ABSTRACT
BACKGROUND:
Despite results of the Intergroup 0116 (INT-0116) study showing an overall survival benefit of adjuvant chemoradiotherapy in gastric adenocarcinoma, its use in the United States remains controversial. The Surveillance Epidemiology of End Results (SEER) database was used to compare cause-specific survival outcomes in resected gastric adenocarcinoma with various adjuvant therapies and patterns of care.
METHODS:
Individual data from 1988 to 2008 were selected for patients with resected, nonmetastatic gastric adenocarcinoma. These patients were stratified by stage (American Joint Committee on Cancer [AJCC], 6th edition), as well as treatment modalities (surgery alone, S; surgery followed by radiotherapy, SR; surgery with chemotherapy, SC; surgery followed by radiotherapy with chemotherapy, SRC; and radiotherapy followed by surgery with chemotherapy, RSC). Overall 21,472 patients (8335 stages IA and 1B; 5944 stage II, 4594 stage III, and 2599 stage IV) were included in this study.
RESULTS:
The median age of the cohort was 66 years, with 63.0% male and 66.4% white. The median number of lymph nodes examined was 17.6. Median survival by stage was 96 months for stage I, 30 months for stage II, 20 months for stage III, and 14 months for stage IV. Using the SRC group as the reference group, for stage I patients, S had the most favorable cause-specific survival (hazard ratio [HR], 0.67; confidence interval, [CI] 0.60–0.76). For patients with stage II, III, or IV, those treated with SRC had the best outcome compared with the other treatment modalities. After 1999, the number of patients treated with surgery alone decreased by at least 14%, whereas the number treated with SRC increased by approximately 12%.
CONCLUSIONS:
This large SEER database analysis showed that stage I patients benefited most from surgery alone, whereas those at more advanced stages benefited most from adjuvant radiotherapy with chemotherapy. This result is consistent with INT-0116 for gastric adenocarcinoma in support of trimodality therapy and is reflected by the increased fraction of patients receiving chemotherapy and adjuvant radiation.
Gastric cancer is the fourth most common cancer worldwide, affecting almost 1 million people per year and resulting in the death of approximately 75% of those diagnosed. In the United States, 21,000 people receive a diagnosis of gastric cancer every year, causing 10,000 deaths.1 Of the stomach cancers diagnosed, 90% are adenocarcinomas, and today, surgical resection remains the primary treatment.2 Patients with a diagnosis of higher stage cancer, who undergo curative gastrectomy have a poor prognosis, with a 5-year survival of less than 35%, thus highlighting the need for adjuvant therapy.3
Clinical trials have been conducted on the efficacy of neoadjuvant and adjuvant therapy for gastric cancer. One of the most influential studies was the Intergroup 0116 (INT-0116) trial demonstrating the efficacy of adjuvant chemoradiation on stages IB–IV M(0). Patients treated with adjuvant 5-fluorouracil [FU] and radiation therapy had an improved median and disease-free survival.4 Another breakthrough study was the MAGIC trial. Patients were randomized to receive either perioperative ECF (epirubicin, cisplatin, and 5-FU) chemotherapy or surgery alone. Those in the treatment arm received a survival benefit when compared with those in the control arm.5 Despite these results, the optimal perioperative treatment for gastric cancer has not been established.
The primary aim of this study was to analyze the patterns of practice today by studying a larger sample of patients. We hypothesized that patients with more extensive gastric cancer who received chemotherapy with adjuvant radiation would outperform other treatment groups. Using the Surveillance and Epidemiology and End Results (SEER) database, we studied the outcomes of patients with gastric cancer who were treated with the following modalities: surgery alone (S), surgery with chemotherapy (SC), surgery followed by radiotherapy (SR), surgery followed by radiotherapy with chemotherapy (SRC), and radiotherapy followed by surgery with chemotherapy (RSC). In the analyses, we used median cause-specific survival as the end point of comparison.
MATERIALS AND METHODS
Data Source
The National Cancer Institute's SEER database was used to select patients. The SEER data comprise patient information gathered from 18 cancer registries and 3 supplemental registries representing approximately 25% of the U.S. population. A data agreement was signed with the SEER program.
Patient Cohort Selection
Using the SEER program, the following patients with gastric cancer were selected for the study: patients with adenocarcinoma, as defined by the International Classification of Disease for Oncology, 3rd Revision, histology codes; patients with nonmetastatic disease according to SEER Extent of Disease codes and the American Joint Committee on Cancer, 6th edition, staging system; those with the stomach as the primary site (C16.0–16.9); those receiving the diagnosis between 1988 and 2008; and those who lived for longer than 3 months, or if deceased, died of stomach cancer only. The final criterion was included to account for immortal time bias, which can artificially increase treatment efficacy.
Stage
In 2004, SEER started to implement the AJCC 6th edition staging system for gastric adenocarcinoma. Therefore, the following SEER Extent of Disease codes were used to form the equivalent AJCC 6th edition tumor stages for patients before 2004: 10–16 (T1), 20 and 40–45 (T2), 50 (T3), and 60–70 (T4). This coding method automatically excludes stage M(1) cancers. Similar methods for nodal staging were performed with the SEER Extent of Disease codes and were classified by number of positive regional nodes: 0 (N0), 1–6 and 97 (N1), 07–15 (N2), and 16–96 (N3).
For cases from 2004 to 2008, The AJCC 6th edition staging system was used to include patients with stage IA, 1B, II, IIIA, IIIB, and IV disease. Patients with stage IV metastatic (M1) disease were excluded from the study.
Variable Definitions
Patients were divided on the basis of their treatment into the 5 groups mentioned in the introduction: surgery alone (S), surgery with chemotherapy (SC), surgery followed by radiotherapy (SR), surgery followed by radiotherapy with chemotherapy (SRC), and radiotherapy followed by surgery with chemotherapy (RSC). SEER uses the North American Association of Central Cancer Registries (NAACCR) item lists to categorize the treatment modality.
Obtaining data regarding a patient's chemotherapy treatment required approval of a SEER custom data request. SEER categorizes chemotherapy into 2 groups of patients: those who received chemotherapy (NAACCR item 1390, code 01) and those who did not or whose treatment was unknown (NAACCR item 1390, code 02). If it was unknown whether a patient received chemotherapy, we assumed he or she had not for this study. The SEER database does not contain information on whether the chemotherapy received was before or after surgery, the type of chemotherapy, or the number of chemotherapeutic agents. The different courses of surgery and radiation therapy were selected with SEER's Radiation Sequence with Surgery codes (NAACCR item 1380, codes 0, 2, or 3). Selecting patients who received only surgery (S) required multiple selection filters that excluded patients who received radiation (NAACCR Item 1360, code 0) or chemotherapy. It included those who underwent surgery at the primary site (NAACCR item 1340, code 0).
Cause-specific survival, defined as death attributed to gastric cancer, was chosen as the end point for this study, primarily because performance status is not easily controlled in the SEER database. This study did not require institutional IRB approval as it did not include patients at the University of California at Los Angeles nor include any patient identifiable information.
Statistical Analysis
Descriptive statistics of clinical factors were reported as means and percentages for entire population and by treatment modality (S, SC, SR, SRC, and RSC). Overall, 21,472 patients (8335 stages IA and 1B; 5944 stage II, 4594 stage III, and 2599 stage IV) were included in this study (Table 1). Univariate and multivariate analyses were performed to evaluate the treatment modalities associated with cause-specific survival, by using the Cox proportional hazards model. Hazard ratios (HRs) with 95% confidence intervals (CIs) are reported. All analyses were performed with SAS version 9.2 (SAS Institute, Cary, NC). P < .05 for 2-sided tests was considered statistically significant.
Table 1.
Patient and tumor characteristics
| Variables | All (N=21,472) |
Treatment type |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery + radiation after + chemo (n=4,768) |
Surgery + radiation after (n=691) |
Surgery + radiation prior + chemo (n=821) |
Surgery only (n=12,696) |
Surgery + chemo (n=2,496) |
||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Race recode (white, black, other) | ||||||||||||
| Black | 2,621 | 12.2 | 629 | 13.2 | 93 | 13.5 | 25 | 3 | 1,571 | 12.4 | 303 | 12.1 |
| Other (American Indian/AK Native, Asian/Pacific Islander) | 4,565 | 21.3 | 997 | 20.9 | 124 | 17.9 | 34 | 4.1 | 2,885 | 22.7 | 525 | 21 |
| Other unspecified (1991+) | 18 | 0.1 | 5 | 0.1 | 1 | 0.1 | 0 | 0 | 9 | 0.1 | 3 | 0.1 |
| White | 14,268 | 66.4 | 3,137 | 65.8 | 473 | 68.5 | 762 | 92.8 | 8,231 | 64.8 | 1,665 | 66.7 |
| Sex | ||||||||||||
| Female | 7,947 | 37 | 1,651 | 34.6 | 231 | 33.4 | 117 | 14.3 | 5,061 | 39.9 | 887 | 35.5 |
| Male | 13,525 | 63 | 3,117 | 65.4 | 460 | 66.6 | 704 | 85.7 | 7,635 | 60.1 | 1,609 | 64.5 |
| Vital status recode (study cutoff used) | ||||||||||||
| Deceased | 12,954 | 60.3 | 3,025 | 63.4 | 499 | 72.2 | 505 | 61.5 | 8,269 | 65.1 | 656 | 26.3 |
| Alive | 8,518 | 39.7 | 1,743 | 36.6 | 192 | 27.8 | 316 | 38.5 | 4,427 | 34.9 | 1,840 | 73.7 |
| Derived AJCC stage group, 6th ed. (2004+) | ||||||||||||
| IA | 3,772 | 17.6 | 52 | 1.1 | 16 | 2.3 | 59 | 7.2 | 3,562 | 28.1 | 83 | 3.3 |
| IB | 4,563 | 21.3 | 707 | 14.8 | 135 | 19.5 | 221 | 26.9 | 3,179 | 25 | 321 | 12.9 |
| II | 5,944 | 27.7 | 1,722 | 36.1 | 237 | 34.3 | 289 | 35.2 | 2,917 | 23 | 779 | 31.2 |
| IIIA | 3,797 | 17.7 | 1,197 | 25.1 | 170 | 24.6 | 177 | 21.6 | 1,659 | 13.1 | 594 | 23.8 |
| IIIB | 797 | 3.7 | 331 | 6.9 | 28 | 4.1 | 12 | 1.5 | 284 | 2.2 | 142 | 5.7 |
| IV | 2,599 | 12.1 | 759 | 15.9 | 105 | 15.2 | 63 | 7.7 | 1,095 | 8.6 | 577 | 23.1 |
| Derived AJCC T, 6th ed. (2004+) | ||||||||||||
| T1 | 4,708 | 21.9 | 268 | 5.6 | 52 | 7.5 | 96 | 11.7 | 3,976 | 31.3 | 316 | 12.7 |
| T2 | 11,155 | 51.9 | 2,864 | 60.1 | 423 | 61.2 | 464 | 56.6 | 6,196 | 48.8 | 1,208 | 48.4 |
| T3 | 3,369 | 15.7 | 1,077 | 22.6 | 121 | 17.5 | 180 | 21.9 | 1,474 | 11.6 | 517 | 20.7 |
| T4 | 2,240 | 10.4 | 559 | 11.7 | 95 | 13.7 | 81 | 9.9 | 1,050 | 8.3 | 455 | 18.2 |
| Derived AJCC N, 6th ed. (2004+) | ||||||||||||
| N0 | 9,641 | 44.9 | 1,723 | 36.2 | 139 | 20.1 | 323 | 39.3 | 7,016 | 55.3 | 440 | 17.6 |
| N1 | 7,663 | 35.7 | 1,576 | 33.1 | 387 | 56 | 425 | 51.8 | 4,057 | 32 | 1,218 | 48.8 |
| N2 | 3,133 | 14.6 | 1,129 | 23.7 | 130 | 18.8 | 62 | 7.6 | 1,248 | 9.8 | 564 | 22.6 |
| N3 | 1,035 | 4.8 | 340 | 7.1 | 35 | 5.1 | 11 | 1.3 | 375 | 3 | 274 | 11 |
RESULTS
This study included 21,472 patients. The median age of the cohort was 66 years, with 63.0% male and 66.4% white (Table 1). Median survival by stage was 96 months for stage I, 30 months for stage II, 20 months for stage III, and 14 months for stage IV. The number of patients organized by therapy was 4,768 for SRC, 2,496 for SC, 691 for SR, 821 for RSC, and 12,696 for S only. SRC was used as the reference group for statistical comparison of survival (HR, 1.00; Table 2). Three- and 5-year survival rates by treatment modality were also calculated (Table 3). For the 3-year survival rate, S (54.3%) was the highest, followed by SRC (46.6%), RSC (43.0%), SR (38.3%), and SC (36.8%). The 5-year survival demonstrated a slightly different trend, with S (38.3%) as the highest, followed by SR (35.2%), RSC (32.5%), SRC (30.7%), and SC (26.1%).
Table 2.
Treatment modalities and HRs by stage
| Treatment modalities | Stage I |
Stage II |
Stage III |
Stage IV |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| SRC | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — |
| SR | 1.07 | (0.84, 1.36) | 1.32* | (1.12, 1.56) | 1.10 | (0.84, 1.36) | 1.25* | (1.07, 1.56) |
| RSC | 1.52* | (1.25, 1.86) | 1.31* | (1.12, 1.54) | 1.08 | (1.25, 1.86) | 1.02 | (0.76, 1.36) |
| S only | 0.67* | (0.60, 0.76) | 1.37* | (1.27, 1.49) | 1.25* | (0.60, 0.76) | 1.51* | (1.36, 1.67) |
| SC | 1.24* | (1.04, 1.48) | 1.30* | (1.18, 1.45) | 1.15* | (1.04, 1.48) | 1.37* | (1.22, 1.54) |
The model was adjusted for age, race, and sex.
P < .05.
Table 3.
Three and Five-Year Survival Rate
| Treatment modalities | 3-year survival rate, % | 5-year survival rate, % | Follow-up time |
||
|---|---|---|---|---|---|
| Mean | Median* | Range | |||
| 1. Surgery + radiation after + chemo | 46.6 | 30.70 | 39.10 | 26 | 4-258 |
| 2. Surgery + radiation after | 38.3 | 35.20 | 38.37 | 19 | 4-260 |
| 3. Surgery + radiation prior +chemo | 43.0 | 32.50 | 32.54 | 22.5 | 4-221 |
| 4. Surgery only | 54.3 | 38.30 | 45.85 | 26 | 4-263 |
| 5. Surgery + chemo | 36.80 | 26.10 | 36.50 | 20 | 4-264 |
1. Surgery followed by radiotherapy with chemotherapy; 2. surgery followed by radiotherapy; 3. radiotherapy followed by surgery with chemotherapy; 4. surgery alone; and 5. surgery with chemotherapy.
Indicates median follow-up time in months.
For stage I patients, those in the S group had the most favorable cause-specific survival (HR, 0.67; CI, 0.60–0.76), followed by SRC, the SC (HR, 1.24; CI, 1.04–1.48) SR (HR, 1.07; CI, 0.84–1.36), and RSC (HR, 1.52; CI, 1.25–1.86). However patients in the S group did not have the highest median survival in months (with range) (99; 94–109), when compared to other groups: SRC (107; 90–129), SR (53; 36–106), RSC (45; 38.0–67.0), and SC (67; 94–105). When stage IA patients were excluded (Figure 1), analyzing stage IB yielded different results. Stage IB (Figure 2) patients receiving SRC treatment demonstrated the highest survival compared with S (HR, 1.29; CI, 1.14–1.46), SC (HR 1.36; CI, 1.12–1.65), RSC (HR, 1.77; CI, 1.43–2.19), and SR (HR, 1.41; CI, 1.10–1.81). The median survival (in months) of stage IB by treatment was SRC, 100; SR, 52; RSC, 45; SC, 63; and S, 70.
Figure 1.
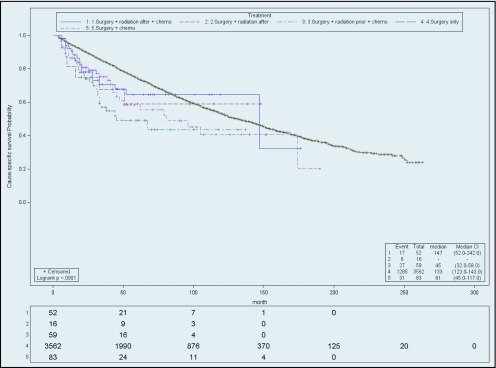
Cause-specific survival of stage IA. Actuarial cause-specific survival estimated for the entire cohort using the Kaplan-Meier method.
Figure 2.
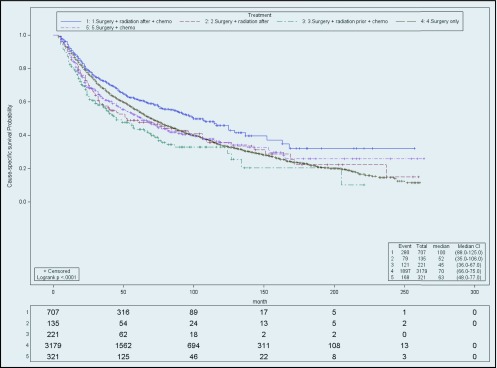
Cause-specific survival of stage IB. Actuarial cause-specific survival estimated for the entire cohort using the Kaplan-Meier method.
SRC had the best outcome compared to others in stage II patients (Figure 3), followed by SC (HR, 1.30; CI 1.18–1.45), RSC (HR, 1.31, CI, 1.12–1.54), SR (HR, 1.32; CI, 1.12–1.56), and S (HR, 1.37; CI, 1.27–1.49). The median survival (months; range) for the SRC patients was the longest (43; 39–48). Stage II patients receiving SC had the second highest median survival (29; 27–32), followed by RSC (28; 24–32), SR (25;19–35), and S (26; 24–27).
Figure 3.
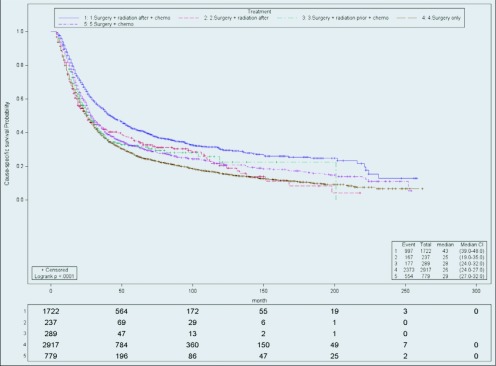
Cause-specific survival of stage II. Actuarial cause-specific survival estimated for the entire cohort using the Kaplan-Meier method.
For stage III patients (Figure 4), SRC remained the most favorable group, followed by RSC (HR, 1.08; CI, 0.90–1.29), SR (HR, 1.10; CI, 0.93–1.31), SC (HR 1.15; CI, 1.04–1.29), and S (HR, 1.25; CI, 1.15–1.35). The SRC group had a median survival of 24 months (range, 23–26). The other groups did not perform as well: RSC (22; 19–25), SC (21; 16–18) SR (18; 17–22), and S (17; 16–18).
Figure 4.
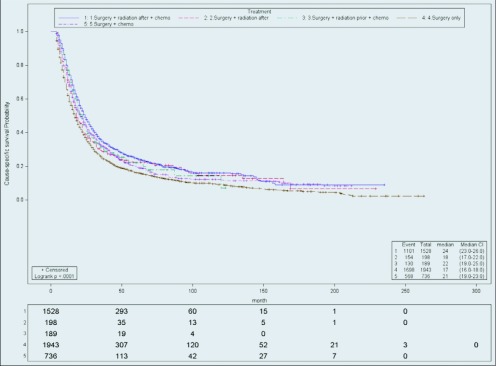
Cause-specific survival of stage III. Actuarial cause-specific survival estimated for the entire cohort using the Kaplan-Meier method.
Last, for nonmetastatic stage IV patients (Figure 5), SRC remained the best group, followed by RSC (HR, 1.02; CI, 0.76–1.36), SR (HR, 1.25; CI, 1.07–1.56), SC (HR, 1.37; CI, 1.22–1.54), and S (HR, 1.51; CI, 1.36–1.67). SRC group median survival was 18 months (range, 16–19). The RSC group performed similarly in median survival, but the results were not statistically significant (months; range) (18; 14–27). The other 3 groups, SC (14; 13–15) SR (3; 11–17), and S (11; 11–12) had worse median survival outcomes.
Figure 5.
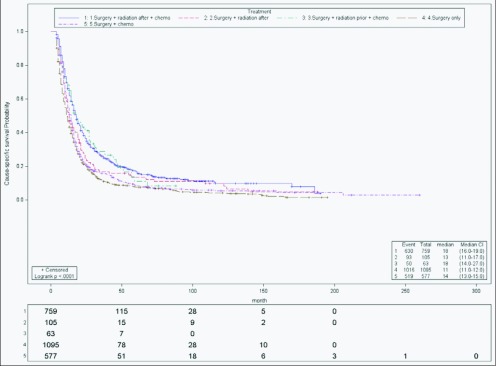
Cause-specific survival of stage IV. Actuarial cause-specific survival estimated for the entire cohort using the Kaplan-Meier method.
A univariate analysis demonstrated that all stages had better outcomes with multimodality therapy, except stage IA. Those who received chemotherapy, radiation, and surgery had a higher median survival than those who did not. The data also illustrate that trimodality therapy became more effective as the stage of the cancer progressed from stage IB.
A multivariate analysis incorporating sex, race, and age at diagnosis demonstrated that age and race had statistically significant effects in all stages. Younger patients received a survival benefit. Compared to Caucasians (HR, 1.00), patients of American Indian/Asian/Pacific Islander ethnicity had more favorable outcomes for every stage (HR < 1.00). As the stage of the cancer progressed, this ethnic group showed outcomes similar to those of the reference Caucasian group but still significantly better. African Americans had outcomes similar to those of the Caucasians, regardless of stage (HR ∼1.00; P > .05). Female patients showed improved outcomes for stages I (HR, 0.8; CI, 0.75–0.86) and II (HR, .84; CI, 0.79–0.90) compared to the male patients (HR, 1.00).
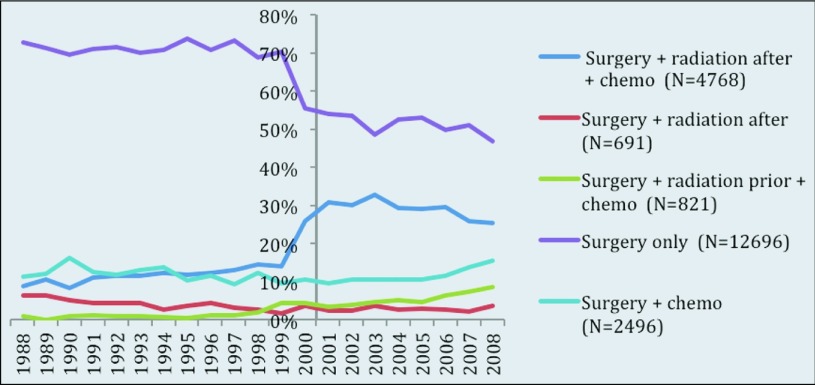
Therapy modality by year was also investigated, to compare the fraction of patients treated from 1988 to 1999 with those treated from 2000 to 2008 (Figure 6). This stratification was based on completion of patient enrollment in INT-0116 in July 1998, and the assumption that early results were known soon thereafter. For patients in the S group, the percentage treated from 1988 to 1999 varied from 69 to 74%. After 1999, the fraction of patients in the S group decreased by at least 13% (range, 47–56%). The opposite trend is noted in the SRC group. Before 2000, SRC patients represented only 8 to 14% of those treated for stomach cancer but from 2000 to 2008, the proportion of SRC patients increased (25–33%). A minimal increase was noted in the RSC group between 1988 and 1999 (0–4%) and 2008 and 2008 (4–9%). All other treatment groups did not display a trend of increase or decline.
Figure 6.
Treatment modality received by year.
DISCUSSION
The goal of this study was to compare different therapy modalities for gastric adenocarcinoma using the SEER database. Although various trials examining the sequence of therapy for gastric cancer have been performed, there is no consensus of optimal multimodality treatment for gastric adenocarcinoma.
In one of the most influential studies, known as the INT-0116 trial (Southwest Oncology Group 9008), the use of postoperative chemoradiation was investigated and compared to surgery alone.4 Patients with resected stage IB–IV M(0) gastric or gastroesophageal junction adenocarcinoma were randomized to receive postoperative chemoradiation (281 patients) with 5-FU/leucovorin/radiation (4500 cGy, 25 fractions) or surgery alone (275 patients). The results showed that patients in the treatment arm had extended median disease-free (30 months; P < .0001) and overall (36 months; P = .005) survival compared with that of the control groups (19 and 27 months, respectively). This correlation did not deteriorate over time. Even though the results showed benefits of adjuvant chemoradiation, in this randomized study, many patients were still treated with surgery alone (69.7% of the patient sample; N = 14,583).
MAGIC was another clinical trial for gastric cancer treatment.5 It tested the efficacy of perioperative chemotherapy vs. (250 patients) surgery alone (253 patients). Patients in the treatment arm received 3 cycles of pre- and postoperative chemotherapy (ECF). The results showed that patients receiving perioperative ECF therapy had higher rates of curative resection (79.3%) than did the control group (70.3%). The perioperative chemotherapy group also demonstrated a higher likelihood of progression-free (HR, 0.66; CI, 0.53-0.81; P < .001) and overall (HR, 0.75; 95% CI, .60-.93; P = .009) survival. Five-year survival rates were 36.3% (CI, 29.5–43.0%) in the perioperative chemotherapy group and 23% (CI, 16.6–29.4%) in the surgery-alone group. One limitation of this study was that 58% of patients in the treatment group did not complete the postoperative chemotherapy because of disease progression, patient request, or postoperative complications. Given these 2 positive studies with different perioperative treatments, the optimal management of gastric cancer remains unclear.
The results from this study are consistent with the INT-0116/SWOG-9008 trial which found the highest median survival in patients with stage IB–IV M(0), who received postoperative radiation therapy with chemotherapy compared with those who underwent surgery alone. However stage IA patients still primarily benefit from surgery alone, but this was not investigated in the INT-0116/SWOG-9008 trial. This SEER retrospective study also compared patients who received surgery and chemotherapy to those who received surgery alone. A similar comparison was made in the MAGIC trial. Patients with stage II–IV disease benefited from chemotherapy, which is consistent with the MAGIC trial.5 However, the 5-year survival rate in this study (26.1%) was lower than that in the MAGIC trial (36%). These differences most likely arise from the variety of regimens and types of chemotherapy given from 1998 to 2008. Nonetheless, stage III and IV patients benefited more from surgery, chemotherapy, and radiation than from surgery and chemotherapy. This finding suggests that in locally advanced disease, surgery with chemoradiation may provide a better survival benefit than chemotherapy.
A recent retrospective study was published by Snyder et al,6 who used SEER to determine the survival benefit of adjuvant radiation and extended lymphadenectomy for gastric cancer. This study of 15,060 patients with histologically confirmed gastric adenocarcinoma demonstrated that patients receiving adjuvant radiation therapy after gastric resection had improved 5-year overall survival for stage IB (P = .002) and higher (P < .001) than did patients who received only surgery. Median overall survival for stage IB, II, IIIA, and IIIB patients treated with radiation therapy was higher (65, 34, 23, and 19 months, respectively) than the control (54, 21, 14, and 11 months, respectively). The extent of lymphadenectomy performed was also explored and categorized by number of lymph nodes removed (>25, 15–25, <15). It also noted that the overall median survival by months (34, 27, and 25, respectively) and 5-year survival (38%, 31%, and 31%, respectively) increased with more extensive lymph node dissection.
This study showed a similar correlation between adjuvant radiation therapy and cause-specific median survival for stages II, III, and IV M(0) (25, 18, and 13 months, respectively). However, we also investigated the use of chemotherapy with adjuvant or neoadjuvant radiation therapy and found that patients treated with chemotherapy with either type of radiation therapy obtained a survival benefit vs. patients who received adjuvant radiation alone. This study also excluded patients who died within 3 months after their initial diagnosis, to account for patients who did not survive long enough to receive radiation therapy. Including these patients in the study artificially increases the efficacy of postoperative radiation therapy and is known as the immortal time bias.7 Although patients with stage I disease receiving postoperative adjuvant radiation therapy had the highest median survival, patients treated with surgery alone still displayed the lowest HR because the group had more patients and a longer follow-up time. When stratified for age and race, surgery alone was most beneficial for patients less than 74 years of age with stage I cancer, consistent with results in previous studies.
Finally, this study did not include the extent of lymph node dissection (LND) performed. The benefit of extensive LND remains controversial. Although initial Japanese investigations show the benefit of D2 gastrectomy,8,9 more recent trials (eg, the Dutch Gastric Center Trial), have shown increased morbidity and mortality with D2 resection.10 In the INT-0116/SWOG-9008 trial, only 10% of patients underwent D2 resection, whereas 54% had D0 resection (gastrectomy with incomplete resection of the N1 nodes). Although extensive LND may increase overall survival, it cannot be assumed that D2 gastrectomy provides a survival benefit, as anatomical resection, not the number of lymph nodes dissected, defines a D2 resection. Therefore, to make the results of this study more applicable, the degree of LND was not investigated.
The current study found that more patients started to receive adjuvant and neoadjuvant radiation with chemotherapy beginning in 2000, whereas the proportion of patients treated with surgery alone decreased. Although the INT-0116 initial results were not published until 2001, the change in practice can be explained. First, the INT-0116 trial was a U.S. cooperative trial. Also, INT-0116 preliminary data and abstract were most likely presented before publication, which could have changed the practices for stomach cancer.
The 3- and 5-year survival rate data (Table 3) imply that RSC was not the most beneficial group. However, there are considerations that should be taken into account. Because it is not further stratified by stage, it is difficult to compare the effectiveness of each treatment modality. Surgery alone had the highest 3- and 5-year survival rates because 30% of the patients in this study were stage IA and benefited most from surgery alone. Also, not all of the results were statistically significant, making treatment comparison further challenging. Because of this, treatment was organized by stage and further analyzed.
The limitations of this retrospective study are related to the capabilities of the SEER database. Although it enables filtering for patients who received chemotherapy, SEER does not provide information on whether it was preoperative, postoperative, or both. It also does not identify the type of chemotherapy given. However, the standard chemotherapy given during the time of the collected data was 5-FU/leucovorin, and so it can be assumed this was the primary chemotherapy given in the current study. Although the sequence of radiation therapy can be selected, the type of radiation therapy given and dosage are unknown. Finally, information regarding local or distant recurrence is not recorded in SEER, and so disease-free survival cannot be calculated. One potential confounding factor in this study was age. Younger patients demonstrated a survival benefit, and the SRC group was the second youngest. It is difficult to make any clear inferences, but should still be considered.
CONCLUSIONS
In summary, this study supports the use of adjuvant radiation with chemotherapy to treat stage IB gastric adenocarcinoma, as seen with increased cause-specific median survival. Patients who received chemoradiation regardless of sequence of therapy survived longer compared to those receiving radiation alone, indicating that chemotherapy helps improve locoregional control of gastric adenocarcinoma. Future studies should include direct comparisons of postoperative chemotherapy (ECF vs. 5-FU/leucovorin) in patients treated with adjuvant chemoradiation for gastric cancer. After 1999, more patients started to receive adjuvant radiation with chemotherapy, indicating a change in practice, mostly resulting from previous trials that demonstrated its efficacy.
ACKNOWLEDGMENTS
Presented at the ASTRO (American Society for Radiation Oncology) Annual Meeting, Atlanta, Georgia, September 22–25, 2013.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Ferlay J, Shin HR, Bray F, et al. : GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer, 2010. Available at: http://globocan.iarc.fr Accessed 29 April 2013 [Google Scholar]
- 2. Fuchs CS, Mayer RJ: Gastric carcinoma. N Engl J Med 333:32–41, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Hundahl SA, Phillips JL, Menck HR: The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy, 5th ed. American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer 88:921, 2000 [PubMed] [Google Scholar]
- 4. Macdonald JS, Smalley SR, Benedetti J, et al. : Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725–730, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Cunningham D, Alum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer: results of MAGIC trial. N Engl J Med 355:11–20, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Synder RA, Castaldo ET, Bailey CE, et al. : Survival benefit of adjuvant radiation therapy for gastric cancer following gastrectomy and extended lymphadenectomy. Int J Surg Oncol 2012:11–307670, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park HS, Gross GP, Makarov DV, et al. : Immortal time bias: a frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys 83:1365–1373, 2012 [DOI] [PubMed] [Google Scholar]
- 8. Maruyama K, Gunven P, Okabayashi K, et al. : Lymph node metastases of gastric cancer: general pattern in 1931 patients. Ann Surg 210:596–602, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sasako M, McCullouch P, Kinoshita T: New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg 82346–351, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Hartgritik HH, van de Velde CH, Putter H, et al. : Extended lymph node dissection for gastric cancer: who may benefit?—final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 22:2069–2077, 2004 [DOI] [PubMed] [Google Scholar]