CASE REPORT
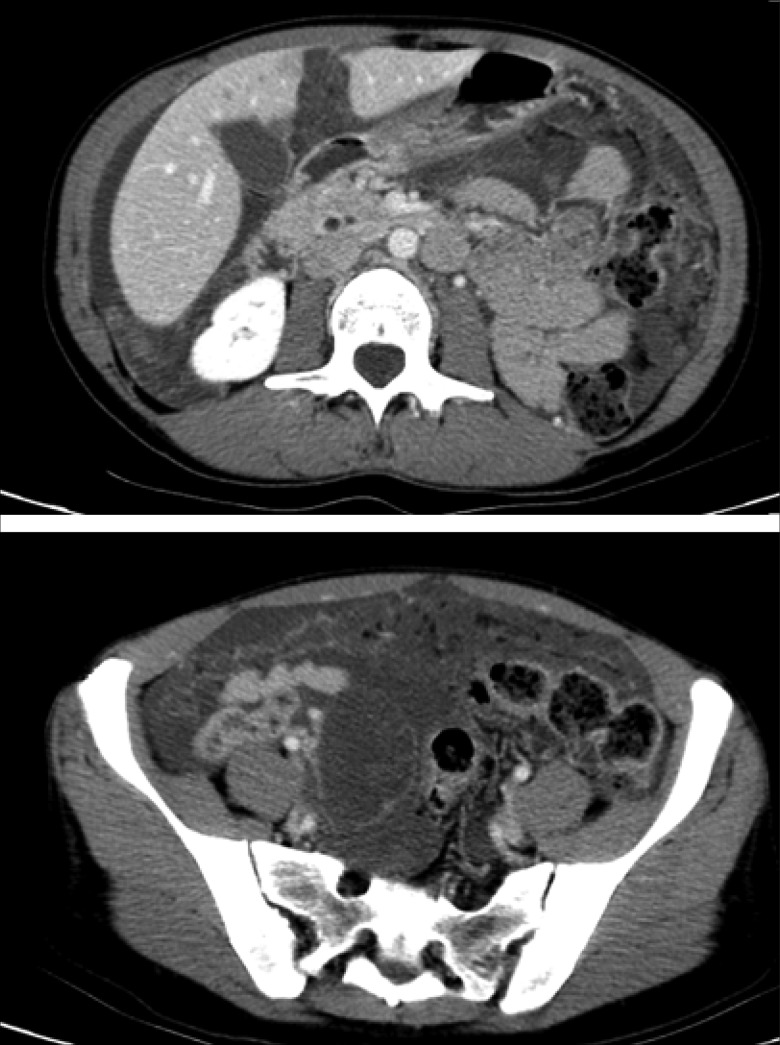
A 53-year-old woman presented in 2010 with increasing abdominal girth and bloating. Computed tomography (CT) showed a multiloculated cystic mass in the right adnexa, as well as peritoneal carcinomatosis, omental cake, and ascites (Figure 1). Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were elevated at 58 ng/mL and 148 U/mL, respectively. The patient underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, appendectomy, and nonoptimal debulking. There was gelatinous material in all four quadrants, with implants throughout the abdomen. Histologic examination showed low-grade mucinous adenocarcinoma of the appendix with associated pseudomyxoma peritonei (PMP; Figure 2). The patient subsequently underwent complete cytoreductive surgery including peritoneal stripping and intraperitoneal hyperthermic chemotherapy. Adjuvant chemotherapy with 5-fluorouracil and oxaliplatin was begun but had to be abandoned after 3 cycles because of poor tolerance. Approximately 18 months after surgery, the patient presented with increasing back pain associated with elevated alkaline phosphatase, CEA, and CA19-9. A bone scan (Figure 3) showed diffuse axial bone metastasis, and a biopsy confirmed the diagnosis of metastatic carcinoma, in keeping with the known appendiceal low-grade mucinous adenocarcinoma (Figure 2). Palliative chemotherapy with oral capecitabine 1000 mg/m2, twice daily for 14 days every 21 days, was initiated, with clinical and biochemical response followed by progression after 9 months of therapy.
Figure 1.
CT scan at presentation showing diffuse intra-abdominal fluid and extensive omental cake.
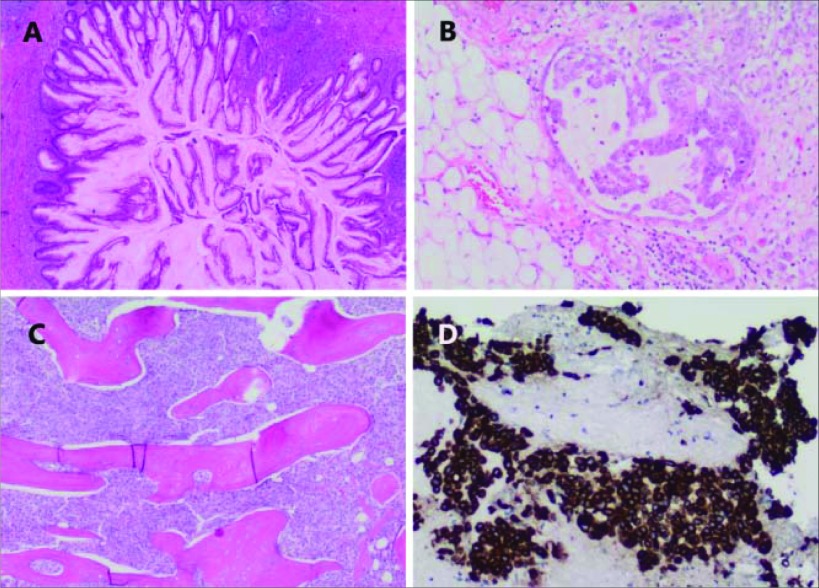
Figure 2.
Histology. Sections of the appendix (A) show prominent neoplastic epithelium with focal infiltration of the appendiceal wall. Cellular mucin deposits (B) are present within the omentum, in keeping with PMP. The core needle biopsy of the ileum (C) shows trabecular bone diffusely infiltrated by metastatic carcinoma with immunoreactivity for cytokeratin 20 (D), supporting metastatic appendiceal carcinoma.
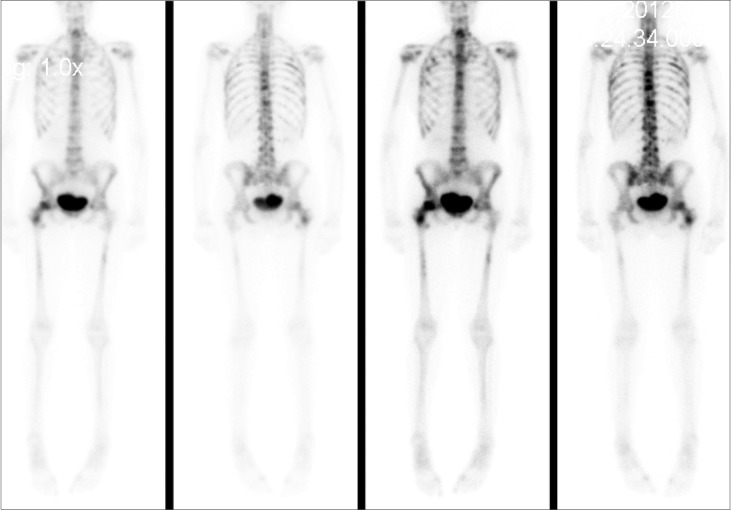
Figure 3.
Whole-body bone scan showing widespread, diffuse, metastatic bone disease.
DISCUSSION
Pseudomyxoma peritonei (PMP) is a rare, slowly progressing neoplasm characterized by extensive mucus accumulation within the abdomen and pelvis and is associated with biologically heterogeneous behavior. Diffuse peritoneal spread occurs in most patients, but distant metastases are infrequent. To the best of our knowledge, this is the first ever reported case of appendiceal PMP metastatic to the bones.
It has been proposed that the term PMP syndrome be applied only to a homogeneous group of histologically benign peritoneal tumors associated with appendiceal mucinous adenomas, a condition currently termed disseminated peritoneal adenomucinosis (DPAM).1 However, others also use the term PMP to describe the peritoneal dissemination of mucus-producing adenocarcinomas of the appendix, large and small bowel, and other sites.1,2 The inconsistent definition of PMP and differing prognoses between histologic subgroups make a comparison of PMP studies problematic.3,4 It is well known that disseminated mucin-producing adenocarcinomas of the appendix (also called peritoneal mucinous carcinomatosis or PMCA) represent a more aggressive subtype of peritoneal mucinous tumors when compared with the more indolent DPAM.3,4
A large, retrospective, multi-institutional review in collaboration with the Peritoneal Surface Oncology Group International reported the results of 2298 patients who underwent cytoreductive surgery (CRS) followed by intraperitoneal (IP) chemotherapy. Multivariate analysis identified PMCA subtype as an independent predictor of poor overall survival (P < .001). The 5-year overall survival rate for patients with DPAM was 81%, compared to only 59% for those with peritoneal PMCA and 78% for those with intermediate features.4
Another series of 109 patients with PMP demonstrated a statistically significant difference in survival among cases classified as DPAM, PMCA with intermediate or discordant features, or PMCA (P < .0001). The age-adjusted 5-year survival rates were 84, 37.6, and 6.7%, respectively.5
Furthermore, among appendiceal adenocarcinomas, histologic subtype appears to be a prognostic indicator. In a retrospective review of 94 patients with appendiceal adenocarcinoma, those with mucinous type (55%) had had a better 5-year survival when compared with those with colonic type (71% vs. 41%; P < .01). Intra-abdominal recurrence was also frequent, and only 27 patients remained disease free by the end of the follow-up. Of note, no patients with mucinous appendiceal adenocarcinoma developed extra-abdominal metastases, and PMCA seemed to have patterns of recurrence similar to those of DPAM.6
Since distant metastasis and visceral involvement are very rare, death is mostly due to loss of intestinal function and obstruction by peritoneal implants. Even in patients with long-term survival, intra-abdominal recurrence is common. In a retrospective review of 97 patients with PMP treated at Memorial Sloan-Kettering Cancer Center, 90% of the 10-year survivors required multiple operations for recurrence, and 77% had evidence of disease either at death or at the completion of follow-up.7
Extra-abdominal metastasis of PMP is a rare event, with lung and pleural disease accounting for most cases.8 Pleural metastases are thought to be an extension of abdominal disease caused by diaphragmatic injury at the time of cytoreductive surgery, direct invasion through the diaphragm, or congenital pleuroperitoneal communication.9–11 PMP spread was once considered unlikely to occur by lymphatic or hematogenous dissemination. However, recent reports of lung metastasis have challenged this assumption. There are at least 11 reported cases of PMP (described as DPAM) metastatic to the lungs.8,12–18 Splenic metastases have also been reported, but, despite resembling metastatic disease, splenic lesions are likely to represent entrapment of mucinous tumor within the splenic surface trabeculae, which extend into the splenic parenchyma.19
A recent retrospective study of 626 cases of appendiceal adenocarcinoma20 included 42 cases of intrathoracic metastases, involving pleura (n = 10), lung (n = 22), or both (n = 10). The authors inferred that lung metastasis from appendiceal adenocarcinoma may be higher than expected. To date, no other case of PMP metastatic to the bones has been described in the literature. We surmise that the bone lesions arose via hematogenous spread.
Standard treatment for PMP consists of repeated surgical debulking for symptomatic intra-abdominal disease. Unfortunately, due to the rarity of PMP, the utility of systemic chemotherapy for unresectable disease remains unknown, and there is no clear evidence supporting the superiority of any particular chemotherapy regimen. In a recent retrospective study from M. D. Anderson describing the use of systemic chemotherapy in 54 patients with PMP, the most commonly prescribed agents were capecitabine and 5-fluorouracil (84%), with or without a platinum drug. Two cases with complete response, 11 with partial response, and 17 with prolonged stable disease were reported, providing a clinical benefit rate of 55%.21 The only published small phase II trial on advanced unresectable PMP suggests activity for capecitabine combined with mitomycin C.22 In this study 15 (38%) of 39 patients with assessable disease appeared to benefit from treatment. Despite the paucity of data on efficacy, medical oncologists typically use combinations of agents similar to those used in the treatment of metastatic colorectal cancer.
In conclusion, the presented case reinforces the potential for metastatic spread of PMP. Because of the lack of knowledge regarding systemic therapies for PMP, studies testing colorectal cancer regimens for use in appendiceal adenocarcinoma and PMP are urgently needed.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1. Sugarbaker PH, Ronnett BM, Archer A, et al. : Pseudomyxoma peritonei syndrome. Adv Surg 30:233–280, 1996 [PubMed] [Google Scholar]
- 2. Hinson FL, Ambrose NS: Pseudomyxoma peritonei. Br J Surg 85:1332–1339, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Ronnett BM, Yan H, Kurman RJ, et al. : Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer 92:85–91, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Baratti D, Kusamura S, Nonaka D, et al. : Pseudomyxoma peritonei: clinical pathological and biological prognostic factors in patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 15:526–534, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Smith JW, Kemeny N, Caldwell C, et al. : Pseudomyxoma peritonei of appendiceal origin: the Memorial Sloan-Kettering Cancer Center experience. Cancer 70:396–401, 1992 [DOI] [PubMed] [Google Scholar]
- 6. Nitecki SS, Wolff BG, Schlinkert R, et al. : The natural history of surgically treated primary adenocarcinoma of the appendix. Ann Surg 219:51–57, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miner TJ, Shia J, Jaques DP, et al. : Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 241:300–308, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geisinger KR, Levine EA, Shen P, et al. : Pleuropulmonary involvement in pseudomyxoma peritonei: morphologic assessment and literature review. Am J Clin Pathol 127:135–143, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Pestieau SR, Esquivel J, Sugarbaker PH: Pleural extension of mucinous tumor in patients with pseudomyxoma peritonei syndrome. Ann Surg Oncol 7:199–203, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Chua TC, Yan TD, Yap ZL, et al. : Thoracic cytoreductive surgery and intraoperative hyperthermic intrathoracic chemotherapy for pseudomyxoma peritonei. J Surg Oncol 99:292–295, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Pestieau SR, Wolk R, Sugarbaker PH: Congenital pleuroperitoneal communication in a patient with pseudomyxoma peritonei. J Surg Oncol 73:174–178, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Berge T: Mucocelle appendicis with pseudomyxoma peritonei and pulmonary metastases. Acta Pathol Microbiol Scand 60:483–486, 1964 [DOI] [PubMed] [Google Scholar]
- 13. Chevillotte G, Choux R, Spik G, et al. : Pseudomyxoma peritonei: a case report with multiple metastasis: ultrastructural study and chemical analysis of mucoid substance. Gastroenterol Clin Biol 7:445–450, 1983 [PubMed] [Google Scholar]
- 14. Kreissig P, Daucourt J, Garnier G: Pseudomyxoma peritonei with pulmonary metastasis (in French). Presse Mede 20:445–1287, 1991 [PubMed] [Google Scholar]
- 15. Mortman KD, Sugarbaker PA, Shmookler BM, et al. : Pulmonary metastases in pseudomyxoma peritonei syndrome. Ann Thorac Surg 64:1434–1436, 1997 [DOI] [PubMed] [Google Scholar]
- 16. Lee BY, Kim HS, Lee SH, et al. : Pseudomyxoma peritonei: extraperitoneal spread to the pleural cavity and lung. J Thorac Imaging 19:123–126, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Khan AA, Tambiah J, Cane P, et al. : Prolonged survival in a patient with recurrent pulmonary metastases secondary to mucinous cystadenocarcinoma of the appendix with pseudomyxomatous peritonei. Ann Thorac Surg 83:1893–1894, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kitai T: Pulmonary metastasis from pseudomyxoma peritonei. Gastroenterol Res Pract 2012:690256, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cabanas J, Gomes da Silva R, Zappa L, et al. : Splenic metastases from mucinous neoplasms of the appendix and colon. Tumori 92:104–112, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hill JS, Rafeeq S, Rice DC, et al. : Clinicopathologic characteristics of patients with appendiceal adenocarcinoma (AA) and intra-thoracic metastasis. J Clin Oncol 30(suppl 4), 2012. (abstr 427) [Google Scholar]
- 21. Shapiro JF, Chase JL, Wolff RA, et al. : Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer 116:316–322, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Farquharson AL, Pranesh N, Witham G, et al. : A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer 99:591–596, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]