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | Academic medical center, Boston, USA (Brigham and Women’s Hospital) 2506 patients, 120 providers |
|
| Interventions | CDSS encouraging deep‐vein thrombosis prophylaxis among high‐risk hospitalized patients | |
| Outcomes | Process adherence (prescribing, other), clinical endpoint (8 outcomes pertaining to pulmonary embolism, venous thromboembolism, deep vein thrombosis, hemorrhage, mortality) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, developed in consultation with users, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Lee 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Leibovici 2013.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Internal medicine wards, academic medical center, Israel (Rabin Medical Center, Beilinson Campus) 1683 patients, 15 wards |
|
| Interventions | CDSS guiding empirical antibiotic treatment of inpatients with moderate to severe bacterial infections using patient‐specific clinical data. It applies a cost‐benefit model to rank antibiotic treatments according to their net benefit and offers advice (including no treatment). | |
| Outcomes | Clinical endpoint (180‐day survival) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, makes care recommendation, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Linder 2009‐1.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care clinics in integrated regional system, USA (Partners HealthCare System) 21 961 episodes of care, 443 clinicians, 27 clinics |
|
| Interventions | Documentation‐based decision support for patients with acute respiratory infections related to diagnosis, antibiotic selection, medication safety, and patient education (‘ARI Smart Form’) | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, makes care recommendation, possible to execute desired action, 'pull' mode of delivery, required provider input of clinical data, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Linder 2009‐2.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with two academic medical centers in a research network, USA (Partners Primary Care Practice‐Based Research Network) 12 207 patients, 521 providers, 26 practices |
|
| Interventions | Smoking status icons, tobacco treatment reminders, and document‐based decision support (‘Smart Form’) that facilitated ordering of medication and fax/e‐mail counseling referrals | |
| Outcomes | Process adherence (prescribing, documentation, other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Lo 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with two academic medical centers, USA (Partners HealthCare System) 2765 patients, 366 providers, 22 practices |
|
| Interventions | Non‐interruptive, real‐time CDSS recommending baseline lab testing when prescribing medications to patients lacking baseline labs | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, makes care recommendation, other concurrent CDSS, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Locatelli 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic and non‐academic nephrology units in Bulgaria, Croatia, Germany, Italy, Latvia, Poland, Romania, Serbia and Montenegro 599 patients, 53 centres |
|
| Interventions | CDSS compiling data from patient visits generating guideline‐based management prompts with arguments for and against the offered option in patients receiving dialysis with renal anemia | |
| Outcomes | Process adherence (prescribing), clinical endpoints (various laboratory targets, including hemoglobin, ferritin levels, transferrin saturation) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, makes care recommendation, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Loo 2011a.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Primary care practice within an academic medical center, Boston, USA (Beth Israel Deaconess Medical Center) 3266 patients, 37 physicians, 2 offices |
|
| Interventions | Reminders for health care proxy designation, osteoporosis screening, and influenza and pneumococcal vaccinations in patients older than 65 years | |
| Outcomes | Process adherence (testing, documentation, vaccination) | |
| Co‐Interventions | Educational: Distribution of educational materials and single education session to providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, other concurrent CDSS, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Loo 2011b.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Primary care practice within an academic medical center, Boston, USA (Beth Israel Deaconess Medical Center) 3324 patients, 37 physicians, 2 offices |
|
| Interventions | Reminders for health care proxy designation, osteoporosis screening, and influenza and pneumococcal vaccinations in patients older than 65 years | |
| Outcomes | Process adherence (testing, documentation, vaccination) | |
| Co‐Interventions | Educational: Distribution of educational materials and single educational session for providers in intervention group Beyond Clinician Education: Dedicated administrative assistant (‘panel manager’) who assisted patients and physicians in completing the four targeted practice behaviors in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, makes care recommendation, other concurrent CDSS, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Mann 2016.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Two urban academic primary care practices, New York, USA 49 patients |
|
| Interventions | Guided physicians through counselling patients with prediabetes on lifestyle modifications | |
| Outcomes | Clinical endpoint (various outcomes pertaining to weight, body mass index, HbA1C, lipids) | |
| Co‐Interventions | Educational: Distribution of educational material to patients in control group; single educational session for providers in intervention group Beyond Clinician Education: Distribution of pedometers to patients in intervention group; audit and feedback for providers in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, developed in consultation with users, makes care recommendation, possible to execute desired action, 'pull' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Martins 2017.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | 14 primary care centers in a health network, Portugal (Western Oporto) 23 432 patients, 123 primary care physicians (average), 9 health center servers |
|
| Interventions | Modified electronic test ordering screen with colored indicators to illustrate high‐ and low‐value screening tests | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | High risk | |
Matheny 2008.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with two academic medical centers, USA (Partners HealthCare System) 2507 episodes of care, 303 providers, 20 outpatient clinics |
|
| Interventions | Electronic reminders delivered at time of office visits to increase rates of appropriate routine medication laboratory monitoring | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Mazzaglia 2016.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practitioners within large research network, Italy (Health Search Network) 25 491 patients, 197 general practitioners |
|
| Interventions | Reminded providers to initiate pharmacological management for patients with high cardiovascular risk and suggested options to mitigate potential drug‐drug interactions | |
| Outcomes | Process adherence (prescribing*) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in both the control and intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, included supporting information on‐screen, makes care recommendation, 'push' mode of delivery, targeted overuse and underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
McCowan 2001.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Outpatient general practices, UK 477 patients, 17 practices |
|
| Interventions | CDSS providing guideline‐concordant suggestions for the management of patients with asthma | |
| Outcomes | Process adherence (other), clinical endpoint (four outcomes pertaining to asthma exacerbation) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, makes care recommendation, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Unclear risk | N/A |
McGinn 2013.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Two large urban ambulatory primary care practices, academic medical center, NY, USA (Mount Sinai Medical Center) 984 patients, 168 providers |
|
| Interventions | Validated clinical prediction rule triggered by presentations suggestive of streptococcal pharyngitis or pneumonia inviting provider to complete risk score calculator with management recommendations given based on the score | |
| Outcomes | Process adherence (prescribing, testing), clinical endpoint (ED and outpatient visits) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in control group; single educational session for providers in intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, developed in consultation with users, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, required provider input of clinical data, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Meigs 2003.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care internal medicine practice at academic medical center, Boston, USA (Massachusetts General Hospital) 598 patients, 26 providers |
|
| Interventions | CDSS displaying recommended target goals of care and last known values of relevant lab testing (e.g. HbA1C, creatinine, lipids) and links to other web‐based care resources | |
| Outcomes | Process adherence (testing), clinical endpoint (various outcomes pertaining to HbA1C, lipids, blood pressure) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, makes care recommendation, 'pull' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Mertens 2015.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | 36 adult primary care clinics, USA (Kaiser Permanente Northern California) 364 physicians |
|
| Interventions | Reminder to screen for alcohol use disorder embedded within larger intervention to provide brief motivational intervention to patients with unhealthy alcohol use and referral to treatment for patients with alcohol use disorder | |
| Outcomes | Process adherence (other) | |
| Co‐Interventions | Educational: Different single educational session for providers in control and intervention groups Beyond Clinician Education: Local opinion leader endorsement and audit and feedback for intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, possible to execute desired action, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | |
Murray 2004.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic ambulatory internal medicine practice, Indianapolis, USA (Regenstrief Health Center) 352 patients |
|
| Interventions | CDSS suggesting evidence‐based recommendations for the treatment of hypertension, including preventive care and monitoring for adverse drug reactions | |
| Outcomes | Process adherence (prescribing). clinical endpoint (blood pressure) | |
| Co‐Interventions | Educational: Distribution of educational materials and multiple (>1) educational sessions for providers in both control and interventions groups Beyond Clinician Education: Patient‐specific encounter form that included problem list and active drugs in both control and interventions groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Myers 2011a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Internal medicine inpatient setting, academic medical center, Philadelphia, USA (Hospital of the University of Pennsylvania) 39 providers |
|
| Interventions | ‘Hard‐stop’ reminder that appeared when entering unapproved abbreviations into the electronic progress notes to force correction | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | N/A |
Myers 2011b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Internal medicine inpatient setting, academic medical center, Philadelphia, USA (Hospital of the University of Pennsylvania) 39 providers |
|
| Interventions | Autocorrection CDSS that automatically replaced an unapproved abbreviation with the acceptable notation embedded within the electronic progress note | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Najafi 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Nendaz 2010.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Inpatient medical setting, Switzerland 721 patients, 4 medical services |
|
| Interventions | Reminder that computed patient‐specific thromboembolic risk score and provided indication for thromboprophylaxis | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, included supporting information on‐screen, interruptive, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | |
Overhage 1996.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General medical ward, academic medical center, Indianapolis, USA (Wishard Memorial Hospital) 1622 episodes of care, 24 care teams |
|
| Interventions | Reminders suggesting orders for 22 preventive care measures in eligible patients | |
| Outcomes | Process adherence (prescribing, testing, vaccination) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: The same reminder(s) appeared on daily printed patient care report in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, makes care recommendation, possible to execute desired action, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | N/A |
Overhage 1997.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General medical ward, academic medical center, Indianapolis, USA (Wishard Memorial Hospital) 2181 patients, 86 providers, 6 provider teams |
|
| Interventions | Guideline‐based reminders to consider implementing additional corollary orders as providers wrote orders for one of 87 selected tests or treatments. This CDSS intended to reduce errors of omission. | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: Drug utilization review program for both control and interventions groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Palen 2010.
| Study characteristics | ||
| Methods | Single cross‐over cluster RCT | |
| Participants | Ambulatory care clinics in an integrated care delivery system, Denver, USA (Kaiser Permanente of Colorado) 1460 patients, 171 providers, 8 clinics |
|
| Interventions | Reminder advising against ordering D‐dimer testing for patient 65 years and older | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | N/A |
| Unit of analysis error | Low risk | |
Paul 2006.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic medical centers in Israel, Germany and Italy 2326 patients, 15 wards, 3 hospitals |
|
| Interventions | CDSS guiding empirical antibiotic treatment of inpatients with moderate to severe bacterial infections using patient‐specific clinical data. It applies a cost‐benefit model to rank antibiotic treatments according to their net benefit and offers advice (including no treatment). | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, makes care recommendation, possible to execute desired action, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Peiris 2015.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practices and Aboriginal Community Controlled Health Services, Sydney region, Australia 38 725 patients, 60 sites |
|
| Interventions | CDSS providing patient‐specific recommendations for management of cardiovascular disease based on patient’s absolute risk | |
| Outcomes | Process adherence (prescribing, testing, documentation, other) | |
| Co‐Interventions | Educational: Distribution of educational materials to patients in intervention group; multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: Audit and feedback in intervention group; sites in both control and intervention arms participating in existing QI initiatives continued with these programs at their discretion |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Appearance differed based on urgency, conveyed patient‐specific information, makes care recommendation, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Persell 2016a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Large adult primary care practice affiliated with an academic medical center, Chicago, USA (Northwestern Medical Faculty Foundation) 206 patient visits, 7 physicians |
|
| Interventions | CDSS triggered by antibiotic prescription for acute respiratory infection that prompted clinicians to provide free‐text justification that would be included in medical record | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in control and intervention groups Beyond Clinician Education: Financial incentives directed at providers in control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | N/A |
Persell 2016b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Large adult primary care practice affiliated with an academic medical center, Chicago, USA (Northwestern Medical Faculty Foundation) 187 patient visits, 7 physicians |
|
| Interventions | CDSS triggered by antibiotic prescription for acute respiratory infection that presented order set containing non‐antibiotic treatments and patient education materials | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in control and intervention groups Beyond Clinician Education: Financial incentives directed at providers in control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, possible to execute desired action, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | N/A |
Persell 2016c.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Large adult primary care practice affiliated with an academic medical center, Chicago, USA (Northwestern Medical Faculty Foundation) 231 patient visits, 8 physicians |
|
| Interventions | CDSS triggered by antibiotic prescription for acute respiratory infection that prompted clinicians to provide free‐text justification that would be included in medical record AND presented order set containing non‐antibiotic treatments and patient education materials | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in control and intervention groups Beyond Clinician Education: Financial incentives directed at providers in control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | N/A |
Persell 2016d.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Large adult primary care practice affiliated with an academic medical center, Chicago, USA (Northwestern Medical Faculty Foundation) 238 patient visits, 8 physicians |
|
| Interventions | CDSS triggered by antibiotic prescription for acute respiratory infection that presented order set containing non‐antibiotic treatments and patient education materials | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in control and intervention groups Beyond Clinician Education: Audit and feedback in intervention group; Financial incentives directed at providers in control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, possible to execute desired action, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | N/A |
Persell 2016e.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Large adult primary care practice affiliated with an academic medical center, Chicago, USA (Northwestern Medical Faculty Foundation) 342 patient visits, 8 physicians |
|
| Interventions | CDSS triggered by antibiotic prescription for acute respiratory infection that prompted clinicians to provide free‐text justification that would be included in medical record | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in control and intervention groups Beyond Clinician Education: Audit and feedback in intervention group; Financial incentives directed at providers in control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | N/A |
Persell 2016f.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Large adult primary care practice affiliated with an academic medical center, Chicago, USA (Northwestern Medical Faculty Foundation) 298 patient visits, 7 physicians |
|
| Interventions | CDSS triggered by antibiotic prescription for acute respiratory infection that prompted clinicians to provide free‐text justification that would be included in medical record AND presented order set containing non‐antibiotic treatments and patient education materials | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Single educational session for providers in control and intervention groups Beyond Clinician Education: Audit and feedback in intervention group; Financial incentives directed at providers in control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | N/A |
Peterson 2007.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Academic medical center, USA 2981 patients, 778 providers |
|
| Interventions | Guided dosing system delivering advice about appropriate initial dosing for high‐risk medications in elderly patients | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, makes care recommendation, possible to execute desired action, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Unclear risk | N/A |
Piazza 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Player 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care clinics in a national network of practices using same EHR, USA (Medical Quality Improvement Consortium) 54 037 patients, 119 providers, 27 offices |
|
| Interventions | Reminder embedded within encounter form suggesting guideline‐based management of gastroesophageal reflux disease (GERD) and atypical GERD | |
| Outcomes | Process adherence (prescribing, other) | |
| Co‐Interventions | Educational: Distribution of educational materials and multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Appearance differed based on urgency, conveyed patient‐specific information, developed by study investigators, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Price 2017.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care provincial research network, BC, Canada (University of British Columbia Department of Family Practice Research Network) 4825 patients, 28 primary care physicians, 8 practices |
|
| Interventions | Informed physicians of potentially inappropriate prescriptions in the elderly by application of 40 Screening Tool of Older People’s Prescriptions (STOPP) rules | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, included supporting information on‐screen, makes care recommendation, other concurrent CDSS, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Ronda 2018.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Rothschild 2007.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic medical center, Boston, USA (Brigham and Women’s Hospital) 453 providers |
|
| Interventions | CDSS encouraging guideline‐concordant orders for the transfusion of blood products | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers and educational sessions for providers in both control and intervention groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, 'push' mode of delivery, required provider input of clinical data, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | |
Safran 1995.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Academic primary care clinic, Boston, USA (Beth Israel Hospital) 349 patients, 136 providers, 5 sites |
|
| Interventions | Reminder to adhere to recommended processes of care in HIV positive patients | |
| Outcomes | Process adherence (other*),clinical endpoint (several outcomes pertaining to hospitalization, outpatient and ED visits) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, makes care recommendation, possible to execute desired action, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Schnipper 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care clinics within a regional academic medical network, USA (Partners HealthCare System) 7009 patients, 239 providers, 10 practices |
|
| Interventions | Documentation‐based reminder for patients with coronary artery disease or diabetes that provided decision support with tailored recommendations for care | |
| Outcomes | Process adherence (prescribing, testing, documentation) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: Local opinion leaders’ endorsement and audit and feedback in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, makes care recommendation, 'pull' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Schriefer 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic family medicine clinic, USA (Mountain Area Health Education Center) 846 patients, 37 physicians, 4 physician teams |
|
| Interventions | Body mass index prompt during office visit in obese patients intended to increase diagnosis of obesity and referral for obesity treatment | |
| Outcomes | Process adherence (prescribing, other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | 'Push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Sequist 2005.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care clinics affiliated with regional academic medical network, USA (Partners HealthCare System) 6243 patients, 194 providers, 20 clinics (4 community health centers, 9 hospital‐based clinics, 7 off‐site practices) |
|
| Interventions | Display of patient‐specific guideline‐concordant recommendations for diabetes and coronary artery disease care | |
| Outcomes | Process adherence (prescribing, other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: Option to print paper reminders for providers in both control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Sequist 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 21 860 patients, 110 physicians, 11 sites |
|
| Interventions | Reminder during office visits for patients overdue for colorectal cancer screening | |
| Outcomes | Process adherence (testing, other) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | N/A |
Sequist 2011.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 7083 patients, 292 providers, 15 health centers |
|
| Interventions | Two reminders that triggered when chief complaint of chest pain was coded in EHR during office visit (one recommended ECG and aspirin for high risk patients, a second recommended against cardiac stress testing for low risk patients) | |
| Outcomes | Process adherence (prescribing, testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse and underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Sequist 2018a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates 3947 patients, 153 primary care physicians |
|
| Interventions | Reminders to improve management of chronic kidney disease for high risk patients (referral to nephrologist, initiation of ACE inhibitor or ARB) | |
| Outcomes | Process adherence (prescribing, other) | |
| Co‐Interventions | Educational: Distribution of educational materials to patients in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, included supporting information on‐screen, makes care recommendation, possible to execute desired action, 'pull' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | N/A |
| Unit of analysis error | Low risk | |
Sequist 2018b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 3744 patients, 153 primary care physicians |
|
| Interventions | Reminders to improve management of chronic kidney disease for low risk patients (initiation of ACE inhibitor or ARB, annual laboratory test monitoring | |
| Outcomes | Process adherence (prescribing, testing) | |
| Co‐Interventions | Educational: Distribution of educational materials to patients in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, included supporting information on‐screen, makes care recommendation, possible to execute desired action, 'pull' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | N/A |
| Unit of analysis error | Low risk | |
Silbernagel 2016.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inpatient units, academic health center, Switzerland (University Hospital Bern) 889 patients |
|
| Interventions | CDSS identified patients with atrial fibrillation who were not on oral anticoagulants (OAC), calculated CHA2DS2‐VASc score, and provided recommendations for OAC prescription | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgment of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | N/A |
Smith 2012.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices, United Kingdom 911 patients, 29 practices |
|
| Interventions | Alert on patient record to flag at‐risk status for severe asthma | |
| Outcomes | Process adherence (prescribing, other), clinical endpoint (five outcomes pertaining to asthma exacerbation) | |
| Co‐Interventions | Educational: Single educational session for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, interruptive, 'push' mode of delivery, targeted overuse and underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Spirk 2017.
| Study characteristics | ||
| Methods | RCT | |
| Participants | General internal medicine wards, academic health center, Switzerland (University Hospital Bern) 1593 patients |
|
| Interventions | Prompted clinicians to evaluate pulmonary embolism risk using risk calculator and recommended thromboprophylaxis for patients at high‐risk | |
| Outcomes | Process adherence (other), Clinical endpoint (mortality, venous thromboembolism, bleeding) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Ambush, considered alert fatigue in design, developed by study investigators, interruptive, makes care recommendation, 'push' mode of delivery, targeted overuse and underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
Stockwell 2015.
| Study characteristics | ||
| Methods | Cluster Crossover RCT | |
| Participants | Community‐based pediatric clinics affiliated with academic medical center, NY, USA (New York–Presbyterian Hospital/ Columbia University Medical Center) 6593 episodes of care, 4 sites |
|
| Interventions | Noninterruptive influenza vaccination reminder using real‐time query of hospital and city immunization information system | |
| Outcomes | Process adherence (documentation, vaccination) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, developed in consultation with users, included supporting information on‐screen, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Strom 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Inpatient setting, academic medical center, Philadelphia, USA (Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center) 96 patients, 1971 providers |
|
| Interventions | Nearly ‘hard stop’ reminder intended to reduce concomitant orders ofwarfarin and trimethoprim‐ sulfamethoxazole | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Considered alert fatigue in design, conveyed patient‐specific information, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Unclear risk | N/A |
| Unit of analysis error | Low risk | |
Szilagyi 2015.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Family medicine and pediatric practices participating in two practice‐based research networks, USA (Greater Rochester PBRN and CORNET) 29 968 patients, 22 practices |
|
| Interventions | CDSS displaying a list of vaccines due at that visit to improve adolescent immunization rates | |
| Outcomes | Process adherence (vaccination) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, developed by study investigators, interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | N/A |
Tamblyn 2003.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices, Quebec, Canada 12 560 encounters, 107 providers |
|
| Interventions | CDSS identifying clinically relevant prescribing problems in the elderly (drug‐disease contraindications, drug interactions, drug‐age contraindications, duration of therapy, and therapeutic duplication) | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, included supporting information on‐screen, makes care recommendation, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Tamblyn 2010.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Primary care research program using same EHR, Quebec, Canada (Medical Office of the 21st Century [MOXXI]) 2293 patients |
|
| Interventions | Cardiovascular medication tracking coupled with a nonadherence alert system for antihypertensive and lipid‐lowering medications | |
| Outcomes | Process adherence (other) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: Electronic drug profile in both control and intervention groups |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
Tamblyn 2015.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices, Quebec, Canada 4447 patients, 81 primary care physicians |
|
| Interventions | Notified clinicians of patients with poorly managed asthma and provided access to guidelines, assessment tools and patient‐specific recommendations (such as home care and monitoring) | |
| Outcomes | Clinical endpoint (inhaled steroids to fast‐acting beta agonist ratio, out‐of‐control asthma incident rate) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgment of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Tamblyn 2018a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Urban primary care practices, Quebec, Canada 1261 patients, 76 primary care physicians |
|
| Interventions | Displayed out‐of‐pocket costs that patients would incur due to new initiation of anti‐hypertensive medication and identified cost savings if switched to an alternative medication | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, required provider input of clinical data, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | N/A |
| Unit of analysis error | Low risk | |
Tamblyn 2018b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Urban primary care practices, Quebec, Canada 2331 patients, 76 primary care physicians |
|
| Interventions | Displayed out‐of‐pocket costs that patients would incur due to continuation of anti‐hypertensive medication and identified cost savings if switched to an alternative medication | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, required provider input of clinical data, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | N/A |
| Unit of analysis error | Low risk | |
Tang 2012.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic general internal medicine clinic, Chicago, USA (Northwestern Medical Faculty Foundation) 2114 patients, 30 providers |
|
| Interventions | Point‐of‐care passive prompt for overweight patients directing providers to open evidence‐based counseling template that, once completed, could open an order set for overweight patients | |
| Outcomes | Process adherence (other) | |
| Co‐Interventions | Educational: Multiple (>1) educational sessions for providers in intervention group Beyond Clinician Education: Endorsement of local opinion leaders in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Taveras 2014.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Pediatric ambulatory practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 378 patients, 9 practices |
|
| Interventions | CDSS triggered at time of well child care visit for a child with a BMI ≥ 95th percentile with links to evidence‐based management of childhood obesity and a pre‐populated standardized note specific for obesity | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Taveras 2015a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Pediatric practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 378 patients, 9 practices |
|
| Interventions | Reminder for documentation and counselling for children with a body mass index equal to or greater than the 95th percentile | |
| Outcomes | Process adherence (documentation), Clinical endpoint (body mass index) | |
| Co‐Interventions | Educational: Distribution of educational materials to patients in both control and intervention groups; Single educational session and access to educational resources such as motivational interviewing strategies for providers in intervention group Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgment of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Taveras 2015b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Pediatric practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 355 patients, 9 practices |
|
| Interventions | Reminder for documentation and counselling for children with a body mass index equal to or greater than the 95th percentile | |
| Outcomes | Process adherence (documentation), Clinical endpoint (body mass index) | |
| Co‐Interventions | Educational: Distribution of educational materials to patients in both control and intervention groups; Single educational session and access to educational resources such as motivational interviewing strategies for providers in intervention group Beyond Clinician Education: Study health coach conducting motivational counseling calls for families in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes – required acknowledgment of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Terrell 2009.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic emergency department, Indianapolis, USA (Wishard Memorial Hospital) 210 episodes of care, 63 providers |
|
| Interventions | Reminder that advised against prescription of nine potentially inappropriate medications in patients ≥ age 65 | |
| Outcomes | Process adherence (Prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS and documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Terrell 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic emergency department, Indianapolis, USA (Wishard Memorial Hospital) 2783 episodes of care, 42 providers |
|
| Interventions | Reminder that provided dosing recommendations for 10 high‐risk medications when renal function was below threshold for dosage adjustment in patients being discharged from the emergency department | |
| Outcomes | Process adherence (prescribing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, interruptive, makes care recommendation, other concurrent CDSS, possible to execute desired action, 'push' mode of delivery, targeted overuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Tierney 2003.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Academic primary care group practice, USA (Indiana University Medical Group‐Primary Care) 378 patients, 4 clinics |
|
| Interventions | CDSS suggesting guideline‐based recommendations for chronic heart failure and ischemic heart disease management | |
| Outcomes | Process adherence (prescribing, vaccination), clinical endpoint (several outcomes pertaining to overall health status, ED visits and hospitalizations due to cardiac disease exacerbations) | |
| Co‐Interventions | Educational: Distribution of educational materials and multiple (>1) educational sessions for providers in both control and interventions groups Beyond Clinician Education: Use of local opinion leaders in intervention group |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Tierney 2005.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practice‐based research network, USA (Indiana University Medical Group‐Primary Care) 363 patients, 4 hospital‐based academic practices |
|
| Interventions | Display of patient‐specific guideline‐based suggestions for management of asthma and chronic obstructive pulmonary disease | |
| Outcomes | Process adherence (prescribing, testing, vaccination), clinical endpoint (several outcomes related to overall health status, medication adherence, emergency visits, hospitalizations) | |
| Co‐Interventions | Educational: Distribution of educational materials and single educational session for providers in both control and interventions groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, included supporting information on‐screen, makes care recommendation, other concurrent CDSS, possible to execute desired action, required provider input of clinical data, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Trick 2009.
| Study characteristics | ||
| Methods | Cluster CCT | |
| Participants | Internal medicine inpatient unit, public hospital, Chicago, USA (Cook County Hospital) 135 patients, 2 teams |
|
| Interventions | CDSS that pre‐selects opt‐out orders for influenza vaccination triggered by an order to discharge the patient | |
| Outcomes | Process adherence (vaccination) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Van Wyk 2008a.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practice clinics, Delft region, the Netherlands 62 536 patients, 46 physicians, 24 clinics |
|
| Interventions | Automatic display of patient‐specific guideline recommendations for the screening and treatment of dyslipidemia | |
| Outcomes | Process adherence (prescribing, testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, makes care recommendation, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Van Wyk 2008b.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practice clinics, Delft region, the Netherlands 56 675 patients, 51 physicians, 23 clinics |
|
| Interventions | User initiated display of patient‐specific guidelines for screening and treatment of dyslipidemia | |
| Outcomes | Process adherence (prescribing, testing) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | No | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, developed by study investigators, makes care recommendation, 'pull' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Walker 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | General practice clinics, Melbourne, Australia 2846 patients, 221 providers, 66 clinics |
|
| Interventions | Reminder that prompted discussion about chlamydia testing with women aged 16‐24 | |
| Outcomes | Process adherence (testing) | |
| Co‐Interventions | Educational: Distribution of educational materials to providers in both intervention and control groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, considered alert fatigue in design, interruptive, makes care recommendation, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Wexler 2010.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Acute general medical service, academic medical center, Boston, USA (Massachusetts General Hospital) 128 patients, 42 residents, 7 teams |
|
| Interventions | Order template facilitating weight‐based dosing of insulin intended to lower mean blood glucose in medical inpatient with type 2 diabetes | |
| Outcomes | Clinical endpoint (hyper‐ and hypo‐glycemia, basal insulin dose) | |
| Co‐Interventions | Educational: Distribution of educational materials and single educational session for providers in both intervention and control groups Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Conveyed patient‐specific information, interruptive, 'push' mode of delivery, required provider input of clinical data, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Wilkinson 2019.
| Study characteristics | ||
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Co‐Interventions | ||
| CDSS Features ‐ Acknowledgement of CDSS Required | ||
| CDSS Features ‐ Other | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
Wright 2012.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care practices affiliated with academic medical center, Boston, USA (Brigham and Women’s Hospital) 79 064 patients, 11 clinics |
|
| Interventions | Reminder using inference rules to suggest adding undocumented problems to the EHR problem list | |
| Outcomes | Process adherence (documentation) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Ambush, considered alert fatigue in design, conveyed patient‐specific information, developed by study investigators, included supporting information on‐screen, interruptive, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | High risk | |
Wu 2018.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Intensive and coronary care units, cardiology wards, and cardiac surgery wards, Guangdong, China (Guangdong General Hospital) 875 patients |
|
| Interventions | Monitored the serum creatinine levels of hospitalized adult patients and alerted physicians to suspected cases of acute kidney injury | |
| Outcomes | Process adherence (other), Clinical endpoint (renal replacement therapy, renal recovery, death) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Developed by study investigators, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
Zanetti 2003.
| Study characteristics | ||
| Methods | Quasi‐RCT | |
| Participants | Cardiac surgery service, academic medical center, Boston, USA (Brigham and Women’s Hospital) 273 patients |
|
| Interventions | CDSS supplemented by audible alarm reminding operating room staff to consider second dose of prophylactic antibiotics for prolonged surgeries | |
| Outcomes | Process adherence (prescribing), clinical endpoint (surgical‐site infection) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Yes ‐ required acknowledgement of the CDSS but not documentation of action taken | |
| CDSS Features ‐ Other | Developed by study investigators, included supporting information on‐screen, interruptive, makes care recommendation, possible to execute desired action, 'push' mode of delivery, targeted underuse | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | N/A |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | N/A |
| Baseline characteristics similar? | Low risk | |
Zera 2015.
| Study characteristics | ||
| Methods | Cluster RCT | |
| Participants | Primary care clinics affiliated with regional academic medical network, USA (Partners HealthCare System) 847 patients, 26 physician clusters |
|
| Interventions | Identified women with a history of gestational diabetes and recommended screening for type 2 diabetes | |
| Outcomes | Process adherence (testing), Clinical endpoint (diabetes diagnosis) | |
| Co‐Interventions | Educational: None Beyond Clinician Education: None |
|
| CDSS Features ‐ Acknowledgement of CDSS Required | Not reported | |
| CDSS Features ‐ Other | Ambush, conveyed patient‐specific information, decision support was complex, developed by study investigators, included supporting information on‐screen, makes care recommendation, other concurrent CDSS, possible to execute desired action, 'push' mode of delivery, targeted underuse, user workflow considered in design | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Baseline characteristics similar? | Low risk | |
| Unit of analysis error | Low risk | |
CPOE: Computerized provider order entry; EMR: electronic medical record; RCT: randomised controlled trial; CCT: Controlled clinical trial; *: Some of the outcomes within this category are continuous (as opposed to dichotomous); **: All of the outcomes within this category are continuous (as opposed to dichotomous).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acevedo 2018 | Not a point‐of‐care reminder |
| Adelman 2013 | Not a computerized decision support system |
| Allen 2016 | Targeted multiple disciplines and physician data could not be isolated |
| ALMohiza 2016 | Not a point‐of‐care reminder |
| Anchala 2015 | Not part of routine care |
| Apkon 2005a | Not part of routine care |
| Åsberg 2010 | Not part of routine care |
| Barkun 2013 | Not a computerized decision support system |
| Beck 2015 | Excluded topic: specialized perfusionist‐directed system |
| Beeckman 2013 | Targeted non‐physicians |
| Bernacki 2015 | Not a computerized decision support system |
| Bhardwaja 2011 | Not an on‐screen computer reminder |
| Bindels 2004 | Not part of routine care |
| Biswas 2018 | Not a point‐of‐care reminder |
| Bosworth 2009 | Ineligible comparison: head‐to‐head designl |
| Caballero‐Ruiz 2017 | Not a point‐of‐care reminder |
| Cannon 2000 | Not a point‐of‐care reminder |
| Chien 2017 | Not directly related to patient care |
| Clarke 2016 | Not on‐screen computerized decision support system |
| Collins 2018 | CDSS present in pre‐randomization phase |
| Colpaert 2012 | Ineligible study design |
| Crosson 2012 | Not a computerized decision support system |
| Curran 2010 | Not a computerized decision support system |
| Curtain 2011 | Targeted non‐physicians |
| Dekarske 2015 | Targeted multiple disciplines and physician data could not be isolated |
| Dexter 2004 | Ineligible comparison: head‐to‐head design |
| Dixon 2017 | Ineligible study design |
| Dragan 2015 | Excluded topic: simulation |
| Duffy 2016 | Targeted non‐physicians |
| Dumont 2012 | Not part of routine care |
| Durieux 2000 | Ineligible study design |
| Dykes 2010 | Targeted non‐physicians |
| Edmiston 2016 | Not a computerized decision support system |
| Eisenstein 2011 | Not a point‐of‐care reminder |
| Elliott 2017 | Targeted non‐physicians |
| Feldstein 2006 | Not a point‐of‐care reminder |
| Fitzgerald 2011 | Not part of routine care |
| Fitzpatrick 2017 | Inappropriate control |
| Flamm 2013 | Ineligible study design |
| Flanagan 1999 | Not a point‐of‐care reminder |
| Foy 2011 | Not on‐screen computerized decision support system |
| Freundlich 2013 | Not directly related to patient care |
| Fricton 2011 | Excluded topic: dental clinics |
| Gallagher 2016 | Not a point‐of‐care reminder |
| Goetz 2013 | Outcome reported in ineligible format |
| Grace 2011 | Not a computerized decision support system |
| Hagiwara 2013 | Excluded topic: simulation |
| Hains 2012 | Not part of routine care |
| Harpole 1997 | Ineligible comparison: head‐to‐head design |
| Heiman 2004 | Not a point‐of‐care reminder |
| Herasevich 2011 | Not a computerized decision support system |
| Holmes 2015 | Targeted non‐physicians |
| Hooper 2012 | Not on‐screen computerized decision support system |
| Humphrey 2011 | Not a point‐of‐care reminder |
| Ignatov 2016 | Excluded topic: specialized system aiding in quantitative cardiotocography interpretation |
| James 1993 | Not a computerized decision support system |
| James 2015 | Not part of routine care |
| Johnson 2010 | Not directly related to patient care |
| Keitel 2017 | Not part of routine care |
| Kim 2017 | Ineligible study design |
| Kollef 2014 | Excluded topic: expert system |
| Kostopoulou 2015 | Excluded topic: simulation |
| Kralj 2003 | Ineligible study design |
| Kuhn 2015 | Ineligible study design |
| Kurian 2009 | Not part of routine care |
| Lee 2009 | Targeted non‐physicians |
| Lee 2016 | Not a computerized decision support system |
| Luders 2010 | Not on‐screen computerized decision support system |
| Luna 2017 | Ineligible comparison: head‐to‐head design |
| Magnus 2012 | Ineligible study design |
| Mainous 2013 | Outcome reported in ineligible format |
| Mann 2011 | Targeted non‐physicians |
| Manns 2012 | Ineligible comparison: head‐to‐head design |
| Martens 2007 | Outcome reported in ineligible format |
| Martí 2017 | Not an on‐screen computerized decision support system |
| Martinez 2018 | Not part of routine care |
| Mayne 2014 | Outcome reported in ineligible format |
| McAvoy 2013 | Not on‐screen computerized decision support system |
| McCormick 2016 | Excluded topic: specialized anesthesiologist‐directed system |
| McDonald 1992 | Not on‐screen computerized decision support system |
| McGreevey 2013 | Excluded topic: order set |
| McGregor 2006 | Not a point‐of‐care reminder |
| Mehta 2016 | Ineligible study design |
| Montgomery 2000 | Not a computerized decision support system |
| Muth 2018 | Not part of routine care |
| Nieuwlaat 2012 | Not a point‐of‐care reminder |
| Ornstein 1991 | Not on‐screen computerized decision support system |
| Palen 2006 | Ineligible comparison or inappropriate control |
| Pang 2015 | Non‐study |
| Panjasawatwong 2015 | Excluded topic: specialized anesthesiologist‐directed system |
| Peremans 2010 | Excluded topic: simulation |
| Pielmeier 2012 | Duplicate publication |
| Poller 1993 | Not a point‐of‐care reminder |
| Raebel 2007 | Targeted non‐physicians |
| Raja 2015 | Not a computerized decision support system |
| Rapoport 2018 | Not part of routine care |
| Rathlev 2016 | Targeted multiple disciplines and physician data could not be isolated |
| Reeve 2008 | Targeted non‐physicians |
| Ribeiro‐Vaz 2012 | Ineligible study design |
| Robbins 2012 | Ineligible comparison: head‐to‐head design |
| Rodriguez‐Aldrete 2016 | Not a point‐of‐care reminder |
| Rood 2005 | Ineligible comparison: head‐to‐head design |
| Roumie 2006 | Not a point‐of‐care reminder |
| Roy 2009 | Not a point‐of‐care reminder |
| Roy 2016 | Insufficient description of CDSS aspect of intervention |
| Safran 1993 | Duplicate publication |
| Schnipper 2010‐2 | Excluded topic: order set |
| Schwarz 2012 | Ineligible comparison: head‐to‐head design |
| Shelley 2015 | Not an on‐screen computerized decision support system |
| Silva 2013 | Not a computerized decision support system |
| Simon 2006 | Ineligible comparison: head‐to‐head design |
| Skinner 2015 | Ineligible comparison: head‐to‐head design |
| Slok 2016 | Targeted multiple disciplines and physician data could not be isolated |
| Strom 2010‐2 | Ineligible comparison: head‐to‐head design |
| Sundaram 2009 | Not on‐screen computerized decision support system |
| Suresh 2018 | Targeted multiple disciplines and physician data could not be isolated |
| Tamblyn 2008 | Ineligible comparison: head‐to‐head design |
| Tamblyn 2012 | Ineligible comparison: head‐to‐head design |
| Thomas 2004 | Not a point‐of‐care reminder |
| Thomas 2018 | Outcome reported in ineligible format |
| Tollitt 2018 | Not a point‐of‐care reminder |
| Tsai 2016 | Inappropriate intervention |
| van Doormaal 2009 | Ineligible study design |
| van Wijk 2001 | Ineligible comparison: head‐to‐head design |
| Weiss 2013 | Inappropriate control |
| Welch 2015 | Not part of routine care |
| Were 2011 | Not on‐screen computerized decision support system |
| Williams 2010 | Ineligible study design |
| Williams 2011 | Targeted non‐physicians |
| Wilson 2015 | Not part of routine care |
| Wipfli 2016 | Excluded topic: simulated scenarios |
| Woller 2018 | Ineligible study design |
| Zhu 2018 | Not a computerized decision support system |
| Ziemer 2006 | Not on‐screen computerized decision support system |
Characteristics of studies awaiting classification [ordered by study ID]
Christakis 2001.
| Methods | Cluster‐RCT |
| Participants | Outpatient pediatric teaching clinic, Seattle, USA (University of Washington) 1339 episodes of care, 38 providers |
| Interventions | Displaying evidence regarding the use and duration of antibiotics for otitis media in children |
| Outcomes | Process adherence (prescribing) |
| Notes | System for delivery of reminder: CPOE |
Christakis 2001a.
| Methods | Cluster RCT |
| Participants | Pediatric primary outpatient teaching clinic, Seattle, USA (University of Washington) 1339 episodes of care, 38 providers |
| Interventions | CDSS presenting real‐time evidence to providers prescribing antibiotics for otitis media |
| Outcomes | Process adherence (prescribing) |
| Notes |
Durieux 2000 ‐ classified, excluded.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Forrest 2013.
| Methods | Cluster RCT |
| Participants | Primary care practice‐based research network, USA (Children’s Hospital of Philadelphia) 91 providers, 12 practices |
| Interventions | Multicomponent CDSS intended to improve adherence to guidelines for acute otitis media and otitis media with effusion (display of relevant clinical information; data gathering tool; and generation of patient‐specific orders for treatment, progress note, and discharge instructions) Co‐intervention (apart from education): Audit and feedback Required acknowledgement of the CDSS only |
| Outcomes | Process adherence (prescribing, other) |
| Notes |
Fortuna 2009.
| Methods | Cluster RCT |
| Participants | Primary care practices affiliated with academic medical center, USA (Harvard Vanguard Medical Associates) 177 providers, 9 sites |
| Interventions | Reminder to decrease prescribing of heavily marketed hypnotic medications by recommending an alternative medication and providing prescribing information and patient education materials Co‐intervention (education only): Distribution of educational materials and single educational session for providers |
| Outcomes | Process adherence (prescribing) |
| Notes |
Kralj 2003 ‐ classified, excluded.
| Methods | Cluster‐CCT |
| Participants | Two community oncology outpatient practices, USA 2170 episodes of care, 2 practices |
| Interventions | Prompting providers to order erythropoietin for patients with haemoglobin < 120 g/dL |
| Outcomes | Process adherence (prescribing) |
| Notes | System for delivery of reminder: EMR with link to CPOE |
Plaza 2005.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Roumie 2006 ‐ classified, excluded.
| Methods | Cluster‐RCT |
| Participants | 2 hospitals, 8 ambulatory clinics, Nashville, USA (Vanderbilt University) 871 patients, 116 providers |
| Interventions | Alert in electronic medical record displaying recent blood pressure value and outlining national recommendations for hypertension treatment and blood pressure goals |
| Outcomes | Process adherence (prescribing), clinical outcomes |
| Notes | System for delivery of reminder: EMR Additional interventions delivered to intervention and control groups: provider education (printed materials delivered via e‐mail) |
Sales 2008.
| Methods | CCT |
| Participants | Hospitals in a regional network within the Veterans Health Administration, USA (Rocky Mountain Network) 5438 patients, 199 providers, 6 hospitals |
| Interventions | Reminders at the point of care to improve lipid measurement and treatment in patients with ischemic heart disease |
| Outcomes | Process adherence (tests, prescribing) |
| Notes |
Tape 1993.
| Methods | Cluster‐CCT |
| Participants | Internal medicine teaching clinic, Omaha, USA (University of Nebraska) 1809 patients, 2 clinics |
| Interventions | Drawing attention to deficiencies in preventive care measures for a given patient |
| Outcomes | Process adherence (test ordering, vaccination) |
| Notes | System for delivery of reminder: EMR Additional interventions delivered to intervention and control groups: provider education (conferences), paper reminders to providers |
Study published in Spanish ‐ awaiting translation. Expected to be eligible for inclusion.
Contributions of authors
KS led the project, including preparing the data abstraction form, screening and dealing with consensus issues, and led the analysis.
AJ participated in screening, data extraction and data analysis.
AM participated in data extraction and screening.
CRR provided support for the analysis.
MPE provided input into overall structure of the review.
JG was involved in the protocol publication and also provided input into the overall structure of the review.
Sources of support
Internal sources
Ottawa Hospital Research Institute, Canada
University of Ottawa, Canada
External sources
Canadian Institutes of Health Research, Canada
CIHR Institute of Health Services and Policy Research, Canada
CIHR Institute of Musculoskeletal Health and Arthritis, Canada
CIHR Institute of Gender and Health, Canada
CIHR Institute of Human Development, Child and Youth Health, Canada
CIHR Institute of Nutrition, Metabolism and Diabetes, Canada
CIHR Institute of Infection and Immunity, Canada
Canadian Foundation for Innovation, Canada
Government of Canada Research Chair in Patient Safety and Quality Improvement, Canada
UK National Institute for Health Research Cochrane Programme Grant scheme, UK
-
Chief Scientist Office of the Scottish Government Health Directorate, UK
The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Government Health Directorate. The views expressed, however, are those of the authors.
Declarations of interest
ME and JG are authors on one included study and three excluded studies in this review. Four authors (AM, ME, CRR, JG) are editors or staff within the Cochrane EPOC Review Group. Editors and staff are required to conduct at least one Cochrane review. This requirement ensures that editors are aware of the processes and commitment needed to conduct reviews. This involvement does not seem to be a source of conflict of interest in the Cochrane EPOC Review Group. Any editor or staff who is a review author is excluded from editorial decisions on the review in which they are contributors.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Abdel‐Kader 2011 {published data only}
- Abdel-Kader K, Fischer G S, Li J, Moore C G, Hess R, Unruh M L. Automated clinical reminders for primary care providers in the care of CKD: a small cluster-randomized controlled trial. Am J Kidney Dis 2011;58:894-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts D L, Abu-Hanna A, Medlock S K, Weert H C. Effectiveness and usage of a decision support system to improve stroke prevention in general practice: A cluster randomized controlled trial. PLoS One 2017;12(2):e0170974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ansari 2003 {published data only}
- Ansari M, Shlipak M G, Heidenreich P A, Van Ostaeyen D, Pohl E C, Browner W S, et al. Improving guideline adherence: a randomized trial evaluating strategies to increase beta-blocker use in heart failure. Circulation 2003;107:2799-804. [DOI] [PubMed] [Google Scholar]
Arts 2017 {published data only}
- Arts D L, Abu-Hanna A, Medlock S K, Weert H C. Effectiveness and usage of a decision support system to improve stroke prevention in general practice: A cluster randomized controlled trial. PLoS One 2017;12(2):e0170974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Awdishu 2016 {published data only}
- Awdishu L, Coates C R, Lyddane A, Tran K, Daniels C E, Lee J, El-Kareh R. The impact of real-time alerting on appropriate prescribing in kidney disease: a cluster randomized controlled trial. J Am Med Inform Assoc 2016;23(3):609-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baandrup 2010 {published data only}
- Baandrup L, Allerup P, Lublin H, Nordentoft M, Peacock L, Glenthoj B. Evaluation of a multifaceted intervention to limit excessive antipsychotic co-prescribing in schizophrenia out-patients. Acta Psychiatr Scand 2010;122:367-74. [DOI] [PubMed] [Google Scholar]
Baer 2013 {published data only}
- Baer H J, Schneider L I, Colditz G A, Dart H, Andry A, Williams D H, et al. Use of a web-based risk appraisal tool for assessing family history and lifestyle factors in primary care. J Gen Intern Med 2013;28:817-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bates 1999 {published data only}
- Bates D W, Kuperman G J, Rittenberg E, Teich J M, Fiskio J, Ma'luf N, et al. A randomized trial of a computer-based intervention to reduce utilization of redundant laboratory tests. Am J Med 1999;106:144-50. [DOI] [PubMed] [Google Scholar]
Beeler 2014 {published data only}
- Beeler P E, Eschmann E, Schumacher A, Studt J D, Amann-Vesti B, Blaser J. Impact of electronic reminders on venous thromboprophylaxis after admissions and transfers. J Am Med Inform Assoc 2014;21:e297-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bell 2010 {published data only}
- Bell L M, Grundmeier R, Localio R, Zorc J, Fiks A G, Zhang X, et al. Electronic health record-based decision support to improve asthma care: a cluster-randomized trial. Pediatrics 2010;125:e770-7. [DOI] [PubMed] [Google Scholar]
Bennett 2018 {published data only}
Bernstein 2017 {published data only}
- Bernstein S L, Rosner J, DeWitt M, Tetrault J, Hsiao A L, Dziura J, Sussman S, O'Connor P, Toll B. Design and implementation of decision support for tobacco dependence treatment in an inpatient electronic medical record: a randomized trial. Transl Behav Med 2017;7(2):185-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beste 2015 {published data only}
- Beste L A, Ioannou G N, Yang Y, Chang M F, Ross D, Dominitz J A. Improved surveillance for hepatocellular carcinoma with a primary care-oriented clinical reminder. Clin Gastroenterol Hepatol 2015;13:172-9. [DOI] [PubMed] [Google Scholar]
Boustani 2012 {published data only}
- Boustani Malaz A, Campbell Noll L, Khan Babar A, Abernathy Greg, Zawahiri Mohammed, Campbell Tiffany, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. Journal of General Internal Medicine 2012;27:561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2019 {published data only}
Chak 2018 {published data only}
- Chak E, Taefi A, Li C S, Chen M S Jr, Harris A M, MacDonald S, Bowlus C. Electronic Medical Alerts Increase Screening for Chronic Hepatitis B: A Randomized, Double-Blind, Controlled Trial. Cancer Epidemiol Biomarkers Prev 2018;27(11):1352-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaturvedi 2019 {published data only}
Co 2010 {published data only}
- Co J P, Johnson S A, Poon E G, Fiskio J, Rao S R, Van Cleave J, et al. Electronic health record decision support and quality of care for children with ADHD. Pediatrics 2010;126:239-46. [DOI] [PubMed] [Google Scholar]
Cote 2008a {published data only}
- Cote Gregory A, Rice John P, Bulsiewicz William, Norvell John P, Christensen Keri, Bobb Anne, et al. Use of physician education and computer alert to improve targeted use of gastroprotection among NSAID users. American Journal of Gastroenterology 2008;103:1097-103. [DOI] [PubMed] [Google Scholar]
Cote 2008b {published data only}
- Cote G A, Rice J P, Bulsiewicz W, Norvell J P, Christensen K, Bobb A, Postelnick M, Howden C W. Use of physician education and computer alert to improve targeted use of gastroprotection among NSAID users. Am J Gastroenterol 2008;103(5):1097-103. [DOI] [PubMed] [Google Scholar]
Davis 2007 {published data only}
- Davis Robert L, Wright Jeffrey, Chalmers Francie, Levenson Linda, Brown Julie C, Lozano Paula, et al. A cluster randomized clinical trial to improve prescribing patterns in ambulatory pediatrics. PLoS Clinical Trials 2007;2:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dean 2015 {published data only}
- Dean N C, Jones B E, Jones J P, Ferraro J P, Post H B, Aronsky D, Vines C G, Allen T L, Haug P J. Impact of an Electronic Clinical Decision Support Tool for Emergency Department Patients With Pneumonia. Ann Emerg Med 2015;66(5):511-20. [DOI] [PubMed] [Google Scholar]
Dexter 2001 {published data only}
- Dexter P R, Perkins S, Overhage J M, Maharry K, Kohler R B, McDonald C J. A computerized reminder system to increase the use of preventive care for hospitalized patients. N Engl J Med 2001;345:965-70. [DOI] [PubMed] [Google Scholar]
Diaz 2018 {published data only}
- Diaz M C G, Werk L N, Crutchfield J H Jr, Handy L K, Franciosi J P, Dent J, Villanueva R, Antico E, Taylor A, Wysocki T. A Provider-Focused Intervention to Promote Optimal Care of Pediatric Patients With Suspected Elbow Fracture. Pediatr Emerg Care 2018. [DOI] [PubMed] [Google Scholar]
Diaz 2019 {published data only}
- Diaz M C G, Wysocki T, Crutchfield J H Jr, Franciosi J P, Werk L N. Provider-Focused Intervention to Promote Comprehensive Screening for Adolescent Idiopathic Scoliosis by Primary Care Pediatricians. Am J Med Qual 2019:182-188. [DOI] [PubMed] [Google Scholar]
Downs 2006 {published data only}
- Downs Murna, Turner Stephen, Bryans Michelle, Wilcock Jane, Keady John, Levin Enid, et al. Effectiveness of educational interventions in improving detection and management of dementia in primary care: cluster randomised controlled study. BMJ 2006;332:692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dregan 2014 {published data only}
- Dregan A, Staa T P, McDermott L, McCann G, Ashworth M, Charlton J, et al. Point-of-care cluster randomized trial in stroke secondary prevention using electronic health records. Stroke 2014;45:2066-71. [DOI] [PubMed] [Google Scholar]
Eccles 2002a {published data only}
- Eccles MP, McColl E, Steen N, Rousseau N, Grimshaw J, Parkin D, et al. Effect of computerized evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomized controlled trial. BMJ 2002;325:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 2002b {published data only}
- Eccles M, McColl E, Steen N, Rousseau N, Grimshaw J, Parkin D, et al. Effect of computerised evidence based guidelines on management of asthma and angina in adults in primary care: cluster randomised controlled trial. BMJ 2002;325:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feder 2011 {published data only}
- Feder Gene, Davies Roxane Agnew, Baird Kathleen, Dunne Danielle, Eldridge Sandra, Griffiths Chris, et al. Identification and Referral to Improve Safety (IRIS) of women experiencing domestic violence with a primary care training and support programme: a cluster randomised controlled trial. Lancet 2011;378:1788-95. [DOI] [PubMed] [Google Scholar]
Field 2009 {published data only}
- Field T S, Rochon P, Lee M, Gavendo L, Baril J L, Gurwitz J H. Computerized clinical decision support during medication ordering for long-term care residents with renal insufficiency. J Am Med Inform Assoc 2009;16:480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fiks 2009 {published data only}
- Fiks A G, Hunter K F, Localio A R, Grundmeier R W, Bryant-Stephens T, Luberti A A, et al. Impact of electronic health record-based alerts on influenza vaccination for children with asthma. Pediatrics 2009;124:159-69. [DOI] [PubMed] [Google Scholar]
Fiks 2013 {published data only}
- Fiks Alexander G, Grundmeier Robert W, Mayne Stephanie, Song Lihai, Feemster Kristen, Karavite Dean, et al. Effectiveness of Decision Support for Families, Clinicians, or Both on HPV Vaccine Receipt. Pediatrics 2013;131:1114-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Filippi 2003 {published data only}
- Filippi A, Sabatini A, Badioli L, Samani F, Mazzaglia G, Catapano A, et al. Effects of an automated electronic reminder in changing the antiplatelet drug-prescribing behavior among Italian general practitioners in diabetic patients: an intervention trial. Diabetes Care 2003;26:1497-500. [DOI] [PubMed] [Google Scholar]
Flottorp 2002a {published data only}
- Flottorp Signe, Oxman Andrew D, Havelsrud Kari, Treweek Shaun, Herrin Jeph. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ 2002;325:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flottorp 2002b {published data only}
- Flottorp S, Oxman A D, Havelsrud K, Treweek S, Herrin J. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ 2002;325(7360):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 2004 {published data only}
- Frank O, Litt J, Beilby J. Opportunistic electronic reminders. Improving performance of preventive care in general practice. Aust Fam Physician 2004;33:87-90. [PubMed] [Google Scholar]
Gill 2009 {published data only}
- Gill J M, Chen Y X, Glutting J J, Diamond J J, Lieberman M I. Impact of decision support in electronic medical records on lipid management in primary care. Popul Health Manag 2009;12:221-6. [DOI] [PubMed] [Google Scholar]
Gill 2011 {published data only}
- Gill J M, Mainous A G 3rd, Koopman R J, Player M S, Everett C J, Chen Y X, et al. Impact of EHR-based clinical decision support on adherence to guidelines for patients on NSAIDs: a randomized controlled trial. Ann Fam Med 2011;9:22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2013 {published data only}
- Gonzales Ralph, Anderer Tammy, McCulloch Charles E, Maselli Judith H, Bloom Frederick J Jr, Graf Thomas R, et al. A cluster randomized trial of decision support strategies for reducing antibiotic use in acute bronchitis. JAMA Internal Medicine 2013;173:267-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goud 2009 {published data only}
- Goud R, Keizer N F, ter Riet G, Wyatt J C, Hasman A, Hellemans I M, et al. Effect of guideline based computerised decision support on decision making of multidisciplinary teams: cluster randomised trial in cardiac rehabilitation. BMJ 2009;338:b1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guiriguet 2016 {published data only}
- Guiriguet C, Munoz-Ortiz L, Buron A, Rivero I, Grau J, Vela-Vallespin C, Vilarrubi M, Torres M, Hernandez C, Mendez-Boo L, Toran P, Caballeria L, Macia F, Castells A. Alerts in electronic medical records to promote a colorectal cancer screening programme: a cluster randomised controlled trial in primary care. Br J Gen Pract 2016;66(648):e483-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 2014 {published data only}
- Gulliford M C, Staa T, Dregan A, McDermott L, McCann G, Ashworth M, et al. Electronic health records for intervention research: a cluster randomized trial to reduce antibiotic prescribing in primary care (eCRT study). Ann Fam Med 2014;12:344-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulliford 2019 {published data only}
Gupta 2014 {published data only}
- Gupta A, Gholami P, Turakhia M P, Friday K, Heidenreich P A. Clinical reminders to providers of patients with reduced left ventricular ejection fraction increase defibrillator referral: a randomized trial. Circ Heart Fail 2014;7:140-5. [DOI] [PubMed] [Google Scholar]
Gurwitz 2008 {published data only}
- Gurwitz Jerry H, Field Terry S, Rochon Paula, Judge James, Harrold Leslie R, Bell Chaim M, et al. Effect of computerized provider order entry with clinical decision support on adverse drug events in the long-term care setting. Journal of the American Geriatrics Society 2008;56:2225-33. [DOI] [PubMed] [Google Scholar]
Hicks 2008 {published data only}
- Hicks L S, Sequist T D, Ayanian J Z, Shaykevich S, Fairchild D G, Orav E J, et al. Impact of computerized decision support on blood pressure management and control: a randomized controlled trial. J Gen Intern Med 2008;23:429-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2010 {published data only}
- Holt T A, Thorogood M, Griffiths F, Munday S, Friede T, Stables D. Automated electronic reminders to facilitate primary cardiovascular disease prevention: randomised controlled trial. Br J Gen Pract 2010;60:e137-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2017 {published data only}
- Holt T A, Dalton A, Marshall T, Fay M, Qureshi N, Kirkpatrick S, Hislop J, Lasserson D, Kearley K, Mollison J, Yu L M, Hobbs F D, Fitzmaurice D. Automated Software System to Promote Anticoagulation and Reduce Stroke Risk: Cluster-Randomized Controlled Trial. Stroke 2017;48(3):787-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoye 2013 {published data only}
- Hoye S, Gjelstad S, Lindbaek M. Effects on antibiotic dispensing rates of interventions to promote delayed prescribing for respiratory tract infections in primary care. Br J Gen Pract 2013;63:e777-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Judge 2006 {published data only}
- Judge J, Field T S, DeFlorio M, Laprino J, Auger J, Rochon P, et al. Prescribers' responses to alerts during medication ordering in the long term care setting. J Am Med Inform Assoc 2006;13:385-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karlsson 2018 {published data only}
- Karlsson L O, Nilsson S, Bang M, Nilsson L, Charitakis E, Janzon M. A clinical decision support tool for improving adherence to guidelines on anticoagulant therapy in patients with atrial fibrillation at risk of stroke: A cluster-randomized trial in a Swedish primary care setting (the CDS-AF study). PLoS Med 2018;15(3):e1002528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenealy 2005 {published data only}
- Kenealy T, Arroll B, Petrie K J. Patients and computers as reminders to screen for diabetes in family practice. Randomized-controlled trial. J Gen Intern Med 2005;20:916-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krall 2004 {published data only}
- Krall M A, Traunweiser K, Towery W. Effectiveness of an electronic medical record clinical quality alert prepared by off-line data analysis. Stud Health Technol Inform 2004;107:135-9. [PubMed] [Google Scholar]
Kucher 2005 {published data only}
- Kucher N, Koo S, Quiroz R, Cooper J M, Paterno M D, Soukonnikov B, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005;352:969-77. [DOI] [PubMed] [Google Scholar]
Lee 2019 {published data only}
Leibovici 2013 {published data only}
- Leibovici L, Kariv G, Paul M. Long-term survival in patients included in a randomized controlled trial of TREAT, a decision support system for antibiotic treatment. J Antimicrob Chemother 2013;68:2664-6. [DOI] [PubMed] [Google Scholar]
Linder 2009‐1 {published data only}
- Linder J A, Schnipper J L, Tsurikova R, Yu T, Volk L A, Melnikas A J, et al. Documentation-based clinical decision support to improve antibiotic prescribing for acute respiratory infections in primary care: a cluster randomised controlled trial. Inform Prim Care 2009;17:231-40. [DOI] [PubMed] [Google Scholar]
Linder 2009‐2 {published data only}
- Linder J A, Rigotti N A, Schneider L I, Kelley J H, Brawarsky P, Haas J S. An electronic health record-based intervention to improve tobacco treatment in primary care: a cluster-randomized controlled trial. Arch Intern Med 2009;169:781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lo 2009 {published data only}
- Lo H G, Matheny M E, Seger D L, Bates D W, Gandhi T K. Impact of non-interruptive medication laboratory monitoring alerts in ambulatory care. J Am Med Inform Assoc 2009;16:66-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Locatelli 2009 {published data only}
- Locatelli F, Covic A, Macdougall I C, Scherhag A, Wiecek A, Group Orama Study. Effect of computer-assisted European Best Practice Guideline implementation on adherence and target attainment: ORAMA results. J Nephrol 2009;22:662-74. [PubMed] [Google Scholar]
Loo 2011a {published data only}
- Loo T S, Davis R B, Lipsitz L A, Irish J, Bates C K, Agarwal K, et al. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011;171:1552-8. [DOI] [PubMed] [Google Scholar]
Loo 2011b {published data only}
- Loo T S, Davis R B, Lipsitz L A, Irish J, Bates C K, Agarwal K, Markson L, Hamel M B. Electronic medical record reminders and panel management to improve primary care of elderly patients. Arch Intern Med 2011;171(17):1552-8. [DOI] [PubMed] [Google Scholar]
Mann 2016 {published data only}
- Mann D M, Palmisano J, Lin J J. A pilot randomized trial of technology-assisted goal setting to improve physical activity among primary care patients with prediabetes. Prev Med Rep 2016;4:107-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martins 2017 {published data only}
- Martins C M, da Costa Teixeira A S, Azevedo L F, Sa L M, Santos P A, do Couto M L, da Costa Pereira A M, Hespanhol A A, da Costa Santos C M. The effect of a test ordering software intervention on the prescription of unnecessary laboratory tests - a randomized controlled trial. BMC Med Inform Decis Mak 2017;17(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matheny 2008 {published data only}
- Matheny M E, Sequist T D, Seger A C, Fiskio J M, Sperling M, Bugbee D, et al. A randomized trial of electronic clinical reminders to improve medication laboratory monitoring. J Am Med Inform Assoc 2008;15:424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mazzaglia 2016 {published data only}
- Mazzaglia G, Piccinni C, Filippi A, Sini G, Lapi F, Sessa E, Cricelli I, Cutroneo P, Trifiro G, Cricelli C, Caputi A P. Effects of a computerized decision support system in improving pharmacological management in high-risk cardiovascular patients: A cluster-randomized open-label controlled trial. Health Informatics J 2016;22(2):232-47. [DOI] [PubMed] [Google Scholar]
McCowan 2001 {published data only}
- McCowan C, Neville R G, Ricketts I W, Warner F C, Hoskins G, Thomas G E. Lessons from a randomized controlled trial designed to evaluate computer decision support software to improve the management of asthma. Med Inform Internet Med 2001;26:191-201. [DOI] [PubMed] [Google Scholar]
McGinn 2013 {published data only}
- McGinn T G, McCullagh L, Kannry J, Knaus M, Sofianou A, Wisnivesky J P, et al. Efficacy of an evidence-based clinical decision support in primary care practices: a randomized clinical trial. JAMA Intern Med 2013;173:1584-91. [DOI] [PubMed] [Google Scholar]
Meigs 2003 {published data only}
- Meigs James B, Cagliero Enrico, Dubey Anil, Murphy-Sheehy Patricia, Gildesgame Catharyn, Chueh Henry, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care 2003;26:750-7. [DOI] [PubMed] [Google Scholar]
Mertens 2015 {published data only}
- Mertens J R, Chi F W, Weisner C M, Satre D D, Ross T B, Allen S, Pating D, Campbell C I, Lu Y W, Sterling S A. Physician versus non-physician delivery of alcohol screening, brief intervention and referral to treatment in adult primary care: the ADVISe cluster randomized controlled implementation trial. Addict Sci Clin Pract 2015;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2004 {published data only}
- Murray Michael D, Harris Lisa E, Overhage J Marc, Zhou Xiao-Hua, Eckert George J, Smith Faye E, et al. Failure of computerized treatment suggestions to improve health outcomes of outpatients with uncomplicated hypertension: results of a randomized controlled trial. Pharmacotherapy:The Journal of Human Pharmacology & Drug Therapy 2004;24:324-37. [DOI] [PubMed] [Google Scholar]
Myers 2011a {published data only}
- Myers J S, Gojraty S, Yang W, Linsky A, Airan-Javia S, Polomano R C. A randomized-controlled trial of computerized alerts to reduce unapproved medication abbreviation use. J Am Med Inform Assoc 2011;18:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Myers 2011b {published data only}
- Myers J S, Gojraty S, Yang W, Linsky A, Airan-Javia S, Polomano R C. A randomized-controlled trial of computerized alerts to reduce unapproved medication abbreviation use. J Am Med Inform Assoc 2011;18(1):17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Najafi 2019 {published data only}
Nendaz 2010 {published data only}
- Nendaz M R, Chopard P, Lovis C, Kucher N, Asmis L M, Dorffler J, et al. Adequacy of venous thromboprophylaxis in acutely ill medical patients (IMPART): multisite comparison of different clinical decision support systems. Journal of Thrombosis & Haemostasis 2010;8:1230-4. [DOI] [PubMed] [Google Scholar]
Overhage 1996 {published data only}
- Overhage J M, Tierney W M, McDonald C J. Computer reminders to implement preventive care guidelines for hospitalized patients. Arch Intern Med 1996;156:1551-6. [PubMed] [Google Scholar]
Overhage 1997 {published data only}
- Overhage J M, Tierney W M, Zhou X H, McDonald C J. A randomized trial of "corollary orders" to prevent errors of omission. J Am Med Inform Assoc 1997;4:364-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palen 2010 {published data only}
- Palen T E, Price D W, Snyder A J, Shetterly S M. Computerized alert reduced D-dimer testing in the elderly. Am J Manag Care 2010;16:e267-75. [PubMed] [Google Scholar]
Paul 2006 {published data only}
- Paul Mical, Andreassen Steen, Tacconelli Evelina, Nielsen Anders D, Almanasreh Nadja, Frank Uwe, et al. Improving empirical antibiotic treatment using TREAT, a computerized decision support system: cluster randomized trial. Journal of Antimicrobial Chemotherapy 2006;58:1238-45. [DOI] [PubMed] [Google Scholar]
Peiris 2015 {published data only}
- Peiris D, Usherwood T, Panaretto K, Harris M, Hunt J, Redfern J, et al. Effect of a computer-guided, quality improvement program for cardiovascular disease risk management in primary health care: the treatment of cardiovascular risk using electronic decision support cluster-randomized trial. Circ Cardiovasc Qual Outcomes 2015;8:87-95. [DOI] [PubMed] [Google Scholar]
Persell 2016a {published data only}
- Persell S D, Doctor J N, Friedberg M W, Meeker D, Friesema E, Cooper A, Haryani A, Gregory D L, Fox C R, Goldstein N J, Linder J A. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2016b {published data only}
- Persell S D, Doctor J N, Friedberg M W, Meeker D, Friesema E, Cooper A, Haryani A, Gregory D L, Fox C R, Goldstein N J, Linder J A. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2016c {published data only}
- Persell S D, Doctor J N, Friedberg M W, Meeker D, Friesema E, Cooper A, Haryani A, Gregory D L, Fox C R, Goldstein N J, Linder J A. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2016d {published data only}
- Persell S D, Doctor J N, Friedberg M W, Meeker D, Friesema E, Cooper A, Haryani A, Gregory D L, Fox C R, Goldstein N J, Linder J A. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2016e {published data only}
- Persell S D, Doctor J N, Friedberg M W, Meeker D, Friesema E, Cooper A, Haryani A, Gregory D L, Fox C R, Goldstein N J, Linder J A. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2016f {published data only}
- Persell S D, Doctor J N, Friedberg M W, Meeker D, Friesema E, Cooper A, Haryani A, Gregory D L, Fox C R, Goldstein N J, Linder J A. Behavioral interventions to reduce inappropriate antibiotic prescribing: a randomized pilot trial. BMC Infect Dis 2016;16:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 2007 {published data only}
- Peterson J F, Rosenbaum B P, Waitman L R, Habermann R, Powers J, Harrell D, et al. Physicians' response to guided geriatric dosing: initial results from a randomized trial. Stud Health Technol Inform 2007;129:1037-40. [PubMed] [Google Scholar]
Piazza 2019 {published data only}
Player 2010 {published data only}
- Player M S, Gill J M, Mainous A G 3rd, Everett C J, Koopman R J, Diamond J J, et al. An electronic medical record-based intervention to improve quality of care for gastro-esophageal reflux disease (GERD) and atypical presentations of GERD. Qual Prim Care 2010;18:223-9. [PubMed] [Google Scholar]
Price 2017 {published data only}
- Price M, Davies I, Rusk R, Lesperance M, Weber J. Applying STOPP Guidelines in Primary Care Through Electronic Medical Record Decision Support: Randomized Control Trial Highlighting the Importance of Data Quality. JMIR Med Inform 2017;5(2):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ronda 2018 {published data only}
Rothschild 2007 {published data only}
- Rothschild Jeffrey M, McGurk Siobhan, Honour Melissa, Lu Linh, McClendon Aubre A, Srivastava Priya, et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion 2007;47:228-39. [DOI] [PubMed] [Google Scholar]
Safran 1995 {published data only}
- Safran C, Rind DM, Davis RB, Sands DZ, Caraballo E, Rippel K, et al. A clinical trial of a knowledge-based medical record. Medinfo 1995;8 Pt 2:1076-80. [PubMed] [Google Scholar]
Schnipper 2010 {published data only}
- Schnipper J L, Linder J A, Palchuk M B, Yu D T, McColgan K E, Volk L A, et al. Effects of documentation-based decision support on chronic disease management. Am J Manag Care 2010;16:SP72-81. [PubMed] [Google Scholar]
Schriefer 2009 {published data only}
- Schriefer S P, Landis S E, Turbow D J, Patch S C. Effect of a computerized body mass index prompt on diagnosis and treatment of adult obesity. Fam Med 2009;41:502-7. [PubMed] [Google Scholar]
Sequist 2005 {published data only}
- Sequist T D, Gandhi T K, Karson A S, Fiskio J M, Bugbee D, Sperling M, et al. A randomized trial of electronic clinical reminders to improve quality of care for diabetes and coronary artery disease. J Am Med Inform Assoc 2005;12:431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sequist 2009 {published data only}
- Sequist T D, Zaslavsky A M, Marshall R, Fletcher R H, Ayanian J Z. Patient and physician reminders to promote colorectal cancer screening: a randomized controlled trial. Arch Intern Med 2009;169:364-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sequist 2011 {published data only}
- Sequist Thomas D, Zaslavsky Alan M, Colditz Graham A, Ayanian John Z. Electronic patient messages to promote colorectal cancer screening: a randomized controlled trial. Archives of Internal Medicine 2011;171:636-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sequist 2018a {published data only}
- Sequist T D, Holliday A M, Orav E J, Bates D W, Denker B M. Physician and patient tools to improve chronic kidney disease care. Am J Manag Care 2018;24(4):e107-e114. [PubMed] [Google Scholar]
Sequist 2018b {published data only}
- Sequist T D, Holliday A M, Orav E J, Bates D W, Denker B M. Physician and patient tools to improve chronic kidney disease care. Am J Manag Care 2018;24(4):e107-e114. [PubMed] [Google Scholar]
Silbernagel 2016 {published data only}
- Silbernagel G, Spirk D, Hager A, Baumgartner I, Kucher N. Electronic Alert System for Improving Stroke Prevention Among Hospitalized Oral-Anticoagulation-Naive Patients With Atrial Fibrillation: A Randomized Trial. J Am Heart Assoc 2016;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2012 {published data only}
- Smith J R, Noble M J, Musgrave S, Murdoch J, Price G M, Barton G R, et al. The at-risk registers in severe asthma (ARRISA) study: a cluster-randomised controlled trial examining effectiveness and costs in primary care. Thorax 2012;67:1052-60. [DOI] [PubMed] [Google Scholar]
Spirk 2017 {published data only}
- Spirk D, Stuck A K, Hager A, Engelberger R P, Aujesky D, Kucher N. Electronic alert system for improving appropriate thromboprophylaxis in hospitalized medical patients: a randomized controlled trial. J Thromb Haemost 2017;15(11):2138-2146. [DOI] [PubMed] [Google Scholar]
Stockwell 2015 {published data only}
- Stockwell M S, Catallozzi M, Camargo S, Ramakrishnan R, Holleran S, Findley S E, et al. Registry-linked electronic influenza vaccine provider reminders: a cluster-crossover trial. Pediatrics 2015;135:e75-82. [DOI] [PubMed] [Google Scholar]
Strom 2010 {published data only}
- Strom B L, Schinnar R, Aberra F, Bilker W, Hennessy S, Leonard C E, et al. Unintended effects of a computerized physician order entry nearly hard-stop alert to prevent a drug interaction: a randomized controlled trial. Arch Intern Med 2010;170:1578-83. [DOI] [PubMed] [Google Scholar]
Szilagyi 2015 {published data only}
- Szilagyi P G, Serwint J R, Humiston S G, Rand C M, Schaffer S, Vincelli P, et al. Effect of provider prompts on adolescent immunization rates: a randomized trial. Acad Pediatr 2015;15:149-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2003 {published data only}
- Tamblyn R, Huang A, Perreault R, Jacques A, Roy D, Hanley J, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ 2003;169:549-56. [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2010 {published data only}
- Tamblyn R, Reidel K, Huang A, Taylor L, Winslade N, Bartlett G, et al. Increasing the detection and response to adherence problems with cardiovascular medication in primary care through computerized drug management systems: a randomized controlled trial. Med Decis Making 2010;30:176-88. [DOI] [PubMed] [Google Scholar]
Tamblyn 2015 {published data only}
- Tamblyn R, Ernst P, Winslade N, Huang A, Grad R, Platt R W, Ahmed S, Moraga T, Eguale T. Evaluating the impact of an integrated computer-based decision support with person-centered analytics for the management of asthma in primary care: a randomized controlled trial. J Am Med Inform Assoc 2015;22(4):773-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2018a {published data only}
- Tamblyn R, Winslade N, Qian C J, Moraga T, Huang A. What is in your wallet? A cluster randomized trial of the effects of showing comparative patient out-of-pocket costs on primary care prescribing for uncomplicated hypertension. Implement Sci 2018;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2018b {published data only}
- Tamblyn R, Winslade N, Qian C J, Moraga T, Huang A. What is in your wallet? A cluster randomized trial of the effects of showing comparative patient out-of-pocket costs on primary care prescribing for uncomplicated hypertension. Implement Sci 2018;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2012 {published data only}
- Tang Joyce W, Kushner Robert F, Cameron Kenzie A, Hicks Brent, Cooper Andrew J, Baker David W. Electronic tools to assist with identification and counseling for overweight patients: a randomized controlled trial. Journal of General Internal Medicine 2012;27:933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taveras 2014 {published data only}
- Taveras E M, Marshall R, Horan C M, Gillman M W, Hacker K, Kleinman K P, et al. Improving children's obesity-related health care quality: process outcomes of a cluster-randomized controlled trial. Obesity (Silver Spring) 2014;22:27-31. [DOI] [PubMed] [Google Scholar]
Taveras 2015a {published data only}
- Taveras E M, Marshall R, Kleinman K P, Gillman M W, Hacker K, Horan C M, Smith R L, Price S, Sharifi M, Rifas-Shiman S L, Simon S R. Comparative effectiveness of childhood obesity interventions in pediatric primary care: a cluster-randomized clinical trial. JAMA Pediatr 2015;169(6):535-42. [DOI] [PubMed] [Google Scholar]
Taveras 2015b {published data only}
- Taveras E M, Marshall R, Kleinman K P, Gillman M W, Hacker K, Horan C M, Smith R L, Price S, Sharifi M, Rifas-Shiman S L, Simon S R. Comparative effectiveness of childhood obesity interventions in pediatric primary care: a cluster-randomized clinical trial. JAMA Pediatr 2015;169(6):535-42. [DOI] [PubMed] [Google Scholar]
Terrell 2009 {published data only}
- Terrell K M, Perkins A J, Dexter P R, Hui S L, Callahan C M, Miller D K. Computerized decision support to reduce potentially inappropriate prescribing to older emergency department patients: a randomized, controlled trial. J Am Geriatr Soc 2009;57:1388-94. [DOI] [PubMed] [Google Scholar]
Terrell 2010 {published data only}
- Terrell Kevin M, Perkins Anthony J, Hui Siu L, Callahan Christopher M, Dexter Paul R, Miller Douglas K. Computerized decision support for medication dosing in renal insufficiency: a randomized, controlled trial. Annals of Emergency Medicine 2010;56:623-9. [DOI] [PubMed] [Google Scholar]
Tierney 2003 {published data only}
- Tierney W M, Overhage J M, Murray M D, Harris L E, Zhou X H, Eckert G J, et al. Effects of computerized guidelines for managing heart disease in primary care. J Gen Intern Med 2003;18:967-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2005 {published data only}
- Tierney W M, Overhage J M, Murray M D, Harris L E, Zhou X H, Eckert G J, et al. Can computer-generated evidence-based care suggestions enhance evidence-based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Serv Res 2005;40:477-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trick 2009 {published data only}
- Trick W E, Das K, Gerard M N, Charles-Damte M, Murphy G, Benson I, et al. Clinical trial of standing-orders strategies to increase the inpatient influenza vaccination rate. Infect Control Hosp Epidemiol 2009;30:86-8. [DOI] [PubMed] [Google Scholar]
Van Wyk 2008a {published data only}
- Wyk J T, Wijk M A, Sturkenboom M C, Mosseveld M, Moorman P W, Lei J. Electronic alerts versus on-demand decision support to improve dyslipidemia treatment: a cluster randomized controlled trial. Circulation 2008;117:371-8. [DOI] [PubMed] [Google Scholar]
Van Wyk 2008b {published data only}
- Wyk J T, Wijk M A, Sturkenboom M C, Mosseveld M, Moorman P W, Lei J. Electronic alerts versus on-demand decision support to improve dyslipidemia treatment: a cluster randomized controlled trial. Circulation 2008;117(3):371-8. [DOI] [PubMed] [Google Scholar]
Walker 2010 {published data only}
- Walker J, Fairley C K, Walker S M, Gurrin L C, Gunn J M, Pirotta M V, et al. Computer reminders for Chlamydia screening in general practice: a randomized controlled trial. Sex Transm Dis 2010;37:445-50. [DOI] [PubMed] [Google Scholar]
Wexler 2010 {published data only}
- Wexler D J, Shrader P, Burns S M, Cagliero E. Effectiveness of a computerized insulin order template in general medical inpatients with type 2 diabetes: a cluster randomized trial. Diabetes Care 2010;33:2181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkinson 2019 {published data only}
Wright 2012 {published data only}
- Wright A, Pang J, Feblowitz J C, Maloney F L, Wilcox A R, McLoughlin K S, et al. Improving completeness of electronic problem lists through clinical decision support: a randomized, controlled trial. J Am Med Inform Assoc 2012;19:555-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2018 {published data only}
- Wu Y, Chen Y, Li S, Dong W, Liang H, Deng M, Chen Y, Chen S, Liang X. Value of electronic alerts for acute kidney injury in high-risk wards: a pilot randomized controlled trial. Int Urol Nephrol 2018;50(8):1483-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanetti 2003 {published data only}
- Zanetti G, Flanagan H L Jr, Cohn L H, Giardina R, Platt R. Improvement of intraoperative antibiotic prophylaxis in prolonged cardiac surgery by automated alerts in the operating room. Infect Control Hosp Epidemiol 2003;24:13-6. [DOI] [PubMed] [Google Scholar]
Zera 2015 {published data only}
- Zera C A, Bates D W, Stuebe A M, Ecker J L, Seely E W. Diabetes Screening Reminder for Women With Prior Gestational Diabetes: A Randomized Controlled Trial. Obstet Gynecol 2015;126(1):109-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Acevedo 2018 {published data only}
- Acevedo A, Lee MT, Garnick DW, Horgan CM, Ritter GA, Panas L, et al. Agency-level financial incentives and electronic reminders to improve continuity of care after discharge from residential treatment and detoxification. Drug and alcohol dependence 2018;183:192-200. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adelman 2013 {published data only}
- Adelman J S, Kalkut G E, Schechter C B, Weiss J M, Berger M A, Reissman S H, et al. Understanding and preventing wrong-patient electronic orders: a randomized controlled trial. J Am Med Inform Assoc 2013;20(2):305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2016 {published data only}
- Allen KD, Yancy WS Jr, Bosworth HB, Coffman CJ, Jeffreys AS, Datta SK, et al. A Combined Patient and Provider Intervention for Management of Osteoarthritis in Veterans: A Randomized Clinical Trial. Annals of internal medicine 2016;164(2):73-83. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
ALMohiza 2016 {published data only}
- ALMohiza MA, Sparto PJ, Marchetti GF, Delitto A, Furman JM, Miller DL, et al. A Quality Improvement Project in Balance and Vestibular Rehabilitation and Its Effect on Clinical Outcomes. Journal of neurologic physical therapy : JNPT 2016;40(2):90-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Anchala 2015 {published data only}
- Anchala R, Kaptoge S, Pant H, Di Angelantonio E, Franco OH, Prabhakaran D. Evaluation of effectiveness and cost-effectiveness of a clinical decision support system in managing hypertension in resource constrained primary health care settings: results from a cluster randomized trial. Journal of the American Heart Association 2015;4(1):e001213. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Apkon 2005a {published data only}
- Apkon M, Mattera J A, Lin Z, Herrin J, Bradley E H, Carbone M, et al. A randomized outpatient trial of a decision-support information technology tool. Arch Intern Med 2005;165(20):2388-94. [DOI] [PubMed] [Google Scholar]
Åsberg 2010 {published data only}
- Åsberg A, Falck P, Undset L H, Dorje C, Holdaas H, Hartmann A, et al. Computer-assisted cyclosporine dosing performs better than traditional dosing in renal transplant recipients: results of a pilot study. Ther Drug Monit 2010;32(2):152-8. [DOI] [PubMed] [Google Scholar]
Barkun 2013 {published data only}
- Barkun A N, Bhat M, Armstrong D, Dawes M, Donner A, Enns R, et al. Effectiveness of disseminating consensus management recommendations for ulcer bleeding: a cluster randomized trial. CMAJ 2013;185(3):E156-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 2015 {published data only}
- Beck JR, Fung K, Lopez H 2nd, Mongero LB, Argenziano M. Real-time data acquisition and alerts may reduce reaction time and improve perfusionist performance during cardiopulmonary bypass. Perfusion 2015;30(1):41-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Beeckman 2013 {published data only}
- Beeckman D, Clays E, Van Hecke A, Vanderwee K, Schoonhoven L, Verhaeghe S. A multi-faceted tailored strategy to implement an electronic clinical decision support system for pressure ulcer prevention in nursing homes: a two-armed randomized controlled trial. Int J Nurs Stud 2013;50(4):475-86. [DOI] [PubMed] [Google Scholar]
Bernacki 2015 {published data only}
- Bernacki R, Hutchings M, Vick J, Smith G, Paladino J, Lipsitz S, et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ open 2015;5(10):e009032. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhardwaja 2011 {published data only}
- Bhardwaja B, Carroll N M, Raebel M A, Chester E A, Korner E J, Rocho B E, et al. Improving prescribing safety in patients with renal insufficiency in the ambulatory setting: the Drug Renal Alert Pharmacy (DRAP) program. Pharmacotherapy 2011;31(4):346-56. [DOI] [PubMed] [Google Scholar]
Bindels 2004 {published data only}
- Bindels R, Hasman A, Wersch J W, Talmon J, Winkens R A. Evaluation of an automated test ordering and feedback system for general practitioners in daily practice. Int J Med Inform 2004;73(9-10):705-12. [DOI] [PubMed] [Google Scholar]
Biswas 2018 {published data only}
- Biswas A, Parikh CR, Feldman HI, Garg AX, Latham S, Lin H, et al. Identification of Patients Expected to Benefit from Electronic Alerts for Acute Kidney Injury. Clinical journal of the American Society of Nephrology : CJASN 2018;13(6):842-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bosworth 2009 {published data only}
- Bosworth H B, Olsen M K, Dudley T, Orr M, Goldstein M K, Datta S K, et al. Patient education and provider decision support to control blood pressure in primary care: a cluster randomized trial. Am Heart J 2009;157(3):450-6. [DOI] [PubMed] [Google Scholar]
Caballero‐Ruiz 2017 {published data only}
- Caballero-Ruiz E, Garcia-Saez G, Rigla M, Villaplana M, Pons B, Hernando ME. A web-based clinical decision support system for gestational diabetes: Automatic diet prescription and detection of insulin needs. International journal of medical informatics 2017;102:35-49. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cannon 2000 {published data only}
- Cannon D S, Allen S N. A comparison of the effects of computer and manual reminders on compliance with a mental health clinical practice guideline. J Am Med Inform Assoc 2000;7(2):196-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chien 2017 {published data only}
- Chien AT, Lehmann LS, Hatfield LA, Koplan KE, Petty CR, Sinaiko AD, et al. A Randomized Trial of Displaying Paid Price Information on Imaging Study and Procedure Ordering Rates. Journal of general internal medicine 2017;32(4):434-48. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2016 {published data only}
- Clarke S, Wilson ML, Terhaar M. Using Clinical Decision Support and Dashboard Technology to Improve Heart Team Efficiency and Accuracy in a Transcatheter Aortic Valve Implantation (TAVI) Program. Studies in health technology and informatics 2016;225:98-102. [PMID: ] [PubMed] [Google Scholar]
Collins 2018 {published data only}
- Collins BN, Lepore SJ, Winickoff JP, Nair US, Moughan B, Bryant-Stephens T, et al. An Office-Initiated Multilevel Intervention for Tobacco Smoke Exposure: A Randomized Trial. Pediatrics 2018;141(Suppl 1):S75-86. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colpaert 2012 {published data only}
- Colpaert K, Hoste E A, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, et al. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med 2012;40(4):1164-70. [DOI] [PubMed] [Google Scholar]
Crosson 2012 {published data only}
- Crosson J C, Ohman-Strickland P A, Cohen D J, Clark E C, Crabtree B F. Typical electronic health record use in primary care practices and the quality of diabetes care. Ann Fam Med 2012;10(3):221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curran 2010 {published data only}
- Curran K, Nichols E, Xie E, Harper R. An intensive insulinotherapy mobile phone application built on artificial intelligence techniques. J Diabetes Sci Technol 2010;4(1):209-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Curtain 2011 {published data only}
- Curtain C, Peterson G M, Tenni P, Bindoff I K, Williams M. Outcomes of a decision support prompt in community pharmacy-dispensing software to promote step-down of proton pump inhibitor therapy. Br J Clin Pharmacol 2011;71(5):780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dekarske 2015 {published data only}
- Dekarske BM, Zimmerman CR, Chang R, Grant PJ, Chaffee BW. Increased appropriateness of customized alert acknowledgement reasons for overridden medication alerts in a computerized provider order entry system. International journal of medical informatics 2015;84(12):1085-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dexter 2004 {published data only}
- Dexter P R, Perkins S M, Maharry K S, Jones K, McDonald C J. Inpatient computer-based standing orders vs physician reminders to increase influenza and pneumococcal vaccination rates: a randomized trial. JAMA 2004;292(19):2366-71. [DOI] [PubMed] [Google Scholar]
Dixon 2017 {published data only}
- Dixon BE, Boockvar KS. Event Notification in Support of Population Health: The Promise and Challenges from a Randomized Controlled Trial. Studies in health technology and informatics 2017;245:1357. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Dragan 2015 {published data only}
- Dragan IF, Newman M, Stark P, Steffensen B, Karimbux N. Using a Simulated Infobutton Linked to an Evidence-Based Resource to Research Drug-Drug Interactions: A Pilot Study with Third-Year Dental Students. Journal of dental education 2015;79(11):1349-55. [PMID: ] [PubMed] [Google Scholar]
Duffy 2016 {published data only}
- Duffy SA, Ronis DL, Ewing LA, Waltje AH, Hall SV, Thomas PL, et al. Implementation of the Tobacco Tactics intervention versus usual care in Trinity Health community hospitals. Implementation science : IS 2016;11(1):147. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dumont 2012 {published data only}
- Dumont C, Bourguignon C. Effect of a computerized insulin dose calculator on the process of glycemic control. Am J Crit Care 2012;21(2):106-15. [DOI] [PubMed] [Google Scholar]
Durieux 2000 {published data only}
- Durieux P, Nizard R, Ravaud P, Mounier N, Lepage E. A clinical decision support system for prevention of venous thromboembolism: effect on physician behavior. JAMA 2000;283(21):2816-21. [DOI] [PubMed] [Google Scholar]
Dykes 2010 {published data only}
- Dykes P C, Carroll D L, Hurley A, Lipsitz S, Benoit A, Chang F, et al. Fall prevention in acute care hospitals: a randomized trial. JAMA 2010;304(17):1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmiston 2016 {published data only}
- Edmiston CE, Krepel CJ, Spencer MP, Ferraz AA, Seabrook GR, Lee CJ, et al. Preadmission Application of 2% Chlorhexidine Gluconate (CHG): Enhancing Patient Compliance While Maximizing Skin Surface Concentrations. Infection control and hospital epidemiology 2016;37(3):254-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Eisenstein 2011 {published data only}
- Eisenstein E L, Kawamoto K, Anstrom K J, Willis J M, Silvey G M, Johnson F S, et al. Clinical and economic results from a randomized trial of clinical decision support in a rural health network. Stud Health Technol Inform 2011;164:77-81. [PubMed] [Google Scholar]
Elliott 2017 {published data only}
- Elliott LS, Henderson JC, Neradilek MB, Moyer NA, Ashcraft KC, Thirumaran RK. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: A prospective pilot randomized controlled trial. PloS one 2017;12(2):e0170905. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldstein 2006 {published data only}
- Feldstein A, Elmer P J, Smith D H, Herson M, Orwoll E, Chen C, et al. Electronic medical record reminder improves osteoporosis management after a fracture: a randomized, controlled trial. J Am Geriatr Soc 2006;54(3):450-7. [DOI] [PubMed] [Google Scholar]
Fitzgerald 2011 {published data only}
- Fitzgerald M, Cameron P, Mackenzie C, Farrow N, Scicluna P, Gocentas R, et al. Trauma resuscitation errors and computer-assisted decision support. Arch Surg 2011;146(2):218-25. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2017 {published data only}
- Fitzpatrick SL, Dickins K, Avery E, Ventrelle J, Shultz A, Kishen E, et al. Effect of an obesity best practice alert on physician documentation and referral practices. Translational behavioral medicine 2017;7(4):881-90. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flamm 2013 {published data only}
- Flamm M, Fritsch G, Hysek M, Klausner S, Entacher K, Panisch S, et al. Quality improvement in preoperative assessment by implementation of an electronic decision support tool. J Am Med Inform Assoc 2013;20(e1):e91-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flanagan 1999 {published data only}
- Flanagan J R, Doebbeling B N, Dawson J, Beekmann S. Randomized study of online vaccine reminders in adult primary care. Proc AMIA Symp 1999:755-9. [PMC free article] [PubMed] [Google Scholar]
Foy 2011 {published data only}
- Foy R, Eccles M P, Hrisos S, Hawthorne G, Steen N, Gibb I, et al. A cluster randomised trial of educational messages to improve the primary care of diabetes. Implement Sci 2011;6:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freundlich 2013 {published data only}
- Freundlich R E, Barnet C S, Mathis M R, Shanks A M, Tremper K K, Kheterpal S. A randomized trial of automated electronic alerts demonstrating improved reimbursable anesthesia time documentation. J Clin Anesth 2013;25(2):110-4. [DOI] [PubMed] [Google Scholar]
Fricton 2011 {published data only}
- Fricton J, Rindal D B, Rush W, Flottemesch T, Vazquez G, Thoele M J, et al. The effect of electronic health records on the use of clinical care guidelines for patients with medically complex conditions. J Am Dent Assoc 2011;142(10):1133-42. [DOI] [PubMed] [Google Scholar]
Gallagher 2016 {published data only}
- Gallagher J, O'Sullivan D, McCarthy S, Gillespie P, Woods N, O'Mahony D, et al. Structured Pharmacist Review of Medication in Older Hospitalised Patients: A Cost-Effectiveness Analysis. Drugs & aging 2016;33(4):285-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goetz 2013 {published data only}
- Goetz M B, Hoang T, Knapp H, Burgess J, Fletcher M D, Gifford A L, et al. Central implementation strategies outperform local ones in improving HIV testing in Veterans Healthcare Administration facilities. J Gen Intern Med 2013;28(10):1311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grace 2011 {published data only}
- Grace S L, Russell K L, Reid R D, Oh P, Anand S, Rush J, et al. Effect of cardiac rehabilitation referral strategies on utilization rates: a prospective, controlled study. Arch Intern Med 2011;171(3):235-41. [DOI] [PubMed] [Google Scholar]
Hagiwara 2013 {published data only}
- Hagiwara M A, Sjoqvist B A, Lundberg L, Suserud B O, Henricson M, Jonsson A. Decision support system in prehospital care: a randomized controlled simulation study. Am J Emerg Med 2013;31(1):145-53. [DOI] [PubMed] [Google Scholar]
Hains 2012 {published data only}
- Hains I M, Ward R L, Pearson S A. Implementing a web-based oncology protocol system in Australia: evaluation of the first 3 years of operation. Intern Med J 2012;42(1):57-64. [DOI] [PubMed] [Google Scholar]
Harpole 1997 {published data only}
- Harpole L H, Khorasani R, Fiskio J, Kuperman G J, Bates D W. Automated evidence-based critiquing of orders for abdominal radiographs: impact on utilization and appropriateness. J Am Med Inform Assoc 1997;4(6):511-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heiman 2004 {published data only}
- Heiman H, Bates D W, Fairchild D, Shaykevich S, Lehmann L S. Improving completion of advance directives in the primary care setting: a randomized controlled trial. Am J Med 2004;117(5):318-24. [DOI] [PubMed] [Google Scholar]
Herasevich 2011 {published data only}
- Herasevich V, Pieper M S, Pulido J, Gajic O. Enrollment into a time sensitive clinical study in the critical care setting: results from computerized septic shock sniffer implementation. J Am Med Inform Assoc 2011;18(5):639-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holmes 2015 {published data only}
- Holmes J F, Freilich J, Taylor S L, Buettner D. Electronic alerts for triage protocol compliance among emergency department triage nurses: a randomized controlled trial. Nurs Res 2015;64(3):226-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hooper 2012 {published data only}
- Hooper M H, Weavind L, Wheeler A P, Martin J B, Gowda S S, Semler M W, et al. Randomized trial of automated, electronic monitoring to facilitate early detection of sepsis in the intensive care unit*. Crit Care Med 2012;40(7):2096-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 2011 {published data only}
- Humphrey L L, Shannon J, Partin M R, O'Malley J, Chen Z, Helfand M. Improving the follow-up of positive hemoccult screening tests: an electronic intervention. J Gen Intern Med 2011;26(7):691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ignatov 2016 {published data only}
- Ignatov PN, Lutomski JE. Quantitative cardiotocography to improve fetal assessment during labor: a preliminary randomized controlled trial. European journal of obstetrics, gynecology, and reproductive biology 2016;205:91-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
James 1993 {published data only}
- James A H, Britt R P. Prospective comparative study of computer programs used for management of warfarin. J Clin Pathol 1993;46(8):781. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2015 {published data only}
- James MT, Garg AX. Acute kidney injury: Do electronic alerts for AKI improve outcomes? Nature reviews. Nephrology 2015;11(6):322-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Johnson 2010 {published data only}
- Johnson K B, Ho Y X, Cala C M, Davison C. Showing Your Work: Impact of annotating electronic prescriptions with decision support results. J Biomed Inform 2010;43(2):321-5. [DOI] [PubMed] [Google Scholar]
Keitel 2017 {published data only}
- Keitel K, Kagoro F, Samaka J, Masimba J, Said Z, Temba H, et al. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): A randomized, controlled non-inferiority trial. PLoS medicine 2017;14(10):e1002411. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2017 {published data only}
- Kim JM, Rivera M, Persing N, Bundy DG, Psoter KJ, Ghazarian SR, et al. Electronic Immunization Alerts and Spillover Effects on Other Preventive Care. Clinical pediatrics 2017;56(9):811-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kollef 2014 {published data only}
- Kollef M H, Chen Y, Heard K, LaRossa G N, Lu C, Martin N R, et al. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med 2014;9(7):424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kostopoulou 2015 {published data only}
- Kostopoulou O, Lionis C, Angelaki A, Ayis S, Durbaba S, Delaney BC. Early diagnostic suggestions improve accuracy of family physicians: a randomized controlled trial in Greece. Family practice 2015;32(3):323-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kralj 2003 {published data only}
- Kralj B, Iverson D, Hotz K, Ashbury F D. The impact of computerized clinical reminders on physician prescribing behavior: evidence from community oncology practice. Am J Med Qual 2003;18(5):197-203. [DOI] [PubMed] [Google Scholar]
Kuhn 2015 {published data only}
- Kuhn L, Reeves K, Taylor Y, Tapp H, McWilliams A, Gunter A, et al. Planning for Action: The Impact of an Asthma Action Plan Decision Support Tool Integrated into an Electronic Health Record (EHR) at a Large Health Care System. Journal of the American Board of Family Medicine : JABFM 2015;28(3):382-93. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kurian 2009 {published data only}
- Kurian B T, Trivedi M H, Grannemann B D, Claassen C A, Daly E J, Sunderajan P. A computerized decision support system for depression in primary care. Prim Care Companion J Clin Psychiatry 2009;11(4):140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee N J, Chen E S, Currie L M, Donovan M, Hall E K, Jia H, et al. The effect of a mobile clinical decision support system on the diagnosis of obesity and overweight in acute and primary care encounters. ANS Adv Nurs Sci 2009;32(3):211-21. [DOI] [PubMed] [Google Scholar]
Lee 2016 {published data only}
- Lee P, Liu JC, Hsieh MH, Hao WR, Tseng YT, Liu SH, et al. Cloud-based BP system integrated with CPOE improves self-management of the hypertensive patients: A randomized controlled trial. Computer methods and programs in biomedicine 2016;132:105-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Luders 2010 {published data only}
- Luders S, Schrader J, Schmieder R E, Smolka W, Wegscheider K, Bestehorn K. Improvement of hypertension management by structured physician education and feedback system: cluster randomized trial. Eur J Cardiovasc Prev Rehabil 2010;17(3):271-9. [DOI] [PubMed] [Google Scholar]
Luna 2017 {published data only}
- Luna DR, Rizzato Lede DA, Rubin L, Otero CM, Ortiz JM, Garcia MG, et al. User-Centered Design Improves the Usability of Drug-Drug Interaction Alerts: A Validation Study in the Real Scenario. Studies in health technology and informatics 2017;245:1085-9. [PMID: ] [PubMed] [Google Scholar]
Magnus 2012 {published data only}
- Magnus M, Herwehe J, Gruber D, Wilbright W, Shepard E, Abrams A, et al. Improved HIV-related outcomes associated with implementation of a novel public health information exchange. Int J Med Inform 2012;81(10):E30-8. [DOI] [PubMed] [Google Scholar]
Mainous 2013 {published data only}
- Mainous A G 3rd, Lambourne C A, Nietert P J. Impact of a clinical decision support system on antibiotic prescribing for acute respiratory infections in primary care: quasi-experimental trial. J Am Med Inform Assoc 2013;20(2):317-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 2011 {published data only}
- Mann E A, Jones J A, Wolf S E, Wade C E. Computer decision support software safely improves glycemic control in the burn intensive care unit: a randomized controlled clinical study. J Burn Care Res 2011;32(2):246-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manns 2012 {published data only}
- Manns B, Tonelli M, Culleton B, Faris P, McLaughlin K, Chin R, et al. A cluster randomized trial of an enhanced eGFR prompt in chronic kidney disease. Clin J Am Soc Nephrol 2012;7(4):565-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martens 2007 {published data only}
- Martens J D, Weijden T, Severens J L, Clercq P A, Bruijn D P, Kester A D, et al. The effect of computer reminders on GPs' prescribing behaviour: a cluster-randomised trial. Int J Med Inform 2007;76 Suppl 3:S403-16. [DOI] [PubMed] [Google Scholar]
Martí 2017 {published data only}
- Martí I R, Allepuz A, Palomar G R, Font F O, Cera M S. Impact of an intervention on the prescription of aliskiren after new evidence on safety reported. Pharmacoepidemiology and drug safety 2017;26(1):91-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Martinez 2018 {published data only}
- Martinez B, Ixen EC, Hall-Clifford R, Juarez M, Miller AC, Francis A, et al. mHealth intervention to improve the continuum of maternal and perinatal care in rural Guatemala: a pragmatic, randomized controlled feasibility trial. Reproductive health 2018;15(1):120. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayne 2014 {published data only}
- Mayne SL, duRivage NE, Feemster KA, Localio AR, Grundmeier RW, Fiks AG. Effect of decision support on missed opportunities for human papillomavirus vaccination. American journal of preventive medicine 2014;47(6):734-44. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McAvoy 2013 {published data only}
- McAvoy B R. Computer-generated reminders influence professional practice. Br J Gen Pract 2013;63(611):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCormick 2016 {published data only}
- McCormick PJ, Levin MA, Lin HM, Sessler DI, Reich DL. Effectiveness of an Electronic Alert for Hypotension and Low Bispectral Index on 90-day Postoperative Mortality: A Prospective, Randomized Trial. Anesthesiology 2016;125(6):1113-20. [PMID: ] [DOI] [PubMed] [Google Scholar]
McDonald 1992 {published data only}
- McDonald C J, Hui S L, Tierney W M. Effects of computer reminders for influenza vaccination on morbidity during influenza epidemics. MD Comput 1992;9(5):304-12. [PubMed] [Google Scholar]
McGreevey 2013 {published data only}
- McGreevey J D 3rd. Order sets in electronic health records: principles of good practice. Chest 2013;143(1):228-35. [DOI] [PubMed] [Google Scholar]
McGregor 2006 {published data only}
- McGregor J C, Weekes E, Forrest G N, Standiford H C, Perencevich E N, Furuno J P, et al. Impact of a computerized clinical decision support system on reducing inappropriate antimicrobial use: a randomized controlled trial. J Am Med Inform Assoc 2006;13(4):378-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2016 {published data only}
- Mehta R, Radhakrishnan NS, Warring CD, Jain A, Fuentes J, Dolganiuc A, et al. The Use of Evidence-Based, Problem-Oriented Templates as a Clinical Decision Support in an Inpatient Electronic Health Record System. Applied clinical informatics 2016;7(3):790-802. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Montgomery 2000 {published data only}
- Montgomery A A, Fahey T, Peters T J, MacIntosh C, Sharp D J. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial. BMJ 2000;320(7236):686-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muth 2018 {published data only}
- Muth C, Uhlmann L, Haefeli WE, Rochon J, den Akker M, Perera R, et al. Effectiveness of a complex intervention on Prioritising Multimedication in Multimorbidity (PRIMUM) in primary care: results of a pragmatic cluster randomised controlled trial. BMJ open 2018;8(2):e017740. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieuwlaat 2012 {published data only}
- Nieuwlaat R, Hubers L M, Spyropoulos A C, Eikelboom J W, Connolly B J, Van Spall H G, et al. Randomised comparison of a simple warfarin dosing algorithm versus a computerised anticoagulation management system for control of warfarin maintenance therapy. Thromb Haemost 2012;108(6):1228-35. [DOI] [PubMed] [Google Scholar]
Ornstein 1991 {published data only}
- Ornstein S M, Garr D R, Jenkins R G, Rust P F, Arnon A. Computer-generated physician and patient reminders. Tools to improve population adherence to selected preventive services. J Fam Pract 1991;32(1):82-90. [PubMed] [Google Scholar]
Palen 2006 {published data only}
- Palen T E, Raebel M, Lyons E, Magid D M. Evaluation of laboratory monitoring alerts within a computerized physician order entry system for medication orders. Am J Manag Care 2006;12(7):389-95. [PubMed] [Google Scholar]
Pang 2015 {published data only}
- Pang C L, Chanouzas D, Thomas M, Baharani J. Improving Acute Kidney Injury (AKI) outcomes through the use of automated electronic alerts. Eur J Intern Med 2015;26(1):73. [DOI] [PubMed] [Google Scholar]
Panjasawatwong 2015 {published data only}
- Panjasawatwong K, Sessler DI, Stapelfeldt WH, Mayers DB, Mascha EJ, Yang D, et al. A Randomized Trial of a Supplemental Alarm for Critically Low Systolic Blood Pressure. Anesthesia and analgesia 2015;121(6):1500-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Peremans 2010 {published data only}
- Peremans L, Rethans J J, Verhoeven V, Coenen S, Debaene L, Meulemans H, et al. Empowering patients or general practitioners? A randomised clinical trial to improve quality in reproductive health care in Belgium. Eur J Contracept Reprod Health Care 2010;15(4):280-9. [DOI] [PubMed] [Google Scholar]
Pielmeier 2012 {published data only}
- Pielmeier U, Rousing M L, Andreassen S, Nielsen B S, Haure P. Decision support for optimized blood glucose control and nutrition in a neurotrauma intensive care unit: preliminary results of clinical advice and prediction accuracy of the Glucosafe system. J Clin Monit Comput 2012;26(4):319-28. [DOI] [PubMed] [Google Scholar]
Poller 1993 {published data only}
- Poller L, Wright D, Rowlands M. Prospective comparative study of computer programs used for management of warfarin. J Clin Pathol 1993;46(4):299-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raebel 2007 {published data only}
- Raebel M A, Charles J, Dugan J, Carroll N M, Korner E J, Brand D W, et al. Randomized trial to improve prescribing safety in ambulatory elderly patients. J Am Geriatr Soc 2007;55(7):977-85. [DOI] [PubMed] [Google Scholar]
Raja 2015 {published data only}
- Raja AS, Ip IK, Dunne RM, Schuur JD, Mills AM, Khorasani R. Effects of Performance Feedback Reports on Adherence to Evidence-Based Guidelines in Use of CT for Evaluation of Pulmonary Embolism in the Emergency Department: A Randomized Trial. AJR. American journal of roentgenology 2015;205(5):936-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rapoport 2018 {published data only}
- Rapoport MJ, Zucchero Sarracini C, Kiss A, Lee L, Byszewski A, Seitz DP, et al. Computer-Based Driving in Dementia Decision Tool With Mail Support: Cluster Randomized Controlled Trial. Journal of medical Internet research 2018;20(5):e194. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rathlev 2016 {published data only}
- Rathlev N, Almomen R, Deutsch A, Smithline H, Li H, Visintainer P. Randomized Controlled Trial of Electronic Care Plan Alerts and Resource Utilization by High Frequency Emergency Department Users with Opioid Use Disorder. The western journal of emergency medicine 2016;17(1):28-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeve 2008 {published data only}
- Reeve J F, Tenni P C, Peterson G M. An electronic prompt in dispensing software to promote clinical interventions by community pharmacists: a randomized controlled trial. Br J Clin Pharmacol 2008;65(3):377-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ribeiro‐Vaz 2012 {published data only}
- Ribeiro-Vaz I, Santos C, da Costa-Pereira A, Cruz-Correia R. Promoting spontaneous adverse drug reaction reporting in hospitals using a hyperlink to the online reporting form: an ecological study in Portugal. Drug Saf 2012;35(5):387-94. [DOI] [PubMed] [Google Scholar]
Robbins 2012 {published data only}
- Robbins G K, Lester W, Johnson K L, Chang Y, Estey G, Surrao D, et al. Efficacy of a clinical decision-support system in an HIV practice: a randomized trial. Ann Intern Med 2012;157(11):757-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez‐Aldrete 2016 {published data only}
- Rodriguez-Aldrete D, Sivanesan E, Banks S, Mavarez A, Arheart K, Eber S, et al. Recurrent Visual Electronic Hand Hygiene Reminders in the Anesthesia Work Area. Infection control and hospital epidemiology 2016;37(7):872-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Rood 2005 {published data only}
- Rood E, Bosman R J, Spoel J I, Taylor P, Zandstra D F. Use of a computerized guideline for glucose regulation in the intensive care unit improved both guideline adherence and glucose regulation. J Am Med Inform Assoc 2005;12(2):172-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roumie 2006 {published data only}
- Roumie C L, Elasy T A, Greevy R, Griffin M R, Liu X, Stone W J, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Ann Intern Med 2006;145(3):165-75. [DOI] [PubMed] [Google Scholar]
Roy 2009 {published data only}
- Roy P M, Durieux P, Gillaizeau F, Legall C, Armand-Perroux A, Martino L, et al. A computerized handheld decision-support system to improve pulmonary embolism diagnosis: a randomized trial. Ann Intern Med 2009;151(10):677-86. [DOI] [PubMed] [Google Scholar]
Roy 2016 {published data only}
- Roy PM, Rachas A, Meyer G, Le Gal G, Durieux P, El Kouri D, et al. Multifaceted Intervention to Prevent Venous Thromboembolism in Patients Hospitalized for Acute Medical Illness: A Multicenter Cluster-Randomized Trial. PloS one 2016;11(5):e0154832. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Safran 1993 {published data only}
- Safran C, Rind D M, Davis R M, Currier J, Ives D, Sands D Z, et al. An electronic medical record that helps care for patients with HIV infection. Proc Annu Symp Comput Appl Med Care 1993:224-8. [PMC free article] [PubMed] [Google Scholar]
Schnipper 2010‐2 {published data only}
- Schnipper J L, Liang C L, Ndumele C D, Pendergrass M L. Effects of a computerized order set on the inpatient management of hyperglycemia: a cluster-randomized controlled trial. Endocr Pract 2010;16(2):209-18. [DOI] [PubMed] [Google Scholar]
Schwarz 2012 {published data only}
- Schwarz E B, Parisi S M, Handler S M, Koren G, Cohen E D, Shevchik G J, et al. Clinical decision support to promote safe prescribing to women of reproductive age: a cluster-randomized trial. J Gen Intern Med 2012;27(7):831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shelley 2015 {published data only}
- Shelley D, VanDevanter N, Cleland CC, Nguyen L, Nguyen N. Implementing tobacco use treatment guidelines in community health centers in Vietnam. Implementation science : IS 2015;10:142. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2013 {published data only}
- Silva B M, Rodrigues J J, Canelo F, Lopes I C, Zhou L. A data encryption solution for mobile health apps in cooperation environments. J Med Internet Res 2013;15(4):e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2006 {published data only}
- Simon S R, Smith D H, Feldstein A C, Perrin N, Yang X, Zhou Y, et al. Computerized prescribing alerts and group academic detailing to reduce the use of potentially inappropriate medications in older people. J Am Geriatr Soc 2006;54(6):963-8. [DOI] [PubMed] [Google Scholar]
Skinner 2015 {published data only}
- Skinner CS, Halm EA, Bishop WP, Ahn C, Gupta S, Farrell D, et al. Impact of Risk Assessment and Tailored versus Nontailored Risk Information on Colorectal Cancer Testing in Primary Care: A Randomized Controlled Trial. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2015;24(10):1523-30. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Slok 2016 {published data only}
- Slok AH, Kotz D, Breukelen G, Chavannes NH, Rutten-van Molken MP, Kerstjens HA, et al. Effectiveness of the Assessment of Burden of COPD (ABC) tool on health-related quality of life in patients with COPD: a cluster randomised controlled trial in primary and hospital care. BMJ open 2016;6(7):e011519. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strom 2010‐2 {published data only}
- Strom B L, Schinnar R, Bilker W, Hennessy S, Leonard C E, Pifer E. Randomized clinical trial of a customized electronic alert requiring an affirmative response compared to a control group receiving a commercial passive CPOE alert: NSAID--warfarin co-prescribing as a test case. J Am Med Inform Assoc 2010;17(4):411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sundaram 2009 {published data only}
- Sundaram V, Lazzeroni L C, Douglass L R, Sanders G D, Tempio P, Owens D K. A randomized trial of computer-based reminders and audit and feedback to improve HIV screening in a primary care setting. Int J STD AIDS 2009;20(8):527-33. [DOI] [PubMed] [Google Scholar]
Suresh 2018 {published data only}
- Suresh S, Saladino RA, Fromkin J, Heineman E, McGinn T, Richichi R, et al. Integration of physical abuse clinical decision support into the electronic health record at a Tertiary Care Children's Hospital. Journal of the American Medical Informatics Association : JAMIA 2018;25(7):833-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2008 {published data only}
- Tamblyn R, Huang A, Taylor L, Kawasumi Y, Bartlett G, Grad R, et al. A randomized trial of the effectiveness of on-demand versus computer-triggered drug decision support in primary care. J Am Med Inform Assoc 2008;15(4):430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tamblyn 2012 {published data only}
- Tamblyn R, Eguale T, Buckeridge D L, Huang A, Hanley J, Reidel K, et al. The effectiveness of a new generation of computerized drug alerts in reducing the risk of injury from drug side effects: a cluster randomized trial. J Am Med Inform Assoc 2012;19(4):635-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2004 {published data only}
- Thomas H V, Lewis G, Watson M, Bell T, Lyons I, Lloyd K, et al. Computerised patient-specific guidelines for management of common mental disorders in primary care: a randomised controlled trial. Br J Gen Pract 2004;54(508):832-7. [PMC free article] [PubMed] [Google Scholar]
Thomas 2018 {published data only}
- Thomas KEH, Kisely S, Urrego F. Electronic Heath Record Prompts May Increase Screening for Secondhand Smoke Exposure. Clinical pediatrics 2018;57(1):27-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tollitt 2018 {published data only}
- Tollitt J, Flanagan E, McCorkindale S, Glynn-Atkins S, Emmett L, Darby D, et al. Improved management of acute kidney injury in primary care using e-alerts and an educational outreach programme. Family practice 2018;35(6):684-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tsai 2016 {published data only}
- Tsai CY, Wang SH, Hsu MH, Li YC. Do false positive alerts in naive clinical decision support system lead to false adoption by physicians? A randomized controlled trial. Computer methods and programs in biomedicine 2016;132:83-91. [PMID: ] [DOI] [PubMed] [Google Scholar]
van Doormaal 2009 {published data only}
- Doormaal J E, den Bemt P M, Zaal R J, Egberts A C, Lenderink B W, Kosterink J G, et al. The influence that electronic prescribing has on medication errors and preventable adverse drug events: an interrupted time-series study. J Am Med Inform Assoc 2009;16(6):816-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Wijk 2001 {published data only}
- Wijk M A, Lei J, Mosseveld M, Bohnen A M, Bemmel J H. Assessment of decision support for blood test ordering in primary care. a randomized trial. Ann Intern Med 2001;134(4):274-81. [DOI] [PubMed] [Google Scholar]
Weiss 2013 {published data only}
- Weiss C H, Dibardino D, Rho J, Sung N, Collander B, Wunderink R G. A clinical trial comparing physician prompting with an unprompted automated electronic checklist to reduce empirical antibiotic utilization. Crit Care Med 2013;41(11):2563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2015 {published data only}
- Welch G, Zagarins S E, Santiago-Kelly P, Rodriguez Z, Bursell S E, Rosal M C, et al. An internet-based diabetes management platform improves team care and outcomes in an urban Latino population. Diabetes Care 2015;38(4):561-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Were 2011 {published data only}
- Were M C, Shen C, Tierney W M, Mamlin J J, Biondich P G, Li X, et al. Evaluation of computer-generated reminders to improve CD4 laboratory monitoring in sub-Saharan Africa: a prospective comparative study. J Am Med Inform Assoc 2011;18(2):150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2010 {published data only}
- Williams E C, Achtmeyer C E, Kivlahan D R, Greenberg D, Merrill J O, Wickizer T M, et al. Evaluation of an electronic clinical reminder to facilitate brief alcohol-counseling interventions in primary care. J Stud Alcohol Drugs 2010;71(5):720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2011 {published data only}
- Williams M, Peterson G M, Tenni P C, Bindoff I K, Curtain C, Hughes J, et al. Drug-related problems detected in Australian Community Pharmacies: The PROMISe Trial. Ann Pharmacother 2011;45(9):1067-76. [DOI] [PubMed] [Google Scholar]
Wilson 2015 {published data only}
- Wilson FP, Shashaty M, Testani J, Aqeel I, Borovskiy Y, Ellenberg SS, et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet (London, England) 2015;385(9981):1966-74. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wipfli 2016 {published data only}
- Wipfli R, Ehrler F, Bediang G, Betrancourt M, Lovis C. How Regrouping Alerts in Computerized Physician Order Entry Layout Influences Physicians' Prescription Behavior: Results of a Crossover Randomized Trial. JMIR human factors 2016;3(1):e15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woller 2018 {published data only}
- Woller SC, Stevens SM, Evans RS, Wray D, Christensen J, Aston VT, et al. Electronic alerts, comparative practitioner metrics, and education improve thromboprophylaxis and reduce venous thrombosis in community hospitals. Research and practice in thrombosis and haemostasis 2018;2(3):481-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2018 {published data only}
- Zhu X, Wong FKY, Wu CLH. Development and evaluation of a nurse-led hypertension management model: A randomized controlled trial. International journal of nursing studies 2018;77:171-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ziemer 2006 {published data only}
- Ziemer D C, Doyle J P, Barnes C S, Branch W T Jr, Cook C B, El-Kebbi I M, et al. An intervention to overcome clinical inertia and improve diabetes mellitus control in a primary care setting: Improving Primary Care of African Americans with Diabetes (IPCAAD) 8. Arch Intern Med 2006;166(5):507-13. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Christakis 2001 {published data only}
- Christakis DA, Zimmerman FJ, Wright JA, Garrison MM, Rivara FP, Davis RL. A randomized controlled trial of point-of-care evidence to improve the antibiotic prescribing practices for otitis media in children. Pediatrics 2001;107:E15. [DOI] [PubMed] [Google Scholar]
Christakis 2001a {published data only}
- Christakis D A, Zimmerman F J, Wright J A, Garrison M M, Rivara F P, Davis R L. A randomized controlled trial of point-of-care evidence to improve the antibiotic prescribing practices for otitis media in children. Pediatrics 2001;107:E15. [DOI] [PubMed] [Google Scholar]
Durieux 2000 ‐ classified, excluded {published data only}
- Durieux P, Nizard R, Ravaud P, Mounier N, Lepage E. A clinical decision support system for prevention of venous thromboembolism: effect on physician behaviour. JAMA 2000;283:2816-21. [DOI] [PubMed] [Google Scholar]
Forrest 2013 {published data only}
- Forrest C B, Fiks A G, Bailey L C, Localio R, Grundmeier R W, Richards T, et al. Improving adherence to otitis media guidelines with clinical decision support and physician feedback. Pediatrics 2013;131:e1071-81. [DOI] [PubMed] [Google Scholar]
Fortuna 2009 {published data only}
- Fortuna R J, Zhang F, Ross-Degnan D, Campion F X, Finkelstein J A, Kotch J B, et al. Reducing the prescribing of heavily marketed medications: a randomized controlled trial. J Gen Intern Med 2009;24:897-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kralj 2003 ‐ classified, excluded {published data only}
- Kralj B, Iverson D, Hotz K, Ashbury FD. The impact of computerized clinical reminders on physician prescribing behavior: evidence from community oncology practice. American Journal of Medical Quality 2003;18:197-203. [DOI] [PubMed] [Google Scholar]
Plaza 2005 {published data only}
- Plaza V, Cobos A, Ignacio-García JM, Molina J, Bergoñón S, García-Alonso F, et al. Cost-effectiveness of an intervention based on the Global Initiative for Asthma (GINA) recommendations using a computerized clinical decision support system: a physicians randomized trial [Coste-efectividad de una intervención basada en las recomendaciones de la Global INitiative for Asthma (GINA), mediante un sistema informatizado de apoyo a la decisión clínica: un ensayo con aleatorización de médicos]. Medicina Clínica 2005;124(6):201-6. [DOI] [PubMed] [Google Scholar]
Roumie 2006 ‐ classified, excluded {published data only}
- Roumie CL, Elasy TA, Greevy R, Griffin MR, Liu X, Stone WJ, Wallston KA, et al. Improving blood pressure control through provider education, provider alerts, and patient education: a cluster randomized trial. Annals of Internal Medicine 2006;145:165-75. [DOI] [PubMed] [Google Scholar]
Sales 2008 {published data only}
- Sales A, Helfrich C, Ho P M, Hedeen A, Plomondon M E, Li Y F, et al. Implementing electronic clinical reminders for lipid management in patients with ischemic heart disease in the veterans health administration: QUERI Series. Implement Sci 2008;3:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tape 1993 {published data only}
- Tape TG, Campbell JR. Computerized medical records and preventive health care: success depends on many factors. American Journal of Medicine 1993;94:619-25. [DOI] [PubMed] [Google Scholar]
Additional references
EPOC 2008
- Cochrane Effective Practice and Organisation of Care Group. EPOC-specific resources for review authors. The Cochrane Collaboration, 2008. Available from http://www.epoc.cochrane.org/en/handsearchers.html (accessed 25 July 2008).
Grimshaw 2003
- Grimshaw J, McAuley LM, Bero LA, Grilli R, Oxman AD, Ramsay C, et al. Systematic reviews of the effectiveness of quality improvement strategies and programmes. Quality and Safety in Health Care 2003;12:298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grimshaw 2004
- Grimshaw JM, Thomas RE, MacLennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment 2004;8:iii-iv, 1-72. [DOI] [PubMed] [Google Scholar]
Kawamoto 2005
- Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. BMJ 2005;330:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shojania 2004a
- Shojania KG, McDonald KM, Wachter RM, Owens DK. Closing the quality gap: a critical analysis of quality improvement strategies. Vol. 1: Series Overview and Methodology. Rockville, MD: Agency for Healthcare Research and Quality, August 2004. [AHRQ Publication NO. 04-0051-1] [PubMed] [Google Scholar]
Shojania 2004b
- Shojania KG, Ranji S, Shaw LK, et al. Closing the quality gap: a critical analysis of quality improvement strategies. Vol. 2: Diabetes Mellitus Care. Rockville, MD: Agency for Healthcare Research and Quality, September 2004. [AHRQ Publication No. 04-0051-2] [PubMed] [Google Scholar]
Shojania 2005
- Shojania KG, Grimshaw JM. Evidence-based quality improvement: the state of the science. Health Affairs (Millwood) 2005;24:138-50. [DOI] [PubMed] [Google Scholar]
Shojania 2006
- Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427-40. [DOI] [PubMed] [Google Scholar]