Abstract
Background. Systemic inflammation has been linked to a failure to normalize CD4+ T-cell numbers in treated human immunodeficiency virus (HIV) infection. Although inflammatory cytokines such as interleukin 6 (IL-6) are predictors of disease progression in treated HIV infection, it is not clear how or whether inflammatory mediators contribute to immune restoration failure.
Methods. We examined the in vitro effects of IL-6 and interleukin 1β (IL-1β) on peripheral blood T-cell cycling and CD127 surface expression.
Results. The proinflammatory cytokine IL-1β induces cell cycling and turnover of memory CD4+ T cells, and IL-6 can induce low-level cycling of naive T cells. Both IL-1β and IL-6 can decrease T-cell surface expression and RNA levels of CD127, the interleukin 7 receptor α chain (IL-7Rα). Preexposure of healthy peripheral blood mononuclear cells (PBMCs) to IL-6 or IL-1β attenuates IL-7–induced Stat5 phosphorylation and induction of the prosurvival factor Bcl-2 and the gut homing integrin α4β7. We found elevated expression of IL-1β in the lymphoid tissues of patients with HIV infection that did not normalize with antiretroviral therapy.
Conclusions. Induction of CD4+ T-cell turnover and diminished T-cell responsiveness to IL-7 by IL-1β and IL-6 exposure may contribute to the lack of CD4+ T-cell reconstitution in treated HIV-infected subjects.
Keywords: HIV, interleukin 6, interleukin 7, interleukin 1 beta, immune failure, inflammation
In successfully treated human immunodeficiency virus (HIV) infection, chronic inflammation often persists and has been identified as a major predictor of morbidity and mortality [1, 2]. Moreover, in treated patients with controlled viremia, immune activation and elevated plasma markers of inflammation have been associated with poor CD4+ T-cell restoration [3, 4]; yet the mechanistic link between persistent immune activation, inflammation, and CD4+ T-cell restoration failure is unknown.
Earlier studies of patients with immune restoration failure found a link between higher indices of inflammation with morbidities and mortalities despite antiretroviral therapy (ART)–induced viral suppression [1, 5, 6]. It is not clear, however, whether the relationship between inflammation and immune failure is causal, and if it is, the mechanisms whereby inflammation might promote persistent CD4+ T-cell restoration failure (or vice versa) are not defined.
An increase in T-cell turnover is characteristic of untreated HIV infection [5–7], and recent data demonstrate elevated frequencies of cycling CD4+ (but not CD8+) T cells in treated HIV-infected patients with immune restoration failure [3, 8]. Loss of CD127, the interleukin 7 receptor α chain (IL-7Rα), and an imputed failure of homeostatic CD4+ T-cell expansion in response to IL-7 have also been identified in treated HIV infection [9, 10] and may contribute to CD4+ T-cell restoration failure [11].
This work asked whether inflammatory cytokines might contribute to CD4+ T-cell restoration failure in treated HIV infection. By treating peripheral blood mononuclear cells (PBMCs) from healthy subjects with the inflammatory cytokines interleukin 6 (IL-6) or interleukin 1β (IL-1β), we could recapitulate much of the immunophenotype seen in patients with immune restoration failure—decreased expression of CD127 [11, 12] and increased T-cell cycling, especially of memory CD4+ T cells [3, 6, 8, 13]. Importantly, IL-1β is expressed throughout all lymph node (LN) compartments in HIV-infected viremic patients and, while reduced with ART, still remains elevated, compared with levels in uninfected controls, providing in vivo support for our in vitro findings.
MATERIALS AND METHODS
Ethics Statement
All subjects provided written informed consent, in accordance with the Declaration of Helsinki. Patient studies were approved by the University Hospitals/Case Medical Center Institutional Review Board.
Cell Culture
PBMCs were purified by centrifugation over Ficoll-Hypaque (GE Healthcare, Sweden) and cultured in complete Roswell Park Memorial Institute 1640 medium with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin, 1% L-glutamine, and 1% sodium pyruvate at 37°C and 5% CO2. Where indicated, PBMCs were stimulated in the presence of recombinant human IL-6 (R&D Systems, Minneapolis, MN), recombinant human IL-1β (R&D Systems), or recombinant human IL-7 (Cytheris, Issy-les-Moulineaux, France). Both IL-6 and IL-1β induced Ki67 optimally at 10 ng/mL. IL-6 could downregulate T-cell CD127 surface expression at concentrations as low as 10 pg/mL, with optimal downregulation at a concentration of 1 ng/mL, and the maximal effect was seen at 2 days (data not shown). Maximal downregulation of CD127 expression by IL-1β was reached at a concentration of 10 ng/mL and at 2 days.
Flow Cytometry
Viable cells were gated using LIVE/DEAD-Aqua or yellow viability dye (Invitrogen, Grand Island, NY). Lymphocytes were identified by forward and side scatter, and T-cell phenotype was assessed using the following fluorochrome-conjugated monoclonal antibodies: anti-CD3 peridinin chlorophyll protein, anti-CD8 allophycocyanin (APC)–Cy7, anti-CD127 fluorescein isothiocyanate (FITC), anti-CD45RA phycoerythrin (PE)–Cy7, anti-CD27 APC, anti-CD197 Alexa Fluor 700 (all from BD Biosciences, San Jose, CA), and anti-CD4 Pacific Blue (Biolegend, San Diego, CA). Anti-α4β7 antibody was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program and was conjugated using an R-PE antibody conjugation kit (Abd Serotec, Oxford, United Kingdom). Cells were stained for 20 minutes in the dark at room temperature, washed, fixed in phosphate-buffered saline containing 2% formaldehyde, and acquired on an LSRII flow cytometer (Becton Dickinson, San Jose, CA). For detection of intracellular Ki67 and Bcl2, cells were surface stained, fixed, and permeabilized with a saponin-based buffer (BD Biosciences), followed by incubation with anti-Ki67-PE and anti-Bcl2-FITC (BD Biosciences, San Jose, CA) for 40 minutes on ice. For detection of phospho-epitopes, fixed cells were permeabilized with a methanol-based buffer (BD Biosciences) and stained with anti-phospho-Akt-PE (S473) and anti-phospho-Stat5 Alexa Fluor 647 (Y694) (BD Biosciences). Data were analyzed using FACSDIVA (version 6.2, BD Biosciences, San Diego, CA) or FlowJo software. Maturation subsets were determined based on CD45RA, CD27, and CCR7 expression.
CD127 RNA Measurement by Real-Time Quantitative Polymerase Chain Reaction (PCR)
Using a cocktail of antibodies targeting CD8, CD14, CD15, CD16, CD19, CD25, CD34, CD36, CD45RO, CD56, CD123, TCRγ/δ, HLA-DR, and glycophorin A, followed by magnetic bead separation (Miltenyi, Auburn, CA), naive CD4+ T cells were separated by negative selection from PBMCs treated for 2 days with IL-6 or IL-1β. Naive CD4+ T-cell purity was consistently >90%. Naive CD4+ T cells were lysed in RLT buffer (Qiagen, Valencia, CA) and stored at −80°C. RNA was isolated with an RNeasy mini kit (Qiagen) and was reverse transcribed using the High-Capacity RNA-to-cDNA kit (Applied Biosystems, Grand Island, NY). Complementary DNA was amplified by StepOnePlus (Applied Biosystems, Carlsbad, CA) real-time quantitative PCR in the presence of SYBR Green (Applied Biosystems). Primers for IL-7 receptor transcripts were 5′-AAAGTTTTAATGCACGAT-3′ and 5′-TGTGCTGGATAAATTCACATGC-3′. Gene expression was normalized to 18S ribosomal RNA, using primers obtained from Applied Biosystems (part 4 308 329).
CFSE Dye Dilution
Cell division was assessed by labeling PBMCs with CFSE dye (Molecular Probes Invitrogen, Grand Island, NY) for 10 minutes at 37°C. Staining was quenched by the addition of FBS for 5 minutes on ice. Cells were then washed and cultured as described above.
Immunohistochemistry and Quantitative Image Analysis
Immunohistochemical staining using rabbit anti-human mature IL-1β (ab2105; Abcam) was performed using a biotin-free polymer approach (Rabbit Polink-1, Golden Bridge International) on 5-µm tissue sections mounted on glass slides, dewaxed, and rehydrated with double-distilled water. Antigen retrieval was performed by heating sections in 0.01% citraconic anhydride at 122°C for 30 seconds. Slides were stained with optimal conditions determined empirically on an IntelliPATH autostainer (Biocare Medical) that consisted of a blocking step (Tris-buffered saline [TBS] with 0.05% Tween-20 and 0.5% casein) for 10 minutes and an endogenous peroxidase block, using 1.5% (v/v) H2O2 in TBS (pH 7.4), for 10 minutes. Rabbit anti-human mature IL-1β was diluted in blocking buffer and incubated for 1 hour at room temperature. Tissue sections were washed, and the Rabbit Polink-1 staining system (Golden Bridge International) was applied for 30 minutes at room temperature. Sections were developed with Impact 3,3′-diaminobenzidine (Vector Laboratories), counterstained with hematoxylin, and mounted in Permount (Fisher Scientific). Stained slides were scanned at high magnification (200×) with ScanScope CS System (Aperio Technologies), yielding high-resolution data for the entire tissue section. Representative regions of interest (500 µm2) were identified and high-resolution images extracted from whole-tissue scans. The percentage of the LN area that stained for IL-1β was quantified using Photoshop CS5 and Fovea tools.
Statistics
Continuous variables were compared between groups, using the Mann–Whitney U test (GraphPad Prism software, version 5.04). P values of <.05 were considered nominally significant.
RESULTS
Both IL-1β and IL-6 Exposure Can Drive CD4+ T Cells Into Cell Cycle
Microbial products such as lipopolysaccharide (LPS) have been strongly linked to immune activation in HIV infection [14, 15], and these elements are recognized to induce proinflammatory cytokines such as IL-6 and IL-1β. To begin to understand the impact of proinflammatory cytokines on T-cell activation and dysfunction in HIV-infected patients, PBMCs from healthy subjects were stimulated with IL-1β (10 ng/mL), IL-6 (10 ng/mL), or, as a positive control, IL-7 (2 ng/mL) for 7 days and examined for intranuclear Ki67. As peak induction of Ki67 by IL-7 was demonstrable after 7 days, we used this duration of culture to test the effects of inflammatory cytokines on cell cycling.
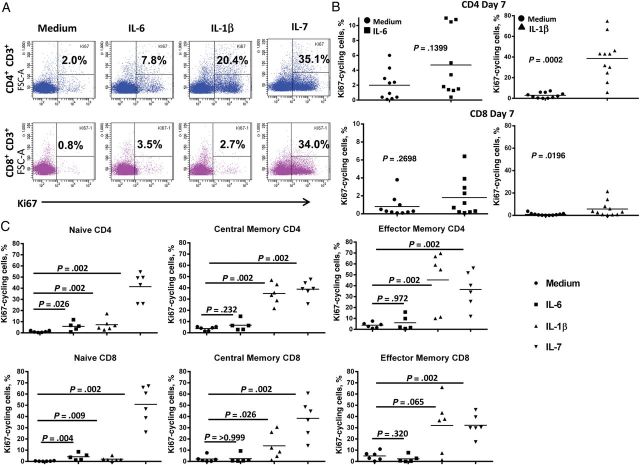
Whereas IL-7 induced dramatic increases in cycling of both CD4+ and CD8+ T cells, IL-1β induced cycling preferentially among CD4+ T cells, and although the increase in cycling induced by IL-1β was substantial, the effect was more subtle after IL-6 exposure (Figure 1A and 1B). Although there was some variability among subjects, the aggregate induction of cycling by IL-1β was significant (P = .0002), whereas the effect of IL-6 was not (P = .1399). As expected, the cells induced to express Ki67 after exposure to IL-7 included naive, central memory, and effector memory CD4+ and CD8+ T cells (Figure 1C). In contrast, cells expressing Ki67 after IL-1β exposure were primarily central and effector memory CD4+ T cells (Figure 1C). Low-level cycling was induced by IL-6 in naive CD4+ and CD8+ T cells (Figure 1C).
Figure 1.
Interleukin 6 (IL-6) and interleukin 1β (IL-1β) induce cell cycle initiation in T cells. A, Peripheral blood mononuclear cells from a healthy subject were stimulated with IL-6 (10 ng/mL), IL-1β (10 ng/mL), or interleukin 7 (IL-7; 2 ng/mL) for 7 days. After 7 days, cells were washed, stained for intranuclear Ki67, and examined by flow cytometry. B, Summary data of Ki67 induction by IL-6 (n = 10) and IL-1β (n = 11). Group data were compared using the Mann–Whitney U test. C, IL-1β–induced cycling is highest in memory T cells; IL-6-induces cycling in naive T cells. Central memory, CD45RA−CCR7+CD27+; effector memory, CD45RA−CCR7−CD27−; naive, CD45RA+CCR7+CD27+.
Cell Cycle Entry Induced by IL-7 or IL-1β Results in Cellular Proliferation, as Detected by Dilution of CFSE Dye
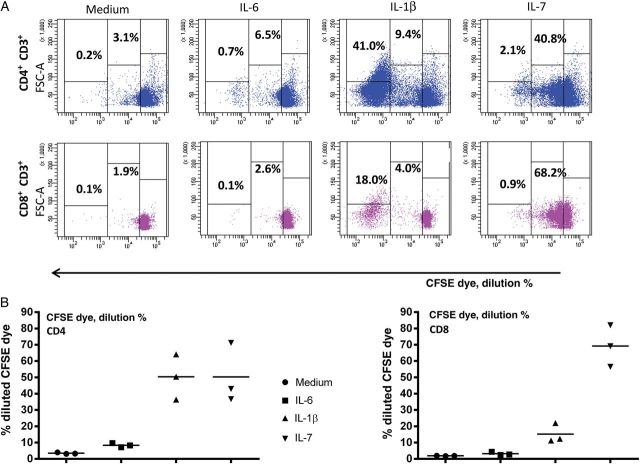
Induction of Ki67 expression is considered evidence of cell cycle entry [16]. Earlier work by others [6] and by our group [17] indicates that in vivo cell cycle entry in HIV infection is associated with an accelerated cellular turnover. To evaluate the effects of inflammatory cytokines on T-cell proliferation, CFSE-labeled PBMCs from healthy controls were stimulated with IL-1β, IL-6, or IL-7 to track cellular division. As expected, IL-7 induced substantial dye dilution, reflective of cellular division, in CD4+ and CD8+ T cells (Figure 2A and 2B). IL-1β induced proliferation of CD4+ T cells and, to a lesser degree, proliferation of CD8+ T cells, whereas IL-6 induced negligible cell division (Figure 2A and 2B). Interestingly, dye dilution induced by IL-1β resulted in most dividing cells undergoing >4 rounds of division, whereas proliferation induced by IL-7 appeared more controlled, with distinct populations undergoing 1 to >4 rounds of division. This suggests that cells induced to expand after IL-1β exposure may more readily become “exhausted” because of many rounds of division. This apparent differential regulation is even more demonstrable when we examined the maturation phenotypes of T cells that had divided in response to these cytokines (Figure 2C and 2D). Naive, central memory, and effector memory CD4+ (Figure 2C) and CD8+ (Figure 2D) T cells underwent 1, 2, 3, or 4 rounds of proliferation after stimulation with IL-7, whereas the many rounds of proliferation induced by IL-1B were restricted predominantly to central and effector memory CD4+ T cells (Figure 2C and 2D).
Figure 2.
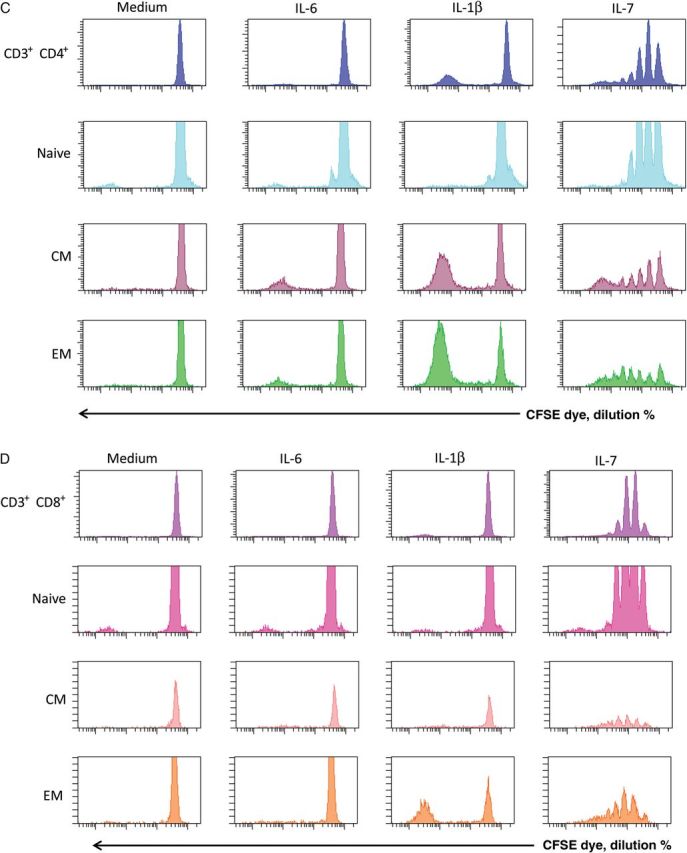
Inflammatory cytokines drive cell proliferation. A, Peripheral blood mononuclear cells from a healthy control were stained with CFSE dye and cultured for 7 days with interleukin 6 (IL-6; 10 ng/mL), interleukin 1β (IL-1β; 10 ng/mL), or interleukin 7 (IL-7; 2 ng/mL). After 7 days, cells were washed, stained for surface markers, and acquired by flow cytometry. B, Summary data of 3 experiments. C, Proliferation of CD4+ T-cell maturation subsets. IL-7–induced proliferation is seen in all CD4+ T-cell maturation subsets, whereas IL-1β–induced proliferation is seen almost exclusively in memory T cells. Data shown are representative of 6 experiments, using 4 different subjects. D, Proliferation of CD8+ T-cell maturation subsets. Data shown are representative of 6 experiments, using 4 different subjects. Maturation subsets were determined as outlined in Figure 1.
IL-1β and IL-6 Downregulate CD127 on CD4+ T Cells In Vitro
In mice, exposure to IL-6 in vitro downregulates T-cell CD127 expression [18]. This appears to be the case in humans, as well, because exposure of PBMCs to IL-6 for 2 days significantly decreased CD127 surface expression on CD4+ T cells but not on CD8+ T cells (Figure 3A). Similar findings were seen when purified T cells were exposed to IL-6, suggesting a direct effect (not shown). Within PBMCs, CD4+ T cells but not CD8+ T cells also downregulated CD127 upon exposure to IL-1β (Figure 3B). The IL-6 effect on CD127 downregulation was restricted to naive CD4+ T cells (Figure 3C), whereas IL-1β tended to affect naive and central memory CD4+ T cells, but this was not significant when the populations were analyzed separately (Figure 3D). CD127 is regulated both transcriptionally and posttranslationally [19]; we next asked whether reductions in CD127 surface expression were associated with reductions at the messenger RNA (mRNA) level. CD127 mRNA was reduced in naive CD4+ T cells in PBMCs treated with IL-1β or IL-6 (Figure 3E). Thus, IL-1β and IL-6 each can downregulate CD127 surface expression in CD4+ T cells, and this is due at least in part to decreased mRNA levels.
Figure 3.
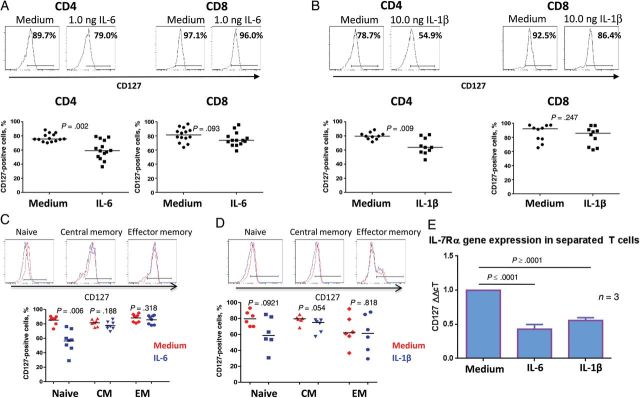
Interleukin 6 (IL-6) and interleukin 1β (IL-1β) downregulate CD127 surface expression on CD4+ T cells. Representative and summaries of CD127 surface expression assessed on CD4+ and CD8+ T cells in peripheral blood mononuclear cells (PBMCs) treated for 2 days in the presence or absence of 1 ng/mL IL-6 (A) or 10 ng/mL IL-1β (B). Representative and summaries of CD127 surface expression in CD4+ T-cell subsets assessed in PBMCs treated in the presence or absence of 1 ng/mL IL-6 (C) or 10 ng/mL of IL-1β (D) for 2 days. E, CD127 messenger RNA expression normalized to 18S ribosomal RNA, as assessed by real-time polymerase chain reaction analysis, in naive CD4+ T cells separated from PBMCs treated for 2 days with or without IL-6 or IL-1β. Data shown represent means and SD of 3 experiments. Statistical comparisons were performed using the Wilcoxon signed rank test.
Preexposure to Either IL-1β or IL-6 Impairs T-Cell Responses to IL-7
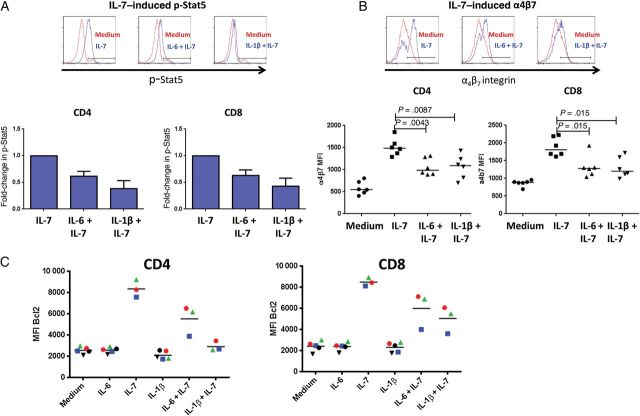
To ascertain whether the downregulation of CD127 was associated with impairment in responsiveness to IL-7, we assessed IL-7–induced phosphorylation of Stat5 and Akt in PBMCs pretreated with IL-6 or IL-1β. Although IL-7–induced Stat5 phosphorylation was unaffected in CD4+ and CD8+ T cells at 15 minutes or 2 days (not shown), maintenance of the p-Stat5 signal was diminished at 5 days after IL-7 stimulation in CD4+ and CD8+ T cells pretreated with IL-1β or IL-6 for 2 days (Figure 4A). Neither IL-1β nor IL-6 affected the IL-7–induced p-Akt signal in either CD4+ or CD8+ T cells (not shown).
Figure 4.
Interleukin 6 (IL-6) and interleukin 1β (IL-1β) impair T-cell responses to interleukin 7 (IL-7). Peripheral blood mononuclear cells (PBMCs) from a healthy donor were treated in medium alone or supplemented with 1 ng/mL IL-6 or 10 ng/mL IL-1β for 2 days, followed by addition of 5 ng/mL IL-7. A, Stat-5 phosphorylation was measured 5 days after IL-7 addition. Representative and summary of 7 independent experiments are shown as means and standard errors. B, Surface staining for the α4β7 heterodimer 5 days after IL-7 addition. C, PBMCs from healthy donors were treated in medium alone or supplemented with 10 ng/mL IL-6 or 10 ng/mL IL-1β for 2 days, and intracellular Bcl2 expression was measured in CD4+ and CD8+ T cells 7 days after addition of IL-7 (2 ng/mL).
CD4+ T-cell repopulation of the gut is impaired in treated HIV infection through mechanisms thought to be related to trafficking defects [20]. Since IL-7 signaling induces a gut-homing phenotype through upregulation of α4β7 integrin [21], we asked whether IL-6 or IL-1β can interfere with this process. IL-1β or IL-6 preexposure attenuated the upregulation of α4β7 on CD4+ and CD8+ T cells 5 days after exposure to IL-7 (but not after 2 days; Figure 4B). Attenuation appeared to be specific for CD127 signaling, because pretreatment with IL-1β or IL-6 had no effect on expression of α4β7 on T cells induced by retinoic acid (not shown). Bcl2 is a prosurvival protein that is upregulated by IL-7 and can inhibit cytochrome c release and subsequent apoptosis [22]. Preexposure of PBMCs to IL-6 or IL-1β for 2 days attenuated the upregulation of Bcl2 seen after 7 days exposure to IL-7 (Figure 4C). Thus, in vitro exposure to IL-1β or IL-6 impairs T-cell responsiveness to IL-7.
Elevated Inflammatory Cytokine Expression in HIV Infection
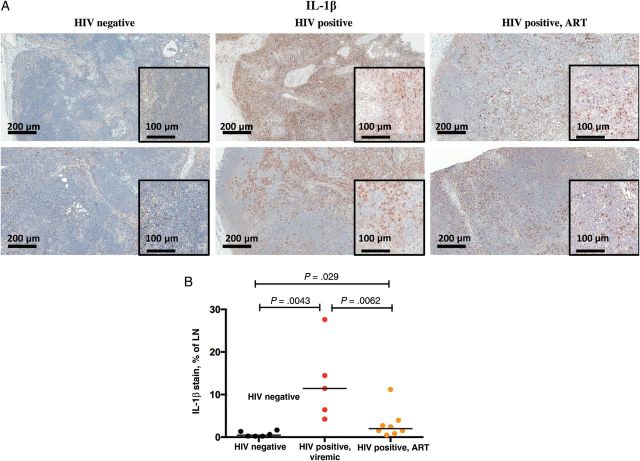
While numerous groups have found elevated levels of IL-6 in the plasma of HIV-infected subjects both before and after ART initiation [1–3, 23–26], information regarding systemic levels of IL-1β is scant [27–29]. Plasma levels of IL-1β were barely detectible, at <1 pg/mL, in both HIV-infected, treated patients and uninfected controls (not shown). We therefore examined LNs of patients and controls for expression of IL-1β. Characteristics of these subjects are shown in Table 1. Expression of mature IL-1β protein was increased in each of 5 LN samples from untreated, HIV-infected, viremic subjects when compared to levels in 6 healthy control LNs (Figure 5B). Mature IL-1β was expressed within all anatomical sites of the LNs in HIV-infected patients but most prominently within medullary cords, sinuses, and the T-cell zone (Figure 5A). Although IL-1β expression was reduced in patients receiving combination ART, it remained significantly elevated, compared with levels in LNs from healthy controls (Figure 5), and all but one of 8 treated patients had IL-1β levels exceeding the median of controls.
Table 1.
Clinical Characteristics of the 6 Healthy Controls and 13 Human Immunodeficiency Virus (HIV)–Infected Subjects Whose Lymph Nodes Were Examined in This Study
| Subject, by Group | ART Status | IL-1β Staining, % of LN | CD4+ T-Cell Count, Cells/mm3 | Plasma HIV RNA, Copies/mL |
|---|---|---|---|---|
| HIV infected, ART recipient | ||||
| 1 | Treated | 2.70 | 726 | 159 |
| 2 | Treated | 0.48 | 470 | 20 |
| 3 | Treated | 1.53 | 132 | 20 |
| 4 | Treated | 11.19 | 311 | 20 |
| 5 | Treated | 2.44 | 495 | 20 |
| 6 | Treated | 0.85 | 920 | 20 |
| 7 | Treated | 3.97 | 980 | 20 |
| 8 | Treated | 1.51 | 485 | 31 |
| Healthy control | ||||
| 1 | NA | 0.27 | ND | ND |
| 2 | NA | 0.64 | ND | ND |
| 3 | NA | 0.22 | ND | ND |
| 4 | NA | 0.22 | ND | ND |
| 5 | NA | 1.35 | ND | ND |
| 6 | NA | 1.65 | ND | ND |
| HIV infected, viremic | ||||
| 1 | Untreated | 4.23 | 476 | 1927 |
| 2 | Untreated | 27.62 | 500 | 6109 |
| 3 | Untreated | 14.47 | 521 | 1862 |
| 4 | Untreated | 6.45 | 950 | 13 888 |
| 5 | Untreated | 11.44 | ND | ND |
Abbreviations: ART, antiretroviral therapy; IL-1β, interleukin 1β; LN, lymph node; NA, not applicable; ND, not done.
Figure 5.
Interleukin 1β (IL-1β) is expressed in lymph nodes from human immunodeficiency virus (HIV)–infected patients and is decreased but not normalized in patients who received antiretroviral therapy (ART). A, Mature IL-1β stained lymph node (LN) sections from 2 healthy controls, 2 viremic HIV-positive patients, and 2 HIV-positive patients receiving ART (100× original magnification; insets, 200×). B, Summary data of LN IL-1β staining from 6 healthy controls, 5 untreated HIV-infected viremic patients, and 8 HIV-positive ART-treated patients.
DISCUSSION
Despite control of viremia, immune activation and inflammation persist in a significant proportion of otherwise effectively treated HIV-infected persons [3]. Among those in whom levels of circulating CD4+ T cells do not increasing to “normal,” activation and inflammation indices are especially elevated and are strongly linked to morbidity and mortality [3, 24, 30–32]. Here, we attempt to explore the relationship between heightened inflammation and CD4+ T-cell restoration failure and demonstrate the in vitro effects of 2 inflammatory cytokines, IL1β and IL6, on T-cell turnover and responsiveness to homeostatic signals.
Untreated HIV infection is characterized by increased T-cell cycling and turnover [5, 6, 13, 17]. With control of viremia, CD4+ and CD8+ T-cell cycling is typically diminished; however, cycling and turnover remains elevated in memory CD4+ T cells among subjects with CD4+ T-cell restoration failure [3, 6]. The drivers of cycling in this setting are not well characterized. Here, we show that IL-1β and, to a lesser degree, IL-6 can drive CD4+ T cells into cell cycle and, in the case of IL-1β, to proliferate. Interestingly, the cycling and proliferation induced by these inflammatory cytokines is distinguishable from the proliferation induced by the homeostatic cytokine IL-7. IL-7 induces cells to undergo distinct rounds of division that is seen in all CD4+ and CD8+ T-cell maturation subsets, whereas cycling and proliferation driven by the inflammatory cytokine IL-1β occurs predominantly among memory CD4+ T cells and typically results in at least 5 rounds of proliferation in almost all cells that divide.
This is the first work that demonstrates an effect of inflammatory cytokines in driving human T-cell turnover. An in vivo study in mice demonstrated enhanced memory T-cell expansion in response to antigenic stimulation if IL-1β was coadministered [33], and partially purified IL-1β could enhance the antigen induced proliferation of human T cells [34].
IL-7 is essential in the maintenance of T-cell homeostasis [19]. In HIV infection, systemic levels of IL-7 are increased, especially as circulating CD4+ T-cell numbers fall [10, 35–37]. Yet despite elevated levels of IL-7, responsiveness to IL-7 is likely impaired in HIV infection. Decreased expression of CD127 is demonstrable on both CD4+ and CD8+ T cells in both viremic HIV infection and in subjects with treatment-controlled viremia [9, 10, 12, 37–40], and in some studies it is linked to CD4+ T-cell restoration failure [9, 10, 12]. In lymphoid tissues, access to IL-7 also may be impaired, because the fibroblastic reticular cell network—a conduit for the trafficking of cytokines—is often disrupted by the deposition of collagen and resultant fibrosis [41–43]. We show here that exposure of PBMCs to IL-1β or IL-6 downregulates CD127 on CD4+ T cells but not on CD8+ T cells and that this effect is likely mediated at least in part at the level of RNA expression. The mechanism of these effects is not clear. The effect of IL-6 on CD127 expression may be mediated through Stat3 signaling because a Stat3 inhibitor (ethyl-1-(4-cyano-2,3,5,6-tetrafluorophenyl)-6,7,8-trifluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate) could block IL-6–mediated CD127 downregulation (data not shown). CD127 gene expression can be promoted by guanine and adenine binding protein and repressed by growth factor independence 1 [19]. The roles of these factors in IL-6– and IL-1β–mediated CD127 downregulation remain to be determined.
IL-7–induced Stat5 phosphorylation is impaired ex vivo in both viremic HIV-infected patients and in HIV-infected ART recipients [44, 45]. Because this might be related to decreased expression of CD127 or a downstream impairment of IL-7 responsiveness, we asked whether exposure of T cells to IL-1β or IL-6 decreases functional responses to IL-7 in vitro. We found that preexposure to IL-1β or IL-6 did not impair the initial induction of IL-7–mediated Stat5 phosphorylation. Yet the ability of T cells to sustain this signal was significantly diminished 5 days after IL-7 addition. IL-7 signaling promotes the expression of the prosurvival factor Bcl2 and the gut-homing integrin α4β7 on T cells, and we demonstrated that the impairment of IL-7 signaling is also associated with diminished induction of Bcl2 and α4β7 in response to IL-7 when cells are preexposed to IL-6 or IL-1β in vitro. These effects may mirror what has been seen in HIV infection, because levels of Bcl2 were lower in T cells from viremic HIV-positive patients [9, 10], as was the IL-7–induced upregulation of Bcl2 [9]. Although these inflammatory cytokines decreased CD127 expression, the attenuated IL-7 responsiveness was not solely attributable to loss of CD127 expression, because responses to IL-7 were blunted in both CD4+ and CD8+ T cells despite a relative preservation of CD127 surface expression on CD8+ T cells after exposure to these inflammatory cytokines. The mechanism for this effect is unclear, but it is plausible that exposure to these inflammatory cytokines may impair signaling events downstream of receptor ligation.
These in vitro effects of IL-1β and IL-6 recapitulate many of the T-cell phenotypes of immune failure in treated HIV infection, such as increased cycling of memory CD4+ (but not CD8+) T cells [3], diminished IL-7 receptor expression on CD4+ T cells [9, 11, 38], and a failure of IL-7 responsiveness even among CD127+ T cells [9, 11, 45].
Although evidence of reduced α4β7 integrin surface expression in treated HIV-infected subjects is lacking, the failure of gut T-cell restoration in treated HIV infection may be attributable to defective T-cell homing to this site [20]. It is plausible that reduced α4β7 expression on T cells may play a role in this defect, because induction of α4β7 expression on T cells from patients with immune failure who were treated with IL-7 is associated with gut T-cell repopulation and decreases in plasma sCD14 and D-dimer levels [46].
These findings support a model wherein the inflammatory environment that characterizes immune failure in treated HIV infection also may contribute to immune pathogenesis via accelerating CD4+ T-cell turnover, impairing homeostatic responses, and impairing restoration of gut T-cell numbers. Although it is well documented that systemic levels of IL-6 are elevated in both treated and untreated HIV infection, we have failed to demonstrate an increase in plasma levels of IL-1β in HIV-infected subjects (not shown). Yet our earlier studies showed increased spontaneous expression of IL-1β in histocultures of LNs from HIV-infected subjects [47], and Doitsh et al have found that abortive infection in the HIV-positive lymphoid tissues is linked to increases in IL-1β expression [48]. In the current study, we confirmed the presence of elevated LN IL-1β expression by immunohistochemical analysis during untreated HIV infection and showed that this typically persists even after therapy. The biologic relevance of this finding is supported by the frequent demonstration of a peripheral blood transcriptional signature of inflammasome activation in patients with CD4+ T-cell restoration failure [49].
These findings suggest that strategies targeting the expression or function of the proinflammatory cytokines IL-1β and IL-6 may have use in treated HIV infection, not only in preventing the clinical complications linked to inflammation, but perhaps also in enhancing CD4+ T-cell restoration. Agents targeting these elements have been approved for treatment of rheumatologic conditions, and in those settings, their toxicities, including risks for infectious complications, are recognized. Whether these targeted immunosuppressive interventions are safer or more effective than more broadly immunosuppressive approaches in HIV infection will require attention to the design and monitoring of clinical intervention studies.
Notes
Financial support. This work was supported by the National Institutes of Health (grants AI 076174 and AI-68636), the CWRU Center for AIDS Research (grant AI 36219), and the Fasenmyer Foundation.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tenorio A, Zheng E, Bosch R, et al. Soluble markers of inflammation and coagulation, but not T cell activation, predict non-AIDS-defining events during suppressive ART [abstract 790]. CROI 3–6 March 2013: 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. [Google Scholar]
- 2.Hunt P, Sinclair E, Rodriguez B, et al. Gut epithelial barrier dysfunction, inflammation, and coagulation predict higher mortality during treated HIV/AIDS [abstract 278]. CROI 5–8 March 2012: 19th Conference on Retroviruses and Opportunistic Infections; Seattle, Washington. [Google Scholar]
- 3.Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204:1217–26. doi: 10.1093/infdis/jir507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–43. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 5.McCune JM, Hanley MB, Cesar D, et al. Factors influencing T-cell turnover in HIV-1-seropositive patients. J Clin Invest. 2000;105:R1–8. doi: 10.1172/JCI8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kovacs JA, Lempicki RA, Sidorov IA, et al. Identification of dynamically distinct subpopulations of T lymphocytes that are differentially affected by HIV. J Exp Med. 2001;194:1731–41. doi: 10.1084/jem.194.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douek DC, Betts MR, Hill BJ, et al. Evidence for increased T cell turnover and decreased thymic output in HIV infection. J Immunol. 2001;167:6663–8. doi: 10.4049/jimmunol.167.11.6663. [DOI] [PubMed] [Google Scholar]
- 8.Marchetti G, Gori A, Casabianca A, et al. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–36. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 9.Colle JH, Moreau JL, Fontanet A, et al. Regulatory dysfunction of the interleukin-7 receptor in CD4 and CD8 lymphocytes from HIV-infected patients--effects of antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;42:277–85. doi: 10.1097/01.qai.0000214823.11034.4e. [DOI] [PubMed] [Google Scholar]
- 10.Rethi B, Fluur C, Atlas A, et al. Loss of IL-7Ralpha is associated with CD4 T-cell depletion, high interleukin-7 levels and CD28 down-regulation in HIV infected patients. AIDS. 2005;19:2077–86. doi: 10.1097/01.aids.0000189848.75699.0f. [DOI] [PubMed] [Google Scholar]
- 11.Colle JH, Moreau JL, Fontanet A, Lambotte O, Delfraissy JF, Theze J. The correlation between levels of IL-7Ralpha expression and responsiveness to IL-7 is lost in CD4 lymphocytes from HIV-infected patients. AIDS. 2007;21:101–3. doi: 10.1097/QAD.0b013e3280115b6a. [DOI] [PubMed] [Google Scholar]
- 12.Marziali M, De Santis W, Carello R, et al. T-cell homeostasis alteration in HIV-1 infected subjects with low CD4 T-cell count despite undetectable virus load during HAART. AIDS. 2006;20:2033–41. doi: 10.1097/01.aids.0000247588.69438.fd. [DOI] [PubMed] [Google Scholar]
- 13.Chattopadhyay PK, Douek DC, Gange SJ, Chadwick KR, Hellerstein M, Margolick JB. Longitudinal assessment of de novo T cell production in relation to HIV-associated T cell homeostasis failure. AIDS Res Hum Retroviruses. 2006;22:501–7. doi: 10.1089/aid.2006.22.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 16.Lopez F, Belloc F, Lacombe F, et al. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12:42–9. doi: 10.1002/cyto.990120107. [DOI] [PubMed] [Google Scholar]
- 17.Sieg SF, Bazdar DA, Lederman MM. S-phase entry leads to cell death in circulating T cells from HIV-infected persons. J Leukoc Biol. 2008;83:1382–7. doi: 10.1189/jlb.0907643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Yu Q, Erman B, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–54. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 20.Mavigner M, Cazabat M, Dubois M, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 2011;122:62–9. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cimbro R, Vassena L, Arthos J, et al. IL-7 induces expression and activation of integrin alpha4beta7 promoting naive T-cell homing to the intestinal mucosa. Blood. 2012;120:2610–9. doi: 10.1182/blood-2012-06-434779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 23.Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lafeuillade A, Poizot-Martin I, Quilichini R, et al. Increased interleukin-6 production is associated with disease progression in HIV infection. AIDS. 1991;5:1139–40. doi: 10.1097/00002030-199109000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Boulware DR, Hullsiek KH, Puronen CE, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–46. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreuzer KA, Dayer JM, Rockstroh JK, Sauerbruch T, Spengler U. The IL-1 system in HIV infection: peripheral concentrations of IL-1beta, IL-1 receptor antagonist and soluble IL-1 receptor type II. Clin Exp Immunol. 1997;109:54–8. doi: 10.1046/j.1365-2249.1997.4181315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reddy MM, Grieco MH. Neopterin and alpha and beta interleukin-1 levels in sera of patients with human immunodeficiency virus infection. J Clin Microbiol. 1989;27:1919–23. doi: 10.1128/jcm.27.9.1919-1923.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadeghi HM, Weiss L, Kazatchkine MD, Haeffner-Cavaillon N. Antiretroviral therapy suppresses the constitutive production of interleukin-1 associated with human immunodeficiency virus infection. J Infect Dis. 1995;172:547–50. doi: 10.1093/infdis/172.2.547. [DOI] [PubMed] [Google Scholar]
- 30.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–90. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armah KA, McGinnis K, Baker J, et al. HIV status, burden of comorbid disease, and biomarkers of inflammation, altered coagulation, and monocyte activation. Clin Infect Dis. 2012;55:126–36. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55:316–22. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ben-Sasson SZ, Hu-Li J, Quiel J, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–24. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu E, Rosenwasser LJ, Dinarello CA, Lareau M, Geha RS. Role of interleukin 1 in antigen-specific T cell proliferation. J Immunol. 1984;132:1311–6. [PubMed] [Google Scholar]
- 35.Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–9. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 36.Fry TJ, Connick E, Falloon J, et al. A potential role for interleukin-7 in T-cell homeostasis. Blood. 2001;97:2983–90. doi: 10.1182/blood.v97.10.2983. [DOI] [PubMed] [Google Scholar]
- 37.Bellistri GM, Casabianca A, Merlini E, et al. Increased bone marrow interleukin-7 (IL-7)/IL-7R levels but reduced IL-7 responsiveness in HIV-positive patients lacking CD4+ gain on antiviral therapy. PLoS One. 2010;5:e15663. doi: 10.1371/journal.pone.0015663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiazyk SA, Fowke KR. Loss of CD127 expression links immune activation and CD4(+) T cell loss in HIV infection. Trends Microbiol. 2008;16:567–73. doi: 10.1016/j.tim.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Paiardini M, Cervasi B, Albrecht H, et al. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J Immunol. 2005;174:2900–9. doi: 10.4049/jimmunol.174.5.2900. [DOI] [PubMed] [Google Scholar]
- 40.MacPherson PA, Fex C, Sanchez-Dardon J, Hawley-Foss N, Angel JB. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28:454–7. doi: 10.1097/00042560-200112150-00008. [DOI] [PubMed] [Google Scholar]
- 41.Zeng M, Smith AJ, Wietgrefe SW, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–9. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schacker TW, Nguyen PL, Martinez E, et al. Persistent abnormalities in lymphoid tissues of human immunodeficiency virus-infected patients successfully treated with highly active antiretroviral therapy. J Infect Dis. 2002;186:1092–7. doi: 10.1086/343802. [DOI] [PubMed] [Google Scholar]
- 44.Bazdar DA, Kalinowska M, Sieg SF. Interleukin-7 receptor signaling is deficient in CD4+ T cells from HIV-infected persons and is inversely associated with aging. J Infect Dis. 2009;199:1019–28. doi: 10.1086/597210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vranjkovic A, Crawley AM, Patey A, Angel JB. IL-7-dependent STAT-5 activation and CD8+ T cell proliferation are impaired in HIV infection. J Leukoc Biol. 2011;89:499–506. doi: 10.1189/jlb.0710430. [DOI] [PubMed] [Google Scholar]
- 46.Sereti I, Estes JD, Thompson WL, et al. Decreases in Colonic and Systemic Inflammation in Chronic HIV Infection after IL-7 Administration. PLoS Pathog. 2014;10:1–12. doi: 10.1371/journal.ppat.1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doitsh G, Galloway NL, Geng X, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–14. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahlers J, Sekaly R-P, Cameron M. Transcriptional and metabolic pathways link chronic inflammation and immune failure in treated HIV-1 infection [abstract 339]. CROI 3–6 March 2013: 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, Georgia. [Google Scholar]