Abstract
Background.
Although the beneficial effects of physical activity (PA) on memory and executive function are well established in older adults, little is known about the relationship between PA and brain microstructure and the contributions of physical functional limitations and chronic diseases. This study examined whether higher PA would be longitudinally associated with greater microstructural integrity in memory- and executive function-related networks and whether these associations would be independent of physical function and chronic diseases.
Methods.
Diffusion tensor imaging was obtained in 2006–2008 in 276 participants (mean age = 83.0 years, 58.7% female, 41.3% black) with PA (sedentary, lifestyle active, and exercise active) measured in 1997–1998. Gait speed, cognition, depressive symptoms, cardiovascular and pulmonary diseases, hypertension, stroke, and diabetes were measured at both time points. Mean diffusivity and fractional anisotropy were computed from normal-appearing gray and white matter in frontoparietal and subcortical networks. Moderating effects of physical function and chronic diseases were tested using hierarchical regression models.
Results.
Compared with the sedentary, the exercise active group had lower mean diffusivity in the medial temporal lobe and the cingulate cortex (β, p values: −.405, .023 and −.497, .006, respectively), independent of age, sex, and race. Associations remained independent of other variables, although they were attenuated after adjustment for diabetes. Associations between PA and other neuroimaging markers were not significant.
Conclusions.
Being exercise active predicts greater memory-related microstructural integrity in older adults. Future studies in older adults with diabetes are warranted to examine the neuroprotective effect of PA in these networks.
Key Words: Brain aging, Physical activity, Neuroimaging, Epidemiology.
Frontoparietal and subcortical networks are highly susceptible to changes in blood oxygenation levels (1,2) due to their watershed vascularization. Loss of structural integrity in these networks, specifically in dorsolateral prefrontal and hippocampal areas, is related to difficulties in memory and executive function and an increased risk of developing dementia (3). Because of exercise-induced vascularization (4–6), these networks may selectively benefit from the exposure to greater amounts of physical activity (PA).
It is well accepted that higher PA is associated with improved brain health (see review (7)) and these effects are stronger for memory and executive function than for motor control (see meta-analysis (8)). Neuroimaging studies indicate positive effects of PA on brain macrostructure and function (9–17). However, most of previous studies were cross-sectional designs and relied on low-resolution imaging and volumetric measurements of the brain. Data on the association between PA and brain microstructure are sparse, with one observational study indicating a positive association between PA and white matter (WM) integrity (14) and one intervention study reporting increases in WM integrity associated with increases with fitness from walking (18). Understanding the longitudinal association with brain microstructure in older adults may have important implications in designing interventions to preserve structural integrity by improving PA. Furthermore, it is important to differentiate the effects of PA on gray matter (GM) and WM to understand the mechanisms of how PA influences brain integrity.
It is also critical to examine the contributions of physical functional limitations and chronic diseases, which are common in older adults. The relationships between PA, physical function, and chronic diseases become increasingly complex in late life. For example, PA may be beneficial for physical function and cardiovascular health, but in turn poor physical function and cardiovascular diseases (CVDs) may limit PA participation (19). Both PA and these potential modifiers are related to brain integrity (14).
This study quantified the associations between PA and microstructural integrity of GM and WM in a well-characterized cohort of very old adults, by accounting for health-related conditions. It was hypothesized that higher PA would be associated with greater microstructural integrity localized in frontal and medial temporal lobes important for memory and executive function compared with other regions, for example regions related to motor control. It was also hypothesized that these associations would be attenuated by physical function and chronic diseases.
Methods
Study Population
Participants were recruited from the Health, Aging and Body Composition Study, which began in March 1997 to assess the relationship between changes in body composition and health outcomes in a cohort of 3,075 community-dwelling older adults (52% women, 42% black) aged 70–79 years (20). A total of 325 participants at the Pittsburgh site received a brain magnetic resonance imaging (MRI) scan. Of 325, 276 had diffusion tensor imaging in 2006–2008 and PA measured in 1997–1998. The study protocol was approved by the University of Pittsburgh and all participants provided informed consent.
MRI Protocol and Markers
MRI scans were obtained at the MR Research Center of the University of Pittsburgh with 3Tesla Siemens TIM TRIO scanners equipped for echo-planer imaging using the protocol previously described (21). Magnetization-prepared rapid gradient echo images and fluid-attenuated inversion recovery images were acquired to obtain volumes of GM, WM, and WM hyperintensities, respectively. Diffusion tensor images were acquired using single-short spin-echo sequence with 12 directions and preprocessed using the FMRIB’s Diffusion Toolbox (22) to remove unwanted distortions (voxel size = 2 mm × 2 mm; slice thickness = 3 mm). There were no pathological findings from MR images for this study as verified by a neuroradiologist.
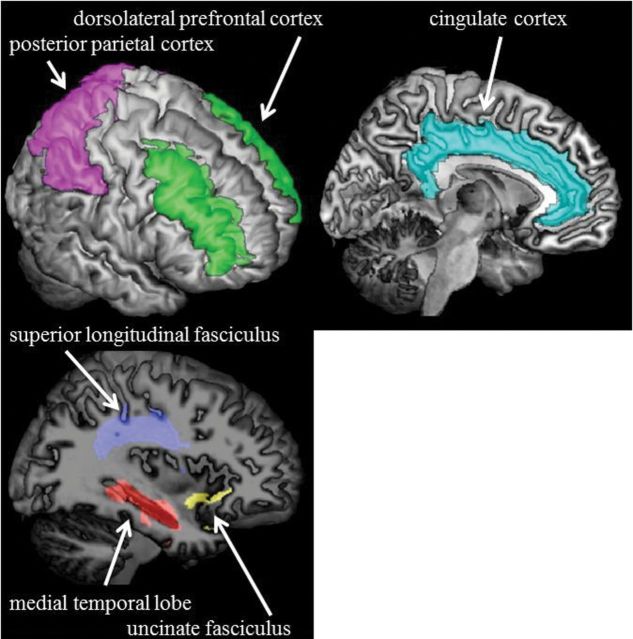
Using the segmentation of GM, WM, and WM hyperintensities, the mean diffusivity (MD) and fractional anisotropy maps were restricted to normal-appearing GM and WM (21). Using Automated Labeling Pathway (23,24), neuroimaging markers in regions and tracts were identified based on the Automated Anatomical Labeling Atlas (25) and the Johns Hopkins University White Matter Atlas (26), respectively. Regions and tracts of interest were selected a priori, including medial temporal lobe, cingulate cortex, dorsolateral prefrontal cortex, posterior parietal cortex, and uncinate and superior longitudinal fasciculi (Figure 1). Other regions were examined as a comparison to the hypothesized regions and tracts, including striatum, primary sensorimotor cortex, and supplementary motor cortex. MD in regions of interest from left and right hemispheres was computed as the mean weighted by GM volume. Fractional anisotropy in tracts of interest from left and right hemispheres was computed as the mean.
Figure 1.
Regions and tracts of interest.
Parenchyma atrophy was calculated by subtracting volumes of GM and WM from intracranial volume. The volume of WM hyperintensities normalized by total brain volume was dichotomized at the median due to a skewed distribution.
Physical Activity
PA was measured in 1997–1998 using a standardized questionnaire (27). Participants were categorized into sedentary, lifestyle active, and exercise active groups as previously defined (Supplementary Table 1) (28,29). Those with lifestyle activities less than 2,719 kcal/wk and exercise activities less than 1,000 kcal/wk were defined as sedentary (n = 39, 14.1%). Those with lifestyle activities more than 2,719 kcal/wk and exercise activities less than 1,000 kcal/wk were defined as lifestyle active (n = 148, 53.6%) and those with exercise activities more than 1,000 kcal/wk, regardless the amount of lifestyle activities, were defined as exercise active (n = 89, 32.2%). In additional sensitivity analyses, the sedentary group was defined by less than 2,719 kcal/wk of lifestyle activities only, because being sedentary most of the day and exercising regularly can also be considered as sedentary (30). Those with exercise activities more than 1,000 kcal/wk and lifestyle activities more than 2,719 kcal/wk were defined as exercise active.
Self-reported time spent walking in minutes per week was measured annually. Change was computed by subtracting walk time at study entry from walk time at time of MRI. The average time spent walking was computed using at least three valid values from study entry to time of MRI.
Other Measures of Interest
Gait speed at usual pace over 3, 4, or 6 m was measured at study entry, years 4 and 6, and time of MRI. Gait speed is a valid and reliable assessment of physical function for older adults (31). Slower gait speed is strongly associated with severity of health conditions and higher mortality risk (32,33). Change was computed by subtracting speed at study entry from speed at time of MRI. Annual percent change was computed by dividing relative change over 9 years by 9.
The Digit Symbol Substitution Test (DSST) was measured at study entry, years 5, 7, 8, and 9, and time of MRI. The modified mini-mental status examination (3MSE) was measured at study entry, years 3, 5, 7, and 9, and time of MRI. The Center for Epidemiologic Studies Depression Scale (CES-D) was measured at study entry, years 3–9, and time of MRI. Changes were computed by subtracting scores at study entry from scores at time of MRI. Annual percent changes were computed by dividing relative changes over 9 years by 9. The annual percent change was 0 when participants had 0 at study entry and time of MRI. For those with 0 values at study entry but nonzero values at time of MRI, 0 values at study entry were added to 1 in order to compute annual percent changes.
Chronic diseases, including prevalent CVD, pulmonary disease, hypertension, and diabetes, were obtained at study entry and time of MRI using prevalent disease algorithms according to self-reported diagnoses by physicians and records of medication use. Prevalent CVD was defined by self-report prevalent coronary heart disease or cerebrovascular disease. Prevalent pulmonary disease was defined by self-report or medication use. Prevalent diabetes and hypertension were defined by self-report and confirmed by medication use. Incident CVD and stroke were ascertained using annual self-report questionnaires from the study entry to time of MRI.
In addition to age, sex, and race obtained at study entry, other characteristics were also measured, including education, body mass index, smoking status, alcohol consumption, pulmonary function, and prevalent knee osteoarthritis defined as consistent knee pain for at least 1 month in the past 12 months. Pulmonary function was assessed by the ratio of forced expiratory volume in the first second and forced vital capacity.
Statistical Analysis
Univariate associations of PA with MD, fractional anisotropy, parenchyma atrophy, and WM hyperintensities were tested using analysis of variance or χ2 tests as appropriate. Change in time spent walking was tested using repeated-measures analysis of variance using 10 time points. Associations with neuroimaging markers with p value less than .10 were tested in multivariate regression models with PA as dummy coded vectors using the sedentary group as referent. Models were adjusted for age, sex, and race that were associated with PA or MRI outcomes and were further adjusted for physical function and chronic diseases at study entry. Models were also adjusted for education because of its known associations with PA and brain health.
The hypothesized moderators, physical function and chronic diseases, were tested using hierarchical multivariate regression models: (i) the moderator (if continuous) was centered on the parent scale, (ii) the interaction term between PA and the moderator was created, and (iii) models were conducted by entering PA and the moderator and then adding the interaction. A significant model change after adding the interaction and the interaction with p value less than .05 indicated a significant moderating effect.
The strength of the associations between PA and neuroimaging markers was tested using forward stepwise analysis with all population characteristics including PA transition using change in time spent walking from study entry to time of MRI or the average time spent walking across 10 years in the model. A p value less than or equal to .10 was used as the criteria for entry into the model and a p value greater than or equal to .05 for removal from the model.
Results
Compared with the parent cohort, the 276 participants in this study were less likely to be sedentary (29). The sedentary group had lower body mass index than the lifestyle active group, and they reported less time spent walking at study entry than the exercise active group. Physical and brain function and chronic diseases at study entry or education did not differ significantly between PA groups (Table 1). There was a significant effect of time on time spent walking across 10 years (p = .022). The linear effect of time was also significant (p < .001). However, there was no significant interaction between time and PA group (p = .193).
Table 1.
Characteristics and Health-Related Conditions Over 9 y by PA
| Total (N = 276) | Sedentary (n = 39, 14.1%) | Lifestyle Active (n = 148, 53.6%) | Exercise Active (n = 89, 32.2%) | p Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age (y) | 72.9±2.7 | 73.1±2.9 | 72.9±2.8 | 72.7±2.6 | .792 |
| Female sex | 162 (58.7) | 22 (56.4) | 94 (63.5) | 46 (51.7) | .192 |
| Black race | 114 (41.3) | 12 (30.8) | 67 (45.3) | 35 (39.3) | .236 |
| Physical function | |||||
| Gait speed (m/s) | 1.28±0.24 | 1.23±0.21 | 1.27±0.25 | 1.33±0.24 | .065 |
| Change in gait speed (m/s) | −0.27±0.26 | −0.30±0.28 | −0.27±0.26 | −0.26±0.24 | .646 |
| Annual % change in gait speed | −2.23±2.06 | −2.63±2.52 | −2.25±2.03 | −2.02±1.87 | .299 |
| Chronic disease conditions | |||||
| Cardiovascular disease | 40 (14.8) | 7 (18.4) | 20 (14.0) | 13 (14.6) | .790 |
| Pulmonary disease | 28 (10.2) | 6 (15.4) | 14 (9.5) | 8 (9.0) | .513 |
| Hypertension | 107 (38.9) | 14 (35.9) | 64 (43.5) | 29 (32.6) | .226 |
| Diabetes | 30 (10.9) | 8 (20.5) | 14 (9.5) | 8 (9.0) | .112 |
| Incident cardiovascular disease | 33 (12.6) | 2 (5.4) | 18 (12.9) | 13 (15.3) | .315 |
| Incident stroke | 9 (3.3) | 0 (0.0) | 7 (4.9) | 2 (2.3) | .268 |
| Cognitive function | |||||
| DSST score (0–90) | 42.4±12.7 | 41.5±13.3 | 41.8±12.8 | 43.8±12.4 | .437 |
| Change in DSST score | −5.9±10.8 | −7.3±10.5 | −6.3±10.9 | −4.6±10.7 | .334 |
| Annual % change in DSST | −.99±6.94 | −2.05±3.47 | −0.77±9.04 | −0.89±2.95 | .586 |
| 3MSE score (0–100) | 92.2±6.3 | 91.3±6.5 | 92.7±5.9 | 91.8±6.8 | .310 |
| Change in 3MSE score | 0.6±5.8 | 1.1±6.5 | −0.1±5.8 | 1.5±5.5 | .116 |
| Annual % change in 3MSE | 0.10±0.73 | 0.16±0.80 | 0.01±0.71 | 0.21±0.72 | .113 |
| Depressive symptoms | |||||
| CES-D score (0–60) | 4.1±4.4 | 4.6±5.0 | 4.3±4.4 | 3.4±4.0 | .203 |
| Change in CES-D score | 2.9±6.3 | 4.4±6.0 | 2.6±6.0 | 2.7±6.8 | .255 |
| Annual % change in CES-D | 21.31±45.72 | 26.32±44.89 | 19.78±39.21 | 21.63±55.58 | .729 |
| Other characteristics related to PA | |||||
| Education > high school | 140 (50.9) | 14 (35.9) | 76 (51.4) | 50 (56.8) | .200 |
| Body mass index, kg/m2 | 27.4±4.6 | 25.5±3.6 | 28.1±4.7 | 26.9±4.5 | .004 |
| Current smokers | 13 (4.7) | 2 (5.1) | 9 (6.1) | 2 (2.2) | .649 |
| Alcohol consumption >7 drinks/wk | 23 (8.3) | 3 (7.7) | 15 (10.1) | 5 (5.6) | .428 |
| Pulmonary function | 77.0±7.4 | 77.8±9.1 | 77.2±7.7 | 76.5±6.3 | .603 |
| Knee osteoarthritis | 18 (6.5) | 1 (2.6) | 12 (8.1) | 2 (5.6) | .421 |
| Time spent walking (min/wk) | |||||
| Time spent walking | 60 (225) | 20 (135) | 40 (143) | 190 (385) | .008 |
| Change in time spent walking | −104.3±380.0 | −13.0±117.1 | −91.5±347.2 | −167.6±488.7 | .092 |
Notes: Values are mean ± SD, N (%), or median (interquartile range). p values were obtained from analysis of variance or χ2 tests as appropriate. 3MSE = modified mini-mental state examination; CES-D = Center for Epidemiologic Studies Depression Scale; DSST = digit symbol substitution test; PA = physical activity.
Neuroimaging markers from total brain did not differ significantly between groups (Table 2). By contrast, PA was significantly associated with MD in medial temporal lobe and cingulate cortex (Table 3, Model 1). Associations followed a dose–response relationship (linear trend p = .012 and .009, respectively). Markers in other regions and tracts did not differ significantly between groups, including striatum, primary sensorimotor cortex, supplementary motor area, and uncinate and superior longitudinal fasciculi (Supplementary Tables 2 and 3).
Table 2.
Neuroimaging Markers From Total Brain and Univariate Associations With PA
| Fractional Anisotropy | Mean Diffusivity* | Parenchyma Atrophy, cm3 | White Matter Hyperintensities† | |
|---|---|---|---|---|
| Total (N = 276) | 0.3581±0.0140 | 1.3057±0.1102 | 908.4±137.0 | 138 (50.0) |
| Sedentary (N = 39, 14.1%) | 0.3552±0.0176 | 1.3326±0.1337 | 892.3±153.3 | 22 (56.4) |
| Lifestyle active (N = 148, 53.6%) | 0.3581±0.0132 | 1.3062±0.1105 | 910.0±129.3 | 78 (52.7) |
| Exercise active (N = 89, 32.2%) | 0.3594±0.0136 | 1.2930±0.0968 | 912.6±143.2 | 38 (42.7) |
| p value | .298 | .174 | .726 | .226 |
Notes: Values are mean ± SD or N (%). p values were obtained from analysis of variance or χ2 tests as appropriate. PA = physical activity.
*Multiplied by 1,000.
†Dichotomized at the median.
Table 3.
Regression Models of PA Predicting MD in Medial Temporal Lobe
| Model 1: Unadjusted | Model 2: Adjusted for Age, Sex, and Race | Model 3: Model 2 + Diabetes | Model 4: Model 2 + Hypertension | |
|---|---|---|---|---|
| β (95% CI), p | ||||
| Sedentary | Reference | Reference | Reference | Reference |
| Lifestyle active | −.192 (−0.544, 0.160), .283 | −.131 (−0.458, 0.196), .433 | −.086 (−0.414, 0.242), .606 | −.148 (−0.471, 0.175), .368 |
| Exercise active | −.431 (−0.806, −0.055), .025 | −.405 (−0.753, −0.057), .023 | −.359 (−0.708, −0.010), .044 | −.389 (−0.733, −0.046), .027 |
| Diabetes | — | — | .362 (0.005, 0.718), .047 | — |
| Hypertension | — | — | — | .347 (0.122, 0.572), .003 |
Note: MD = mean diffusivity; PA = physical activity.
In multivariate regression models of PA predicting MD in medial temporal lobe, the exercise active group had lower MD than the sedentary group, independent of age, sex, and race (Table 3, Model 2). The association was attenuated after adjustment for diabetes (Table 3, Model 3 vs 2; ∆β = 11.4%) and hypertension (Table 3, Model 4 vs 2; ∆β = 4.0%), but remained significant. The interactions between PA and diabetes or hypertension were not significant (p > .05). Adjustment for gait speed, other chronic diseases, or education did not substantially attenuate these associations (all ∆β < 10%). In stepwise analyses, the association remained significant (β = −.407, p = .024) with age, sex, and hypertension retained in the model.
In multivariate regression models of PA predicting MD in cingulate cortex, the exercise active group had lower MD than the sedentary group, independent of age, sex, and race (Table 4, Model 2). The association was attenuated after adjustment for diabetes (Table 4, Model 3 vs 2; ∆β = 23.3%), but remained significant. The interaction of PA and diabetes was not significant (p = .677). Adjustment for gait speed, other chronic diseases, or education did not substantially attenuate these associations (all ∆β < 10%). In stepwise analyses, the association remained significant (β = −.381, p = .037) with age, race, diabetes, and DSST score retained in the model.
Table 4.
Regression Models of PA Predicting MD in Cingulate Cortex
| Model 1: Unadjusted | Model 2: Adjusted for Age, Sex, and Race | Model 3: Model 2 + Diabetes | |
|---|---|---|---|
| β (95% CI), p | |||
| Sedentary | Reference | Reference | Reference |
| Lifestyle active | −.395 (−0.745, −0.044), .028 | −.328 (−0.660, 0.004), .053 | −.253 (−0.592, 0.086), .131 |
| Exercise active | −.536 (−0.910, −0.162), .005 | −.497 (−0.851, −0.144), .006 | −.421 (−0.781, −0.061), .019 |
| Diabetes | — | — | .602 (0.234, 0.970), .001 |
Note: MD = mean diffusivity; PA = physical activity.
When the sedentary group was defined by the amount of lifestyle activities only, associations with MD in medial temporal lobe and cingulate cortex remained similar (β, p values: −.314, .059 and −.506, .006, respectively).
Discussion
In this cohort of adults aged 70–79, a relatively large proportion of participants engaged in lifestyle and exercise activities. Our findings suggest that being exercise active, such as walking for exercise, exercising, or doing recreational activities, for at least 1,000 kcal every week, may be optimal for microstructural integrity in GM among older adults with a range of physical and brain function and chronic diseases.
This study extends prior investigations on the dose–response relationship between PA and brain structure in older adults (11,14). First, the application of high-resolution diffusion tensor imaging allowed the identification of focal associations at the microstructural level. Although previous studies identified the hippocampus as a region related to PA (10,11), these studies relied on low-resolution imaging and volumetric measurements of the brain. Examining specific networks of the microstructure can help understand the mechanisms underlying the neuroprotective effects of PA in old age. Second, this study included a comprehensive characterization of health-related conditions at multiple time points, which could affect PA and were important contributors to brain integrity. Our findings indicate that diabetes may have a moderating effect on the association of PA with microstructural integrity. It has been proposed that PA can protect against cognitive dysfunction and brain neurodegeneration by reducing CVD conditions, including diabetes (34). The moderating effect of diabetes needs to be further explored to formulate individualized prescriptions of PA to promote brain health in older adults.
Contrary to our expectations, we did not find an association of PA with WM integrity. These null findings appeared inconsistent with previous reports. Recent cross-sectional studies indicated that higher PA and fitness were associated with greater WM integrity (14,35), and one intervention study reported increases in fitness from walking was associated with elevated WM integrity in young older adults (18). It is possible that being exercise active may not impact WM integrity a decade later, because the evolution of WM degeneration is stronger for very old adults than for young older adults and it may override the short-term but potentially beneficial effects of PA. It is also possible that the association was not detected due to the small sample.
A strength of this study was the availability of repeated measures of time spent walking to estimate the potential for cross-over between groups (eg, the sedentary may have become exercise active and vice versa). Although less comprehensive than the PA measure that we used as main independent variable, time spent walking has been applied in other investigations (11,12), and it was positively associated with PA type (p = .008) in this study. Extreme transitions appeared unlikely in this cohort, because change in time spent walking over time did not significantly differ among three groups as shown in the secondary analysis of existing data. On average, the exercise active group reported more time spent walking than the sedentary group across 10 years (p < .001).
Because brain MRI was not obtained at study entry, a possible reverse causality between PA and brain integrity cannot be ruled out. Those with a more favorable neuroimaging profile at study entry could have engaged in higher PA. However, adjustment for cognitive function at study entry, a surrogate marker of brain integrity, did not modify these associations.
One of the limitations is using PA as a categorical variable, which is less sensitive to small variations in PA-related behaviors compared with a continuous variable. We chose this coding because it has been previously validated in the parent cohort (28,29). Furthermore, self-reported PA may be less sensitive in measuring low intensity activities compared with objective measures. For example, the SenseWear Armband is more valid and accurate in detecting low intensity activities than self-report measures. Future neuroimaging studies applying objective measures are warranted to quantify small amounts and small variations of PA. Another methodological limitation is that the presence of crossing fibers may affect the estimate of fractional anisotropy in WM (36). However, our approach is based on predefined anatomic tracts of interest, which is less susceptible to this limitation than tractography. Lastly, this cohort may not well represent the general population of older adults due to voluntary participation and MRI eligibility. While possible, we also noted that our analytic sample and the participants seen in 2006–2008 without a brain MRI shared similar baseline characteristics.
Conclusions
As the number of very old adults rises, so does the incidence of cognitive impairment and dementia. A major public health priority is to identify strategies to prevent or delay the progression of brain abnormalities using effective interventions for older people. This study suggests that being exercise active may help preserve brain microstructural integrity in memory-related networks among community-dwelling older adults. Future studies are warranted to explore the moderating effect of diabetes on the neuroprotective effect of PA.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106, K23-AG028966-01; NIA grants R01-AG028050, R01-AG029232, P30-AG024827; and National Institute of Nursing Research (NINR) grant R01-NR012459. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute on Aging.
Supplementary Material
References
- 1. Pantano P, Baron JC, Lebrun-Grandié P, Duquesnoy N, Bousser MG, Comar D. Regional cerebral blood flow and oxygen consumption in human aging. Stroke. 1984;15(4):635–641 [DOI] [PubMed] [Google Scholar]
- 2. Lu H, Xu F, Rodrigue KM, et al. Alterations in cerebral metabolic rate and blood supply across the adult lifespan. Cereb Cortex. 2011;21(6):1426–1434. :10.1093/cercor/bhq224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. :10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 4. Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87(14):5568–5572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerr AL, Steuer EL, Pochtarev V, Swain RA. Angiogenesis but not neurogenesis is critical for normal learning and memory acquisition. Neuroscience. 2010;171:214–226. :10.1016/j.neuroscience.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 6. Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046 [DOI] [PubMed] [Google Scholar]
- 7. Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol (1985). 2006;101(4):1237–1242. :10.1152/japplphysiol.00500.2006 [DOI] [PubMed] [Google Scholar]
- 8. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14(2):125–130 [DOI] [PubMed] [Google Scholar]
- 9. Benedict C, Brooks SJ, Kullberg J, et al. Association between physical activity and brain health in older adults. Neurobiol Aging. 2013;34(1):83–90. :10.1016/j.neurobiolaging.2012.04.013 [DOI] [PubMed] [Google Scholar]
- 10. Bugg JM, Head D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol Aging. 2011;32(3):506–514. :10.1016/j.neurobiolaging.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erickson KI, Raji CA, Lopez OL, et al. Physical activity predicts gray matter volume in late adulthood: the Cardiovascular Health Study. Neurology. 2010;75(16):1415–1422. :10.1212/WNL.0b013e3181f88359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho AJ, Raji CA, Becker JT, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32(9):1371–1382. :10.1002/hbm.21113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rovio S, Spulber G, Nieminen LJ, et al. The effect of midlife physical activity on structural brain changes in the elderly. Neurobiol Aging. 2010;31(11):1927–1936. :10.1016/j.neurobiolaging.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 14. Gow AJ, Bastin ME, Muñoz Maniega S, et al. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology. 2012;79(17):1802–1808. :10.1212/WNL.0b013e3182703fd2 [DOI] [PubMed] [Google Scholar]
- 15. Colcombe SJ, Erickson KI, Scalf PE, et al. Aerobic exercise training increases brain volume in aging humans. J Gerontol A Biol Sci Med Sci. 2006;61(11):1166–1170 [DOI] [PubMed] [Google Scholar]
- 16. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. :10.1073/pnas.1015950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosano C, Venkatraman VK, Guralnik J, et al. Psychomotor speed and functional brain MRI 2 years after completing a physical activity treatment. J Gerontol A Biol Sci Med Sci. 2010;65(6):639–647. :10.1093/gerona/glq038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013;34:2972–2985. :10.1002/hbm.22119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165 [DOI] [PubMed] [Google Scholar]
- 20. Simonsick EM, Newman AB, Nevitt MC, et al. ; Health ABC Study Group. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56(10):M644–M649 [DOI] [PubMed] [Google Scholar]
- 21. Rosano C, Aizenstein HJ, Newman AB, et al. ; Health ABC Study. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62(1):307–313. :10.1016/j.neuroimage.2012.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(suppl 1):S208–S219. :10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- 23. Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148(2–3):133–142. :10.1016/j.pscychresns.2006.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biol Psychiatry. 2005;58(4):290–296. :10.1016/j.biopsych.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 25. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. :10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- 26. Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. :10.1148/radiol.2301021640 [DOI] [PubMed] [Google Scholar]
- 27. Taylor HL, Jacobs DR, Jr, Schucker B, Knudsen J, Leon AS, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31(12):741–755 [DOI] [PubMed] [Google Scholar]
- 28. Brach JS, Simonsick EM, Kritchevsky S, Yaffe K, Newman AB; Health, Aging and Body Composition Study Research Group. The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J Am Geriatr Soc. 2004;52(4):502–509. :10.1111/j.1532-5415.2004.52154.x [DOI] [PubMed] [Google Scholar]
- 29. Peterson MJ, Giuliani C, Morey MC, et al. ; Health, Aging and Body Composition Study Research Group. Physical activity as a preventative factor for frailty: the health, aging, and body composition study. J Gerontol A Biol Sci Med Sci. 2009;64(1):61–68. :10.1093/gerona/gln001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Craft LL, Zderic TW, Gapstur SM, et al. Evidence that women meeting physical activity guidelines do not sit less: an observational inclinometry study. Int J Behav Nutr Phys Act. 2012;9:122. :10.1186/1479-5868-9-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–M231 [DOI] [PubMed] [Google Scholar]
- 32. Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304–1309 [DOI] [PubMed] [Google Scholar]
- 33. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. :10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. :10.1016/j.tins.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 35. Marks BL, Madden DJ, Bucur B, et al. Role of aerobic fitness and aging on cerebral white matter integrity. Ann N Y Acad Sci. 2007;1097:171–174. :10.1196/annals.1379.022 [DOI] [PubMed] [Google Scholar]
- 36. Budde MD, Annese J. Quantification of anisotropy and fiber orientation in human brain histological sections. Front Integr Neurosci. 2013;7:3. :10.3389/fnint.2013.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.