Abstract
Background. Ebola hemorrhagic fever (EHF) outbreaks occur sporadically in Africa and result in high rates of death. The 2000–2001 outbreak of Sudan virus–associated EHF in the Gulu district of Uganda led to 425 cases, of which 216 were laboratory confirmed, making it the largest EHF outbreak on record. Serum specimens from this outbreak had been preserved in liquid nitrogen from the time of collection and were available for analysis.
Methods. Available samples were tested using a series of multiplex assays to measure the concentrations of 55 biomarkers. The data were analyzed to identify statistically significant associations between the tested biomarkers and hemorrhagic manifestations, viremia, and/or death.
Results. Death, hemorrhage, and viremia were independently associated with elevated levels of several chemokines and cytokines. Death and hemorrhage were associated with elevated thrombomodulin and ferritin levels. Hemorrhage was also associated with elevated levels of soluble intracellular adhesion molecule. Viremia was independently associated with elevated levels of tissue factor and tissue plasminogen activator. Finally, samples from nonfatal cases had higher levels of sCD40L.
Conclusions. These novel associations provide a better understanding of EHF pathophysiology and a starting point for researching new potential targets for therapeutic interventions.
Keywords: Ebola virus, biomarkers, hemorrhage, Gulu, hemorrhagic fever virus
Ebola hemorrhagic fever (EHF) was first identified in 1976 during an outbreak in the Democratic Republic of the Congo (formerly known as Zaire). Since then, 21 additional outbreaks among humans have been recorded in sub-Saharan Africa. The largest of these outbreaks took place in the Gulu district of Uganda in 2000–2001 and was caused by Sudan virus (SUDV). This outbreak resulted in 425 cases, 216 of which were laboratory confirmed, and a 53% overall case-fatality rate [1].
The genus Ebolavirus includes 5 different viruses: SUDV, Tai Forest virus (TAFV), Reston virus (RESTV), Ebola virus (EBOV), and Bundibugyo virus (BDBV). EHF caused by EBOV has the highest fatality (57%–90%), followed by SUDV (41%–65%) and BDBV (40%); to date, RESTV infection has been asymptomatic in humans, and TAFV has only been identified in 2 human infections, both of which were nonfatal [2–4]. Clinical EHF is characterized by abrupt onset of fever, fatigue, headache, myalgia, and gastrointestinal distress 3–13 days after exposure to virus [5]. Many patients develop hemorrhagic manifestations, which has led to the term “hemorrhagic fever.”
The samples collected during the Gulu outbreak have already yielded extensive information about EHF pathophysiology, including the observation that aspartate aminotransferase (AST), D-dimer, blood urea nitrogen (BUN), and creatinine levels are higher than normal, while calcium and albumin levels are lower than normal in samples from fatal EHF cases [6]. Elevated levels of the cytokines interleukin 6 (IL-6), interleukin 8 (IL-8), interleukin 10 (IL-10), and macrophage inflammatory protein 1β (MIP-1β) were also associated with fatal outcomes [7]. Additionally, human leukocyte antigen B67 (HLA-B67), HLA-B15, and marked CD8 lymphopenia were associated with fatal outcome, and HLA-B7 and HLA-B14 were associated with nonfatal outcome [8, 9].
The goal of our study was to better understand the pathophysiology of EHF by correlating hemorrhagic manifestations and death with specific biomarkers. We measured levels of markers of immune function, endothelial activation, and coagulation in available patient serum samples, to determine whether alterations in levels of these biomarkers were associated with hemorrhagic manifestations, viremia, or death.
METHODS
Study Design
During the 2000–2001 Gulu outbreak, an international response team, including Centers for Disease Control and Prevention (CDC) staff, responded with clinical and technical assistance. Serum samples were frozen in liquid nitrogen in the field and have been stored that way since the time of the outbreak. For our analyses, samples were chosen to represent sex ratios, hemorrhagic manifestations, and death rates consistent with those observed during the outbreak (Table 1). Specimens were prioritized for novel analyses; serum chemistry analyses were performed on samples of sufficient volume. All samples were inactivated by γ irradiation before use.
Table 1.
Patient Characteristics
| Characteristic | This Study (n = 86) | Gulu Outbreak (n = 425) |
|---|---|---|
| Age, y, range | 6–60 | 0.25–81 |
| Sex | ||
| Female | 56 (65) | 267 (63) |
| Male | 30 (35) | 158 (37) |
| Any hemorrhagic manifestation | 31 (36) | 128 (30) |
| Death | 41 (48) | 224 (53) |
Data are no. (%) of patients, unless otherwise indicated. Gulu outbreak data are from Okware et al [1].
Institutional Review Board (IRB)
IRB approval was obtained before initiating the study, and an exemption was granted by the CDC Human Research Protection Office.
Luminex-Based Assays
The following assays were purchased from Affymetrix (Santa Clara, CA) and performed according to the manufacturer's instructions: a 26-plex assay for granulocyte macrophage colony stimulating factor (GM-CSF), GROα, interferon α2 (IFN-α2), IFN-β, IFN-γ, IL-10, interleukin 12p70 (IL-12p70), IL-12p40, interleukin 1α (IL-1α), IL-1β, IL-1 receptor antagonist (IL-1RA), interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), IL-6, IL-8, IFN-γ–inducible protein 10 (IP-10), monocyte chemoattractant protein 1 (MCP-1), macrophage colony-stimulating factor (MCSF), MIP-1α, MIP-1β, soluble CD40 ligand (sCD40L), soluble E-selectin (sE-selectin), soluble Fas ligand (sFasL), tumor necrosis factor α (TNF-α), and vascular endothelial growth factor A (VEGF-A); a 2-plex assay for D-dimer and tissue plasminogen activator (TPA); a 5-plex assay for plasminogen activator inhibitor-1 (PAI-1), serum amyloid antigen (SAA), regulated on activation, normal T-cell expressed and secreted (RANTES), soluble intracellular adhesion molecule 1 (sICAM-1), and soluble vascular cell adhesion molecule (sVCAM-1); and single-plex assays for C reactive protein (CRP) and fibrinogen. Assays for ferritin and cortisol were performed as single-plex assays according to manufacturer's instructions (Millipore, Billerica, MA). 2-plex assays were performed for tissue factor (TF) and thrombomodulin according to the manufacturer's instructions (Millipore). For samples with values outside the upper end of the standard curve, additional dilutions were made as necessary to obtain accurate values for all analytes.
Enzyme-Linked Immunosorbent Assays (ELISAs)
Mannose-binding lectin (MBL; Hycult Biotech, Plymouth Meeting, PA) and total immunoglobulin G (IgG; eBioscience, San Diego, CA) ELISAs were performed according to the manufacturers' instructions. For samples with values outside the upper end of the standard curve, additional dilutions were made as necessary to obtain accurate values for all analytes.
Serum Chemistry Analyses
A Piccolo comprehensive metabolic reagent disc was run on the Piccolo xpress Chemistry Analyzer (Abaxis, Union City, CA) to determine serum chemistry values for alanine aminotransferase (ALT), albumin, AST, alkaline phosphatase, calcium, chloride, creatinine, glucose, potassium, sodium, total bilirubin, total CO2, total protein, and BUN. Only 132 of the 187 samples had sufficient volume for serum chemistry analyses.
Human Immunodeficiency Virus (HIV) Testing
The Multispot HIV-1/HIV-2 Rapid Test (BioRad, Hercules, CA) was used to assess the HIV status of the patient cohort. Two patients (ages 7 and 19 years) did not have sufficient sample for analysis.
Viremia Analysis via Measurement of RNA Copy Number
Total RNA was isolated from serum using the MagMAX-96 Viral RNA Isolation Kit on the MagMAX Express-96 Magnetic Particle Processor (Ambion, Grand Island, NY). In parallel, serial dilutions of a normal human serum specimen inoculated with a known titer of SUDV was subjected to RNA isolation and used to generate a standard curve. Real-time polymerase chain reaction was then performed as previously described [10].
Statistical Analysis
Data on sex, age, date of symptom onset, viremia levels, hemorrhagic manifestations, HIV status, patient survivability, and date of sample collection were available for the 86 patients included in our study. Only samples taken 0–15 days from symptom onset were analyzed, to represent the normal course of the illness.
Analysis of variance (ANOVA) was conducted for each of the 55 analytes, using sex, age, day from symptom onset, viremia, death, hemorrhaging, and HIV status as the independent variables. All variables were converted to categorical variables (meaning that there was limited number of outcomes [ie, viremic vs not viremic]) because of the number of samples and the distribution of the numerical data, with the date from symptom onset converted to 3 categories (0–5 days, 6–10 days, and 11–15 days). An initial overall α of 0.25 was set, and the Bonferroni inequality was used to arrive at a critical P value of .0045 for the individual analytes. Using these criteria, 29 of the 55 analytes had statistically significant differences. Knowing the impact of multiple testing on the power of tests, the technique of Yoav and Hochbergs was used to control the false discovery rate to balance the power and type 1 error of the analyses [11]; this correction resulted in 10 additional analytes meeting the criteria for statistical significance. The independent variables were analyzed to measure colinearity, but no significant variance inflation was observed.
Ultimately, our study included 161 samples, each described by 9 categorical variables. Each sample was analyzed for 41 different analytes (one sample was insufficient to perform a subsequent analysis of 2 analytes). An analysis of metabolic information was attempted for all samples, but only 132 samples had sufficient volume for analyzing 14 additional analytes.
Eight analytes (IL-12p70, IL-12p40, IL-2, IL-4, IL-5, sFasL, IFN-γ, and IFN-α2) had statistically significant correlations but were excluded from analysis because ≥30% of the samples had a value below the limit of detection. We therefore did not attempt to make conclusions based on data that could have been affected by degradation over time.
Model Selection
After the initial ANOVA, 27 significant analytes were selected for further analysis. Stepwise regression for each significant analyte was used to find the best model, excluding insignificant variables at an α of 0.05. Particular attention was paid to those analytes in which age, death, viremia, hemorrhaging, and HIV status were significant. Ten analytes were significant for age, 16 for death, 7 for viremia, 8 for hemorrhaging, and 2 for HIV status. We compiled the analytes that were significantly different for these variables and calculated the means and standard error for each interval.
Finally, 2 additional statistical tests were performed to measure the relationships between death and both viremia levels and the presence of hemorrhaging. The distribution of the viremia levels was clearly nonnormal, so the Mann–Whitney U test was used to determine differences in viremia for patients with fatal and those with nonfatal outcomes. Patients with no measurable viremia were excluded from analysis. The survivability of patients with and those without hemorrhaging was also examined, but no significant differences were found.
RESULTS
Study Population
The study included 86 EHF patients from the 2000–2001 Gulu outbreak that were representative of the outbreak as a whole. As noted in previous outbreaks, more women were affected than men, likely because women are commonly caretakers, a known risk factor for contracting EHF. Thirty-six percent of the patients in this study had hemorrhagic manifestations (defined as any of the following: vomiting blood, blood in the stool, or bleeding from the gums, skin, or eyes), and approximately half of the patients died, consistent with reported death rates for SUDV (Table 1). One limitation to our study was that the limited sample volume influenced our selection of samples because we only selected patients for whom there were sufficient sample volume to complete the desired testing. This may have biased our data slightly toward the nonfatal group.
In the filovirus field, it has generally been assumed that immune system deficiencies associated with HIV infection would likely negatively impact EHF outcome. As the HIV status of EHF patients has never been determined previously, HIV testing was conducted on patients included in this study. None of the patients <18 years of age were HIV positive. Of the 67 adults (ages 18–60 years), 12 (17.9%) were HIV positive. This rate is roughly consistent with data on HIV prevalence in the Gulu region of Uganda during the time of the outbreak; 12% of women attending antenatal clinics were reported to be HIV positive [12]. Of the 12 HIV-positive adults, 5 were male and 7 were female. Although CD4+ T-cell counts were unknown in these patients, 50% of the HIV-positive adults (3 males and 3 females) had fatal outcomes. This mortality rate was approximately the same as that in the total adult population, suggesting that an individual's HIV status did not significantly affect outcome during SUDV infection.
Study Design
Fifty-five different biomarkers were assessed in 187 serum samples in this study. Thirty-six patients had ≥2 serial samples collected 1–15 days after onset of symptoms; composite data rather than individual patient data are presented, to provide the most objective data for analysis. Supplementary Table 1 provides the statistical significance for each biomarker tested and its relationship to the examined variables. The data presented in this article represent the statistically significant associations observed between a given biomarker and death, hemorrhagic manifestations, or viremia.
Serum Chemistry Analysis and Viremia
Serum chemistry analyses were performed on many patients as part of their clinical management during the 2000–2001 outbreak. These data were still available, allowing for a direct assessment of the stability of several metabolic analytes after 12 years of storage in liquid nitrogen. Many analytes were strikingly stable, as shown by a maximal coefficient of variance of 7.4% between the original measurement and the repeat measurement for ALT, AST, BUN, creatinine, total protein, and albumin levels (Table 2). The more labile components, CO2 and electrolytes, were excluded from our analysis.
Table 2.
Coefficient of Variance (CV) Between Repeat Chemistry Measurements After 12 Years of Storage in Liquid Nitrogen
| CV | ALT | AST | BUN | CRE | TP | ALB |
|---|---|---|---|---|---|---|
| Percentage difference, mean (range) | 2.3 (0–7.4) | 1.5 (0–4.4) | 1.7 (0–7.1) | 2.2 (0–5.4) | 0.96 (0–2.9) | 0.75 (0–2.8) |
Abbreviations: ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRE, creatinine; TP, total protein.
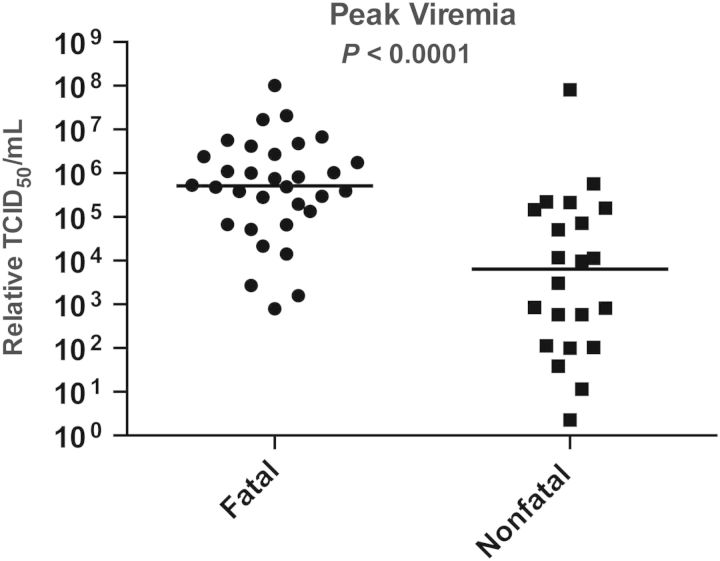
Previously reported associations between fatal outcomes and AST, BUN, creatinine, and albumin levels [6] were again seen in our study (data not shown) [6]. Higher levels of viremia were associated with a fatal outcome, as previously demonstrated [10] (Figure 1).
Figure 1.
Viremia as a function of clinical outcome. Patients with fatal outcomes had higher viral loads. TCID50, median tissue-culture infective dose.
Cytokines and Chemokines
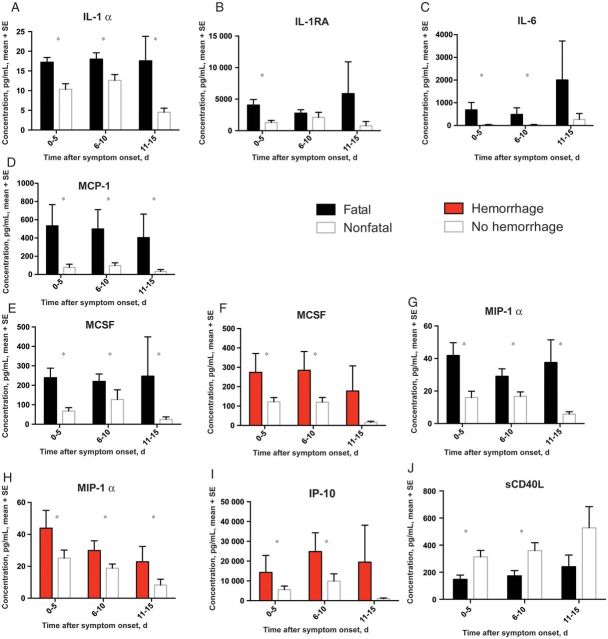
Cytokines and chemokines are a diverse, extensively studied group of proteins that modulate the immune response. We analyzed 25 different cytokines and chemokines. IL-1α, IL-1RA, IL-6, MCP-1, MCSF, and MIP-1α levels were all elevated in patients with a fatal outcome (Figure 2A–E and 2G). Previously published data have shown associations between MIP-1β, IL-6, IL-8, and IL-10 levels and fatal outcomes in SUDV-infected patients [7]. All of these prior associations were observed in our study. However, IL-8 levels were only elevated very late in the infection in patients with a fatal outcome (data not shown); MIP-1β levels tended to be higher in patients with fatal outcomes but did not meet the cutoff for statistical significance; and IL-10 levels demonstrated an age-specific association with death. GRO-α levels, similar to IL-8 levels, were only elevated late during infection in patients who died (data not shown). IL-1α, MCSF, and IP-10 levels were elevated in samples with documented viremia, compared with samples with no detectable virus, but this finding was independent of their relationship with death (data not shown). MCSF, MIP-1α, and IP-10 levels were also elevated in patients who developed hemorrhagic manifestations (Figure 2F, 2H, and 2I). Unexpectedly, elevated levels of sCD40L correlated with nonfatal outcomes, a first for any report of EHF (Figure 2J).
Figure 2.
Cytokines and chemokines associated with a fatal outcome (black bars) or hemorrhagic manifestations (red bars). *P ≤ .05. Abbreviations: IL-1α, interleukin 1α; IL-1RA, interleukin 1 receptor antagonist; IL-6, interleukin 6; IP-10, interferon γ–inducible protein 10; MCP-1, monocyte chemoattractant protein 1; MCSF, macrophage colony-stimulating factor; sCD40L, soluble CD40 ligand; SE, standard error.
Biomarkers of Inflammation
The acute phase response refers to a constellation of host responses that occur following infection and in other inflammatory diseases [13]. These responses are classically triggered by proinflammatory cytokines, such as IL-6, IL-1, IFN-γ, and TNF-α. Increased liver production of CRP and SAA and decreased production of albumin are common consequences of the acute-phase reaction. In addition, hypergammaglobulinemia and hyperferritinemia are often seen. These markers of inflammation are used clinically in many disease processes to help in diagnosis and to follow a patient's response to therapy. CRP levels were within the normal range in all samples and were excluded from further analysis. Ferritin levels were elevated in patients with fatal outcomes and in those with hemorrhagic manifestations (Figure 3A and 3B). Ferritin levels were also associated with viremia, independent of the associations with death and hemorrhage (data not shown). Ferritin levels were higher in HIV-positive patients than in HIV-negative patients (data not shown); this has been previously noted specifically in association with advanced HIV disease [14, 15]. HIV-positive patients also had higher total IgG levels than HIV-negative patients (data not shown), a well-documented phenomenon [16]. Finally, levels of sICAM, a marker of endothelial activation [17], were elevated in patients with hemorrhagic manifestations (Figure 3C)
Figure 3.

Markers of inflammation associated with a fatal outcome (black bars) or hemorrhagic manifestations (red bars). *P ≤ .05. Abbreviations: SE, standard error; sICAM, soluble intracellular adhesion molecule 1.
Biomarkers of Coagulopathy
The frequency of hemorrhagic manifestations during EHF warranted an examination of the measureable factors that control hemostasis. The delicate balance of coagulation and fibrinolysis is depicted in a simplified model in Figure 4. In this study, PAI-1, fibrinogen, TPA, D-dimer, thrombomodulin, and TF levels were measured in all patient samples. TPA, D-dimer, thrombomodulin, and TF levels were all statistically significant in our analysis. Thrombomodulin levels were elevated in patients with fatal outcomes and in patients with hemorrhagic manifestations (Figure 4A and 4B). D-dimer levels were elevated in patients with a fatal outcome (Figure 4C), as previously reported [6]. TPA levels were higher in viremic patients (Figure 4D). TF levels were within the normal range in all samples tested but were slightly higher in samples with detectable viremia than in those without detectable viremia (Figure 4E). Notably, TF levels were not associated with hemorrhagic manifestations or death.
Figure 4.
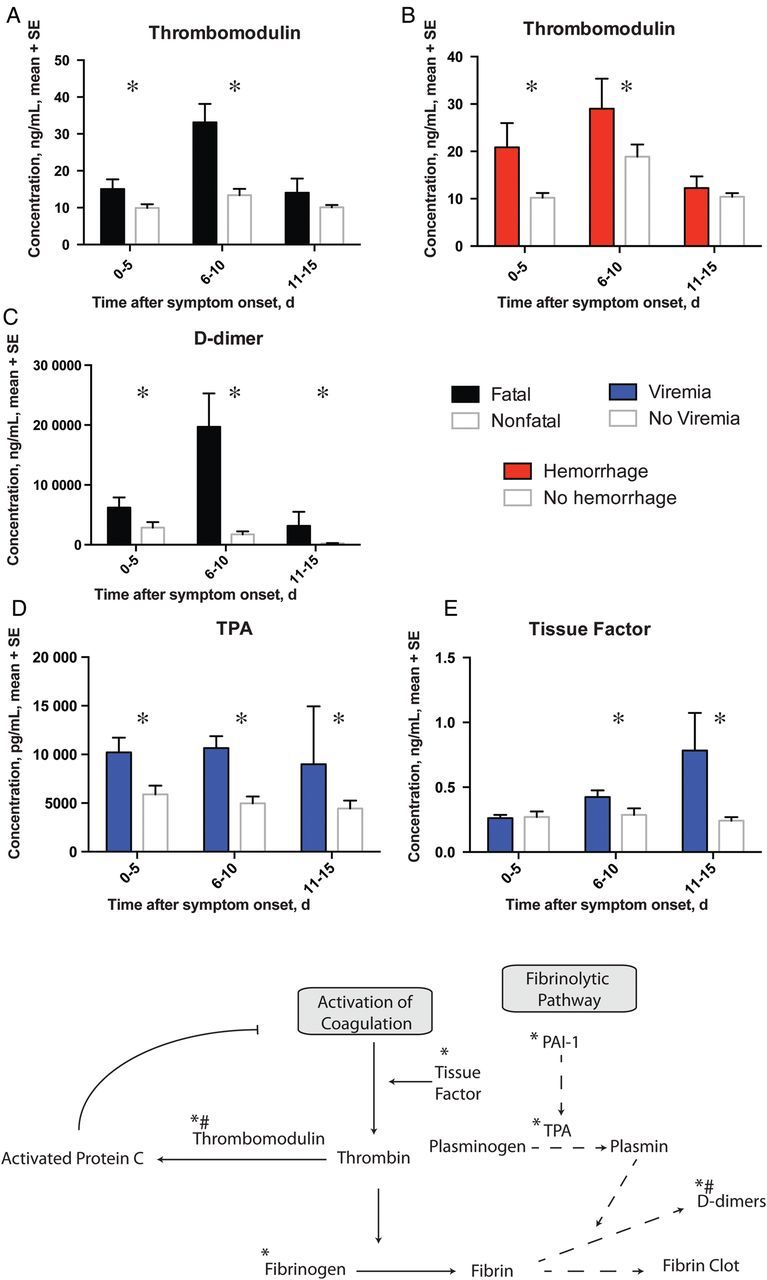
Markers of coagulation and fibrinolytic pathways associated with a fatal outcome (black bars), hemorrhagic manifestations (red bars) or viremia (blue bars). *P ≤ .05. A simplified model of the coagulation/fibrinolytic pathway is also depicted. Proteins that are known to play a major role in the pathways are noted. Arrows indicate activation while bars indicate inhibition. *, analytes assessed in the study; #, analytes associated with death.
DISCUSSION
We found associations between multiple proinflammatory cytokines and increased death rates, as previously shown in humans [7, 18–20] and EBOV-infected nonhuman primates [21], consistent with the idea of systemic inflammation contributing to a fatal outcome. We also found several novel associations, most strikingly, the association between increased sCD40L levels and nonfatal outcomes. Since sCD40L is largely produced by activated platelets [22], increased sCD40L levels could represent ongoing repair of altered endothelium by activated platelets. Additionally, nitric oxide (NO) inhibits platelet activation [23], and elevated NO levels have been reported in fatal EHF cases [9]. Therefore, the deleterious effects of NO could be related to inhibiting platelet activation, in addition to its vasodilatory effects. Effective repair of altered endothelium by activated platelets could lead to improved hemodynamic stability in patients and, thus, improved clinical outcomes.
Ferritin, another biomarker we examined, is a well-established acute-phase reactant that has been associated with severe disease in both dengue and Crimean Congo hemorrhagic fevers [24, 25]. Ferritin is the intracellular storage form of iron, and its levels increase during times of stress or inflammation. Ferritin levels were higher in samples from patients with EHF who had fatal outcomes, detectable viremia, and hemorrhagic manifestations; thus, ferritin could prove to be a useful clinical maker of EHF severity.
The striking clinical manifestation of hemorrhage during historic EHF outbreaks led to the naming of the disease as Ebola hemorrhagic fever, which has resulted in the perception that hemorrhage occurs in most patients and is associated with fatal outcomes. However, published data from the 3 largest EHF outbreaks, caused by EBOV (Kikwit, Democratic Republic of the Congo, 1995), SUDV (Gulu, Uganda, 2000), and BDBV (Bundibugyo, Uganda, 2007), demonstrate hemorrhagic manifestations in only 41%, 30%, and 46.5% of total cases, respectively [1, 3, 26]. Furthermore, no significant correlation between hemorrhage and death has been found in these same outbreaks of EBOV and BDBV infections. Consistently, we found no statistically significant differences in survival between patients with and those without hemorrhage (P = .23). Therefore, it is not surprising that, of the 6 different coagulation and fibrinolysis markers we assessed, only thrombomodulin was associated with both fatal outcome and hemorrhagic manifestations. Thrombomodulin is a marker of endothelial activation/dysfunction and, as discussed below, has many functions besides inhibiting coagulation. There is a common perception, not only in EHF but also in routine sepsis, that correction of coagulopathy will improve survival outcomes. However, therapeutic interventions that have focused on targeting coagulopathy in nonhuman primate models of EBOV only moderately increased survival [27, 28], and use of activated protein C in septic patients in the intensive care unit has been similarly disappointing [29]. A large focus has been placed on understanding the role of TF in EHF, given the marked increases in TF expression seen in monocytes in nonhuman primates infected with EBOV [30]. However, when TF levels were examined in patients from this outbreak, which was caused by SUDV, none of the patients had elevated levels of TF outside of the normal range, and there were no associations between TF levels and death or hemorrhage. These data are limited by our ability to only sample plasma TF versus monocyte-associated TF, although plasma and monocyte TF levels correlated in a human study of thrombosis [31]. Finally, the nonhuman primate studies were performed with EBOV, whereas the patients in this study were infected with SUDV, so these TF differences could be virus or host related.
Thrombomodulin is released by an activated endothelium and functions as an anticoagulant by activating protein C when present in the microenvironment on the surface of endothelial cells. In our study, thrombomodulin levels were elevated in samples from patients who died and from those with hemorrhagic manifestations, the latter being consistent with its anticoagulant function. Thrombomodulin has several protein domains that are responsible for its anticoagulant activity, but it also contains a C-type lectin domain that has antiinflammatory activities, including maintaining endothelial integrity [32]. The fact that EBOV GP protein binds to other C-type lectins [33–35] makes the thrombomodulin C-lectin domain an attractive subject for further study. If thrombomodulin's C-lectin domain–mediated functions are inhibited by association with viral GP, this could provide a possible explanation for how these viruses are able to disrupt endothelial function without causing direct cytopathic effects. Alternatively, overactivation of endothelial cells, leading to shedding of thrombomodulin into the plasma, could result in a loss of thrombomodulin's stabilizing function at endothelial junctions, contributing to vascular leakage.
MCSF, MIP-1α, IP-10, sICAM, and thrombomodulin levels were all associated with hemorrhage in our study, and on the basis of findings in the literature, their activities can all be correlated with leukocyte/endothelium interactions. In one possible model, these cytokines could recruit leukocytes to areas of inflammation, and production of adhesion molecules such as ICAM would facilitate leukocyte adhesion, rolling, and diapedesis. Activated endothelial cells would then release thrombomodulin into the bloodstream, so it would no longer be present in the microenvironment to activate protein C and coagulation, nor would it be present to stabilize the endothelium. This would leave an activated, leukocyte-enriched, procoagulant endothelium, causing dysregulated hemostasis, which could manifest clinically as hemorrhage.
Future pathogenesis models could evaluate the possible role of activated platelets in improving clinical outcomes by therapeutic administration of platelets, inhibitors of NO synthetase, or active sCD40L to infected animals. Finally, the potential roles of thrombomodulin C-lectin domains in modulating EHF outcomes should be further studied at the molecular level to determine whether viral glycoproteins interact with thrombomodulin. If this proves to be the case, evaluating the role of thrombomodulin C-lectin domains in animal models would be warranted.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgment. We thank Tatyana Klimova, PhD, for editing the manuscript.
Financial support. This work was supported by the PIDS/St. Jude Fellowship (to A. K . M.), the National Institutes of Health Loan Repayment Award (to A. K. M.), and the Atlanta Pediatric Scholars Program is an NIH K12 (grant HD072245 to A. K. M.).
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Okware SI, Omaswa FG, Zaramba S, et al. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002;7:1068–75. doi: 10.1046/j.1365-3156.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- 2.Fields BN, Knipe DM, Howley PM. Fields’ virology. 5th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 3.MacNeil A, Farnon EC, Wamala J, et al. Proportion of deaths and clinical features in Bundibugyo Ebola virus infection, Uganda. Emerg Infect Dis. 2010;16:1969–72. doi: 10.3201/eid1612.100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roddy P, Howard N, Van Kerkhove MD, et al. Clinical manifestations and case management of Ebola haemorrhagic fever caused by a newly identified virus strain, Bundibugyo, Uganda, 2007–2008. PLoS One. 2012;7:e52986. doi: 10.1371/journal.pone.0052986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kortepeter MG, Bausch DG, Bray M. Basic clinical and laboratory features of filoviral hemorrhagic fever. J Infect Dis. 2011;204(Suppl 3):S810–6. doi: 10.1093/infdis/jir299. [DOI] [PubMed] [Google Scholar]
- 6.Rollin PE, Bausch DG, Sanchez A. Blood chemistry measurements and D-Dimer levels associated with fatal and nonfatal outcomes in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S364–71. doi: 10.1086/520613. [DOI] [PubMed] [Google Scholar]
- 7.Hutchinson KL, Rollin PE. Cytokine and chemokine expression in humans infected with Sudan Ebola virus. J Infect Dis. 2007;196(Suppl 2):S357–63. doi: 10.1086/520611. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez A, Wagoner KE, Rollin PE. Sequence-based human leukocyte antigen-B typing of patients infected with Ebola virus in Uganda in 2000: identification of alleles associated with fatal and nonfatal disease outcomes. J Infect Dis. 2007;196(Suppl 2):S329–36. doi: 10.1086/520588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez A, Lukwiya M, Bausch D, et al. Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol. 2004;78:10370–7. doi: 10.1128/JVI.78.19.10370-10377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol. 2004;78:4330–41. doi: 10.1128/JVI.78.8.4330-4341.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hochberg BYaY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc. 1995;57:289–300. [Google Scholar]
- 12.Fabiani M, Nattabi B, Opio AA, et al. A high prevalence of HIV-1 infection among pregnant women living in a rural district of north Uganda severely affected by civil strife. Trans R Soc Trop Med Hyg. 2006;100:586–93. doi: 10.1016/j.trstmh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello C, Porat R. The acute phase response. In: Goldman L, Ausiello DA, editors. Cecil textbook of medicine. 22nd ed. Philadelphia, PA: Saunders; 2004. pp. 1733–5. [Google Scholar]
- 14.Ellaurie M, Rubinstein A. Ferritin levels in pediatric HIV-1 infection. Acta Paediatr. 1994;83:1035–7. doi: 10.1111/j.1651-2227.1994.tb12978.x. [DOI] [PubMed] [Google Scholar]
- 15.Riera A, Gimferrer E, Cadafalch J, Remacha A, Martin S. Prevalence of high serum and red cell ferritin levels in HIV-infected patients. Haematologica. 1994;79:165–7. [PubMed] [Google Scholar]
- 16.Blomback M, Kjellman H, Schulman S, Egberg N, Bottiger B, Wiechel B. Immunoglobulin levels in haemophiliacs at HIV seroconversion and during follow up. Infection. 1987;15:248–52. doi: 10.1007/BF01644125. [DOI] [PubMed] [Google Scholar]
- 17.Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence. 2013;4:1–10. doi: 10.4161/viru.24530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wauquier N, Becquart P, Padilla C, Baize S, Leroy EM. Human fatal zaire ebola virus infection is associated with an aberrant innate immunity and with massive lymphocyte apoptosis. PLoS Negl Trop Dis. 2010;e837:1–10. doi: 10.1371/journal.pntd.0000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villinger F, Rollin PE, Brar SS, et al. Markedly elevated levels of interferon (IFN)-gamma, IFN-alpha, interleukin (IL)-2, IL-10, and tumor necrosis factor-alpha associated with fatal Ebola virus infection. J Infect Dis. 1999;179(Suppl 1):S188–91. doi: 10.1086/514283. [DOI] [PubMed] [Google Scholar]
- 20.Baize S, Leroy EM, Georges AJ, et al. Inflammatory responses in Ebola virus-infected patients. Clin Exp Immunol. 2002;128:163–8. doi: 10.1046/j.1365-2249.2002.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley LE, Young HA, Jahrling PB, Geisbert TW. Proinflammatory response during Ebola virus infection of primate models: possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett. 2002;80:169–79. doi: 10.1016/s0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 22.Henn V, Steinbach S, Buchner K, Presek P, Kroczek RA. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coexpressed CD40. Blood. 2001;98:1047–54. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 23.Schafer A, Wiesmann F, Neubauer S, Eigenthaler M, Bauersachs J, Channon KM. Rapid regulation of platelet activation in vivo by nitric oxide. Circulation. 2004;109:1819–22. doi: 10.1161/01.CIR.0000126837.88743.DD. [DOI] [PubMed] [Google Scholar]
- 24.Chaiyaratana W, Chuansumrit A, Atamasirikul K, Tangnararatchakit K. Serum ferritin levels in children with dengue infection. Southeast Asian J Trop Med Public Health. 2008;39:832–6. [PubMed] [Google Scholar]
- 25.Barut S, Dincer F, Sahin I, Ozyurt H, Akkus M, Erkorkmaz U. Increased serum ferritin levels in patients with Crimean-Congo hemorrhagic fever: can it be a new severity criterion? Int J Infect Dis. 2010;14:e50–4. doi: 10.1016/j.ijid.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Bwaka MA, Bonnet MJ, Calain P, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 27.Hensley LE, Stevens EL, Yan SB, et al. Recombinant human activated protein C for the postexposure treatment of Ebola hemorrhagic fever. J Infect Dis. 2007;196(Suppl 2):S390–9. doi: 10.1086/520598. [DOI] [PubMed] [Google Scholar]
- 28.Geisbert TW, Hensley LE, Jahrling PB, et al. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: a study in rhesus monkeys. Lancet. 2003;362:1953–8. doi: 10.1016/S0140-6736(03)15012-X. [DOI] [PubMed] [Google Scholar]
- 29.Marti-Carvajal AJ, Sola I, Gluud C, Lathyris D, Cardona AF. Human recombinant protein C for severe sepsis and septic shock in adult and paediatric patients. Cochrane Database Syst Rev. 2012;12:CD004388. doi: 10.1002/14651858.CD004388.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisbert TW, Young HA, Jahrling PB, Davis KJ, Kagan E, Hensley LE. Mechanisms underlying coagulation abnormalities in Ebola hemorrhagic fever: overexpression of tissue factor in primate monocytes/macrophages is a key event. J Infect Dis. 2003;188:1618–29. doi: 10.1086/379724. [DOI] [PubMed] [Google Scholar]
- 31.Vieira LM, Dusse LM, Fernandes AP, et al. Monocytes and plasma tissue factor levels in normal individuals and patients with deep venous thrombosis of the lower limbs: potential diagnostic tools? Thromb Res. 2007;119:157–65. doi: 10.1016/j.thromres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Huang HC, Shi GY, Jiang SJ, et al. Thrombomodulin-mediated cell adhesion: involvement of its lectin-like domain. J Biol Chem. 2003;278:46750–9. doi: 10.1074/jbc.M305216200. [DOI] [PubMed] [Google Scholar]
- 33.Simmons G, Reeves JD, Grogan CC, et al. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305:115–23. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol. 2002;76:6841–4. doi: 10.1128/JVI.76.13.6841-6844.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takada A, Fujioka K, Tsuiji M, et al. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J Virol. 2004;78:2943–7. doi: 10.1128/JVI.78.6.2943-2947.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.