Abstract
Arachidonic acid metabolism leads to the generation of key lipid mediators which play a fundamental role during inflammation. The inhibition of enzymes involved in arachidonic acid metabolism has been considered as a synergistic anti-inflammatory effect with enhanced spectrum of activity. A series of 1,3-diphenyl-2-propen-1-one derivatives were investigated for anti-inflammatory related activities involving inhibition of secretory phospholipase A2, cyclooxygenases, soybean lipoxygenase, and lipopolysaccharides-induced secretion of interleukin-6 and tumor necrosis factor-alpha in mouse RAW264.7 macrophages. The results from the above mentioned assays exhibited that the synthesized compounds were effective inhibitors of pro-inflammatory enzymes and cytokines. The results also revealed that the chalcone derivatives with 4-methlyamino ethanol substitution seem to be significant for inhibition of enzymes and cytokines. Molecular docking experiments were carried out to elucidate the molecular aspects of the observed inhibitory activities of the investigated compounds. Present findings increase the possibility that these chalcone derivatives might serve as a beneficial starting point for the design and development of improved anti-inflammatory agents.
Keywords: anti-inflammatory, tumor necrosis factor-alpha, lipopolysaccharides, molecular docking
Introduction
Pro-inflammation is a common phenomenon that is linked to various diseases including cardiovascular diseases and cancer.1–3 The pro-inflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) are involved in the pathogenesis of various inflammatory disorders including rheumatoid arthritis, inflammatory bowel disease, osteoarthritis, psoriasis, endotoxemia and/or toxic shock syndrome.4–12 Apart from pro-inflammatory characteristics, these cytokines have a wide range of functions for maintaining the normal cellular physiology, for instance, TNF-α can induce apoptosis and secretion of cytokines such as IL-1, IL-6, and IL-10; it can also activate T cells and other inflammatory cells. On the other hand, an excess of TNF-α and IL-6 is attributed to the development of various human diseases including inflammatory disorders. The treatment of rheumatoid arthritis has been successful in several clinical trials by targeting the inhibition of cytokines, particularly TNF-α. The inhibition of TNF-α, pro-inflammatory cytokines and the over-expressions of cytokines has been recognized as an attractive target for the design and development of novel anti-inflammatory agents.13–16
Secretory phospholipase A2 (sPLA2) is an enzyme that catalyzes the hydrolysis of ester bond at the sn-2 position of glycerophospholipids. The released fatty-acid, such as arachidonic acid, may be enzymatically metabolized into strong pro-inflammatory mediators, referred to as eicosanoids (prostaglandins [PGs], leukotrienes, and thromboxanes), whereas lysophospholipid, the other product of the sPLA2 catalyzed reaction in the case of lyso-platelet-activating-factor (lyso-PAF), might be converted by the PAF-acetyltransferase into PAF, another renowned pro-inflammatory mediator. Because of the involvement of lipid mediators in miscellaneous pathological processes, the suppression of their production has long been well-thought-out as therapeutical strategies.17
The cyclooxygenases (COX-1 and COX-2) are important isozymes that catalyze the complex biotransformation of arachidonic acid into PGs and thromboxanes, which are ultimately responsible for many physiological and pathophysiological responses.18,19 The COX-1 isozyme facilitates homeostatic functions including cyto-protection of the gastric mucosa, start of labor pain, regulation of renal blood flow, and platelet aggregation. Recently, experimental results have identified a likely involvement of COX-1 in angiogenesis, therefore providing the basis for the development of COX-1 inhibitors.20,21 On the other hand, COX-2 isozyme is principally responsible for the production of inflammatory PGs that induce pain, swelling, and fever.22–24 Apart from its ability to induce peripheral inflammation, the expression of COX-2 isozyme is up-regulated in several human cancers such as gastric, breast, lung, colon, esophageal, prostate, and hepatocellular carcinomas.25,26
Natural and synthetic flavonoids have been drawing attention owing to their wide range of biological activities. Chalcones belong to the group of chemical compounds that are linked to various pharmacological activities. Recently, we summarized the biological properties of chalcones.27,28 Previous reports have also demonstrated the anti-inflammatory activity of chalcone derivatives by the modulation of pro-inflammatory gene expression of COX-2, inducible nitric oxide synthase, and numerous essential cytokines.29–32 Recent reports indicate the importance of chalcones as anti-inflammatory agents involved in inhibition of cell migration and inhibition of TNF-α production in a mouse model.33 Chalcones are also excellent skeletons for modification of drug design and development. Recent findings by different groups of researchers suggested that some chalcones, such as the promising anti-inflammatory agents, exhibited their potential in the therapy of inflammatory and immune diseases.33–35 Chalcone derivatives have been extensively reported to inhibit NO synthesis and inducible NO synthetase and COX-2 protein expression in lipopolysaccharide (LPS) stimulated cells.34,36 However, few endeavors were proposed on evaluating the inhibitory effect of chalcone derivatives against TNF-α and IL-6 expression or their structure–activity relationship.
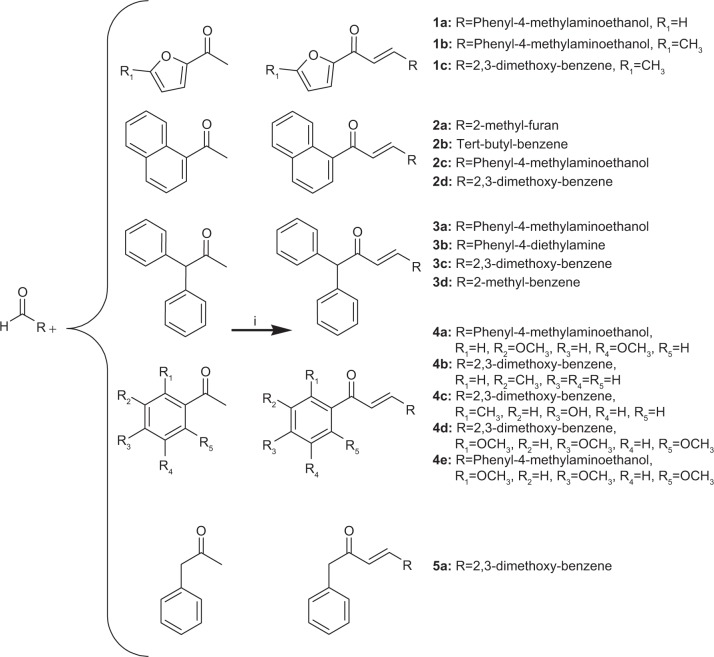
In our search for potential anti-inflammatory drug candidates, we have synthesized novel chalcone derivatives and investigated their immunomodulatory effects.37,38 In the present study we have investigated the in vitro anti-inflammatory activity of a series of novel chalcone derivatives (Figure 1) by evaluation of their effects on COX, lipoxygenase (LOX), and pro-inflammatory cytokines. Molecular docking experiments were performed to explain the molecular basis of the observed inhibitory activities of the investigated compounds.
Figure 1.
Synthesis of chalcone derivatives (i = NaOH, ethanol).
Materials and methods
Chemicals and reagents
All chemicals, reagents used in this project were purchased from Sigma-Aldrich Co., (St Louis, MO, USA), Merck Millipore (Billerica, MA, USA) and Acros Organics (Thermo Fisher Scientific, Waltham, MA, USA) (above 98% purity) and used without additional purification. The chemicals used in sPLA2 assay comprised of 1,2-bis(heptanoylthio)-phosphatidylcholine, 5,5-dithiobis (2-nitrobenzoic acid) (DTNB), and recombinant human phospholipase A2 (PLA2)-V from Cayman Chemicals (Ann Arbor, MI, USA). CaCl2, KCl, and HCl were procured from Merck Millipore. COX inhibition activity was determined using a COX Inhibitor Screening Kit purchased from Cayman Chemicals. The levels of TNF-α and IL-6 in the media were calculated by an enzyme-linked immunosorbent assay (ELISA) using mouse TNF-α (catalogue no 500850) and mouse IL-6 (catalogue no 583371), ELISA kits were obtained from Cayman Chemicals. Mouse RAW264.7 macrophages were obtained from Abcam plc (Cambridge, UK). Cell culture chemicals and fetal bovine serum (FBS) were obtained from Sigma-Aldrich Co., and FBS was heat-inactivated for 30 minutes at 65°C. LPS supplied by Sigma-Aldrich Co., was dissolved in phosphate buffered saline (PBS). The synthetic compounds were dissolved in dimethyl sulfoxide (DMSO).
sPLA2-V activity assay
Human recombinant sPLA2-V was used as an enzyme source. The activity of sPLA2 enzyme was determined by a photometric assay based on the Ellman’s method (Table 1).39 Briefly, the hydrolysis of sn-2 ester bond of the substrate 1,2-bis(heptanoylthio)-glycerophosphocholine by sPLA2-V led to the exposure of free thiols. The alteration of DTNB to 2-nitro-5-thiobenzoic acid was activated by these thiols, which was detected photometrically at 405 nm. Afterwards, the assay was progressed in an aqueous buffer solution (pH 7.5) comprising KCl (94 mM), CaCl2 (9 mM), Tris (24 mM), and Triton-X 100 (280 μM). Prior to the assay, substrate and sPLA2-V were re-suspended in assay buffer, and aqueous solution of Tris-HCl (pH 8) was used to dissolve DTNB. Enzyme and DTNB produced final concentrations of 100 ng/mL and 87 μM, respectively. Assays were performed in 96-well microliter plates, at room temperature containing substrate solution, DTNB, and the respective test substance. Activity of the enzyme was determined 100% by only adding substrate and enzyme. DMSO was used as negative control at a concentration (1.7% v/v) at which it did not affect the assay.
Table 1.
Inhibition of secretory phospholipase A2-V (sPLA2-V), COX-1, COX-2 and LOX-15 activities by synthetic chalcone derivatives
| Compound | sPLA2-V IC50 (μM) | COX-1 IC50 (μM) | COX-2 IC50 (μM) | LOX IC50 (μM) |
|---|---|---|---|---|
| 1a | 12.38±0.62 | 20.42±0.55 | 59.57±1.90 | 59.42±0.82 |
| 1b | 4.32±0.48 | 25.86±0.75 | 10.09±1.78 | 45.13±0.53 |
| 1c | 19.18±1.68 | ND | 56.17±1.46 | 79.51±0.12 |
| 2a | 33.58±3.50 | 23.24±0.66 | 153.6±3.56 | 09.07±0.50 |
| 2b | 12.58±2.69 | 51.51±0.89 | 70.71±1.36 | 65.76±1.89 |
| 2c | 115.65±4.23 | 188.60±1.50 | ND | ND |
| 2d | 12.90±0.76 | 21.08±0.55 | 36.15±1.75 | 92.34±0.71 |
| 3a | 38.88±1.30 | 35.70±0.51 | 84.66±1.16 | ND |
| 3b | 11.02±0.60 | 29.55±0.19 | 51.25±1.29 | 45.53±0.81 |
| 3c | 25.96±1.24 | 14.65±0.85 | 15.40±0.70 | ND |
| 3d | ND | 58.52±0.68 | ND | 95.18±2.14 |
| 4a | 14.09±2.59 | 41.25±0.24 | 4.78±0.73 | 32.06±0.51 |
| 4b | 73.81±3.10 | 164.04±2.15 | 58.75±1.58 | 25.86±1.22 |
| 4c | 18.45±0.32 | 81.96±1.54 | ND | 53.76±1.74 |
| 4d | 74.75±3.13 | 245.44±2.19 | 35.70±1.51 | 115.65±5.67 |
| 4e | 14.12±0.74 | 48.24±0.96 | 08.38±1.44 | 52.52±0.12 |
| 5a | 4.82±0.32 | 50.01±0.57 | ND | ND |
| Indomethacin* | – | 0.24±0.05 | 3.28±0.09 | – |
| Dexamethasone | 0.65±0.15 | – | – | – |
| NDGA** | – | – | – | 9.55±0.27 |
Notes:
30 μM concentration;
16 μM concentration. Values are the mean ± SD; n=3.
Abbreviations: COX, cyclooxygenase; LOX, lipoxygenase; SD, standard deviation; ND, not determined; NDGA, nordihydroguaiaretic acid; IC50, half maximal inhibitory concentration.
COX assay
The effects of test compounds on COX-1 and COX-2 were determined by quantifying prostaglandin E2 (PGE2) using a COX Inhibitor Screening kit (Cayman Chemicals).40 The reaction mixtures were prepared in 100 mM Tris-HCl buffer (pH 8.0) containing 1 μM heme and COX-1 (ovine) or COX-2 (human recombinant) and pre-incubated for 10 minutes in a water bath (37°C). Arachidonic acid (10 μL) was added to initiate the reaction (final concentration in reaction mixture 100 μM). After 2 minutes, the reaction was completed by adding 1 M HCl and lastly, PGE2 level was measured by ELISA method. The test compounds were dissolved in DMSO and diluted with 100 mM potassium phosphate buffer (pH 7.4) to the desired concentration. After transferring to a 96-well plate coated with a mouse anti-rabbit immunoglobulin G (IgG), the tracer PG acetylcholine esterase and primary antibody (mouse anti-PGE2) were added. Subsequently, plates were incubated at room temperature overnight, reaction mixtures were removed, and the wells were washed with 10 mM potassium phosphate buffer comprising 0.05% Tween 20 (Cayman Chemicals). Afterwards, Ellman’s reagent (Cayman Chemicals) (200 μL) was added to all wells and the plate was incubated at room temperature in the dark for 60 minutes, until the control wells yielded an Optical density (OD) =0.3–0.8 at 412 nm. A standard curve with PGE2 was generated from the same plate which was used for the quantification of PGE2 levels produced in the presence of test samples. Results were shown as percentage relative to a control (solvent treated samples). All the steps were performed in triplicate and values normally agreed within 10%.
LOX activity assay
The effect of α, β-unsaturated carbonyl based compounds on LOX enzyme was investigated by a standard colorimetric assay.33 Soybean LOX served as enzyme source. A buffer solution (0.1 M) of Tris-HCl (pH 7) was used to perform the assay. Prior to assay, LOX enzyme was re-suspended in buffer. The substrate was dissolved in equal amounts of potassium hydroxide, vortexed, and diluted to obtain the concentration of 1 mM. The assay was performed in 96-well micro-liter plates containing substrate, enzyme, and test substance at room temperature. The maximum (100%) activity of enzyme was calculated by adding the substrate and enzyme only. For determination of activity, 90 μL of LOX and 10 μL of the respective sample was added. The reaction was started by adding substrate solution to all wells for 5 minutes. Subsequently, to stop enzyme catalysis 100 μL of chromogen solution was added to all wells for 5 minutes. Later, the absorbance was measured at 490 nm using Tecan® Infinite Pro 200 microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
Cell treatment and ELISA assay for TNF-α and IL-6
Mouse RAW264.7 macrophages were incubated in Dulbecco’s Modified Eagle’s Medium supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C with 5% CO2. The macrophages were pre-treated with test compounds (at 10 μM concentration) or vehicle control for 2 hours and then treated with LPS (0.5 μg/mL) for 22 hours. After the treatment, the culture media and cells were separately collected. The media collected were centrifuged at 1,000 rpm for 10 minutes. The TNF-α and IL-6 levels in the media were determined using mouse TNF-α and mouse IL-6 ELISA kits according to the manufacturer’s instructions. After centrifugation, supernatant was separated and stored at −80°C until use. The washing of the cells was done with PBS and harvested later on with cell lysis buffer (Tris-HCl 20 mM, NP40 1%, NaCl 150 mM, EDTA 2 mM, Na3VO4 200 mM, SDS 0.1%, NaF 20 mM). The mixed liquid was shaken forcefully for 10 minutes in lysis buffer at 0°C. After centrifugation at 12,000 rpm for 30 minutes at 4°C, total protein was collected and the concentrations were determined by using Bio-Rad protein assay reagents (Bio-Rad Laboratories Inc., Hercules, CA, USA). The total quantity of inflammatory factor in the media was normalized to total protein amount of the viable cell pellets.
Computational details
Molecular homology models of receptors
Homology models of COX-2 and PLA2-V receptors were built using Internal Coordinate Mechanics, ICM, program (Molsoft LLC).41 The high resolution structure of naproxen-COX-2 complex with pdb code 3ntl was used as template for the homology model of COX-2.42 It has 88% sequence identity, while the protein database structure with the pdb code 3g8h was used as template for PLA2 -V.43 It has 48% sequence identity with PLA2-V. Figure 2 shows structures of models of COX-2, PLA2-V, and templates. The model of PLA2-V consists of two beta sheets and three helixes with five to six disulfide bridges, while the template has seven disulfide bridges. The last disulfide bridge is due to an extra cysteine residue that the template has at the C-terminal and at position 49 (see multiple alignment used for model building). PLA2-V lack these cysteine residues.
Figure 2.
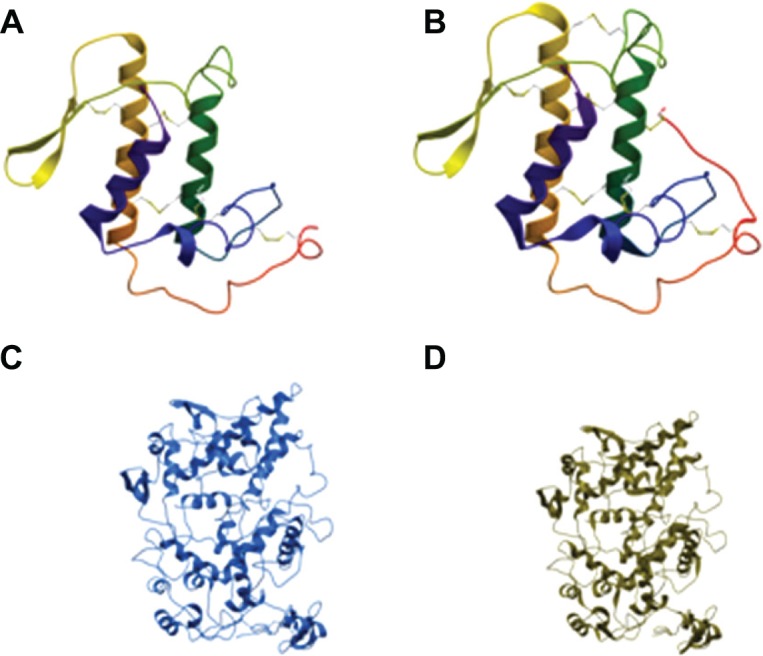
Structures of models of COX-2, phospholipase A2-V and templates.
Notes: (A) Showing model of phospholipase A2-V. (B) Model of template 3g8h. (C) Model of COX-2. (D) Model of template 3ntl1.
Abbreviation: COX, cyclooxygenase.
Structure verification/validation of models built
The structure verification server at the University of California, Los Angeles (UCLA) facility (http://nih-server.mbi.ucla.edu/SAVES/) was used for structure verification. This server uses the programs: PROCHECK, WHAT_CHECK, VERIFY_3D, ERRAT, and PROVE software. All the software check different properties of protein structure. We used PROCHECK, VERIFY_3D, and ERRAT for our validation.
The VERIFY_3D program uses potential based on the relative burial of residues and local secondary structure. The ERRAT program analyzes the statistics of non-bonded interactions between different atom types. By comparison with statistics from high quality structures, the error values have been calibrated to give confidence limits. The PROVE program calculates the volumes of atoms in macromolecules. The mean volumes of buried atoms and the corresponding standard deviations are computed. The deviation of the atomic volume from the standard values is evaluated by the volume Z-score. The PROCHECK geometry validation program compares the geometry (standard Ramachandran plot) of the model with that of high quality X-ray structures.
The verification methods fall into two major classes, one class mainly focusing on the local environment (VERIFY_3D), and one class mainly focusing on local geometry (ERRAT and PROCHECK). The environment-oriented method VERIFY_3D gave scoring values for the model that were approximately similar to that of the template. The scoring values of VERIFY_3D, ERRAT, and PROVE are in percentages and should in general be as high as possible. For the geometry-oriented methods the scoring differences between the model and the template were small, while the ERRAT method, which focuses on non-bonded interactions, gave a better score for the model than for the template.
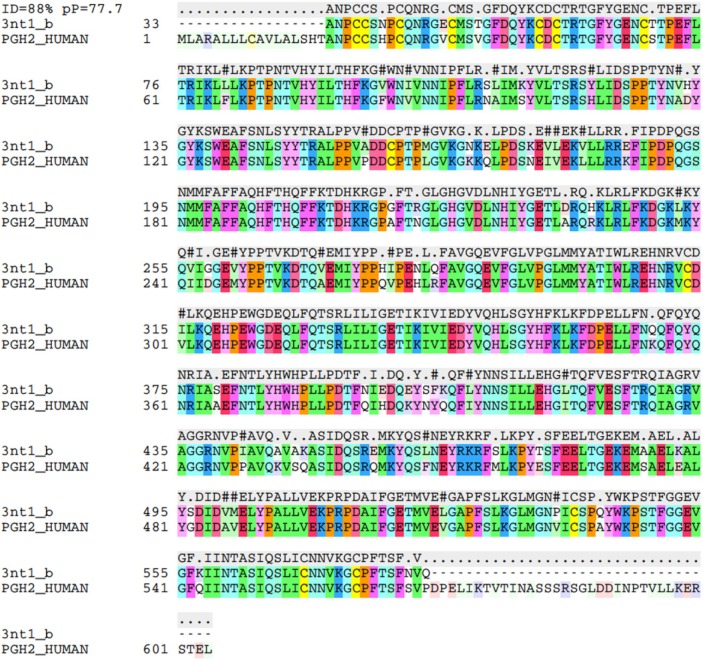
For PROCHECK, the model (COX-2) had 433 (90.8%) of residues in most favored regions, 43 residues (9%) in additionally allowed regions, none in generously allowed region and only one residue (HIS 398) in disallowed region. For VERIFY_3D, 87.52% of the residues had an average 3D_1D score >0.2. For ERRAT, the model has an overall quality factor of 97.58. The template with pdb code 3ntl, however, had 432, or 90.6% of residues in most favored regions, 43 (9%) in additionally allowed regions, none in generously allowed region and two residues (GLU398 and SER496) in disallowed region. For the template model, VERIFY_3D showed 86.23% of the residues having an average 3D_1D score >0.2. For ERRAT, the 3nt1 had an overall quality factor of 96.83. These values show that we have a very reliable model. Most of the values are actually better than the template values (Figure 3).
Figure 3.

Amino acid sequence alignment for COX-2.
Abbreviation: COX, cyclooxygenase.
Structural validation of the PLA2-V model built
The result for PROCHECK had 83 residues (88.3%) in most favored regions, 11 (11.7%) residues in additional allowed regions and none in disallowed regions (Figure 4). For VERIFY_3D, 95.65% of the residues had an average 3D_1D score >0.2 and ERRAT values of 83.96. The template with pdb code 3g8h, however, had 91 (88.3%) residues in most favored regions, 12 in additionally allowed region and none in disallowed regions. For template model, VERIFY_3D showed, 88.62% of the residues had an average 3D_1D score >0.2 and ERRAT values of 96.5. This also confirms that we have a reliable and very good model for PLA2-V. The PROCHECK values are the same while the VERIFY_3D values are better with the model than the template. Comparison of structures of templates and homology models by structural verification methods indicates that the homology structure built is very reliable (Table 2).
Figure 4.
Amino acid sequence alignment for phospholipase A2-V.
Table 2.
Quality scores of the phospholipase A2-V and COX-2 models using different quality control programs available at the Structure Analysis and Verification (SAVS) server
| Structure | VERIFY_3Da | ERRATb | PROCHECKc |
|---|---|---|---|
| Model (COX-2) | 87.5 | 95.6 | 90.8 |
| Template (3ntl1) | 86.2 | 96.8 | 90.6 |
| Model (phospholipase A2-V) | 95.6 | 83.9 | 88.3 |
| Template (3g8h) | 88.6 | 96.5 | 88.3 |
Notes:
Percentage of residues with average 3D-1D score >0.2.
Percentage of residues with scoring below the 95% limit.
Percentage of residues found in the core region of the Ramachandran plot. Structure Analysis and Verification (SAVS) server at UCLA facility (http://nihserver.mbi.ucla.edu/SAVES/).
Abbreviation: COX, cyclooxygenase.
Docking of ligands into receptors/enzymes
All the ligands were modeled using the ICM program.44 Charges were assigned and they were docked into the respective receptors/enzymes. Two of the enzymes did not have X-ray derived structures that could be used for docking studies. Homology models of these enzymes (PLA2-V and COX-2) were built and used for the docking studies. For COX-2, a homology model were built using the pdb structure with pdb code 3ntl as template while for PLA2-V, pdb structure with pdb code 3g8h was used as template to build a homology model. Soybean LOX-15 and COX-1 had X-ray derived structures. For soybean LOX-15, the soybean LOX-15 with pdb structure code 1yge was used for docking studies while for COX-1, the COX-1 with pdb structure code 3n8y was used. Before docking of ligands, all crystallographic water and small molecule inhibitors were removed, hydrogen atoms were added and a semi-rigid docking was done. In this method, the ligands were fully flexible and the receptors’ binding sites were fixed. The ten best binding poses were retained after docking runs and analyzed.
Statistical analysis
All the experiments were performed thrice and the data obtained are presented as the mean ± standard error of mean. The half maximal inhibitory concentration (IC50) values were calculated from at least three determinations by using Graph Pad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA). All the data were analyzed using a one-way analysis of variance for multiple comparisons. P<0.05 was considered to be statistically significant.
Results and discussion
Chemistry
All the compounds were synthesized, characterized, and formerly reported (Figure 1).37 Chalcone derivatives were synthesized by the reaction of equimolar aldehyde and ketone in the presence of a base via Claisen-Schmidt condensation. The successful synthesis of all the novel compounds was confirmed by several analytical techniques comprising infrared spectroscopy, 1H NMR, 13C NMR, mass spectroscopy, and elemental analysis.37
Inhibition of sPLA2-V
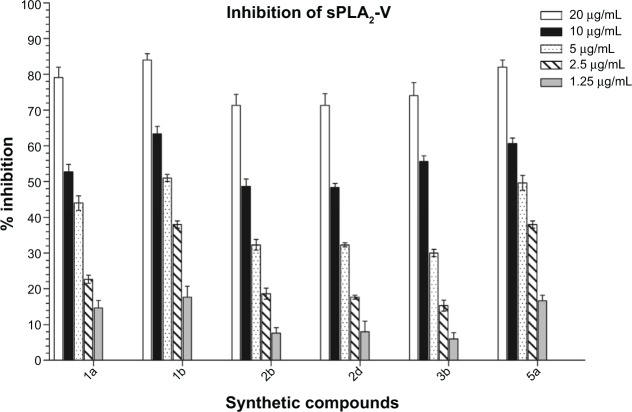
Inflammation can be prevented by the inhibition of PLA2. Thus, all compounds (at 1.25 to 20 μg/mL) were evaluated in vitro by enzymatic photometric assay based on Ellman’s method for their inhibitory potency against sPLA2 and the results obtained are presented in Table 1. Dexamethasone served as positive control. A number of previous studies have shown that the compounds bearing α, β-unsaturated carbonyl group like chalcone derivatives decreased activities of PLA2, and various isoforms of PLA2 enzymes displayed more than 70% homology.40,45,46 The compounds 1a, 1b, 2b, 2d, 3b, and 5a, were strong inhibitors of sPLA2-V in a dose dependent manner (Figure 5), with IC50 values ranging from 4.32–12.90 μM. Among all the chalcone derivatives, 1b was the strongest inhibitor of sPLA2-V with IC50 4.32 μM. Major anti-inflammatory agents, steroids, and COX, were proven to have serious side effects so now attention is toward natural products that are a valuable source of novel bioactive anti-inflammatory agents. Recent evidence has demonstrated that curcumin exhibited potent anti-inflammatory activities, which may help to prevent or even treat sepsis, as well as cancer and diabetes.47 Curcumin showed a protective effect in sepsis-induced acute lung injury and organ dysfunction in a rat model.47 But in our previous studies48 curcumin showed less sPLA2-V inhibitory activity (IC50 11.10 μM) as compared to synthetic compound 1b, so these synthetic compounds that are derivatives of natural chalcones can be suitable anti-inflammatory candidates, more effective than curcumin.
Figure 5.
Concentration dependent inhibitory effects of chalcone derivatives on activity of secretory phospholipase A2-V (sPLA2-V).
Notes: Graph represents the percentage inhibition by most active compounds at different concentrations. The data shown are an average of three independent experiments and values are mean ± standard deviation.
Inhibition of COX-1 and COX-2
The inhibition of COX-1 and COX-2 is one of the anti-inflammatory mechanisms. The COX inhibitory activity of chalcone derivatives was investigated for both isoforms of COX ie, COX-1 and COX-2 at 40 μg/mL. COX-1 and COX-2 inhibitory activity was assessed by an in vitro COX-inhibitor screening assay.40 The IC50 were calculated and the results are presented in Table 1. The results exhibit that these novel compounds have affinity toward COX-2. Compounds 1b, 3c, 4a, and 4e displayed strong COX-2 inhibitory activity with the extent of inhibition in the range of 80.74%–92.55%. Indomethacin was used as positive control. Most of chalcone derivatives showed better COX-2 inhibitory activity with IC50 ranging from 4.78–15.40 μM. The derivative 4a exhibited strongest inhibition of COX-2.
In previous studies, a selective COX-1 inhibitor was equipotent to COX-2 selective inhibitor (celecoxib) in inhibiting PG formation in an inflammatory exudate, however did not reduce increased PG levels in the cerebrospinal fluid, whereas non-selective COX-1/COX-2 inhibition seemed to be more effective in decreasing signs of inflammation.49,50 These findings and other data propose that the functions of COX-1 and COX-2 might be more complicated than initially estimated and that COX-1 inhibition may contribute to inhibition of PG production in inflammatory exudates. Hence, a combined inhibition of COX-1 and COX-2 may lead to more efficient inhibition of chronic inflammation as compared to selective inhibition of COX-1 or COX-2.
The chalcone analogs were less efficient in suppressing the activity of COX-1 in contrast to COX-2. The compounds 1a, 1b, 2a, 2d, and 3c exhibited COX-1 inhibitory activity with IC50 less than 25 μM. The compound 3c exhibited highest COX-1 inhibition with an IC50 value of 14.65 μM. The compounds inhibited COX activity in a dose-dependent manner; there was an increase in the inhibitory activity of compounds with a rise in the concentration. These results suggest that chalcone derivatives act as inhibitors of COX enzymes and exhibit anti-inflammatory effects.
In vitro inhibition of soybean LOX
The inhibitors of LOX have gained consideration as potential agents for the treatment of inflammation, allergic diseases, and cancer. Owing to this reason, we assessed the capability of our synthesized chalcone derivatives to inhibit soybean LOX. LOX is inhibited by non-steroidal anti-inflammatory drugs in the same manner to that of rat mast cell LOX, and can be used as a reliable screen for such activity.51 The inhibitory effect of our novel compounds (at a concentration of 2.5 to 40 μg/mL) on LOX was determined and drafted in Table 1. The initial screening discovered that compounds 2a and 4b were strong inhibitors of LOX with percentage inhibitions in the range of 65%–84%. DMSO served as negative control and did not alter the activity of the enzyme. The IC50 of the compounds 2a and 4b were 9.07 and 25.86 μM, respectively. Among all the compounds the compound 2a exhibited strongest LOX inhibitory activity, with IC50 less than positive control. All the compounds inhibited LOX activity in a dose-dependent way.
Inhibition of TNF-α and IL-6 release in LPS-stimulated macrophages
LPS is a significant structural constituent of the outer membrane of gram-negative bacteria, and the literature suggests its role as an immunostimulator that induces systemic inflammation response, and particularly, the expression of pro-inflammatory cytokines, for instance TNF-α and IL-6.52 In this study, chalcone derivatives were evaluated for their inhibitory activity against LPS-induced TNF-α and IL-6 release in mouse RAW264.7 macrophages. The macrophages were pre-treated with the tested compounds (at 10 μM concentration) for 2 hours and incubated afterwards with 0.5 μg/mL LPS for 22 hours. The levels of TNF-α and IL-6 in media were detected by ELISA and normalized by protein concentration of cells collected in homologous culture plates.
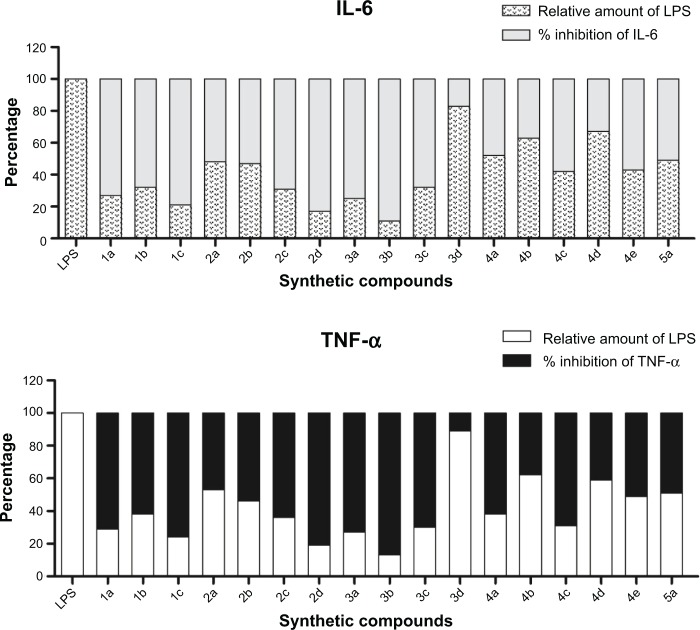
The results of the anti-inflammatory assay of compounds are presented in Figure 6. All the tested compounds inhibited LPS-induced TNF-α and IL-6 expression to different degrees, with compounds 1a, 1c, 2d, 3a, 3b, and 3c displaying the highest inhibitory activities against LPS-induced TNF-α expression (percentage inhibition 70%–87%), moreover, the compounds 1a, 1c, 2d, 3a, and 3b exhibited strong inhibitory effects against IL-6 release as compared to LPS-control (73%–89%). Among the tested compounds, 2d and 3b were the strongest inhibitors of LPS-induced TNF-α and IL-6 release and their inhibitory rates reached 89% in contrast to LPS-control.
Figure 6.
Chalcone derivatives inhibited LPS-induced TNF-α and IL-6 secretion in RAW264.7 macrophages.
Notes: The results are expressed as percent of LPS control. Each bar represents mean ± SE of three independent experiments.
Abbreviations: LPS, lipopolysaccharides; TNF-α, tumor necrosis factor-alpha; IL, interleukin; SE, standard error.
Interactions of ligands and various receptors/enzymes
Ligand interactions with COX-1
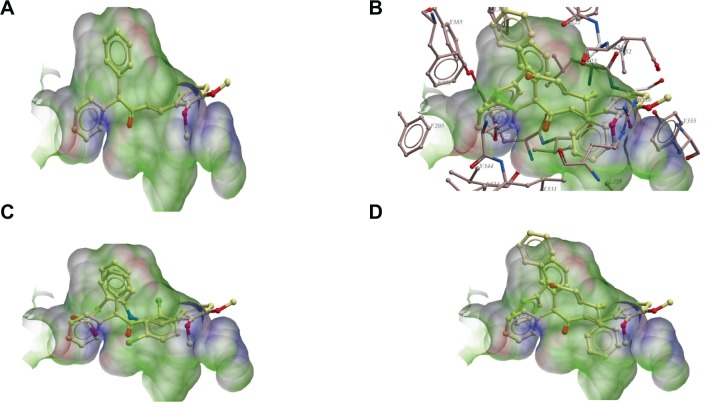
Chalcone derivative 3c has the best interaction with COX-1 because of the interaction of its aromatic rings with the aromatic and hydrophobic residues of COX-1. These are F205, Y389, F381, L384, E387, F518, Y348, V344, V349, l352, and L534 (Figure 7). These residues have maximum interactions with 3c. The other aromatic ring on its other side also makes aromatic–aromatic interactions with Y355, and hydrophobic-aromatic interaction with I523. R120 also makes electrostatic interaction with the ether oxygen of 3c. It is interesting to note that a comparison of diclofenac interactions with COX-1 in the X-ray structure shows similar interactions with these residues. A superimposition of diclofenac on the docking pose of 3c showed a good overlay on 3c. The observed interactions of 3c with COX-1 are a little different from those observed with 3d. They both have similar interactions with these residues but the aromatic interaction of the aromatic ring on 3c is better with Y355 and L352 than 3d. This is also true regarding its interaction with R120. Compound 4a was observed having a hydrogen bond interaction with S530. Of course 3c has better interaction with the highly aromatic pocket involving F205, Y389, F381, L384, E387, F518, Y348, V344, V349, l352, and L534. These observations indicate why 3c has the best interaction with COX-1 compared with other ligands (Figure 7).
Figure 7.
Ligands in the active site of COX-1.
Notes: (A) Showing 3c in the active site of COX-1 (without active site residues). (B) Showing 3c and 3d in the active site with the active site residues of COX-1. (C) Showing 3c and diclofenac in the active site of COX-1 (without active site residues). (D) Showing 3c and 3d in the active site of COX-1 without the active site residues.
Abbreviation: COX, cyclooxygenase.
Ligand interactions with COX-2
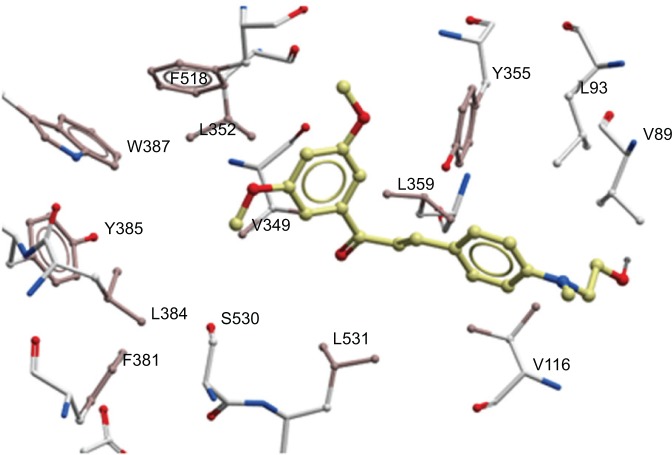
Analyses of the interactions of 4a with COX-2 showed why it is one of the best compounds having strongest inhibition against COX-2. Docking pose of 4a in COX-2 is very close to those observed with celecox (it aligns nicely on celecox) as seen in 3nt1. These interactions are not observed in the docking poses of 3c, 2a, or 4e. Compound 4a occupied the same pocket as celecox interacting with V523, F518, L352, and Y355 (Figure 8). Compound 4a’s other aromatic ring interacts with V89, L93, Y355 (at the border, so interacting with the two aromatic rings), and V116. This observation is unique with 4a interacting with COX-2 model. Another docking position of 4a that is similar is close to the best docking position of 4e (position is not the one that is occupying the same as celecox). Chalcone derivative 4e in this position has the same interactions as 4a and is almost overlaid. In this pose, interaction with the hydrophobic/aromatic pocket of Y385, V349, F381, L384, F518, L352, V349, and Y348 is observed.
Figure 8.
Showing chalcone derivative 4a in the active site of COX-2 with focus on the hydrophobic/aromatic interactions with residues.
Abbreviation: COX, cyclooxygenase.
Ligand interaction with soybean LOX-15
Analyses of the docking positions of 2a and 4b show very good similarities in their interactions with soybean LOX-15, which explains why they have good interactions with soybean LOX-15 as compared with 4d and 2d (Figure 9A). These docking positions of 2a and 4b on one hand and 2d and 4d on the other hand show that they interact on different sites in the active site of soybean LOX-15 (Figure 9B). This is confirmed by the comparison of their docked positions with X-ray position of small molecule dihydrobenzoic in the pdb structure code 1n8q. Analyses of all the four docking positions and X-ray structure show that 2a and 4b are docked on the same position as the X-ray solved molecule dihydrobenzoic acid in 1n8q. In these interactions, dihydrobenzoic acid, 2a, and 4b all had hydrophobic/aromatic interactions with residues H499, H690, H504, and W500 in soybean LOX-15. These interactions are not observed with 4d and 2d. The furan ring in 2a and aromatic ring in 4b interacted with these residues. Compound 2a had better interaction than 4b because it had aromatic/hydrophobic interactions with its naphthalene ring with V354, I553, Q697, V750, I 751, and V754. It also interacted with H494. Compound 2a has been observed to have better interactions with all these residues compared to 4b because of its double aromatic ring (naphthalene) rather than the single aromatic ring in 4b. Compounds 4d and 2d also had interactions with all these residues (V354, H494, I553, Q697, V750, I751, V754). However, they do not have the interaction with H499, H690, H504, and W500 as observed in 1n8q.
Figure 9.
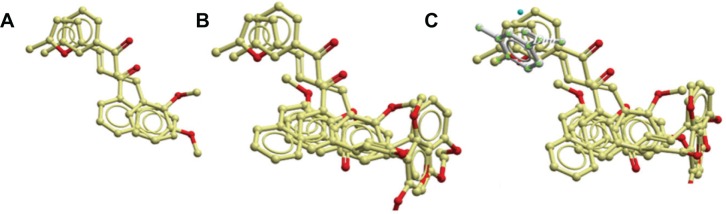
Ligand interactions with soybean lipoxygenase-15.
Notes: (A) Showing 2a and 4b in the active site of soybean lipoxygenase-15 without active site residues. (B) Showing 2a, 4b, 2d, and 4d in the active site of soybean lipoxygenase-15 without active site residues. (C) Showing 2a and 4b, dihydrobenzoic acid, and 2d and 4d in the active site of soybean lipoxygenase-15 without active site residues. Dihydrobenzoic acid is observed on the same side with 2a and 4b.
Ligand interaction with sPLA2-V
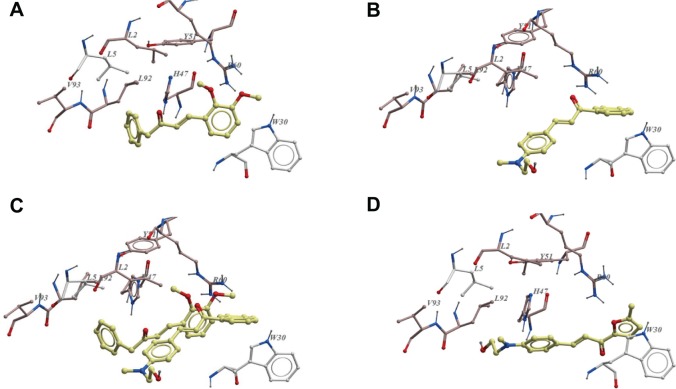
Compound 2c has the weakest binding to PLA2-V, while 5a and 1b had the strongest binding. Analyses of docked positions of 5a, 1b, and 2c in PLA2-V show that these compounds were binding slightly differently. Docked positions of 5a and 1b (strong binders of PLA2-V) are almost completely superimposable in their binding poses while 2c’s binding pose is not (Figure 10). Analysis of interaction of small molecule inhibitor 2-carbaoylmethly-5-propyl-octahydro-indol-7 acetic acid in PA2G5 (pdb code 1oxl) shows that it had hydrophobic interactions with L2, F5, I9, A18, I19, W31, and Y52. Chalcone derivative 5a, however, has even better hydrophobic/aromatic interactions with L2, L5, 19, Y21, Y27, W30, H47, Y51, L92, V93, and L96, and had hydrogen bond interactions with R60 and K55 (Figure 10). The main difference between 5a and 1b and 2c is mainly the hydrophobic/aromatic interactions with L2, L5, 19, Y21, Y27, Y51, H47, L92, V93, and L96.
Figure 10.
Ligand interactions with phospholipase A2-V.
Notes: (A) Showing 5a in the active site of phospholipase A2-V active site residues. (B) Showing 2c in the active site of phospholipase A2-V active site residues. Here 2c is observed away from the better interactions with the hydrophobic residues. (C) A pose of 2c and 5a in the active site of phospholipase A2-V. (D) Compound 1b in the active site of phospholipase A2-V.
Conclusion
A novel series of chalcones was evaluated for its potential inhibitory activities against the sPLA2-V, COX-1, COX-2, LOX, and release of pro-inflammatory cytokines such as IL-6 and TNF-α. Inhibition of these enzymes and cytokines by chalcones highlights the anti-inflammatory characteristics of these compounds. The current investigation illustrates that most of these synthesized molecules can be considered as lead molecules for optimization and effective anti-inflammatory agents. Additional studies regarding toxicity and in vivo anti-inflammatory activities are needed for better understanding.
Acknowledgments
This work was supported by the Research Incentive Fund of Universiti Kebangsaan Malaysia (Grant no UKM-DLP-2013-013).
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Yang H, Gu J, Lin X, et al. Profiling of Genetic Variations in Inflammation Pathway Genes in Relation to Bladder Cancer Predisposition. Clin Cancer Res. 2008;14(7):2236–2244. doi: 10.1158/1078-0432.CCR-07-1670. [DOI] [PubMed] [Google Scholar]
- 2.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 3.Esch T, Stefano G. Proinflammation: a common denominator or initiator of different pathophysiological disease processes. Med Sci Monit. 2002;8(5):HY1–HY9. [PubMed] [Google Scholar]
- 4.Dinarello CA. Inflammatory cytokines: interleukin-1 and tumor necrosis factor as effector molecules in autoimmune diseases. Curr Opin Immunol. 1991;3(6):941–948. doi: 10.1016/s0952-7915(05)80018-4. [DOI] [PubMed] [Google Scholar]
- 5.Arend WP, Dayer JM. Cytokines and cytokine inhibitors or antagonists in rheumatoid arthritis. Arthritis Rheum. 1990;33(3):305–315. doi: 10.1002/art.1780330302. [DOI] [PubMed] [Google Scholar]
- 6.Dayer JM, Demczuk S. Cytokines and other mediators in rheumatoid arthritis. Springer Semin Immunopathol. 1984;7(4):387–413. doi: 10.1007/BF00201968. [DOI] [PubMed] [Google Scholar]
- 7.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50(4):515–596. [PubMed] [Google Scholar]
- 8.Loyau G, Pujol JP. The role of cytokines in the development of osteoarthritis. Scand J Rheumatol Suppl. 1990;81:8–12. doi: 10.3109/03009749009096939. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham B. Interleukin-1, immune activation pathways, and different mechanisms in osteoarthritis and rheumatoid arthritis. Ann Rheum Dis. 1991;50(6):395–400. doi: 10.1136/ard.50.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickoloff BJ. The immunologic and genetic basis of psoriasis. Arch Dermatol. 1999;135(9):1104–1110. doi: 10.1001/archderm.135.9.1104. [DOI] [PubMed] [Google Scholar]
- 11.Saklatvala J, Davis W, Guesdon F. Interleukin 1 (IL1) and tumour necrosis factor (TNF) signal transduction. Philos Trans R Soc Lond B Biol Sci. 1996;351(1336):151–157. doi: 10.1098/rstb.1996.0011. [DOI] [PubMed] [Google Scholar]
- 12.Sacca R, Cuff CA, Ruddle NH. Mediators of inflammation. Curr Opin Immunol. 1997;9(6):851–857. doi: 10.1016/s0952-7915(97)80189-6. [DOI] [PubMed] [Google Scholar]
- 13.Matsui T, Kondo T, Nishita Y, et al. Highly potent inhibitors of TNF-alpha production. Part I: discovery of new chemical leads and their structure-activity relationships. Bioorg Med Chem. 2002;10(12):3757–3786. doi: 10.1016/s0968-0896(02)00381-4. [DOI] [PubMed] [Google Scholar]
- 14.Kim KJ, Lee JS, Kwak MK, et al. Anti-inflammatory action of mollugin and its synthetic derivatives in HT-29 human colonic epithelial cells is mediated through inhibition of NF-kappaB activation. Eur J Pharmacol. 2009;622(1–3):52–57. doi: 10.1016/j.ejphar.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Esposito E, Cuzzocrea S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr Med Chem. 2009;16(24):3152–3167. doi: 10.2174/092986709788803024. [DOI] [PubMed] [Google Scholar]
- 16.Mendis E, Kim MM, Rajapakse N, Kim SK. Suppression of cytokine production in lipopolysaccharide-stimulated mouse macrophages by novel cationic glucosamine derivative involves down-regulation of NF-kappaB and MAPK expressions. Bioorg Med Chem. 2008;16(18):8390–8396. doi: 10.1016/j.bmc.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Aitdafoun M, Mounier C, Heymans F, Binisti C, Bon C, Godfroid JJ. 4-Alkoxybenzamidines as new potent phospholipase A2 inhibitors. Biochem Pharmacol. 1996;51(6):737–742. doi: 10.1016/0006-2952(95)02172-8. [DOI] [PubMed] [Google Scholar]
- 18.Kurumbail RG, Stevens AM, Gierse JK, et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–648. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 19.Marnett LJ. The COXIB experience: a look in the rearview mirror. Annu Rev Pharmacol Toxicol. 2009;49:265–290. doi: 10.1146/annurev.pharmtox.011008.145638. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura T, Itoh M, Noda T, Matsuura M, Wakabayashi K. Combined effects of cyclooxygenase-1 and cyclooxygenase-2 selective inhibitors on intestinal tumorigenesis in adenomatous polyposis coli gene knockout mice. Int J Cancer. 2004;109(4):576–580. doi: 10.1002/ijc.20012. [DOI] [PubMed] [Google Scholar]
- 21.Sano H, Noguchi T, Miyajima A, Hashimoto Y, Miyachi H. Anti-angiogenic activity of basic-type, selective cyclooxygenase (COX)-1 inhibitors. Bioorg Med Chem Lett. 2006;16(11):3068–3072. doi: 10.1016/j.bmcl.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS. Progress in COX-2 inhibitors: a journey so far. Curr Med Chem. 2010;17(15):1563–1593. doi: 10.2174/092986710790979980. [DOI] [PubMed] [Google Scholar]
- 23.Singh P, Mittal A. Current status of COX-2 inhibitors. Mini Rev Med Chem. 2008;8(1):73–90. doi: 10.2174/138955708783331577. [DOI] [PubMed] [Google Scholar]
- 24.Langford RM, Mehta V. Selective cyclooxygenase inhibition: its role in pain and anaesthesia. Biomed Pharmacother. 2006;60(7):323–328. doi: 10.1016/j.biopha.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterol. 1994;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 26.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23(2):254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 27.Bukhari SN, Jasamai M, Jantan I. Synthesis and biological evaluation of chalcone derivatives (mini review) Mini Rev Med Chem. 2012;12(13):1394–1403. doi: 10.2174/13895575112091394. [DOI] [PubMed] [Google Scholar]
- 28.Bukhari SN, Jantan I, Jasamai M. Anti-inflammatory trends of 1,3-diphenyl-2-propen-1-one derivatives. Mini Rev Med Chem. 2013;13(1):87–94. [PubMed] [Google Scholar]
- 29.Bandgar BP, Gawande SS, Bodade RG, Totre JV, Khobragade CN. Synthesis and biological evaluation of simple methoxylated chalcones as anticancer, anti-inflammatory and antioxidant agents. Bioorg Med Chem. 2010;18(3):1364–1370. doi: 10.1016/j.bmc.2009.11.066. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Rivera A, Aguilar-Mariscal H, Romero-Ceronio N, Roa-de la Fuente LF, Lobato-García CE. Synthesis and anti-inflammatory activity of three nitro chalcones. Bioorg Med Chem Lett. 2013;23(20):5519–5522. doi: 10.1016/j.bmcl.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 31.Kil JS, Son Y, Cheong YK, et al. Okanin, a chalcone found in the genus Bidens, and 3-penten-2-one inhibit inducible nitric oxide synthase expression via heme oxygenase-1 induction in RAW264.7 macrophages activated with lipopolysaccharide. J Clin Biochem Nutr. 2012;50(1):53–58. doi: 10.3164/jcbn.11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramesh B, Sumana T. Synthesis and Anti-Inflammatory Activity of Pyrazolines. J Chem. 2010;7(2):514–516. [Google Scholar]
- 33.Bharate SB, Mahajan TR, Gole YR, et al. Synthesis and evaluation of pyrazolo[3,4-b]pyridines and its structural analogues as TNF-alpha and IL-6 inhibitors. Bioorg Med Chem. 2008;16(15):7167–7176. doi: 10.1016/j.bmc.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 34.Lorenzo P, Alvarez R, Ortiz MA, Alvarez S, Piedrafita FJ, de Lera AR. Inhibition of IkappaB kinase-beta and anticancer activities of novel chalcone adamantyl arotinoids. J Med Chem. 2008;51(17):5431–5440. doi: 10.1021/jm800285f. [DOI] [PubMed] [Google Scholar]
- 35.Rao YK, Fang SH, Tzeng YM. Synthesis and biological evaluation of 3′,4′,5′-trimethoxychalcone analogues as inhibitors of nitric oxide production and tumor cell proliferation. Bioorg Med Chem. 2009;17(23):7909–7914. doi: 10.1016/j.bmc.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan B, Johnson TE, Lad R, Xing C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J Med Chem. 2009;52(22):7228–7235. doi: 10.1021/jm901278z. [DOI] [PubMed] [Google Scholar]
- 37.Bukhari SN, Tajuddin Y, Benedict VJ, et al. Synthesis and Evaluation of Chalcone Derivatives as Inhibitors of Neutrophils’ Chemotaxis, Phagocytosis and Production of Reactive Oxygen Species. Chem Biol Drug Des. 2014;83(2):198–206. doi: 10.1111/cbdd.12226. [DOI] [PubMed] [Google Scholar]
- 38.Bukhari SN, Jantan I, Wai LK, Lajis NH, Abbas F, Jasamai M. Synthesis and effects of pyrazolines and isoxazoles on the phagocytic chemotaxis and release of reactive oxygen species by zymosan stimulated human neutrophils. Med Chem. 2013;9(8):1091–1098. doi: 10.2174/1573406411309080011. [DOI] [PubMed] [Google Scholar]
- 39.Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7(2):88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 40.Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxy-genase inhibition. J Med Chem. 2007;50(7):1425–1441. doi: 10.1021/jm0613166. [DOI] [PubMed] [Google Scholar]
- 41.Cardozo T, Totrov M, Abagyan R. Homology modeling by the ICM method. Proteins. 1995;23(3):403–414. doi: 10.1002/prot.340230314. [DOI] [PubMed] [Google Scholar]
- 42.Duggan KC, Walters MJ, Musee J, et al. Molecular basis for cyclooxy-genase inhibition by the non-steroidal anti-inflammatory drug naproxen. J Bio Chem. 2010;285(45):34950–34959. doi: 10.1074/jbc.M110.162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saul FA, Prijatelj-Žnidaršič P, Vulliez-le Normand B, et al. Comparative structural studies of two natural isoforms of ammodytoxin, phospholipases A2 from Vipera ammodytes ammodytes which differ in neurotoxicity and anticoagulant activity. J Struct Biol. 2010;169(3):360–369. doi: 10.1016/j.jsb.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 44.Strynadka NC, Eisenstein M, Katchalski-Katzir E, et al. Molecular docking programs successfully predict the binding of a beta-lactamase inhibitory protein to TEM-1 beta-lactamase. Nat Struct Biol. 1996;3(3):233–239. doi: 10.1038/nsb0396-233. [DOI] [PubMed] [Google Scholar]
- 45.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J Pharmacol Sci. 2004;96(3):229–245. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Xu W, Shao H, Xie Y, Wang J. Design and Synthesis of Novel Pyrazole-based Lp-PLA2 Inhibitors. Chinese Journal of Chemistry. 2011;29(10):2039–2048. [Google Scholar]
- 47.Zhang Y, Zhao C, He W, et al. Discovery and evaluation of asymmetrical monocarbonyl analogs of curcumin as anti-inflammatory agents. Drug Des Devel Ther. 2014;8:373–382. doi: 10.2147/DDDT.S58168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad W, Kumolosasi E, Jantan I, Bukhari SNA, Jasamai M. Effects of Novel Diarylpentanoid Analogues of Curcumin on Secretory Phospholipase A2, Cyclooxygenases, Lipo-oxygenase, and Microsomal Prostaglandin E Synthase-1. Chem Biol Drug Des. 2014;83(6):670–681. doi: 10.1111/cbdd.12280. [DOI] [PubMed] [Google Scholar]
- 49.Seibert K, Zhang Y, Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Willingale HL, Gardiner NJ, McLymont N, Giblett S, Grubb BD. Prostanoids synthesized by cyclo-oxygenase isoforms in rat spinal cord and their contribution to the development of neuronal hyperexcitability. Br J Pharmacol. 1997;122(8):1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katsori AM, Chatzopoulou M, Dimas K, et al. Curcumin analogues as possible anti-proliferative and anti-inflammatory agents. Eur J Med Chem. 2011;46(7):2722–2735. doi: 10.1016/j.ejmech.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 52.Stoll LL, Denning GM, Weintraub NL. Endotoxin, TLR4 signaling and vascular inflammation: potential therapeutic targets in cardiovascular disease. Curr Pharm Des. 2006;12(32):4229–4245. doi: 10.2174/138161206778743501. [DOI] [PubMed] [Google Scholar]