Abstract
Human immunodeficiency virus type 1 (HIV-1)–infected individuals, despite receipt of antiretroviral therapy (ART), often have impaired vaccine responses. We examined the role that immune activation and cellular phenotypes play in influenza A(H1N1) vaccine responsiveness in HIV-infected subjects receiving ART. Subjects received the H1N1 vaccine (15-µg dose; Novartis), and antibody titers at baseline and after immunization were evaluated. Subjects were classified as responders if, by week 3, seroprotection guidelines were met. Responders had higher percentages of baseline naive T cells and lower percentages of terminally differentiated T cells, compared with nonresponders. Additionally, the naive CD4+ T-cell percentage and age were negatively correlated. Preservation of naive T-cell populations by starting therapy early could impact vaccine responses against influenza virus and other pathogens, especially as this population ages.
Keywords: HIV-1, influenza, H1N1, chronic inflammation, activation, naive T cells, CD4+ T cells, vaccination, anti-retroviral therapy
Apart from the severity of the 2009 influenza A(H1N1) pandemic, seasonal influenza can lead to serious illness in elderly individuals, people with chronic infections, and immunocompromised individuals [1]. Human immunodeficiency virus type 1 (HIV-1) infection induces changes in cytokine secretion, increased levels of apoptosis, exhaustion, and senescence [2]. As this population ages, so does their immune system, further compounding defects of the immune system, such as defects to the innate arm of immunity [3]. The data indicating that HIV–infected individuals have a higher frequency of influenza virus infection are limited [4], but several studies have demonstrated that HIV–infected patients are more likely to have severe or prolonged influenza virus infections [5]. The widespread use of highly active antiretroviral therapy (HAART) has decreased the rate of complications of influenza in HIV–infected individuals but has not returned the rate to that of the HIV-uninfected population [5]. As a consequence of this, treatment guidelines recommend yearly influenza vaccination for all patients with HIV infection [6].
HIV–infected individuals respond poorly to influenza vaccination. Poor responses have been associated with CD4+ T-cell counts and HIV RNA levels [7]. Despite immune reconstitution that is enough to prevent the development of opportunistic infections, HIV-positive subjects receiving ART are shown to have poor antibody responses and memory B-cell responses [8]. To achieve seroprotection in these individuals, often higher or multiple doses are necessary [9].
The purpose of this study was to understand what dysregulation might be occurring in HIV–infected individuals that prevents them from responding to influenza vaccination. Understanding the immune dysregulation in these individuals can help elucidate better preventive strategies against influenza virus and maybe other pathogens and improve the immunogenicity of vaccine regimens in this population. We have a particular interest in evaluating prevaccination baseline characteristics that would serve as predictors of their vaccine response. Specifically, we are interested in the CD4+ T-cell responses, which provide help to B cells.
METHODS
Vaccine
Subjects received a single 15-µg dose of the monovalent, unadjuvanted, inactivated, split virus H1N1 vaccine (Novartis, Basel, Switzerland). Each participant had baseline studies performed at the time of enrollment followed by the intramuscular administration of the 2009 H1N1 vaccine (0.5 mL) to one of the deltoid muscles. Two telephone calls and serological response evaluations were completed 21–28 days after vaccination.
Subjects
HIV–infected individuals >18 years of age who had an indication to receive the H1N1 vaccine were included in the study. Individuals with a known allergy to eggs or other components of the vaccine, a history of severe reactions to previous immunization with seasonal influenza vaccine, a known case of H1N1 influenza during the spring of 2009, or past receipt of the novel H1N1 vaccine were excluded. A total of 120 subjects were included in the study, which was described elsewhere by McKittrick et al [9]. All patients provided informed consent. Forty-six subjects had frozen peripheral blood mononuclear cells available to use for the purpose of this study. These 46 subjects had baseline hemagglutination inhibition (HAI) titers of <40, were receiving ART, and were aged 26–77 years (median age, 48 years); 69.6% were male, 63% were black, 10.9% were Hispanic/Latino, 23.9% were white, and 2.1% were Asian/Pacific Islander. Furthermore, they had an average CD4+ T-cell count (±SD) of 542 ± 306.8 cells/µL, and an average nadir CD4+ T-cell count (±SD) of 193 ± 187.2 cells/µL; the HIV RNA load was <400 copies/mL in 90% and below the limit of quantification in 85%. At week 3, 61% of the subjects met the guidelines for seroprotection.
Hemagglutination Inhibition Assay
Antibody titers of the 120 subjects were measured using an HAI assay as previously described [10]. The HAI assays were done by McKittrick et al at Bioqual [9]. For the 46 subjects in this study, if they had a week 3 titer of >1:40 and a 4-fold increase in their HAI titer, they were classified as seroprotected and responders to vaccination, while those with titers of <1:40 and/or a <4-fold increase in their HAI titer were classified as nonresponders.
Flow Cytometry
Samples from 46 of the 120 subjects were available and were examined for their memory and activation phenotypes, using multiparameter flow cytometry. Antibodies were as follows: CD3 (fluorescein isothiocyanate), CD4 (allophycocyanin [APC]–Cy7), CD8 (AF700), CD27 (APC), and HLA-DR (phycoerythrin [PE]–Cy5) from BD-Pharmingen; CD45RO (ECD) from Beckman Coulter; CD38 (PE-Cy7) from eBioscience; CD25 (PE) from Becton-Dickinson; CD127 (V450) from BD Horizon; and Viability Dye (Aqua) from Invitrogen. Forty-six subjects were analyzed, and cellular (CD27 and CD45RO) and activation (HLA-DR and CD38) phenotypes were compared using an unpaired t test between responders and nonresponders. Cell surface staining was done at 4°C for 30–45 minutes in the dark, and gating was done as described in Supplementary Figure 1.
Predictors of Response
A multivariate logistic regression model was used to examine the predictors of response, which included viral load, prevaccination CD4+ T-cell count, nadir CD4+ T-cell count, age, and naive and terminally differentiated CD4+ and CD8+ T cells. A multivariate logistic regression model was also used to examine the effect of age on the percentage of naive CD4+ T cells and total activated CD4+ T cells.
RESULTS
Flow Cytometry
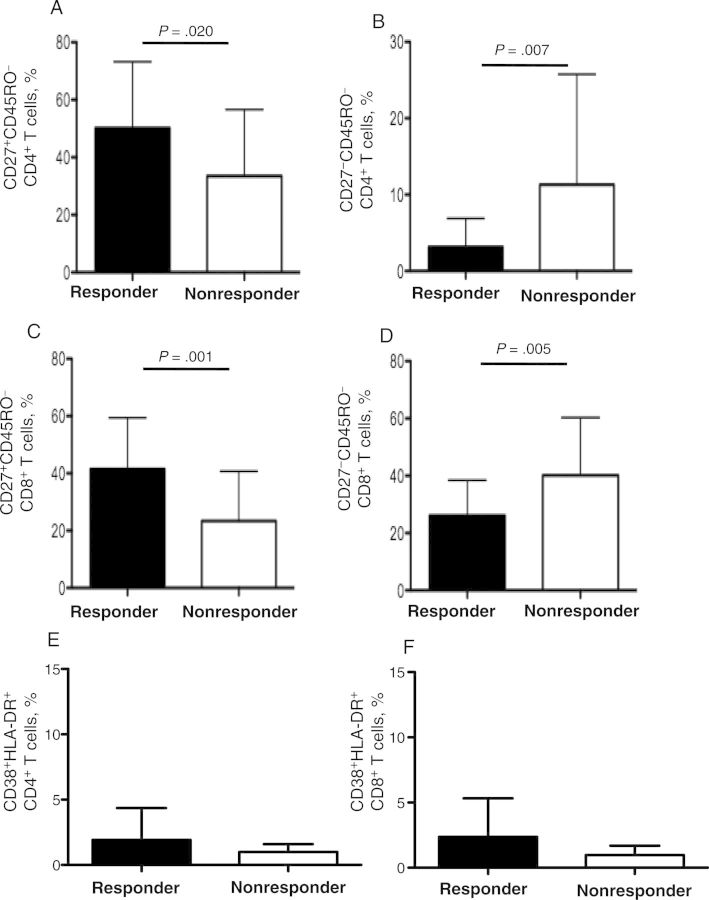
At week 3 after vaccination, 59% of the subjects in this study were responders (ie, they had HAI titers of ≥1:40 and a 4-fold HAI titer increase). We assessed the T-cell phenotype, including level of T-cell activation, of responders and nonresponders. We found that responders showed a higher percentage of baseline naive CD4+ T cells (50.2% vs 33.5%; P = .02; Figure 1A) and CD8+ T cells (41.5% vs 23.4%; P = .001; Figure 1C) and a lower percentage of terminally differentiated CD4+ T cells (3.2% vs 11.3%; P = .007; Figure 1B) and CD8+ T cells (26.1% vs 40.2%; P = .005; Figure 1D) than nonresponders.
Figure 1.
Peripheral blood mononuclear cells were stained for markers of differentiation and analyzed via multiparameter flow cytometry. A and B, Mean percentages (±SD) of naive (CD27+CD45RO−; 50.2% vs 33.5%; P = .02; A) and terminally differentiated effector (CD27−CD45RO−; 3.2% vs 11.3%; P = .007; B) CD4+ T cells in the CD4+ T-cell population among responders and nonresponders. C and D, Mean percentages (±SD) of naive (CD27+CD45RO−; 41.5% vs 23.4%; P = .001; C) and terminally differentiated effector (CD27−CD45RO−; 26.1% vs 40.2%; P = .005; D) CD8+ T cells in the CD8+ T-cell population among responders and nonresponders. E and F, Mean percentages (±SD) of activated (CD38+HLA-DR+) CD4+ T cells (1.56% ± 1.63% [n = 27] vs 1.95% ± 2.87% [n = 19]; P = .7; E) and CD8+ T cells (1.91% ± 1.79% [n = 27] vs 2.3% ± 3.63% [n = 19]; P = .8; F) among responders and nonresponders.
We did not observe a difference between responders and nonresponders on the baseline (day 0, before vaccination) frequency of the cellular activation markers HLA-DR+CD38+ for both CD4+ T cells (mean [±SD], 1.56% ± 1.63% [n = 27] vs 1.95% ± 2.87% [n = 19]; P = .688; Figure 1E) and CD8+ T cells (mean [±SD], 1.91% ± 1.79% [n = 27] vs 2.3% ± 3.63% [n = 19]; P = .8409; Figure 1F).
Predictors of Vaccine Response
In a logistic regression model that included the viral load, prevaccination CD4+ T-cell count, nadir CD4+ T-cell count, age, and percentages of naive and terminally differentiated CD4+ and CD8+ T cells, the frequencies of naive CD4+ T cells (n = 46; P = .024; R2 = 0.111) and terminally differentiated CD8+ T cells (n = 46; P = .0004; R2 = 0.251) were the only independent predictors of response.
Since age is known to put individuals at risk for severe influenza virus infection, we examined whether age had an effect on the CD4+ T-cell population. We noticed that age was significantly negatively correlated with the total percentage of naive CD4+ T cells (n = 46; P = .003; R2 = −0.182; Figure 2A). Furthermore, since HIV infection leads to depletion of the CD4+ T-cell population, we examined whether the nadir CD4+ T-cell count was related to the naive CD4+ T-cell population after the start of ART. There was a significant positive correlation between the nadir CD4+ T-cell count and the percentage of naive CD4+ T cells (n = 46; P = .01; R2 = 0.141; Figure 2B).
Figure 2.
Multivariate regression analysis examining linear regression between the percentage of naive (CD27+CD45RO−) CD4+ T cells in the CD4+ T-cell population and age (n = 46; R2 = −0.182; P = .003; A) and between the percentage of naive CD4+ T cells in the CD4+ T-cell population and the nadir CD4+ T-cell count (n = 46; R2 = 0.141; P = .01; B).
DISCUSSION
The baseline frequency of naive and terminally differentiated T cells were directly and inversely, respectively, associated with seroprotection after H1N1 vaccination (Figure 1A–D). However, responders and nonresponders did not differ significantly in their levels of baseline activation (Figure 1E–F).
Our study therefore demonstrates the potential roles that the frequencies of naive and terminally differentiated T cells play in vaccine responses and underscores the importance of preserving the naive T-cell population to ensure vaccination responses and presumably response to natural infection.
Patients with HIV infection have been characterized with premature immunosenescence and persistent ongoing inflammation or so-called inflammaging. This is similar to what is observed in older individuals but occurs decades earlier in patients living with HIV. This phenomenon occurs despite well-controlled viral replication with the use of HAART [2]. This immunosenescence has been associated with a lack of CD4+ T-cell recovery after the initiation of HAART [11], cancers, AIDS progression [2], and death. We hypothesized that this phenomenon would be associated with lack of vaccine response [2, 11].
In elderly individuals, the reduced numbers of naive T cells can potentially impact how likely an HIV-infected individual will achieve seroprotection after influenza vaccination. In the elderly population, there is a loss of thymic tissue, T-cell numbers, and stimulation sensitivity of the naive CD4+ T-cell population [12]. This depletion of the CD4+ T-cell subset and reduction in thymic function are also aspects that are shown to affect HIV–infected individuals [13]. Maintaining a healthy T-cell population is important since T-cell–mediated protection helps avoid issues with influenza virus infection when antibodies cannot provide sterilizing immunity [14]. It is possible that in addition to the immune dysregulation caused by HIV infection, age may be a compounding factor that influences whether an individual will respond to influenza vaccination and should be taken into consideration to provide better immunization strategies for these individuals.
Since age increases the risk for more-severe disease, the Centers for Disease Control and Prevention suggests that certain vaccines, such as pneumococcal and shingles vaccines, be given to elderly individuals aged ≥60 years [15]. Furthermore, as with HIV-negative elderly individuals, older HIV-infected individuals exhibit more activation and inflammatory cytokines/chemokines [2] and fewer naive CD4+ T cells (Figures 1A and 2A) than the general population, despite good virological control. Therefore, these findings suggest that it is necessary to administer some vaccines (such as shingles vaccine) earlier than currently recommended, before further immunological damage occurs.
In summary, our study suggests that the baseline frequency of naive T cells may affect the vaccine response to influenza in HIV-infected individuals, especially as they age. Strategies targeting the preservation of the naive population or the use of immunomodulators could potentially improve the vaccine response and potentially decrease the morbidity associated with these infections. It may be important to initiate ART early, especially since we saw a positive association with the nadir CD4+ T-cell count and naive CD4+ T-cell count (Figure 2B). Furthermore, response to vaccination may be used as a surrogate marker of immune recovery in patients with HIV infection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. L. A. R. thanks her thesis committee (Drs Luis Montaner, David Weiner, Michael Betts, Hildegund Ertl, and Ron Collman), for their advice and support; and all authors thank Edward Thompson and the Human Immunology Core, for flow cytometry advice.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases Center for AIDS Research of the University of Pennsylvania (grant P30-AI-045008-14).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Panel on Opportunistic Infections in HIV-Infected Adults and Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf. Accessed 20 January 2014.
- 2.Appay V, Almeida JR, Sauce D, Autran B, Papagno L. Accelerated immune senescence and HIV-1 infection. Exp Gero. 2007;42:432–7. doi: 10.1016/j.exger.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Panda A, Arjona A, Sapey E, et al. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–33. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen JP, Macauley C. Susceptibility to influenza A in HIV-positive patients. JAMA. 1989;261:245. doi: 10.1001/jama.1989.03420020097023. [DOI] [PubMed] [Google Scholar]
- 5.Neuzil KM, Coffey CS, Mitchel EF, Jr, Griffin MR. Cardiopulmonary hospitalizations during influenza season in adults and adolescents with advanced HIV infection. J Acquir Immune Defic Syndr. 2003;34:304–7. doi: 10.1097/00126334-200311010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Aberg JA, Gallant JE, Anderson J, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: recommendations of the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2004;39:609–29. doi: 10.1086/423390. [DOI] [PubMed] [Google Scholar]
- 7.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malaspina A, Moir S, Orsega SM, et al. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J Infect Dis. 2005;191:1442–50. doi: 10.1086/429298. [DOI] [PubMed] [Google Scholar]
- 9.McKittrick N, Frank I, Jacobson JM, et al. The Center for AIDS Research. Improved immunogenicity with high-dose seasonal influenza vaccine in HIV-infected persons: a single-center, parallel, randomized trial. Ann Intern Med. 2013;158:19–26. doi: 10.7326/0003-4819-158-1-201301010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Kendal AP, Skehel JJ, Pereira MS. Concepts and procedures for laboratory-based influenza surveillance. Atlanta: Centers for Disease Control and Prevention; 1982. pp. B17–35. [Google Scholar]
- 11.Hunt P, Sinclair E, Epling L, et al. T cell senescence and proliferation defects persist in treated HIV-infected individuals maintaining viral suppression and are associated with poor CD4+ T cell recovery [abstract 316]. Program and abstracts of the Conference on Retrovirus and Opportunistic Infections; 16–19 February; San Francisco, CA. 2010. [Google Scholar]
- 12.Provinciali M, Moresi R, Donnini A, Lisa RM. Reference values for CD4+ and CD8+ T lymphocytes with naïve or memory phenotype and their association with mortality in the elderly. Gerentology. 2009;55:314–21. doi: 10.1159/000199451. [DOI] [PubMed] [Google Scholar]
- 13.Hazenberg MD, Hamann D, Schultemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol. 2000;1:285–9. doi: 10.1038/79724. [DOI] [PubMed] [Google Scholar]
- 14.McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–9. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention, Department of Health and Human Services. Recommended immunizations for adults—by age and by medical condition; 2013; http://www.cdc.gov/vaccines/schedules/downloads/adult/adult-schedule-easy-read-bw.pdf. Accessed 7 October 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.