Abstract
The Herpevac Trial evaluated a herpes simplex virus type 2 (HSV-2) glycoprotein D (gD2) subunit vaccine to prevent genital herpes. Unexpectedly, the vaccine protected against genital HSV-1 infection but not genital HSV-2 infection. We evaluated sera from 30 women seronegative for HSV-1 and HSV-2 who were immunized with gD2 in the Herpevac Trial. Neutralizing antibody titers to HSV-1 were 3.5-fold higher than those to HSV-2 (P < .001). HSV-2 gC2 and gE2 on the virus blocked neutralization by gD2 antibody, while HSV-1 gC1 and gE1 did not block neutralization by gD2 antibody. The higher neutralizing antibody titers to HSV-1 offer an explanation for the Herpevac results, and shielding neutralizing domains provides a potential mechanism.
Keywords: HSV-1, HSV-2, vaccine, glycoprotein D, glycoprotein C, glycoprotein E, neutralizing antibody, Herpevac Trial
Two publications reporting use of a herpes simplex virus type 2 (HSV-2) glycoprotein D2 (gD2) subunit vaccine adjuvanted with monophosphoryl lipid A (MPL) and aluminum hydroxide (alum) demonstrated mixed efficacy against HSV genital disease [1, 2]. The first report enrolled couples discordant for genital HSV-2 infection. In a subset analysis, the gD2 vaccine provided approximately 70% protection against HSV-2 genital disease in women seronegative for both HSV-1 and HSV-2 but not in women seropositive for HSV-1 or in men of any serostatus [2]. The results were sufficiently compelling to initiate the Herpevac Trial for Women, which enrolled >8000 women seronegative for both HSV-1 and HSV-2 [1]. The Herpevac Trial subjects were not restricted to discordant couples. Unexpectedly, the gD2 vaccine provided significant protection against HSV-1 but not HSV-2 genital infection and disease [1]. Sixty percent of genital disease in control subjects was caused by HSV-1, which has emerged as the leading cause of primary genital disease [1, 3]. Few HSV-1 infections were detected in the first report, owing to selection of HSV-2–discordant couples.
HSV-2 gD2 is required for virus entry into cells [4]. In choosing gD2 as an immunogen, a goal is to induce potent neutralizing antibodies to block entry. Enzyme-linked immunosorbent assay (ELISA) antibody titers but not cellular immune responses to gD2 were highly correlated with protection in the Herpevac Trial [5]. A possible explanation for protection against HSV-1 but not HSV-2 may relate to the relative ease of blocking entry, as measured by neutralization of the 2 viruses. Therefore, we evaluated the hypothesis that neutralizing antibody titers were higher to HSV-1 than to HSV-2 in the Herpevac Trial.
METHODS
Antibody Assays
We measured neutralizing antibodies by 2 methods. One approach evaluated the 50% end point neutralizing titer by incubating serial 2-fold dilutions of heat-inactivated serum with 100 plaque-forming units (PFU) of virus for 1 hour at 37°C, inoculating the virus-antibody mix onto Vero cells, overlaying with methylcellulose, and counting plaques at 72 hours [6]. The second assay measured the log10 titer reduction by using 106 PFU of virus mixed with a 1:40 dilution of heat-inactivated serum for 1 hour at 37°C and measuring plaques at 72 hours [7]. The log10 neutralization was calculated as the difference in titer, using serum from immunized individuals and nonimmune human serum as a control. ELISA was performed at a 1:500 serum dilution, using baculovirus-expressed gD1 (bac-gD1[306t]) or gD2 (bac-gD2[306t]) as antigen [6, 8].
Sera
Subjects were immunized at 0, 1, and 6 months [1, 2]. Paired sera were obtained before the first immunization and at month 7 from 35 Herpevac Trials subjects; 30 had received 20 µg of gD2 with MPL/alum, whereas 5 received hepatitis A vaccine as a control [1]. An additional 13 sera were obtained at month 7 from subjects seronegative for both HSV-1 and HSV-2 who were enrolled in GSK gD2 vaccine trials between 1995 and 1997. Ten had received gD2 with MPL/alum vaccine, and 3 were MPL/alum controls. These sera were stored at −80°C. Seven of 13 sera were from subjects enrolled at the University of Pennsylvania in HSV-007 (3 controls and 4 subjects immunized with 20 µg gD2 with MPL/alum) [2]. Five of 13 sera were from subjects enrolled in a dose-ranging study that used 20, 40, or 80 µg of gD2 with MPL/alum (HSV-014), and 1 subject was immunized with 20 µg gD2 with MPL/alum to evaluate diluent volumes (HSV-015).
Virus Strains
Four low-passage HSV-1 and 3 low-passage HSV-2 clinical isolates were used for neutralization assays. The gC and gE deletion strains were derived from low-passage HSV-1 and HSV-2 wild-type (WT) viruses NS and 2.12, respectively [9–11]. The University of Pennsylvania Institutional Review Board approved this study.
Statistics
Methods for calculating statistical significance are described in the legends of Figures 1 and 2.
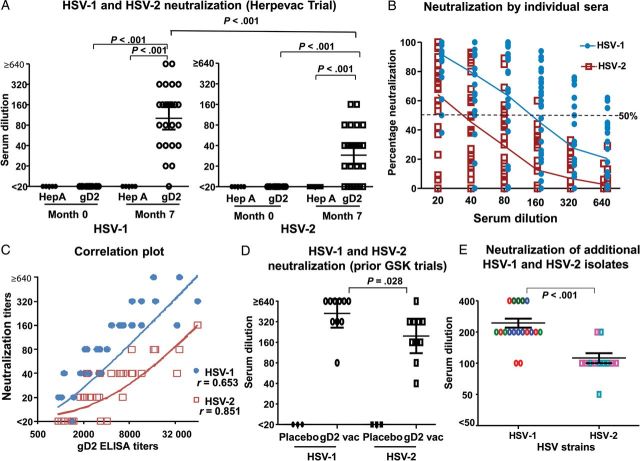
Figure 1.
A, Fifty percent end point neutralizing antibody titers of herpes simplex virus type 1 (HSV-1) and HSV-2 Herpevac Trial sera obtained at months 0 and 7 from hepatitis A (HepA; n = 5) and glycoprotein D2 (gD2; n = 30) immunized subjects. B, Neutralization at each serum dilution of gD2-immunized subjects (n = 30). The dotted line represents 50% neutralization. The solid lines represent the mean percentage neutralization at each dilution. The curves were compared by 2-way analysis of variance and the Bonferroni test for repeated measures. The values at each point are significantly different, except for 1:640. C, Correlation of gD2 enzyme-linked immunosorbent assay (ELISA) titers with HSV-1 (P < .001) or HSV-2 (P < .001) neutralizing antibody titers of subjects immunized with gD2 (n = 30). P values were calculated using the Pearson correlation for nonparametric data and a 2-tailed analysis. D, Fifty percent end point neutralizing antibody titers to HSV-1 or HSV-2 by sera obtained between 1995 and 1997 at month 7 from placebo recipient (n = 3) or gD2-immunized subjects (n = 10; 4 from HSV-007 and 6 from HSV-014 and 015). E, The same 6 HSV-014 and 015 sera were tested against 3 low-passage HSV-1 strains and 2 low-passage HSV-2 strains (each strain represented by a different color). Note that the y-axis scale differs in panel E. P values for panels A and D were calculated by 1-way analysis of variance, using the Dunn multiple comparisons test, whereas the P value in panel E was calculated by the Mann–Whitney test. In panels A, D, and E, data are geometric mean titers with 95% confidence intervals.
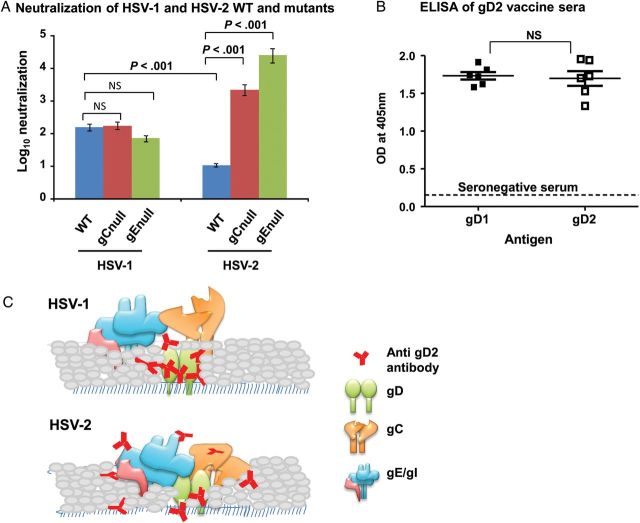
Figure 2.
A, Log10 neutralization of wild-type (WT), gCnull, and gEnull mutant viruses by sera from gD2 HSV-014 and 015 vaccinees (n = 6). Error bars represent standard errors of the mean. B, Enzyme-linked immunosorbent assay (ELISA) titers at a 1:500 dilution of vaccine sera (n = 6). The dotted line shows the ELISA value of serum specimens seronegative for both herpes simplex virus type 1 (HSV-1) and HSV-2. Results represent geometric mean titers with 95% confidence intervals. P values in panels A and B were calculated by 1-way analysis of variance with Tukey posttest analysis for pair-wise comparison. C, HSV-1 glycoprotein C1 (gC1) and gE1 fail to block antibody access to gD1. D, HSV-2 gC2 and gE2 shield antibody access to gD2. Abbreviation: NS, not significant.
RESULTS
Sera from subjects enrolled in the Herpevac Trial were selected by biostatisticians to include 10 each with high, intermediate, and low gD2 ELISA titers to reflect the distribution of ELISA responses in the entire study population [1]. Selected subjects were not infected with HSV before month 7. The geometric mean 50% end point neutralization titer of sera taken at month 7 was 1:101 for HSV-1, compared with 1:29 for HSV-2 (3.5-fold difference; Figure 1A). The mean percentage neutralization at each serum dilution was higher against HSV-1 than against HSV-2 (Figure 1B). A significant correlation was detected between gD2 ELISA titers (provided by the Herpevac investigators) and neutralizing antibody titers to HSV-1 and HSV-2 for the 30 subjects immunized with gD2 (Figure 1C) [5]. We evaluated neutralizing antibody titers of 13 additional sera from subjects enrolled in GSK gD2 vaccine trials between 1995 and 1997, including 3 sera from placebo-immunized subjects and 10 from subjects immunized with gD2 with MPL/alum. The geometric mean neutralizing antibody titer was 1:422 against HSV-1 and 1:97 against HSV-2 (4.4-fold difference; Figure 1D). Six of the 10 sera (those from HSV-014 and 015) were tested for neutralizing antibody titers against 3 additional low-passage HSV-1 strains and 2 additional low-passage HSV-2 strains. The geometric mean neutralizing antibody titer was 1:242 against HSV-1 and 1:106 against HSV-2 (2.3-fold difference; Figure 1E).
As a potential explanation for the greater neutralization of HSV-1 than HSV-2, we hypothesized that HSV-2 gC2 and gE2 may shield neutralizing domains on HSV-2 gD2 more effectively than gC1 and gE1 shield domains on HSV-1 gD1. This hypothesis is based on our prior studies demonstrating that gC1 and gE1 block access of neutralizing antibodies to HSV-1 glycoproteins involved in virus entry [12]. We compared neutralization of WT HSV-1 and HSV-2 with gC or gE mutant strains derived from the WT viruses. Six sera from gD2-immunized subjects (from HSV-014 and 015; Figure 1E) were evaluated. Neutralization was significantly greater against WT HSV-1 (2.2 log10) than against WT HSV-2 (1.0 log10; Figure 2A), confirming results using the 50% end point neutralizing titer (Figure 1). The gD2 vaccine sera reduced the titer of HSV-1 gCnull (NS-gCnull) virus by 2.2 log10 and HSV-1 gEnull (NS-gEnull) virus by 1.8 log10, which are not statistically different from findings for WT HSV-1 strain NS, indicating that gC1 and gE1 do not block neutralization by gD2 antibodies (Figure 2A). Strikingly, gC2 and gE2 had a significantly greater effect on blocking HSV-2 strain 2.12 neutralization, since the gD2 vaccine sera reduced the titer of HSV-2 gCnull (2.12-gC2null) virus by 3.3 log10 and HSV-2 gEnull (gE2-del) virus by 4.4 log10 (Figure 2A). Importantly, ELISA revealed that antibody produced by immunization with the gD2 vaccine reacted equally well to soluble, purified gD1 and gD2 proteins (Figure 2B), suggesting that antibody bound comparably to both glycoproteins.
DISCUSSION
The gD2 amino acid sequence included in the vaccine has 84% identity with gD1 [5]; therefore, cross-protection against HSV-1 is not surprising. However, it is surprising that neutralizing titers were significantly higher to HSV-1 than to HSV-2. The impressive difference in neutralizing antibody titers provides a plausible explanation for the protection against HSV-1 infection and disease reported in the Herpevac Trial. The low HSV-2 mean neutralizing titer of 1:29 at month 7 may explain the lack of protection observed against HSV-2.
The neutralizing titers of Herpevac Trial sera cannot be compared with sera from the 1995–1997 studies since Herpevac samples, not the 1995–1997 samples, were intentionally selected to include subjects with high, medium, and low ELISA titers. In addition, higher antigen doses were used in some of the 1995–1997 studies. Nevertheless, the sera can be compared for neutralizing titers to HSV-1 and HSV-2, since the same serum sample was tested against both viruses. Neutralizing titers were significantly higher to HSV-1 than to HSV-2 in sera from subjects immunized with the gD2 vaccine prepared years earlier than the Herpevac trial, which suggests that variations in vaccine lots cannot explain the higher HSV-1 titers. Higher neutralizing titers to HSV-1 than to HSV-2 were noted using a total of 4 HSV-1 and 3 HSV-2 low-passage isolates, which supports the generalizability of the observation.
We previously reported that HSV-1 gC1 and gE1 did not block neutralization by gD antibodies; however, gC1 and gE1 blocked neutralization when gD antibodies were combined with neutralizing antibodies to other glycoproteins involved in entry, including gB and gH/gL. We interpreted these results as indicating that gC1 and gE1 block domains of interaction between gD1 and other entry molecules [12]. Here we hypothesized that gC2 and gE2 shield gD2 from neutralizing antibodies, as a mechanism to explain the neutralizing antibody results. The ratio of gC1 to gD1 molecules on HSV-1 strain NS is 1:14, which may help explain the lack of blocking by gC1 [13]. Similar evaluations have not been performed with HSV-2; therefore, comparing the stoichiometry of glycoproteins on HSV-1 and HSV-2 is not yet possible.
Glycosylation of envelope proteins prevents antibody access to neutralizing domains on HIV-1 gp120 and influenza hemagglutinin [14]. Our results suggest that neighboring glycoproteins also may block antibody access. A hypothetical model depicting differences between gC and gE of HSV-1 and HSV-2 in blocking antibody access is shown in Figure 2C and 2D. Other potential mechanisms to explain greater neutralization of HSV-1 than HSV-2 include the possibility that epitopes recognized by antibody on gD1 may be more essential for virus entry than analogous epitopes on gD2 or that fewer gD molecules are expressed on HSV-1, making it easier to neutralize.
In the Herpevac Trial, ELISA titers correlated with protection against HSV-1, while CD4+ or CD8+ T-cell responses did not [1, 5]. We demonstrated here that ELISA titers correlated with neutralizing antibody titers; however, it is possible that other antibody responses are also important, including antibody-dependent cellular cytotoxicity and mucosal antibodies. T-cell responses that were not measured in the trial may also be important for protection.
Our results suggest that a vaccine containing gD2 antigen will likely induce higher neutralizing antibody titers to HSV-1 than to HSV-2, which is encouraging in terms of potential benefits of a vaccine that includes gD2. However, approaches are required to improve the neutralizing titers to HSV-2. One possibility is to induce antibodies to gD2 domains that are not shielded, while other possibilities include using higher concentrations of antigen, using more-potent adjuvants, or preventing antibody and complement immune evasion by the virus to enhance the efficacy of the antibodies produced [6].
Notes
Acknowledgments. We thank Gary Cohen and Roselyn Eisenberg for providing gD1 and gD2 antigens.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (subcontract of grant N01-AI-45250, to Saint Louis University) and GlaxoSmithKline.
Potential conflicts of interest. R. B. served on a data safety monitoring board for GSK on an unrelated vaccine. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–61. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 3.Wald A. Genital HSV-1 infections. Sex Transm Infect. 2006;82:189–90. doi: 10.1136/sti.2006.019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitbeck JC, Peng C, Lou H, et al. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–93. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belshe RB, Heineman TC, Bernstein DI, et al. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis. 2013 doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Awasthi S, Lubinski JM, Shaw CE, et al. Immunization with a vaccine combining herpes simplex virus 2 (HSV-2) glycoprotein C (gC) and gD subunits improves the protection of dorsal root ganglia in mice and reduces the frequency of recurrent vaginal shedding of HSV-2 DNA in guinea pigs compared to immunization with gD alone. J Virol. 2011;85:10472–86. doi: 10.1128/JVI.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judson KA, Lubinski JM, Jiang M, et al. Blocking immune evasion as a novel approach for prevention and treatment of herpes simplex virus infection. J Virol. 2003;77:12639–45. doi: 10.1128/JVI.77.23.12639-12645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Awasthi S, Lubinski JM, Friedman HM. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine. 2009;27:6845–53. doi: 10.1016/j.vaccine.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hook LM, Lubinski JM, Jiang M, Pangburn MK, Friedman HM. Herpes simplex virus type 1 and 2 glycoprotein C prevents complement-mediated neutralization induced by natural immunoglobulin M antibody. J Virol. 2006;80:4038–46. doi: 10.1128/JVI.80.8.4038-4046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang F, Tang W, McGraw HM, Bennett J, Enquist LW, Friedman HM. Herpes simplex virus type 1 glycoprotein E is required for axonal localization of capsid, tegument, and membrane glycoproteins. J Virol. 2005;79:13362–72. doi: 10.1128/JVI.79.21.13362-13372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F, Zumbrun EE, Huang J, Si H, Makaroun L, Friedman HM. Herpes simplex virus type 2 glycoprotein E is required for efficient virus spread from epithelial cells to neurons and for targeting viral proteins from the neuron cell body into axons. Virology. 2010;405:269–79. doi: 10.1016/j.virol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hook LM, Huang J, Jiang M, Hodinka R, Friedman HM. Blocking antibody access to neutralizing domains on glycoproteins involved in entry as a novel mechanism of immune evasion by herpes simplex virus type 1 glycoproteins C and E. J Virol. 2008;82:6935–41. doi: 10.1128/JVI.02599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handler CG, Eisenberg RJ, Cohen GH. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–70. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Julien JP, Lee PS, Wilson IA. Structural insights into key sites of vulnerability on HIV-1 Env and influenza HA. Immunol Rev. 2012;250:180–98. doi: 10.1111/imr.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]