Abstract
Background
This is an updated version of the Cochrane review published in Issue 4, 1998. Combining drugs from different classes with different modes of action may offer opportunity to optimise efficacy and tolerability, using lower doses of each drug to achieve the same degree of pain relief. Previously we concluded that addition of codeine to paracetamol provided additional pain relief, but at expense of additional adverse events. New studies have been published since. This review sought to evaluate efficacy and safety of paracetamol plus codeine using current data, and compare findings with other analgesics evaluated similarly.
Objectives
Assess efficacy of single dose oral paracetamol plus codeine in acute postoperative pain, increase in efficacy due to the codeine component, and associated adverse events.
Search methods
We searched CENTRAL, MEDLINE, EMBASE, the Oxford Pain Relief Database in October 2008 for this update.
Selection criteria
Randomised, double‐blind, placebo‐controlled trials of paracetamol plus codeine, compared with placebo or the same dose of paracetamol alone, for relief of acute postoperative pain in adults.
Data collection and analysis
Two authors assessed trial quality and extracted data. The area under the “pain relief versus time” curve was used to derive proportion of participants with paracetamol plus codeine and placebo or paracetamol alone experiencing least 50% pain relief over four‐to‐six hours, using validated equations. Number‐needed‐to‐treat‐to‐benefit (NNT) was calculated using 95% confidence intervals (CIs). Proportion of participants using rescue analgesia over a specified time period, and time to use of rescue analgesia, were sought as additional measures of efficacy. Information on adverse events and withdrawals were collected.
Main results
Twenty‐six studies, with 2295 participants, were included comparing paracetamol plus codeine with placebo. Significant dose response was seen for the outcome of at least 50% pain relief over four‐to‐six hours, with NNTs of 2.2 (95% CI 1.8 to 2.9) for 800 to 1000 mg paracetamol plus 60 mg codeine, 3.9 (2.9 to 4.5) for 600 to 650 mg paracetamol plus 60 mg codeine, and 6.9 (4.8 to 12) for 300 mg paracetamol plus 30 mg codeine. Time to use of rescue medication was over four hours with paracetamol plus codeine and two hours with placebo. The NNT to prevent remedication was 5.6 (4.0 to 9.0) for 600 mg paracetamol plus 60 mg codeine over four to six hours. Adverse events increased of mainly mild to moderate severity with paracetamol plus codeine than placebo.
Fourteen studies, with 926 participants, were included in the comparison of paracetamol plus codeine with the same dose of paracetamol alone. Addition of codeine increased proportion of participants achieving at least 50% pain relief over four‐to‐six hours by 10 to 15%, increased time to use of rescue medication by about one hour, and reduced proportion of participants needing rescue medication by about 15% (NNT to prevent remedication 6.9 (4.2 to 19). Adverse events were mainly mild to moderate in severity and incidence did not differ between groups.
Authors' conclusions
This update confirms previous findings that combining paracetamol with codeine provided clinically useful levels of pain relief in about 50% of patients with moderate to severe postoperative pain, compared with under 20% with placebo. New information for remedication shows that the combination extended the duration of analgesia by about one hour compared to treatment with the same dose of paracetamol alone. At higher doses, more participants experienced adequate pain relief, but the amount of information available for the 1000 mg paracetamol plus 60 mg codeine dose was small, and based on limited information.
Plain language summary
Single dose oral paracetamol (acetaminophen) plus codeine for postoperative pain relief in adults
Pain is commonly experienced after surgical procedures, and is not always well controlled. Combining analgesics from different classes has the potential to provide adequate pain relief with reduced dose‐dependent adverse events. This review assessed data from twenty‐six studies comparing paracetamol plus codeine with placebo, and fourteen studies comparing paracetamol plus codeine with the same dose of paracetamol alone. The combination provided effective pain relief for about 40% of participants experiencing moderate to severe pain after an operation with 600 to 650 mg paracetamol plus 60 mg codeine, the dose most commonly used in these studies, and about 50% of participants with 800 to 1000 mg paracetamol plus 60 mg codeine, the dose most commonly used in clinical practice. The addition of codeine provided effective pain relief to about 10% more participants than the same dose of paracetamol alone. These single dose studies did not associate paracetamol plus codeine with any serious side effects.
Background
This is an update of a review published in The Cochrane Library in Issue 4, 1998 on 'Single dose paracetamol (acetaminophen), with and without codeine, for postoperative pain' (Moore 1998a). The title now states that the review is limited to adults. The previous review looked at three sets of comparisons: paracetamol versus placebo, paracetamol with codeine versus placebo, and paracetamol with codeine versus paracetamol. This review updates only the paracetamol with codeine versus placebo or paracetamol alone comparisons. A separate review looks at paracetamol versus placebo only (Toms 2008).
Acute pain occurs as a result of tissue damage commonly accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. The management of postoperative pain and inflammation is a critical component of patient care. This is one of a series of reviews whose aim is to present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level.
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants is small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following four to six hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome in this setting. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over four to six hours (Moore 2005). Patients often remain in the hospital or clinic for at least the first six hours following the intervention, with measurements supervised, although they may then go home to make their own measurements in trials of longer duration.
Paracetamol
Paracetamol (acetaminophen) was first identified as the active metabolite of two older antipyretic drugs, acetanilide and phenacetin in the late nineteenth century but the drug was not added to the British Pharmacopoeia until 1963 (PIC 2008). Since then it has become one of the most popular antipyretic and analgesic drugs worldwide, and is also often used in combination with other drugs.
The lack of significant anti‐inflammatory activity for paracetamol implies a mode of action distinct from that of non‐steroidal anti‐inflammatory drugs (NSAIDs) yet, despite years of use and research, the mechanisms of action of paracetamol are not fully understood. NSAIDs act by inhibiting the activity of cyclooxygenase (COX). COX (now recognised to consist of two isoforms, COX‐1 and COX‐2) catalyses the production of prostaglandins responsible for pain and inflammation. Paracetamol has previously been shown to have no significant effects on COX‐1 or COX‐2 (Schwab 2003), but is now being considered as a selective COX‐2 inhibitor (Hinz 2008). Significant paracetamol‐induced inhibition of prostaglandin production has been demonstrated in tissues in the brain, spleen, and lung (Flower 1972; Botting 2000). A 'COX‐3 hypothesis' wherein the efficacy of paracetamol is attributed to its specific inhibition of a third cyclooxygenase isoform enzyme, COX‐3 (Botting 2000; Chandrasekharan 2002; PIC 2008) now has little credibility, and a central mode action of paracetamol is thought to be likely (Graham 2005).
Despite a low incidence of adverse effects, paracetamol has a recognised potential for hepatotoxicity and is thought to be responsible for approximately half of all cases of liver failure in the UK (Hawton 2001). Acute paracetamol hepatotoxicity at therapeutic doses is extremely unlikely despite reports of so‐called therapeutic misadventure (Prescott 2000). In recent years legislative changes restricting pack sizes and the maximum number of tablets permitted in over‐the‐counter sales were introduced in the UK (CSM 1997) on the basis of evidence that poisoning is lower in countries that restrict availability (Hawton 2001; Gunnell 1997). The contribution of these changes to any observed reductions in incidence of liver failure or death, which are inconvenient and more costly (particularly to chronic pain sufferers), remains uncertain (Hawkins 2007). There have been concerns over the safety of paracetamol in patients with compromised hepatic function (those with severe alcoholism, cirrhosis or hepatitis) but these have not been substantiated (PIC 2008; Dart 2000).
Paracetamol is the analgesic of choice for adult patients in whom salicylates or other NSAIDs are contraindicated. Such patients include asthmatics, those with salicylate allergies, and those with a history of peptic ulcer. Compared with available alternatives, it is associated with few problems in all but the most compromised of renal patients, and few drug‐drug interactions. Paracetamol is useful for children with febrile viral illnesses, in whom aspirin is contraindicated due to the risk of Reye's syndrome (swelling of the brain that may lead to coma and death).
Paracetamol with codeine
Paracetamol, NSAIDs and opioids have different mechanisms of action and side effect profiles, and combining drugs from these main classes may offer the opportunity to optimise efficacy and tolerability. It is thought that reduced doses of two (or more) drugs from different classes, given together, can provide adequate pain relief, acting via different targets, while reducing dose dependent adverse events (Edwards 2002). The combination of paracetamol and an opioid is an attractive choice, particularly for individuals for whom NSAIDs are contraindicated. In contrast to most other classes of analgesic, opioids do not cause direct organ damage in long‐term use, but they have limited tolerability due to their common, troublesome side effects (constipation, somnolence, dry mouth, nausea and dizziness), and must be used with care in the elderly, or individuals with renal impairment.
Paracetamol with codeine is an established, cheap and widely available combination. The earlier Cochrane review concluded that the combination is effective for postoperative pain, but additional trials have since been published. This review aims to provide more robust estimates of both efficacy and harm, in a format which facilitates direct comparison with other analgesics.
Objectives
To assess the efficacy and adverse effects of single dose paracetamol with codeine for acute postoperative pain using methods that permit comparison with other analgesics evaluated in the same way, using wider criteria of efficacy recommended by an in‐depth study at the individual patient level (Moore 2005).
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were full publications of double blind trials of a single dose oral paracetamol with codeine compared with placebo or the same dose of paracetamol alone for the treatment of moderate to severe postoperative pain in adults, with at least ten participants randomly allocated to each treatment group. Multiple dose studies were included if appropriate data from the first dose were available, and cross‐over studies were included provided that data from the first arm were presented separately.
Studies were excluded if they were:
posters or abstracts not followed up by full publication;
reports of trials concerned with pain other than postoperative pain (including experimental pain);
trials using healthy volunteers;
trials where pain relief was assessed by clinicians, nurses or carers (i.e., not patient‐reported);
trials of less than four hours' duration or which failed to present data over four to six hours post‐dose.
Types of participants
Studies of adult participants (15 years and older) with postoperative pain of moderate to severe intensity following day surgery or inpatient surgery were included. For studies using a visual analogue scale (VAS), pain intensity was assumed to be of at least moderate intensity when the VAS score was greater than 30 mm (Collins 1997).Trials of patients with postpartum pain were included provided the pain investigated resulted from episiotomy or Caesarean section (with or without uterine cramp). Trials investigating pain due to uterine cramps alone were excluded.
Types of interventions
Orally administered paracetamol with codeine and matched placebo or the same dose of paracetamol administered for relief of postoperative pain.
Types of outcome measures
Information extracted from the studies included:
patient characteristics;
pain model;
patient reported pain at baseline (physician, nurse, or carer reported pain will not be included in the analysis);
patient‐reported pain relief and/or pain intensity expressed hourly over four to six hours using validated pain scales (pain intensity and pain relief in the form of visual analogue scales or categorical scales, or both), or reported total pain relief (TOTPAR) or summed pain intensity difference (SPID) at four to six hours;
patient‐reported global evaluation of efficacy (PGE), using a standard categorical rating scale;
number of participants using rescue medication, and the time of assessment;
time to use of rescue medication;
withdrawals ‐ all cause, adverse event;
adverse events ‐ participants experiencing one or more, and any serious adverse event, and the time of assessment.
Search methods for identification of studies
The following databases were searched:
Cochrane CENTRAL (November 2002 for original search and 2002 to August 2008 for the update);
MEDLINE via Ovid (1966 to November 2002 for the original review and 2002 to August 2008 for the update);
EMBASE via Ovid (1966 to November 2002 for the original review and 2002 to August 2008 for the update);
Oxford Pain Database (Jadad 1996a).
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy, and Appendix 3 for the Cochrane CENTRAL search strategy.
Reference lists of retrieved studies were also manually searched. Other databases searched for the original review were not searched for the update.
Language
No language restriction was applied.
Unpublished studies
Abstracts, conference proceedings and other grey literature were not searched. Manufacturers were not contacted.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies that might be included in the updated review.
Quality assessment
Two review authors independently assessed the included studies for quality using a five‐point scale (Jadad 1996b).
The scale used is as follows:
Is the study randomised? If yes ‐ one point;
Is the randomisation procedure reported and is it appropriate? If yes add one point, if no deduct one point;
Is the study double blind? If yes then add one point;
Is the double blind method reported and is it appropriate? If yes add 1 point, if no deduct one point;
Are the reasons for patient withdrawals and dropouts described? If yes add one point.
The results are described under 'Risk of bias in included studies' and are detailed in 'Characteristics of included studies'.
Data management
Data were extracted by two review authors and recorded on a standard data extraction form. Data suitable for pooling were entered into RevMan 5.0.13.
Data analysis
QUOROM guidelines were followed (Moher 1999). For efficacy analyses we used the number of participants in each treatment group who were randomised, received medication, and provided at least one valid post‐baseline assessment. For safety analyses we used number of participants randomised to each treatment group who took the study medication. Analyses were planned for different doses (where there were at least 200 participants). Sensitivity analyses were planned for pain model (dental versus other postoperative pain), trial size (39 or fewer versus 40 or more per treatment arm), and quality score (two versus three or more).
Primary outcome: Number of participants achieving at least 50% pain relief
For each study, mean TOTPAR (total pain relief) or SPID (summed pain intensity difference) for active and placebo groups were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). The proportion of participants in each treatment group who achieved at least 50%maxTOTPAR was calculated using verified equations (Moore 1996; Moore 1997a; Moore 1997b). These proportions were then converted into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. Information on the number of participants with at least 50%maxTOTPAR for active treatment and placebo was then used to calculate relative benefit (RR) and number‐needed‐to‐treat to benefit (NNT). Pain measures accepted for the calculation of TOTPAR or SPID were:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
visual analogue scales (VAS) for pain relief;
VAS for pain intensity.
If none of these measures were available, numbers of participants reporting "very good or excellent" on a five‐point categorical global scale with the wording "poor, fair, good, very good, excellent" were taken as those achieving at least 50% pain relief (Collins 2001).
Details of the scales used to measure pain and pain relief are in the glossary (Appendix 4).
Secondary outcomes:
1. Use of rescue medication. Numbers of participants requiring rescue medication were used to calculate numbers‐needed‐to‐treat to prevent (NNTp) use of rescue medication for treatment and placebo groups. Median (or mean) time to use of rescue medication was used to calculate the weighted mean of the median (or mean) for the outcome. Where fewer than 50% of participants required rescue medication at the time of censorship, the median time was taken as the time of censorship. This will give a conservative estimate for time to use of rescue medication. Weighting was by number of participants.
2.Adverse events. Numbers of participants reporting adverse events for each treatment group were used to calculate relative risk (RR) and numbers needed to treat to harm (NNH) estimates for:
any adverse event;
any serious adverse event (as reported in the study);
withdrawal due to an adverse event.
3.Other withdrawals. Withdrawals for reasons other than lack of efficacy (participants using rescue medication ‐ see above) and adverse events were noted.
Relative benefit and risk estimates were calculated with 95% confidence intervals (CI) using a fixed‐effect model (Morris 1995). NNT or NNH with 95% CI were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the relative benefit did not include the number one.
Homogeneity of studies was assessed visually (L'Abbé 1987). The z test (Tramèr 1997) was used to determine if there was a significant difference between NNTs for different doses of active treatment, or between groups in the sensitivity analyses.
Results
Description of studies
This review examines only trials of paracetamol plus codeine versus placebo or paracetamol plus codeine versus paracetamol. Trials of paracetamol alone versus placebo are to be found in a separate review (Toms 2008).
Included studies
Paracetamol with codeine versus placebo
Twenty‐six studies with 2295 participants met our inclusion criteria for the comparison of paracetamol plus codeine versus placebo (Bentley 1987; Bjune 1996; Bourne 2005; Chang 2001; Chang 2005; Cooper 1981; Cooper 1988; Cooper 1991; Desjardins 1986; Dionne 1994; Forbes 1982; Forbes 1983; Forbes 1986; Forbes 1989; Forbes 1990a; Forbes 1990b; Forbes 1994; Heidrich 1985; Honig 1984; Malmstrom 2004; Pande 1996c; Smith 2004; Stubhaug 1995; Sunshine 1986; Turek 1988; Ziccardi 2000)). In total 1357 participants were given paracetamol plus codeine and 938 placebo. The original review included 21 studies reporting 22 comparisons (1407 participants). Two of these comparisons had group sizes of less than 10 (Pande 1996a; Pande 1996b) and hence did not meet our inclusion criteria. We found six new studies with 908 participants (Bourne 2005; Chang 2001; Chang 2005; Malmstrom 2004; Smith 2004; Ziccardi 2000), and excluded six new studies (Chung 2004; De los Santos 1998; Maalaki 2002; Macleod 2002; Peter 2001; Raeder 2001) for this update for Issue 1, 2009. Details of all studies are in the Characteristics of included studies or the Characteristics of excluded studies tables.
Paracetamol with codeine versus paracetamol
Fourteen studies with 926 participants met our inclusion criteria for the comparison of paracetamol plus codeine versus paracetamol (Bentley 1987; Bjune 1996; Breivik 1999; Cooper 1981; Cooper 1988; Cooper 1991; Dionne 1994; Forbes 1982; Forbes 1983; Forbes 1989; Forbes 1990b; Gertzbein 1986; Honig 1984; Sunshine 1986). In total 462 participants were given paracetamol with codeine and 464 paracetamol alone. Twelve studies (794 participants) were included in the original review and two (132 participants) are new to this update (Bjune 1996 and Breivik 1999) for Issue 1, 2009. Although Bjune 1996 did appear in the original review, the paracetamol plus codeine arm used 800 mg of paracetamol with 60 mg codeine and the paracetamol only arm used 1000 mg paracetamol alone. Hence it was only analysed for its paracetamol versus placebo comparison as the doses of paracetamol are different. We considered the dose of paracetamol in the paracetamol only arm to be sufficiently similar to that in the paracetamol plus codeine arm, and hence we included this comparison in our analysis. We excluded six new studies (Chung 2004; De los Santos 1998; Maalaki 2002; Macleod 2002; Peter 2001; Raeder 2001). Details of all studies are in the Characteristics of included studies or the Characteristics of excluded studies tables.
Dose
Paracetamol with codeine versus placebo
Paracetamol 1000 mg with codeine 60 mg was used in two studies (Bentley 1987; Stubhaug 1995) and Paracetamol 800 mg with codeine 60 mg was used in one study (Bjune 1996): for the purposes of this analysis these doses will be classed as one group.
Paracetamol 600 to 650 mg with codeine 60 mg was used in 17 studies (Chang 2001; Chang 2005; Cooper 1981; Cooper 1988; Cooper 1991; Dionne 1994; Forbes 1982; Forbes 1983; Forbes 1989; Forbes 1990a; Forbes 1990b; Honig 1984; Malmstrom 2004; Pande 1996c; Sunshine 1986; Turek 1988; Ziccardi 2000).
Paracetamol 300 mg with codeine 30 mg was used in six studies (Bourne 2005; Desjardins 1986; Forbes 1986; Forbes 1994; Heidrich 1985; Smith 2004).
Paracetamol with codeine versus paracetamol
Paracetamol 1000 mg with 60 mg codeine versus 1000 mg paracetamol alone was used in three studies (Breivik 1999; Bentley 1987; Gertzbein 1986). Paracetamol 800 mg with 60 mg codeine versus 1000 mg paracetamol alone was used in one comparison (Bjune 1996): for the purposes of this analysis these doses will be classed as one group.
Paracetamol 600 mg to 650 mg plus 60 mg codeine versus paracetamol 600 mg to 650 mg alone was used in 10 treatment arms (Cooper 1981; Cooper 1988; Cooper 1991; Dionne 1994; Forbes 1982; Forbes 1983; Forbes 1989; Forbes 1990b; Honig 1984; Sunshine 1986).
Pain model
Paracetamol with codeine versus placebo
Eighteen studies enrolled patients with dental pain following extraction of at least one impacted third molar (Bentley 1987; Chang 2001; Chang 2005; Cooper 1981; Cooper 1988; Cooper 1991; Desjardins 1986; Dionne 1994; Forbes 1982; Forbes 1986; Forbes 1989; Forbes 1990a; Forbes 1990b; Forbes 1994; Malmstrom 2004; Pande 1996c; Sunshine 1986; Ziccardi 2000).
Eight studies enrolled patients with pain following other surgical procedures. Four were mixed general surgery (Forbes 1983; Honig 1984; Smith 2004; Turek 1988), three were orthopaedic surgery (Bourne 2005; Heidrich 1985; Stubhaug 1995) and one was Caesarean section (Bjune 1996).
Paracetamol with codeine versus paracetamol
Ten studies (Bentley 1987; Breivik 1999; Cooper 1981; Cooper 1988; Cooper 1991; Dionne 1994; Forbes 1982; Forbes 1989; Forbes 1990b; Sunshine 1986) enrolled patients with dental pain following extraction of at least one impacted third molar.
Four studies (Forbes 1983; Bjune 1996; Gertzbein 1986; Honig 1984) enrolled patients with pain following general surgical procedures (including one study with Caesarean section).
Study duration
Paracetamol with codeine versus placebo
Study duration was four hours in five studies, five hours in one study, six hours in 15 studies, 12 hours in three studies, and 24 hours in two studies.
Paracetamol with codeine versus paracetamol
Study duration was four hours in four studies, six hours in five studies, eight hours in one study and 12 hours three studies.
Details of included and excluded studies are in the corresponding "Characteristics of studies" tables.
Risk of bias in included studies
Paracetamol with codeine versus placebo
Ten studies were given a quality score of five (Bourne 2005; Forbes 1983; Forbes 1986; Forbes 1989; Forbes 1990a; Forbes 1990b; Forbes 1994; Malmstrom 2004; Smith 2004; Sunshine 1986), ten a score of four (Bjune 1996; Chang 2001; Chang 2005; Cooper 1981; Cooper 1988; Desjardins 1986; Forbes 1982; Pande 1996c; Stubhaug 1995; Ziccardi 2000), five a score of three (Bentley 1987; Cooper 1991; Dionne 1994; Honig 1984; Turek 1988), and one a score of two (Heidrich 1985).
Paracetamol with codeine versus paracetamol
Five studies were given a quality score of five (Breivik 1999; Forbes 1983; Forbes 1989; Forbes 1990b; Sunshine 1986), four a score of four (Bjune 1996; Cooper 1981; Cooper 1988; Forbes 1982), and five a score of three (Bentley 1987; Cooper 1991; Dionne 1994; Gertzbein 1986; Honig 1984).
Further details are in 'Characteristics of included studies'.
Effects of interventions
Number of participants achieving at least 50% pain relief
Paracetamol with codeine versus placebo
800 to 1000 mg paracetamol with 60 mg codeine versus placebo
Three studies provided data: 121 participants were treated with paracetamol plus codeine and 71 with placebo (Table 1; Figure 1; Summary of results A).
The proportion of participants experiencing at least 50% pain relief over four to six hours with 800 mg to 1000 mg of paracetamol plus 60 mg codeine was 53% (64/121).
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with placebo was 7% (5/71).
The relative benefit of treatment compared with placebo was 6.3 (2.9 to 14).
The NNT for at least 50% pain relief over four to six hours was 2.2 (1.8 to 2.9). For every two participants treated with paracetamol plus codeine, one would experience at least 50% pain relief who would not have done so with placebo.
1. Summary of outcomes ‐ analgesia and use of rescue medication.
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: v good or excellent | Median time to use (hr) | Number using |
| Bentley 1987 | (1) Paracetamol+codeine 1000/60 mg, n=41 (2) Paracetamol 1000 mg, n=41 (3) Codeine 60 mg, n=21 (4) Placebo, n=17 |
TOTPAR 5: (1) 11.5 (2) 8.7 (4) 4.9 |
(1) 27/41 (2) 19/41 (4) 4/17 |
No data | (1) 4.1 (2) 3.3 (4) 1.4 |
at 4 hr: (1) 44 (2) 68 (4) 81 |
| Bjune 1996 | (1) Paracetamol+codeine 800/60 mg, n=50 (2) Paracetamol 1000 mg, n=50 (3) Placebo, n=25 |
TOTPAR 6: severe pain (1) 10.5 (2) 6.4 (3) 0 moderate pain (1) 6.5 (2) 8.0 (3) 1.5 |
(1) 16/44 (2) 12/43 (3) 0/21 |
No usable data | No data | No data |
| Bourne 2005 | (1) Paracetamol+codeine 300/30 mg, n=55 (2) Paracetamol+tramadol 325/37.5 mg, n=49 (3) Placebo, n=49 |
SPID 4: (1) 2.8 (3) 2.1 Baseline PI: (1) 2.1 (2) 2.2 |
(1) 24/55 (3) 15/49 |
No usable data | No data | at 4 hr: (1) 18 (3) 37 |
| Breivik 1999 | (1) Paracetamol+codeine 1000/60 mg n=24 (2) Paracetamol 1000 mg, n=22 (3) Diclofenac 100 mg, n=22 (4) Paracetamol+diclofenac 1000/100 mg, n=24 (5) Paracetamol+diclofenac+codeine 1000/100/60 mg, n=24 |
TOTPAR 6: (1) 15.5 (2) 12.1 |
(1) 17/23 (2) 12/22 |
No data | No data | at 6 hr: (1) 17 (2) 38 |
| Chang 2001 | (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50mg n=182 (3) Placebo n=31 |
TOTPAR 6: (1) 7.0 (3) 3.4 |
(1) 49/180 (3) 2/31 |
(1) 27/180 (3) 0/31 |
(1) 2.3 (3) 1.6 |
No data |
| Chang 2005 | (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50 mg n=180 (3) Placebo n=30 |
TOTPAR 6: (1) 10.7 (3) 6.7 |
(1) 86/180 (3) 8/30 |
(1) 123/180 (3) 3/30 |
(1) 6.5 (3) 3.2 |
at 24 hr: (1) 86 (3) 73 |
| Cooper 1981 | (1) Paracetamol+codeine 650/60 mg, n=42 (2) Paracetamol 650 mg, n=37 (3) Paracetamol+d‐propoxyphene 650/100 mg, n=42 (4) Ibuprofen 200 mg, n=42 (5) Placebo, n=37 |
TOTPAR 4: (1) 8.4 (2) 8.2 (5) 3.4 |
(1) 24/42 (2) 21/37 (5) 6/37 |
No usable data | Mean: (1) 3.1 (2) 3.5 (5) 2.9 |
at 4 hr: (1) 12 (2) 5 (5) 54 |
| Cooper 1988 | (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=36 (3) Meclofenamate Na 100 mg, n=36 (2) Placebo, n=40 |
TOTPAR 6: (1) 12.0 (2) 8.0 (4) 6.3 |
(1) 17/31 (2) 12/36 (4) 9/40 |
(1) 15/31 (2) 12/36 (4) 8/40 |
No data | at 6 hr: (1) 58 (2) 78 (4) 82 |
| Cooper 1991 | (1) Paracetamol+codeine 650/60 mg, n=39 (2) Paracetamol 650 mg, n=37 (3) Zomepirac 100 mg, n=23 (4) Flurbiprofen 50 mg, n=42 (5) Flurbiprofen 100 mg, n=41 (6) Placebo, n=44 |
TOTPAR 6: (1) 10.1 (2) 6.8 (6) 5.7 |
(1) 17/39 (2) 10/37 (6) 9/44 |
(1) 14/39 (2) 3/37 (6) 2/44 |
Mean: (1) 3.5 (2) 3.2 (6) 3.1 |
at 6 hr: (1) 80 (2) 95 (6) 84 |
| Desjardins 1986 | (1) Paracetamol+codeine 300/30 mg, n=39 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/30 mg, n=43 (3) Placebo, n=41 |
TOTPAR 6: (1) 7.8 (3) 5.1 |
(1) 12/39 (3) 7/41 |
No usable data | Mean: (1) 3.4 (3) 3.0 |
No data |
| Dionne 1994 | (1) Paracetamol+codeine 650/60 mg, n=24 (2) Paracetamol 650 mg, n=27 (3) Flurbiprofen 50 mg, n=25 (4) Flurbiprofen 100 mg, n=22 (5) Placebo, n=25 |
TOTPAR 6: (1) 16.9 (2) 18.4 (5) 14.9 |
(1) 20/24 (2) 24/27 (5) 18/25 |
No usable data | No data | No data |
| Forbes 1982 | (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=34 (3) Diflusinal 500 mg, n=32 (4) Diflusinal 1000 mg, n=32 (5) Placebo, n=30 |
TOTPAR 4: (1) 9.7 (2) 8.9 (5) 3.8 |
(1) 21/31 (2) 15/34 (5) 6/30 |
No usable data | (1) 5.3 (2) 3.5 (5) 2.4 |
at 6 hr: (1) 56 (2) 70 (5) 82 |
| Forbes 1983 | (1) Paracetamol+codeine 600/60 mg, n=26 (2) Paracetamol 600 mg, n=26 (3) Diflusinal 500 mg, n=26 (4) Diflusinal 1000 mg, n=28 (5) Placebo, n=26 |
TOTPAR 6: (1) 13.5 (2) 11.2 (5) 5.4 |
(1) 16/26 (2) 13/26 (5) 5/26 |
No usable data | (1) 5.3 (2) 4.0 (5) 2.4 |
at 6 hr: (1) 63 (2) 73 (5) 86 |
| Forbes 1986 | (1) Paracetamol+codeine 300/30 mg, n=43 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/15 mg, n=41 (3) Placebo, n=38 |
TOTPAR 6: (1) 7.4 (3) 2.4 |
(1) 13/43 (2) 1/38 |
No usable data | Mean: (1) 4.5 (2) 3.0 |
No data |
| Forbes 1989 | (1) Paracetamol+codeine 600/60 mg, n=17 (2) Paracetamol 600 mg, n=22 (3) Flurbiprofen 100 mg, n=26 (4) Placebo, n=23 |
TOTPAR 6: (1) 10.5 (2) 4.5 (3) 2.0 |
(1) 8/17 (2) 1/22 (4) 0/23 |
No usable data | (1) 5.1 (2) 2.8 (4) 1.7 |
at 6 hr: (1) 64 (2) 82 (4) 91 |
| Forbes 1990a | (1) Paracetamol+codeine 600/60 mg, n=27 (2) Aspirin 650 mg, n=32 (3) Ketorolac 10 mg, n=37 (4) Placebo, n=32 |
TOTPAR 6: (1) 8.6 (4) 2.9 |
(1) 9/27 (4) 1/32 |
No usable data | Mean: (1) 4.3 (4) 3.1 |
at 6 hr: (1) 85 (4) 84 |
| Forbes 1990b | (1) Paracetamol+codeine 600/60 mg, n=38 (2) Paracetamol 600 mg, n=36 (3) Ketorolac 10 mg, n=31 (4) Ketorolac 20 mg, n=35 (5) ibuprofen 400 mg, n=32 (6) Placebo, n=34 |
TOTPAR 6: (1) 6.2 (2) 5.8 (6) 1.9 |
(1) 9/38 (2) 7/36 (6) 0/34 |
No usable data | (1) 2.6 (2) 3.0 (6) 1.8 |
at 6 hr: (1) 82 (2) 81 (6) 97 |
| Forbes 1994 | (1) Paracetamol+codeine 300/30 mg, n=93 (2) Paracetamol+hydrocodone bitartrate 500/7.5 mg, n=94 (3) Placebo, n=45 |
TOTPAR 6: (1) 6.6 (3) 3.4 |
(1) 23/93 (3) 3/45 |
No usable data | Mean: (1) 3.9 (3) 3.4 |
at 6 hr: (1) 77 (3) 87 |
| Gertzbein 1986 | (1) Paracetamol+codeine 1000/60 mg, n=45 (2) Paracetamol 1000 mg, n=45 |
TOTPAR 5: (1) 11.5 (2) 10.2 |
(1) 30/45 (2) 25/45 |
No usable data | Mean: (1) 3.8 (2) 3.6 |
No data |
| Heidrich 1985 | (1) Paracetamol+codeine 300/30 mg, n=40 (2) Ibuprofen 400 mg, n=40 (3) Placebo, n=40 |
TOTPAR 6: (1) 5.5 (3) 3.3 |
(1) 8/40 (3) 3/40 |
No data | Mean: (1) 4.7 (3) 3.5 |
(1) 85 (3) 90 |
| Honig 1984 | (1) Paracetamol+codeine 600/60 mg, n=30 (2) Paracet 600 mg, n=28 (3) Codeine 60 mg, n=28 (4) Placebo, n=30 |
TOTPAR 6: (1) 10.6 (2) 8.9 (4) 5.9 |
(1) 14/30 (2) 11/28 (4) 6/30 |
(1) 12/30 (2) 8/28 (4) 4/30 |
No data | at 6 hr: (1) 33 (2) 43 (4) 53 |
| Malmstrom 2004 | (1) Paracetamol+codeine 600/60 mg, n=50 (2) Etoricoxib 120 mg, n=50 (3) Naproxen sodium 550 mg, n=50 (4) Placebo, n=50 |
TOTPAR 6: (1) 9.2 (4) 4.2 |
(1) 20/50 (4) 6/50 |
at 8 hr: (1) 24/50 (4) 7/50 |
(1) 3.6 (4) 1.6 |
at 24 hr: (1) 76 (4) 99 |
| Pande 1996a | (1) Paracetamol+codeine 600/60 mg, n=23 (2) Placebo, n=26 |
SPID 6: (1) 3.1 (2) 0 Baseline PI: 2.5 |
(1) 6/23 (2) 0/26 |
No usable data | No data | No data |
| Smith 2004 | 1)Paracetamol+codeine 300/30 mg, n=109 2) Paracetamol+tramadol 325/37.5 mg, n=98 3) Placebo n=98 |
SPID 4: (1) 2.7 (3) 2.0 |
(1) 43/109 (3) 27/98 |
No usable data | No data | at 4 hr: (1) 26 (3) 40 |
| Stubhaug 1995 | (1) Paracetamol+codeine 1000/60 mg, n=36 (2) Tramadol 50 mg, n=33 (3) Tramadol 100 mg, n=35 (4) Placebo, n=33 |
VAS SPID 6: (1) 204 (4) 17 Baseline PI: (1) 67 (4) 66 |
(1) 21/36 (4) 1/33 |
No usable data | (1) >6 (4) 2.8 |
at 6 hr: (1) 36 (4) 82 |
| Sunshine 1986 | (1) Paracetamol+codeine 650/60 mg, n=31 (2) Paracetamol 650 mg, n=30 (3) Flurbiprofen 50 mg, n=31 (4) Flurbiprofen 100 mg, n=29 (5) Zomepirac 100 mg, n=31 (6) Placebo, n=30 |
TOTPAR 6: (1) 13.4 (2) 11.1 (6) 8.3 |
(1) 19/31 (2) 15/30 (6) 10/30 |
No usable data | No data | at 6 hr: (1) 30 (2) 47 (6) 43 |
| Turek 1988 | (1) Paracetamol+codeine 650/60 mg, n=39 (2) Ketoprofen 50 mg, n=41 (3) Ketoprofen 150 mg, n=39 (4) Placebo, n=41 |
TOTPAR 6: (1) 8.1 (4) 4.6 |
(1) 14/39 (4) 8/41 |
No usable data | Mean: (1) 3.5 (4) 2.2 |
at 6 hr: (1) 67 (4) 83 |
| Ziccardi 2000 | (1) Paracetamol+codeine 300/30 mg, n=49 (2) Ibuprofen+hydrocodone 200/75 mg, n=49 (3) Placebo, n=27 |
TOTPAR 6: (1) 9.8 (3) 3.5 |
(1) 21/49 (3) 2/27 |
No usable data | (1) 3.0 (3) 1.0 |
No data |
1.

Forest plot of comparison: 3 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, outcome: 1.1 Participants with at least 50% pain relief over 4 to 6 hours.
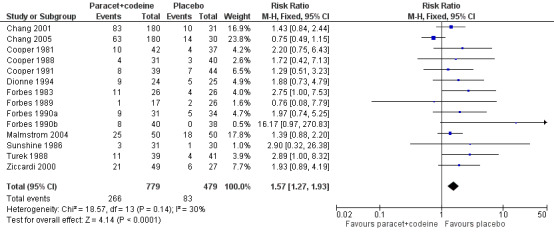
600 to 650 mg paracetamol with 60 mg codeine versus placebo
Seventeen studies provided data: 857 participants were treated with 600/650 mg paracetamol plus 60 mg codeine and 556 with placebo (Table 1; Figure 2; Summary of results A).
The proportion of participants experiencing at least 50% pain relief over four to six hours with 600/650 mg paracetamol plus 60 mg codeine was 43% (370/857).
The proportion of patients experiencing at least 50% pain relief over four to six hours with placebo was 17% (96/556).
The relative benefit of treatment compared with placebo was 2.6 (2.2 to 3.2).
The NNT for at least 50% pain relief over four to six hours was 3.9 (3.3 to 4.7). For every four participants treated with 600/650 mg paracetamol plus 60 mg codeine, one would experience at least 50% pain relief who would not have done so with placebo.
2.

Forest plot of comparison: 5 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over 4 to 6 hours.
300 mg paracetamol with 30 mg codeine versus placebo
Six studies provided data: 379 participants were treated with 300 paracetamol plus 30 mg codeine and 311 with placebo (Table 1; Figure 3; Summary of results A).
The proportion of participants experiencing at least 50% pain relief over four to six hours with 300 mg paracetamol plus 30 mg codeine was 32% (123/379).
The proportion of patients experiencing at least 50% pain relief over four to six hours with placebo was 18% (56/311).
The relative benefit of treatment compared with placebo was 1.9 (1.4 to 2.5).
The NNT for at least 50% pain relief over four to six hours was 6.9 (4.8 to 12). For every seven participants treated with 300 mg paracetamol plus 30 mg codeine, one would experience at least 50% pain relief who would not have done so with placebo.
3.

Forest plot of comparison: 7 Paracetamol 300 mg plus codeine 30 mg versus placebo, outcome: 5.1 Participants with at least 50% pain relief over 4 to 6 hours.
| Summary of results A: participants achieving at least 50% pain relief over 4 to 6 hours: paracetamol plus codeine versus placebo | |||||
| Dose (mg) | Studies | Participants | Paracetamol + codeine (%) | Placebo (%) | NNT (95%CI) ≥50% PR |
| 800‐1000/60 mg | 3 | 192 | 53 | 7 | 2.2 (1.8 to 2.9) |
| 600‐650/60 mg | 17 | 1413 | 43 | 17 | 3.9 (3.3 to 4.7) |
| 300/30 mg | 6 | 690 | 32 | 18 | 6.9 (4.8 to 12) |
The 800 mg to 1000 mg paracetamol plus 60 mg codeine dose was significantly superior to 600 mg to 650 mg paracetamol plus 60 mg codeine (z = 3.42, P < 0.0067), and to 300 mg paracetamol plus 30 mg codeine (z = 4.94, P < 0.0006). Since the total number of participants in the highest dose group is relatively small, the extent of the difference between the groups is not robust. There was borderline significance between 600 to 650 mg paracetamol plus 60 mg codeine and 300 mg paracetamol plus 30 mg codeine (z = 2.78, P < 0.0056).
Paracetamol with codeine versus paracetamol
800 to 1000 mg paracetamol plus 60 mg codeine versus same dose paracetamol alone
Four studies provided data: 109 participants were treated with 1000 mg paracetamol plus 60 mg codeine, 44 with 800 mg paracetamol plus 60 mg codeine, and 151 with 1000 mg paracetamol alone (Table 1; Figure 4; Summary of results B).
The proportion of patients experiencing at least 50% pain relief over four to six hours with 800 mg to 1000 mg paracetamol plus 60 mg codeine was 59% (90/153).
The proportion of participants experiencing at least 50% pain relief over four to six hours with 1000 mg paracetamol alone was 42% (68/151).
The relative benefit of combination treatment compared with paracetamol alone was 1.3 (1.1 to 1.6).
The NNT for at least 50% pain relief over four to six hours was 6.1 (3.6 to 19). For every six participants treated with 800 mg to 1000 mg paracetamol plus 60 mg codeine, one would experience at least 50% pain relief who would not have done so with the same dose of paracetamol alone.
4.
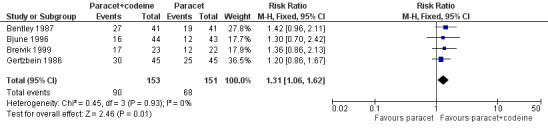
Forest plot of comparison: 4 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, outcome: 2.1 Participants with at least 50% pain relief over 4 to 6 hours.
Omitting the study that compared 800 mg paracetamol plus 60 mg codeine with 1000 mg paracetamol alone, gave a slightly lower (better) NNT, but did not significantly change the result.
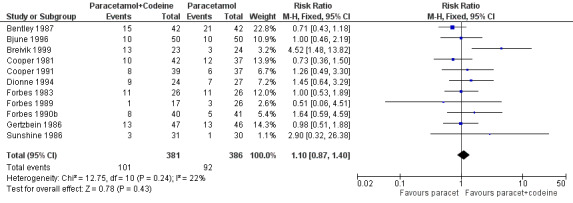
600 to 650 mg paracetamol plus 60 mg codeine versus same dose paracetamol alone
Ten studies provided data: 309 participants were treated with 600 mg to 650 mg paracetamol plus 60 mg codeine and 313 with 600‐650 mg paracetamol alone (Table 1; Figure 5; Summary of results B).
The proportion of participants experiencing at least 50% pain relief over four to six hours with 600 mg to 650 mg paracetamol plus 60 mg codeine was 53% (165/309).
The proportion of participants experiencing at least 50% pain relief over four to six hours with 600/650 mg paracetamol alone was 41% (129/313).
The relative benefit of combination treatment compared with paracetamol alone was 1.3 (1.1 to 1.5).
The NNT for at least 50% pain relief over four to six hours was 8.2 (5.0 to 23). For every eight participants treated with 600 mg to 650 mg paracetamol plus 60 mg codeine, one would experience at least 50% pain relief who would not have done so with the same dose of paracetamol alone.
5.
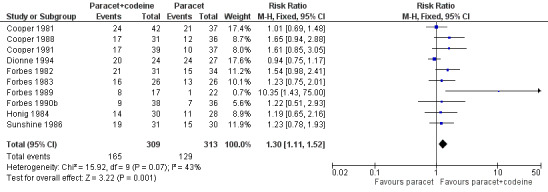
Forest plot of comparison: 6 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600 to 650 mg, outcome: 4.1 Participants with at least 50% pain relief over 4 to 6 hours.
| Summary of results B: participants achieving at least 50% pain relief over 4 to 6 hours: paracetamol plus codeine versus the same dose of paracetamol alone | |||||
| Dose (mg) | Studies | Participants | Paracetamol + codeine (%) | Paracetamol (%) | NNT (95%CI) ≥50% PR |
| 800‐1000/60 | 4 | 304 | 59 | 42 | 6.1 (3.6 to 19) |
| 1000/60 | 3 | 217 | 68 | 48 | 5.1 (3.1 to 15) |
| 600‐650/60 | 10 | 622 | 53 | 41 | 8.2 (5.0 to 23) |
The addition of 60 mg codeine to effective doses of paracetamol increases the number of participants achieving effective pain relief by 10 to 15%. There was no clear dose response.
Sensitivity analysis
Sensitivity analyses were carried out on the primary outcome of number of participants achieving at least 50% pain relief.
Paracetamol with codeine versus placebo
Study quality
Only one study (Heidrich 1985) had a quality score of two. A formal sensitivity analysis by quality was therefore not performed. Removing this single study from the analysis for paracetamol 300 mg plus codeine 30 mg versus placebo did not change the results for relative benefit or NNT.
Pain model
For 600 mg to 650 mg paracetamol plus 60 mg codeine in dental studies only, the relative benefit of treatment compared with placebo was 2.7 (2.2 to 3.4), and the NNT for at least 50% pain relief over four to six hours was 3.9 (3.3 to 4.8). For other surgical trials only, the relative benefit of treatment compared with placebo was 2.4 (1.5 to 3.7), and the NNT for at least 50% pain relief over four to six hours was 3.7 (2.5 to 7.2).
For 300 mg paracetamol plus 30 mg codeine in dental trials only, the relative benefit of treatment compared with placebo was 3.3 (1.8 to 6.2), and the NNT for at least 50% pain relief over four to six hours was 5.4 (3.7 to 9.7). For other surgical trials only, the relative benefit of treatment compared with placebo was 1.5 (1.1 to 2.1), and the NNT for at least 50% pain relief over four to six hours was 7.9 (4.6 to 27).
There were insufficient data to analyse the 1000 mg paracetamol plus 60 mg codeine dose separately. For both comparisons, the 95% CIs were overlapping, indicating no statistically significant differences between studies in dental and other surgery for this outcome.
| Sensitivity analysis ‐ effect of pain model: paracetamol plus codeine versus placebo | |||||
| Study characteristics | Studies | Participants | Paracetamol + codeine (%) | Placebo (%) | NNT (95%CI) ≥50% PR |
| 600‐650/60 mg dental | 14 | 1221 | 43 | 17 | 3.9 (3.3 to 4.8) |
| 600‐650/60 mg other surgical | 3 | 192 | 46 | 20 | 3.7 (2.5 to 7.2) |
| 300/60 mg dental | 3 | 299 | 27 | 9 | 5.4 (3.7 to 9.7) |
| 300/60 mg other surgical | 3 | 391 | 37 | 24 | 7.9 (4.6 to 27) |
Study size
Five studies, with 629 participants, had more than 40 patients in both the paracetamol plus codeine and placebo arms. The NNT for at least 50% pain relief over 4 to 6 hours was 6.7 (4.6 to 12). Four of these five studies used 300 mg paracetamol plus 30 mg codeine.
Ten studies with 572 participants had fewer than 40 patients in both the paracetamol with codeine and placebo arms. The NNT for at least 50% pain relief over four to six hours was 3.0 (2.4 to 3.8). All of these studies used 600 mg paracetamol plus 60 mg codeine or more.
There was no overlap in the 95% CIs for the larger and smaller studies, indicating a statistically significant difference between these two groups of studies. However, the comparison is confounded by dose, since for the larger group size, four of these five studies used 300 mg paracetamol plus 30 mg codeine in contrast to the smaller group size, where all of the studies used a dose of 600 mg paracetamol plus 60 mg codeine or more.
Paracetamol with codeine versus paracetamol
Study quality
All trials scored three or more on the Oxford pain score, hence a sensitivity analysis was not possible.
Pain model
The primary outcome of at least 50% pain relief was compared in dental versus other surgical pain models. There were insufficient data to compare the results of different pain models separately for each dose.
Study size
Three studies, with 259 participants, had more than 40 patients in both the paracetamol with codeine and paracetamol alone arms. All three studies used 800 mg to 1000 mg paracetamol plus 60 mg codeine versus 1000 mg paracetamol. The NNT for at least 50% pain relief over four to six hours was 7.1 (4.1 to 150).
Ten studies, with 588 participants, had fewer than 40 patients in both the paracetamol with codeine and paracetamol only arms. Nine of these studies used 600 mg to 650 mg paracetamol plus 60 mg codeine versus the same dose of paracetamol, and one used 1000 mg paracetamol plus 60 mg codeine versus the same dose of paracetamol. The NNT for at least 50% pain relief over four to six hours was 6.4 (4.3 to 13).
No significant effect of size on the primary outcome was demonstrated using 40 participants per treatment arm as the threshold.
| Sensitivity analysis ‐ effect of study size: paracetamol plus codeine versus the same dose of paracetamol alone | |||||
| Study characteristics | Studies | Participants | Paracetamol + codeine (%) | Paracetamol alone (%) | NNT (95%CI) 50% PR |
| ≥40 participants in active and comparator groups | 3 | 259 | 56 | 43 | 7.1 (4.1 to 150) |
| <40 participants in active and comprator groups | 10 | 588 | 54 | 39 | 6.4 (4.3 to 13) |
Use of rescue medication
Number of participants using rescue medication over 4 to 6 hours
Paracetamol with codeine versus placebo
In total 16 studies, with 1313 participants, reported the number of participants taking rescue medication over four to six hours (Table 1). For 600 mg to 650 mg paracetamol plus 60 mg codeine, 10 studies with 657 participants reported this outcome. The weighted mean proportion was 59% (198/320) in the combination group versus 80% (270/337) in the placebo group, giving a NNTp of 4.8 (3.6 to 7.2) (Figure 6). For 300 mg paracetamol with 30 mg codeine, four studies with 529 participants reported this outcome. The weighted mean proportion was 48% (143/297) in the combination group versus 57% (132/232) in the placebo group. The NNTp was not calculated (Figure 7; Summary of results C). There were insufficient data to analyse the 1000 mg paracetamol plus 60 mg codeine dose separately.
6.
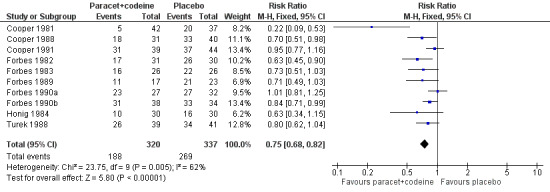
Forest plot of comparison: 5 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, outcome: 3.4 Participants using rescue medication over 4 to 6 hours.
7.
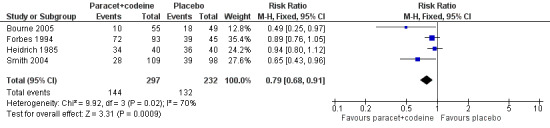
Forest plot of comparison: 7 Paracetamol 300 mg plus codeine 30 mg versus placebo, outcome: 5.4 Participants using rescue medication over 4 to 6 hours.
Five people would need to be treated with 600 to 650 mg paracetamol plus 60 mg codeine to prevent one needing rescue medication over four to six hours, who would have needed it with placebo.
Paracetamol with codeine versus paracetamol
Nine studies, with 563 participants, reported the proportion taking rescue medication over four to six hours (Table 1). For 600 mg to 650 mg paracetamol plus 60 mg codeine, seven studies with 436 participants reported this outcome. The weighted mean proportion was 50% (108/216) in the combination group versus 65% (142/220) in the paracetamol alone group, giving a NNTp of 6.9 (4.2 to 19) (Figure 8; Summary of results C). There was insufficient data to analyse the 1000 mg paracetamol plus 60 mg codeine dose separately.
8.
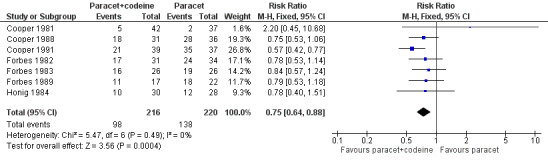
Forest plot of comparison: 6 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600 to 650 mg, outcome: 4.4 Participants using rescue medication over 4 to 6 hours.
Adding 60 mg codeine to 600 mg to 650 mg paracetamol reduced the number of participants requiring rescue medication over four to six hours by 15%. Seven people would need to be treated with the combination to prevent one requiring rescue medication over four to six hours, who would have needed it with 600 mg to 650 mg paracetamol alone.
| Summary of results C: number of participants using rescue medication in 4 to 6 hours | |||||
| Dose | Studies | Participants | Paracetamol + codeine (%) | Placebo (%) | NNTp |
| 600‐650/60 mg | 10 | 657 | 62 | 80 | 5.6 (4.0 to 9.0) |
| 300/30 mg | 4 | 529 | 48 | 57 | not calculated |
| Studies | Participants | Paracetamol + codeine (%) | Paracetamol (%) | NNTp | |
| 600‐650/60 mg | 7 | 436 | 50 | 65 | 6.9 (4.2 to 19) |
Time to use of rescue medication
Paracetamol with codeine versus placebo
Eighteen studies reported time to use of rescue medication (all of those reporting the number remedicating) (Table 1). Eight studies reported the median time, eight studies the mean time, and two studies reported both median and mean times to use of rescue medication. There were insufficient data to analyse either the mean or median time to use of rescue medication by dose.
The range of values for median time was 2.3 to 6.5 hours for combination treatment and 1.0 to 3.2 hours for placebo, and for mean time was 3.1 to 7.0 hours for combination treatment and 2.2 to 3.5 hours for placebo. The weighted mean of the median time to use of rescue medication was 4.3 hours for paracetamol plus codeine (all doses) versus 2.0 hours for placebo. The weighted mean of the mean time to use of rescue medication was 4.1 hours for paracetamol (all doses) versus 2.4 hours for placebo (Summary of results D).
Half of the participants required rescue medication by 4.3 hours when treated with paracetamol plus codeine, compared to 2.0 hours when treated with placebo.
Paracetamol with codeine versus paracetamol
Eight studies reported time to use of rescue medication, five of which reported the median, and three the mean time (Table 1). There were insufficient data to analyse either the mean or median time to use of rescue medication by dose.
The range of values for median time was 4.1 to more than eight hours for combination treatment and 2.8 to 8.0 hours for paracetamol alone, and the range for mean time was 3.1 to 3.8 hours for combination treatment and 3.2 to 3.6 hours for paracetamol alone. The weighted mean of the median time to remedication was 5.4 hours for paracetamol plus codeine (all doses) versus 4.1 hours for paracetamol alone. The weighted mean of the mean time to remedication was 3.5 hours for paracetamol plus codeine (all doses) versus 3.4 hours for paracetamol alone (Summary of results D).
Half of the participants required rescue medication by 5.4 hours when treated with paracetamol plus codeine, compared to 4.1 hours when treated with the same dose of paracetamol alone.
| Summary of results D ‐ weighted mean time to use of rescue medication | ||
| Weighted mean | Paracetamol + codeine | Placebo |
| Median (hr) | 4.3 | 2.0 |
| Mean (hr) | 4.1 | 2.4 |
| Paracetamol + codeine | Paracetamol | |
| Median (hr) | 5.4 | 4.1 |
| Mean (hr) | 3.5 | 3.4 |
Adverse events
The time over which adverse events were collected varied from four hours to seven days. It was unclear in some reports whether the adverse event reports covered the duration of the trial, or whether they included any adverse events occurring between the end of the trial and a follow‐up visit some days later. Few studies reported whether adverse event data continued to be collected after rescue medication had been taken. Reported adverse events were mainly mild and transient, and there were no serious adverse events reported.
Paracetamol with codeine versus placebo
Twenty studies reported numbers of participants with one or more adverse events (Table 2). For all doses together, the NNH for any adverse event for paracetamol plus codeine compared to placebo was 8.9 (6.6 to 14) (Figure 9), and for 600 mg to 650 mg paracetamol plus 60 mg codeine compared to placebo the NNH was 6.0 (4.6 to 8.3) (Figure 10). For the higher and lower doses the relative risk was not significant.
2. Summary of outcomes ‐ adverse events and withdrawals.
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| Bentley 1987 | (1) Paracetamol+codeine 1000/60 mg, n=41 (2) Paracetamol 1000 mg, n=41 (3) Codeine 60 mg, n=21 (4) Placebo, n=17 |
(1) 15/42 (2) 21/42 (4) 9/19 |
No data | None reported | 1 pt lost to follow up, 4 had invalid data |
| Bjune 1996 | (1) Paracetamol+codeine 800/60 mg, n=50 (2) Paracetamol 1000 mg, n=50 (3) Placebo, n=25 |
(1) 10/50 (2) 10/50 (3) 1/25 |
None | None reported | 6 paracetamol+codeine pts, 7 paracetamol pts, 4 placebo pts had invalid data |
| Bourne 2005 | (1) Paracetamol+codeine 300/30 mg, n = 55 (2) Paracetamol+tramadol 325/37.5 mg, n = 49 (3) Placebo, n = 49 |
No single dose data | No single dose data | No single dose data | None |
| Breivik 1999 | (1) Paracetamol+codeine 1000/60 mg n=24 (2) Paracetamol 1000mg, n=22 (3) Diclofenac 100 mg, n=22 (4) Paracetamol+diclofenac 1000/100 mg, n=24 (5) Paracetamol+diclofenac+codeine 1000/100/60 mg, n=24 |
(1) 13/23 (2) 3/24 |
None | None | 1 pt in paracetamol+codeine group lost to follow up, 4 pts (2 paracetamol, 2 diclofenac) had invalid data |
| Chang 2001 | (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50 mg n=182 (3) Placebo n=31 |
At 24 hr: (1) 83/180 (2) 10/31 |
None | None | 6 pts lost to follow up, 1 para/codeine group vomited medication |
| Chang 2005 | (1) Paracetamol+codeine 600/60 mg n=180 (2) Rofecoxib 50 mg n=180 (3) Placebo n=30 |
At 24 hr: (1) 63/180 (3) 14/30 |
None | None | 1 rofecoxib pt lost to follow up |
| Cooper 1981 | (1) Paracet+codeine 650/60 mg, n=42 (2) Paracetamol 650 mg, n=37 (3) Paracetamol+d‐propoxyphene 650/100 mg, n=42 (4) Ibuprofen 200 mg, n=42 (5) Placebo, n=37 |
(1) 10/42 (2) 12/37 (5) 4/37 |
None | None | 31 pts had invalid data |
| Cooper 1988 | (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=36 (3) Meclofenamate Na 100 mg, n=36 (4) Placebo, n=40 |
15 pts in total reported adverse events | None | None | 11 pts lost to follow up, 3 had invalid data |
| Cooper 1991 | (1) Paracetamol+codeine 650/60 mg, n=39 (2) Paracetamol 650 mg, n=37 (3) Zomepirac 100 mg, n=23 (4) Flurbiprofen 50 mg, n=42 (5) Flurbiprofen 100 mg, n=41 (6) Placebo, n=44 |
(1) 8/39 (2) 6/37 (6) 7/44 |
None | None reported | 3 lost to follow up, 5 had invalid data |
| Desjardins 1986 | (1) Paracetamol+codeine 300/30 mg, n=39 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/30 mg, n=43 (3) Placebo, n=41 |
(1) 2/39 (3) 4/41 |
None | None | 14 pts did not medicate, lost to follow up, invalid data |
| Dionne 1994 | (1) Paracetamol+codeine 650/60 mg, n=24 (2) Paracetamol 650 mg, n=27 (3) Flurbiprofen 50 mg, n=25 (4) Flurbiprofen 100 mg, n=22 (5) Placebo, n=25 |
(1) 9/24 (2) 7/27 (5) 5/25 |
None | None reported | 11 pts had invalid data |
| Forbes 1982 | (1) Paracetamol+codeine 600/60 mg, n=31 (2) Paracetamol 600 mg, n=34 (3) Diflusinal 500 mg, n=32 (4) Diflusinal 1000 mg, n=32 (5) Placebo, n=30 |
15% in total reported adverse events | None | None | 4 pts lost to follow up, 11 had invalid data |
| Forbes 1983 | (1) Paracetamol+codeine 600/60 mg, n=26 (2) Paracetamol 600 mg, n=26 (3) Diflusinal 500 mg, n=26 (4) Diflusinal 1000 mg, n=28 (5) Placebo, n=26 |
(1) 11/26 (2) 11/26 (5) 4/26 |
None reported | None | None |
| Forbes 1986 | (1) Paracetamol+codeine 300/30 mg, n=43 (2) Aspirin+butalbital+caffeine+codeine 325/50/40/15 mg, n=41 (3) Placebo, n=38 |
(1) 6/46 (2) 9/46 |
None | None | 1 pt lost to follow up, 17 had invalid data |
| Forbes 1989 | (1) Paracetamol+codeine 600/60 mg, n=17 (2) Paracetamol 600 mg, n=22 (3) Flurbiprofen 100 mg, n=26 (4) Placebo, n=23 |
(1) 1/17 (2) 3/26 (4) 2/26 |
None | None | 10 pts had invalid data |
| Forbes 1990a | (1) Paracetamol+codeine 600/60 mg, n=27 (2) Aspirin 650 mg, n=32 (3) Ketorolac 10 mg, n=37 (4) Placebo, n=32 |
(1) 9/31 (4) 5/34 |
None | None | 1 lost to follow up, 14 had invalid data |
| Forbes 1990b | (1) Paracetamol+codeine 600/60 mg, n=38 (2) Paracetamol 600 mg, n=36 (3) Ketorolac 10 mg, n=31 (4) Ketorolac 20 mg, n=35 (5) Ibuprofen 400 mg, n=32 (6) Placebo, n=34 |
(1) 8/40 (2) 5/41 (6) 0/38 |
None | None | 3 pts lost to follow up, 27 had invalid data |
| Forbes 1994 | (1) Paracetamol+codeine 300/30 mg, n=93 (2) Paracetamol+hydrocodone bitartrate 500/7.5 mg, n=94 (3) Placebo, n=45 |
(1) 18/107 (3) 10/65 |
None | None | 1 pt lost to follow up, 59 had invalid data |
| Gertzbein 1986 | (1) Paracetamol+codeine 1000/60 mg, n=45 (2) Paracetamol 1000 mg, n=45 |
(1) 13/47 (2) 13/46 |
None | None | 1 pt withdrew consent, 2 pts had invalid data (2 paracetamol+codeine group, 1 paracetamol group) |
| Heidrich 1985 | (1) Paracetamol+codeine 300/30 mg, n=40 (2) Ibuprofen 400 mg, n=40 (3) Placebo, n=40 |
No sig diff between groups | No data | None | No data |
| Honig 1984 | (1) Paracetamol+codeine 600/60 mg, n=30 (2) Paracetamol 600 mg, n=28 (3) Codeine 60 mg, n=28 (4) Placebo, n = 30 |
No data | None reported | None reported | None reported |
| Malmstrom 2004 | (1) Paracetamol+codeine 600/60 mg, n=50 (2) Etoricoxib 120 mg, n=50 (3) Naproxen sodium 550 mg, n=50 (4) Placebo, n=50 |
Within 10 days: (1) 25/50 (4) 18/50 |
None | None | 4 pts lost to follow up (2 paracetamol+codeine group, 1 placebo group) |
| Pande 1996a | (1) Paracetamol+codeine 600/60 mg, n=23 (2) Placebo, n=26 |
no data | None | None reported | None reported |
| Smith 2004 | (1)Paracetamol+codeine 300/30 mg, n = 109 (2) Paracetamol+tramadol 325/37.5 mg, n = 98 (3) Placebo n = 98 |
No single dose data | No single dose data | No single dose data | 1 pt had invalid data |
| Stubhaug 1995 | (1) Paracetamol+codeine 1000/60 mg, n=36 (2) Tramadol 50 mg, n=33 (3) Tramadol 100 mg, n=35 (4) Placebo, n=33 |
(1) 10/37 (2) 15/36 |
None | None reported | 7 pts had invalid data |
| Sunshine 1986 | (1) Paracetamol+codeine 650/60 mg, n=31 (2) Paracetamol 650 mg, n=30 (3) Flurbiprofen 50 mg, n=31 (4) Flurbiprofen 100 mg, n=29 (5) Zomepirac 100 mg, n=31 (6) Placebo, n=30 |
(1) 3/31 (2) 1/30 (6) 1/30 |
None | None reported | None |
| Turek 1988 | (1) Paracetamol+codeine 650/60 mg, n=39 (2) Ketoprofen 50 mg, n=41 (3) Ketoprofen 150 mg, n=39 (4) Placebo, n=41 |
(1) 11/39 (4) 4/41 |
None | None | 1 placebo pt had invalid data |
| Ziccardi 2000 | (1) Paracetamol+codeine 300/30 mg, n=49 (2) Ibuprofen+hydrocodone 200/75 mg, n=49 (3) Placebo, n= 27 |
(1) 21/49 (3) 6/27 |
None | No data | None reported |
9.
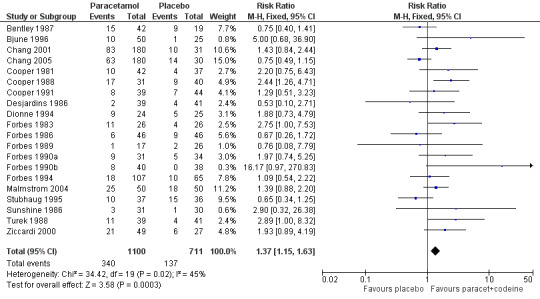
Forest plot of comparison: 8 Paracetamol plus codeine (all doses) versus placebo, outcome: 8.1 Participants with at least one adverse event.
10.
Forest plot of comparison: 5 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, outcome: 3.5 Participants with any adverse event.
Paracetamol with codeine versus paracetamol
Eleven studies reported numbers of participants with one or more adverse events (Table 2). There were no significant differences in relative risk and NNH for any adverse event between paracetamol plus codeine and paracetamol alone at any dose, or for all doses combined (Figure 11).
11.
Forest plot of comparison: 9 Paracetamol plus codeine (all doses) versus paracetamol alone, outcome: 9.1 Participants with any adverse event.
| Summary of results E ‐ participants with one or more adverse events | |||||
| Dose (mg) | Studies | Participants | Paracetamol + codeine (%) | Placebo (%) | NNH (95%CI) any AE |
| All doses | 20 | 1811 | 31 | 19 | 8.6 (6.4 to 13) |
| 800‐1000/60 mg | 3 | 209 | 27 | 31 | not calculated |
| 600‐650/60 mg | 14 | 1187 | 35 | 18 | 6.0 (4.6 to 8.3) |
| 300/30 mg | 3 | 344 | 15 | 14 | not calculated |
| Paracetamol + codeine (%) | Paracetamol (%) | ||||
| All doses | 11 | 767 | 27 | 24 | not calculated |
| 800‐1000/60 mg | 4 | 324 | 31 | 29 | not calculated |
| 600‐650/60 mg | 7 | 443 | 23 | 20 | not calculated |
Withdrawals
Participants who took rescue medication were classified as withdrawals due to lack of efficacy, and are reported under 'use of rescue medication' above.
Data on other withdrawals were generally poorly reported, probably because these were single dose studies where withdrawals for reasons other than lack of efficacy are uncommon. Some studies reported participants who had invalid data due to inadequate baseline pain, who violated a protocol, or took rescue medication within the first hour, as withdrawals or exclusions. Whether these should be included in the intention to treat population is arguable. Attrition due to invalid data is unlikely to affect results.
No study reported any withdrawals due to adverse events.
Discussion
Since the previous review of paracetamol with codeine in acute pain a number of new studies of good methodological quality have been published. For the comparison with placebo, new studies added 459 participants treated with 600 mg paracetamol plus 60 mg codeine, and 109 treated with 300 mg paracetamol plus 30 mg codeine. This more than doubles the number of participants for the 600 plus 60 mg dose analysis, and adds 75% more to the 300 plus 30 mg analysis. No new trials compared 1000 mg paracetamol plus 60 mg codeine with placebo. For the comparison with paracetamol alone, only 74 new participants treated with 800 or 1000 mg paracetamol plus 60 mg codeine were added, but even this almost doubled the number available for analysis at this dose (Moore 1998a).
The primary measure of efficacy was the proportion of patients achieving at least 50% pain relief over four to six hours. This is now generally regarded as a useful level of pain relief in acute pain, and also in chronic pain conditions such as arthritis (Moore 2008) and neuropathic pain (Straube 2008). It has the advantage that it also highlights that not all of those given an analgesic have useful pain relief, and that interventions do not work in everyone. Participants not having a useful level of pain relief are important because therapeutic failure is to be avoided. Figures and tables therefore provide percentages of patients with outcomes, as well as statistical comparisons.
The variation in placebo and active response rates was large, but this degree of variation is common in pain studies (McQuay 1996), and occurs because of the random play of chance (Moore 1998b). Paracetamol plus codeine was significantly better than placebo, with a clear dose response for the primary outcome of at least 50% pain relief over four to six hours. At doses of 300 mg paracetamol plus 30 mg codeine about 30% of participants achieved this level of pain relief, rising to about 40% with 600 mg to 650 mg paracetamol plus 60 mg codeine, and 50% with 800 to 1000 mg paracetamol plus 60 mg codeine. Corresponding NNTs were 6.9 (4.8 to 12), 3.9 (3.3 to 4.7) and 2.2 (1.8 to 2.9). These results are consistent with those found in the earlier review, but should be more robust because of the increased number of participants. Seven people would need to be treated with 600 mg paracetamol plus 60 mg codeine, and two with 1000 mg paracetamol plus 60 mg codeine, for one more to experience at least 50% pain relief, who would not have done so with placebo.
Because the same methods of analyses have been used, it is possible to compare the NNT for a single dose of oral paracetamol plus codeine with that of a single dose of other analgesics.
Analgesics with comparable efficacy to 1000 mg paracetamol plus 60 mg codeine include celecoxib 400 mg (NNT 2.5 (2.2 to 2.9)) (Derry 2008), diclofenac 100 mg (NNT 1.8 (1.5 to 2.1)) (Collins 1999), and ibuprofen 400 mg (NNT 2.7 (2.5 to 3.0)) (Collins 1999).
Paracetamol combination analgesics with comparable efficacy to 1000 mg paracetamol plus 60 mg codeine include 650 mg paracetamol plus 10 mg oxycodone (NNT 2.6 (2.0 to 3.5)), and 650 mg paracetamol plus 75 mg tramadol (NNT 2.6 (2.3 to 3.0)).
Analgesics with comparable efficacy to 600 mg to 650 mg paracetamol plus 60 mg codeine include celecoxib 200 mg (NNT 4.2 (3.4 to 5.6)) (Derry 2008), paracetamol 1000 mg (NNT 3.6 (3.2 to 4.1)) (Toms 2008), and aspirin 600 mg to 650 mg (NNT 4.4 (4.0 to 4.9) (Oldman 1999).
Paracetamol combination analgesics with comparable efficacy to 600 mg to 650 mg paracetamol plus 60 mg codeine include 650 mg paracetamol plus 65 mg dextropropoxyphene HCl (NNT 4.4 (3.5 to 5.6)).
Analgesics with lower efficacy in single dose studies include codeine 60 mg alone (NNT 17 (11 to 48)) and tramadol 50 mg (NNT 8.3 (6.0 to 13)).
Analgesics with superior efficacy include etoricoxib 180/240 mg (NNT 1.5 (1.3 to 1.7) and etoricoxib 120 mg (NNT 1.9 (1.7 to 2.1)) (Clarke in press).
A current listing of reviews of analgesics in the single dose postoperative pain model can be found at www.medicine.ox.ac.uk/bandolier.
The comparison of combination therapy with the same dose of paracetamol alone demonstrated that the addition of codeine increased the number of participants with at least 50% pain relief by over 10%. No statistically significant difference was demonstrated between 800 mg to 1000 mg paracetamol plus 60 mg codeine and 600 mg to 650 mg paracetamol plus 60 mg codeine, although the higher dose did give a lower (better) NNT of 6.1 (3.6 to 19) compared to 8.2 (5.0 to 23). Six people would need to be treated with 1000 mg paracetamol plus 60 mg codeine for one more to experience at least 50% pain relief, who would not have done so with the same dose of paracetamol alone. One of the four studies contributing to the higher dose analysis compared 800 mg paracetamol plus 60 mg codeine with 1000 mg paracetamol alone, rather than 800 mg (exactly the same dose). Omitting this study from the analysis gave a lower (better) NNT of 5.1 (3.1 to 15), but did not significantly change the result. The higher dose of paracetamol in the comparator arm would be expected, if anything, to reduce the estimate of efficacy for the combination therapy arm.
We have effective analgesics, but clinical practice finds it difficult to use effective analgesics effectively. More immediately relevant outcomes are needed than relative benefit and even NNTs. One such outcome is the time before participants with adequate pain relief require additional analgesic because the pain has returned. This can be measured in terms of the mean or median time to remedication, or the percentage of participants needing more analgesic over a particular time. This update includes both these outcomes. The previous review did not report data on participants using rescue medication, and not all studies (17/28) provided this information, which restricted analysis, particularly by dose.
The median time to use of rescue medication varied greatly between trials, particularly for the active treatment arms, but was generally longer for paracetamol plus codeine than for placebo or paracetamol alone. The weighted mean of the median time to use of rescue medication (all doses of paracetamol plus codeine) at 4.3 hours is equal to or shorter than most non‐selective NSAIDs (diclofenac 50 mg 3.8 hours, ibuprofen 400 mg 5.3 hours, naproxen 9.8 hours) and much shorter than etoricoxib 120 mg and rofecoxib 50 mg (20 hours or more). The addition of codeine to paracetamol extended the duration of action by about one hour. There were insufficient data to analyse this by dose, as not all studies reporting number of patients remedicating also reported this outcome. Most of the data was for the 600 mg to 650 mg paracetamol plus 60 mg codeine dose, for which about 60% of participants receiving the combination therapy used rescue medication over four to six hours, compared to about 80% of those receiving placebo. Five participants would need to be treated with this dose of the combination to prevent one using rescue medication over four to six hours, who would have done so if treated with placebo. The addition of 60 mg codeine to 600 mg to 650 mg paracetamol decreased the number of participants using rescue medication by about 15%; seven people would need to be treated with 600 mg to 650 mg paracetamol plus 60 mg codeine for one fewer to need rescue medication over four to six hours, who would have done so if treated with the same dose of paracetamol alone.
Longer duration of action is desirable in an analgesic, particularly in a postoperative setting where the patient may experience postoperative nausea, or be dependent on a third party to respond to a request for rescue medication. Duration of pain relief and requirement of rescue medication information have only recently been recognised as important outcomes (Moore 2005), and a fuller evaluation of the importance of these outcomes will depend on more data being collected from other, ongoing, systematic reviews.
Assessment of adverse events is limited in single dose studies as the size and duration of the trials permits only the simplest analysis, as has been emphasised previously (Edwards 1999). Combining results was potentially hampered by the different periods over which the data was collected. There was also an uncertainty about whether adverse event data continued to be collected after rescue medication had been taken. This could disproportionately inflate adverse events in the placebo groups, which tended to use more rescue medication. Most adverse events were reported as mild to moderate in intensity, and were most likely to be related to the anaesthetic or surgical procedure (e.g. nausea, vomiting and somnolence). Although the original review compared individual adverse events, we deemed there to be insufficient data in for this analysis to be valid.
There was a significant difference between paracetamol plus codeine and placebo for numbers of participants experiencing any adverse event in the hours immediately following a single dose of the study medication for all doses together and for 600 mg to 650 mg paracetamol plus 60 mg codeine. Analyses for higher and lower doses did not show a significant difference, but numbers of participants in these groups were small. There was no significant difference between paracetamol plus codeine and the same dose of paracetamol alone.
No serious adverse events or withdrawals due to adverse events were reported. It is important to recognise that adverse event analysis after single dose oral administration will not reflect possible adverse events occurring with use of drugs for longer periods of time. In addition, the relatively small numbers of participants, even when all the trials were combined, and short duration of studies is insufficient to detect rare but serious adverse events, which typically occur with longer use, and usually less frequently than one in 1000.
All studies were of adequate or good methodological quality. The sensitivity analyses did not demonstrate an effect of dental model on NNT for 50% pain relief in the comparison of paracetamol plus codeine and placebo, where there were sufficient data to analyse by dose. For the comparison of combination therapy with placebo, studies with fewer than 40 participants in each treatment arm had a higher active response rate and lower (better) NNT than those with more than 40 per treatment arm, but this comparison was confounded by dose, with the smaller treatment arms using 600 mg paracetamol plus 60 mg codeine (nine studies) or 1000 mg paracetamol plus 60 mg codeine (one study), and the larger treatment arms using 300 mg paracetamol plus 30 mg codeine (four studies) or 600 mg paracetamol plus 60 mg codeine (one study). The comparison of combination therapy with paracetamol alone was also confounded by dose, but did not demonstrate a difference between smaller and larger studies.
The main limitation is that these were single‐dose studies, and they could be criticised because pain relief, even in the acute setting, usually requires multiple dosing. That is true, but, in very general terms, pain is pain, and these single dose studies have been used for over 60 years to establish that a drug is actually an analgesic. The relative effectiveness of drugs and other interventions in this setting translates well to other settings like migraine, or musculoskeletal pain. It is unfortunate that so little data were available for the 1000 mg paracetamol plus 60 mg codeine dose. This is the most commonly used dose in clinical practice in some parts of the world, including the UK, but is under‐represented in these studies. The information that is available indicates that this dose is superior to the lower doses.
Authors' conclusions
Implications for practice.
The data found in this update for Issue 1, 2009 further supports previous findings although more research is still required. The combination of paracetamol and codeine is an effective analgesic in postoperative pain with a low incidence of adverse events. At a dose of 1000 mg paracetamol plus 60 mg codeine it provides effective analgesia for over half of patients with moderate to severe postoperative pain following various types of surgery. The addition of codeine increases the proportion of patients with effective pain relief by over 10%, but increases the number of patients experiencing adverse events. Associated adverse events were of mild or moderate intensity, and did not lead to withdrawal.
Implications for research.
Additional data for the higher dose is needed to confirm the dose response demonstrated in this review, and provide a more robust estimate of efficacy. More consistent data on use of rescue medication would provide better estimates of duration of analgesia, which in turn may help to decide which analgesics are most effective in the clinical setting. While more recent studies were generally of good quality, and efficacy data, where collected, was well reported, the quality of adverse event reporting remains problematical.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 8 June 2016 | Review declared as stable | See Published notes. |
| 15 September 2011 | Review declared as stable | The authors of this review ran a quick search in April 2011 and agreed that this review would not need updating for at least five years. |
| 13 May 2009 | Amended | Contact details updated. |
| 29 August 2008 | New citation required but conclusions have not changed | Analysis completed. Similar results. New analyses for use of rescue medication |
| 22 August 2008 | New search has been performed | New studies added. For paracetamol plus codeine versus placebo, six new studies (908 participants, 65% more) were included and six new and two old studies were excluded. For paracetamol plus codeine versus paracetamol alone, two new studies (132 participants, 14% more) were included and six new studies were excluded. Some references previously included in this review have also now been removed as they relate to when this review was joined with the 'Single dose oral paracetamol (acetaminophen) for postoperative pain in adults' review. |
| 23 May 2008 | Amended | Converted to new review format. |
| 25 January 2002 | Amended | New studies found but not yet included or excluded |
Notes
2011: This review is an update of a previous review of single dose paracetamol and codeine in postoperative pain. Originally paracetamol plus codeine was part of a larger review that included paracetamol alone, published in 1998. The original review was split into two components and both have been updated in 2008.
2016: A restricted search in June 2016 only identified one relevant study (Gatoulis 2012). However, we judged that this study was unlikely to change the conclusions. Therefore, this review has now been stabilised for five years following discussion with the authors and editors. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Gatoulis SC, Voelker M, Fisher M: Assessment of the efficacy and safety profiles of aspirin and acetaminophen with codeine: results from 2 randomized, controlled trials in individuals with tension‐type headache and postoperative dental pain; Clinical therapeutics 2012; 34(1):138‐48.
Acknowledgements
Sally Collins, Dawn Carroll and Jayne Rees were authors on the original review; Martin Tramèr, Marie Bisercic and Anna Oldman translated reports, and Clare Abbott helped with obtaining a mountain of papers. Sources of support for the original review were: Anglia and Oxford RHA, UK, NHS Research and Development Health Technology Evaluation Programmes, UK, and European Union Biomed 2 Grant no. BMH4 CT95 0172, UK.
Appendices
Appendix 1. MEDLINE search strategy
1. acetaminophen [single term MESH] AND codeine [single term MESH]
2. (acetaminophen OR paracetamol) AND codeine
3. OR/1‐2
4. PAIN, POSTOPERATIVE [single term MeSH]
5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")) [in title, abstract or keywords]
6. ((post‐surgical adj4 pain$) or (“post surgical” adj4 pain$) or (post‐surgery adj4 pain$))[in title, abstract or keywords]
7. ((“pain‐relief after surg$”) or (“pain following surg$”) or (“pain control after”)) [in title, abstract or keywords]
8. ((“post surg$” or post‐surg$) AND (pain$ or discomfort)) [in title, abstract or keywords]
9. ((pain$ adj4 “after surg$”) or (pain$ adj4 “after operat$”) or (pain$ adj4 “follow$ operat$”) or (pain$ adj4 “follow$ surg$”))[in title, abstract or keywords]
10. ((analgesi$ adj4 “after surg$”) or (analgesi$ adj4 “after operat$”) or (analgesi$ adj4 “follow$ operat$”) or (analgesi$ adj4 “follow$ surg$”))
11. OR/4‐10
12. randomized controlled trial.pt.
13. controlled clinical trial.pt.
14. randomized controlled trials.sh.
15. random allocation.sh.
16. double‐blind method.sh.
17. clinical trial.pt.
18. exp clinical trials/
19. (clin$ adj25 trial$) [in title, abstract or keywords]
20. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)) [in title, abstract or keywords]
21. placebos.sh.
22. placebo$ [in title, abstract or keywords]
23. random$ [in title, abstract or keywords]
24. research design.sh.
25. OR/12‐24
26. 3 AND 11 AND 25
Appendix 2. EMBASE search strategy
1. acetaminophen [single term MESH] AND codeine [single term MESH]
2. (acetaminophen OR paracetamol) AND codeine
3. OR/1‐2
4. PAIN, POSTOPERATIVE [single term MeSH]
5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$"))
6. ((post‐surgical adj4 pain$) or (“post surgical” adj4 pain$) or (post‐surgery adj4 pain$))
7. ((“pain‐relief after surg$”) or (“pain following surg$”) or (“pain control after”))
8. ((“post surg$” or post‐surg$) AND (pain$ or discomfort))
9. ((pain$ adj4 “after surg$”) or (pain$ adj4 “after operat$”) or (pain$ adj4 “follow$ operat$”) or (pain$ adj4 “follow$ surg$”))
10. ((analgesi$ adj4 “after surg$”) or (analgesi$ adj4 “after operat$”) or (analgesi$ adj4 “follow$ operat$”) or (analgesi$ adj4 “follow$ surg$”))
11. OR/4‐10
12. clinical trials [exp MESH term]
13. controlled clinical trials [exp MESH term]
14. randomized controlled trial [exp MESH term]
15. double‐blind procedure [single term MESH]
16. (clin$ adj25 trial$)
17. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$))
18. placebo$
19. random$
20. OR/12‐19
21. 3 AND 11 AND 20
Appendix 3. Cochrane search strategy
1. acetaminophen [single term MESH] AND codeine [single term MESH]
2. (acetaminophen OR paracetamol) AND codeine [ti, ab, kw]
3. OR/1‐2
4. PAIN, POSTOPERATIVE [single term MeSH]
5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")) [ti, ab, kw]
6. ((post‐surgical adj4 pain$) or (“post surgical” adj4 pain$) or (post‐surgery adj4 pain$)) [ti, ab, kw]
7. ((“pain‐relief after surg$”) or (“pain following surg$”) or (“pain control after”)) [ti, ab, kw]
8. ((“post surg$” or post‐surg$) AND (pain$ or discomfort)) [ti, ab, kw]
9. ((pain$ adj4 “after surg$”) or (pain$ adj4 “after operat$”) or (pain$ adj4 “follow$ operat$”) or (pain$ adj4 “follow$ surg$”)) [ti, ab, kw]
10. ((analgesi$ adj4 “after surg$”) or (analgesi$ adj4 “after operat$”) or (analgesi$ adj4 “follow$ operat$”) or (analgesi$ adj4 “follow$ surg$”)) [ti, ab, kw]
11. OR/4‐10
12. clinical trials [exp MESH term]
13. controlled clinical trials [exp MESH term]
14. randomized controlled trial [exp MESH term]
15. double‐blind procedure [single term MESH]
16. (clin$ adj25 trial$) [ti, ab, kw]
17. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)) [ti, ab, kw]
18. placebo$ [ti, ab, kw]
19. random$ [ti, ab, kw]
20. OR/12‐19
21. 3 AND 11 AND 20
Appendix 4. Glossary
Categorical rating scale:
The commonest is the five category scale (none, slight, moderate, good or lots, and complete). For analysis numbers are given to the verbal categories (for pain intensity, none=0, mild=1, moderate=2 and severe=3, and for relief none=0, slight=1, moderate=2, good or lots=3 and complete=4). Data from different subjects is then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
VAS:
Visual analogue scale: lines with left end labelled "no relief of pain" and right end labelled "complete relief of pain", seem to overcome this limitation. Patients mark the line at the point which corresponds to their pain. The scores are obtained by measuring the distance between the no relief end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms and provide many points from which to choose. More concentration and coordination are needed, which can be difficult post‐operatively or with neurological disorders.
TOTPAR:
Total pain relief (TOTPAR) is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the composite trapezoidal rule. The trapezoidal rule is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
SPID:
Summed pain intensity difference (SPID) is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See “Measuring pain” in Bandolier’s Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13.1 (Moore 2003)
Data and analyses
Comparison 1. Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.34 [2.93, 13.73] |
| 2 Participants with any adverse event | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.58, 1.39] |
1.1. Analysis.
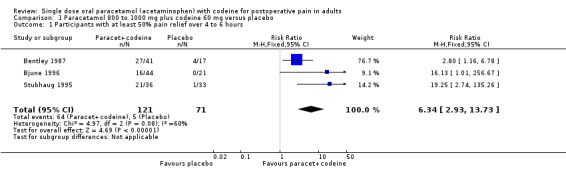
Comparison 1 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
1.2. Analysis.
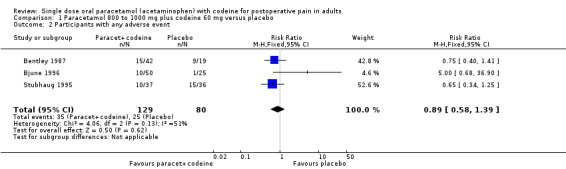
Comparison 1 Paracetamol 800 to 1000 mg plus codeine 60 mg versus placebo, Outcome 2 Participants with any adverse event.
Comparison 2. Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.06, 1.62] |
| 2 Participants using rescue medication over 4 to 6 hours | 2 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.41, 0.89] |
| 3 Participants with any adverse event | 4 | 324 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.70, 1.81] |
2.1. Analysis.
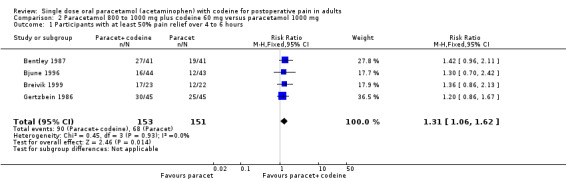
Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
2.2. Analysis.
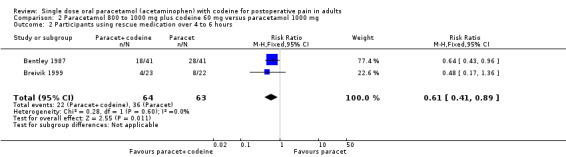
Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 2 Participants using rescue medication over 4 to 6 hours.
2.3. Analysis.
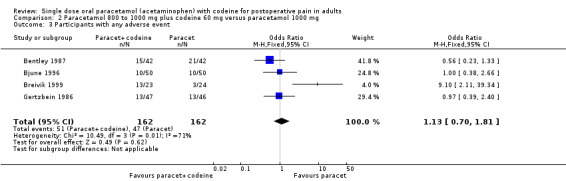
Comparison 2 Paracetamol 800 to 1000 mg plus codeine 60 mg versus paracetamol 1000 mg, Outcome 3 Participants with any adverse event.
Comparison 3. Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 17 | 1413 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.17, 3.21] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental | 14 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.18, 3.35] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery | 3 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.49, 3.73] |
| 4 Participants using rescue medication over 4 to 6 hours | 10 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.82] |
| 5 Participants with any adverse event | 14 | 1258 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.27, 1.93] |
3.1. Analysis.
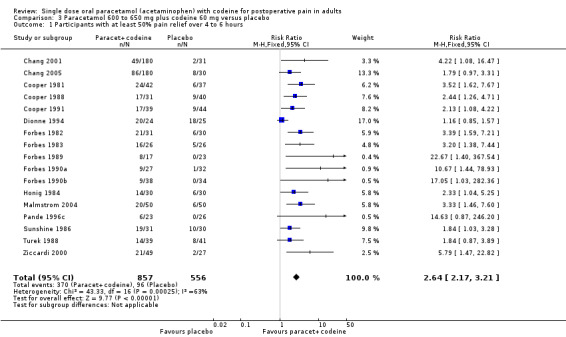
Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
3.2. Analysis.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
3.3. Analysis.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery.
3.4. Analysis.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.
3.5. Analysis.

Comparison 3 Paracetamol 600 to 650 mg plus codeine 60 mg versus placebo, Outcome 5 Participants with any adverse event.
Comparison 4. Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 10 | 622 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental | 8 | 512 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.11, 1.57] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery | 2 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.83, 1.77] |
| 4 Participants using rescue medication over 4 to 6 hours | 7 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 5 Participants with any adverse event | 7 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.57] |
4.1. Analysis.
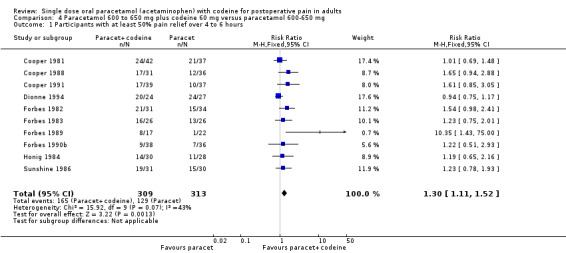
Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
4.2. Analysis.
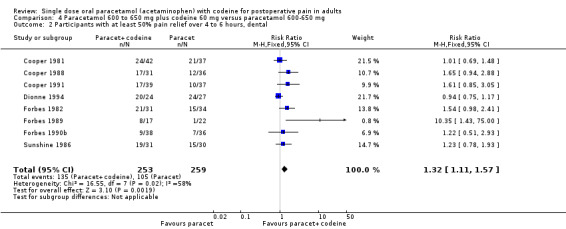
Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
4.3. Analysis.
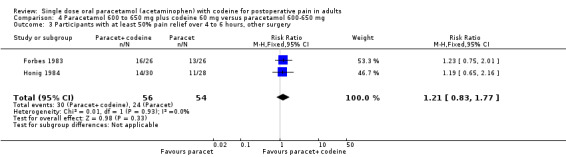
Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery.
4.4. Analysis.
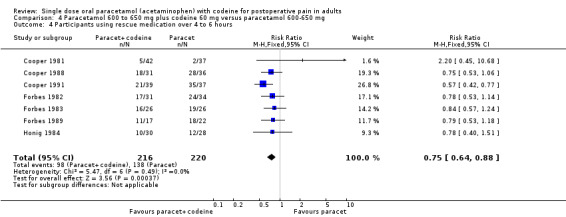
Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 4 Participants using rescue medication over 4 to 6 hours.
4.5. Analysis.

Comparison 4 Paracetamol 600 to 650 mg plus codeine 60 mg versus paracetamol 600‐650 mg, Outcome 5 Participants with any adverse event.
Comparison 5. Paracetamol 300 mg plus codeine 30 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 6 | 690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.42, 2.47] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours, dental | 3 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.31 [1.76, 6.23] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.11, 2.05] |
| 4 Participants using rescue medication over 4 to 6 hours | 4 | 529 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.68, 0.91] |
| 5 Participants with any adverse event | 3 | 344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.50, 1.45] |
5.1. Analysis.
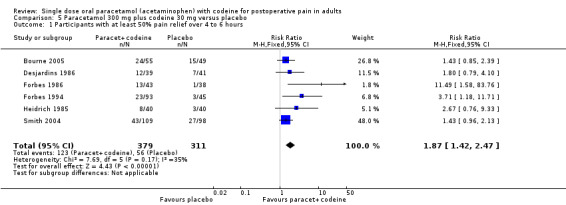
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
5.2. Analysis.
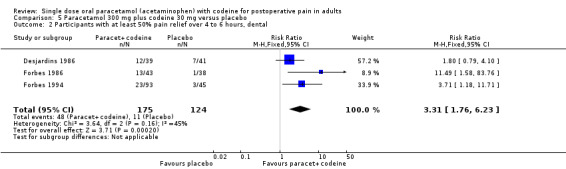
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours, dental.
5.3. Analysis.
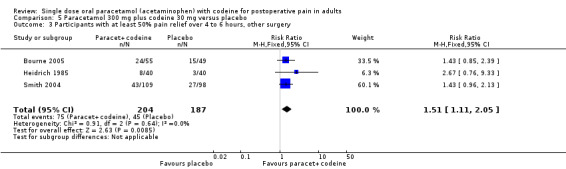
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, other surgery.
5.4. Analysis.
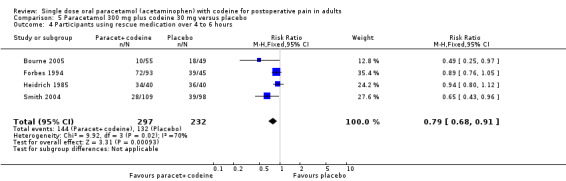
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 4 Participants using rescue medication over 4 to 6 hours.
5.5. Analysis.
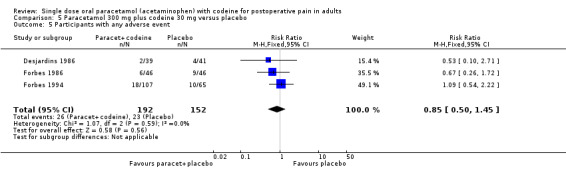
Comparison 5 Paracetamol 300 mg plus codeine 30 mg versus placebo, Outcome 5 Participants with any adverse event.
Comparison 6. Sensitivity analysis (paracetamol with codeine versus placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fewer than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours | 10 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [2.31, 3.92] |
| 2 More than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours | 5 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.21, 2.03] |
6.1. Analysis.
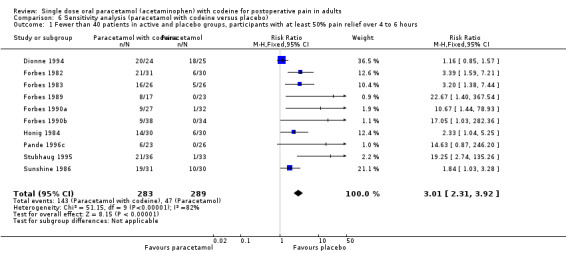
Comparison 6 Sensitivity analysis (paracetamol with codeine versus placebo), Outcome 1 Fewer than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours.
6.2. Analysis.
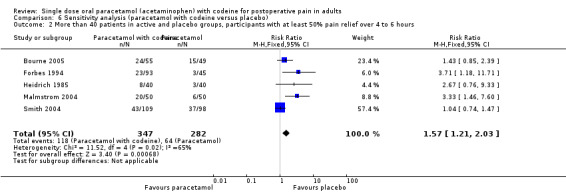
Comparison 6 Sensitivity analysis (paracetamol with codeine versus placebo), Outcome 2 More than 40 patients in active and placebo groups, participants with at least 50% pain relief over 4 to 6 hours.
Comparison 7. Sensitivity analysis (paracetamol with codeine versus paracetamol).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fewer than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours | 10 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.15, 1.60] |
| 2 More than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours | 3 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.02, 1.65] |
7.1. Analysis.
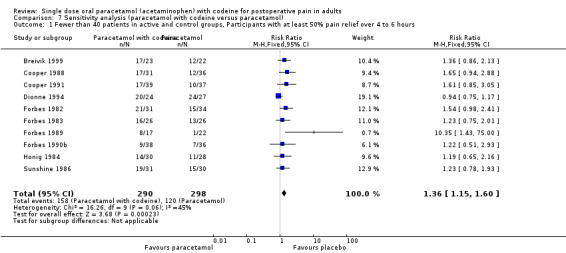
Comparison 7 Sensitivity analysis (paracetamol with codeine versus paracetamol), Outcome 1 Fewer than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours.
7.2. Analysis.
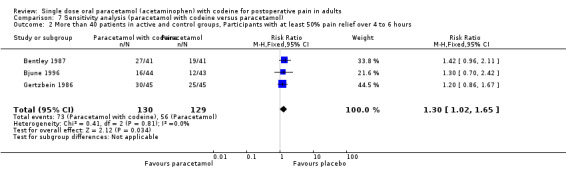
Comparison 7 Sensitivity analysis (paracetamol with codeine versus paracetamol), Outcome 2 More than 40 patients in active and control groups, Participants with at least 50% pain relief over 4 to 6 hours.
Comparison 8. Paracetamol plus codeine (all doses) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with any adverse event | 20 | 1811 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.15, 1.63] |
8.1. Analysis.
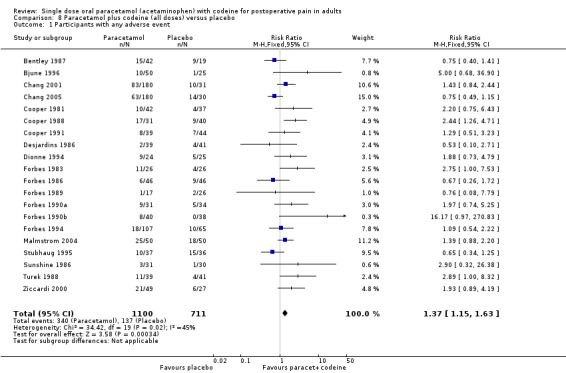
Comparison 8 Paracetamol plus codeine (all doses) versus placebo, Outcome 1 Participants with any adverse event.
Comparison 9. Paracetamol plus codeine (all doses) versus paracetamol alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with any adverse event | 11 | 767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.87, 1.40] |
9.1. Analysis.
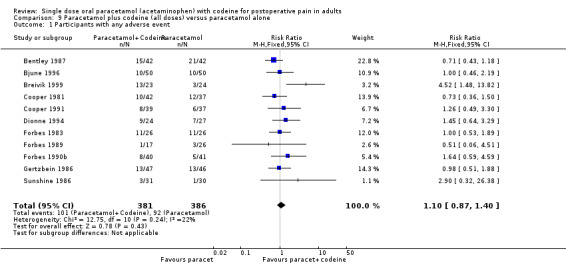
Comparison 9 Paracetamol plus codeine (all doses) versus paracetamol alone, Outcome 1 Participants with any adverse event.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bentley 1987.
| Methods | RCT, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline the hourly to 4 hours |
|
| Participants | Impacted third molar extraction Mean age mid 20's N = 128 |
|
| Interventions | Paracetamol+codeine 1000/60 mg, n = 41 Paracetamol 1000 mg, n = 41 Codeine 60 mg, n = 21 Placebo, n = 17 |
|
| Outcomes | PI: non std 10 point scale PR: std 5 point scale Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 | |
Bjune 1996.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate (more than 40 mm on 100 mm VAS) to severe (greater than 60 mm on a std 100 mm VAS) intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Caesarean section Age: 27 ‐ 37 years N = 125 |
|
| Interventions | Paracetamol+codeine 800/60 mg, n = 50 Paracetamol 1000 mg, n = 50 Placebo, n = 25 |
|
| Outcomes | PI: std 4 point scale and std 100 mm VAS ("no pain" to "unbearable pain") PR: std 5 point scale Number of patients reporting any adverse event and serious adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Bourne 2005.
| Methods | RCT, DB single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes the hourly to 4 hours. Multiple dose phase continued after initial 4 hours |
|
| Participants | Orthopaedic surgery Mean age 46 years N = 153 M = 83, F = 70 |
|
| Interventions | Paracetamol+codeine 300/30 mg, n = 55 Paracetamol+tramadol 325/37.5 mg, n = 49 Placebo, n = 49 |
|
| Outcomes | PI: std 4 point scale PR: non std 6 point scale Number of patients using rescue medication Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Breivik 1999.
| Methods | RCT, DB single oral dose, dummy, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline the every 30 minutes to 8 hours |
|
| Participants | Impacted third molar extraction Mean age 25 years N = 120 M = 44, F = 76 |
|
| Interventions | Paracetamol+codeine 1000/60 mg, n = 24 Paracetamol 1000 mg, n = 22 Diclofenac 100 mg, n = 22 Paracetamol+diclofenac 1000/100 mg, n = 24 Paracetamol+diclofenac+codeine 1000/100/60 mg, n = 24 |
|
| Outcomes | PI: std 100 mm VAS PR: std 5 point scale PGE: non std 4 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Chang 2001.
| Methods | RCT, DB single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Impacted third molar extraction Mean age 21 years N = 292 M = 122, F = 371 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 180 Rofecoxib 50mg, n = 182 Placebo, n = 31 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
Chang 2005.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at: 0, 0.5, 1, 2, 3, 4, 5, 6, 24 hours |
|
| Participants | Impacted third molar extraction Mean age 19 years N = 390 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 180 Rofecoxib 50 mg, n = 180 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 pt scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1.5 hours |
|
Cooper 1981.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 4 hours |
|
| Participants | Impacted third molar extraction Mean age early 20s N = 248 |
|
| Interventions | Paracetamol 650 mg, n = 37 Paracetamol+codeine 650/60 mg, n = 42 Paracetamol+d‐propoxyphene 650/100 mg, n = 42 Ibuprofen 200 mg, n = 42 Placebo, n = 37 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 pt scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1988.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 4 hours |
|
| Participants | Impacted third molar extraction Age 18‐57 years N =165 |
|
| Interventions | Paracetamol 600 mg, n = 36 Paracetamol+codeine 600/60 mg, n = 31 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Cooper 1991.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Impacted tooth removal Age "young adults' n=247 |
|
| Interventions | Paracetamol 650 mg, n = 37 Paracetamol+codeine 650/60 mg, n = 39 Zomepirac 100 mg, n = 23 Flurbiprofen 50 mg, n = 42 Flurbiprofen 100 mg, n = 41 Placebo, n = 44 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Desjardins 1986.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes, then hourly to 6 hours |
|
| Participants | Oral Surgery Age 18+ years N = 137 |
|
| Interventions | Paracetamol+codeine 300/30 mg, n = 39 Aspirin+butalbital+caffeine+codeine 325/50/40/30 mg, n = 43 Placebo, n = 41 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Dionne 1994.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached moderate to severe intensity Pain assessed at baseline then hourly to 6 hours |
|
| Participants | Impacted third molar removal
Age 16+ years N = 135 |
|
| Interventions | Paracetamol 500 mg, n = 72 Piroxicam 20 mg, n = 76 Piroxicam cyclodextrin =20 mg, n = 74 Placebo, n = 76 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R1, DB1, W1 | |
Forbes 1982.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 12 hours |
|
| Participants | Impacted third molar removal Age 15+ years N = 177 |
|
| Interventions | Paracetamol 600 mg, n = 34 Paracetamol+codeine 600/60 mg, n = 31 Diflusinal 500 mg, n = 32 Diflusinal 1000 mg, n = 32 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R1, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1983.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 ,90, 120 mins then hourly to 12 hours |
|
| Participants | General, gynaecological or orthopaedic surgery Age 19+ years N = 132 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 26 Paracetamol 600 mg, n = 26 Diflusinal 500 mg, n = 26 Diflusinal 1000 mg, n = 28 Placebo, n = 26 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Forbes 1986.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline the hourly to 6 hours |
|
| Participants | Removal of impacted 3rd molar Age 15+ years N = 146 |
|
| Interventions | Paracetamol+codeine 300/30 mg, n = 43 Aspirin+butalbital+caffeine+codeine 325/50/40/15 mg, n = 41 Placebo, n = 38 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Forbes 1989.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 12 hours |
|
| Participants | Impacted third molar removal Age 15+ years N = 107 |
|
| Interventions | Paracetamol 600 mg, n = 22 Paracetamol+codeine 600/60 mg, n = 17 Flurbiprofen 100 mg, n = 26 Placebo, n = 23 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1990a.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 6 hours |
|
| Participants | Removal of impacted 3rd molar (1 or more) Age 15+ years N = 162 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 27 Aspirin 650 mg, n = 32 Ketorolac 10 mg, n = 37 Placebo, n = 32 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1990b.
| Methods | RCT, DB, single oral dose, 6 parallel groups. Followed by multiple dose phase Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at baseline then hourly to 6 hours |
|
| Participants | Removal of impacted 3rd molar (1 or more) Age 15+ years N = 206 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 38 Paracetamol 600 mg, n = 36 Ketorolac 10 mg, n = 31 Ketorolac 20 mg, n = 35 ibuprofen 400 mg, n = 32 Placebo, n = 34 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Forbes 1994.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes the hourly to 6 hours |
|
| Participants | Removal of impacted 3rd molar Age 15+ years N = 324 |
|
| Interventions | Paracetamol+codeine 300/30 mg, n = 93 Paracetamol+hydrocodone bitartrate 500/7.5 mg, n = 94 Placebo, n = 45 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Patients asked to refrain from rescue medication for 2 hours |
|
Gertzbein 1986.
| Methods | RCT, DB, single oral dose, 2 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 5 hours |
|
| Participants | Elective orthopaedic or general surgery Age 16 ‐ 65 years N = 116 |
|
| Interventions | Paracetamol+codeine 1000/60 mg, n = 45 Paracetamol 1000 mg, n = 45 |
|
| Outcomes | PI: std 4 point scale and std 100mm VAS PR: std 5 point scale PGE: non std 4 point scale Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Heidrich 1985.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Orthopaedic surgery Age 18‐65 years N = 120 |
|
| Interventions | Paracetamol+codeine 300/30 mg, n = 40 Ibuprofen 400 mg, n = 40 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W0 | |
Honig 1984.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 mins then hourly to 6 hours |
|
| Participants | Elective abdominal, orthopaedic, rectal, thoracic and vascular surgery Age 19‐87 years N = 116 |
|
| Interventions | Paracetamol 600 mg, n = 28 Paracetamol+codeine 600/60 mg, n = 28 Codeine 60 mg, n = 28 Placebo, n = 25 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of serious adverse events |
|
| Notes | Oxford Quality Score R1, DB2, W0 | |
Malmstrom 2004.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60, 90, 120 minutes, then hourly to 8 hours, then at 10, 12, 20 and 24 hours |
|
| Participants | Impacted third molar removal Mean age 23 years N = 201 M = 97, F = 104 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 50 Naproxen sodium 550mg, n = 50 Etoricoxib 120mg, n = 50 Placebo, n = 50 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score:R2, DB2, W1 | |
Pande 1996c.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes, the hourly to 6 hours |
|
| Participants | Removal of impacted third molar N = 100 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 23 Placebo, n = 26 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Smith 2004.
| Methods | RCT, DB single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 4 hours |
|
| Participants | Orthopaedic surgery Mean age 47 years N = 305 M = 215, F = 90 |
|
| Interventions | Paracetamol+codeine 300/30 mg, n = 109 Paracetamol+tramadol 325/37.5 mg, n = 98 Placebo, n = 98 |
|
| Outcomes | PI: std 4 point scale PR: non std 6 point scale Number of patients using rescue medication |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
Stubhaug 1995.
| Methods | RCT, DB, single oral dose, 4 parallel groups Baseline pain was >60 mm on VAS scale (severe) Pain assessed at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Orthopaedic surgery Age: Adults N = 144 |
|
| Interventions | Paracetamol+codeine 1000/60 mg, n = 36 Tramadol 50 mg, n = 33 Tramadol 100 mg, n = 35 Placebo, n = 33 |
|
| Outcomes | PI: std 100 mm VAS Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events |
|
| Notes | Oxford Quality score: R1, DB2, W1 | |
Sunshine 1986.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes, then hourly to 6 hours |
|
| Participants | Removal of impacted third molar Age 16+ years N =182 |
|
| Interventions | Paracetamol 650 mg, n = 30 Paracetamol+codeine 650/60 mg, n = 31 Flurbiprofen 50 mg, n = 31 Flurbiprofen 100 mg, n = 29 Zomepirac 100 mg, n = 31 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event 'Overall improvement': non std 7 point scale |
|
| Notes | Oxford Quality Score R2, DB2, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Turek 1988.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60 minutes then hourly to 6 hours |
|
| Participants | Elective surgery ‐ mainly orthopaedic, abdominal, gynaecological and urological Age 18+ years N = 161 |
|
| Interventions | Paracetamol+codeine 650/60 mg, n = 39 Ketoprofen 50 mg, n = 41 Ketoprofen 150 mg, n = 39 Placebo, n = 41 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale (patients reporting "very good" or "excellent") Time to use of rescue medication Number of patients using rescue medication Number of patients reporting any adverse event and serious adverse events Number of patients withdrawing due to adverse event |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Patients asked to refrain from rescue medication for 1 hour |
|
Ziccardi 2000.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain reached a moderate to severe intensity Pain assessed at 0, 30, 60, 90 and 120 minutes then hourly to 8 hours |
|
| Participants | Impacted third molar extraction Mean age 25 years N = 125 M = 50, F = 75 |
|
| Interventions | Paracetamol+codeine 600/60 mg, n = 49 Ibuprofen+hydrocodone 400/150 mg, n = 49 Placebo, n = 27 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non std 5 point scale Time to use of rescue medication Number of patients reporting any adverse event and serious adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W0 | |
DB: double blind; F: female; M: male; N: total number of participants in study; n: number of participants in treatment arm; R: randomisation RCT: randomised controlled trial; PI: pain intensity; PR: pain relief; PGR: patient global response; std: standard; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Becker 1990 | Pain only assessed for 2 hours after administration of the interventions |
| Behotas 1992 | Interventions were given when pain was "of sufficient intensity that analgesia would normally be given" and data was only presented for pain relief over the first hour |
| Chung 2004 | No appropriate control (Paracetamol with codeine vs codeine CR) |
| Cooper 1984 | Inadequate description of method. Excluded as did not state whether allocation was randomised or if the studies (summary of five trials) were double blind |
| De los Santos 1998 | No appropriate control (Lysine Clonixinate (LC) vs Paracetamol with codeine) |
| Forbes 1981 | Inadequate description of method. Excluded as did not state whether allocation was randomised |
| Fulkerson 1986 | No appropriate control (Diflunisal v paracetamol plus codeine) |
| Jacobson 1987 | No appropriate control (paracetamol plus codeine v paracetamol plus dextropropoxyphene) |
| Levin 1974 | PI scale was 5 point and therefore not validated for the data extraction method. No results for pain relief which would allow the calculation of TOTPAR were presented. Global evaluation was in the opinion of the investigator and not the patient |
| Maalaki 2002 | No appropriate control (Paracetamol with codeine vs. ibuprofen) |
| Macleod 2002 | Insufficient baseline pain (not of moderate or severe intensity, less than 30 mm on 100 mm VAS). No placebo group |
| Matthews 1984 | Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain |
| Ottinger 1990 | No appropriate control (paracetamol plus codeine v flurbiprofen) |
| Oullette 1986 | No appropriate control (paracetamol plus codeine v naproxen) |
| Pande 1996a | Fewer than 10 patients in the treatment arm |
| Pande 1996b | Fewer than 10 patients in the treatment arm |
| Peter 2001 | No appropriate control (ibuprofen vs. paracetamol with codeine) |
| Petti 1985 | Single blind study |
| Quiding 1982a | Patients instructed to take tablets "when pain relief was needed". Mean baseline pain minus 2 standard deviations was less than 30 mm for all interventions (>30 mm equates to at least moderate pain), therefore it is probable that patients with mild pain were included |
| Quiding 1983 | Patients instructed to take tablets "when pain relief was needed". Mean baseline pain minus 2 standard deviations was less than 30 mm for all interventions (>30 mm equates to at least moderate pain), therefore it is probable that patients with mild pain were included |
| Quiding 1984 | No appropriate control (paracetamol v codeine) |
| Raeder 2001 | No appropriate control (ibuprofen vs paracetamol with codeine) |
| Scoren 1987 | No appropriate control (paracetamol plus codeine v APC v naproxen) |
| Skjelbred 1977 | No appropriate control (paracetamol v aspirin) |
| Sunshine 1988 | Outline of 5 studies. Study 3 and 4 compare paracetamol plus codeine to placebo. Study 4 is a duplicate of an included RCT. Study 3 cannot be included as the report fails to state whether the allocation to each intervention was randomised |
| Sveen 1975 | Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain |
| Torabinejad 1994 | Intervention administered immediately after surgery before anaesthetic wore off. Therefore inadequate baseline pain |
| Vangen 1988 | No appropriate control (paracetamol plus codeine v ketorolac) |
| Wittenberg 1984 | No appropriate control (paracetamol plus codeine v ibuprofen) |
Contributions of authors
LT and SD identified studies for inclusion in this update and carried out data extraction, analysis and writing. RAM was involved with planning, analysis and writing, and acted as arbitrator. HJM was involved in planning and writing.
Sources of support
Internal sources
Oxford Pain Research Funds, UK.
External sources
-
NHS Cochrane Collaboration Programme Grant Scheme, UK.
Funding
European Union Biomed 2 Grant no. BMH4 CT95 0172, UK.
Declarations of interest
LT has no interests to declare. RAM and HJM have consulted for various pharmaceutical companies. RAM and HJM and have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. SD, RAM and HJM have received research support from charities, government and industry sources at various times: no support other than the declared financial support was received for this work.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bentley 1987 {published data only}
- Bentley KC, Head TW. The additive analgesic efficacy of acetaminophen, 1000 mg, and codeine, 60 mg, in dental pain. Clinical Pharmacology and Therapeutics 1987;42(6):634‐40. [DOI] [PubMed] [Google Scholar]
Bjune 1996 {published data only}
- Bjune K, Stubhaug A, Dodgson MS, Breivik H. Additive analgesic effect of codeine and paracetamol can be detected in strong, but not moderate, pain after Caesarean section. Baseline pain‐intensity is a determinant of assay‐sensitivity in a postoperative analgesic trial. Acta Anaesthesiologica Scandinavica 1996;40(4):399‐407. [DOI] [PubMed] [Google Scholar]
Bourne 2005 {published data only}
- Bourne MH, Rosenthal NR, Xiang J, Jordan D, Kamin M. Tramadol/acetaminophen tablets in the treatment of postsurgical orthopedic pain. The American Journal of Orthopedics 2005;24(12):592‐7. [PubMed] [Google Scholar]
Breivik 1999 {published data only}
- Breivik EK, Barkvoll P, Skovlund E. Combining diclofenac with acetaminophen or acetaminophen‐codeine after oral surgery: A randomized, double‐blind single‐dose study. Clinical Pharmacology and Therapeutics 1999;66(6):625‐35. [DOI] [PubMed] [Google Scholar]
Chang 2001 {published data only}
- Chang DJ, Fricke JR, Bird SR, Bohidar NR, Dobbins TW, Geba GP. Rofecoxib versus codeine/acetaminophen in postoperative dental pain: a double‐blind, randomized, placebo‐ and active comparator‐controlled clinical trial. Clinical Therapeutics 2001;23(9):1446‐55. [DOI] [PubMed] [Google Scholar]
Chang 2005 {published data only}
- Chang DJ, Bird SR, Bohidar NR, King T. Analgesic efficacy of rofecoxib compared with codeine/acetaminophen using a model of acute dental pain. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontology 2005;100(4):e74‐80. [DOI: 10.1016/j.tripleo.2005.04.026] [DOI] [PubMed] [Google Scholar]
Cooper 1981 {published data only}
- Cooper SA, Breen JF, Giuliani RL. The relative efficacy of indoprofen compared with opioid analgesic combinations. Journal of Oral Surgery 1981;39(1):21‐5. [PubMed] [Google Scholar]
Cooper 1988 {published data only}
- Cooper SA, Firestein A, Cohn P. Double blind comparison of meclofenamate sodium with acetaminophen, acetaminophen with codeine and placebo for relief of postsurgical dental pain. The Journal of Clinical Dentistry 1988;1(2):31‐4. [PubMed] [Google Scholar]
Cooper 1991 {published data only}
- Cooper SA, Kupperman A. The analgesic efficacy of flurbiprofen compared to acetaminophen with codeine. The Journal of Clinical Dentistry 1991;2(3):70‐4. [PubMed] [Google Scholar]
Desjardins 1986 {published data only}
- Desjardins PJ, Cooper SA, Finizio T. Efficacy of low dose combination analgesics: acetaminophen/codeine, aspirin/butalbital/caffeine/codeine, and placebo in oral surgery pain. Anesthesia Progress 1986;33(3):143‐6. [PMC free article] [PubMed] [Google Scholar]
Dionne 1994 {published data only}
- Dionne RA, Snyder J, Hargreaves KM. Analgesic efficacy of flurbiprofen in comparison with acetaminophen, acetaminophen plus codeine, and placebo after impacted third molar removal. Journal of Oral Maxillofacial Surgery 1994;52(9):919‐24. [DOI] [PubMed] [Google Scholar]
Forbes 1982 {published data only}
- Forbes JA, Beaver WT, White EH, White RW, Neilson GB, Shackleford RW. Diflunisal. A new oral analgesic with an unusually long duration of action. The Journal of the American Medical Association 1982;248(17):2139‐42. [DOI] [PubMed] [Google Scholar]
Forbes 1983 {published data only}
- Forbes JA, Kolodny AL, Beaver WT, Shackleford RW, Scarlett VR. A 12 hour evaluation of the analgesic efficacy of diflunisal, acetaminophen, and acetaminophen codeine combination, and placebo in postoperative pain. Pharmacotherapy 1983;3(2 Pt 2):47S‐54S. [PubMed] [Google Scholar]
Forbes 1986 {published data only}
- Forbes JA, Jones KF, Smith WK, Gongloff CM. Analgesic effect of an aspirin codeine butalbital caffeine combination and an acetaminophen codeine combination in postoperative oral surgery pain. Pharmacotherapy 1986;6(5):240‐7. [DOI] [PubMed] [Google Scholar]
Forbes 1989 {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Yorio CC, Selinger LR, Rosenmertz SK, et al. Evaluation of flurbiprofen, acetaminophen, an acetaminophen‐codeine combination, and placebo in postoperative oral surgery pain. Pharmacotherapy 1989;9(5):322‐30. [DOI] [PubMed] [Google Scholar]
Forbes 1990a {published data only}
- Forbes JA, Butterworth GA, Burchfield WH, Beaver WT. Evaluation of ketorolac, aspirin, and an acetaminophen‐codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6 Pt 2):77S‐93S. [PubMed] [Google Scholar]
Forbes 1990b {published data only}
- Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6 Pt 2):94S‐105S. [PubMed] [Google Scholar]
Forbes 1994 {published data only}
- Forbes JA, Bates JA, Edquist IA, Burchfield WH, Smith FG, Schwartz MK, et al. Evaluation of two opioid‐acetaminophen combinations and placebo in postoperative oral surgery pain. Pharmacotherapy 1994;14(2):139‐46. [PubMed] [Google Scholar]
Gertzbein 1986 {published data only}
- Gertzbein SD, Tile M, McMurty RY, Kellam JF, Hunter GA, Keith RG, et al. Analysis of the analgesic efficacy of acetaminophen 1000 mg, codeine phosphate 60 mg, and the combination of acetaminophen 1000 mg and codeine phosphate 60 mg in the relief of postoperative pain. Pharmacotherapy 1986;6(3):104‐7. [DOI] [PubMed] [Google Scholar]
Heidrich 1985 {published data only}
- Heidrich G, Slavic Svircev V, Kaiko RF. Efficacy and quality of ibuprofen and acetaminophen plus codeine analgesia. Pain 1985;22(4):385‐97. [DOI] [PubMed] [Google Scholar]
Honig 1984 {published data only}
- Honig S, Murray KA. An appraisal of codeine as an analgesic: single dose analysis. The Journal of Clinical Pharmacology 1984;24(2‐3):96‐102. [DOI] [PubMed] [Google Scholar]
Malmstrom 2004 {published data only}
- Malmstrom K, Kotey P, Coughlin H, Desjardins PJ. A randomized, double‐blind, parallel‐group study comparing the analgesic effect of etoricoxib to placebo, naproxen sodium, and acetaminophen with codeine using the dental impaction pain model. The Clinical Journal of Pain 2004;20(3):147‐55. [DOI] [PubMed] [Google Scholar]
Pande 1996c {published data only}
- Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the K‐receptor agonist, Enadoline, in dental surgery pain. Clinical Neuropharmacology 1996;19(1):92‐7. [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith AB, Ravikumar TS, Kamin M, Jordan D, Xiang J, Capss‐ Study Group, et al. Combination tramadol plus acetaminophen for postsurgical pain. American Journal of Surgery 2004;187(4):521‐7. [DOI] [PubMed] [Google Scholar]
Stubhaug 1995 {published data only}
- Stubhaug A, Grimstad J, Breivik H. Lack of analgesic effect of 50 mg and 100 mg oral tramadol after orthopaedic surgery: a randomized, double‐blind, placebo and standard active drug comparison. Pain 1995;62(1):111‐8. [DOI] [PubMed] [Google Scholar]
Sunshine 1986 {published data only}
- Sunshine A, Marrero I, Olson N, McCormick N, Laska EM. Comparative study of flurbiprofen, zomepirac sodium, acetaminophen plus codeine, and acetaminophen for the relief of postsurgical dental pain. The American Journal of Medicine 1986;80(3A):50‐4. [DOI] [PubMed] [Google Scholar]
Turek 1988 {published data only}
- Turek MD, Baird WM. Double blind parallel comparison of ketoprofen (Orudis), acetaminophen plus codeine, and placebo in postoperative pain. The Journal of Clinical Pharmacology 1988;28(12 Suppl):S23‐8. [DOI] [PubMed] [Google Scholar]
Ziccardi 2000 {published data only}
- Ziccardi VB, Desjardins PJ, Daly‐DeJoy E, Seng GF. Single‐dose vicoprofen compared with acetaminophen with codeine and placebo in patients with acute postoperative pain after third molar extractions. Journal of Oral and Maxillofacial Surgery : official journal of the American Association of Oral and Maxillofacial Surgeons 2000;58(6):622‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Becker 1990 {published data only}
- Becker J, Beckmann J, Bertelt C, Gundert‐Remy U, Rohmel J, Ohlendorf D. Double blind biometric study on postoperative effects of analgesics [Doppelblindstudie uber postoperative analgetikawirkungen]. Deutsche Zahnarztliche Zeitschrift 1990;45(1):36‐8. [PubMed] [Google Scholar]
Behotas 1992 {published data only}
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, Lienhart A. Analgesic efficacy of ibuprofen for post‐episiotomy pain [Effets antalgiques de L'ibuprofene dans les doulers apres episiotomie]. Annales Françaises d'Anesthèsie et de Rèanimation 1992;11(1):22‐6. [DOI] [PubMed] [Google Scholar]
Chung 2004 {published data only}
- Chung F, Tong D, Miceli PC, Reiz J, Harsanyi Z, Darke AC, Payne LW. Controlled‐release codeine is equivalent to acetaminophen plus codeine for post‐cholecystectomy analgesia. Canadian Journal of Anaesthesia 2004;51(3):216‐21. [DOI] [PubMed] [Google Scholar]
Cooper 1984 {published data only}
- Cooper SA. Five studies on ibuprofen for postsurgical dental pain. The American Journal of Medicine 1984;Suppl:70‐7. [DOI] [PubMed] [Google Scholar]
De los Santos 1998 {published data only}
- Santos AR, MartÌ MI, Espinosa D, Girolamo G, Vinacur JC, Casadei A. Lysine clonixinate vs. paracetamol/codeine in postepisiotomy pain. Acta Physiologica, Pharmacologica et Therapeutica Latinoamericana 1998;48(1):52‐8. [PubMed] [Google Scholar]
Forbes 1981 {published data only}
- Forbes JA, Bowser MW, Calderazzo JP, Foor VM. An evaluation of the analgesic efficacy of three opioid‐analgesic combinations in postoperative oral surgery pain. Journal of Oral Surgery 1981;39:108‐12. [PubMed] [Google Scholar]
Fulkerson 1986 {published data only}
- Fulkerson JP, Folcik MA. Analgesia following arthroscopic surgery: comparison of Diflunisal and acetaminophen with codeine. Arthroscopy 1986;2(2):108‐10. [DOI] [PubMed] [Google Scholar]
Jacobson 1987 {published data only}
- Jacobson J, Bertilson SO. Analgesic efficacy of paracetamol/codeine and paracetamol/dextropropoxyphene in pain after episiotomy and ruptures in connection with childbirth. The Journal of International Medical Research 1987;15(2):89‐95. [DOI] [PubMed] [Google Scholar]
Levin 1974 {published data only}
- Levin HM, Bare WW, Berry FN, Miller JM. Acetaminophen with codeine for the relief of severe pain in postpartum patients. Current Therapeutic Research 1974;16(9):921‐7. [PubMed] [Google Scholar]
Maalaki 2002 {published data only}
- Maaliki H, Church L. Which is better for the management of postpartum perineal pain: Ibuprofen or acetaminophen with codeine?. The Journal of Family Practice 2002;51(3):207. [PubMed] [Google Scholar]
Macleod 2002 {published data only}
- Macleod AG, Ashford B, Voltz M, Williams B, Cramond T, Gorta L, et al. Paracetamol versus paracetamol‐codeine in the treatment of post‐operative dental pain: a randomized, double‐blind, prospective trial. Australian Dental Journal 2002;47(2):147‐51. [DOI] [PubMed] [Google Scholar]
Matthews 1984 {published data only}
- Matthews RW, Scully CM, Levers BG. The efficacy of diclofenac sodium (Voltarol) with and without paracetamol in the control of post surgical dental pain. British Dental Journal 1984;157(10):357‐9. [DOI] [PubMed] [Google Scholar]
Ottinger 1990 {published data only}
- Ottinger ML, Kinney KW, Black JR, Wittenberg M. Comparison of flurbiprofen and acetaminophen with codeine in postoperative foot pain. Journal of the American Podiatry Association 1990;80(5):266‐70. [DOI] [PubMed] [Google Scholar]
Oullette 1986 {published data only}
- Ouellette RD, Feinberg A, Laraja R, et al. Naproxen sodium vs acetaminophen plus codeine in postsurgical pain. Current Therapeutic Research, Clinical and Experimental 1986;39(5):839‐45. [Google Scholar]
Pande 1996a {published data only}
- Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. Analgesic efficacy of the kappa‐receptor agonist, enadoline, in dental surgery pain. Clinical Neuropharmacology 1996;19(1):92‐7. [DOI] [PubMed] [Google Scholar]
Pande 1996b {published data only}
- Pande AC, Pyke RE, Greiner M, Wideman GL, Benjamin R, Pierce MW. Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clinical Neuropharmacology 1996;19(5):451‐6. [DOI] [PubMed] [Google Scholar]
Peter 2001 {published data only}
- Peter EA, Janssen PA, Grange CS, Douglas MJ. Ibuprofen versus acetaminophen with codeine for the relief of perineal pain after childbirth: a randomized controlled trial. Canadian Medical Association Journal 2001;165(9):1203‐9. [PMC free article] [PubMed] [Google Scholar]
Petti 1985 {published data only}
- Petti A. Postoperative pain relief with pentazocine and acetaminophen: comparison with other analgesic combinations and placebo. Clinical Therapeutics 1985;8(1):126‐33. [PubMed] [Google Scholar]
Quiding 1982a {published data only}
- Quiding H, Persson G, Ahlstrom U, Bangens S, Hellem S, Johansson G, et al. Paracetamol plus supplementary doses of codeine. An analgesic study of repeated doses. The Journal of Clinical Pharmacology 1982;23(4):315‐9. [DOI] [PubMed] [Google Scholar]
Quiding 1983 {published data only}
- Quiding H, Haggquist SO. Visual analogue scale and the analysis of analgesic action. The Journal of Clinical Pharmacology 1983;24(4):475‐8. [DOI] [PubMed] [Google Scholar]
Quiding 1984 {published data only}
- Quiding H, Oikarinen V, Sane J, Sjoblad AM. Analgesic efficacy after single and repeated doses of codeine and acetaminophen. The Journal of Clinical Pharmacology 1984 Jan;24(1):27‐34. [DOI] [PubMed] [Google Scholar]
Raeder 2001 {published data only}
- Raeder JC, Steine S, Vatsgar TT. Oral ibuprofen versus paracetamol plus codeine for analgesia after ambulatory surgery. Anesthesia and Analgesia 2001;92(6):1470‐2. [DOI] [PubMed] [Google Scholar]
Scoren 1987 {published data only}
- Scoren RD, Corn H, Rhodes P, Schwarz M, Segal PL, Marks MH. Pain following periodontal surgery treatment with a nonnarcotic analgesic compared with two codeine combinations. Current Therapeutic Research, Clinical and Experimental 1987;42(3):463‐71. [Google Scholar]
Skjelbred 1977 {published data only}
- Skjelbred P, Album B, Lokken P. Acetylsalicylic acid vs paracetamol: effects on postoperative course. European Journal of Clinical Pharmacology 1977;12(4):257‐64. [DOI] [PubMed] [Google Scholar]
Sunshine 1988 {published data only}
- Sunshine A, Olson NZ. Analgesic efficacy of ketoprofen in postpartum, general surgery, and chronic cancer pain. The Journal of Clinical Pharmacology 1988;28:S47‐S54. [DOI] [PubMed] [Google Scholar]
Sveen 1975 {published data only}
- Sveen K, Gilhuus MoeO. Paracetamol/codeine in relieving pain following removal of impacted mandibular third molars. International Journal of Oral Surgery 1975;4(6):258‐66. [DOI] [PubMed] [Google Scholar]
Torabinejad 1994 {published data only}
- Torabinejad M, Dorn SO, Eleazer PD, Frankson M, Jouhari B, Mullin RK, et al. Effectiveness of various medications on postoperative pain following root canal obturation. Journal of Endodontics 1994;20(9):427‐31. [DOI] [PubMed] [Google Scholar]
Vangen 1988 {published data only}
- Vangen O, Doessland S, Lindbaek E. Comparative study of ketorolac and paracetamol/codeine in alleviating pain following gynaecological surgery. The Journal of International Medical Research 1988;16(6):443‐51. [DOI] [PubMed] [Google Scholar]
Wittenberg 1984 {published data only}
- Wittenberg M, Kinney KW, Black JR. Comparison of ibuprofen and acetaminophen codeine in postoperative foot pain. Journal of the American Podiatry Association 1984;74(5):233‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Botting 2000
- RM Botting. Mechanism of action of acetaminophen: is there a cyclooxygenase 3?. Clinical Infectious Diseases 2000;31(5):S203‐S210. [DOI] [PubMed] [Google Scholar]
Chandrasekharan 2002
- NV Chandrasekharan. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;99:13926‐13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke in press
- Clarke R, Derry S, Moore RA, McQuay HJ. Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews In press. [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Collins 1999
- Collins SL, Moore RA, McQuay HJ, Wiffen PJ, Edwards JE. Single dose oral ibuprofen and diclofenac for postoperative pain. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD001548] [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith L, McQuay HJ. Seeking a simple measure of analgesia for mega trials: is simple global assessment good enough?. Pain 2001;91:189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. British Medical Journal 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
CSM 1997
- Committee on Safety of Medicines. Medicines Control Agency. Paracetamol and aspirin. Current pproblems in Pharmacovigilance 1997;23:9. [Google Scholar]
Dart 2000
- Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. The American Journal of Medicine 2000;7(2):123‐34. [DOI] [PubMed] [Google Scholar]
Derry 2008
- Derry S, Barden J, McQuay HJ, Moore RA. Single dose oral celecoxib for postoperative pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004233] [DOI] [PubMed] [Google Scholar]
Edwards 1999
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427‐37. [DOI: ] [DOI] [PubMed] [Google Scholar]
Edwards 2002
- Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta‐analysis of single‐dose oral tramadol plus acetaminophen in acute postoperative pain. Journal of Pain and Symptom Management 2002;23(2):121–130. [DOI: 10.1016/S0885-3924(01)00404-3] [DOI] [PubMed] [Google Scholar]
Flower 1972
- RJ Flower, JR Vane. Inhibition of prostaglandin synthetase in brain explains the anti‐pyretic activity of paracetamol (4‐acetamidophenol). Nature 1972;240:410‐411. [DOI] [PubMed] [Google Scholar]
Graham 2005
- Graham GG, Scott KF. Mechanism of action of paracetamol. American Journal of Therapeutics 2005;12(1):46‐55. [DOI] [PubMed] [Google Scholar]
Gunnell 1997
- D Gunnell, K Hawton, V Garnier, C Bismuth, J Fagg. Use of paracetamol for suicide and non‐fatal poisoning in the UK and France: are restrictions on availability justified?. Journal of Epidemiology and Community Health 1997;51:175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawkins 2007
- Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature. Drug Safety 2007;30(6):465‐79. [DOI] [PubMed] [Google Scholar]
Hawton 2001
- K Hawton, E Townsend, J Deeks, L Appleby, D Gunnell, O Bennewith, J Cooper. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ 2001;322:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinz 2008
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase‐2 inhibitor in man. FASEB J 2008;22(2):383‐90. [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66:239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
McQuay 1996
- McQuay H, Carroll D, Moore A. Variation in the placebo effect in randomised controlled trials of analgesics: all is as blind as it seems. Pain 1996;64(2):331‐5. [DOI: 10.1016/0304-3959(95)00116-6] [DOI] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta‐analyses. Lancet 1999;27(354):1896‐900. [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI] [PubMed] [Google Scholar]
Moore 1998b
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything‐‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Moore 2008
- Moore RA, Moore OA, Derry S, McQuay HJ. Numbers needed to treat calculated from responder rates give a better indication of efficacy in osteoarthritis trials than mean pain scores. Arthritis Research and Therapy 2008;10(2):R39. [DOI: 10.1186/ar2394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratio and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldman 1999
- Oldman A, Smith LA, Collins S, Carroll D, Wiffen PJ, McQuay HJ, Rees J, Moore A. Single dose oral aspirin for acute pain. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD002067] [DOI] [PubMed] [Google Scholar]
PIC 2008
- Paracetamol Information Centre. www.pharmweb.net Accessed 3 July 2008.
Prescott 2000
- LF Prescott. Therapeutic misadventure with paracetamol: Fact or fiction?. American Journal of Therapeutics 2000;7(2):99‐114. [DOI] [PubMed] [Google Scholar]
Schwab 2003
- JM Schwab, HJ Schluesener, S Laufer. COX‐3: just anothet COX or the solitary elusive target of paracetamol?. The Lancet 2003;361:981‐982. [DOI] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrolment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66:266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramèr 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta‐analysis: a case study. BMJ 1997;315:635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Moore 1997
- Moore A, Collins S, Carroll D, McQuay H. Paracetamol with and without codeine in acute pain: a quantitative systematic review. Pain 1997;70:193‐201. [DOI] [PubMed] [Google Scholar]
Moore 1998a
- Moore A, Collins S, Carroll D, McQuay H, Edwards J. Single dose paracetamol (acetaminophen), with and without codeine, for postoperative pain. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001547] [DOI] [PubMed] [Google Scholar]


