Abstract
Background
This review updates a 1999 Cochrane review showing that ibuprofen at various doses was effective in postoperative pain in single dose studies designed to demonstrate analgesic efficacy. New studies have since been published. Ibuprofen is one of the most widely used non‐steroidal anti‐inflammatory (NSAID) analgesics both by prescription and as an over‐the‐counter medicine. Ibuprofen is used for acute and chronic painful conditions.
Objectives
To assess analgesic efficacy of ibuprofen in single oral doses for moderate and severe postoperative pain in adults.
Search methods
We searched Cochrane CENTRAL, MEDLINE, EMBASE and the Oxford Pain Relief Database for studies to May 2009.
Selection criteria
Randomised, double blind, placebo‐controlled trials of single dose orally administered ibuprofen (any formulation) in adults with moderate to severe acute postoperative pain.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. Pain relief or pain intensity data were extracted and converted into the dichotomous outcome of number of participants with at least 50% pain relief over 4 to 6 hours, from which relative risk and number‐needed‐to‐treat‐to‐benefit (NNT) were calculated. Numbers of participants using rescue medication over specified time periods, and time to use of rescue medication, were sought as additional measures of efficacy. Information on adverse events and withdrawals were collected.
Main results
Seventy‐two studies compared ibuprofen and placebo (9186 participants). Studies were predominantly of high reporting quality, and the bulk of the information concerned ibuprofen 200 mg and 400 mg. For at least 50% pain relief compared with placebo the NNT for ibuprofen 200 mg (2690 participants) was 2.7 (2.5 to 3.0) and for ibuprofen 400 mg (6475 participants) it was 2.5 (2.4 to 2.6). The proportion with at least 50% pain relief was 46% with 200 mg and 54% with 400 mg. Remedication within 6 hours was less frequent with higher doses, with 48% remedicating with 200 mg and 42% with 400 mg. The median time to remedication was 4.7 hours with 200 mg and 5.4 hours with 400 mg. Sensitivity analysis indicated that pain model and ibuprofen formulation may both affect the result, with dental impaction models and soluble ibuprofen salts producing better efficacy estimates. Adverse events were uncommon, and not different from placebo.
Authors' conclusions
The very substantial amount of high quality evidence demonstrates that ibuprofen is an effective analgesic in treating postoperative pain. NNTs for 200 mg and 400 mg ibuprofen did not change significantly from the previous review even when a substantial amount of new information was added. New information is provided on remedication.
Plain language summary
A single dose of ibuprofen administered orally to treat acute postoperative pain in adults
Ibuprofen at 200 mg and 400 mg produces a high level of pain relief in about half of those with moderate or severe acute postoperative pain. This is a good result compared with most other analgesics tested in a very well researched model of pain used for demonstrating that drugs can actually produce pain relief. There were no more adverse events than with placebo.
Background
This review is an update of a previously published review in The Cochrane Database of Systematic Reviews on 'Single dose oral ibuprofen and diclofenac for postoperative pain' (Collins 1999). In this update it refers to ibuprofen only, and the title now states that the review is limited to adults. An updated review of single dose oral diclofenac in acute postoperative pain in adults has also been published (Derry P 2009).
Acute pain occurs as a result of tissue damage either accidentally due to an injury or as a result of surgery. Acute postoperative pain is a manifestation of inflammation due to tissue injury. The management of postoperative pain and inflammation is a critical component of patient care. This is one of a series of reviews whose aim is to present evidence for relative analgesic efficacy through indirect comparisons with placebo, in very similar trials performed in a standard manner, with very similar outcomes, and over the same duration. Such relative analgesic efficacy does not in itself determine choice of drug for any situation or patient, but guides policy‐making at the local level.
Recently published reviews include paracetamol (Toms 2008), celecoxib (Derry 2008), naproxen (Derry C 2009) and parecoxib (Lloyd 2009).
Single dose trials in acute pain are commonly short in duration, rarely lasting longer than 12 hours. The numbers of participants is small, allowing no reliable conclusions to be drawn about safety. To show that the analgesic is working it is necessary to use placebo (McQuay 2005). There are clear ethical considerations in doing this. These ethical considerations are answered by using acute pain situations where the pain is expected to go away, and by providing additional analgesia, commonly called rescue analgesia, if the pain has not diminished after about an hour. This is reasonable, because not all participants given an analgesic will have significant pain relief. Approximately 18% of participants given placebo will have significant pain relief (Moore 2006), and up to 50% may have inadequate analgesia with active medicines. The use of additional or rescue analgesia is hence important for all participants in the trials.
Clinical trials measuring the efficacy of analgesics in acute pain have been standardised over many years. Trials have to be randomised and double blind. Typically, in the first few hours or days after an operation, patients develop pain that is moderate to severe in intensity, and will then be given the test analgesic or placebo. Pain is measured using standard pain intensity scales immediately before the intervention, and then using pain intensity and pain relief scales over the following 4 to 6 hours for shorter acting drugs, and up to 12 or 24 hours for longer acting drugs. Pain relief of half the maximum possible pain relief or better (at least 50% pain relief) is typically regarded as a clinically useful outcome. For patients given rescue medication it is usual for no additional pain measurements to be made, and for all subsequent measures to be recorded as initial pain intensity or baseline (zero) pain relief (baseline observation carried forward). This process ensures that analgesia from the rescue medication is not wrongly ascribed to the test intervention. In some trials the last observation is carried forward, which gives an inflated response for the test intervention compared to placebo, but the effect has been shown to be negligible over 4 to 6 hours (Moore 2005). Patients usually remain in the hospital or clinic for at least the first 6 hours following the intervention, with measurements supervised, although they may then be allowed home to make their own measurements in trials of longer duration.
Clinicians prescribe non‐steroidal anti‐inflammatory drugs (NSAIDs) on a routine basis for a range of mild‐to‐moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (FitzGerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclooxygenase‐dependent prostanoid formation (Hawkey 1999). Since NSAIDs do not depress respiration and do not impair gastro‐intestinal motility as do opioids (BNF 2002) they are clinically useful for treating pain after minor surgery and day surgery, and have an opiate‐sparing effect after more major surgery (Grahame‐Smith 2002).
Ibuprofen was developed in the 1960s and is used extensively throughout the world for relief of pain and inflammation in both acute and chronic conditions. It is available over the counter in most countries, usually as 200 mg tablets, with 1200 mg as the recommended maximum daily dose for adults. Under medical supervision, up to 3200 mg daily may be taken, divided into three doses. The lysine salt of ibuprofen is more soluble in water, with some theoretical advantage for faster onset after oral administration, and with the possibility that it could be used intravenously. Intravenous ibuprofen lysine has been used for closure of patent ductus arteriosis in newborns (Aranda 2006). Topical formulations are also available over the counter, and are dealt with in other separate reviews.
In UK primary care in 2007 there were 4.5 million prescriptions for ibuprofen, most commonly for 400 mg tablets (2.6 million), but only 6800 for ibuprofen lysine (PACT 2007). These numbers do not include over the counter sales, which are considerable, with over seven million packs sold annually in the UK in 2000, about 46,000 kg by weight (Sheen 2002).
A major concern regarding the use of conventional NSAIDs postoperatively is the possibility of bleeding from both the operative site (because of the inhibition of platelet aggregation) (Forrest 2002) and from the upper gastrointestinal tract, (especially in patients stressed by surgery, the elderly, frail, or dehydrated). Other potentially serious adverse events include acute liver injury, acute renal injury, heart failure, and adverse reproductive outcomes (Hernandez‐Diaz 2001). However, such complications are more likely to occur with chronic use and NSAIDs generally present fewer risks if used in the short term, as in the treatment of postoperative pain (Rapoport 1999).
The previous review included 35 studies in 34 reports with 3591 participants. Ibuprofen was shown to be an effective analgesic at 200 mg and 400 mg, with numbers‐needed‐to‐treat‐to‐benefit (NNTs) for at least 50% pain relief over 4 to 6 hours of 3.3 (95% confidence interval (CI) 2.8 to 4.0) and 2.7 (2.5 to 3.0) respectively. Adverse events were generally mild and transient and did not differ from placebo. A number of new studies are now available. The increased numbers of studies and participants gives more robust estimates of outcomes, and permits more detailed analysis of subgroups. This review has also looked at use of rescue medication as an additional measure of efficacy.
Objectives
To evaluate the analgesic efficacy and safety of oral ibuprofen in the treatment of acute postoperative pain, using methods that permit comparison with other analgesics evaluated in the same way, using criteria of efficacy recommended by an in‐depth study at the individual patient level (Moore 2005).
Methods
Criteria for considering studies for this review
Types of studies
Studies were included if they were full publications of double blind trials of a single dose oral ibuprofen against placebo for the treatment of moderate to severe postoperative pain in adults, with at least 10 participants randomly allocated to each treatment group. Multiple dose studies were included if appropriate data from the first dose were available, and cross‐over studies were included provided that data from the first arm were presented separately.
Studies were excluded if they were:
posters or abstracts not followed up by full publication;
reports of trials concerned with pain other than postoperative pain (including experimental pain);
studies using healthy volunteers;
studies where pain relief was assessed by clinicians, nurses or carers (i.e. not patient‐reported);
studies of less than 4 hours' duration or which failed to present data over 4 to 6 hours post‐dose.
Types of participants
Studies of adult participants (15 years old or above) with established moderate to severe postoperative pain were included. For studies using a visual analogue scale (VAS), pain of at least moderate intensity was assumed when the VAS score was greater than 30 mm (Collins 1997). Studies of participants with postpartum pain were included provided the pain investigated resulted from episiotomy or Caesarean section (with or without uterine cramp). Studies investigating participants with pain due to uterine cramps alone were excluded.
Types of interventions
Orally administered ibuprofen with matched placebo administered as a single oral dose for post‐operative pain.
Types of outcome measures
Data collected included the following.
characteristics of participants;
pain model;
patient‐reported pain at baseline (physician, nurse, or carer reported pain will not be included in the analysis);
patient‐reported pain relief and/or pain intensity expressed hourly over 4 to 6 hours using validated pain scales (pain intensity and pain relief in the form of visual analogue scales (VAS) or categorical scales, or both), or reported total pain relief (TOTPAR) or summed pain intensity difference (SPID) at 4 to 6 hours;
patient‐reported global assessment of treatment (PGE), using a standard five‐point scale;
number of participants using rescue medication, and the time of assessment;
time to use of rescue medication;
withdrawals ‐ all cause, adverse event;
adverse events ‐ participants experiencing one or more, and any serious adverse event, and the time of assessment.
Search methods for identification of studies
For the earlier review the following electronic databases were searched using a sensitive search strategy:
The Cochrane Library (August 1996);
The Specialised Register of the Cochrane Pain, Palliative and Supportive Care group (December 1996);
MEDLINE (1966 to December 1996);
EMBASE (1980 to January 1997);
Biological Abstracts (Jan 1985 to December 1996;
Oxford Pain database (Jadad 1996a).
For this update the following electronic databases were searched.
Cochrane CENTRAL (Issue 2, 2009);
MEDLINE via Ovid (1996 to May 2009);
EMBASE via Ovid (1996 to May 2009);
See Appendix 1 for the MEDLINE search strategy, Appendix 2 for the EMBASE search strategy and Appendix 3 for the CENTRAL search strategy.
Additional studies were sought in reference lists of retrieved articles and reviews.
Language
No language restriction was applied.
Unpublished studies
Abstracts, conference proceedings and other grey literature were not searched, but known unpublished studies from a different review were included.
Data collection and analysis
Selection of studies
Two review authors independently assessed and agreed the search results for studies that might be included in the updated review. Disagreements were resolved by consensus or referral to a third review author.
Quality assessment
Two review authors independently assessed the included studies for quality using a five‐point scale (Jadad 1996b).
The scale used is as follows. Is the study randomised? If yes give one point. Is the randomisation procedure reported and is it appropriate? If yes add one point, if no deduct one point. Is the study double blind? If yes then add one point. Is the double blind method reported and is it appropriate? If yes add one point, if no deduct one point. Are the reasons for patient withdrawals and dropouts described? If yes add one point.
The results are described in the 'Methodological quality of included studies' section below, and 'Characteristics of included studies' table.
Data management
Data were extracted by two review authors and recorded on a standard data extraction form. Data suitable for pooling were entered into RevMan 5.
Data analysis
QUOROM guidelines were followed (Moher 1999). For efficacy analyses we used the number of participants in each treatment group who were randomised, received medication, and provided at least one post‐baseline assessment. For safety analyses we used number of participants who received study medication in each treatment group. Analyses were planned for different doses. Sensitivity analyses were planned for pain model (dental versus other postoperative pain), trial size (39 or fewer versus 40 or more per treatment arm), and quality score (two versus three or more), and formulation (standard tablet versus more soluble tablet or liquid preparations). A minimum of two studies and 200 participants were required for any analysis (Moore 1998).
Primary outcome:
Number of participants achieving at least 50% pain relief
For each study, mean TOTPAR (total pain relief) or SPID (summed pain intensity difference) for active and placebo groups were converted to %maxTOTPAR or %maxSPID by division into the calculated maximum value (Cooper 1991). The proportion of participants in each treatment group who achieved at least 50%maxTOTPAR was calculated using verified equations (Moore 1996; Moore 1997a; Moore 1997b). These proportions were then converted into the number of participants achieving at least 50%maxTOTPAR by multiplying by the total number of participants in the treatment group. Information on the number of participants with at least 50%maxTOTPAR for active treatment and placebo was then used to calculate relative benefit (RB) and NNT. Pain measures accepted for the calculation of TOTPAR or SPID were:
five‐point categorical pain relief (PR) scales with comparable wording to "none, slight, moderate, good or complete";
four‐point categorical pain intensity (PI) scales with comparable wording to "none, mild, moderate, severe";
Visual analogue scales (VAS) for pain relief;
VAS for pain intensity.
If none of these measures were available, numbers of participants reporting "very good or excellent" on a five‐point categorical global scale with the wording "poor, fair, good, very good, excellent" were taken as those achieving at least 50% pain relief (Collins 2001).
Further details of the scales and derived outcomes are in the glossary (Appendix 4).
Secondary outcomes:
1. Use of rescue medication. Numbers of participants requiring rescue medication were used to calculate relative risk (RR) and numbers needed to treat to prevent (NNTp) use of rescue medication for treatment and placebo groups. Median (or mean) time to use of rescue medication was used to calculate the weighted mean of the median (or mean) for the outcome. Weighting was by number of participants.
2. Adverse events. Numbers of participants reporting adverse events for each treatment group were used to calculate RR and numbers needed to treat to harm (NNH) estimates for:
any adverse event;
any serious adverse event (as reported in the study);
withdrawal due to an adverse event.
3. Withdrawals. Withdrawals for reasons other than lack of efficacy (participants using rescue medication ‐ see above) and adverse events were noted, as were exclusions from analysis where data were presented.
RB or RR estimates were calculated with 95% Confidence Interval (CI) using a fixed‐effect model (Morris 1995). NNT, NNTp and NNH with 95% CI were calculated using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the RB did not include the number one.
Homogeneity of studies was assessed visually (L'Abbé 1987). The z test (Tramèr 1997) was used to determine if there was a significant difference between NNTs for different doses of active treatment, or between groups in the sensitivity analyses.
Results
Description of studies
This review included 72 studies in abstract, 9186 participants. The previous review identified 34 reports of 35 studies, in which 2214 participants were treated with ibuprofen and 1377 with placebo. This updated review identified a total of 65 published reports of 67 studies, and one published report of five unpublished studies (Edwards 2002), in which a total of 5804 participants were treated with ibuprofen and 3382 with placebo. Details of the studies are in the 'Characteristics of included studies' table. Three new studies were excluded (Cooper 1996b; Doyle 2002; Schleier 2007), please see the 'Characteristics of excluded studies' table for further details.
In an new search in May 2009, four additional studies were identified. Two were subsequently excluded after reading the full text (Akural 2009; Chopra 2009), and two are awaiting classification (Daniels 2009; Kleinert 2008). These studies are not included in this analysis.
Ibuprofen 50 mg was used in three studies, 100 mg in four studies, 200 mg in 20 studies (25 treatment arms), 400 mg in 61 studies (67 treatment arms), 600 mg in three studies (four treatment arms), and 800 mg in one study.
Most studies had treatment arms using standard formulation tablets, but nine used tablets of a more soluble salt of ibuprofen (lysine or arginine) or a "soluble" or liquid preparation (De Miguel Rivero 1997; Hersh 2000; Laveneziana 1996; Mehlisch 1995; Nelson 1994; Olson 2001; Pagnoni 1996; Parker 1986; Wahl 1997). Six studies included treatment arms using both standard tablets and a more soluble preparation (Black 2002; Desjardins 2002; Mehlisch 2002; Seymour 1991 (study 1); Seymour 1991 (study 2); Seymour 1996).
Fifty‐seven studies were in participants with dental pain following surgical extraction of one or more impacted third molars, 10 studies were in participants with pain following obstetric or gynaecological surgery (seven), abdominal or gynaecological surgery (two), and abdominal or pelvic surgery (one), two studies were in participants with pain following orthopaedic surgery, and one study each in general surgery, tonsillectomy, and hernia repair.
Study duration was 4 hours in nine studies, 5 hours in two studies, 6 hours in 42 studies, 7 hours in one study, 8 hours in nine studies, 12 hours in six studies, and 24 hours in three studies.
Risk of bias in included studies
Methodological quality of included studies
All included studies were both randomised and double blind. Twenty‐one studies were given a score of five, 32 a score of four, 16 a score of three, and three a score of two. Details are in the 'Characteristics of included studies' table.
Effects of interventions
All studies contributed data for analysis of the primary efficacy outcome.
Number of participants achieving at least 50% pain relief
(Table 1; Summary of results A)
1. Summary of outcomes: analgesia and use of rescue medication.
| Analgesia | Rescue medication | |||||
| Study ID | Treatment | PI or PR | Number with 50% PR | PGE: v good or excellent | Median time to use (h) | % using |
| Ahlstrom 1993 | (1) Ibuprofen 400 mg, n = 32 (3) Placebo n = 30 |
SPID 6:
(1) 188 mm (3) 32 mm |
(1) 19/32 (3) 2/30 |
at 6 h: (1) 75% (3) 17% |
No data | at 6 h: (1) 31 (3) 77 |
| Arnold 1990 | (1) Ibuprofen 400 mg, n = 15 (2) Ketoprofen 25 mg, n = 14 (3) Ketoprofen 100 mg, n = 16 (4) Placebo, n = 14 |
TOTPAR 6: (1) 4.2 (4) 1.5 |
(1) 2/15 (4) 0/14 |
No usable data | Mean: (1) 3.9 (4) 2.4 |
at 6 h: (1) 67 (4) 83 |
| Bakshi 1994 | (1) Ibuprofen 400 mg, n = 80 (2) Diclofenac (dispersible) 50 mg, n = 83 (3) Placebo, n = 82 |
TOTPAR 6: (1) 14.9 (3) 8.9 |
(1) 57/80 (3) 31/82 |
No usable data | Mean: (1) 5.3 (3) 3.4 |
at 6 h: (1) 28 (3) 65 |
| Black 2002 | (1) Ibuprofen 200 mg, n = 100 (2) Ibuprofen 400 mg, n = 100 (3) Ibuprofen arginate 200 mg, n = 100 (4) Ibuprofen arginate 400 mg, n = 99 (5) Placebo, n = 99 |
TOTPAR 6:
(1) 12.6 (2) 14.9 (3) 13.1 (4) 15.0 (5) 6.9 |
(1) 58/100 (2) 71/100 (3) 61/100 (4) 71/99 (5) 26/99 |
No usable data | (1) 4.2 (2) 5.2 (3) 4.0 (4) 4.5 (5) 1.3 |
No data |
| Cheung 2007 | (1) Ibuprofen 440 mg, n = 57 (2) Celecoxib 400 mg, n = 57 (3) Placebo, n = 57 |
TOTPAR 6: (1) 14.9 (3) 3.7 |
(1) 40/57 (3) 5/57 |
No data | (1) 11 (3) 1.9 |
at 24 h: (1) 72 (3) 86 |
| Cooper 1977 | (1) Ibuprofen 200 mg n = 38 (2) Ibuprofen 400 mg, n = 40 (3) Aspirin 325 mg, n = 37 (4) Aspirin 650 mg, n = 37 (5) Placebo, n= 40 |
TOTPAR 4: (1) 7.32 (2) 6.27 (5) 3.32 |
(1) 17/38 (2) 20/40 (5) 6/40 |
No data | No data | No data |
| Cooper 1982 | (1) Ibuprofen 400 mg, n =38 (2) Ibuprofen 400 mg + Codeine 60 mg, n = 41 (3) Aspirin 650 mg, n = 38 (4) Aspirin 650 mg + codeine 60 mg, n = 45 (5) Codeine 60 mg, n = 41 (6) Placebo, n = 46 |
TOTPAR 4: (1) 8.4 (6) 2.7 |
(1) 22/38 (2) 27/41 (6) 5/46 |
No usable data | Mean: (1) 3.8 (6) 2.4 |
No data |
| Cooper 1988a | (1) Ibuprofen 400 mg, n = 37 (2) Ketoprofen 100 mg, n = 39 (3) Ketoprofen 25 mg, n = 42 (4) Placebo, n = 43 |
TOTPAR 6: (1) 11.3 (4) 4.7 |
(1) 19/37 (4) 6/43 |
at 6 h: (1) 12/37 (4) 2/43 |
(1) 4.0 (4) 3.0 |
at 6 h: (1) 65 (4) 79 |
| Cooper 1989 | (1) Ibuprofen 400 mg, n = 61 (2) Paracetamol 1000 mg, n = 59 (3) Placebo, n = 64 |
TOTPAR 6: (1) 13.1 (3) 4.7 |
(1) 37/61 (3) 9/64 |
at 6 h: (1) 32/61 (3) 4/64 |
(1) 5.5 (3) 2.3 Mean: (1) 4.5 (3) 3.3 |
at 6 h: (1) 52 (3) 78 |
| Cooper 1996a | (1) Ibuprofen 200 mg, n = 19 (2) Misoprostal 200 mg, n = 18 (3) Ibuprofen 200 mg + misoprostal 200 mg, n = 20 (4) Placebo, n = 13 |
TOTPAR 6: (1) 5.3 (3) 5.1 (4) 1.2 |
(1) 3/19 (3) 3/18 (4) 0/33 |
No usable data | Mean: (1) 3.0 (3) 2.8 (4) 1.8 |
No data |
| De Miguel Rivero 1997 | (1) Ibuprofen arginine 400 mg, n = 36 (2) Magnesic dipyrone, 2 g (IM), n = 33 (3) Placebo, n = 34 |
VAS SPID 5: (1) 187 mm (3) 120 mm |
(1) 24/36 (3) 15/34 |
at 5 h: (1) 20/36 (3) 7/34 |
Mean: (1) 3.5 (3) 1.8 |
at 5 h: (1) 14 (3) 12 |
| Desjardins 2002 | (1) Ibuprofen 200 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Ibuprofen arginine 200 mg, n = 49 (4) Ibuprofen arginine 400 mg, n = 50 (5) Placebo, n = 24 |
TOTPAR 6: (1) 5.4 (2) 7.3 (3) 5.8 (4) 7.9 (5) 1.7 |
(1) 9/50 (2) 15/52 (3) 10/49 (4) 16/49 (5) 0/23 |
No data | (1) 2.6 (2) 4.0 (3) 3.0 (4) 4.0 (5) 1.5 |
No data |
| Dionne 1998 | (1) S(+)‐Ibuprofen 200 mg, n = 51 (2) S(+)‐Ibuprofen 400 mg, n = 50 (3) Ibuprofen (racemic) 400 mg, n = 50 (4) Placebo, n = 25 |
TOTPAR 6: (1) 13.0 (2) 14.9 (3) 11.5 (4) 3.5 |
(1) 31/51 (2) 35/40 (3) 26/50 (4) 2/25 |
No data | (1) 5.8 (2) 6.1 (3) 5.4 (4) 1.8 |
No data |
| Ehrich 1999 | (1) Ibuprofen 400 mg, n = 20 (2) Rofecoxib 50 mg, n = 32 (3) Rofecoxib 500 mg, n = 20 (4) Placebo, n = 32 |
TOTPAR 6: (1) 15.1 (4) 2.7 |
(1) 14/20 (4) 1/32 |
No usable data | (1) >6 (4) 1.6 |
No usable data |
| Forbes 1984 | (1) Ibuprofen 400 mg, n = 28 (2) Fendosal 200 mg, n = 29 (3) Aspirin 650 mg, n = 24 (4) Placebo n = 28 |
TOTPAR 6: (1) 15.8 (4) 3.8 |
(1) 21/28 (4) 3/28 |
No usable data | (1) 8.3 (4) 2.7 Mean: (1) 8.5 (4) 4.5 |
at 12 h: (1) 75 (4) 89 |
| Forbes 1990 | (1) Ibuprofen 400 mg, n = 32 (2) Ketorolac 10 mg, n = 31 (3) Ketorolac 20 mg, n = 35 (4) Paracetamol 600 mg, n = 36 (5) Paracetamol 600 mg + codeine 60 mg, n = 38 (6) Placebo, n = 34 |
TOTPAR 6: (1) 10.5 (6) 1.9 |
(1) 15/32 (6) 0/34 |
No usable data | (1) 4.7 (6) 1.9 Mean: (1) 4.6 (6) 2.9 |
at 6 h: (1) 58 (6) 97 |
| Forbes 1991a | (1) Ibuprofen 50 mg, n = 57 (2) Ibuprofen 100 mg, n = 49 (3) Ibuprofen 200 mg, n = 48 (4) Ibuprofen 100 mg + Caffeine 100 mg, n = 49 (5) Ibuprofen 200 mg + Caffeine 100 mg, n = 44 (6) Placebo n = 51 |
TOTPAR 6: (1) 7.0 (2) 7.0 (3) 8.7 (4) 9.3 (5) 12.6 (6) 2.2 |
(1) 16/57 (2) 13/49 (3) 18/48 (4) 19/49 (5) 26/44 (6) 0/51 |
No usable data | Mean: 1) 4.9 (2) 4.8 (3) 5.1 (4) 5.4 (5) 6.1 (6) 3.0 |
at 8 h: (1) 79 (2) 78 (3) 79 (4) 69 (5) 57 (6) 94 |
| Forbes 1991b | (1) Ibuprofen 400 mg, n = 37 (2) Bromfenac 5 mg, n = 39 (3) Bromfenac 10 mg, n = 43 (4) Bromfenac 25 mg, n = 42 (5) Aspirin 650 mg, n = 41 (6) Placebo, n = 39 |
TOTPAR 6: (1) 11.0 (6) 2.5 |
(1) 18/37 (6) 3/39 |
No usable data | (1) 6.9 (6) 1.8 Mean: (1) 5.7 (6) 2.8 |
at 6 h: (1) 42 (6) 96 at 8 h: (1) 57 (6) 97 |
| Forbes 1992 | (1) Ibuprofen 400 mg, n = 38 (2) Bromfenac 10 mg, n = 43 (3) Bromfenac 25 mg, n = 41 (4) Bromfenac 50 mg, n = 42 (5) Bromfenac 100 mg, n = 40 (6) Aspirin 650 mg, n = 38 (7) Placebo, n = 38 |
TOTPAR 6: (1) 11.8 (7) 2.1 |
(1) 20/38 (7) 0/38 |
No usable data | (1) 7.3 (7) 1.8 Mean: (1) 6.3 (7) 2.7 |
at 6 h: (1) 38 (7) 92 at 8 h: 1) 58 (7) 97 |
| Frame 1989 | (1) Ibuprofen 400 mg, n = 42 (2) Dihydrocodeine 30 mg, n = 43 (3) Placebo, n = 38 |
TOTPAR 5: (1) 11.1 (3) 1.9 |
(1) 26/42 (3) 0/38 |
No data | No usable data | at 5 h: (1) 40 (3) 89 |
| Fricke 1993 | (1) Ibuprofen 400 mg, n = 81 (2) Naproxen Na 440 mg, n = 81 (3) Placebo, n = 39 |
TOTPAR 6: (1) 10.9 (3) 2.9 |
(1) 40/81 (3) 2/39 |
No usable data | (1) 6.0 (3) 1.1 |
at 12 h: (1) 78 (3) No data |
| Gay 1996 | (1) Ibuprofen 400 mg, n = 41 (2) DKP.TRIS 5 mg, n = 41 (3) DKP.TRIS 10 mg, n = 42 (4) DKP.TRIS 20 mg, n = 41 (5) Placebo, n = 39 |
TOTPAR 6: (1) 13.6 (5) 5.2 |
(1) 26/41 (5) 7/39 |
No usable data | Mean: (1) 5.04 (5) 3.65 |
at 6 h: (1) 27 (5) 67 |
| Heidrich 1985 | (1) Ibuprofen 400 mg, n = 40 (2) Paracetamol 300 + codeine 30 mg, n = 40 (3) Placebo, n = 40 |
VAS TOTPAR 6: (1) 234 mm (3) 104 mm |
(1) 15/40 (3) 5/40 |
No data | No data | No data |
| Hersch 1993a | (1) Ibuprofen 200 mg, n = 51 (2) Ibuprofen 400 mg, n = 49 (3) Meclofenamate 100 mg, n = 52 (4) Meclofenamate 50 mg, n = 51 (5) Placebo, n = 51 |
TOTPAR 6: (1) 10.3 (2) 8.0 (5) 1.7 |
(1) 17/51 (2) 22/49 (5) 0/51 |
at 8 h: (1) 24/51 (2) 14/49 (5) 6/51 |
Mean: (1) 3.1 (2) 4.2 (5) 1.5 |
at 8 h: (1) 94 (2) 94 (5) 98 |
| Hersch 1993b | (1) Ibuprofen 400 mg, n = 12 (2) Codeine 60 mg, n = 16 (3) Placebo, n = 16 |
TOTPAR 6: (1) 15.7 (3) 9.0 |
(1) 9/12 (3) 6/16 |
No usable data | Mean: (1) 5.0 (3) 4.0 |
No data |
| Hersh 2000 | (1) Ibuprofen liquigel 200 mg, n = 61 (2) Ibuprofen liquigel 400 mg, n = 59 (3) Paracetamol 1000 mg, n = 63 (4) Placebo, n = 27 |
TOTPAR 6: (1) 14.7 (2) 16.6 (4) 5.2 |
(1) 43/61 (2) 47/59 (4) 5/27 |
at 6 h: (1) 38/61 (2) 41/59 (4) 4/27 |
(1) > 6 (2) > 6 (4) 1.6 |
at 6 h: (1) 31 (2) 23 (4) 75 |
| Hill 2001 | (1) Ibuprofen 400 mg, n = 49 (2) Pregabalin 50 mg, n = 49 (3) Pregabalin 300 mg, n = 50 (4) Placebo, n = 50 |
TOTPAR 6: (1) 10.1 (4) 3.8 |
(1) 22/49 (4) 5/50 |
No usable data | (1) 4.1 (4) 2.0 |
at 6 h: (1) 61 (4) 81 |
| Jain 1986 | (1) Ibuprofen 100 mg, n = 39 (2) Ibuprofen 200 mg, n = 47 (3) Ibuprofen 400 mg, n = 49 (4) Aspirin 650 mg, n = 45 (5) Placebo, n = 47 |
SPID 6: (1) 1.5 (2) 2.3 (3) 3.0 (5) ‐1.7 |
(1) 3/39 (2) 7/47 (3) 9/49 (5) 0/47 |
No usable data | Mean: (1) 3.9 (2) 4.2 (3) 4.0 (5) 2.1 |
at 6 h: (1) 74 (2) 67 (3) 59 (5) 96 |
| Jain 1988 | (1) Ibuprofen 400 mg, n = 49 (2) Ibuprofen 200 mg + caffeine 100 mg, n = 50 (3) Placebo, n = 48 |
TOTPAR 6: (1) 14.4 (2) 13.9 (3) 8.6 |
(1) 33/49 (2) 33/50 (3) 17/48 |
No usable data | No data | at 6 h: (1) 20 (2) 24 (3) 49 |
| Johnson 1997 | (1) Ibuprofen 400 mg, n = 48 (2) Paracetamol 650 mg + oxycodone 10 mg, n = 47 (3) Bromfenac 100 mg, n = 48 (4) Bromfenac 50 mg, n = 47 (5) Placebo, n = 48 |
TOTPAR 6: (1) 7.7 (5) 5.5 |
(1) 15/48 (5) 9/48 |
No usable data | (1) 3.4 (5) 2.7 |
at 6 h: (1) 79 (5) 89 |
| Kiersch 1993 | (1) Ibuprofen 200 mg, n = 81 (2) Naproxen Na 220 mg, n = 80 (3) Placebo, n = 42 |
TOTPAR 6: (1) 10.3 (3) 3.7 |
(1) 37/81 (3) 4/42 |
at 12 h: (1) 34/81 (3) 4/42 |
(1) 8.0 (3) 2.0 |
at 12 h: (1) 63 (3) 90 |
| Laska 1986 | (1) Ibuprofen 400 mg, n = 39 (2) Ibuprofen 600 mg, n = 36 (3) Ibuprofen 800 mg, n = 39 (4) Aluminium ibuprofen 400 mg, n = 39 (5) Placebo, n = 37 |
SPID 6: (1) 13.9 (2) 14.1 (3) 13.4 (5) 5.3 |
(1) 39/39 (2) 36/36 (3) 39/39 (5) 14/37 |
No data | No data | No usable data |
| Laveneziana 1996 | (1) Ibuprofen arginine soluble 400 mg, n = 42 (2) Ketorolac 30 mg, n = 41 (3) Placebo, n = 41 |
VAS SPID 6: (1) 233 mm (3) 204 mm |
(1) 29/42 (3) 24/41 |
No usable data | (1) 1.2 (3) 1.2 |
at 6 h: (1) 36 (3) 41 |
| Malmstrom 1999 | (1) Ibuprofen 400 mg, n = 46 (2) Rofecoxib 50 mg, n = 90 (3) Celecoxib 200 mg, n = 91 (4) Placebo, n = 45 |
TOTPAR 6: (1) 15.2 (4) 3.7 |
(1) 33/46 (4) 4/45 |
No usable data | (1) 8.9 (4) 1.5 |
at 24 h: (1) 76 (4) 91 |
| Malmstrom 2002 | (1) Ibuprofen 400 mg, n = 45 (2) Rofecoxib 50 mg, n = 151 (3) Celecoxib 400 mg, n = 151 (4) Celecoxib 200 mg, n = 90 (5) Placebo, n = 45 |
TOTPAR 6: (1) 11.7 (5) 1.0 |
(1) 24/45 (5) 0/45 |
No usable data | (1) 10.0 (5) 1.6 |
at 24 h: (1) 87 (5) 98 |
| Malmstrom 2004 | (1) Ibuprofen 400 mg, n = 48 (2) Etoricoxib 60 mg, n = 75 (3) Etoricoxib 120 mg, n = 76 (4) Etoricoxib 180 mg, n = 74 (5) Etoricoxib 240 mg, n = 76 (6) Placebo, n = 49 |
TOTPAR 6: (1) 14.1 (6) 3.4 |
(1) 32/48 (6) 4/49 |
No usable data | (1) 10.1 (6) 2.1 |
at 24 h: (1) 81 (6) 82 |
| McQuay 1996 | (1) Ibuprofen 200 mg, n = 31 (2) Ibuprofen 400 mg, n = 30 (3) Ibuprofen 200 mg + caffeine 50 mg, n = 30 (4) Ibuprofen 200 mg + caffeine 100 mg, n = 30 (5) Ibuprofen 200 mg + caffeine 200 mg, n = 29 (6) Placebo, n = 11 |
TOTPAR 6: (1) 3.0 (2) 7.0 (3) 10.3 (4) 9.5 (5) 5.5 (6) 0 |
(1) 2/31 (2) 6/30 (3) 8/30 (4) 14/30 (5) 12/29 (6) 0/11 |
No usable data | No data | No data |
| Medve 2001 | (1) Ibuprofen 200 mg, n = 240 (2) Tramadol 37.5 mg, n = 238 (3) Paracetamol 325 mg, n = 240 (4) Tramadol 37.5 mg + paracetamol 325 mg, n = 240 (5) Placebo, n = 239 |
Data taken from IPMA: (1) 114/240 (5) 5/239 |
No usable data | (1) 5.4 (5) 2.0 |
No data | |
| Mehlisch 1990 | (1) Ibuprofen 400 mg, n = 306 (2) Paracetamol 1000 mg, n = 306 (3) Placebo, n = 85 |
SPID 6: (1) 5.8 (3) 1.2 |
(1) 124/306 (3) 5/85 |
No data | No data | at 6 h: (1) 41 (3) 75 |
| Mehlisch 1995 | (1) Ibuprofen lysine 400 mg, n = 98 (2) Paracetamol 1000 mg, n = 101 (3) Placebo, n = 40 |
TOTPAR 6: (1) 14.4 (3) 2.6 |
(1) 67/98 (3) 1/40 |
at 6 h: (1) 65/98 (3) 1/40 |
(1) > 6 (3) 1.4 |
at 6 h: (1) 26 (3) 88 |
| Mehlisch 2002 | (1) Ibuprofen 200 mg, n = 100 (2) Ibuprofen 400 mg, n = 100 (3) Ibuprofen arginine 200 mg, n = 100 (4) Ibuprofen arginine 400 mg, n = 100 (5) Placebo, n = 100 |
TOTPAR 6: (1) 10.0 (2) 12.4 (3) 13.6 (4) 13.3 (5) 4.5 |
(1) 44/100 (2) 57/100 (3) 64/100 (4) 62/100 (5) 13/100 |
(1) 3.8 (2) 4.2 (3) 4.5 (4) 4.4 (5) 2.3 |
at 4 h: (1) 32 (2) 47 (3) 27 (4) 33 (5) No data |
|
| Morrison 1999 | (1) Ibuprofen 400 mg, n = 51 (2) Rofecoxib 50 mg, n = 50 (3) Placebo, n = 50 |
TOTPAR 6: (1) 9.3 (3) 4.2 |
(1) 20/51 (3) 6/50 |
No usable data | (1) 6.1 (3) 2.4 |
at 24 h: (1) 82 (3) 92 |
| Nelson 1994 | (1) Ibuprofen lysine 200 mg, n = 77 (2) Aspirin 500 mg, n = 65 (3) Placebo, n = 40 |
TOTPAR 6: (1) 12.3 (3) 5.6 |
(1) 44/77 (3) 8/40 |
at 6 h: (1) 39/77 (3) 6/40 |
(1) >6 (3) 2.9 |
at 6 h: (1) 44 (3) 70 |
| Nørholt 1998 | (1) Ibuprofen 400 mg, n = 26 (2) Placebo, n = 31 |
TOTPAR 4: (1) 11.7 (2) 4.5 |
(1) 22/26 (2) 8/31 |
No data | No data | at 4 h: (1) 15 (2) 71 |
| Olson 2001 | (1) Ibuprofen liquigel 400 mg, n = 67 (2) Ketoprofen 25 mg, n = 67 (3) Paracetamol 1000 mg, n = 66 (4) Placebo, n = 39 |
TOTPAR 6: (1) 17.4 (4) 4.3 |
(1) 57/67 (4) 5/39 |
at 6 h: (1) 52/67 (4) 4/49 |
(1) > 6 (4) 1.3 |
at 6 h: (1) 21 (4) 79 |
| Pagnoni 1996 | (1) Ibuprofen arginine soluble 400 mg, n = 30 (2) Ketorolac (IM) 30 mg, n = 30 (3) Placebo, n = 32 |
VAS SPID 6: (1) 279 (3) 114 |
(1) 13/30 (3) 5/32 |
at 6 h: (1) 5/30 (3) 0/32 |
(1) 2.1 (3) 1.9 |
at 6 h: (1) 43 (3) 66 |
| Parker 1986 | (1) Ibuprofen syrup 600 mg, n = 44 (2) Aspirin syrup 600 mg, n = 33 (3) Placebo, n = 33 |
TOTPAR 4: (1) 10.4 (3) 8.8 |
(1) 33/44 (3) 20/33 |
No data | No data | No data |
| Schachtel 1989 | (1) Ibuprofen 400 mg, n = 36 (2) Paracetamol 1000 mg, n = 37 (3) Placebo, n = 38 |
TOTPAR 4: (1) 10.4 (3) 5.5 |
(1) 27/36 (3) 13/38 |
No data | No data | at 4 h: (1) 22 (3) 58 |
| Schou 1998 | (1) Ibuprofen 50 mg, n = 51 (2) Ibuprofen 100 mg, n = 53 (3) Ibuprofen 200 mg, n = 49 (4) Ibuprofen 400 mg, n = 49 (5) Placebo, n = 56 |
TOTPAR 6: (1) 11.8 (2) 11.2 (3) 15.5 (4) 17.2 (5) 7.3 |
(1) 27/51 (2) 27/53 (3) 36/49 (4) 41/49 (5) 16/56 |
No data | (1) 5.5 (2) >6 (3) >6 (4) >6 (5) 3.7 |
Up to 6 h: (1) 54 (2) 48 (3) 36 (4) 16 (5) 66 |
| Schwartz 2007 | (1) Ibuprofen 400 mg, n = 15 (2) MK‐0703 12.5 mg, n = 31 (3) MK‐0703 50 mg, n = 28 (4) MK‐0703 100 mg, n = 31 (5) Placebo, n = 16 |
No data | Not available | at 8 h: (1) 5/15 (5) 0/16 |
(1) 7.1 (5) 1.6 |
at 8 h: (1) 80 (5) 100 |
| Seymour 1991 (study 1) | (1) Ibuprofen tablets 400 mg, n = 31 (2) Ibuprofen liquid in gelatin capsules 400 mg, n = 32 (3) Placebo n = 32 |
VAS SPID 6: (1) 243 mm (2) 233 mm (3) 120 mm |
(1) 20/31 (2) 22/32 (3) 10/32 |
No usable data | Mean: (1) 3.6 (2) 3.5 (3) 2.1 |
at 6 h: (1) 39 (2) 44 (3) 69 |
| Seymour 1991 (study 2) | (1) Ibuprofen tablets 400 mg, n = 30 (2) Ibuprofen soluble 400 mg, n = 32 (3) Placebo, n = 30 |
VAS SPID 6: (1) 214 mm (2) 228 mm (3) 86 mm |
(1) 20/30 (2) 8/30 (3) 7/30 |
No usable data | Mean: (1) 3.24 (2) 3.15 (3) 1.40 |
at 6 h: (1) 60 (2) 72 (3) 93 |
| Seymour 1996 | (1) Ibuprofen tablets 200 mg, n = 18 (2) Ibuprofen soluble 200 mg, n = 17 (3) Ibuprofen tablets 400 mg, n = 15 (4) Ibuprofen soluble 400 mg, n = 16 (5) Ibuprofen tablets 600 mg, n = 17 (6) Ibuprofen soluble 600 mg, n = 17 (7) Placebo, n = 19 |
VAS SPID 6: (1) 230 mm (2) 148 mm (3) 258 mm (4) 238 mm (5) 140 mm (6) 198 mm (7) 44 mm |
(1) 7/18 (2) 9/17 (3) 11/15 (4) 11/16 (5) 11/17 (6) 8/17 (7) 2/19 |
No usable data | (1) 3.0 (2) 1.6 (3) 2.8 (4) 2.1 (5) 2.0 (6) 1.5 (7) 0.8 |
at 6 h: (1) 88 (2) 88 (3) 67 (4) 81 (5) 100 (6) 88 (7) 100 |
| Seymour 1998 | (1) Ibuprofen 400 mg, n = 76 (2) Aceclofenac 150 mg, n = 71 (3) Placebo, n = 70 |
VAS TOTPAR 4: (1) 151 mm (3) 46 mm |
(1) 27/76 (3) 3/70 |
No usable data | (1) 3.5 (3) 1.6 |
at 6 h: (1) 55 (3) 86 |
| Seymour 1999 | (1) Ibuprofen 400 mg, n = 41 (2) WAG 994 1 mg, n = 42 (3) Placebo, n = 39 |
TOTPAR 6: (1) 10.4 (3) 5.1 |
(1) 19/41 (3) 7/39 |
No usable data | (1) 5.2 (3) 2.0 |
at 6 h: (1) 56 (3) 100 |
| Seymour 2000 | (1) Ibuprofen 200 mg, n = 59 (2) Buffered ketoprofen 12.5 mg, n = 61 (3) Placebo, n = 60 |
TOTPAR 6: (1) 6.4 (3) 4.1 |
(1) 14/59 (3) 7/60 |
No usable data | (1) 2.0 (3) 1.9 |
at 6 h: (1) 83 (3) 98 |
| Singla 2005 | (1) Ibuprofen 400 mg, n = 175 (2) Ibuprofen 400 mg + oxycodone 5 mg, n = 169 (3) Oxycodone 5 mg, n = 52 (4) Placebo, n = 60 |
TOTPAR 6: (1) 10.0 (4) 6.4 |
(1) 77/175 (4) 14/60 |
No usable data | (1) 4.0 (4) 2.3 |
at 6 h: (1) 71 (4) No data |
| Sunshine 1983 | (1) Ibuprofen 400 mg, n = 30 (2) Aspirin 600 mg, n = 30 (3) Zomepirac 100 mg, n = 30 (4) Placebo, n = 30 |
SPID 4: (1) 6.0 (4) 1.0 |
(1) 21/30 (4) 3/30 |
No data | No data | at 4 h: (1) 0 (4) 17 |
| Sunshine 1987 | (1) Ibuprofen 400 mg, n = 38 (2) Ibuprofen 200 mg + codeine 30 mg, n = 40 (3) Ibuprofen 400 mg + codeine 60 mg, n = 40 (4) Codeine 60 mg, n = 37 (5) Placebo, n = 40 |
SPID 4: (1) 4.8 (5) 3.4 |
(1) 16/38 11/40 |
No usable data | No usable data | at 4 h: (1) 13 (5) 50 |
| Sunshine 1996 | (1) Ibuprofen 50 mg, n = 51 (2) Ibuprofen 100 mg, n = 51 (3) Ibuprofen 200 mg, n = 50 (4) Ibuprofen 100 mg + caffeine 100 mg, n = 50 (5) Ibuprofen 200 mg + caffeine 100 mg, n = 50 (6) Placebo, n = 50 |
TOTPAR 6: (1) 4.7 (2) 8.2 (3) 13.9 (4) 10.9 (5) 14.9 (6) 2.2 |
(1) 7/51 (2) 17/51 (3) 33/50 (4) 24/50 (5) 36/50 (6) 0/50 |
No usable data | No data | at 6 h: (1) 4 (2) 0 (3) 0 (4) 0 (5) 2 (6) 32 |
| Sunshine 1997 | (1) Ibuprofen 400 mg, n = 40 (2) Ibuprofen 400 mg + hydrocodone 15 mg, n = 40 (3) Placebo, n = 39 |
TOTPAR 6: (1) 9.7 (3) 2.7 |
(1) 17/40 (3) 1/39 |
No usable data | No usable data | at 6 h: (1) 25 (3) 82 |
| Sunshine 1998 | (1) Ibuprofen 200 mg, n = 35 (2) Ketoprofen 6.25 mg, n = 35 (3) Ketoprofen 12.5 mg, n = 35 (4) Ketoprofen 25 mg, n = 35 (5) Placebo, n = 35 |
TOTPAR 6: (1) 12.5 (5) 3.6 |
(1) 20/35 (5) 3/35 |
No usable data | No usable data | No usable data |
| Unpublished from Edwards 2002 | (1) Ibuprofen 400 mg, n = 339 (2) Placebo, n = 339 |
Individual patient meta‐analysis | (1) 145/339 (2) 11/339 |
No usable data | No usable data | at 8 h: (1) 43/339 (2) 121/337 |
| Van Dyke 2004 | (1) Ibuprofen 400 mg, n = 186 (2) Ibuprofen 400 mg + oxycodone 5 mg, n = 187 (3) Oxycodone 5 mg, n = 63 (4) Placebo, n = 62 |
TOTPAR 6: (1) 12.9 (4) 4.8 |
(1) 112/186 (4) 9/62 |
No usable data | 1) > 6 (4) 2.0 |
at 6 h: (1) 38 (4) 84 |
| Wahl 1997 | (1) Ibuprofen lysinate 342 mg (= 200 mg Ibu), n = 74 (2) Paracetamol 200 mg + aspirin 250 mg + caffeine 50 mg, n = 73 (3) Placebo, n = 42 |
TOTPAR 6: (1) 11.6 (3) 2.5 |
(1) 39/74 (3) 1/42 |
No usable data | No data | at 6 h: (1) 42 (3) 81 |
| Wideman 1999 (study 1) | (1) Ibuprofen 200 mg, n = 60 (2) Ibuprofen 200 mg, + hydrocodone 7.5 mg, n = 59 (3) Hydrocodone 7.5 mg, n = 61 (4) Placebo, n = 60 |
TOTPAR 6: (1) 4.9 (4) 3.5 |
(1) 9/60 (4) 5/60 |
No data | No data | No data |
| Wideman 1999 (study 2) | (1) Ibuprofen 400 mg, n = 50 (2) Ibuprofen 400 mg + hydrocodone 15 mg, n = 50 (3) Hydrocodone 15 mg, n = 50 (4) Placebo, n = 51 |
TOTPAR 6: (1) 9.7 (4) 3.0 |
(1) 21/50 (4) 3/51 |
No data | (1) 4.2 (4) 1.8 |
at 8 h: (1) 69 (4) 100 |
| Zelenakas 2004 | (1) Ibuprofen 400 mg, n = 51 (2) Lumiracoxib 100 mg, n = 51 (3) Lumiracoxib 400 mg, n = 50 (4) Placebo, n = 50 |
TOTPAR 6: (1) 11.6 (4) 4.2 |
(1) 27/51 (4) 6/50 |
No usable data | (1) ˜8 (4) ˜2 |
at 12 h: (1) 73 (4) 92 |
Ibuprofen 50 mg versus placebo
Three studies with 316 participants provided data (Forbes 1991a; Schou 1998; Sunshine 1996) (Analysis 1.1).
1.1. Analysis.
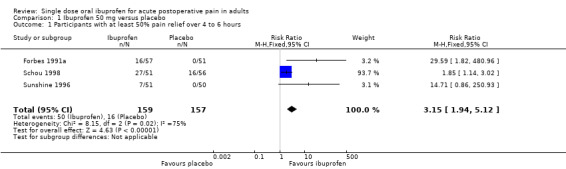
Comparison 1 Ibuprofen 50 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with Ibuprofen 50 mg was 31% (50/159; range 14% to 53%).
The proportion of participants experiencing at least 50% pain relief with placebo was 10% (16/157; range 0% to 29%).
The RB of treatment compared with placebo was 3.2 (1.9 to 5.1), giving an NNT for at least 50% pain relief over 4 to 6 hours of 4.7 (3.3 to 8.0).
Ibuprofen 100 mg versus placebo
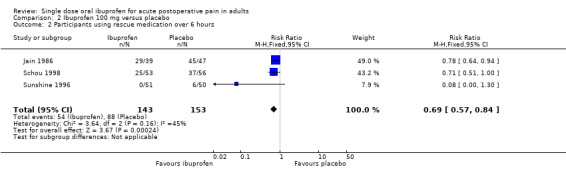
Four studies with 396 participants provided data (Forbes 1991a; Jain 1986; Schou 1998; Sunshine 1996) (Analysis 2.1).
2.1. Analysis.
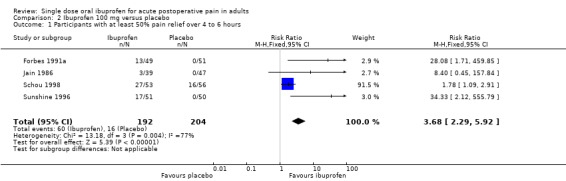
Comparison 2 Ibuprofen 100 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with Ibuprofen 100 mg was 31% (60/192; range 8% to 51%).
The proportion of participants experiencing at least 50% pain relief with placebo was 8% (16/204; range 0% to 29%).
The RB of treatment compared with placebo was 3.7 (2.3 to 5.9), giving an NNT for at least 50% pain relief over 4 to 6 hours of 4.3 (3.2 to 6.4).
Ibuprofen 200 mg versus placebo
Twenty studies (25 treatment arms) with 2690 participants provided data (Analysis 3.1; Figure 1)
3.1. Analysis.
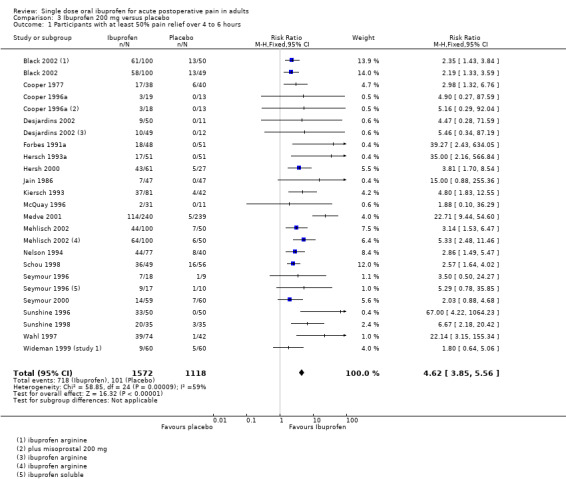
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
1.
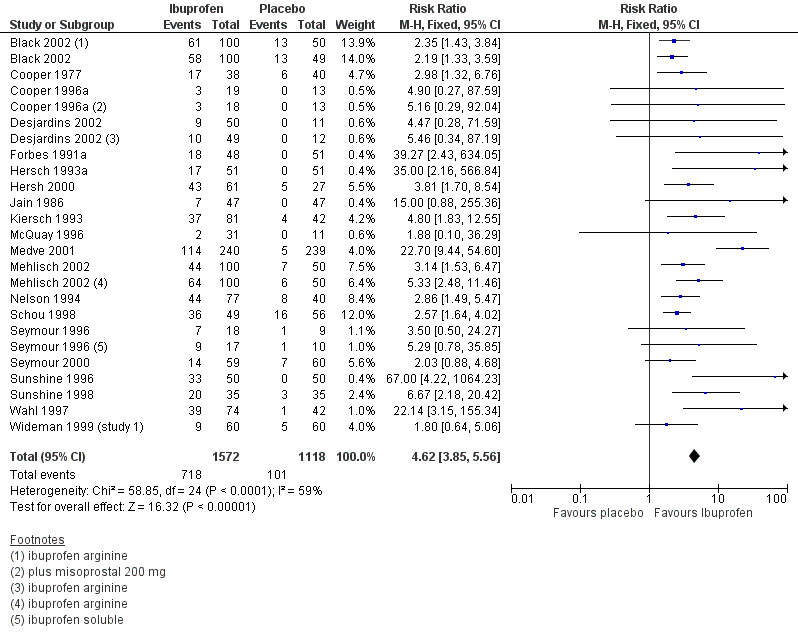
Forest plot of comparison: 3 Ibuprofen 200 mg versus placebo, outcome: 3.1 Participants with at least 50% pain relief over 4 to 6 hours.
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with Ibuprofen 200 mg was 46% (718/1572; range 6% to 73%).
The proportion of participants experiencing at least 50% pain relief with placebo was 9% (101/1118; range 0% to 29%).
The RB of treatment compared with placebo was 4.6 (3.9 to 5.6), giving an NNT for at least 50% pain relief over 4 to 6 hours of 2.7 (2.5 to 3.0).
Ibuprofen 400 mg versus placebo
Sixty‐one studies (67 treatment arms) with 6475 participants provided data (Analysis 4.1; Figure 2)
4.1. Analysis.
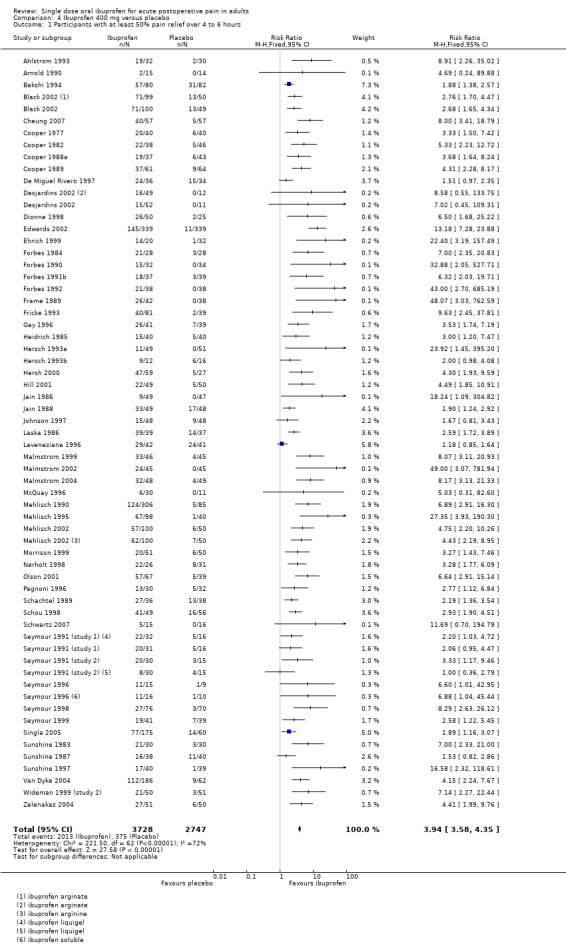
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
2.
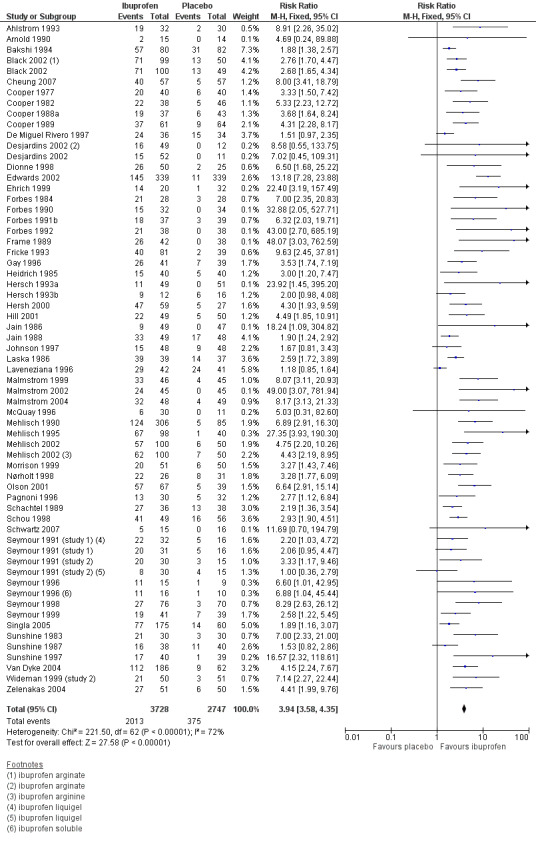
Forest plot of comparison: 4 Ibuprofen 400 mg versus placebo, outcome: 4.1 Participants with at least 50% pain relief over 4 to 6 hours.
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with Ibuprofen 400 mg was 54% (2013/3728; range 13% to 100%).
The proportion of participants experiencing at least 50% pain relief with placebo was 14% (375/2747; range 0% to 59%).
The RB of treatment compared with placebo was 3.9 (3.6 to 4.4), giving an NNT for at least 50% pain relief over 4 to 6 hours of 2.5 (2.4 to 2.6).
Ibuprofen 600 mg versus placebo
Three studies (four treatment arms) with 203 participants provided data (Laska 1986; Parker 1986; Seymour 1996) (Analysis 5.1).
5.1. Analysis.
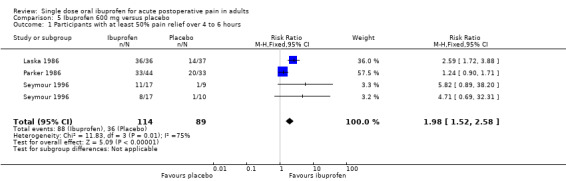
Comparison 5 Ibuprofen 600 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
The proportion of participants experiencing at least 50% pain relief over 4 to 6 hours with Ibuprofen 200 mg was 77% (88/114; range 47% to 100%).
The proportion of participants experiencing at least 50% pain relief with placebo was 40% (36/89; range 10% to 61%).
The RB of treatment compared with placebo was 2.0 (1.5 to 2.6), giving an NNT for at least 50% pain relief over 4 to 6 hours of 2.7 (2.0 to 4.2).
Only one treatment arm used ibuprofen 800 mg (Laska 1986) (Analysis 6.1).
6.1. Analysis.
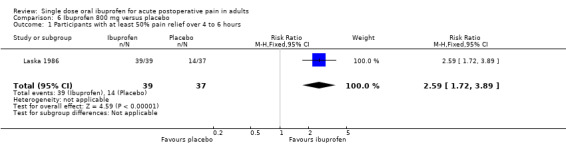
Comparison 6 Ibuprofen 800 mg versus placebo, Outcome 1 Participants with at least 50% pain relief over 4 to 6 hours.
A general trend for better efficacy (lower NNT) with increasing dose was seen. The result for 800 mg ibuprofen was compatible with this trend, and is added for completeness even though there were fewer than 200 participants. (200 mg versus 100 mg z = 3.25, P = 0.001; 400 mg versus 200 mg z = 1.74, P = 0.082; 400 mg versus 100 mg z = 4.15, P < 0.0001).
| Summary of results A: Number of participants with ≥ 50% pain relief over 4 to 6 hours | |||||
| Dose | Studies | Participants | Ibuprofen (%) | Placebo (%) | NNT (95%CI) |
| 50 mg | 3 | 316 | 31 | 10 | 4.7 (3.3 to 8.0) |
| 100 mg | 4 | 396 | 31 | 8 | 4.3 (3.2 to 6.4) |
| 200 mg | 20 | 2690 | 46 | 9 | 2.7 (2.5 to 3.0) |
| 400 mg | 61 | 6475 | 54 | 14 | 2.5 (2.4 to 2.6) |
| 600 mg | 3 | 203 | 77 | 40 | 2.7 (2.0 to 4.2) |
| 800 mg | 1 | 76 | 100 | 38 | 1.6 (1.3 to 2.2) |
Sensitivity analysis of primary outcome
(Summary of results B)
Methodological quality
Only three studies (Cooper 1996a; Heidrich 1985; Hersch 1993a) were given quality scores of two, so no sensitivity analysis was carried out for this criterion. Removing these three studies from the analyses did not alter the results.
Pain model; dental versus other surgery
Ibuprofen 200 mg
3.2. Analysis.
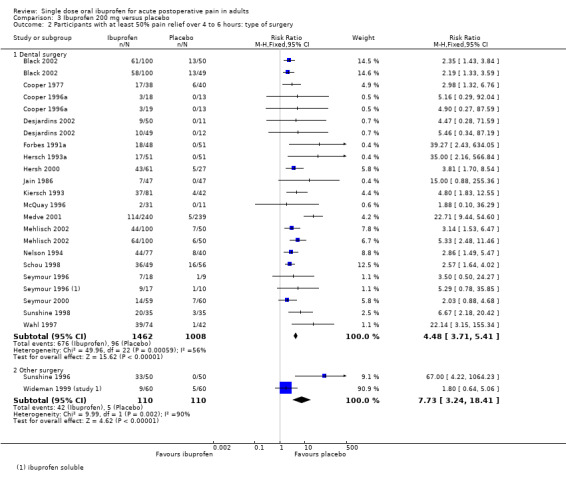
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours: type of surgery.
Eighteen studies reporting the primary outcome were in dental pain (Analysis 3.2.1). The proportion of participants with at least 50% pain relief was 47% (680/1462) for ibuprofen 200 mg, and 10% (100/1008) for placebo. The RB was 4.5 (3.7 to 5.4), and the NNT was 2.7 (2.5 to 3.0).
Two studies reporting the primary outcome were in other types of surgery (episiotomy, abdominal and gynaecological surgery) (Analysis 3.2.2). The proportion of participants with at least 50% pain relief was 38% (42/110) for ibuprofen 200 mg, and 5% (5/110) for placebo. The RB was 7.7 (3.2 to 18), and the NNT was 3.0 (2.3 to 4.2).
The 95% CI for NNT in dental and other surgery overlap, indicating that there was no significant difference for this outcome between dental and other types of surgery in these studies at this dose.
Ibuprofen 400 mg
4.2. Analysis.
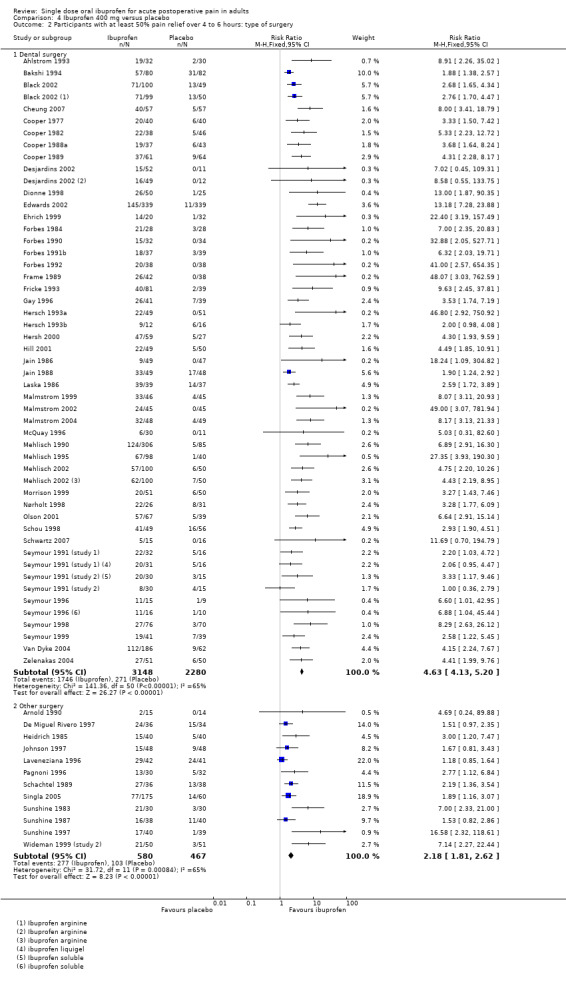
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 2 Participants with at least 50% pain relief over 4 to 6 hours: type of surgery.
3.
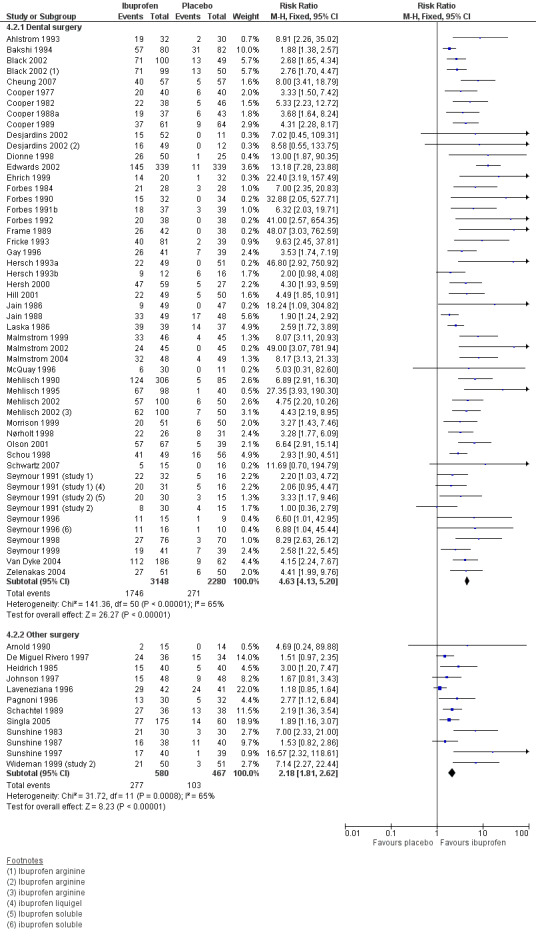
Forest plot of comparison: 4 Ibuprofen 400 mg versus placebo, outcome: 4.2 Participants with at least 50% pain relief over 4 to 6 hours: type of surgery.
Forty‐nine studies reporting the primary outcome were in dental pain (Analysis 4.2.1). The proportion of participants with at least 50% pain relief was 55% (1746/3148) for ibuprofen 400 mg, and 12% (271/2280) for placebo. The RB was 4.3 (3.8 to 4.9), and the NNT was 2.3 (2.2 to 2.4).
Twelve studies reporting the primary outcome were in other types of surgery (including general, orthopaedic, abdominal, obstetric and gynaecological surgery) (Analysis 4.2.2). The proportion of participants with at least 50% pain relief was 48% (277/580) for ibuprofen 400 mg, and 22% (103/467) for placebo. The RB was 2.2 (1.8 to 2.6), and the NNT was 3.9 (3.2 to 5.0).
The 95% CIs for RB and NNT in dental and other surgery do not overlap, indicating that there was a significant difference for this outcome between dental and other types of surgery in these studies at 400 mg (z = 5.86, P < 0.0001).
There were insufficient data to compare different pain models at other doses of ibuprofen.
Dose response in dental studies
A significant difference was seen in dental studies between ibuprofen 200 mg and 400 mg (z = 3.52, P < 0.0005) and also between 400 mg and 600/800 mg (z = 2.02, P = 0.04), although with limited data.
Salt preparation: standard ibuprofen versus ibuprofen lysine, arginine and "soluble"
Ibuprofen 200 mg
3.3. Analysis.
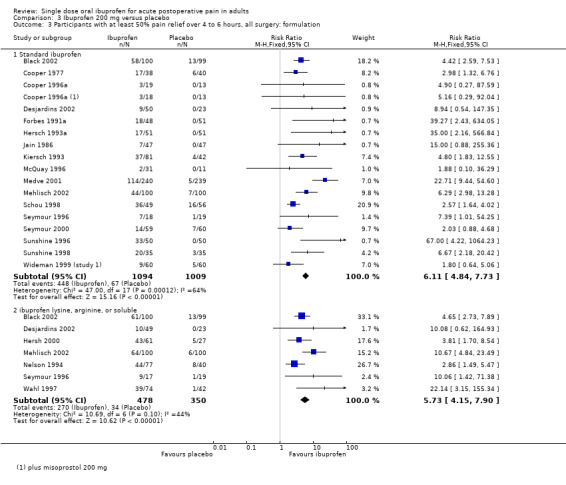
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, all surgery: formulation.
3.4. Analysis.
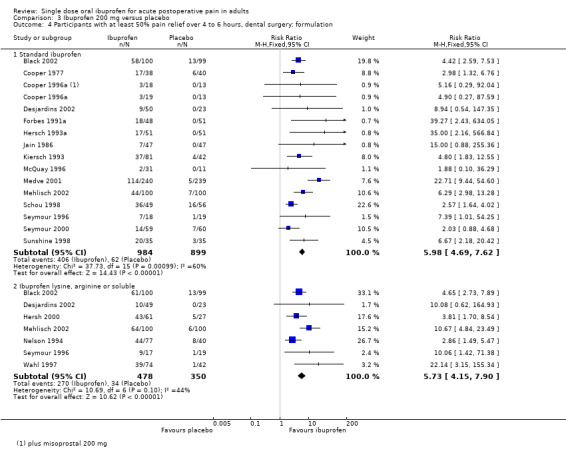
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 4 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: formulation.
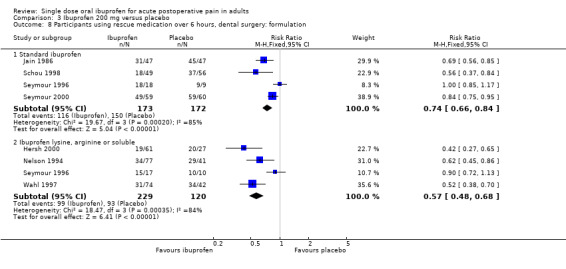
In all types of surgery, 17 studies used standard ibuprofen (Analysis 3.3.1). The proportion of participants with at least 50% pain relief was 41% (448/1094) for ibuprofen 200 mg, and 7% (67/1009) for placebo; the RB was 6.1 (4.8 to 7.7), and the NNT was 2.9 (2.7 to 3.2). In dental surgery only, 15 studies used standard ibuprofen (Analysis 3.4.1). The proportion of participants with at least 50% pain relief was 41% (406/984) for ibuprofen 200 mg, and 7% (62/899) for placebo; the RB was 5.9 (4.7 to 7.6), and the NNT was 2.9 (2.6 to 3.2).
Seven studies, all in dental surgery, used the lysine or arginine salts, or a preparation described as "soluble", all of which are thought to be more soluble and more readily absorbed (Analysis 3.3.2, Analysis 3.4.2). The corresponding proportion of participants with at least 50% pain relief was 56% (270/478) for ibuprofen 200 mg, and 10% (34/350) for placebo; the RB was 5.7 (4.2 to 7.9), and the NNT was 2.1 (1.9 to 2.4).
The more soluble salts of ibuprofen had significantly lower (better) NNTs than the standard preparation when all surgery was combined (z = 3.85, P < 0.0001) and in dental studies only (z = 3.77, P < 0.0002).
Ibuprofen 400 mg
(Analysis 4.3; Figure 4; Analysis 4.4; Figure 5)
4.3. Analysis.
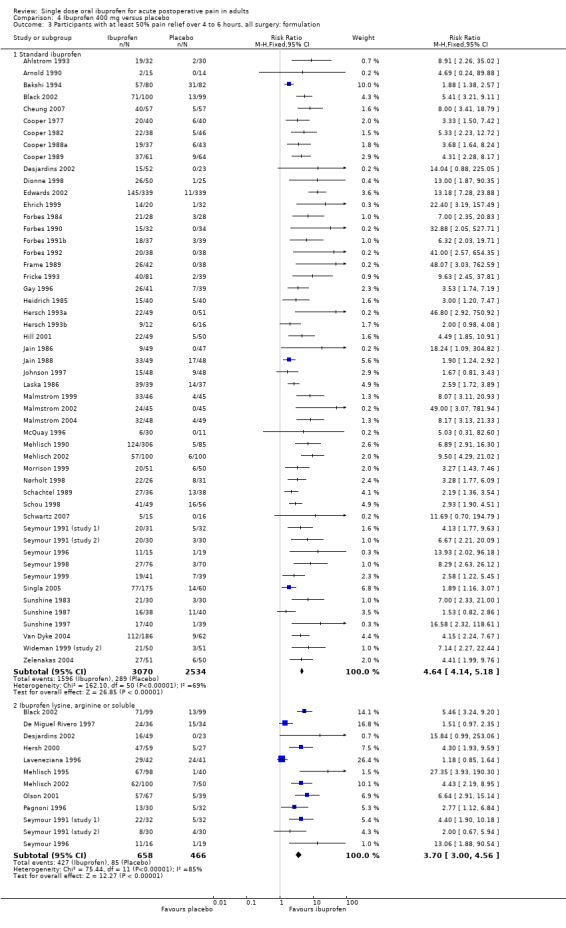
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 3 Participants with at least 50% pain relief over 4 to 6 hours, all surgery: formulation.
4.
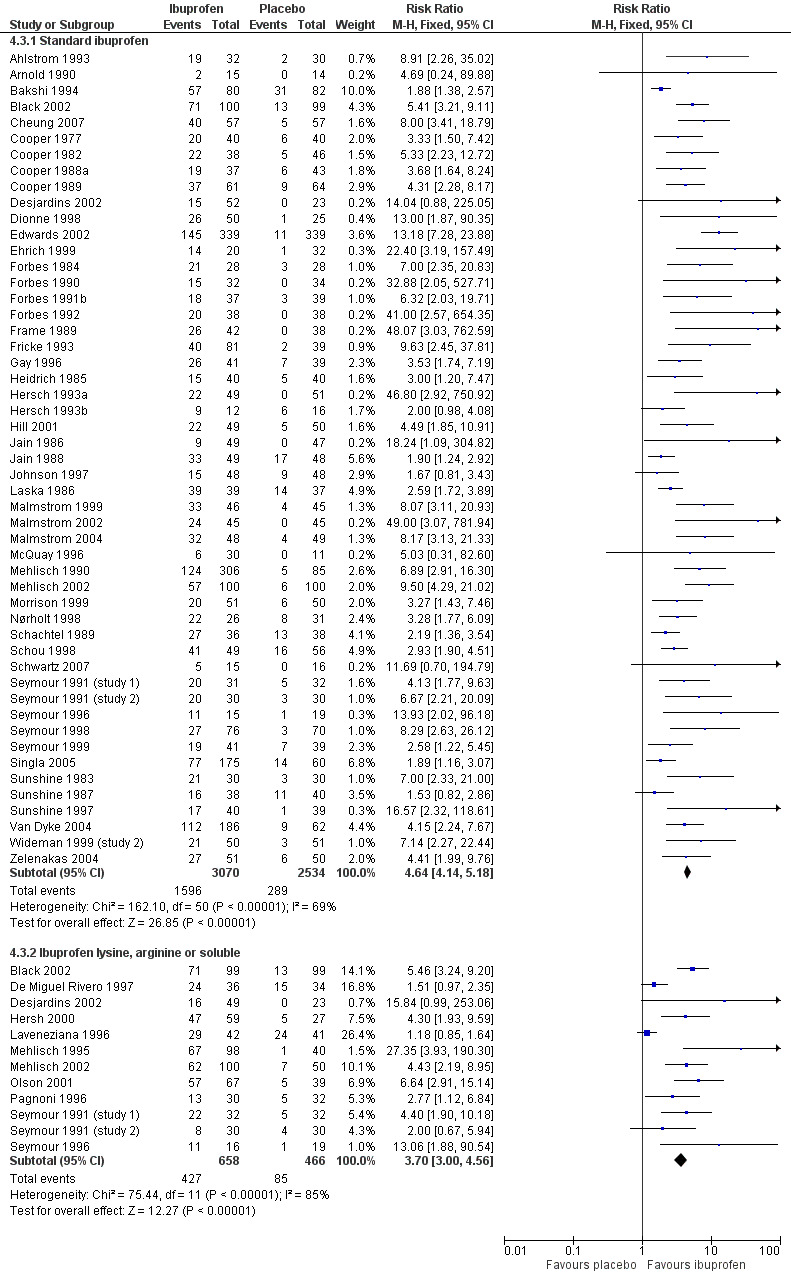
Forest plot of comparison: 4 Ibuprofen 400 mg versus placebo, outcome: 4.3 Participants with at least 50% pain relief over 4 to 6 hours, all surgery: formulation.
4.4. Analysis.
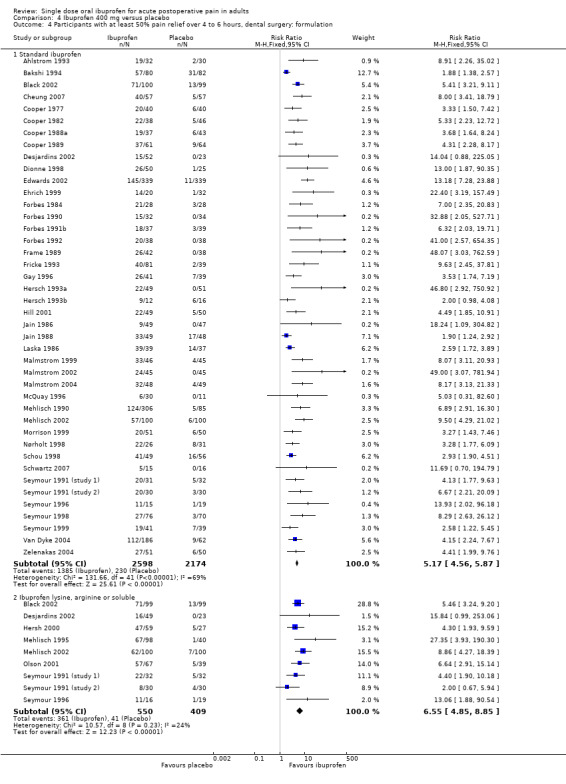
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 4 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: formulation.
5.
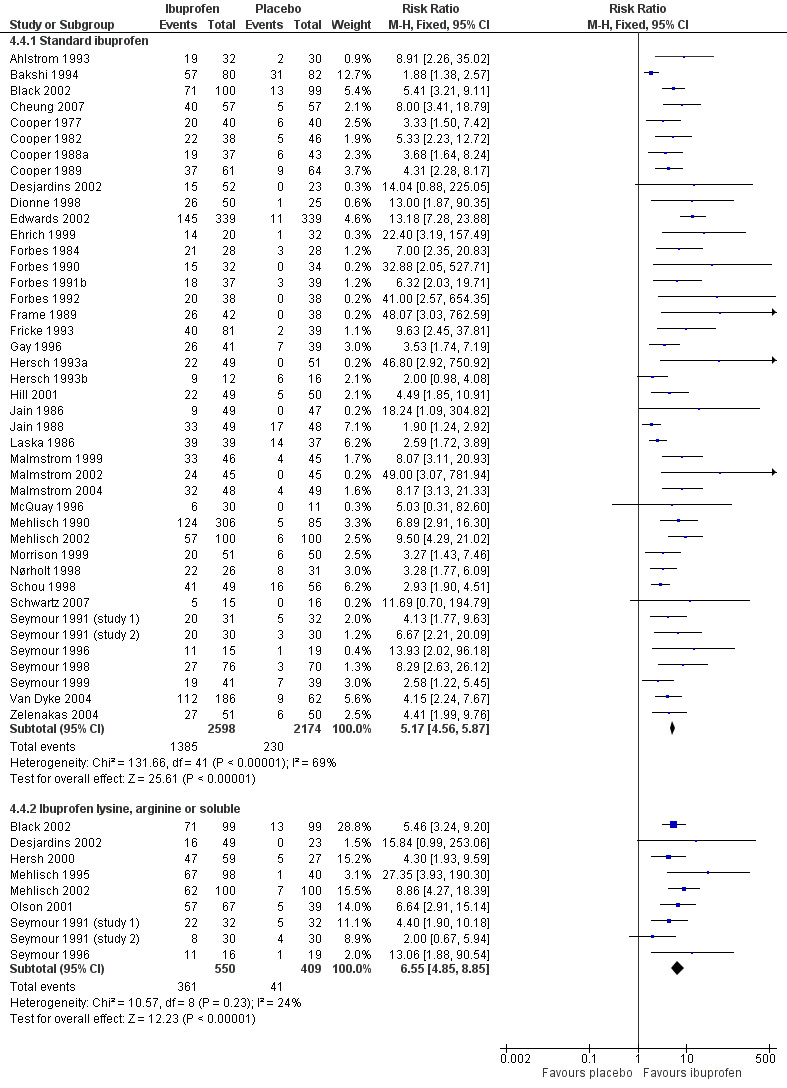
Forest plot of comparison: 4 Ibuprofen 400 mg versus placebo, outcome: 4.4 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: formulation.
In all types of surgery, 55 studies used standard ibuprofen (Analysis 4.3.1). The proportion of participants with at least 50% pain relief was 52% (1596/3070) for ibuprofen 400 mg, and 11% (289/2534) for placebo; the RB was 4.6 (4.1 to 5.2), and the NNT was 2.5 (2.4 to 2.7). In dental surgery only, 46 studies used standard ibuprofen (Analysis 4.4.1). The proportion of participants with at least 50% pain relief was 53% (1385/2598) for ibuprofen 400 mg, and 11% (230/2174) for placebo; the RB was 5.2 (4.6 to 5.9), and the NNT was 2.3 (2.2 to 2.5).
In all types of surgery, 12 studies used the lysine or arginine salts, or a preparation described as "soluble" (Analysis 4.3.2). The corresponding proportion of participants with at least 50% pain relief was 65% (427/658) for ibuprofen 400 mg, and 18% (85/466) for placebo; the RB was 3.7 (3.0 to 4.6), and the NNT was 2.1 (1.9 to 2.4). In dental surgery, nine studies used lysine, arginine or "soluble" salts (Analysis 4.4.2). The corresponding proportion of participants with at least 50% pain relief was 66% (361/550) for ibuprofen 400 mg, and 10% (41/409) for placebo; the RB was 6.5 (4.8 to 8.9), and the NNT was 1.8 (1.7 to 2.0).
The more soluble salts of ibuprofen had significantly lower (better) NNTs than the standard preparation when all surgery was combined (z = 2.16, P = 0.03) and in dental studies only (z = 4.64, P < 0.0001).
There were insufficient data to analyse the salt preparation for other doses of ibuprofen.
Study size: 40 or more participants per treatment arm versus fewer than 40
The two largest data sets, ibuprofen 200 mg and 400 mg, were used to investigate the effect of study size on the primary outcome. The analysis was further restricted to dental studies only, since these are clinically the most homogeneous studies.
Ibuprofen 200 mg
3.5. Analysis.
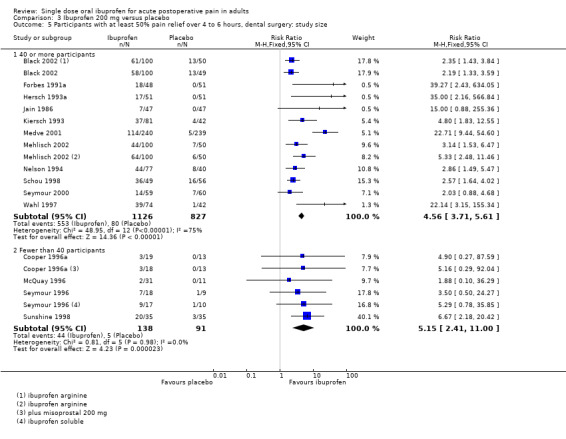
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 5 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: study size.
Eleven studies had 40 or more participants in both treatment arms (Black 2002; Forbes 1991a; Hersch 1993b; Jain 1986; Kiersch 1993; Medve 2001; Mehlisch 2002; Nelson 1994; Schou 1998; Seymour 2000; Wahl 1997) (Analysis 3.5.1). The proportion of participants with at least 50% pain relief was 49% (553/1126) for ibuprofen 200 mg, and 10% (80/827) for placebo; the RB was 4.6 (3.7 to 5.6), and the NNT was 2.5 (2.3 to 2.8). Four studies had fewer than 40 participants in both treatment arms (Cooper 1996a; McQuay 1996; Seymour 1996; Sunshine 1998) (Analysis 3.5.2). The proportion of participants with at least 50% pain relief was 34% (44/138) for ibuprofen 200 mg, and 5% (5/91) for placebo; the RB was 5.1 (2.4 to 11), and the NNT was 3.9 (2.8 to 6.1).
Ibuprofen 400 mg
4.5. Analysis.
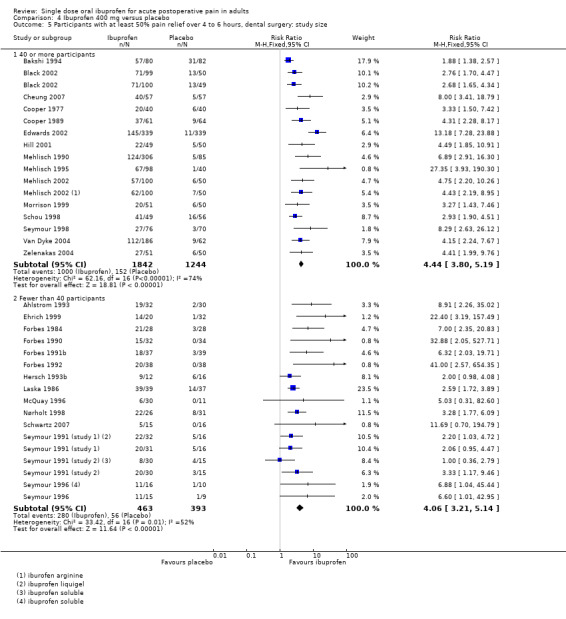
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 5 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: study size.
Nineteen studies had 40 or more participants in both treatment arms (Bakshi 1994; Black 2002; Cheung 2007; Cooper 1977; Cooper 1989; Hill 2001; Mehlisch 1990; Mehlisch 1995; Mehlisch 2002; Morrison 1999; Schou 1998; Seymour 1998; Edwards 2002; Van Dyke 2004; Zelenakas 2004) (Analysis 4.5.1). The proportion of participants with at least 50% pain relief was 54% (1000/1842) for ibuprofen 400 mg, and 12% (152/1244) for placebo; the RB was 4.4 (3.8 to 5.2), and the NNT was 2.4 (2.2 to 2.6). Fourteen studies had fewer than 40 participants in both treatment arms (Ahlstrom 1993; Ehrich 1999; Forbes 1984; Forbes 1990; Forbes 1991b; Forbes 1992; Hersch 1993b; Laska 1986; McQuay 1996; Nørholt 1998; Schwartz 2007; Seymour 1991 (study 1); Seymour 1991 (study 2); Seymour 1996) (Analysis 4.5.2). The proportion of participants with at least 50% pain relief was 60% (280/463) for ibuprofen 400 mg, and 14% (56/393) for placebo; the RB was 4.1 (3.2 to 5.2), and the NNT was 2.2 (1.9 to 2.5).
There was no consistent or statistically significant effect of study size in this group of studies, using 40 participants per treatment arm as the cut‐off.
| Summary of results B: Sensitivity analyses using number of participants with ≥ 50% pain relief over 4 to 6 hours | |||||
| Criterion | Studies | Participants | Ibuprofen (%) | Placebo (%) | NNT (95%CI) |
| Dental surgery 200 mg | 18 | 2470 | 47 | 10 | 2.7 (2.5 to 3.0) |
| Other surgery 200 mg | 2 | 220 | 39 | 5 | 3.0 (2.3 to 4.2) |
| Dental surgery 400 mg | 49 | 5428 | 55 | 12 | 2.3 (2.2 to 2.4) |
| Other surgery 400 mg | 12 | 1047 | 48 | 22 | 3.9 (3.2 to 5.0) |
| Dental 600/800 mg | 2 | 165 | 86 | 29 | 1.7 (1.4 to 2.3) |
| Standard 200 mg, all surgery | 17 | 2103 | 41 | 7 | 2.9 (2.6 to 3.2) |
| "Soluble" salts 200 mg, all surgery | 7 | 828 | 56 | 10 | 2.1 (1.9 to 2.4) |
| Standard 400 mg, all surgery | 55 | 5604 | 52 | 11 | 2.5 (2.4 to 2.7) |
| "Soluble" salts 400 mg, all surgery | 12 | 1124 | 65 | 18 | 2.1 (1.9 to 2.4) |
| Standard 200 mg, dental surgery | 15 | 1883 | 41 | 7 | 2.9 (2.6 to 3.2) |
| "Soluble" salts 200 mg, dental surgery | 7 | 828 | 56 | 10 | 2.1 (1.9 to 2.4) |
| Standard 400 mg, dental surgery | 46 | 4772 | 53 | 11 | 2.3 (2.2 to 2.5) |
| "Soluble" salts 400 mg, dental surgery | 9 | 959 | 66 | 10 | 1.8 (1.7 to 2.0) |
| 40 + participants, dental surgery 200 mg | 11 | 1953 | 49 | 10 | 2.5 (2.3 to 2.8) |
| < 40 participants, dental surgery 200 mg | 4 | 229 | 32 | 5 | 3.9 (2.8 to 6.1) |
| 40 + participants, dental surgery 400 mg | 19 | 3086 | 54 | 12 | 2.4 (2.2 to 2.6) |
| < 40 participants, dental surgery 400 mg | 14 | 856 | 60 | 14 | 2.2 (1.9 to 2.5) |
Use of rescue medication
Proportion of participants using rescue medication (Summary of results C)
The majority of studies reporting this outcome did so after 6 hours. A minority reported at shorter times (4 and 5 hours) or longer times (8, 12 and 24 hours) (Table 1). We analysed data for 6 hours because there were sufficient data to permit analysis by dose, and because longer times are likely to exceed the expected duration of effect of ibuprofen (plasma half life 2 hours).
Two studies using ibuprofen 50 mg reported the proportion of participants using rescue medication over 6 hours (Schou 1998; Sunshine 1996). The weighted mean proportion was 29% (30/102) with ibuprofen and 50% (53/106) with placebo, giving an NNTp of 4.9 (3.0 to 13) (Analysis 1.2).
Three studies using ibuprofen 100 mg reported the proportion of participants using rescue medication over 6 hours (Jain 1986; Schou 1998; Sunshine 1996). The weighted mean proportion was 38% (54/143) with ibuprofen and 64% (88/153) with placebo, giving an NNTp of 3.8 (2.7 to 6.5) (Analysis 2.2). In the two studies in dental pain, the weighted mean proportion was 59% (54/92) with ibuprofen and 80% (82/103) with placebo, giving an NNTp of 4.8 (2.3 to 12).
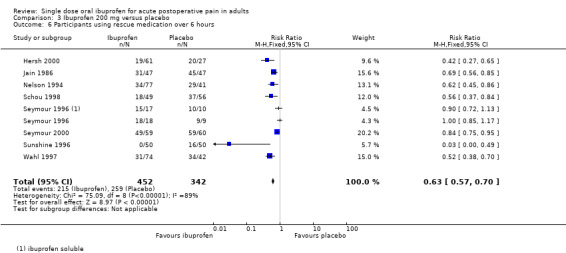
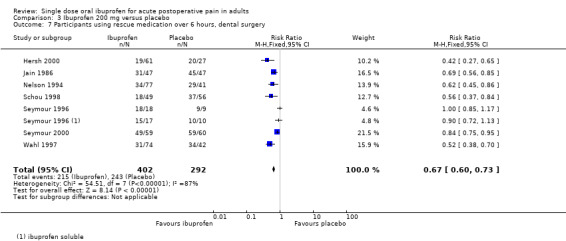
Eight studies using ibuprofen 200 mg reported the proportion of participants using rescue medication over 6 hours. The weighted mean proportion was 48% (215/452) with ibuprofen and 76% (259/342) with placebo, giving an NNTp of 3.6 (2.9 to 4.6) (Analysis 3.6). In the seven studies in dental pain, the weighted mean proportion was 53% (215/402) with ibuprofen and 83% (243/292) with placebo, giving an NNTp of 3.4 (2.8 to 4.3) (Analysis 3.7).
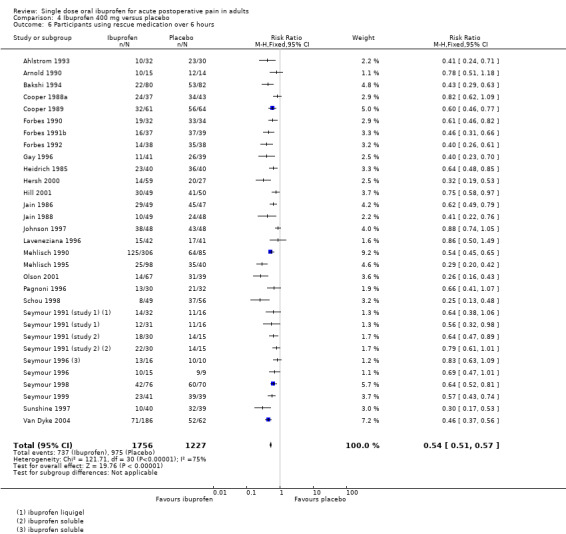
Twenty‐eight studies using ibuprofen 400 mg reported the proportion of participants using rescue medication over 6 hours. The weighted mean proportion was 42% (737/1756) with ibuprofen and 79% (975/1227) with placebo, giving an NNTp of 2.7 (2.5 to 2.9) (Analysis 4.6). In the 22 studies in dental pain, the weighted mean proportion was 41% (628/1541) with ibuprofen and 80% (814/1013) with placebo, giving an NNTp of 2.5 (2.3 to 2.8) (Analysis 4.7).
1.2. Analysis.
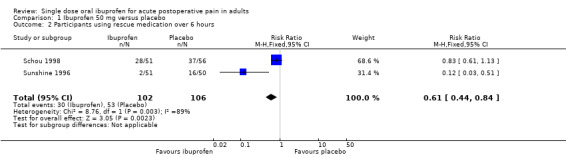
Comparison 1 Ibuprofen 50 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
2.2. Analysis.
Comparison 2 Ibuprofen 100 mg versus placebo, Outcome 2 Participants using rescue medication over 6 hours.
3.6. Analysis.
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 6 Participants using rescue medication over 6 hours.
3.7. Analysis.
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 7 Participants using rescue medication over 6 hours, dental surgery.
4.6. Analysis.
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 6 Participants using rescue medication over 6 hours.
4.7. Analysis.
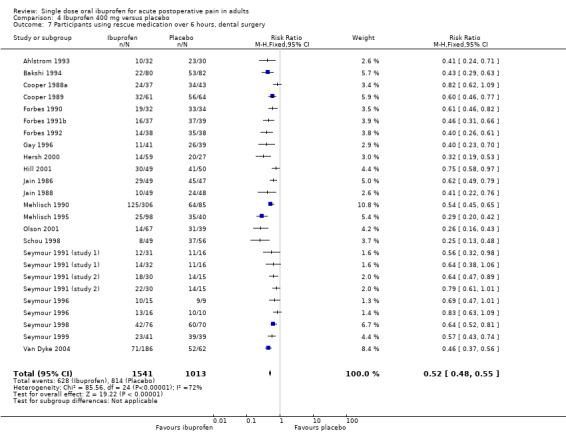
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 7 Participants using rescue medication over 6 hours, dental surgery.
Only one study (Seymour 1996) using ibuprofen 600 mg reported the proportion of participants using rescue medication, so no analysis was possible for the higher doses.
There was a trend towards a lower (better) NNTp with higher dose for all surgery combined, and for the dental studies alone (200 mg versus 400 mg all surgery: z = 2.53, P = 0.01; 200 mg versus 400 mg dental surgery z = 2.63, P = 0.009).
Dental surgery: effect of formulation
3.8. Analysis.
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 8 Participants using rescue medication over 6 hours, dental surgery: formulation.
4.8. Analysis.
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 8 Participants using rescue medication over 6 hours, dental surgery: formulation.
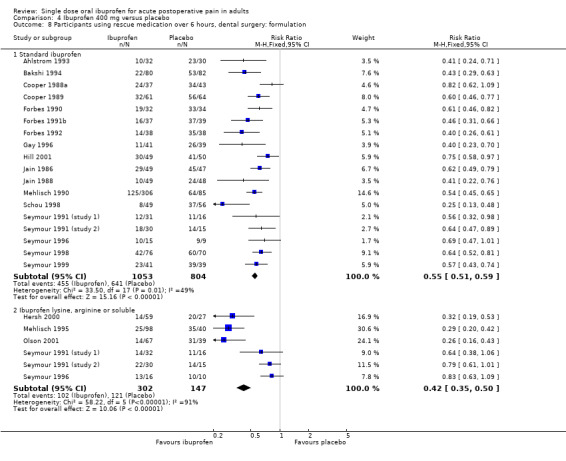
In four dental studies using standard ibuprofen 200 mg, the weighted mean proportion of participants using rescue medication over 6 hours was 67% (116/173) with ibuprofen and 87% (150/172) with placebo, giving an NNTp of 5.0 (3.5 to 8.7) (Analysis 3.8.1). In four dental studies using soluble preparations of ibuprofen 200 mg, the weighted mean proportion of participants using rescue medication over 6 hours was 43% (99/229) with ibuprofen and 78% (93/120) with placebo, giving an NNTp of 2.9 (2.3 to 4.1) (Analysis 3.8.2).
In 18 dental studies using standard ibuprofen 400 mg, the weighted mean proportion of participants using rescue medication over 6 hours was 42% (455/1053) with ibuprofen and 80% (693/866) with placebo, giving an NNTp of 2.7 (2.4 to 3.0) (Analysis 4.8.1). In six dental studies using soluble preparations of ibuprofen 400 mg, the weighted mean proportion of participants using rescue medication over 6 hours was 34% (102/302) with ibuprofen and 78% (121/147) with placebo, giving an NNTp of 2.1 (1.8 to 2.5) (Analysis 4.8.2).
At both doses fewer participants needed rescue medication over 6 hours with the soluble preparations than the standard preparation. The difference in NNTp was statistically significant for the 400 mg dose (z = 2.39, P = 0.017).
| Summary of results C: Weighted mean proportion using rescue medication over 6 hours | |||||
| Dose | Studies | Participants | Ibuprofen (%) | Placebo (%) | NNTp (95%CI) |
| 50 mg | 2 | 208 | 29 | 50 | 4.9 (3.0 to 13) |
| 100 mg | 3 | 296 | 38 | 64 | 3.8 (2.7 to 6.5) |
| 200 mg | 8 | 794 | 48 | 76 | 3.6 (2.9 to 4.6) |
| 400 mg | 28 | 2983 | 42 | 79 | 2.7 (2.5 to 3.0) |
| 100 mg, dental surgery | 2 | 195 | 59 | 80 | 4.8 (2.3 to 12) |
| 200 mg, dental surgery | 7 | 694 | 53 | 83 | 3.4 (2.8 to 4.3) |
| 400 mg, dental surgery | 22 | 2554 | 41 | 80 | 2.5 (2.3 to 2.8) |
| Standard 200 mg, dental surgery, | 4 | 345 | 67 | 87 | 5.0 (3.5 to 8.7) |
| "Soluble" salts 200 mg, dental surgery | 4 | 349 | 43 | 78 | 2.9 (2.3 to 4.1) |
| Standard 400 mg, dental surgery, | 18 | 1857 | 43 | 80 | 2.7 (2.5 to 3.1) |
| "Soluble" salts 400 mg, dental surgery | 6 | 449 | 34 | 82 | 2.1 (1.8 to 2.5) |
Time to use of rescue medication (Summary of results D)
Thirty‐four studies reported the median time, and 17 the mean time to use of rescue medication (Table 1).
In 10 studies (1807 participants) the weighted mean of the median time to use of rescue medication was 4.7 hours for ibuprofen 200 mg and 2.1 hours for placebo.
In 31 studies (3548 participants) the weighted mean of the median time to use of rescue medication was 5.6 hours for ibuprofen 400 mg and 1.9 hours for placebo.
In four studies (345 participants) the weighted mean of the mean time to use of rescue medication was 3.9 hours for ibuprofen 200 mg and 2.2 hours for placebo.
In 16 studies (1313 participants) the weighted mean of the median time to use of rescue medication was 4.6 hours for ibuprofen 400 mg and 2.8 hours for placebo.
| Summary of results D: Weighted median and mean time to use of rescue medication | ||||
| Median time | ||||
| Dose | Studies | Participants | Ibuprofen | Placebo |
| 200 mg | 10 | 1807 | 4.7 | 2.1 |
| 400 mg | 31 | 3548 | 5.6 | 1.9 |
| Mean time | ||||
| 200 mg | 4 | 345 | 3.9 | 2.2 |
| 400 mg | 16 | 1313 | 4.6 | 2.8 |
Adverse events (Summary of results E)
Any adverse event
Most studies collected adverse event data over 4 to 8 hours, but a few collected at 12 and 24 hours, and one at 14 days (Malmstrom 2004). Adverse events were generally described as mild and transient (Table 2).
2. Summary of outcomes: adverse events and withdrawals.
| Adverse events | Withdrawals | ||||
| Study ID | Treatment | Any | Serious | Adverse event | Other |
| Ahlstrom 1993 | (1) Ibuprofen 400 mg, n = 32 (2) Diclofenac (drinkable) 50 mg, n = 35 (3) Placebo n = 30 |
at 6 h: (1) 3/32 (3) 2/30 |
None | None | 30 excluded for various protocol violations |
| Arnold 1990 | (1) Ibuprofen 400 mg, n = 15 (2) Ketoprofen 25 mg, n = 14 (3) Ketoprofen 100 mg, n = 16 (4) Placebo, n = 14 |
at 6 h: (1) 6/15 (4) 3/14 |
None | None | No data |
| Bakshi 1994 | (1) Ibuprofen 400 mg, n = 80 (2) Diclofenac (dispersible) 50 mg, n = 83 (3) Placebo, n = 82 |
at 6 h: (1) 6/80 (3) 5/82 |
None | None | 12 exclusions: 9 with insufficient baseline pain, 2 remedicated early, 1 completed diary incorrectly |
| Black 2002 | (1) Ibuprofen 200 mg, n = 100 (2) Ibuprofen 400 mg, n = 100 (3) Ibuprofen arginate 200 mg, n = 100 (4) Ibuprofen arginate 400 mg, n = 99 (5) Placebo, n = 99 |
at 6 h: (1) 31/100 (2) 41/100 (3) 51/100 (4) 36/99 (5) 48/99 |
(3) 1/100 (dysphagia and pharyngitis after 60 min assessment) | (3) 1/100 | 4 exclusions from efficacy analysis: 2 from Ibu Arg groups vomited soon after taking drug, 1 ibu arg 200 mg and 1 placebo took prohibited medication |
| Cheung 2007 | (1) Ibuprofen 440 mg, n = 57 (2) Celecoxib 400 mg, n = 57 (3) Placebo, n = 57 |
at 24 h: (1) 35/57 (3) 39/57 |
None | (3) 3/57 (vomiting and anxiety) | (3) 1/57 (withdrew consent) |
| Cooper 1977 | (1) Ibuprofen 200 mg n = 38 (2) Ibuprofen 400 mg, n = 40 (3) Aspirin 325 mg, n = 37 (4) Aspirin 650 mg, n = 37 (5) Placebo, n = 40 |
No data | None | None | Exclusions: 17 provided uninterpretable data, 12 took confounding medication, 10 were lost to follow up, 9 did not need medication, 5 fell asleep |
| Cooper 1982 | (1) Ibuprofen 400 mg, n =38 (2) Ibuprofen 400 mg + Codeine 60 mg, n = 41 (3) Aspirin 650 mg, n = 38 (4) Aspirin 650 mg + codeine 60 mg, n = 45 (5) Codeine 60 mg, n = 41 (6) Placebo, n = 46 |
at 4 h: (1) 11/38 (6) 5/46 |
None | None | Exclusions: 30 were lost to follow up, 15 did not need medication, 11 remedicated early, 6 missed more the 1 evaluation, 3 medicated with slight pain, 1 did not take all the medication, 1 medicated over 24 hrs after surgery |
| Cooper 1988a | (1) Ibuprofen 400 mg, n = 37 (2) Ketoprofen 100 mg, n = 39 (3) Ketoprofen 25 mg, n = 42 (4) Placebo, n = 43 |
at 6 h: (1) 10/40 (4) 7/45 |
None reported | None reported | Exclusions: 20 did not need medication, 13 were lost to follow up, 7 had various protocol violations |
| Cooper 1989 | (1) Ibuprofen 400 mg, n = 61 (2) Paracetamol 1000 mg, n = 59 (3) Placebo, n = 64 |
at 6 h: (1) 5/63 (3) 7/64 |
None | None | Exclusions: 2 were lost to follow up, 2 did not need medication, 4 missed more than 1 evaluation, 1 had insufficient baseline pain, 1 failed to complete the diary at the appropriate time |
| Cooper 1996a | (1) Ibuprofen 200 mg, n = 19 (2) Misoprostal 200 mg, n = 18 (3) Ibuprofen 200 mg + misoprostal 200 mg, n = 20 (4) Placebo, n = 13 |
No usable data All transient and mild |
None reported | No data | No data |
| De Miguel Rivero 1997 | (1) Ibuprofen arginine 400 mg, n = 36 (2) Magnesic dipyrone, 2 g (IM), n = 33 (3) Placebo, n = 34 |
at 5 h: (1) 1/36 (3) 1/34 |
None | None | Exclusions: 3 did not need medication |
| Desjardins 2002 | (1) Ibuprofen 200 mg, n = 50 (2) Ibuprofen 400 mg, n = 52 (3) Ibuprofen arginine 200 mg, n = 49 (4) Ibuprofen arginine 400 mg, n = 50 (5) Placebo, n = 24 |
at 6 h: (1) 4/50 (2) 4/52 (3) 3/49 (4) 7/50 (5) 1/24 |
None | None | Exclusions from efficacy analysis: 1 (placebo) for protocol violation, 1 (placebo) for vomiting after receiving study drug and 1 (Ibu arg 400) for having a seizure 11 hours post‐dose |
| Dionne 1998 | (1) S(+)‐Ibuprofen 200 mg, n = 51 (2) S(+)‐Ibuprofen 400 mg, n = 50 (3) Ibuprofen (racemic) 400 mg, n = 50 (4) Placebo, n = 25 |
No usable data | None reported | None | Exclusions: 4 had neither partial or bony impaction, 1 remedicated early |
| Ehrich 1999 | (1) Ibuprofen 400 mg, n = 20 (2) Rofecoxib 50 mg, n = 32 (3) Rofecoxib 500 mg, n = 20 (4) Placebo, n = 32 |
No usable data Any events mild and transient |
None | No data | No data Exclusions: 2 remedicated early |
| Forbes 1984 | (1) Ibuprofen 400 mg, n = 28 (2) Fendosal 200 mg, n = 29 (3) Aspirin 650 mg, n = 24 (4) Placebo n = 28 |
at 12 h: (1) 5/29 (4) 3/30 All transitory and did not require treatment |
None | None | Exclusions: 2 remedicated early, 2 remedicated with some pain relief, 2 took rescue medication not test drug |
| Forbes 1990 | (1) Ibuprofen 400 mg, n = 32 (2) Ketorolac 10 mg, n = 31 (3) Ketorolac 20 mg, n = 35 (4) Paracetamol 600 mg, n = 36 (5) Paracetamol 600 mg + codeine 60 mg, n = 38 (6) Placebo, n = 34 |
at 6 h: (1) 8/43 (6) 0/38 All transitory and did not require treatment |
None | None | Exclusions; 3 were lost to follow up, 1 lost report card, 27 remedicated early or while still experiencing some pain relief from study medication, 7 failed to follow instructions, 3 did not complete the forms |
| Forbes 1991a | (1) Ibuprofen 50 mg, n = 57 (2) Ibuprofen 100 mg, n = 49 (3) Ibuprofen 200 mg, n = 48 (4) Ibuprofen 100 mg + Caffeine 100 mg, n = 49 (5) Ibuprofen 200 mg + Caffeine 100 mg, n = 44 (6) Placebo n = 51 |
at 8 h: (1) 10/63 (2) 5/62 (3) 6/60 (4) 12/58 (5) 8/58 (6) 8/61 All transitory and did not require treatment |
None | None | Exclusions from efficacy analysis: 33 did not need medication, 14 remedicated early, 1 ate caffeine containing food, 2 medicated for a headache, 1 rated only one side of mouth, 1 form completed by relative, 3 lacked consistency, 22 evaluated at incorrect time, 3 incomplete forms |
| Forbes 1991b | (1) Ibuprofen 400 mg, n = 37 (2) Bromfenac 5 mg, n = 39 (3) Bromfenac 10 mg, n = 43 (4) Bromfenac 25 mg, n = 42 (5) Aspirin 650 mg, n = 41 (6) Placebo, n = 39 |
at 8 h: (1) 7/43 (6) 3/47 All transitory and did not require treatment |
None | None | Exclusions: 7 were lost to follow up, 12 did not need medication, 24 remedicated early or while still experiencing some pain relief from study medication, 2 lacked consistency, 1 did not complete the form, 1 took only part of the med |
| Forbes 1992 | (1) Ibuprofen 400 mg, n = 38 (2) Bromfenac 10 mg, n = 43 (3) Bromfenac 25 mg, n = 41 (4) Bromfenac 50 mg, n = 42 (5) Bromfenac 100 mg, n = 40 (6) Aspirin 650 mg, n = 38 (7) Placebo, n = 38 |
at 8 h: (1) 4/45 (7) 2/46 All transitory and did not require treatment |
None | None | Exclusions; 3 did not return form, 14 did not need medication, 28 remedicated early or while still experiencing some pain relief from study medication, 2 lacked consistency, 2 did not complete form, 2 took only part of medication, 5 took back up medication, 2 evaluated at incorrect time |
| Frame 1989 | (1) Ibuprofen 400 mg, n = 42 (2) Dihydrocodeine 30 mg, n = 43 (3) Placebo, n = 38 |
at 5 h: (1) 4/42 (3) 3/38 |
None reported | (3) 1/38 (postoperative bleed) | Exclusions: 9 did not take the medication, 7 were lost to follow up, 1 was asleep so did not complete the forms, 1 had postoperative complications so did not complete the form |
| Fricke 1993 | (1) Ibuprofen 400 mg, n = 81 (2) Naproxen Na 440 mg, n = 81 (3) Placebo, n = 39 |
at 12 h: (1) 8/81 (3) 1/39 |
None | (1) 1/81 (soreness and swelling at 8 hrs) | Exclusions: 5 did not need medication, 1 took study medication twice ‐ excluded from efficacy analysis |
| Gay 1996 | (1) Ibuprofen 400 mg, n = 41 (2) DKP.TRIS 5 mg, n = 41 (3) DKP.TRIS 10 mg, n = 42 (4) DKP.TRIS 20 mg, n = 41 (5) Placebo, n = 39 |
at 6 h: (1) 3/41 (5) 4/41 |
None | None | Exclusion from efficacy analysis: 2 remedicated early |
| Heidrich 1985 | (1) Ibuprofen 400 mg, n = 40 (2) Paracetamol 300 + codeine 30 mg, n = 40 (3) Placebo, n = 40 |
No usable data Overall occurrence ±15%, no difference between groups |
None reported | None | No data |
| Hersch 1993a | (1) Ibuprofen 200 mg, n = 51 (2) Ibuprofen 400 mg, n = 49 (3) Meclofenamate 100 mg, n = 52 (4) Meclofenamate 50 mg, n = 51 (5) Placebo, n = 51 |
at 8 h: (1) 6/49 (2) 4/51 (5) 9/51 All transitory and did not require treatment |
None reported | None | No data |
| Hersch 1993b | (1) Ibuprofen 400 mg, n = 12 (2) Codeine 60 mg, n = 16 (3) Placebo, n = 16 |
No data | None reported | None reported | Exclusions: 19 lost to follow up, 11 did not need medication, 3 excluded for various protocol violations |
| Hersh 2000 | (1) Ibuprofen liquigel 200 mg, n = 61 (2) Ibuprofen liquigel 400 mg, n = 59 (3) Paracetamol 1000 mg, n = 63 (4) Placebo, n = 27 |
at 6 h: (1) 7/61 (2) 4/59 (4) 7/27 |
None | None | None |
| Hill 2001 | (1) Ibuprofen 400 mg, n = 49 (2) Pregabalin 50 mg, n = 49 (3) Pregabalin 300 mg, n = 50 (4) Placebo, n = 50 |
at 12 h: (1) 6/49 (4) 8/50 |
None | None | None |
| Jain 1986 | (1) Ibuprofen 400 mg, n = 49 (2) Ibuprofen 200 mg, n = 47 (3) Ibuprofen 100 mg, n = 39 (4) Aspirin 650 mg, n = 45 (5) Placebo, n = 47 |
at 6 h: (1) 10/49 (2) 6/47 (3) 13/39 (5) 12/47 |
None reported | None reported | None |
| Jain 1988 | (1) Ibuprofen 400 mg, n = 49 (2) Ibuprofen 200 mg + caffeine 100 mg, n = 50 (3) Placebo, n = 48 |
at 6 h: (1) 2/49 (2) 5/50 (3) 1/48 |
None reported | None reported | Exclusions: 11 remedicated early, 2 received confounding agents, 1 was under 18 yrs old |
| Johnson 1997 | (1) Ibuprofen 400 mg, n = 48 (2) Paracetamol 650 mg + oxycodone 10 mg, n = 47 (3) Bromfenac 100 mg, n = 48 (4) Bromfenac 50 mg, n = 47 (5) Placebo, n = 48 |
No usable data CNS AEs at 8 h: (1) 5/48 (5) 2/48 |
None reported | None reported | Exclusions: 2 had invalid data |
| Kiersch 1993 | (1) Ibuprofen 200 mg, n = 81 (2) Naproxen Na 220 mg, n = 80 (3) Placebo, n = 42 |
at 12 h: (1) 16/81 (3) 5/43 |
None | (1) 1/81 | Exclusions: 2 had protocol violations |
| Laska 1986 | (1) Ibuprofen 400 mg, n = 39 (2) Ibuprofen 600 mg, n = 36 (3) Ibuprofen 800 mg, n = 39 (4) Aluminium ibuprofen 400 mg, n = 39 (5) Placebo, n = 37 |
at 6 h: (1) 0/39 (2) 1/36 (3) 0/39 (4) 1/39 (5) 3/37 |
None reported | None | Exclusions: 4 remedicated early, 1 vomited within 5 mins of taking the study medication. 4 with moderate pain dropped to keep populations homogeneous (author letter) |
| Laveneziana 1996 | (1) Ibuprofen arginine soluble 400 mg, n = 42 (2) Ketorolac 30 mg, n = 41 (3) Placebo, n = 41 |
None | None | None | Exclusions: 1 had insufficient pain |
| Malmstrom 1999 | (1) Ibuprofen 400 mg, n = 46 (2) Rofecoxib 50 mg, n = 90 (3) Celecoxib 200 mg, n = 91 (4) Placebo, n = 45 |
No usable data | None reported | (4) 1/45 (excessive bleeding) | None 4 patients lost to follow up at post‐study visit |
| Malmstrom 2002 | (1) Ibuprofen 400 mg, n = 45 (2) Rofecoxib 50 mg, n = 151 (3) Celecoxib 400 mg, n = 151 (4) Celecoxib 200 mg, n = 90 (5) Placebo, n = 45 |
at 24 h: (1) 8/45 (5) 12/45 |
None | None | None |
| Malmstrom 2004 | (1) Ibuprofen 400 mg, n = 48 (2) Etoricoxib 60 mg, n = 75 (3) Etoricoxib 120 mg, n = 76 (4) Etoricoxib 180 mg, n = 74 (5) Etoricoxib 240 mg, n = 76 (6) Placebo, n = 49 |
Up to 14 days: (1) 17/48 (6) 24/49 |
None | (1) 1/48 (vomiting) | None |
| McQuay 1996 | (1) Ibuprofen 200 mg, n = 31 (2) Ibuprofen 400 mg, n = 30 (3) Ibuprofen 200 mg + caffeine 50 mg, n = 30 (4) Ibuprofen 200 mg + caffeine 100 mg, n = 30 (5) Ibuprofen 200 mg + caffeine 200 mg, n = 29 (6)Placebo, n = 11 |
at 8 h: (1) 4/31 (2) 1/30 (3) 2/30 (4) 0/29 (5) 2/30 (6) 1/11 |
None reported | None | Exclusions: 3 with protocol violations |
| Medve 2001 | (1) Ibuprofen 200 mg, n = 240 (2) Tramadol 37.5 mg, n = 238 (3) Paracetamol 325 mg, n = 240 (4) Tramadol 37.5 mg + paracetamol 325 mg, n = 240 (5) Placebo, n = 239 |
No usable data Generally transient and mild to moderate in severity |
None reported | No data | No details for 3 exclusions |
| Mehlisch 1990 | (1) Ibuprofen 400 mg, n = 306 (2) Paracetamol 1000 mg, n = 306 (3) Placebo, n = 85 |
at 6 h: (1) 31/306 (3) 12/85 |
None reported | None | Exclusions: 4 were lost to follow up, 4 entered in the trial twice (1st entry only was analysed for efficacy but both were included in safety analysis) and 1 excluded for failing to meet inclusion criteria |
| Mehlisch 1995 | (1) Ibuprofen lysine 400 mg, n = 98 (2) Paracetamol 1000 mg, n = 101 (3) Placebo, n = 40 |
at 6 h: (1) 12/98 (3) 4/40 |
None | None | Exclusions: 1 failed to complete diary |
| Mehlisch 2002 | (1) Ibuprofen 200 mg, n = 100 (2) Ibuprofen 400 mg, n = 100 (3) Ibuprofen arginine 200 mg, n = 100 (4) Ibuprofen arginine 400 mg, n = 100 (5) Placebo, n = 100 |
at 6 h: (1) 28/100 (2) 27/100 (3) 27/100 (4) 26/100 (5) 27/100 |
None | None | Exclusions: 3 from efficacy analysis for protocol violation |
| Morrison 1999 | (1) Ibuprofen 400 mg, n = 51 (2) Rofecoxib 50 mg, n = 50 (3) Placebo, n = 50 |
at 24 h: (1) 13/51 (3) 17/50 |
None | None | None |
| Nelson 1994 | (1) Ibuprofen lysine 200 mg, n = 77 (2) Aspirin 500 mg, n = 65 (3) Placebo, n = 40 |
at 6 h: (1) 16/75 (3) 11/40 |
None | None | Exclusions: 2 remedicated early, 1 did not record baseline pain intensity |
| Nørholt 1998 | (1) Ibuprofen 400 mg, n = 26 (2) Placebo, n = 31 |
No data | No data | None | None |
| Olson 2001 | (1) Ibuprofen liquigel 400 mg, n = 67 (2) Ketoprofen 25 mg, n = 67 (3) Paracetamol 1000 mg, n = 66 (4) Placebo, n = 39 |
at 6 h: (1) 7/67 (4) 2/39 |
None | None | None |
| Pagnoni 1996 | (1) Ibuprofen arginine soluble 400 mg, n = 30 (2) Ketorolac (IM) 30 mg, n = 30 (3) Placebo, n = 32 |
None | None | None | None |
| Parker 1986 | (1) Ibuprofen syrup 600 mg, n = 44 (2) Aspirin syrup 600 mg, n = 33 (3) Placebo, n = 33 |
No usable data | None reported | (3) 1/33 (probably in multiple dose phase) | Exclusions: 29 for which there is no further data |
| Schachtel 1989 | (1) Ibuprofen 400 mg, n = 36 (2) Paracetamol 1000 mg, n = 37 (3) Placebo, n = 38 |
at 4 h: None |
None | None | Exclusions: 4 remedicated early |
| Schou 1998 | (1) Ibuprofen 50 mg, n = 51 (2) Ibuprofen 100 mg, n = 53 (3) Ibuprofen 200 mg, n = 49 (4) Ibuprofen 400 mg, n = 49 (5) Placebo, n = 56 |
No usable data 18 patients reported mild transient AEs ‐ no details of groups |
None | None | Exclusions: 46 due to insufficient baseline pain, 3 withdrew (reasons not related to AE), 5 failed to attend follow‐up, 5 lost self‐report measure, 3 took study prohibited additional analgesia, 3 did not require surgery, 2 remedicated early, 1 had concomitant surgical removal of maxillary third molar |
| Schwartz 2007 | (1) Ibuprofen 400 mg, n = 15 (2) MK‐0703 12.5 mg, n = 31 (3) MK‐0703 50 mg, n = 28 (4) MK‐0703 100 mg, n = 31 (5) Placebo, n = 16 |
No usable data 38 patients in total reported AEs, no details of groups |
None | None | None |
| Seymour 1991 (study 1) | (1) Ibuprofen tablets 400 mg, n = 31 (2) Ibuprofen liquid in gelatin capsules 400 mg, n = 32 (3) Placebo n = 32 |
at 6 h: (1) 0/31 (2) 0/32 (3) 1/32 |
None | None | No data |
| Seymour 1991 (study 2) | (1) Ibuprofen tablets 400 mg, n = 30 (2) Ibuprofen soluble 400 mg, n = 32 (3) Placebo, n = 30 |
at 6 h: None |
None | None | No data |
| Seymour 1996 | (1) Ibuprofen tablets 200 mg, n = 18 (2) Ibuprofen soluble 200 mg, n = 17 (3) Ibuprofen tablets 400 mg, n = 15 (4) Ibuprofen soluble 400 mg, n = 16 (5) Ibuprofen tablets 600 mg, n = 17 (6) Ibuprofen soluble 600 mg, n = 17 (7) Placebo, n = 19 |
No data | No data | No usable data 4 reported adverse effects, 3 had received ibuprofen (did not clarify which dose) and 1 had taken placebo |
25 had inadequate baseline pain intensity |
| Seymour 1998 | (1) Ibuprofen 400 mg, n = 76 (2) Aceclofenac 150 mg, n = 71 (3) Placebo, n = 70 |
at 6 h: (1) 5/76 (3) 3/68 |
None reported | None reported | Exclusions: 2 patients in group 2 and 2 patients in group 3 not accounted for |
| Seymour 1999 | (1) Ibuprofen 400 mg, n = 41 (2) WAG 994 1 mg, n = 42 (3) Placebo, n = 39 |
No data | None | No data | No data |
| Seymour 2000 | (1) Ibuprofen 200 mg, n = 59 (2) Buffered ketoprofen 12.5 mg, n = 61 (3) Placebo, n = 50 |
at 6 h: (1) 5/59 (3) 3/60 |
None | None | Exclusions: 2 remedicated early, one in Ibuprofen group and one in placebo group |
| Singla 2005 | (1) Ibuprofen 400 mg, n = 175 (2) Ibuprofen 400 mg + oxycodone 5 mg, n = 169 (3) Oxycodone 5 mg, n = 52 (4) Placebo, n = 60 |
at 6 h: (1) 74/175 (4) 33/60 |
None | (1) 1/175 (4) 0/60 |
1 patient in Ibu group excluded due to protocol violation |
| Sunshine 1983 | (1) Ibuprofen 400 mg, n = 30 (2) Aspirin 600 mg, n = 30 (3) Zomepirac 100 mg, n = 30 (4) Placebo, n = 30 |
None | None | None | None |
| Sunshine 1987 | (1) Ibuprofen 400 mg, n = 38 (2) Ibuprofen 200 mg + codeine 30 mg, n = 40 (3) Ibuprofen 400 mg + codeine 60 mg, n = 40 (4) Codeine 60 mg, n = 37 (5) Placebo, n = 40 |
at 4 h: (1) 0/38 (5) 0/40 |
None | None | Exclusions: 1 had not complied with the washout period, 4 did not complete the evaluations |
| Sunshine 1996 | (1) Ibuprofen 50 mg, n = 51 (2) Ibuprofen 100 mg, n = 51 (3) Ibuprofen 200 mg, n = 50 (4) Ibuprofen 100 mg + caffeine 100 mg, n = 50 (5) Ibuprofen 200 mg + caffeine 100 mg, n = 50 (6) Placebo, n = 50 |
at 6 h: (1) 1/51 (2) 4/51 (3) 1/50 (4) 2/50 (5) 4/50 (6) 0/50 |
None | None | Exclusions: 3 for protocol violations |
| Sunshine 1997 | (1) Ibuprofen 400 mg, n = 40 (2) Ibuprofen 400 mg + hydrocodone 15 mg, n = 40 (3) Placebo, n = 39 |
at 6 h: (1) 5/40 (3) 4/40 |
None | None | Exclusions: One from placebo group refused to cooperate and was excluded from the study |
| Sunshine 1998 | (1) Ibuprofen 200 mg, n = 35 (2) Ketoprofen 6.25 mg, n = 35 (3) Ketoprofen 12.5 mg, n = 35 (4) Ketoprofen 25 mg, n = 35 (5) Placebo, n = 35 |
Up to 6 h: (1) 2/35 (5) 3/35 |
None | None | Exclusions: 2 remedicated early, 1 vomited study drug, 1 withdrew consent |
| Unpubl 2002 (Edwards 2002) |
(1) Ibuprofen 400 mg, n = 339 (2) Placebo, n = 339 |
at 6 h: (1) 41/339 (2) 58/337 |
None | No usable data | Total withdrawals not due to lack of efficacy (1) 2/339 (2) 3/337 |
| Van Dyke 2004 | (1) Ibuprofen 400 mg, n = 186 (2) Ibuprofen 400 mg + oxycodone 5 mg, n = 187 (3) Oxycodone 5 mg, n = 63 (4) Placebo, n = 62 |
at 6 h: (1) 20/186 (4) 7/62 |
None | None | Exclusions: 1 had inadequate baseline pain (1) 1/186 no reason given |
| Wahl 1997 | (1) Ibuprofen lysinate 342 mg (= 200 mg Ibu), n = 74 (2) Paracetamol 200 mg + aspirin 250 mg + caffeine 50 mg, n = 73 (3) Placebo, n = 42 |
at 6 h: (1) 7/74 (3) 5/42 |
None | None | Exclusions: 12 withdrew consent, 13 did not require analgesia after surgery, 6 failed to complete their study lists, and 1 may not have taken study medication correctly |
| Wideman 1999 (study 1) | (1) Ibuprofen 200 mg, n = 60 (2) Ibuprofen 200 mg, + hydrocodone 7.5 mg, n = 59 (3) Hydrocodone 7.5 mg, n = 61 (4) Placebo, n = 60 |
at 8 h: (1) 6/60 (4) 1/60 |
None | None | None |
| Wideman 1999 (study 2) | (1) Ibuprofen 400 mg, n = 50 (2) Ibuprofen 400 mg + hydrocodone 15 mg, n = 50 (3) Hydrocodone 15 mg, n = 50 (4) Placebo, n = 51 |
at 8 h: (1) 7/50 (4) 6/51 |
None | None | None |
| Zelenakas 2004 | (1) Ibuprofen 400 mg, n = 51 (2) Lumiracoxib 100 mg, n = 51 (3) Lumiracoxib 400 mg, n = 50 (4) Placebo, n = 50 |
at 12 h: (1) 5/51 (4) 10/50 |
(1) 0/51 (4) 1/50 (deep vein thrombosis) |
(1) 1/51 (postoperative bleed) | (1) 1/51 (? withdrew consent) |
Two studies using ibuprofen 50 mg reported on the number of participants with at least one adverse event (Forbes 1991a; Sunshine 1996): 10% (11/114) with ibuprofen, and 7% (8/111) with placebo (Analysis 1.3).
Three studies using ibuprofen 100 mg reported on the number of participants with at least one adverse event (Forbes 1991a; Jain 1986; Sunshine 1996): 14% (22/152) with ibuprofen, and 13% (20/158) with placebo (Analysis 2.3).
Fourteen studies using ibuprofen 200 mg reported on the number of participants with at least one adverse event (Black 2002; Desjardins 2002; Forbes 1991a; Hersch 1993a; Hersh 2000; Jain 1986; McQuay 1996; Mehlisch 2002; Nelson 1994; Seymour 2000; Sunshine 1996; Sunshine 1998; Wahl 1997; Wideman 1999 (study 1)): 19% (208/1102) with ibuprofen, and 19% (137/706) with placebo (Analysis 3.9).
Forty studies using ibuprofen 400 mg reported on the number of participants with at least one adverse event: 17% (476/2870) with ibuprofen, and 16% (326/1997) with placebo (Analysis 4.9).
1.3. Analysis.
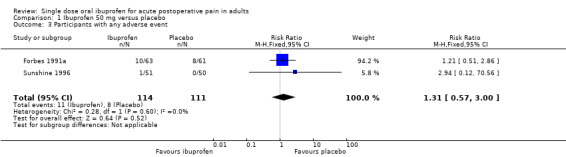
Comparison 1 Ibuprofen 50 mg versus placebo, Outcome 3 Participants with any adverse event.
2.3. Analysis.
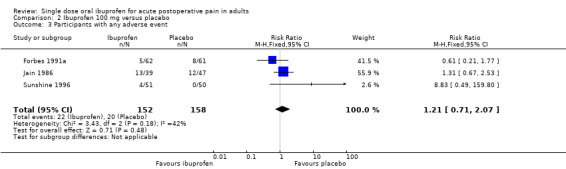
Comparison 2 Ibuprofen 100 mg versus placebo, Outcome 3 Participants with any adverse event.
3.9. Analysis.
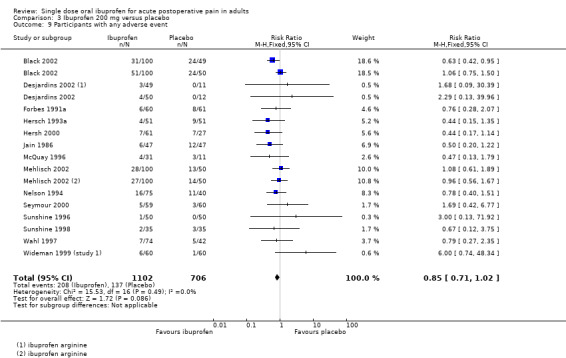
Comparison 3 Ibuprofen 200 mg versus placebo, Outcome 9 Participants with any adverse event.
4.9. Analysis.
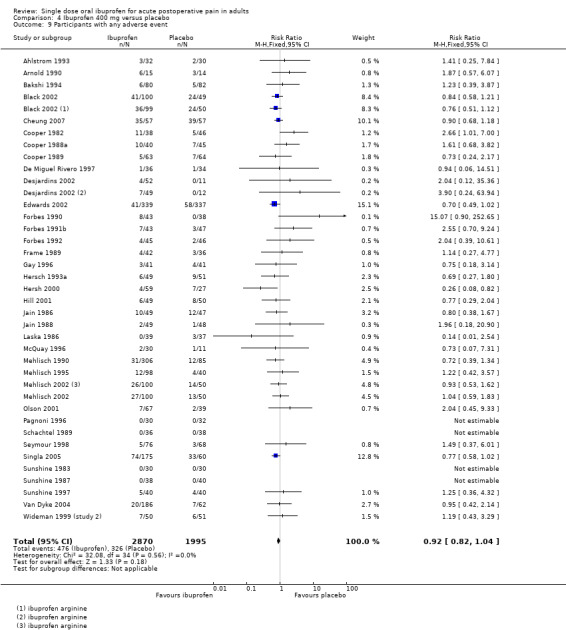
Comparison 4 Ibuprofen 400 mg versus placebo, Outcome 9 Participants with any adverse event.
For doses of ibuprofen of 50 mg to 400 mg there was no significant difference in participants experiencing any adverse event compared with placebo. Two studies using 600 mg and 800 mg ibuprofen also showed no difference from placebo, but these had small amounts of data.
| Summary of results E: Participants with at least one adverse event | |||||
| Dose | Studies | Participants | Ibuprofen (%) | Placebo (%) | NNH (95%CI) |
| 50 mg | 2 | 225 | 10 | 7 | not calculated |
| 100 mg | 3 | 310 | 14 | 13 | not calculated |
| 200 mg | 14 | 1808 | 19 | 19 | not calculated |
| 400 mg | 40 | 4867 | 17 | 16 | not calculated |
Serious adverse event
Two studies reported serious adverse events. Black 2002 reported one participant treated with ibuprofen arginine 200 mg who had dysphagia and pharyngitis after the 60 minute assessment, and Zelenakas 2004 reported one participant treated with placebo who had deep vein thrombosis (DVT).
Withdrawals
(Table 2)
Participants who took rescue medication were classified as withdrawals due to lack of efficacy, and details are reported under "Use of rescue medication" above.
Withdrawals and exclusions were not reported consistently, particularly in older studies. Exclusions may not be of any particular consequence in single dose acute pain studies, where most exclusions result from patients not having moderate or severe pain (McQuay 1982). Withdrawals were sometimes reported without stating which treatment groups these referred to, or when withdrawals occurred, i.e., before assessment of analgesia at 4 to 6 hours, or at some other point before the end of the trial. Where details were given, withdrawals or exclusions were usually due to protocol violations or adverse events related to the surgical procedure.
A small number of withdrawals due to adverse events were reported. Amongst participants treated with ibuprofen 400 mg, two withdrew due to postoperative bleeding (Malmstrom 1999; Zelenakas 2004), one due to soreness and swelling (Fricke 1993), and two due to vomiting (Malmstrom 2004; Singla 2005). One participant treated with ibuprofen 200 mg withdrew due to a headache, although the headache was present before dosing with the study drug (Kiersch 1993). In the five unpublished studies (Edwards 2002), no more than three participants per treatment group withdrew because of adverse events; no further details were given. Amongst participants treated with placebo, three withdrew due to vomiting and anxiety (Cheung 2007) and one due to postoperative bleeding (Frame 1989). One other placebo participant withdrew due to an adverse event that probably occurred during the multiple dose phase of the study (Parker 1986).
Discussion
The original review of 35 studies in 34 reports included 2214 participants on ibuprofen and 1377 on placebo. This updated review doubles the number of studies, and more than doubles the number of participants, with 72 studies in 66 reports including 5804 participants on ibuprofen and 3382 on placebo. Most of the additional information came from 200 mg and 400 mg ibuprofen doses, and the bulk of the information is for these doses. There was a small amount of additional data for 50 mg and 100 mg, but not for doses higher than 400 mg, although 600 mg is a common dose of ibuprofen in some countries. The new information does not substantially change the result for the primary efficacy outcome, but provides more robust estimates with narrower confidence intervals, and permits more detailed subgroup analysis. Additionally, more attention has been paid to use of and time to additional analgesic requirement.
Overall studies were of good methodological quality. Three studies scored only the minimum for inclusion, one point each for stating they were randomised and double blind. It is possible that additional points were lost because of poor reporting rather than poor methods, and excluding these studies from the primary analysis did not change the results. No formal sensitivity analysis was possible. Almost all of the trials were of sufficiently high reporting quality to minimise reporting bias, and the amount of information such as to minimise any possible effect of publication bias.
NNTs for the primary outcome of at least 50% pain relief over 4 to 6 hours showed a trend for increasing efficacy with increasing dose over the range 100 to 400 mg. At doses of 50 and 100 mg, around 30% of those treated experience at least 50% pain relief, compared to about 10 to 15% with placebo. At 200 mg and 400 mg 46% and 55% experience this level of pain relief, giving NNTs of 2.7 (2.5 to 3.0) and 2.5 (2.4 to 2.7) respectively. Limited data for 600 mg and 800 mg are compatible with this trend. In dental studies only, the dose response was more marked, with a statistically significant difference between 200 mg and 400 mg (P < 0.0005), and between 400 mg and 600/800 mg (P = 0.04), although with limited data. Dose response from indirect analyses like these has been confirmed by dose response within trials (McQuay 2007).
Indirect comparisons of NNTs for at least 50% pain relief over 4 to 6 hours in reviews of other analgesics using identical methods indicate that ibuprofen 200 mg has equivalent efficacy to naproxen 500/550 mg (2.7 (2.3 to 3.2)) (Derry C 2009) and lumiracoxib 400 mg (2.7 (2.2 to 3.5)) (Roy 2007), while ibuprofen 400 mg has equivalent efficacy to aspirin 1200 mg (2.4 (1.9 to 3.2)) (Oldman 1999) and oxycodone 10 mg plus paracetamol 650 mg (2.5 (2.0 to 3.3)) (Edwards 2000). Both doses are better than paracetamol 1000 mg (3.6 (3.2 to 4.1)) (Toms 2008), but worse than rofecoxib (2.2 (1.9 to 2.4) (Barden 2005). A current listing of reviews of analgesics in the single dose postoperative pain model can be found at www.medicine.ox.ac.uk/bandolier/index.html.
Comparison of dental and other types of surgery demonstrated lower (better) NNTs for at least 50% pain relief compared with placebo in dental studies. This difference was statistically significant for ibuprofen 400 mg (P < 0.0001), but not 200 mg, where there were only two studies (220 participants) in non‐dental surgery. It has previously been difficult to demonstrate a difference in efficacy between dental and other types of surgery (Barden 2004), although a recent review of diclofenac did demonstrate a similar difference with limited amounts of data (Derry P 2009). It may be that there is indeed a difference, but previous data sets have been too small to show statistical significance. In this review "other" types of surgery were diverse, including orthopaedic, abdominal and hernia surgery, tonsillectomy and episiotomy. Both the extent of the surgery and the context (e.g. perinatal hormonal changes) may influence the perception of pain and make this a highly heterogeneous group. There have never been sufficient data for any one type of "other" surgery to compare with dental surgery.
We carried out all further sensitivity analyses on the large and clinically more homogenous data set of dental studies using ibuprofen 200 and 400 mg. Study size had no statistically significant or consistent effect on efficacy, although as expected, smaller studies gave more variable results (Moore 1998).
A number of studies used ibuprofen preparations that are more soluble than standard ibuprofen, and were developed primarily to speed up absorption and onset of action. We combined these preparations for comparison of efficacy with standard ibuprofen. At both 200 mg and 400 mg the soluble preparations had better efficacy in dental studies (P < 0.0002 and P < 0.0001 respectively). Whether soluble formulations provide important clinical benefits, and whether the pharmacodynamic results accord with pharmacokinetic properties of the different formulations is beyond the scope of this review, but it is important to note the power of the systematic review to reveal these differences.
It has been suggested that data on use of rescue medication, whether as a proportion of participants requiring it, or the median time to use of it, might be helpful in assessing the usefulness of an analgesic, and possibly distinguishing between different doses (Moore 2005). This review demonstrated a non‐significant trend for fewer participants to need rescue medication within 6 hours with higher doses of ibuprofen over the range 50 to 400 mg. For dental studies only, the trend was more obvious, with about 60% using rescue medication with ibuprofen 100 mg, 50% with 200 mg, and 40% with 400 mg, compared with about 80% with placebo over the 6 hour period. It was also possible to demonstrate that the proportion of participants using rescue medication was lower in those treated with "soluble" salts than with standard ibuprofen for the 200 mg and 400 mg doses.
Additionally, the median time to use of rescue medication increased with higher doses, from 4.7 hours with 200 mg to 5.6 hours with 400 mg. Both of these results indicate that the higher doses give more prolonged pain relief than lower doses. Longer duration of action may be advantageous in some circumstances. In a postoperative setting, where patients may feel nauseated, a longer time before remedication is needed may be of benefit to the patient, and it may also reduce demands on time for nursing staff. Duration of action may also be a useful outcome with which to compare different analgesics. The full implications of the importance of remedication as an outcome awaits completion of other reviews, allowing examination of a substantial body of evidence.
Reporting of data for adverse events, withdrawals (other than lack of efficacy) or exclusions, and handling of missing data was not always complete, although it did appear to be better in the more recent studies. Adverse events were collected using various methods (questioning, patient diary) over different periods of time. This may have included periods after the use of rescue medication, which may cause its own adverse events. Poor reporting of adverse events in acute pain trials has been noted before (Edwards 1999). The usefulness of single dose studies for assessing adverse events is questionable, but it is non‐the‐less reassuring that there was no difference between ibuprofen (at any dose) and placebo for occurrence of any adverse event, and that serious adverse events and adverse event withdrawals were rare, and generally not thought to be related to the test drug. Long term multiple dose studies should be used for meaningful analysis of adverse events since, even in acute pain settings, analgesics are likely to be used in multiple doses. The difficulty will be that the postoperative setting is one in which there are many sequelae of surgery and anaesthesia that manifest as adverse events, like nausea, vomiting, or abdominal discomfort, while others, like headache, can be caused by acute caffeine withdrawal over the postoperative period. The main issue is that of rare but serious adverse events, and these are more likely to be found in large observational studies.
Lack of information about withdrawals or exclusions may have led to an overestimate of efficacy, but the effect is probably not significant because it is as likely to be related to poor reporting as poor methods. In single dose studies most exclusions occur for protocol violations such as failing to meet baseline pain requirements, or failing to return for post treatment visits after the acute pain results are concluded. Where patients are treated with a single dose of medication and observed, often "on site" for the duration of the trial, it might be considered unnecessary to report on "withdrawals" if there were none. For missing data it has been shown that over the 4 to 6 hour period, there is no difference between baseline observation carried forward, which gives the more conservative estimate, and last observation carried forward (Moore 2005).
Authors' conclusions
Implications for practice.
This updated review does not change the overall primary estimate of efficacy, the NNT for at least 50% pain relief over 4 to 6 hours compared with placebo, but does demonstrate differences in efficacy with different formulations, and provides additional estimates of efficacy in terms of use of rescue medication.
A single dose of ibuprofen 400 mg is an effective analgesic, providing at least 50% pain relief to over half of the treated patients with acute, moderate to severe, postoperative pain. The NNT of 2.5 for at least 50% pain relief compares favourably with other analgesics commonly used for postoperative pain. In single dose, it is associated with a low rate of adverse events, similar to that with placebo. Lower doses provide slightly lower levels of analgesia. The 200 mg dose has a shorter duration of action. The more soluble salts of ibuprofen appear to offer better analgesia for a longer time. The amount of information available for 200 mg and 400 mg dwarfs almost all other analgesics except paracetamol and aspirin.
Implications for research.
The most important implication for research is to clarify the apparent difference in efficacy between the standard and more soluble preparations of ibuprofen. A preparation with better efficacy than standard ibuprofen may present an opportunity to provide equivalent analgesia at a reduced dose, and potentially improve safety in longer term use. It should always be the goal to use the lowest dose of a drug that provides the desired clinical effect, and lower doses are likely to be associated with fewer adverse events in clinical practice.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 7 July 2017 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 25 April 2012 | Review declared as stable | Although new studies on Ibuprofen may be published, they are unlikely to impact on the results of this review and so the authors suggest there should be no need to update this review for at least five years. |
| 8 February 2011 | Amended | Contact details updated. |
| 6 October 2010 | Amended | Contact details updated. |
| 11 May 2009 | New citation required and conclusions have changed | Information from 37 new studies with 5595 participants was added, giving a total of 72 studies and 9186 participants. NNTs for at least 50% pain relief over 4 to 6 hours were not significantly changed. Additional information on the proportion of participants requiring rescue medication, and median or mean time to use of rescue medication, are provided, with higher doses giving slightly better results. Pain model and ibuprofen formulation may both affect the result, with dental impaction models and soluble ibuprofen salts producing better efficacy estimates. A dose response was demonstrated in dental pain. |
| 11 May 2009 | New search has been performed | The original review published in 1999 was updated and an additional updated search was run prior to publication from January 2009 to May 2009 which found four new studies; two were subsequently excluded, and two are awaiting classification. |
| 23 May 2008 | Amended | Converted to new review format. |
| 25 January 2002 | Amended | New studies found but not yet included or excluded |
Notes
We performed a restricted search in June 2017. We are aware of some additional relevant studies, but given the existing wealth of information, the large numbers of studies and participants, the stability of the efficacy estimate over time, and the fact that due attention has been given to issues over formulation, it is unlikely that any update will change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
Sally Collins, Phil Wiffen and Jayne Rees (Edwards) were authors on the earlier review. For the earlier review Clare Abbott at the Cairns Library, Churchill Hospital, and Catherine Strong (Novartis medical information services) and Darren Bloore (Knoll library) helped with obtaining papers. Laska provided additional information on his study.
Appendices
Appendix 1. Search strategy for MEDLINE via Ovid
1. Ibuprofen.sh 2. (ibuprofen OR brufen OR propionic acid OR isobutylphenyl propionic acid).ti,ab,kw. 3. OR/1‐2 4. Pain, postoperative.sh 5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")).ti,ab,kw. 6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)).ti,ab,kw. 7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")).ti,ab,kw. 8. (("post surg$" or post‐surg$) AND (pain$ or discomfort)).ti,ab,kw. 9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")).ti,ab,kw. 10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")).ti,ab,kw. 11. OR/4‐10 12. randomized controlled trial.pt. 13. controlled clinical trial.pt. 14. randomized.ab. 15. placebo.ab. 16. drug therapy.fs. 17. randomly.ab. 18. trial.ab. 19. groups.ab. 20. OR/12‐19 21. humans.sh. 22. 20 AND 21 23. 3 AND 11 AND 22
Appendix 2. Search strategy for EMBASE via Ovid
1. Ibuprofen.sh 2. (ibuprofen OR brufen OR propionic acid OR isobutylphenyl propionic acid).ti,ab,kw. 3. OR/1‐2 4. Postoperative pain.sh 5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")).ti,ab,kw. 6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)).ti,ab,kw. 7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")).ti,ab,kw. 8. (("post surg$" or post‐surg$) AND (pain$ or discomfort)).ti,ab,kw. 9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")).ti,ab,kw. 10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")).ti,ab,kw. 11. OR/4‐10 12. clinical trials.sh 13. controlled clinical trials.sh 14. randomized controlled trial.sh 15. double‐blind procedure.sh 16. (clin$ adj25 trial$).ab 17. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ab 18. placebo$.ab 19. random$.ab 20. OR/12‐19 21. 3 AND 11 AND 20
Appendix 3. Search strategy for Cochrane CENTRAL
1. MESH descriptor Ibuprofen 2. (ibuprofen OR brufen OR propionic acid OR isobutylphenyl propionic acid).ti,ab,kw. 3. OR/1‐2 4. MESH descriptor Pain, Postoperative 5. ((postoperative adj4 pain$) or (post‐operative adj4 pain$) or post‐operative‐pain$ or (post$ NEAR pain$) or (postoperative adj4 analgesi$) or (post‐operative adj4 analgesi$) or ("post‐operative analgesi$")):ti,ab,kw. 6. ((post‐surgical adj4 pain$) or ("post surgical" adj4 pain$) or (post‐surgery adj4 pain$)):ti,ab,kw. 7. (("pain‐relief after surg$") or ("pain following surg$") or ("pain control after")):ti,ab,kw. 8. (("post surg$" or post‐surg$) AND (pain$ or discomfort)):ti,ab,kw. 9. ((pain$ adj4 "after surg$") or (pain$ adj4 "after operat$") or (pain$ adj4 "follow$ operat$") or (pain$ adj4 "follow$ surg$")):ti,ab,kw. 10. ((analgesi$ adj4 "after surg$") or (analgesi$ adj4 "after operat$") or (analgesi$ adj4 "follow$ operat$") or (analgesi$ adj4 "follow$ surg$")):ti,ab,kw. 11. OR/4‐10 12. Clinical trials:pt. 13. Controlled Clinical Trial:pt. 14. Randomized Controlled Trial.pt. 15. MESH descriptor Double‐Blind Method 16. (clin$ adj25 trial$):ti,ab,kw. 17. ((doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)):ti,ab,kw. 18. placebo$:ti,ab,kw. 19. random$:ti,ab,kw. 20. OR/12‐19 21. 3 AND 11 AND 20
Appendix 4. Glossary
Categorical rating scale:
The commonest is the five category scale (none, slight, moderate, good or lots, and complete). For analysis numbers are given to the verbal categories (for pain intensity, none = 0, mild = 1, moderate = 2 and severe = 3, and for relief none = 0, slight = 1, moderate = 2, good or lots = 3 and complete = 4). Data from different subjects is then combined to produce means (rarely medians) and measures of dispersion (usually standard errors of means). The validity of converting categories into numerical scores was checked by comparison with concurrent visual analogue scale measurements. Good correlation was found, especially between pain relief scales using cross‐modality matching techniques. Results are usually reported as continuous data, mean or median pain relief or intensity. Few studies present results as discrete data, giving the number of participants who report a certain level of pain intensity or relief at any given assessment point. The main advantages of the categorical scales are that they are quick and simple. The small number of descriptors may force the scorer to choose a particular category when none describes the pain satisfactorily.
VAS:
Visual analogue scale: lines with left end labelled "no relief of pain" and right end labelled "complete relief of pain", seem to overcome this limitation. Patients mark the line at the point which corresponds to their pain. The scores are obtained by measuring the distance between the no relief end and the patient's mark, usually in millimetres. The main advantages of VAS are that they are simple and quick to score, avoid imprecise descriptive terms and provide many points from which to choose. More concentration and coordination are needed, which can be difficult post‐operatively or with neurological disorders.
TOTPAR:
Total pain relief (TOTPAR) is calculated as the sum of pain relief scores over a period of time. If a patient had complete pain relief immediately after taking an analgesic, and maintained that level of pain relief for six hours, they would have a six‐hour TOTPAR of the maximum of 24. Differences between pain relief values at the start and end of a measurement period are dealt with by the composite trapezoidal rule. This is a simple method that approximately calculates the definite integral of the area under the pain relief curve by calculating the sum of the areas of several trapezoids that together closely approximate to the area under the curve.
SPID:
Summed pain intensity difference (SPID) is calculated as the sum of the differences between the pain scores over a period of time. Differences between pain intensity values at the start and end of a measurement period are dealt with by the trapezoidal rule.
VAS TOTPAR and VAS SPID are visual analogue versions of TOTPAR and SPID.
See "Measuring pain" in Bandolier's Little Book of Pain, Oxford University Press, Oxford. 2003; pp 7‐13 (Moore 2003).
Data and analyses
Comparison 1. Ibuprofen 50 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 3 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [1.94, 5.12] |
| 2 Participants using rescue medication over 6 hours | 2 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.84] |
| 3 Participants with any adverse event | 2 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.57, 3.00] |
Comparison 2. Ibuprofen 100 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 4 | 396 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.68 [2.29, 5.92] |
| 2 Participants using rescue medication over 6 hours | 3 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.57, 0.84] |
| 3 Participants with any adverse event | 3 | 310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.71, 2.07] |
Comparison 3. Ibuprofen 200 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 20 | 2690 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [3.85, 5.56] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours: type of surgery | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Dental surgery | 18 | 2470 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.48 [3.71, 5.41] |
| 2.2 Other surgery | 2 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.73 [3.24, 18.41] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, all surgery: formulation | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Standard ibuprofen | 17 | 2103 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.11 [4.84, 7.73] |
| 3.2 ibuprofen lysine, arginine, or soluble | 7 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.73 [4.15, 7.90] |
| 4 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: formulation | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Standard ibuprofen | 15 | 1883 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [4.69, 7.62] |
| 4.2 Ibuprofen lysine, arginine or soluble | 7 | 828 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.73 [4.15, 7.90] |
| 5 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: study size | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 40 or more participants | 11 | 1953 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.56 [3.71, 5.61] |
| 5.2 Fewer than 40 participants | 4 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.15 [2.41, 11.00] |
| 6 Participants using rescue medication over 6 hours | 8 | 794 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.57, 0.70] |
| 7 Participants using rescue medication over 6 hours, dental surgery | 7 | 694 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.60, 0.73] |
| 8 Participants using rescue medication over 6 hours, dental surgery: formulation | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Standard ibuprofen | 4 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.66, 0.84] |
| 8.2 Ibuprofen lysine, arginine or soluble | 4 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.48, 0.68] |
| 9 Participants with any adverse event | 14 | 1808 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.71, 1.02] |
Comparison 4. Ibuprofen 400 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 57 | 6475 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.94 [3.58, 4.35] |
| 2 Participants with at least 50% pain relief over 4 to 6 hours: type of surgery | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Dental surgery | 45 | 5428 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.63 [4.13, 5.20] |
| 2.2 Other surgery | 12 | 1047 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [1.81, 2.62] |
| 3 Participants with at least 50% pain relief over 4 to 6 hours, all surgery: formulation | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Standard ibuprofen | 51 | 5604 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.64 [4.14, 5.18] |
| 3.2 Ibuprofen lysine, arginine or soluble | 12 | 1124 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [3.00, 4.56] |
| 4 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: formulation | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Standard ibuprofen | 42 | 4772 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [4.56, 5.87] |
| 4.2 Ibuprofen lysine, arginine or soluble | 9 | 959 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.55 [4.85, 8.85] |
| 5 Participants with at least 50% pain relief over 4 to 6 hours, dental surgery: study size | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 40 or more participants | 15 | 3086 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [3.80, 5.19] |
| 5.2 Fewer than 40 participants | 14 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.06 [3.21, 5.14] |
| 6 Participants using rescue medication over 6 hours | 28 | 2983 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.51, 0.57] |
| 7 Participants using rescue medication over 6 hours, dental surgery | 22 | 2554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.48, 0.55] |
| 8 Participants using rescue medication over 6 hours, dental surgery: formulation | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Standard ibuprofen | 18 | 1857 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.51, 0.59] |
| 8.2 Ibuprofen lysine, arginine or soluble | 6 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.35, 0.50] |
| 9 Participants with any adverse event | 36 | 4865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.82, 1.04] |
Comparison 5. Ibuprofen 600 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 3 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.52, 2.58] |
Comparison 6. Ibuprofen 800 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Participants with at least 50% pain relief over 4 to 6 hours | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [1.72, 3.89] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahlstrom 1993.
| Methods | RCT, DB, DD, single oral dose, 3 parallel groups Medication administered when baseline pain was of least moderate intensity Pain assessed at 0, 20, 40, 60 mins then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 127 (97 valid for analysis) M/F not given Mean age 25 years |
|
| Interventions | Ibuprofen 400 mg, n = 32 Diclofenac (drinkable) 50 mg, n= 35 Placebo n= 30 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Arnold 1990.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins then hourly up to 6 hours. |
|
| Participants | General surgery (including gynaecological and orthopaedic) N = 59 M = 35, F = 24 Age: 22 ‐ 70 years |
|
| Interventions | Ibuprofen 400 mg, n = 15 Ketoprofen 25 mg, n = 14 Ketoprofen 100 mg, n = 16 Placebo, n = 14 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals due to AE |
|
| Notes | Oxford Quality Score: R2, DB2, W0 Rescue medication permitted ‐ no further details. |
|
Bakshi 1994.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of severe intensity Pain assessed at 0, 20, 40, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 257 (245 valid for analysis) M = 151, F = 94 Mean age 28 years |
|
| Interventions | Ibuprofen 400 mg, n = 80 Diclofenac (dispersible) 50 mg, n = 83 Placebo, n = 82 |
|
| Outcomes | PI: std 100 mm VAS PR: std 5 point scale PGE: non‐std 4 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication of patient's choice permitted after 1 hour. |
|
Black 2002.
| Methods | RCT, DB, DD, single then multiple oral dose, 5 parallel groups multicentre Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 5, 10, 15, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 498 M = 219, F = 279 Mean age 22 years |
|
| Interventions | Ibuprofen 200 mg, n = 100 Ibuprofen 400 mg, n = 100 Ibuprofen arginate 200 mg, n = 100 Ibuprofen arginate 400 mg, n = 99 Placebo, n = 99 |
|
| Outcomes | PI: std 5 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication was 2nd dose (or active treatment if in placebo group) as required. |
|
Cheung 2007.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity (≥ 50 mm) Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 12 hours, then at 16, 24 hours. |
|
| Participants | Third molar extraction N = 171 M = 77, F = 94 Mean age 22 years |
|
| Interventions | Ibuprofen 440 mg, n = 57 Celecoxib 400 mg, n = 57 Placebo, n = 57 |
|
| Outcomes | PI: std 4 point scale PR: std 5point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication (not ibuprofen or celecoxib) permitted after 1 hour. |
|
Cooper 1977.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline then hourly up to 4 hours. |
|
| Participants | Third molar extraction N = 245 (192 analysed) M = 83, F = 109 Age 16‐35 years |
|
| Interventions | Ibuprofen 200 mg n = 38 Ibuprofen 400 mg, n = 40 Aspirin 325 mg, n = 37 Aspirin 650 mg, n = 37 Placebo, n= 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Cooper 1982.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline then hourly up to 4 hours. |
|
| Participants | Third molar extraction N = 316 (249 analysed) M = 83, F = 166 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n =38 Ibuprofen 400 mg + Codeine 60 mg, n = 41 Aspirin 650 mg, n = 38 Aspirin 650 mg + codeine 60 mg, n = 45 Codeine 60 mg, n = 41 Placebo, n = 46 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Cooper 1988a.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline then hourly up to 4 hours. |
|
| Participants | Third molar extraction N = 201 M = 59, F = 102 (161 analysed) Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 37 Ketoprofen 100 mg, n = 39 Ketoprofen 25 mg, n = 42 Placebo, n = 43 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Cooper 1989.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 4 hours. |
|
| Participants | Third molar extraction N = 194 (184 analysed for efficacy, 190 for safety) M = 51, F = 133 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 61 Paracetamol 1000 mg, n = 59 Placebo, n = 64 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 1 hour. |
|
Cooper 1996a.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 70 M = 31, F = 39 Mean age 22 years |
|
| Interventions | Ibuprofen 200 mg, n = 19 Misoprostal 200 mg, n = 18 Ibuprofen 200 mg + misoprostal 200 mg, n = 20 Placebo, n = 13 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB1, W0 Rescue medication permitted after 2 hours. |
|
De Miguel Rivero 1997.
| Methods | RCT, DB, DD, single oral or intramuscular dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 10, 20, 30, 45, 60, 90, 120 mins, then hourly up to 5 hours. |
|
| Participants | Total hip replacement N = 103 (106 randomised, 3 did not need medication) M = 47, F = 56 Mean age 62 years |
|
| Interventions | Ibuprofen arginine 400 mg, n = 36 Magnesic dipyrone, 2 g (IM), n = 33 Placebo, n = 34 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Desjardins 2002.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 5, 10, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 225 M = 103, F = 122 Mean age 25 years |
|
| Interventions | Ibuprofen 200 mg, n = 50 Ibuprofen 400 mg, n = 52 Ibuprofen arginate 200 mg, n = 49 Ibuprofen arginate 400 mg, n = 50 Placebo, n = 24 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol plus codeine) permitted after 1.5 hours. |
|
Dionne 1998.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 181 (176 analysed for efficacy) M = 50, F = 126 Mean age 22 years |
|
| Interventions | S(+)‐Ibuprofen 200 mg, n = 51 S(+)‐Ibuprofen 400 mg, n = 50 Ibuprofen (racemic) 400 mg, n = 50 Placebo, n = 25 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale and 100 mm VAS Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted ‐ no further details. |
|
Edwards 2002.
| Methods | Five RCTs, DB, single oral dose, parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 8 hours. |
|
| Participants | Third molar extraction N = 339 M/F nor given Age not given |
|
| Interventions | Ibuprofen 400 mg, n = 338 Placebo, n = 339 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | R2, DB2, W1 Rescue medication permitted ‐ no further details. |
|
Ehrich 1999.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 104 M = 97, F = 5 Mean age 25 years |
|
| Interventions | Ibuprofen 400 mg, n = 20 Rofecoxib 50 mg, n = 32 Rofecoxib 500 mg, n = 20 Placebo, n = 32 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W0 Rescue medication (paracetamol) permitted at any time. |
|
Forbes 1984.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 12 hours. |
|
| Participants | Third molar extraction N = 113 M = 52, F = 57 Mean age 21 years |
|
| Interventions | Ibuprofen 400 mg, n = 28 Fendosal 200 mg, n = 29 Aspirin 650 mg, n = 24 Placebo, n= 28 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Forbes 1990.
| Methods | RCT, DB, single then multiple oral dose, 6 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 6 hours |
|
| Participants | Third molar extraction N = 269 (206 analysed for efficacy, 244 for safety) M = 104, F = 102 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 32 Ketorolac 10 mg, n = 31 Ketorolac 20 mg, n = 35 Paracetamol 600 mg, n = 36 Paracetamol 600 mg + codeine 60 mg, n = 38 Placebo, n = 34 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Forbes 1991a.
| Methods | RCT, DB, single oral dose, 6 parallel groups, multicentre Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 8 hours. |
|
| Participants | Third molar extraction N = 362 (298 analysed for efficacy, 362 for safety) M = 121, F = 177 Mean age 22 years |
|
| Interventions | Ibuprofen 50 mg, n = 57 Ibuprofen 100 mg, n = 49 Ibuprofen 200 mg, n = 48 Ibuprofen 100 mg + Caffeine 100 mg, n = 49 Ibuprofen 200 mg + Caffeine 100 mg, n = 44 Placebo, n = 51 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 2 hours. |
|
Forbes 1991b.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 8 hours |
|
| Participants | Third molar extraction N = 276 (241 analysed for efficacy, 269 for safety) M = 100, F = 141 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 37 Bromfenac 5 mg, n = 39 Bromfenac 10 mg, n = 43 Bromfenac 25 mg, n = 42 Aspirin 650 mg, n = 41 Placebo, n = 39 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Forbes 1992.
| Methods | RCT, DB, single oral dose, 7 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 8 hours. |
|
| Participants | Third molar extraction N = 324 (280 analysed for efficacy, 317 for safety) M = 119, F = 161 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 38 Bromfenac 10 mg, n = 43 Bromfenac 25 mg, n = 41 Bromfenac 50 mg, n = 42 Bromfenac 100 mg, n = 40 Aspirin 650 mg, n = 38 Placebo, n = 38 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Frame 1989.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 5 hours |
|
| Participants | Third molar extraction N = 139 (123 analysed for efficacy) M = 56, F = 92 Mean age 24 years |
|
| Interventions | Ibuprofen 400 mg, n = 42 Dihydrocodeine 30 mg, n = 43 Placebo, n = 38 |
|
| Outcomes | PI: non‐std 9 point scale PR: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication (2nd dose or active drug if placebo group) permitted after 2 hours. |
|
Fricke 1993.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 20, 30, 40, 60 mins, then hourly up to 12 hours. |
|
| Participants | Third molar extraction N = 202 (201 analysed for efficacy) M = 77, F = 124 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 81 Naproxen Na 440 mg, n = 81 Placebo, n = 39 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication permitted after 2 hour. |
|
Gay 1996.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at "regular intervals" up to 6 hours. |
|
| Participants | Third molar extraction N = 206 (204 analysed for efficacy) M = 86, F = 118 Mean age 24 years |
|
| Interventions | Ibuprofen 400 mg, n = 41 Dexketoprofen 5 mg, n = 41 Dexketoprofen 10 mg, n = 42 Dexketoprofen 20 mg, n = 41 Placebo, n = 39 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Heidrich 1985.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Orthopaedic surgery N = 120 M = 72, F = 48 Mean age 31 years |
|
| Interventions | Ibuprofen 400 mg, n = 40 Paracetamol 300 + codeine 30 mg, n = 40 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale and 100 mm VAS Numbers of participants using rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W0 No details about rescue medication. |
|
Hersch 1993a.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 8 hours. |
|
| Participants | Third molar extraction N = 254 M/F not given Age 16+ years |
|
| Interventions | Ibuprofen 200 mg, n = 51 Ibuprofen 400 mg, n = 49 Meclofenamate 100 mg, n = 52 Meclofenamate 50 mg, n = 51 Placebo, n = 51 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W0 Rescue medication permitted after 1 hour. |
|
Hersch 1993b.
| Methods | RCT, DB, pre‐surgery placebo then single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30 60 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 103 (81 analysed) M/F not given Age not given |
|
| Interventions | All participants received preoperative placebo, then: Ibuprofen 400 mg, n = 12 Codeine 60 mg, n = 16 Placebo, n = 16 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Hersh 2000.
| Methods | RCT, DB, DD, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity (≥50 mm) Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 210 M = 66, F = 144 Mean age 24 years |
|
| Interventions | Ibuprofen liquigel 200 mg, n = 61 Ibuprofen liquigel 400 mg, n = 59 Paracetamol 1000 mg, n = 63 Placebo, n = 27 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol+hydrocodone) permitted after 1 hour |
|
Hill 2001.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60, mins, then hourly up to 12 hours. |
|
| Participants | Third molar extraction N = 198 M = 82, F = 116 Mean age 26 years |
|
| Interventions | Ibuprofen 400 mg, n = 49 Pregabalin 50 mg, n = 49 Pregabalin 300 mg, n = 50 Placebo, n = 50 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication permitted ‐ no further details |
|
Jain 1986.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 241 (227 analysed for efficacy) M = 100, F = 127 Mean age 24 years |
|
| Interventions | Ibuprofen 400 mg, n = 49 Ibuprofen 200 mg, n = 47 Ibuprofen 100 mg, n = 39 Aspirin 650 mg, n = 45 Placebo, n = 47 |
|
| Outcomes | PI: non‐std 4 point scale and 100 mm VAS PR: non‐std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 1 hr. |
|
Jain 1988.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Episiotomy N = 161 (147 analysed) All F Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 49 Ibuprofen 200 mg + caffeine 100 mg, n = 50 Placebo, n = 48 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Johnson 1997.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Obstetric and gynaecological surgery N = 238 (236 analysed) All F Mean age 41 years |
|
| Interventions | Ibuprofen 400 mg, n = 48 Paracetamol 650 mg + oxycodone 10 mg, n = 47 Bromfenac 100 mg, n = 48 Bromfenac 50 mg, n = 47 Placebo, n = 48 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (2nd dose or investigator's choice) permitted after 1 hour. |
|
Kiersch 1993.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 20, 30, 40, 60 mins, then hourly up to 12 hours. |
|
| Participants | Third molar extraction N = 205 (203 analysed for efficacy) M = 90, F = 113 Mean age 25 years |
|
| Interventions | Ibuprofen 200 mg, n = 81 Naproxen Na 220 mg, n = 80 Placebo, n = 42 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication permitted after 2 hours. |
|
Laska 1986.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 200 (191 analysed for efficacy) M/F not given Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 39 Ibuprofen 600 mg, n = 36 Ibuprofen 800 mg, n = 39 Aluminium ibuprofen 400 mg, n = 39 Placebo, n = 37 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Laveneziana 1996.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Inguinal hernia N = 124 All M Mean age 50 years |
|
| Interventions | Ibuprofen arginine soluble 400 mg, n = 42 Ketorolac 30 mg, n = 41 Placebo, n = 41 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Malmstrom 1999.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 5, 10, 15, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 272 M = 100, F = 172 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 46 Rofecoxib 50 mg, n = 90 Celecoxib 200 mg, n = 91 Placebo, n = 45 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication (paracetamol ±hydrocodone) permitted after 1.5 hours. |
|
Malmstrom 2002.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 8 hours, then at 10, 12 and 24 hours. |
|
| Participants | Third molar extraction N = 482 M = 124, F = 358 Mean age 22 years |
|
| Interventions | Ibuprofen 400 mg, n = 45 Rofecoxib 50 mg, n = 151 Celecoxib 400 mg, n = 151 Celecoxib 200 mg, n = 90 Placebo, n = 45 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication (paracetamol ± hydrocodone) permitted after 1.5 hours. |
|
Malmstrom 2004.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 8 hours, then at 12 and 24 hours. |
|
| Participants | Third molar extraction N = 398 M = 147, F = 251 Mean age 25 years |
|
| Interventions | Ibuprofen 400 mg, n = 48 Etoricoxib 60 mg, n = 75 Etoricoxib 120 mg, n = 76 Etoricoxib 180 mg, n = 74 Etoricoxib 240 mg, n = 76 Placebo, n = 49 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication (paracetamol ± hydrocodone) permitted at any time. |
|
McQuay 1996.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed up to 6 hours (time points not specified). |
|
| Participants | Third molar extraction N = 161 M = 59, F = 102 Mean age 25 years |
|
| Interventions | Ibuprofen 200 mg, n = 31 Ibuprofen 400 mg, n = 30 Ibuprofen 200 mg + caffeine 50 mg, n = 30 Ibuprofen 200 mg + caffeine 100 mg, n = 30 Ibuprofen 200 mg + caffeine 200 mg, n = 29 Placebo, n = 11 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale and 100 mm VAS PGE: std 5 point scale Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 45 mins. |
|
Medve 2001.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 8 hours. |
|
| Participants | Third molar extraction N = 1197 M = 476, F = 721 Mean age 21 years |
|
| Interventions | Ibuprofen 200 mg, n = 240 Tramadol 37.5 mg, n = 238 Paracetamol 325 mg, n = 240 Tramadol 37.5 mg + paracetamol 325 mg, n = 240 Placebo, n = 239 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Time to use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W0 Rescue medication permitted after 2 hours. |
|
Mehlisch 1990.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Various oral surgery procedures N = 705 (697 analysed for efficacy) M = 277, F = 420 Mean age 31 years |
|
| Interventions | Ibuprofen 400 mg, n = 306 Paracetamol 1000 mg, n = 306 Placebo, n = 85 |
|
| Outcomes | PI: std 4 point scale PR: non‐std 4 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication permitted (time not specified). |
|
Mehlisch 1995.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 240 (239 analysed for efficacy) M = 85, F = 155 Mean age 25 years |
|
| Interventions | Ibuprofen lysine 400 mg, n = 98 Paracetamol 1000 mg, n = 101 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 1 hour (but were encouraged to wait for 4 hrs). |
|
Mehlisch 2002.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 5, 10, 15, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 500 M/F not given Mean age 26 years |
|
| Interventions | Ibuprofen 200 mg, n = 100 Ibuprofen 400 mg, n = 100 Ibuprofen arginine 200 mg, n = 100 Ibuprofen arginine 400 mg, n = 100 Placebo, n = 100 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Morrison 1999.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 5, 10, 15, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 151 M = 75, F = 76 Mean age 18 years |
|
| Interventions | Ibuprofen 400 mg, n = 51 Rofecoxib 50 mg, n = 50 Placebo, n = 50 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1 Rescue medication (paracetamol+hydrocodone) permitted after 1.5 hours. |
|
Nelson 1994.
| Methods | RCT, DB, DD, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 183 (180 analysed for efficacy) M = 72, F = 111 Mean age 24 years |
|
| Interventions | Ibuprofen lysine 200 mg, n = 77 Aspirin 500 mg, n = 65 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 2 hours. |
|
Nørholt 1998.
| Methods | RCT, DB, single oral dose, 2 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 4 hours. |
|
| Participants | Third molar extraction N = 57 M = 21, F = 36 Mean age 24 years |
|
| Interventions | Ibuprofen 400 mg, n = 26 Placebo, n = 31 |
|
| Outcomes | PI: non‐std 5 point scale PR: 5 point scale ‐ reverse wording Numbers of participants using rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol) permitted. |
|
Olson 2001.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 239 M = 76, F = 163 Mean age 23 years |
|
| Interventions | Ibuprofen liquigel 400 mg, n = 67 Ketoprofen 25 mg, n = 67 Paracetamol 1000 mg, n = 66 Placebo, n = 39 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication (standard analgesic) permitted. |
|
Pagnoni 1996.
| Methods | RCT, DB, DD, single oral dose, 3 parallel groups Medication administered when baseline pain intensity was at least 55 mm Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Caesarean section N = 92 All F Mean age 32 years |
|
| Interventions | Ibuprofen arginine soluble 400 mg, n = 30 Ketorolac (IM) 30 mg, n = 30 Placebo, n = 32 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (ketoprofen IM) permitted after 1 hour. |
|
Parker 1986.
| Methods | RCT, DB, single oral dose then multiple doses, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 4 hours. |
|
| Participants | Tonsillectomy N = 139 (110 analysed) M/F not given Age range 16 ‐ 66 years |
|
| Interventions | Ibuprofen syrup 600 mg, n = 44 Aspirin syrup 600 mg, n = 33 Placebo, n = 33 |
|
| Outcomes | PI: non‐std 9 point scale PR: std 5 point scale PGE: std 5 point scale Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W0 Rescue medication (oral or IM) permitted. |
|
Schachtel 1989.
| Methods | RCT, DB, single oral dose then multiple doses, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 4 hours. |
|
| Participants | Episiotomy N = 115 (111 analysed) Mean age 27 years |
|
| Interventions | Ibuprofen 400 mg, n = 36 Paracetamol 1000 mg, n = 37 Placebo, n = 38 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1 Rescue medication permitted after 1 hour. |
|
Schou 1998.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at baseline, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 280 (258 analysed for efficacy) M = 132, F = 126 Mean age 26 years |
|
| Interventions | Ibuprofen 50 mg, n = 51 Ibuprofen 100 mg, n = 53 Ibuprofen 200 mg, n = 49 Ibuprofen 400 mg, n = 49 Placebo, n = 56 |
|
| Outcomes | PI: non‐std 5 point scale PR: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol) permitted after 1 hour. |
|
Schwartz 2007.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity. Pain assessed at 0, 15, 30, 75, 120 mins, then hourly up to 8 hours, then at 12 and 24 hours. |
|
| Participants | Third molar extraction N = 121 M = 65, F = 56 Mean age 23 years |
|
| Interventions | Ibuprofen 400 mg, n = 15 MK‐0703 12.5 mg, n = 31 MK‐0703 50 mg, n = 28 MK‐0703 100 mg, n = 31 Placebo, n = 16 [MK‐0703 is a Cox‐2 selective inhibitor] |
|
| Outcomes | PI: std 4 point scale PR: non‐std 4 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1 Rescue medication (hydrocodone bitartrate plus paracetamol as needed) permitted after 1.5 hours |
|
Seymour 1991 (study 1).
| Methods | RCT, DB, DD, single oral dose, 3 parallel groups Medication administered when baseline pain was more than 30 mm. Pain assessed at 0, 10, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 187 (study 1 and study 2) M = 60, F = 127 Mean age 26 years |
|
| Interventions | Ibuprofen tablets 400 mg, n = 31 Ibuprofen liquid in gelatin capsules 400 mg, n = 32 Placebo n = 32 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals due to adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0 Rescue medication (Co‐codamol) permitted. |
|
Seymour 1991 (study 2).
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain intensity was more than 30 mm. Pain assessed at 0, 10, 20, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 187 (study 1 and study 2) M = 60, F = 127 Mean age 26 years |
|
| Interventions | Ibuprofen tablets 400 mg, n = 30 Ibuprofen soluble 400 mg, n = 32 Placebo, n = 30 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals due to adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0 Rescue medication (Co‐codamol) permitted. |
|
Seymour 1996.
| Methods | RCT, DB, single oral dose, 7 parallel groups Medication administered when baseline pain intensity was at least 30 mm Pain assessed at 0, 10, 20, 30, 45, 60, 75, 90, 120, 150, 180 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 123 (119 analysed) M = 41, F = 78 age range 18‐40 years |
|
| Interventions | Ibuprofen tablets 200 mg, n = 18 Ibuprofen soluble 200 mg, n = 17 Ibuprofen tablets 400 mg, n = 15 Ibuprofen soluble 400 mg, n = 16 Ibuprofen tablets 600 mg, n = 17 Ibuprofen soluble 600 mg, n = 17 Placebo, n = 19 |
|
| Outcomes | PI: std 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol) permitted. |
|
Seymour 1998.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120, 150 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 217 M = 102, F = 115 Mean age 25 years |
|
| Interventions | Ibuprofen 400 mg, n = 76 Aceclofenac 150 mg, n = 71 Placebo, n = 70 |
|
| Outcomes | PI: std 100 mm VAS PR: std 100 mm VAS PGE: non‐std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals due to adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (Co‐proxamol) permitted. |
|
Seymour 1999.
| Methods | RCT, DB, DD, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 122 M = 40, F = 82 Mean age 26 years |
|
| Interventions | Ibuprofen 400 mg, n = 41 WAG 994 1 mg, n = 42 Placebo, n = 39 (WAG is an adenosine agonist) |
|
| Outcomes | PI: std 100 mm VAS PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W0 Rescue medication permitted. |
|
Seymour 2000.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 180 M = 58, F = 122 Mean age 27 years |
|
| Interventions | Ibuprofen 200 mg, n = 59 Buffered ketoprofen 12.5 mg, n = 61 Placebo, n = 50 |
|
| Outcomes | PI: std 4 point scale and 100 mm VAS PR: std 5 point scale PGE: non‐std 4 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (co‐codamol) permitted after 1 hour. |
|
Singla 2005.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Abdominal or pelvic surgery N = 455 All F Mean age 42 years |
|
| Interventions | Ibuprofen 400 mg, n = 175 Ibuprofen 400 mg + oxycodone 5 mg, n = 169 Oxycodone 5 mg, n = 52 Placebo, n = 60 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1 Rescue medication permitted. |
|
Sunshine 1983.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 4 hours. |
|
| Participants | Episiotomy N = 120 All F Mean age 24 years |
|
| Interventions | Ibuprofen 400 mg, n = 30 Aspirin 600 mg, n = 30 Zomepirac 100 mg, n = 30 Placebo, n = 30 |
|
| Outcomes | PI: std 4 point scale PR: non‐std 5 point scale PGE: non‐std 4 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Sunshine 1987.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 4 hours. |
|
| Participants | Episiotomy , caesarean section or gynaecological surgery N = 200 All F Mean age 26 years |
|
| Interventions | Ibuprofen 400 mg, n = 38 Ibuprofen 200 mg + codeine 30 mg, n = 40 Ibuprofen 400 mg + codeine 60 mg, n = 40 Codeine 60 mg, n = 37 Placebo, n = 40 |
|
| Outcomes | PI: std 4 point scale PR: non‐std 5 point scale PGE: non‐std 4 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Sunshine 1996.
| Methods | RCT, DB, single oral dose, 6 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Episiotomy N = 305 All F Mean age 23 years |
|
| Interventions | Ibuprofen 50 mg, n = 51 Ibuprofen 100 mg, n = 51 Ibuprofen 200 mg, n = 50 Ibuprofen 100 mg + caffeine 100 mg, n = 50 Ibuprofen 200 mg + caffeine 100 mg, n = 50 Placebo, n = 50 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non‐std 4 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Sunshine 1997.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 30, 60 mins, then hourly up to 6 hours. |
|
| Participants | Caesarian section or gynaecological surgery N = 120 All F Mean age 28 years |
|
| Interventions | Ibuprofen 400 mg, n = 40 Ibuprofen 400 mg + hydrocodone 15 mg, n = 40 Placebo, n = 39 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted. |
|
Sunshine 1998.
| Methods | RCT, DB, single oral dose, 5 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 179 (175 analysed for efficacy) M = 58, F = 117 Mean age 22 years |
|
| Interventions | Ibuprofen 200 mg, n = 35 Ketoprofen 6.25 mg, n = 35 Ketoprofen 12.5 mg, n = 35 Ketoprofen 25 mg, n = 35 Placebo, n = 35 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale and 100 mm VAS PGE: non‐std 4 point scale Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol) permitted after 1 hour. |
|
Van Dyke 2004.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 498 M = 219, F = 279 Mean age 25 years |
|
| Interventions | Ibuprofen 400 mg, n = 186 Ibuprofen 400 mg + oxycodone 5 mg, n = 187 Oxycodone 5 mg, n = 63 Placebo, n = 62 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1 Rescue medication permitted after 2 hours. |
|
Wahl 1997.
| Methods | RCT, DB, single oral dose, 3 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 6 hours. |
|
| Participants | Third molar extraction N = 164 (163 analysed for efficacy) M = 88, F = 75 Mean age 27 years |
|
| Interventions | Ibuprofen lysinate 342 mg (=200 mg Ibu), n = 74 Paracetamol 200 mg + aspirin 250 mg + caffeine 50 mg, n = 73 Placebo, n = 42 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: non‐std 6 point scale Numbers of participants using rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Rescue medication permitted. |
|
Wideman 1999 (study 1).
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 20, 40, 60, 80, 100, 120, 150, 180 mins, then hourly up to 7 hours. |
|
| Participants | Abdominal or gynaecological surgery N = 240 All F Mean age 39 years |
|
| Interventions | Ibuprofen 200 mg, n = 60 Ibuprofen 200 mg, + hydrocodone 7.5 mg, n = 59 Hydrocodone 7.5 mg, n = 61 Placebo, n = 60 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Wideman 1999 (study 2).
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 20, 40, 60, 80, 100, 120, 150, 180 mins, then hourly up to 8 hours. |
|
| Participants | Abdominal or gynaecological surgery N = 201 All F Mean age 40 years |
|
| Interventions | Ibuprofen 400 mg, n = 50 Ibuprofen 400 mg + hydrocodone 15 mg, n = 50 Hydrocodone 15 mg, n = 50 Placebo, n = 51 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication permitted after 1 hour. |
|
Zelenakas 2004.
| Methods | RCT, DB, single oral dose, 4 parallel groups Medication administered when baseline pain was of moderate to severe intensity Pain assessed at 0, 15, 30, 45, 60, 90, 120 mins, then hourly up to 12 hours. |
|
| Participants | Third molar extraction N = 202 M = 77, F = 125 Mean age 22 years |
|
| Interventions | Ibuprofen 400 mg, n = 51 Lumiracoxib 100 mg, n = 51 Lumiracoxib 400 mg, n = 50 Placebo, n = 50 |
|
| Outcomes | PI: std 4 point scale PR: std 5 point scale and 100 mm VAS PGE: std 5 point scale Numbers of participants using rescue medication Time to use of rescue medication Numbers with any adverse event Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 Rescue medication (paracetamol+hydrocodone) permitted after 1 hour. |
|
DB ‐ double blind; DD ‐ double dummy; PGE ‐ patient global evaluation of efficacy; PI ‐ pain intensity; PR ‐ pain relief; R ‐ randomised; RCT ‐ randomised controlled trial; std ‐ standard; W ‐ withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahlstrom 1989 | No placebo control. |
| Akural 2009 | Medication administered preoperativley. |
| Anaokar 1993 | No placebo control. |
| Apaydin 1994 | No placebo control. |
| Apiou 1988 | No placebo control. |
| Aranda 1989 | No placebo control. |
| Averbuch 2000 | Not an original report of trials, and unable to ensure that participants (total of 75 treated with ibuprofen) were not in another included study. |
| Bailey 1993 | No placebo control. |
| Behotas 1992 | Baseline pain intensity was not moderate to severe. |
| Bhounsule 1990 | Did not state whether patients had a baseline pain of at least moderate intensity. |
| Biehl 1981 | No placebo control. |
| Bloomfield 1974 | Used 5 point pain intensity scale which is not validated for the data extraction method. |
| Calanchini 1991 | No placebo control. |
| Carlos 1984 | Could not be obtained despite attempts to contact the authors, ordering through the British Library and help from the librarians at Novartis and Knoll pharmaceuticals. |
| Carrillo 1990 | Did not state when the interventions were administered but as the pain levels were recorded for the first 4 hours following surgery it may be assumed that they were given immediately postoperatively. Therefore insufficient baseline pain. |
| Chopra 2009 | Medication administered at 1 hour, irrespective of baseline pain. |
| Cooper 1984 | Inadequate description of method. Did not state whether interventions were randomly allocated or studies were double‐blind. |
| Cooper 1988b | Inadequate description of method. Did not state whether interventions were randomly allocated. |
| Cooper 1993 | No placebo control. |
| Cooper 1996b | Intervention administered irrespective of postoperative baseline pain. |
| Darsow 1988 | No direct pain outcome measured over the first 4‐6 hours (recorded motility etc in the days following surgery). |
| Dorfmann 1991 | Inadequate description of method. Did not say whether the allocation was randomised, did not say when the interventions were administered postoperatively, no mention of the level of baseline pain and did not define the pain measurement used. |
| Doyle 2002 | No usable data for ibuprofen treatment arm. |
| El‐Tanany 1993 | No placebo control. |
| Fleiss 1979 | Take medication "if experience pain". Cannot assume that all the patients included had a baseline pain of >moderate intensity. |
| Forbes 1991 | Used a controlled release formulation of ibuprofen. |
| Frezza 1985 | Could not be obtained despite attempts to contact the authors, ordering through the British Library and help from the librarians at Novartis and Knoll pharmaceuticals. |
| Gallardo 1980 | Baseline pain intensity not moderate to severe. |
| Gallardo 1981 | Data was only collected for three hours. |
| Garwood 1983 | No placebo control. |
| Giles 1981 | No placebo control. |
| Giles 1985 | Did not state which scale was used. |
| Hazra 1982 | Baseline pain intensity not moderate to severe. |
| Henderson 1994 | No placebo control. |
| Henrikson 1982 | Only presented the data for the placebo arm for the first hour. |
| Henrikson 1985 | No placebo control. |
| Hopkinson 1980 | Five point pain intensity scale and 5 point pain relief scale (including "worse") neither of which are validated for the data extraction method used. Global evaluation was the opinion of the investigators rather than the patient. |
| Hultin 1978 | Cross‐over study with the first dose administered exactly 1 hour after the local anaesthetic rather than when the patient experienced at least moderate pain. |
| Hyrkas 1992 | Intervention administered preoperatively. Therefore inadequate baseline pain. |
| Hyrkas 1993 | Intervention administered preoperatively. Therefore inadequate baseline pain. |
| Hyrkas 1994 | Intervention administered preoperatively. Therefore inadequate baseline pain. |
| Iles 1980 | Data was only presented for one hour after administration of the interventions. |
| Iqbal 1986 | Could not be obtained despite attempts to contact the authors, ordering through the British Library and help from the librarians at Novartis and Knoll pharmaceuticals. |
| Iwabuchi 1980 | No placebo control. |
| Joubert 1977 | Could not be obtained despite attempts to contact the authors, ordering through the British Library and help from the librarians at Novartis and Knoll pharmaceuticals. |
| Katharia 1992 | Multiple dose study with no separate analysis of the first dose. |
| Khan 1992 | No placebo control. |
| Kittala 1972 | Intervention routinely administered to all participants irrespective of level of baseline pain. |
| Klein 1994 | Abstract. |
| Mastronardi 1988 | No placebo control. |
| Matthews 1984 | First dose was administered immediately postoperatively irrespective of patients level of pain. |
| McEvoy 1996 | No placebo control. |
| McQuay 1993 | No placebo control. |
| Movilia 1990 | First dose was administered immediately postoperatively irrespective of patients level of pain. |
| Nakanishi 1990 | No placebo control. |
| Negm 1989 | Included participants who took the medication when they were experiencing only mild pain. |
| Rondeau 1980 | Baseline pain intensity not moderate to severe. |
| Rossi 1981 | Inadequate description of method. Did not state whether study was double blind. Also data was only recorded for three hours. |
| Schleier 2007 | No placebo control. |
| Shimura 1981 | No placebo control. |
| Squires 1981 | Intervention administered preoperatively. Therefore inadequate baseline pain. |
| Tai 1992 | No placebo control. |
| Tani 1974 | No placebo control. |
| Tesseroli 1986 | The only measure of pain which was in the opinion of the patient rather than the investigator was the pain intensity VAS. At baseline, the mean VAS minus 1.96 x SD was less than 30 mm, therefore some patients included may have had a baseline pain intensity of less than moderate. |
| Troullos 1990 | Intervention administered preoperatively. Therefore inadequate baseline pain. |
| Turcotte 1986 | Baseline pain intensity not moderate to severe. |
| Van Der Zwan 1982 | No direct pain outcome measurement used, pain assessed by analgesic intake. |
| Van Wering 1972 | No placebo control. |
| Vigneron 1977 | Could not be obtained despite attempts to contact the authors, ordering through the British Library and help from the librarians at Novartis and Knoll pharmaceuticals. |
| Vogel 1984 | Combined the data from separate arms of a cross‐over trial into one data set. |
| Von Mayer 1980 | Multiple dose study with no mention of the level of baseline pain. |
| Walker 1976 | No placebo control. |
| Walton 1990 | Intervention administered preoperatively. Therefore inadequate baseline pain. |
| Walton 1993 | First dose was given im during surgery, then oral doses were given postoperatively at specified times rather than when patients had baseline pain of at least moderate intensity. |
| Weber 1990 | No placebo control. |
| Winter 1978 | Baseline pain intensity not moderate to severe. |
| Wuolijoki 1987 | Interventions were administered either pre‐operatively or immediately post‐operatively. Therefore insufficient baseline pain. |
Characteristics of studies awaiting assessment [ordered by study ID]
Daniels 2009.
| Methods | RCT, DB, DD single dose |
| Participants | Third molar extraction, experiencing moderate to severe postoperative pain |
| Interventions | Sodium ibuprofen 512 mg, ibuprofen/poloxamer 400 mg/60 mg, paracetamol 1000 mg, placebo |
| Outcomes | Pain intensity and pain relief, onset of pain relief, tolerability |
| Notes |
Kleinert 2008.
| Methods | RCT, DB, single dose |
| Participants | Mandibular third molar extraction, experiencing moderate to severe postoperative pain |
| Interventions | Tapentodol HCl 25 mg, 50 mg, 75 mg, 100 mg, 200 mg, morphine sulphate 60 mg, ibuprofen 400 mg, placebo |
| Outcomes | Pain relief, adverse events |
| Notes |
Differences between protocol and review
There are no major differences between the protocol and review.
Contributions of authors
CD and SD carried out searches, identified studies for inclusion, and carried out data extraction and analysis. SD entered the data into RevMan. RAM was involved with analysis and writing. HJM acted as arbitrator and was involved with writing. SD will be responsible for conducting the next update of this review.
Sources of support
Internal sources
Oxford Pain Research funds, UK.
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
NIHR Biomedical Research Centre Programme, UK.
Declarations of interest
RAM and HJM have consulted for various pharmaceutical companies. RAM and HJM have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM, HJM and SD have received research support from charities, government and industry sources at various times. Support for this review came from Oxford Pain Research, the NHS Cochrane Collaboration Programme Grant Scheme, and NIHR Biomedical Research Centre Programme.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Ahlstrom 1993 {published data only}
- Ahlstrom U, Bakshi R, Nilsson P, Wahlander L. The analgesic efficacy of diclofenac dispersible and ibuprofen in postoperative pain after dental extraction. European Journal of Clinical Pharmacology 1993;44(6):587‐8. [DOI] [PubMed] [Google Scholar]
Arnold 1990 {published data only}
- Arnold JD. Ketoprofen, ibuprofen, and placebo in the relief of postoperative pain. Advances in Therapy 1990;7(5):264‐75. [Google Scholar]
Bakshi 1994 {published data only}
- Bakshi R, Frenkel G, Dietlein G, Meurer Witt B, Schneider B, et al. A placebo‐controlled comparative evaluation of diclofenac dispersible versus ibuprofen in postoperative pain after third molar surgery. The Journal of Clinical Pharmacology 1994;34(3):225‐30. [DOI] [PubMed] [Google Scholar]
Black 2002 {published data only}
- Black P, Max MB, Desjardins P, Norwood T, Ardia A, Pallotta T. A randomized, double‐blind, placebo‐controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clinical Therapeutics 2002;24(7):1072‐89. [DOI] [PubMed] [Google Scholar]
Cheung 2007 {published data only}
- Cheung R, Krishnaswami S, Kowalski K. Analgesic efficacy of celecoxib in postoperative oral surgery pain: a single‐dose, two‐center, randomized, double‐blind, active‐ and placebo‐controlled study. Clinical Therapeutics 2007;29(Suppl):2498‐510. [DOI] [PubMed] [Google Scholar]
Cooper 1977 {published data only}
- Cooper SA, Needle SE, Kruger GO. Comparative analgesic potency of aspirin and ibuprofen. Journal of Oral Surgery 1977;35(11):898‐903. [PubMed] [Google Scholar]
Cooper 1982 {published data only}
- Cooper SA, Engel J, Ladov M, Precheur H, Rosenheck A, Rauch D. Analgesic efficacy of an ibuprofen codeine combination. Pharmacotherapy 1982;2(3):162‐7. [DOI] [PubMed] [Google Scholar]
Cooper 1988a {published data only}
- Cooper SA, Berrie R, Cohn P. Comparison of ketoprofen, ibuprofen, and placebo in a dental surgery pain model. Advances in Therapy 1988;5:43‐53. [Google Scholar]
Cooper 1989 {published data only}
- Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double blind, placebo controlled study. The Journal of Clinical Pharmacology 1989;29(11):1026‐30. [DOI] [PubMed] [Google Scholar]
Cooper 1996a {published data only}
- Cooper SA, Cowan A, Tallarida RJ, Hargreaves K, Roszkowski M, Jamali F, et al. The analgesic interaction of misoprostol with nonsteroidal anti‐Inflammatory drugs. American Journal of Therapeutics 1996;3(4):261‐7. [DOI] [PubMed] [Google Scholar]
De Miguel Rivero 1997 {published data only}
- Miguel Rivero C, Garcia Araujo C, Mella Sousa M. Comparative efficacy of oral ibuprofen‐arginine, intramuscular magnesic dipyrone and placebo in patients with postoperative pain following total hip replacement. Clinical Drug Investigation 1997;14(4):276‐85. [Google Scholar]
Desjardins 2002 {published data only}
- Desjardins P, Black P, Papageorge M, Norwood T, Shen DD, Norris L, et al. Ibuprofen arginate provides effective relief from postoperative dental pain with a more rapid onset of action than ibuprofen. European Journal of Clinical Pharmacology 2002;58(6):387‐94. [DOI] [PubMed] [Google Scholar]
Dionne 1998 {published data only}
- Dionne RA, McCullagh L. Enhanced analgesia and suppression of plasma B‐endorphin by the S(+)‐isomer of ibuprofen. Clinical Pharmacology and Therapeutics 1998;63(5):694‐701. [DOI] [PubMed] [Google Scholar]
Edwards 2002 {published data only}
- Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: individual patient data meta‐analysis of single‐dose oral tramadol plus acetaminophen in acute postoperative pain. Journal of Pain and Symptom Management 2002;23(2):121‐30. [DOI: 10.1016/S0885-3924(01)00404-3] [DOI] [PubMed] [Google Scholar]
Ehrich 1999 {published data only}
- Ehrich EW, Dallob A, Lepeleire I, Hecken A, Riendeau D, Yuan W, et al. Characterization of rofecoxib as a cyclooxygenase‐2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clinical Pharmacology and Therapeutics 1999;65(3):336‐47. [DOI] [PubMed] [Google Scholar]
Forbes 1984 {published data only}
- Forbes JA, Barkaszi BA, Ragland RN, Hankle JJ. Analgesic effect of fendosal, ibuprofen and aspirin in postoperative oral surgery pain. Pharmacotherapy 1984;4(6):385‐91. [DOI] [PubMed] [Google Scholar]
Forbes 1990 {published data only}
- Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen codeine combination in postoperative oral surgery pain. Pharmacotherapy 1990;10(6(Pt 2)):94S‐105S. [PubMed] [Google Scholar]
Forbes 1991a {published data only}
- Forbes JA, Beaver WT, Jones KF, Kehm CJ, Smith WK, Gongloff CM, et al. Effect of caffeine on ibuprofen analgesia in postoperative oral surgery pain. Clinical Pharmacology and Therapeutics 1991;49(6):674‐84. [DOI] [PubMed] [Google Scholar]
Forbes 1991b {published data only}
- Forbes JA, Edquist IA, Smith FG, Schwartz MK, Beaver WT. Evaluation of bromfenac, aspirin, and ibuprofen in postoperative oral surgery pain. Pharmacotherapy 1991;11(1):64‐70. [PubMed] [Google Scholar]
Forbes 1992 {published data only}
- Forbes JA, Beaver WT, Jones KF, Edquist IA, Gongloff CM, Smith WK, et al. Analgesic efficacy of bromfenac, ibuprofen, and aspirin in postoperative oral surgery pain. Clinical Pharmacology and Therapeutics 1992;51(3):343‐52. [DOI] [PubMed] [Google Scholar]
Frame 1989 {published data only}
- Frame JW, Evans CR, Flaum GR, Langford R, Rout PG. A comparison of ibuprofen and dihydrocodeine in relieving pain following wisdom teeth removal. British Dental Journal 1989;166(4):121‐4. [DOI] [PubMed] [Google Scholar]
Fricke 1993 {published data only}
- Fricke JR, Halladay SC, Francisco CA. Efficacy and safety of naproxen sodium and ibuprofen for pain relief after oral surgery. Current Therapeutic Research 1993;54(6):619‐27. [Google Scholar]
Gay 1996 {published data only}
- Gay C, Planas E, Donado M, Martinez JM, Artigas R, Torres F, et al. Analgesic efficacy of low doses of dexketoprofen in the dental pain model: A randomised, double‐blind, placebo‐controlled study. Clinical Drug Investigation 1996;11(6):320‐30. [Google Scholar]
Heidrich 1985 {published data only}
- Heidrich G, Slavic Svircev V, Kaiko RF. Efficacy and quality of ibuprofen and acetaminophen plus codeine analgesia. Pain 1985;22(4):385‐97. [DOI] [PubMed] [Google Scholar]
Hersch 1993a {published data only}
- Hersh EV, Cooper S, Betts N, Wedell D, MacAfee K, Quinn P, et al. Single dose and multidose analgesic study of ibuprofen and meclofenamate sodium after third molar surgery. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology 1993;76(6):680‐7. [DOI] [PubMed] [Google Scholar]
Hersch 1993b {published data only}
- Hersh EV, Ochs H, Quinn P, MacAfee K, Cooper SA. Narcotic receptor blockade and its effect on the analgesic response to placebo and ibuprofen after oral surgery. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology 1993;75(5):539‐46. [DOI] [PubMed] [Google Scholar]
Hersh 2000 {published data only}
- Hersh EV, Levin LM, Cooper SA, Doyle G, Waksman J, Wedell D, et al. Ibuprofen liquigel for oral surgery pain. Clinical Therapeutics 2000;22(11):1306‐18. [DOI] [PubMed] [Google Scholar]
Hill 2001 {published data only}
- Hill CM, Balkenohl M, Thomas DW, Walker R, Math? H, Murray G. Pregabalin in patients with postoperative dental pain. European Journal of Pain 2001;5(2):119‐24. [DOI] [PubMed] [Google Scholar]
Jain 1986 {published data only}
- Jain AK, Ryan JR, McMahon FG, Kuebel JO, Walters PJ, Noveck C. Analgesic efficacy of low dose ibuprofen in dental extraction pain. Pharmacotherapy 1986;6(6):318‐22. [DOI] [PubMed] [Google Scholar]
Jain 1988 {published data only}
- Jain AK, Mcmahon FG, Ryan JR, Narcisse C. A double‐blind study of ibuprofen 200 mg in combination with caffeine 100 mg, ibuprofen 400 mg, and placebo in episiotomy pain. Current Therapeutic Research 1988;43(4):762‐79. [Google Scholar]
Johnson 1997 {published data only}
- Johnson GH, Wagoner JD, Brown J, Cooper SA. Bromfenac sodium, acetaminophen/oxycodone, ibuprofen, and placebo for relief of postoperative pain. Clinical Therapeutics 1997;19(3):507‐19. [DOI] [PubMed] [Google Scholar]
Kiersch 1993 {published data only}
- Kiersch TA, Halladay SC, Koschik M. A double‐blind, randomized study of naproxen sodium, ibuprofen, and placebo in postoperative dental pain. Clinical Therapeutics 1993;15(5):845‐54. [PubMed] [Google Scholar]
Laska 1986 {published data only}
- Laska EM, Sunshine A, Marrero I, Olson N, Siegel C, McCormick N. The correlation between blood levels of ibuprofen and clinical analgesic response. Clinical Pharmacology & Therapeutics 1986;40(1):1‐7. [DOI] [PubMed] [Google Scholar]
Laveneziana 1996 {published data only}
- Laveneziana D, Riva A, Bonazzi M, Cipolla M, Migliavacca S. Comparative efficacy of oral ibuprofen arginine and intramuscular ketorolac in patients with postoperative pain. Clinical Drug Investigation 1996;11:8‐14. [Google Scholar]
Malmstrom 1999 {published data only}
- Malmstrom K, Daniels S, Kotey P, Seidenberg BC, Desjardins PJ. Comparison of rofecoxib and celecoxib, two cyclooxygenase‐2 inhibitors, in postoperative dental pain: a randomized, placebo‐ and active‐comparator‐controlled clinical trial. Clinical Therapeutics 1999;21(10):1653‐63. [DOI] [PubMed] [Google Scholar]
Malmstrom 2002 {published data only}
- Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B. A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single‐center, randomized, double‐blind, placebo‐ and active‐comparator‐controlled, parallel‐group, single‐dose study using the dental impaction pain model. Clinical Therapeutics 2002;24(10):1549‐60. [DOI] [PubMed] [Google Scholar]
Malmstrom 2004 {published data only}
- Malmstrom K, Sapre A, Couglin H, Agrawal NG, Mazenko RS, Fricke JR Jr. Etoricoxib in acute pain associated with dental surgery: a randomized, double‐blind, placebo‐ and active comparator‐controlled dose‐ranging study. Clinical Therapeutics 2004;26(5):667‐79. [DOI] [PubMed] [Google Scholar]
McQuay 1996 {published data only}
- McQuay HJ, Angell K, Carroll D, Moore RA, Juniper RP. Ibuprofen compared with ibuprofen plus caffeine after third molar surgery. Pain 1996;66:247‐51. [DOI] [PubMed] [Google Scholar]
Medve 2001 {published data only}
- Medve RA, Wang J, Karim R. Tramadol and acetaminophen tablets for dental pain. Anesthesia Progress 2001;48(3):79‐81. [PMC free article] [PubMed] [Google Scholar]
Mehlisch 1990 {published data only}
- Mehlisch DR, Sollecito WA, Helfrick JF, Leibold DG, Markowitz R, Schow CEJr, et al. Multicenter clinical trial of ibuprofen and acetaminophen in the treatment of postoperative dental pain. he Journal of the American Dental Association 1990;121(2):257‐63. [DOI] [PubMed] [Google Scholar]
Mehlisch 1995 {published data only}
- Mehlisch DR, Jasper RD, Brown P, Korn SH, McCarroll K, Murakami AA. Comparative study of ibuprofen lysine and acetaminophen in patients with postoperative dental pain. Clinical Therapeutics 1995;17:852‐60. [DOI] [PubMed] [Google Scholar]
Mehlisch 2002 {published data only}
- Mehlisch DR, Ardia A, Pallotta T. Analgesic efficacy of the cyclooxygenase‐2‐specific inhibitor rofecoxib in post‐dental surgery pain: a randomized, controlled trial. Clinical Therapeutics 2002;21(6):943‐53. [DOI] [PubMed] [Google Scholar]
Morrison 1999 {published data only}
- Morrison BW, Christensen S, Yuan W, Brown J, Amlani S, Seidenberg B. Analgesic efficacy of the cyclooxygenase‐2‐specific inhibitor rofecoxib in post‐dental surgery pain: a randomized, controlled trial. Clinical Therapeutics 1999;21(6):943‐53. [DOI] [PubMed] [Google Scholar]
Nelson 1994 {published data only}
- Nelson SL, Brahim JS, Korn SH, Greene SS, Suchower LJ. Comparison of single‐dose ibuprofen lysine, acetylsalicylic acid, and placebo for moderate‐to‐severe postoperative dental pain. Clinical Therapeutics 1994;16(3):458‐65. [PubMed] [Google Scholar]
Nørholt 1998 {published data only}
- Nørholt SE, Aagaard E, Svensson P, Sindet‐Pedersen S. Evaluation of trismus, bite force, and pressure algometry after third molar surgery: a placebo‐controlled study of ibuprofen. Journal of Oral and Maxillofacial Surgery 1998;56(4):420‐7. [DOI] [PubMed] [Google Scholar]
Olson 2001 {published data only}
- Olson NZ, Otero AM, Marrero I, Tirado S, Cooper S, Doyle G, Jayawardena S, Sunshine A. Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain. Journal of Clinical Pharmacology 2001;41(11):1238‐47. [DOI] [PubMed] [Google Scholar]
Pagnoni 1996 {published data only}
- Pagnoni B, Ravanelli A, Degrai L, Rossi R, Tiengo M. Clinical efficacy of ibuprofen arginine in the management of postoperative pain associated with suction termination of pregnancy. A double blind placebo controlled study. Clinical Drug Investigation 1996;11:27‐32. [Google Scholar]
Parker 1986 {published data only}
- Parker DA, Gibbin KP, Noyelle RM. Syrup formulations for post tonsillectomy analgesia: a double blind study comparing ibuprofen, aspirin and placebo. The Journal of Laryngology and Otology 1986;100(9):1055‐60. [DOI] [PubMed] [Google Scholar]
Schachtel 1989 {published data only}
- Schachtel BP, Thoden WR, Baybutt RI. Ibuprofen and acetaminophen in the relief of postpartum episiotomy pain. The Journal of Clinical Pharmacology 1989;29(6):550‐3. [DOI] [PubMed] [Google Scholar]
Schou 1998 {published data only}
- Schou S, Nielsen H, Nattestad A, Hillerup S, Ritzau M, Branebjerg PE, et al. Analgesic dose‐response relationship of Ibuprofen 50, 100, 200 and 400 mg after surgical removal of third molars: a single‐dose, randomized, placebo‐controlled, and double‐blind study of 304 patients. The Journal of Clinical Pharmacology 1998;38:447‐54. [DOI] [PubMed] [Google Scholar]
Schwartz 2007 {published data only}
- Schwartz JI, Kotey PN, Fricke JR Jr, Gottesdiener K. MK‐0703 (a cyclooxygenase‐2 inhibitor) in acute pain associated with dental surgery: a randomized, double‐blind, placebo‐ and active comparator‐controlled dose‐ranging study. American Journal of Therapeutics 2007;14(1):13‐9. [DOI] [PubMed] [Google Scholar]
Seymour 1991 (study 1) {published data only}
- Seymour RA, Hawkesford JE, Weldon M, Brewster D. An evaluation of different ibuprofen preparations in the control of postoperative pain after third molar surgery. British Journal of Clinical Pharmacology 1991;31(1):83‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 1991 (study 2) {published data only}
- Seymour RA, Hawkesford JE, Weldon M, Brewster D. An evaluation of different ibuprofen preparations in the control of postoperative pain after third molar surgery. British Journal of Clinical Pharmacology 1991;31(1):83‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 1996 {published data only}
- Seymour RA, Ward Booth P, Kelly PJ. Evaluation of different doses of soluble ibuprofen and ibuprofen tablets in postoperative dental pain. The British Journal of Oral and Maxillofacial Surgery 1996;34(1):110‐4. [DOI] [PubMed] [Google Scholar]
Seymour 1998 {published data only}
- Seymour RA, Frame J, Negus TW, Hawkesford JE, Marsden J, Matthew IR. The comparative efficacy of aceclofenac and ibuprofen in postoperative pain after third molar surgery. British Journal of Oral and Maxillofacial Surgery 1998;36(5):375‐9. [DOI] [PubMed] [Google Scholar]
Seymour 1999 {published data only}
- Seymour RA, Hawkesford JE, Hill CM, Frame J, Andrews C. The efficacy of a novel adenosine agonist (WAG 994) in postoperative dental pain. British Journal of Clinical Pharmacology 1999;47(6):675‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seymour 2000 {published data only}
- Seymour RA, Watkinson H, Hawkesford JE, Moore U. The efficacy of buffered ketoprofen in postoperative pain after third molar surgery. European Journal of Clinical Pharmacology 2000;55(11‐12):801‐6. [DOI] [PubMed] [Google Scholar]
Singla 2005 {published data only}
- Singla N, Pong A, Newman K, MD‐10 Study Group. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of pain after abdominal or pelvic surgery in women: a randomized, double‐blind, placebo‐ and active‐controlled parallel‐group study. Clinical Therapeutics 2005;27(1):45‐57. [DOI] [PubMed] [Google Scholar]
Sunshine 1983 {published data only}
- Sunshine A, Olson NZ, Laska EM, Zighelboim I, Castro A, Sarrazin C. Ibuprofen, zomepirac, aspirin, and placebo in the relief of postepisiotomy pain. Clinical Pharmacology and Therapeutics 1983;34(2):254‐8. [DOI] [PubMed] [Google Scholar]
Sunshine 1987 {published data only}
- Sunshine A, Roure C, Olson N, Laska EM, Zorrilla C, Rivera J. Analgesic efficacy of two ibuprofen codeine combinations for the treatment of postepisiotomy and postoperative pain. Clinical Pharmacology and Therapeutics 1987;42(4):374‐80. [DOI] [PubMed] [Google Scholar]
Sunshine 1996 {published data only}
- Sunshine A, Zighelboim I, Bartizek RD. A double‐blind, placebo‐controlled, single‐dose comparison study of ibuprofen, and ibuprofen in combination with caffeine, in the treatment of postopisiotomy pain. Royal Society of Medicine 1996;218:105‐88. [Google Scholar]
Sunshine 1997 {published data only}
- Sunshine A, Olson NZ, O'Neill E, Ramos I, Doyle R. Analgesic efficacy of a hydrocodone with ibuprofen combination compared with ibuprofen alone for the treatment of acute postoperative pain. The Journal of Clinical Pharmacology 1997;37:908‐15. [DOI] [PubMed] [Google Scholar]
Sunshine 1998 {published data only}
- Sunshine A, Olson NZ, Marrero I, Tirado S. Onset and duration of analgesia for low‐dose ketoprofen in the treatment of postoperative dental pain. Journal of Clinical Pharmacology 1998;38(12):1155‐64. [PubMed] [Google Scholar]
Van Dyke 2004 {published data only}
- Dyke T, Litkowski LJ, Kiersch TA, Zarringhalam NM, Zheng H, Newman K. Combination oxycodone 5 mg/ibuprofen 400 mg for the treatment of postoperative pain: a double‐blind, placebo‐ and active‐controlled parallel‐group study. Clinical Therapeutics 2004;26(12):2003‐14. [DOI] [PubMed] [Google Scholar]
Wahl 1997 {published data only}
- Wahl G, Becker J, Keller U. A comparison between a paracetamol/acetylsalicylic acid/caffeine combination and ibuprofen lysinate. Clinical Drug Investigation 1997;13(3):121‐7. [Google Scholar]
Wideman 1999 (study 1) {published data only}
- Wideman GL, Keffer M, Morris E, Doyle RT, Jiang JG, Beaver WT. Analgesic efficacy of a combination of hydrocodone with ibuprofen in postoperative pain. Clinical Pharmacology and Therapeutics 1999;65(1):66‐76. [DOI] [PubMed] [Google Scholar]
Wideman 1999 (study 2) {published data only}
- Wideman GL, Keffer M, Morris E, Doyle RT, Jiang JG, Beaver WT. Analgesic efficacy of a combination of hydrocodone with ibuprofen in postoperative pain. Clinical Pharmacology and Therapeutics 1999;65(1):66‐76. [DOI] [PubMed] [Google Scholar]
Zelenakas 2004 {published data only}
- Zelenakas K, Fricke JR Jr, Jayawardene S, Kellstein D. Analgesic efficacy of single oral doses of lumiracoxib and ibuprofen in patients with postoperative dental pain. International Journal of Clinical Practice 2004;58(3):251‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahlstrom 1989 {published data only}
- Ahlstrom U, Wahlander LA. Double‐blind comparison of two different dosage recommendations for pain after surgical removal of a lower wisdom tooth. Current Therapeutic Research 1989;45(3):495‐501. [Google Scholar]
Akural 2009 {published data only}
- Akural EI, Järvimäki V, Länsineva A, Niinimaa A, Alahuhta S. Effects of combination treatment with ketoprofen 100 mg + acetaminophen 1000 mg on postoperative dental pain: A single‐dose, 10‐hour, randomized, double‐blind, active‐ and placebo‐controlled clinical trial. Clinical Therapeutics 2009;31(3):560‐8. [DOI: 10.1016/j.clinthera.2009.03.017] [DOI] [PubMed] [Google Scholar]
Anaokar 1993 {published data only}
- Anaokar SM, Parulekar SV, Thatte UM, Dahanukar SA. A multiple dose comparison of ketorolac tromethamine with ibuprofen for analgesic activity. Journal of Postgraduate Medicine 1993;39(2):74‐6. [PubMed] [Google Scholar]
Apaydin 1994 {published data only}
- Apaydin A, Ozyuvaci H, Ordulu M, Disci R. Postoperative pain relief by single dose diclofenac kalium and etodolac. A comparative clinical study. Ağrı (Algoloji) Derneği'nin Yayın organıdır = The Journal of the Turkish Society of Algology 1994;6(4):28‐34. [Google Scholar]
Apiou 1988 {published data only}
- Apiou, Benque, Bertrand, Charon, Fourel, Guidicelli, et al. Multicentric study of fenalgic 400 in odonto‐stomatology [Etude multicentrique du fenalgic 400 en odonto‐stomatologie]. Gazette Medicale 1988;95(11):64‐6. [Google Scholar]
Aranda 1989 {published data only}
- Aranda B, Bor YM. Comparative study between the efficacy of aluminium acetotartrate poultice and diclofenac in the postoperative treatment of degenerative knees [Etude du cataplasme d'acetotartrate d'alumine versus diclofenac dans le traitmente post‐operatoire des genoux degeneratifs]. Comptes Rendus de Therapeutique et de Pharmacologie Clinique 1989;7(80):11‐7. [Google Scholar]
Averbuch 2000 {published data only}
- Averbuch M, Katzper M. Baseline pain and response to analgesic medications in the postsurgery dental pain model. The Journal of Clinical Pharmacology 2000;40:133‐7. [DOI] [PubMed] [Google Scholar]
Bailey 1993 {published data only}
- Bailey BMW, Zaki G, Rotman H, Woodwards RT. A double‐blind comparative study of soluble aspirin and diclofenac dispersible in the control of postextraction pain after removal of impacted third molars. International Journal of Oral and Maxillofacial Surgery 1993;22(4):238‐41. [DOI] [PubMed] [Google Scholar]
Behotas 1992 {published data only}
- Behotas S, Chauvin A, Castiel J, Martin A, Boureau F, Barrat J, et al. Analgesic effect of ibuprofen in pain after episiotomy [Effets antalgiques de l'ibuprofene dans les douleurs apres episiotomie]. Annales Françaises d'anesthèsie et de Rèanimation 1992;11(1):22‐6. [DOI] [PubMed] [Google Scholar]
Bhounsule 1990 {published data only}
- Bhounsule SA, Nevreker PR, Agshikar NV, Manindra NP, Dhume VG. A comparison of four analgesics in post‐episiotomy pain. Indian Journal of Physiology and Pharmacology 1990;34(1):34‐8. [PubMed] [Google Scholar]
Biehl 1981 {published data only}
- Biehl G. Preventing postoperative edema with Irritren. Results of a double blind study [Prophylaxe postoperativer Schwellungen mit Irritren. Ergebnisse einer Dopelblind‐Studie]. Fortschritte der Medizin 1981;99(19):745‐8. [PubMed] [Google Scholar]
Bloomfield 1974 {published data only}
- Bloomfield SS, Barden TP, Mitchell J. Comparative efficacy of ibuprofen and aspirin in episiotomy pain. Clinical Pharmacology and Therapeutics 1974;15(6):565‐70. [DOI] [PubMed] [Google Scholar]
Calanchini 1991 {published data only}
- Calanchini B, Gazzaniga A, Ziegler SJ. Pharmacologic and therapeutic properties of a new ibuprofen formula [Pharmakologische und therapeutische eigenschaften einer neuen ibuprofen‐ formulierung]. Therapiewoche Schweiz 1991;7(8):554‐60. [Google Scholar]
Carlos 1984 {published data only}
- Carlos DE. Comparative study of the efficacy of diclofenac NA, meperidine HCL and nalbuphine HCL in post‐operative analgesia. Philippine Journal of Internal Medicine 1984;22(1):51‐5. [Google Scholar]
Carrillo 1990 {published data only}
- Carrillo JS, Calatayud J, Manso FJ, Barberia E, Martinez JM, Donado M. A randomized double blind clinical trial on the effectiveness of helium neon laser in the prevention of pain, swelling and trismus after removal of impacted third molars. International Dental Journal 1990;40(1):31‐6. [PubMed] [Google Scholar]
Chopra 2009 {published data only}
- Chopra D, Rehan HS, Mehra P, Kakkar AK. A randomized, double‐blind, placebo‐controlled study comparing the efficacy and safety of paracetamol, serratiopeptidase, ibuprofen and betamethasone using the dental impaction pain model. International Journal of Oral and Maxillofacial Surgery 2009;38(4):350‐5. [DOI: 10.1016/j.ijom.2008.12.013] [DOI] [PubMed] [Google Scholar]
Cooper 1984 {published data only}
- Cooper SA. Five studies on ibuprofen for postsurgical dental pain. The American Journal of Medicine 1984;Suppl:70‐7. [DOI] [PubMed] [Google Scholar]
Cooper 1988b {published data only}
- Cooper SA. Ketoprofen in oral surgery pain: a review. The Journal of Clinical Pharmacology 1988;28(12 Suppl):S40‐6. [DOI] [PubMed] [Google Scholar]
Cooper 1993 {published data only}
- Cooper SA, Quinn PD, MacAfee K, Hersh EV, Sullivan D, Lamp C. Ibuprofen controlled‐release formulation. A clinical trial in dental impaction pain. Oral Surgery, Oral Medicine, and Oral Pathology 1993;75(6):677‐83. [DOI] [PubMed] [Google Scholar]
Cooper 1996b {published data only}
- Cooper SA. Lornoxicam: analgesic efficacy and safety of a new oxicam derivative. Advances in Therapy 1996;13(1):67‐77. [Google Scholar]
Darsow 1988 {published data only}
- Darsow F, Witt M. Treatment of postoperative synovitis following meniscus operations with ibuprofen [Zur Behandlung der postoperativen Synovialitis nach Meniskusoperationen mit Ibuprofen]. Beiträge zur Orthopädie und Traumatologie 1988 Dec;35(12):616‐20. [PubMed] [Google Scholar]
Dorfmann 1991 {published data only}
- Dorfmann H. Controlled therapeutic trial of diclofenac in meniscectomy under arthroscopy [Essai therapeutique controle du diclofenac dans les meniscectomies sous arthroscopie]. Revue du Rhumatisme et des Maladies Osteo‐Articulaires 1991;58(1):59‐61. [PubMed] [Google Scholar]
Doyle 2002 {published data only}
- Doyle G, Jayawardena S, Ashraf E, Cooper SA. Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. Journal of Clinical Pharmacology 2002;42(8):912‐9. [DOI] [PubMed] [Google Scholar]
El‐Tanany 1993 {published data only}
- El‐Tanany H, Boghdady W. A double blind comparative study of Diclofenac‐K and Glafenine in the management of acute dental pain. Cairo Dental Journal 1993;9(2):117‐20. [Google Scholar]
Fleiss 1979 {published data only}
- Fleiss JL, Chilton NW, Wallenstein S. Ridit analysis in dental clinical studies. Journal of Dental Research 1979;58(11):2080‐4. [DOI] [PubMed] [Google Scholar]
Forbes 1991 {published data only}
- Forbes JA, Moore EM, Allen HW, Beaver WT. Evaluation of an ibuprofen controlled‐release tablet and placebo in postoperative oral surgery pain. Pharmacotherapy 1991;11(3):242‐8. [PubMed] [Google Scholar]
Frezza 1985 {published data only}
- Frezza R, Bolognesi P, Bernardi F. Comparison of the action of 3 non‐steroidal anti‐inflammatory agents in the control of post‐operative pain. Effectiveness of NSAID against pain [Confronto sull'azione di tre antinfiammatori non steroidei nel controllo de dolore odontoiatrico post‐chirurgico. Efficaci i FANS contro il dolore]. Attualita Dentale 1985;1(30):40‐2. [PubMed] [Google Scholar]
Gallardo 1980 {published data only}
- Gallardo F, Rossi E. Double blind evaluation of naproxen and ibuprofen in periodontal surgery. Pharmacology and Therapeutics in Dentistry 1980;5(3‐4):69‐72. [PubMed] [Google Scholar]
Gallardo 1981 {published data only}
- Gallardo F, Lobo R, Pino M, Martino LE. Double blind evaluation of naproxen and Ibuprofen in oral surgery outpatients. IRCS Medical Science 1981;9:440‐1. [Google Scholar]
Garwood 1983 {published data only}
- Garwood AJ, Lownie JF, Cleaton Jones PE, Butz SJ. The effect of ibuprofen (Brufen) following the removal of impacted third molar teeth. The Journal of the Dental Association of South Africa = Die Tydskrif van die Tandheelkundige Vereniging van Suid‐Afrika 1983;38(12):739‐42. [PubMed] [Google Scholar]
Giles 1981 {published data only}
- Giles AD. Analgesia following dental surgery: a comparison of brufen and distalgesic. The British Journal of Oral Surgery 1981;19(2):105‐11. [DOI] [PubMed] [Google Scholar]
Giles 1985 {published data only}
- Giles AD, Pickvance NJ. Combination analgesia following oral surgery; administered pre‐operatively double blind comparison of ibuprofen, codeine phosphate and two combination ratios. Clinical Trials Journal 1985;22(4):300‐13. [Google Scholar]
Hazra 1982 {published data only}
- Hazra DK, Elhence IP. Double blind comparative trial to study efficacy of various analgesic formulations. Clinician 1982;46(5):218‐22. [Google Scholar]
Henderson 1994 {published data only}
- Henderson RC. A double‐blind comparison of diclofenac potassium and naproxen sodium in the treatment of pain due to orthopedic skeletal surgery. Today's Therapeutic Trends 1994;12(S1):81‐95. [Google Scholar]
Henrikson 1982 {published data only}
- Henrikson PA, Thilander H, Wahlander LA. Absorption and effect of diclofenac‐sodium after surgical removal of a lower wisdom tooth. Current Therapeutic Research 1982;31(1):20‐6. [Google Scholar]
Henrikson 1985 {published data only}
- Henrikson PA, Thilander H, Wahlander LA. Voltaren as an analgesic after surgical removal of a lower wisdom tooth. International Journal of Oral Surgery 1985;14(4):333‐8. [DOI] [PubMed] [Google Scholar]
Hopkinson 1980 {published data only}
- Hopkinson JH. Ibuprofen versus propoxyphene hydrochloride and placebo in the relief of postepisiotomy pain. Current Therapeutic Research 1980;27(1):55‐63. [Google Scholar]
Hultin 1978 {published data only}
- Hultin M, Olander KJ. A clinical trial of the analgesic properties of Voltaren (diclofenac sodium). Scandinavian Journal of Rheumatology. Supplement 1978;22:42‐5. [DOI] [PubMed] [Google Scholar]
Hyrkas 1992 {published data only}
- Hyrkas T, Ylipaavalniemi P, Oikarinen VJ, Hampf G. Postoperative pain prevention by a single‐dose formulation of diclofenac producing a steady plasma concentration. Journal of Oral Maxillofacial Surgery 1992;50(2):124‐7. [DOI] [PubMed] [Google Scholar]
Hyrkas 1993 {published data only}
- Hyrkas T, Ylipaavalniemi P, Oikarinen VJ, Paakkari I. A comparison of diclofenac with and without single‐dose intravenous steroid to prevent postoperative pain after third molar removal. Journal of Oral Maxillofacial Surgery 1993;51(6):634‐6. [DOI] [PubMed] [Google Scholar]
Hyrkas 1994 {published data only}
- Hyrkas T. Effect of preoperative single doses of diclofenac and methylprednisolone on wound healing. Scandinavian journal of plastic and reconstructive surgery and hand surgery 1994;28(4):275‐8. [DOI] [PubMed] [Google Scholar]
Iles 1980 {published data only}
- Iles JD. Relief of postoperative pain by ibuprofen: a report of two studies. Canadian Journal of Surgery 1980;23(3):288‐90. [PubMed] [Google Scholar]
Iqbal 1986 {published data only}
- Iqbal KM, Biswas GK, Mondo SK, Afzalunnessa B. Assessment of post operative analgesia: A comparative study of pethidine and diclofenac sodium. Journal of Bangladesh College of Physicians and Surgeons 1986;4(1):1‐7. [Google Scholar]
Iwabuchi 1980 {published data only}
- Iwabuchi T, Soma S. [Use of Voltaren for relief of post‐ extraction pain]. Shikai Tenbo = Dental outlook 1980;55(2):367‐70. [PubMed] [Google Scholar]
Joubert 1977 {published data only}
- Joubert JJ. An assessment of the efficacy and tolerability of Voltaren in the treatment of inflammation after extraction of teeth. The Journal of the Dental Association of South Africa 1977;32:581‐3. [PubMed] [Google Scholar]
Katharia 1992 {published data only}
- Katharia SK, Singh SP, Kulshreshtha VK. A clinical assessment of ibuprofen and a new herbal compound in oral surgical procedures. Indian Journal of Pharmacology 1992;24(1):29‐31. [Google Scholar]
Khan 1992 {published data only}
- Khan AA, Malik S, Zuberi NIS. Double‐blind study on emorfazone and ibuprofen in dental pain and inflammation. Journal of the Pakistan Medical Association 1992;42:17‐8. [PubMed] [Google Scholar]
Kittala 1972 {published data only}
- Kittala S, Aizawa M, Tokue I, Suge Y. Efficacy of GP‐45840 on after‐pains. Clinical trial with double blind method. Shinryo To Shinyaku. (Shinryo To Shinyaku) Medical Consultation and New Remedies 1972;9(6):1123. [Google Scholar]
Klein 1994 {published data only}
- Klein RL, Woll S. A postoperative analgesia comparison of intramuscular and oral ketorolac (Tromethamine) with ibuprofen in patients with foot and ankle surgery. Regional Anaesthesia 1994;19(2S):67. [Google Scholar]
Mastronardi 1988 {published data only}
- Mastronardi P, D'Onofrio M, Scanni E, Pinto M, Frontespezi S, Ceccarelli MG, et al. Analgesic activity of flupirtine maleate: a controlled double blind study with diclofenac sodium in orthopaedics. The Journal of International Medical Research 1988;16(5):338‐48. [DOI] [PubMed] [Google Scholar]
Matthews 1984 {published data only}
- Matthews RW, Scully CM, Levers BG. The efficacy of diclofenac sodium (Voltarol) with and without paracetamol in the control of post surgical dental pain. British Dental Journal 1984;157(10):357‐9. [DOI] [PubMed] [Google Scholar]
McEvoy 1996 {published data only}
- McEvoy A, Livingstone JI, Cahill CJ. Comparison of diclofenac sodium and morphine sulphate for postoperative analgesia after day case inguinal hernia surgery. Annals of the Royal College of Surgeons England 1996;78(4):363‐6. [PMC free article] [PubMed] [Google Scholar]
McQuay 1993 {published data only}
- McQuay HJ, Carroll D, Guest PG, Robson S, Wiffen PJ, Juniper RP. A multiple dose comparison of ibuprofen and dihydrocodeine after third molar surgery. The British Journal of Oral and Maxillofacial Surgery 1993;31(2):95‐100. [DOI] [PubMed] [Google Scholar]
Movilia 1990 {published data only}
- Movilia P, Restelli L, Miriano F, Vaiani G, Grossi E. Analgesic activity of nabumetone in postoperative pain. Drugs 1990;40 Supp(5):71‐4. [DOI] [PubMed] [Google Scholar]
Nakanishi 1990 {published data only}
- Nakanishi M, Mizukawa N, Koyama S, Takagi S, Nishijima K. Clinical evaluation of Amfenac sodium compared with Diclofenac sodium. Journal of Oral Therapeutics and Pharmacology 1990;9(2):85‐92. [Google Scholar]
Negm 1989 {published data only}
- Negm MM. Management of endodontic pain with nonsteroidal anti inflammatory agents: a double blind, placebo controlled study. Oral Surgery, Oral Medicine, and Oral Pathology 1989;67(1):88‐95. [DOI] [PubMed] [Google Scholar]
Rondeau 1980 {published data only}
- Rondeau PL, Yeung E, Nelson P. Dental surgery pain analgesic. Journal Canadian Dental Association 1980;46(7):433‐9. [PubMed] [Google Scholar]
Rossi 1981 {published data only}
- Rossi E, Gallardo F. Analgesic efficacy of naproxen and Ibuprofen in patients undergoing mucogingival surgery. IRCS Medical Science 1981;9:272‐3. [Google Scholar]
Schleier 2007 {published data only}
- Schleier P, Prochnau A, Schmidt‐Westhausen AM, Peters H, Becker J, Latz T, et al. Ibuprofen sodium dihydrate, an ibuprofen formulation with improved absorption characteristics, provides faster and greater pain relief than ibuprofen acid. International Journal of Clinical Pharmacology and Therapeutics 2007;45(2):89‐97. [DOI] [PubMed] [Google Scholar]
Shimura 1981 {published data only}
- Shimura T, Otaka Y, Ando N, Nagao H. [Voltaren tablets for the pain following tooth extraction ‐ studies of its analgesic effects]. Shikai Tenbo = Dental outlook 1981;58(1):175‐83. [PubMed] [Google Scholar]
Squires 1981 {published data only}
- Squires DJ, Masson EL. A double blind comparison of ibuprofen, ASA codeine caffeine compound and placebo in the treatment of dental surgery pain. The Journal of International Medical Research 1981;9(4):257‐60. [DOI] [PubMed] [Google Scholar]
Tai 1992 {published data only}
- Tai YMA, Baker R. Comparison of controlled‐release ketoprofen and diclofenac in the control of post‐surgical dental pain. Journal of the Royal Society of Medicine 1992;85(1):16‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tani 1974 {published data only}
- Tani I. Therapeutic results of Voltaren tablets an analgesic anti‐inflammatory agent in pain following tonsillectomy. (Shinryo To Shinyaku) Medical Consultation and New Remedies 1974;11(8):274. [Google Scholar]
Tesseroli 1986 {published data only}
- Tesseroli de Siqueira JT, Potenza BJ, Basta D. Evaluation of the analgesic efficacy of potassium diclofenac and placebo in postoperative treatment of dental surgery. Multicentre, double blind study [Avaliacao do eficacia analgesicado diclofenaco potassico e placebo no pos‐operatorio de cirurgias para extracoes dentarias. Estudo multicentrico, dupl‐cego]. Revista Paulista de Odontologia 1986;6:2‐7. [Google Scholar]
Troullos 1990 {published data only}
- Troullos ES, Hargreaves KM, Butler DP, Dionne RA. Comparison of nonsteroidal anti inflammatory drugs, ibuprofen and flurbiprofen, with methylprednisolone and placebo for acute pain, swelling, and trismus. Journal of Oral Maxillofacial Surgery 1990;48(9):945‐52. [DOI] [PubMed] [Google Scholar]
Turcotte 1986 {published data only}
- Turcotte JY. Ibuprofen pre and postoperatively in oral surgery [L'ibuprofene en post et en pre‐operatoire en chirurgie buccale]. Journal Canadian Dental Association 1986;52(4):325‐8. [PubMed] [Google Scholar]
Van Der Zwan 1982 {published data only}
- Zwan J, Boering G, Wesseling H, et al. The lower third molar and antiphlogistics. Effects of betamethasone, ibuprofen, indomethacin, naproxen, niflumic acid, oxyphenylbutazone, tranexamic acid and glafenine on the patient's condition after surgical removal of a lower third molar. International Journal of Oral Surgery 1982;11(6):340‐50. [DOI] [PubMed] [Google Scholar]
Van Wering 1972 {published data only}
- Wering RF, Bleker OP. Oral analgesia in post partum pain: a comparison of ibuprofen ("brufen") and dextropropoxyphene. Current Medical Research and Opinion 1972;1(1):49‐52. [DOI] [PubMed] [Google Scholar]
Vigneron 1977 {published data only}
- Vigneron JR, Thys R. Study of the anti‐inflammatory and analgesic actions of diclofenac in traumatology and orthopedic surgery [Etude de l'action anti‐inflammatoire et antalgique du diclofenac en traumatologie et chirurgie orthopedique]. Revue Medicale de Liege 1977;32(1):10‐14. [PubMed] [Google Scholar]
Vogel 1984 {published data only}
- Vogel RI, Gross JI. The effects of nonsteroidal anti inflammatory analgesics on pain after periodontal surgery. Journal of the American Dental Association 1984;109(5):731‐4. [DOI] [PubMed] [Google Scholar]
Von Mayer 1980 {published data only}
- Mayer M, Weiss P. Antiphlogistic and analgesic effect of diclofenac sodium after maxillofacial interventions in a double‐blind trial [Uber die antiphlogistische und analgetische Wirkung von Diclofenac‐Na nach kieferchirurgischen Eingriffen im Doppelblindversuch]. Deutsche Zahnarztliche Zeitschrift 1980;35(5):559‐63. [PubMed] [Google Scholar]
Walker 1976 {published data only}
- Walker JE, Kay LW. Idarac v ibuprofen in the relief of dental pain. The British journal of Clinical Practice 1976;30(2):43‐5. [PubMed] [Google Scholar]
Walton 1990 {published data only}
- Walton GM, Rood JP. A comparison of ibuprofen and ibuprofen codeine combination in the relief of post operative oral surgery pain. British Dental Journal 1990;169(8):245‐7. [DOI] [PubMed] [Google Scholar]
Walton 1993 {published data only}
- Walton GM, Rood JP, Snowdon AT, Rickwood D. Ketorolac and diclofenac for postoperative pain relief following oral surgery. The British Journal of Oral and Maxillofacial Surgery 1993;31(3):158‐60. [DOI] [PubMed] [Google Scholar]
Weber 1990 {published data only}
- Weber F, Wentorp W, Hadjianghelou O. Ibuprofen for pain treatment following dental surgery. A comparative prospective randomized study [Schmerztherapie mit Ibuprofen nach zahnarztlich‐chirurgischen eingriffen. Eine vergleichende prospektiv randomisierte studie]. Deutsche Zeitschrift für Mund‐, Kiefer‐ und Gesichts‐Chirurgie 1990;14(3):206‐9. [PubMed] [Google Scholar]
Winter 1978 {published data only}
- Winter LJr, Bass E, Recant B, Cahaly JF. Analgesic activity of ibuprofen (Motrin) in postoperative oral surgical pain. Oral Surgery, Oral Medicine, and Oral Pathology 1978;45(2):159‐66. [DOI] [PubMed] [Google Scholar]
Wuolijoki 1987 {published data only}
- Wuolijoki E, Oikarinen VJ, Ylipaavalniemi P, Hampf G, Tolvanen M. Effective postoperative pain control by preoperative injection of diclofenac. European Journal of Clinical Pharmacology 1987;32(3):249‐52. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Daniels 2009 {published data only}
- Daniels S, Reader S, Berry P, Goulder M. Onset of analgesia with sodium ibuprofen, ibuprofen acid incorporating poloxamer and acetaminophen‐‐a single‐dose, double‐blind, placebo‐controlled study in patients with post‐operative dental pain. European Journal of Clinical Pharmacology 2009;65(4):343‐53. [DOI: 10.1007/s00228-009-0614-y] [DOI] [PubMed] [Google Scholar]
Kleinert 2008 {published data only}
- Kleinert R, Lange C, Steup A, Black P, Goldberg J, Desjardins P. Single dose analgesic efficacy of tapentadol in postsurgical dental pain: the results of a randomized, double‐blind, placebo‐controlled study. Anesthesia and Analgesia 2008;107(6):2048‐55. [DOI: 10.1213/ane.0b013e31818881ca] [DOI] [PubMed] [Google Scholar]
Additional references
Aranda 2006
- Aranda JV, Thomas R. Systematic review: intravenous ibuprofen in preterm newborns. Seminars in Perinatology 2006;30(3):114‐20. [DOI: 10.1053/j.semperi.2006.04.003] [DOI] [PubMed] [Google Scholar]
Barden 2004
- Barden J, Edwards JE, McQuay HJ, Moore RA. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain 2004;107(1‐2):86‐90. [DOI] [PubMed] [Google Scholar]
Barden 2005
- Barden J, Rees J, Moore RA, McQuay HJ. Single dose oral rofecoxib for postoperative pain. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004604.pub2] [DOI] [PubMed] [Google Scholar]
BNF 2002
- Non‐steroidal anti‐inflammatory drugs. In: Mehta DK editor(s). British National Formulary. 43. Vol. March, London: BMJ Publishing Group Ltd, 2002:482. [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72:95‐7. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins SL, Edwards J, Moore RA, Smith LA, McQuay HJ. Seeking a simple measure of analgesia for mega‐trials: is a single global assessment good enough?. Pain 2001;91(1‐2):189‐94. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. British Medical Journal 1995;310:452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 1991
- Cooper SA. Single‐dose analgesic studies: the upside and downside of assay sensitivity. In: Max MB, Portenoy RK, Laska EM editor(s). The design of analgesic clinical trials. Advances in Pain Research and Therapy. Vol. 18, New York: Raven Press, 1991:117‐24. [Google Scholar]
Derry 2008
- Derry S, Moore RA, McQuay HJ. Single dose oral celecoxib for acute postoperative pain. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004233] [DOI] [PubMed] [Google Scholar]
Derry C 2009
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral naproxen and naproxen sodium for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004234.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry P 2009
- Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edwards 1999
- Edwards JE, McQuay HJ, Moore RA, Collins SL. Reporting of adverse effects in clinical trials should be improved: lessons from acute postoperative pain. Journal of Pain and Symptom Management 1999;18(6):427‐37. [DOI] [PubMed] [Google Scholar]
Edwards 2000
- Edwards JE, Moore RA, McQuay HJ. Single dose oxycodone and oxycodone plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002763] [DOI] [PubMed] [Google Scholar]
FitzGerald 2001
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. [DOI] [PubMed] [Google Scholar]
Forrest 2002
- Forrest JB, Camu F, Greer IA, Kehlet H, Abdalla M, Bonnet F. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. British Journal of Anaesthesia 2002;88(2):227‐33. [DOI] [PubMed] [Google Scholar]
Grahame‐Smith 2002
- Grahame‐Smith DG, Aronson JK. Oxford textbook of clinical pharmacology and drug therapy. 3. Oxford: Oxford University Press, 2002. [ISBN: 13: 978‐0‐19‐263234‐0] [Google Scholar]
Hawkey 1999
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. [DOI] [PubMed] [Google Scholar]
Hernandez‐Diaz 2001
- Hernández‐Díaz S, García‐Rodríguez LA. Epidemiologic assessment of the safety of conventional nonsteroidal anti‐inflammatory drugs. The American Journal of Medicine 2001;110(Suppl 3A):20S‐7S. [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66:239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107:224‐33. [DOI] [PubMed] [Google Scholar]
Lloyd 2009
- Lloyd R, Derry S, Moore RA, McQuay HJ. Intravenous parecoxib for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004771.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1982
- McQuay HJ, Bullingham RE, Moore RA, Evans PJ, Lloyd JW. Some patients don't need analgesics after surgery. Journal of the Royal Society of Medicine 1982;75(9):705‐8. [PMC free article] [PubMed] [Google Scholar]
McQuay 2005
- McQuay HJ, Moore RA. Placebo. Postgraduate Medical Journal 2005;81:155‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Moore RA. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271‐8. [DOI: 10.1111/j.1365-2125.2006.02723.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1999
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of meta‐analyses of randomised controlled trials: the QUOROM statement. Lancet 1999;354:1896‐900. [DOI] [PubMed] [Google Scholar]
Moore 1996
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 1996;66(2‐3):229‐37. [DOI] [PubMed] [Google Scholar]
Moore 1997a
- Moore A, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Verification from independent data. Pain 1997;69(1‐2):127‐30. [DOI] [PubMed] [Google Scholar]
Moore 1997b
- Moore A, Moore O, McQuay H, Gavaghan D. Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: Use of pain intensity and visual analogue scales. Pain 1997;69(3):311‐5. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything‐large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2005
- Moore RA, Edwards JE, McQuay HJ. Acute pain: individual patient meta‐analysis shows the impact of different ways of analysing and presenting results. Pain 2005;116(3):322‐31. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore A, McQuay H. Bandolier's Little Book of Making Sense of the Medical Evidence. Oxford: Oxford University Press, 2006. [ISBN: 0‐19‐856604‐2] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldman 1999
- Oldman A, Smith LA, Collins S, Carroll D, Wiffen PJ, McQuay HJ, et al. Single dose oral aspirin for acute pain. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD002067] [DOI] [PubMed] [Google Scholar]
PACT 2007
- Prescription Cost Analysis. England, 2007. [ISBN: 978‐1‐84636‐210‐1]
Rapoport 1999
- Rapoport RJ. The Safety of NSAIDs and Related Drugs for the Management of Acute Pain: Maximizing Benefits and Minimizing Risks. Cancer Control 1999;6(2 Suppl 1):18‐21. [DOI] [PubMed] [Google Scholar]
Roy 2007
- Roy YM, Derry S, Moore RA. Single dose oral lumiracoxib for postoperative pain. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006865] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheen 2002
- Sheen CL, Dillon JF, Bateman DN, Simpson KJ, MacDonald TM. Paracetamol pack size restriction: the impact on paracetamol poisoning and the over‐the‐counter supply of paracetamol, aspirin and ibuprofen. Pharmacoepidemiology and Drug Safety 2002;11(4):329‐31. [DOI] [PubMed] [Google Scholar]
Toms 2008
- Toms L, McQuay HJ, Derry S, Moore RA. Single dose oral paracetamol (acetaminophen) for postoperative pain in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD004602.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramèr 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315:635‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Collins 1998
- Collins SL, Moore RA, McQuay HJ, Wiffen PJ. Oral ibuprofen and diclofenac in postoperative pain: a quantitative systematic review. European Journal of Pain 1998;2:285‐91. [DOI] [PubMed] [Google Scholar]
Collins 1999
- Collins SL, Moore RA, McQuay HJ, Wiffen PJ, Edwards JE. Single dose oral ibuprofen and diclofenac for postoperative pain. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001548] [DOI] [PubMed] [Google Scholar]
McQuay 1998
- McQuay HJ, Moore RA. An evidence‐based resource for pain relief. Oxford: Oxford University Press, 1998. [Google Scholar]


