Abstract
Background
At present, it is unknown whether the use of nutrient-rich dairy proteins improves the markers of sarcopenia syndrome. Therefore, our proposal was to investigate whether adding 210 g of ricotta cheese daily would improve skeletal muscle mass, handgrip strength, and physical performance in non-sarcopenic older subjects.
Subjects and methods
This was a single-blind randomized clinical trial that included two homogeneous, randomized groups of men and women over 60 years of age. Participants in the intervention group were asked to consume their habitual diet but add 210 g of ricotta cheese (IG/HD + RCH), while the control group was instructed to consume only their habitual diet (CG/HD). Basal and 12-week follow-up measurements included appendicular skeletal muscle mass (ASMM) by dual-energy X-ray absorptiometry, handgrip strength by a handheld dynamometer, and physical performance using the short physical performance battery (SPPB) and the stair-climb power test (SCPT). The main outcomes were relative changes in ASMM, strength, SPPB, and SCPT.
Results
ASMM increased in the IG/HD + RCH (0.6±3.5 kg), but decreased in the CG/HD (−1.0±2.6). The relative change between groups was statistically significant (P=0.009). The relative change in strength in both groups was negative, but the loss of muscle strength was more pronounced in CG/HD, though in this regard statistical analysis found only a tendency (P=0.07). The relative change in the balance-test scores was positive for the IG/HD + RCH, while in the CG/HD it was negative, as those individuals had poorer balance. In this case, the relative change between groups did reach statistical significance.
Conclusion
The addition of 210 g of ricotta cheese improves ASMM and balance-test scores, while attenuating the loss of muscle strength. These results suggest that adding ricotta cheese to the habitual diet is a promising dietetic strategy that may improve the markers of sarcopenia in subjects without a pronounced loss of ASMM or sarcopenia.
Keywords: nutritional intervention, nutrient-rich dairy proteins, ricotta cheese, markers of sarcopenia, elderly
Introduction
Sarcopenia is a syndrome characterized by the progressive, generalized loss of skeletal muscle mass and strength, with a risk of such adverse outcomes as physical disability, poor quality of life, and even death.1 The causes of loss of skeletal muscle are unclear, but may include a lower basal rate of protein synthesis in aged muscle and/or an increased rate of protein breakdown, lower sensitivity to insulin-induced stimulation of protein synthesis, and a reduced sensitivity to amino acid feeding.2 Also, an insufficient amount and inadequate distribution of dietary protein could contribute to the age-related loss of skeletal muscle and lead to sarcopenia.3,4 However, as other, nondietary factors are also involved, sarcopenia syndrome is considered a multifactorial disorder.1 Regardless of its causes, dietary protein intake and protein and essential amino acid supplementation seem to play an important role in preventing and reversing the loss of skeletal muscle and functionality in both healthy and ill older people.5,6
Another option for reversing this condition consists in using nutrient-rich meat or dairy proteins as a dietary strategy to counteract the loss of skeletal muscle and sarcopenia, based on the suggestion that dietary protein supplementation boosts muscle protein synthesis and increases whole-body lean mass. An increase in skeletal muscle-protein synthesis after ingestion of lean beef has been reported in both young and elderly subjects.7,8 More recently, similar results were reported for minced beef consumption in older adults.9
Milk proteins on the other hand, specifically casein and whey, are the highest-quality proteins, and a recent study found that fluid skim milk promotes greater muscle-protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage,10 as the former was efficiently converted into an increase in muscle mass in healthy young men.11 It is clear that the intake of nutrient-rich meat proteins provides a positive net-protein balance, while nutrient-rich dairy proteins result in greater accretion of protein synthesis and muscle-mass gain in combination with resistance exercise in young people.11 In the geriatric population, some clinical intervention studies using nutrient-rich egg or dairy proteins, such as ricotta cheese and milk, have failed to show an effect on muscle mass and strength,12–14 but it is important to note that new findings on protein supplementation using high amounts of protein in the form of milk-protein concentrate provide fresh evidence that such supplementation may be considered a promising strategy for recovering muscle mass, strength, and functionality in elderly populations.6,15
We recently reported that a mixed group of sarcopenic men and women who added 210 g of ricotta cheese daily for a period of 12 weeks (18 g of protein) to their habitual diet did not significantly improve appendicular skeletal muscle mass (ASMM) or strength compared to a control group. However, all subjects included in that study were already sarcopenic.14 It has been suggested that the response of skeletal muscle to protein supplementation depends on the stage of skeletal muscle mass, so in the case of subjects who have suffered accelerated and pronounced loss of muscle mass, ie, sarcopenic older adults, it is likely that they will be less responsive to the anabolic stimulus of protein supplementation compared to nonsarcopenic subjects.16 Considering this hypothesis, and the findings recently reported by Tieland et al6 we used the same nutrition-intervention protocol published previously14 to investigate whether adding 210 g of nutrient-rich dairy proteins improves the recommended markers for sarcopenia in nonsarcopenic older men and women subjects.
Subjects and methods
Subjects
A total of 132 apparently healthy (ie, free of major chronic diseases and conditions that affect the primary response variables assessed) older men and women subjects were considered as potential participants. Subjects were recruited through visits to homes and retirement clubs, as well as through telephone calls and posted announcements.
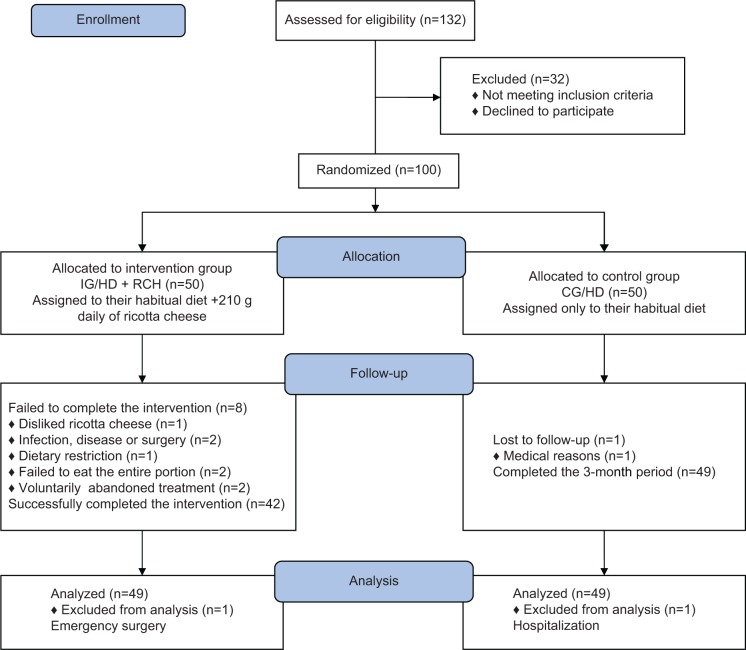
After medical screening and the application of inclusion and exclusion criteria, 100 volunteers were considered for the nutrition-intervention protocol. All volunteers underwent dual-energy X-ray absorptiometry (DXA) measurements, and blood samples were drawn to determine various biochemical parameters, including markers of renal function. None of the subjects had sarcopenia, as defined by a low relative ASMM below two standard deviations from the mean value of the ASMM of a young Mexican adult population.17 All volunteers were free of physical disabilities, as assessed by the instrumental activities of daily living scale.18 Also, they were free of type 2 diabetes and kidney disease. Thirty-two volunteers were excluded due to self-reported type 2 diabetes or glucose levels ≥126 g/dL. Also excluded from the study were those with microalbuminuria, those who refused to eat ricotta cheese due to gastrointestinal problems caused by consuming dairy products, those who simply preferred not to participate, those who consumed fewer than three meals a day, and those with protein supplementation and a body composition that exceeded the margins of the DXA bed (Figure 1).
Figure 1.
Participant flow during the trial.
Abbreviations: IG/HD + RCH, intervention group – ricotta cheese + habitual diet; CG/HD, control group – habitual diet.
Ethics
The protocol was reviewed and approved by the Ethics Committee of the Research Center for Food and Development. All volunteers received a complete explanation of the intervention study, expressed their commitment to maintain their lifestyle during the intervention, and signed the written informed consent.
Baseline and follow-up measurements
Blood samples were drawn after an overnight fast, and collected in ethylenediaminetetraacetic acid and serum tubes. Samples were centrifuged at 1,000 g at 40°C for 15 minutes. Aliquots of plasma and serum were frozen in liquid nitrogen and stored at −80°C. Hemoglobin was measured by HemoCue® (Ängelholm, Sweden), and fasting glucose by the glucose oxidase phenol 4-aminoantipyrine peroxidase Randox technique. Lipid profile was also measured using Randox TL100 kit (Crumlin, UK). Kidney function was evaluated by the glomerular filtration rate following the Cockcroft–Gault formula,19 and by microalbumin in urine by NycoCard Microalbumin Single Test (Axis-Shield plc, Oslo, Norway). In addition, blood pressure was measured and diagnoses of hypertension evaluated on the basis of both self-reports and the use of medication. Measurements were assessed in the context of the subject’s medical history in order to apply our inclusion and exclusion criteria. Finally, some laboratory tests were repeated at the end of the intervention to identify possible adverse effects.
Anthropometry and markers of sarcopenia
Body weight was measured using a digital electronic scale (ADN HV-200K; Japan) with subjects barefoot and lightly clothed. Standing height was recorded using a Seca stadiometer (222; Germany). Body mass index (BMI) was also calculated.
Body composition assessment
Fat mass, lean tissue, and total bone mineral content were measured by DXA using a GE Lunar DPX-MD + (Lunar Radiation, Madison, WI, USA), as reported previously.14,20 ASMM was determined on the basis of the DXA scans following the recommended anatomical landmarks, and represents the sum of nonfat plus nonbone tissue in both arms and legs.21 The DXA machine was calibrated daily in accordance with the manufacturer’s guidelines.
Maximum handgrip or muscle strength
Handgrip strength was measured using a Takei Smedley handgrip dynamometer (Takei Scientific Instruments, Niigata, Japan). Measurements were taken according to the manufacturer’s recommendations, and results were obtained as previously published.14
Physical performance
Each subject’s physical performance was assessed using the short physical performance battery (SPPB), which assesses balance, gait speed, strength, and endurance by examining an individual’s ability to stand with feet together in side-by-side, semitandem, and tandem positions, the time required to walk 8 feet (2.44 m), and the time required to rise from a chair and return to a seated position five times. Scores were calculated following the procedures published by Guralnik et al.22
In addition, the stair-climb power test (SCPT) was assessed as a clinical measure of leg-power impairment.23 For this trial, all volunteers were instructed to safely ascend a 12-step flight of stairs (height of each step =14.2 cm) as quickly as possible. They were allowed to use the handrail for safety reasons. Timing began after the countdown “ready, set, go” on the word “go”, and was stopped when both of the participant’s feet reached the top step. Time was measured by a stopwatch to the nearest 0.01 second and the average of two trials was obtained, though subjects who had greater difficulty in climbing the stairs were only asked to perform this task once. The SCPT score was calculated according to the formula published by Bean et al.23
Experimental design
This study was a single-blind randomized clinical trial with follow-up using the same protocol as reported previously.14 Briefly, for this study, volunteers were randomly assigned 1:1 to the intervention or control group using sampling with replacement (Figure 1). Once the groups were formed, a person from outside the project conducted a randomized procedure to assign the corresponding treatment. The assessment personnel were blind to this randomized procedure. Participants in the intervention group were asked to consume their habitual diet but add 210 g of ricotta cheese (IG/HD + RCH; divided into three equal portions of 70 g, ingested at breakfast, lunch and dinner), while subjects in the control group were instructed to consume only their habitual diet (CG/HD). Both groups were treated under similar conditions during the intervention protocol, and were asked to maintain normal physical activity and eating patterns. As part of the study, all volunteers were asked not to engage in any intentional effort to gain or lose weight for the ensuing 3 months. The elapsed time between baseline measurements and the beginning of the intervention trial was 2 weeks, and the recording of follow-up measurements began after the last day of the nutrition-intervention protocol. Data were collected at the Research Center for Food and Development. Finally, assessment personnel were blind to both the randomization process and treatment regimen assigned to the participants.
Outcomes
Primary outcomes were ASMM, maximum handgrip or muscle strength and SPPB and SCPT scores. Primary response variables were the relative changes in the markers of sarcopenia, ie, ASMM, strength, SPPB, and SCPT scores. Relative changes for all variables were calculated as: ([follow-up value − baseline value]/baseline value) ×100.24
Statistical methods
Sample size was calculated based on the differences in muscle strength reported in a previous study with sarcopenic older men and women subjects.14 Calculations were made for a total sample of 96 subjects (48 subjects per group) in order to obtain a statistical difference of an increase of 0.9 kg of muscle strength between groups, assuming an alpha of 5% and a power of 80%. Mean values and values of relative change are reported. Balance in the two randomized groups (IG/HD + RCH versus CG/HD) was confirmed by evaluating intergroup differences using an independent t-test. Similarly, differences in the aforementioned intergroup outcomes were analyzed using an independent t-test under the intention-to-treat strategy. A P-value ≤0.05 indicated statistical significance. Normality was tested by the D’Agostino–Kurtosis test. All analyses were performed using NCSS software (Kaysville, UT, USA).
Results
The total sample pool of 132 subjects over 60 years of age was screened with the result that 100 subjects fulfilled the inclusion criteria. Therefore, the total intervention sample consisted of 50 women and 50 men with a mean age of 70.2±7.0 years and a mean body weight of 71.0±11.3 kg. The mean BMI was 27.1±3.5 kg/m2. All volunteers were apparently healthy (as explained in the Subjects section). The randomization procedure was successful in that it resulted in homogeneity between the two groups with respect to several variables, including age, anthropometric measurements, body composition components (eg, ASMM), maximum handgrip strength, and the physical performance test variables. Table 1 shows no significant differences between groups.
Table 1.
Age, anthropometry, body composition, strength, physical performance scores, and biochemistry parameters in both groups
| IG/HD + RCH | CG/HD | P-value | |
|---|---|---|---|
| Men/women, n | 25/25 | 25/25 | |
| Age, years | 70.8±7.6 | 69.6±6.4 | 0.40 |
| Weight, kg | 70.7±11.7 | 71.3±10.9 | 0.81 |
| Height, m | 1.6±0.1 | 1.6±0.1 | 0.97 |
| BMI, kg/m2 | 26.9±3.3 | 27.3±3.8 | 0.61 |
| Fat, kg | 25.8±8.0 | 25.6±7.9 | 0.92 |
| Truncal fat, kg | 15.0±4.6 | 15.3±4.6 | 0.78 |
| ASMM, kg | 17.6±4.1 | 17.9±4.1 | 0.63 |
| ASMMI, kg/m2 | 6.6±1.0 | 6.8±1.0 | 0.36 |
| Total lean tissue, kg | 40.6±8.5 | 41.6±8.3 | 0.55 |
| Total mass, kg | 68.8±11.6 | 69.9±10.7 | 0.62 |
| BMC, kg | 2.5±0.5 | 2.5±0.5 | 0.54 |
| Strength, kg | 24.1±9.4 | 24.1±8.6 | 0.98 |
| SPPB, score | 10.7±1.6 | 10.9±1.4 | 0.47 |
| Balance, score | 2.9±0.4 | 3.0±0.2 | 0.08 |
| Gait speed, m/s | 0.9±0.2 | 0.8±0.2 | 1.00 |
| Five chair rise, seconds | 10.6±3.7 | 11.5±2.6 | 0.15 |
| SCPT, W | 205.3±60.3 | 210.9±54.1 | 0.63 |
| Glucose, mg/dL | 96.9±10.7 | 97.1±10.2 | 0.92 |
| GFR, mL/min/1.73 m2 | 71.7±20.3 | 65.5±21.3 | 0.14 |
| Cholesterol, mg/dL | 210.3±39.3 | 217±39.7 | 0.40 |
| Triglycerides, mg/dL | 134.7±46.1 | 157.6±80.4 | 0.08 |
| Microalbumin, mg/L | 21.7±24.1 | 18.9±7.2 | 0.77 |
| Hemoglobin, g/dL | 14.0±1.5 | 14.3±1.3 | 0.18 |
| SBP, mmHg | 120.1±10.2 | 123.2±9.2 | 0.11 |
| DBP, mmHg | 79.0±8.6 | 81.7±8.7 | 0.12 |
Notes: Data are presented as means ± standard deviation. SPPB score ranges from 0 to 12. The score for each SPPB component ranges from 0 to 4.
Abbreviations: BMI, body mass index; ASMM, appendicular skeletal muscle mass; ASMMI, ASMM index; BMC, bone mineral content; SPPB, short physical performance battery; SCPT, stair-climb power test; GFR, glomerular filtration rate; IG/HD + RCH, intervention group – ricotta cheese + habitual diet; CG/HD, control group – habitual diet; SBP, systolic blood pressure; DBP, diastolic blood pressure.
With respect to adherence to the intervention protocol, nine volunteers withdrew from the study: eight from the IG/HD + RCH, and one from the CG/HD. For the intention-to-treat analyses, seven dropouts from the IG/HD + RCH were willing to undergo final assessments. Since there were no differences in the primary outcome measures between the intention-to-treat analysis and the per-protocol analysis, all data were analyzed according to the intention-to-treat principle.
Outcomes
The main outcomes were relative changes in ASMM, maximum handgrip or muscle strength, and SPPB and SCPT test scores. Table 2 shows the effect of adding nutrient-rich dairy proteins, specifically 210 g of ricotta cheese/day, on ASMM and maximum handgrip strength, as well as physical performance-test scores compared to the CG/HD. The relative change in ASMM was significant between groups after the 12-week follow-up period. The relative change in ASMM was positive in the IG/HD + RCH (0.6±3.5 kg), but negative in the CG/HD (−1.0±2.6) (P=0.009). Both total lean tissue and lean tissue in the legs experienced a positive and significant relative change in the intervention group, while the relative change in lean tissue in the arms was negative and significant between groups; however, the loss of lean tissue in the arms was less pronounced in the IG/HD + RCH (Table 2).
Table 2.
Relative changes in body weight, and markers of sarcopenia at baseline and 12 weeks of follow-up
| IG/HD + RCH
|
CG/HD
|
P-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Relative change (%) | Baseline | Follow-up | Relative change (%) | ||
| Men/women, n | 25/24 | 25/24 | 24/25 | 24/25 | |||
| Weight, kg | 70.3±11.7 | 70.8±12.0 | 0.6±2.6 | 71.6±10.8 | 71.4±10.8 | −0.3±2.5 | 0.06 |
| Fat, kg | 25.6±7.9 | 26.0±8.5 | 1.6±6.1 | 25.7±7.9 | 26.1±8.0 | 1.7±6.5 | 0.91 |
| Truncal fat, kg | 14.9±4.6 | 15.2±4.8 | 1.5±7.4 | 15.3±4.7 | 15.5±4.6 | 1.2±6.4 | 0.81 |
| TLT, kg | 40.6±8.6 | 40.7±8.4 | 0.4±3.0 | 41.7±8.4 | 41.3±8.7 | −0.9±2.7 | 0.02 |
| LTA, kg | 4.4±1.2 | 4.4±1.2 | −1.2±3.5 | 4.5±1.1 | 4.4±1.1 | −3.2±4.2 | 0.02 |
| LTL, kg | 13.1±3.0 | 13.3±2.9 | 1.3±4.1 | 13.4±3.1 | 13.4±3.0 | −0.28±3.2 | 0.03 |
| ASMM, kg | 17.6±4.2 | 17.6±4.1 | 0.6±3.5 | 18.0±4.1 | 17.8±4.1 | −1.0±2.6 | 0.009 |
| ASMMI, kg/m2 | 6.6±1.0 | 6.7±0.9 | 0.7±3.43 | 6.8±1.0 | 6.7±1.0 | −1.1±2.6 | 0.004 |
| Total mass, kg | 68.6±11.6 | 69.2±11.9 | 0.9±2.6 | 70.1±10.7 | 70.0±10.8 | −0.2±2.6 | 0.05 |
| Strength, kg | 24.1±9.5 | 23.8±9.3 | −0.6±10.8 | 24.1±8.7 | 23.1±8.8 | −4.5±10.8 | 0.07 |
| SPPB, score | 10.7±1.7 | 10.8±1.5 | 2.4±9.9 | 10.9±1.4 | 11.0±1.3 | 1.2±9.3 | 0.55 |
| Balance, score | 2.9±0.4 | 2.9±0.3 | 3.7±17.1 | 3.0±0.2 | 2.9±0.3 | −2.4±12.7 | 0.05 |
| Gait speed, m/s | 5.1±1.0 | 4.6±1.0 | 6.3±23.7 | 5.2±1.2 | 4.5±0.8 | 8.6±22.3 | 0.63 |
| Five chair rise, seconds | 10.5±3.7 | 10.6±3.2 | −0.8±16.0 | 11.4±2.6 | 11.3±2.4 | −0.2±15.1 | 0.83 |
| SCPT, W | 204.2±60.4 | 203.5±57.1 | 0.5±9.8 | 211.3±54.6 | 203.9±52.2 | −2.8±11.4 | 0.10 |
Notes: Data are presented as means ± standard deviation. SPPB score ranges from 0 to 12. The score for each SPPB component ranges from 0 to 4.
Abbreviations: TLT, total lean tissue; LTA, lean tissue in arms; LTL, lean tissue in legs; ASMM, appendicular skeletal muscle mass; ASMMI, ASMM index; SPPB, short physical performance battery; SCPT, stair-climb power test; IG/HD + RCH, intervention group – ricotta cheese + habitual diet; CG/HD, control group – habitual diet.
The relative change in maximum handgrip or muscle strength was not significant between groups, though it did show a clear tendency toward significance (P=0.07). Table 2 also shows that the loss of muscle strength expressed in terms of the relative change was less pronounced in the IG/HD + RCH than CG/HD.
With respect to the physical performance tests, the present study showed no effect of the nutrient-rich dairy proteins on the relative change in the summary performance scores calculated by totaling the partial results from the three tests. However, separate analyses showed that the relative change in balance-test scores was statistically significant between the groups after the 12-week follow-up period. The relative change in balance-test scores in the IG/HD + RCH was positive, while in the CG/HD it was negative (P≤0.05). Results are shown in Table 2.
Assessment of possible adverse effects
The relative change in triglycerides and high-density lipid cholesterol between groups was negative but not statistically different; however, the relative change in total cholesterol was negative in both groups and did reach statistical significance. The relative change in glomerular filtration rate was positive and significant between groups, and was more pronounced in the CG/HD than in the IG/HD + RCH (P<0.001) (Table 3).
Table 3.
Relative changes in some markers of adverse effects at baseline and 12 weeks of follow-up
| IG/HD + RCH
|
CG/HD
|
P-value | |||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | Relative change (%) | Baseline | Follow-up | Relative change (%) | ||
| Men/women, n | 25/24 | 25/24 | 24/25 | 24/25 | |||
| Cholesterol, mg/dL | 210.7±39.6 | 191.3±39.1 | −8.8±11.4 | 217.1±40.1 | 181.2±37.8 | −15.8±13.9 | 0.001 |
| Triglycerides, mg/dL | 135.6±46.1 | 129.4±55.9 | −1.1±38.0 | 158.1±81.2 | 135.7±63.2 | −6.1±37.6 | 0.52 |
| HDL-C, mg/dL | 58.1±13.3 | 59.2±13.4 | 3.2±16.9 | 57.6±14.8 | 60.4±16.4 | 5.1±13.9 | 0.55 |
| Glucose, mg/dL | 97.1±10.8 | 100.4±11.7 | 3.8±9.5 | 97.5±10.0 | 103.0±11.0 | 6.3±12.3 | 0.30 |
| GFR, mL/min/1.73 m2 | 71.4±20.4 | 78.5±20.2 | 13.1±23.4 | 66.1±21.2 | 82.5±21.7 | 27.6±16.1 | 0.001 |
Note: Data are presented as means ± standard deviation.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; GFR, glomerular filtration rate; IG/HD + RCH, intervention group – ricotta cheese + habitual diet; CG/HD, control group – habitual diet.
Discussion
The present study shows that adding nutrient-rich dairy proteins, specifically 210 g of ricotta cheese, for 12 weeks significantly improved total mass as measured by DXA, ASMM, and balance-test scores. Also, it showed a clear tendency toward significance in attenuating the loss of muscle strength in the IG/HD + RCH of free-living and apparently healthy, nonsarcopenic older men and women subjects. It is important to note that neither total mass, total lean tissue, lean tissue in legs, nor the ASMM improvements were associated with significant increases in total and truncal fat (Table 2).
The gains in total lean tissue, lean tissue in arms, and ASMM could be related to the amount of protein added to the habitual diet of the IG/HD + RCH of apparently healthy, nonsarcopenic older men and women subjects. Nowadays, it is widely recognized that dietary protein supplementation boosts muscle-protein synthesis and increases whole-body lean mass, a finding that may be due to the stimulation of protein synthesis induced by adding nutrient-rich dairy proteins or 18.12 g of protein from the ricotta cheese per day in the IG/HD + RCH. With respect to the amount of protein added to the habitual diet, it is important to mention that other researchers using similar amounts of protein have reported similar results. For example, Paddon-Jones et al25 used specially designed supplements, such as essential amino acids and whey protein, to show that both 15 g of essential amino acids and 15 g of whey protein increase muscle-protein synthesis in healthy, older subjects (65–79 years).
Also, our findings are likely related to the fact that increasing total protein intake increases whole-body protein synthesis and improves nitrogen balance, which leads to gaining more lean tissue. In their studies of malnourished and frail elderly subjects, Bos et al26 and Chevalier et al27 point out that an increase in the total amount of dietary protein from 0.5 to 2.0 g/kg/day produces higher rates of whole-body protein synthesis and improves nitrogen balance. In the present single-blind, randomized clinical trial we did not apply any dietary study; however, we have local evidence of dietary protein intake in older people, since we recently reported on older men and women volunteers who ingested 0.9 g of protein/kg of body weight/day.3 Considering that body weight was similar in the cited study and in our study groups, we estimated that adding ricotta cheese increased protein intake from 0.9 g to 1.2 g of protein/kg of body weight/day. In the present experimental protocol, the results related to skeletal muscle in nonsarcopenic subjects rely on an increase in regular protein intake with meals.
Considering the results for the primary response variables, particularly ASMM in both groups, in the previously published clinical trial14 and the present study, the gain of muscle mass seems to be dependent on the stage of skeletal muscle. In both clinical trials, the main value of ASMM at baseline was 15.7±3.0 and 15.3±2.8 kg in the sarcopenic older men and women subjects in the intervention and control groups, respectively, while the nonsarcopenic older men and women subjects in the present trial had 17.6±4.1 and 17.9±4.1 kg of ASMM in the IG/HD + RCH and CG/HD, respectively. The same nutritional intervention protocol was followed in both clinical trials. Considering the comments by Wolfe,16 it is probable that the subjects included in this trial were more responsive to the anabolic stimulus of the high protein intake provided by the ricotta cheese than the sarcopenic subjects included in the earlier randomized clinical trial;14 however, we cannot ignore the possible effect of age on responsiveness to the anabolic stimulus of protein supplementation, as the elderly participants included in this trial were on average 6 years younger than the group studied in the earlier clinical trial. These findings suggest that supplementation in the form of nutrient-rich dairy proteins should be provided before sarcopenia syndrome develops.
With respect to the relative change in maximum handgrip or muscle strength, there were no significant differences between the groups, though this variable showed a clear tendency (P=0.07). While it did not reach the level of significance, the loss of muscle strength reflected in the relative change in the IG/HD + RCH was attenuated compared to the loss in the CG/HD (Table 2). It is important to clarify that the sample size for this trial was calculated to determine differences in muscle strength reported in a previous published study,14 not in lean tissues. In spite of the increase in muscle mass, no significant effect on strength was found. It is widely recognized that increases in muscle strength do not depend solely on muscle mass, and that the relation between strength and muscle mass is not linear.28,29 Other key factors that likely affect muscle strength in elderly people include alternative forms of administering the nutrients,30,31 the amounts administered,6 and sample size. Reversing strength loss is of great clinical importance in the geriatric population, since it is also recognized that the risk of falling is significantly higher in subjects with reduced muscle strength.32
Despite the gain in ASMM following 12 weeks of dietary protein supplementation in nonsarcopenic older men and women subjects, the results did not show between-group significance in the relative change on the SPPB. However, separate analyses did reveal an increase in the relative change on the balance-test score (balance-test score for this SPPB component ranges from 0 to 4) in the IG/HD + RCH (3.7±17.1), while in the CG/HD this was negative (−2.4±12.7) (P≤0.05). The results of adding dietary protein supplementation on physical performance seem to be promising in terms of preventing physical disabilities. The increases in the balance-test scores could have a substantial clinical impact on functionality. We are aware of the strong relation between muscle mass and balance in the older population,33 and of the risk of falling due to poor walking and standing balance.34 The improvements in physical performance, particularly the balance-test scores, may be attributable to the gain in ASMM, though other studies have shown an improvement in physical performance (SPPB) in the absence of any gain in muscle mass.6 In light of recent evidence and our results, new knowledge of the possible mechanism relating protein supplementation using nutrient-rich dairy proteins or specifically designed supplements to functionality is needed.
The overall results of the kidney-function assessment in this study show that the relative change in the glomerular filtration rate was positive in response to the increase in daily protein ingestion of 18.1 g achieved with the ricotta cheese. This increment in the glomerular filtration rate reached the normal range and increased in both groups, but doubled in the CG/HD group (Table 3). Therefore, the improvements in skeletal muscle and balance-test scores were not accompanied by any collateral effects on renal function during the intervention period. These results are in line with findings reported earlier14 and others reported more recently.6
The results of the present clinical trial should be interpreted with caution. As mentioned earlier, the primary limitation was that calculations for the sample were based on gains in muscle strength and not lean tissue in a group of sarcopenic older men and women.14 Also, it is possible that the amount of protein given to volunteers was insufficient to impact strength. A recent study showed an improvement in muscle strength in frail subjects who were supplemented with 30 g of protein per day. However, most people would surely find it difficult to persuade older individuals to consume such a large amount of protein by eating ricotta cheese on a routine basis. Also, we cannot neglect the importance of assessing muscle strength using the leg press in addition to handgrip strength, as most of the ASMM gained in the IG/HD + RCH group was found to occur in the legs. Second, not all subjects completed the protocol of the intervention trial, despite the research staff’s best efforts to assure treatment compliance. Nevertheless, statistical analysis was carried out considering the total sample (except for two volunteers, one in each group) in order to comply with the recommendations for clinical trials.
Finally, one additional limitation is that this study did not include biopsies of skeletal muscle and biochemistry markers or hormones of muscle-mass gain, such as insulin-like growth factor 1. However, DXA is an accurate and precise method for assessing lean tissue. Therefore, adding these markers of changes in skeletal muscle should help to give a physiological explanation of the results obtained by adding nutrient-rich dairy proteins. In addition, future studies exploring the effects of high amounts of other common high-quality food proteins, such as egg, milk, beef, and soy, on functionality in older people will be conducted. To ensure that nutrient-rich dairy proteins, together with the habitual diet, achieve the desired effect, between 25 g and 30 g at each meal would seem to be a convenient amount to explore. Today, we know that it is not only the total amount of dietary proteins that is key for ensuring functional muscle mass but also the distribution at each meal. A study by our group3 and another recently published work35 showed that older adults did not consume this amount at breakfast and dinner. Therefore, future dietary intervention studies should look at both the total amount and the distribution of proteins to achieve a better impact on sarcopenia syndrome.
Conclusion
The addition of nutrient-rich dairy proteins, specifically 210 g of ricotta cheese, improved ASMM and physical performance, mainly on the balance-test scores, while also attenuating the loss of muscle strength in the absence of health complaints, fat-mass gain, and side effects on kidney function. Therefore, our results suggest that adding ricotta cheese to the habitual diet may be a promising dietetic strategy for improving ASMM and physical performance in subjects with no pronounced loss of ASMM. As a result, this dietetic strategy could potentially prevent sarcopenia syndrome in the geriatric population.
Acknowledgments
This project was funded by CONACYT, Mexico (S0008-2010-1-140157). We are grateful to the study participants and to Daniel David Robles Ochoa, Bertha Isabel Pacheco Moreno, Karina Muro, and Reynaldo Landeros for their technical assistance. Also, we wish to thank Luis Huesca Reynoso for his help in the randomization procedures.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Cruz-Jentoft A, Bayens JP, Bauer JM. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips SM. Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Sci. 2012;92:174–178. doi: 10.1016/j.meatsci.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 3.Valenzuela RE, Ponce JA, Morales-Figueroa GG, Muro KA, Carreón VR, Alemán-Mateo H. Insufficient amounts and inadequate distribution of dietary protein intake in apparently healthy older adults in a developing country: implications for dietary strategies to prevent sarcopenia. Clin Interv Aging. 2013;8:1143–1148. doi: 10.2147/CIA.S49810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malafarina V, Uriz-Otano F, Iniesta R, Gil-Guerrero L. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc. 2013;14:10–17. doi: 10.1016/j.jamda.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Tieland M, van de Rest O, Dirks ML, et al. Protein supplementation improves physical performance in frail elderly people: a randomized, single-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:720–726. doi: 10.1016/j.jamda.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109:1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 9.Pennings B, Groen BB, van Dijk JW, et al. Minced beef is more rapidly digested and absorbed than beef steak, resulting in greater postprandial protein retention in older men. Am J Clin Nutr. 2013;98:121–128. doi: 10.3945/ajcn.112.051201. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 11.Hartman JW, Tang JE, Wilkinson SB, et al. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 12.Iglay HB, Apolzan JW, Gerrard DE, Eash JK, Anderson JC, Campbell WW. Moderately increased protein intake predominantly from egg sources does not influence whole body, regional, or muscle composition responses to resistance training in older people. J Nutr Health Aging. 2009;13:108–114. doi: 10.1007/s12603-009-0016-y. [DOI] [PubMed] [Google Scholar]
- 13.Björkman MP, Pilvi TK, Kekkonen RA, Korpela R, Tilvis RS. Similar effects of leucine rich and regular dairy products on muscle mass and functions of older polymyalgia rheumatica patients: a randomized crossover trial. J Nutr Health Aging. 2011;15:462–467. doi: 10.1007/s12603-010-0276-6. [DOI] [PubMed] [Google Scholar]
- 14.Alemán-Mateo H, Macías L, Esparza-Romero J, Astiazaran-García H, Blancas AL. Physiological effects beyond the significant gain in muscle mass in sarcopenic elderly men: evidence from a randomized clinical trial using a protein-rich food. Clin Interv Aging. 2012;7:225–234. doi: 10.2147/CIA.S32356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tieland M, Dirks ML, van der Zwaluw N, et al. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, single-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13:713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 16.Wolfe RR. Perspective: Optimal protein intake in the elderly. J Am Med Dir Assoc. 2013;14:65–66. doi: 10.1016/j.jamda.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Alemán-Mateo H, Ruiz Valenzuela RE. Skeletal muscle mass indices in healthy young Mexican adults aged 20–40 years: implications for diagnoses of sarcopenia in the elderly population. Scientific World Journal. 2014;2014:672158. doi: 10.1155/2014/672158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 19.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 20.Aleman-Mateo H, Lee SY, Javed F, et al. Elderly Mexicans have less muscle and greater total and truncal fat compared to African–Americans and Caucasians with the same BMI. J Nutr Health Aging. 2009;13:919–923. doi: 10.1007/s12603-009-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heymsfield SB, Smith R, Aulet M, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 23.Bean JF, Kiely DK, LaRose S, Alian J, Frontera WR. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch Phys Med Rehabil. 2007;88:604–609. doi: 10.1016/j.apmr.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 25.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Bos C, Benamouzig R, Bruhat A, et al. Nutritional status after short-term dietary supplementation in hospitalized malnourished geriatric patients. Clin Nutr. 2001;20:225–233. doi: 10.1054/clnu.2000.0387. [DOI] [PubMed] [Google Scholar]
- 27.Chevalier S, Gougeon R, Nayar K, et al. Frailty amplifies the effects of aging on protein metabolism: role of protein intake. Am J Clin Nutr. 2003;78:422–429. doi: 10.1093/ajcn/78.3.422. [DOI] [PubMed] [Google Scholar]
- 28.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Baumgartner RN, Ross R, Rosenberg IH, Roubenoff R. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol. 2004;159:413–421. doi: 10.1093/aje/kwh058. [DOI] [PubMed] [Google Scholar]
- 30.Katsanos CS, Chinkes DL, Paddon-Jones D, Zhang XJ, Aarsland A, Wolfe RR. Whey protein ingestion in elderly results in greater muscle protein accrual than ingestion of its constituent essential amino acid content. Nutr Res. 2008;28:651–658. doi: 10.1016/j.nutres.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Børsheim E, Bui QU, Tissier S, Kobayashi H, Ferrando AA, Wolfe RR. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin Nutr. 2008;27:189–195. doi: 10.1016/j.clnu.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Ger Soc. 2004;52:1121–1129. doi: 10.1111/j.1532-5415.2004.52310.x. [DOI] [PubMed] [Google Scholar]
- 33.Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 34.de Rekeneire N, Visser M, Peila R, et al. Is a fall just a fall: correlates of falling in healthy older persons. The Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:841–846. doi: 10.1046/j.1365-2389.2003.51267.x. [DOI] [PubMed] [Google Scholar]
- 35.Tieland M, Borgonjen-Van den Berg KJ, van Loon LJ, de Groot LC. Dietary protein intake in community-dwelling, frail, and institutionalized elderly people: scope for improvement. Eur J Nutr. 2012;51:173–179. doi: 10.1007/s00394-011-0203-6. [DOI] [PubMed] [Google Scholar]