Abstract
Patient: Male, 23
Final Diagnosis: Rabdomyolysis
Symptoms: Cardiac arrest • cardiac arrhythmia • hypercalcemia
Medication: —
Clinical Procedure: —
Specialty: —
Objective:
Unusual clinical course
Background:
Rhabdomyolysis is frequently complicated by multiple electrolyte abnormalities, including hyperkalemia, hyperphosphatemia, and hypo/hypercalcemia. Hypercalcemia can be severe and life-threatening.
Case Report:
A 23-year-old white male suffered severe trauma to his lower extremities after a motor vehicle accident, leading to severe muscle damage, cardiac arrhythmia, cardiac arrest, and oliguric acute kidney injury (AKI), requiring hemodialysis treatment. As expected, he was hypocalcemic during the oliguric phase but during the diuretic phase he developed severe symptomatic hypercalcemia requiring hemodialysis treatment in spite of volume replacement and administration of pamidronate. Hypercalcemia reached a peak of 17.1 mg/dL, corrected for serum albumin and urine output was as high as 11.9 liters daily. Hypercalcemia lasted for 3 weeks and then it returned back to normal levels. Plasma levels of 25-OH and 1–25(OH)2 vitamin D were low, intact parathyroid hormone level was appropriately suppressed, and 24-hour urine calcium was 1194 mg (normal up to 350 mg/daily). Mobilization of calcium from calcium phosphate deposits in the injured muscles seems to be the main reason for hypercalcemia and hypercalciuria in rhabdomyolysis-induced AKI.
Conclusions:
Hypercalcemia is not uncommon during the recovery phase of ATN. Unattended, it can cause severe morbidity and even mortality. Fluid administration, pamidronate, and calcium-free dialysis are some methods used to correct severe hypercalcemia. Over time, hypercalcemia improves in almost all cases.
MeSH Keywords: Acute Kidney Injury, Hypercalcemia, Rhabdomyolysis
Background
Rhabdomyolysis is a common phenomenon that results from muscle breakdown due to trauma, extreme exertion, or myotoxins, causing the release of intracellular contents including potassium, calcium, and phosphorus [1–3], which results in acute kidney injury (AKI) in 10–65% of cases [4–10]. Early hypocalcemia is often seen due to precipitation of calcium with phosphate released from damaged muscle cells, and occasionally hypercalcemia can be seen during the recovery phase due to mobilization of calcium phosphate deposits [11,12]. Herein we present a case of rhabdomyolysis complicated by AKI and profound hypercalcemia and hypercalciuria.
Case Report
A 23-year-old white male suffered severe trauma to his lower extremities after a motor vehicle accident, leading to rhabdomyolysis and oliguric acute renal injury requiring renal replacement therapy. He was admitted to the intensive care unit of another health care facility where he was found to have severe hyperkalemia leading to 5 episodes of cardiac arrest. He was stabilized and then transferred to the Veterans Hospital for continuation of care. In the VA hospital he had severe damage of lower extremities, including compartment syndrome, which eventually culminated in an above the knee amputation of his left lower extremity. While in the intensive care unit, he was oliguric and uremic, and hemodialysis treatment was continued. About 3 weeks after his initial injury, his renal function began to recover; he entered the polyuric phase of AKI, and came off dialysis. During this phase, his serum calcium
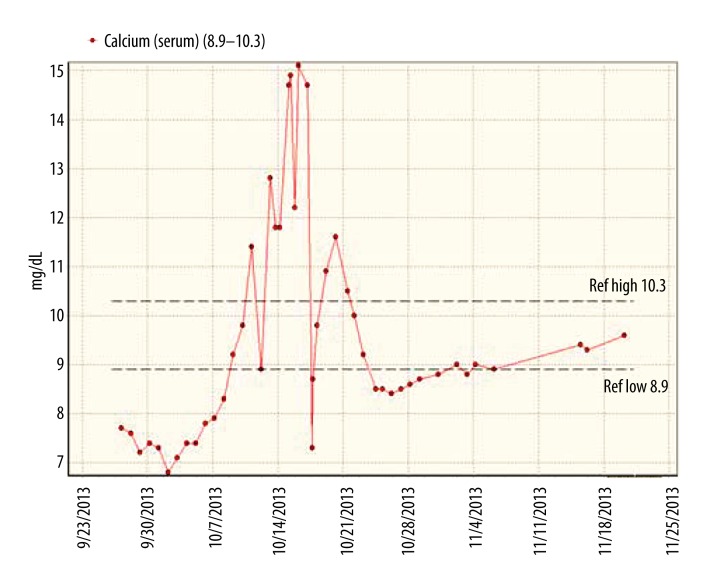
started to rise, reaching a peak of 17.1mg/d, corrected for serum albumin (Figure 1), causing nausea, weakness, drowsiness, and polyuria, with urine output up to 11 liters per day. He was treated with intravenous fluids, calcitonin, and pamidronate but he continued to be hypercalcemic. Therefore, he was treated one time with calcium-free dialysate, which he tolerated well and brought down his serum calcium from 15.1 to 7.3 mg/dL, uncorrected for serum albumin. Overall hypercalcemia lasted for about 3 weeks and gradually decreased to normal levels. During this time, plasma levels of 25-OH and 1–25(OH)2 vitamin D levels were 12 ng/mL (normal >30 ng/mL) and <8 ng/mL (normal >30 ng/mL), respectively. Intact parathyroid hormone level was appropriately suppressed and was less than 2.5 pg/mL. A 24-hour urine collection showed excretion of 1194 mg of calcium (normal up to 350 mg/day). A technetium pyrophosphate scan (Figure 2) showed extensive calcium deposition in his left thigh muscles, where he had severe muscle injury and compartment syndrome.
Figure 1.
Serum calcium levels during the oliguric and diuretic phases of AKI. Serum Ca is demonstrated in mg/dL on the ordinate and the timeline by date on the abscissa. Arrow indicates use of calcium free dialysate and its impact on serum calcium.
Figure 2.
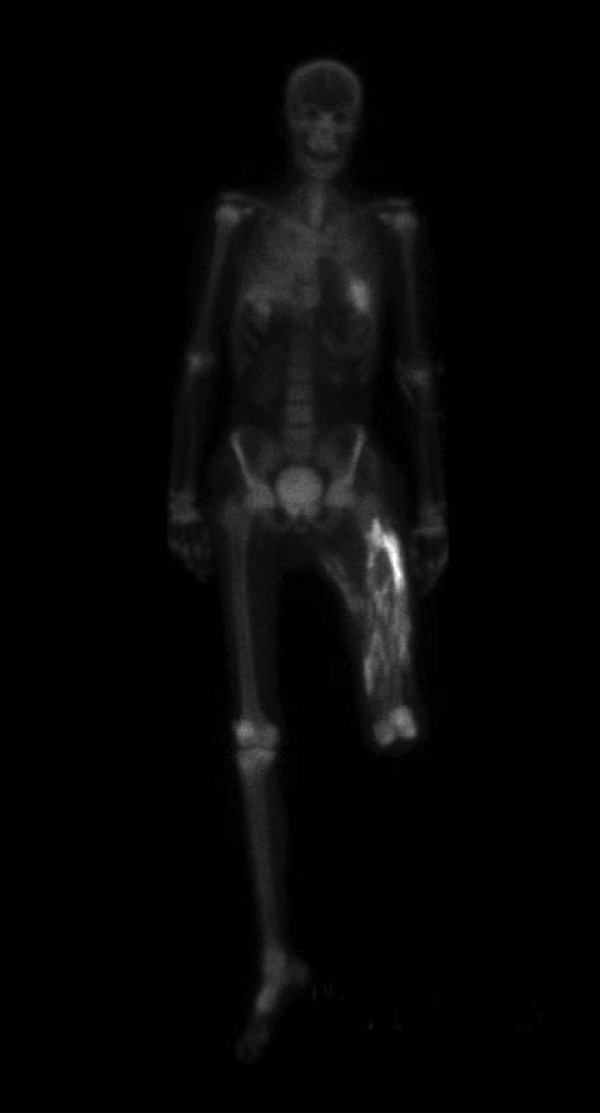
Heavy deposition of calcium in the injured tissues of the left thigh. The left leg has already been amputated.
Discussion
Rhabdomyolysis occurs when there is an insult to muscle cells, such as trauma, extreme exertion, ischemia, infection, metabolic derangements, or exposure to myotoxins [13–19], which triggers a chain of events causing progressive derangements in intracellular calcium homeostasis and eventual cell death. In health, the cytoplasmic calcium concentration is very tightly regulated by various calcium pumps/exchangers (e.g., calcium channels, 2Na+/Ca2+ exchanger, and Ca2+ ATPase pump). Calcium is transported from the cytoplasm into the sarcoplasmic reticulum via the calcium ATPase pump, where it is released on stimulation from neural impulses causing muscle contraction. Calcium is also pumped from the cytoplasm to the extracellular space by the 2Na+/Ca2+ exchanger, which moves sodium down its concentration gradient into the cell while moving calcium out of the cell against its concentration gradient. The net result is that the intracellular cytoplasmic ionized calcium concentration is 10 000 times lower than the extracellular ionized calcium concentration [13,20]. In rhabdomyolysis, the 2 main mechanisms that trigger the cascade of events resulting in the demise of the cell are: 1) a lack of ATP, which causes dysfunction of the Na+/K+ ATPase and Ca2+ ATPase, thus causing accumulation of cytosolic calcium; and 2) loss of integrity of the sarcoplasmic reticulum membrane, which also causes increase in cytoplasmic calcium concentration. The increase in cytosolic calcium concentration activates neutral proteases or phospholipases, which damage cell membranes. The release of intracellular contents to the extracellular space further compounds the problem by damaging nearby cells. The inflammatory response to this insult causes ischemia and progressive muscle damage [13]. As the muscle cells are damaged, the intracellular contents, which include potassium, phosphorus, uric acid, and creatinine kinase, are released [1–3]. Since potassium is a predominantly intracellular cation with 70% of the intracellular potassium in muscle cells [21], rhabdomyolysis can cause significant hyperkalemia, with resultant arrhythmias, as seen in this patient. The release of intracellular phosphorus can cause early hypocalcemia due to formation of calcium phosphate deposits on the injured muscles [15], as seen in our patient. During the recovery phase of AKI, the calcium phosphate deposits mobilize, causing hypercalcemia in up to one-third of patients [11,12]. However, it is uncommon to see hypercalcemia as severe as in this case. A review of the 60 current PubMed indexed case reports on hypercalcemia associated with rhabdomyolysis (with available abstracts and/or full-text articles) reveals that this is the third-highest calcium level associated with rhabdomyolysis in the current literature. The highest level of hypercalcemia associated with rhabdomyolysis was 20 mg/dL [22]. There have only been 5 other cases of hypercalcemia with serum calcium level >15 mg/dL in the past 40 years [23–26]. Of the cases in the past 20 years in which the duration of hypercalcemia was reported, the average was 8.6 days, with a median of 10 days. This demonstrates the importance of recognizing the severity of hypercalcemia, which can be associated with rhabdomyolysis. The long duration of hypercalcemia in our case may have been because he was young, as well as the severity of his injuries.
The mechanism of hypercalcemia seems to be the dissolution of deposited calcium phosphate during the diuretic phase of acute kidney injury. Various studies have evaluated parathyroid hormone and vitamin D response to hypocalcemia during the oliguric and to hypercalcemia during the polyuric phase, and the response seems to be appropriate [23]. The fact that our patient had suppressed level of intact parathyroid hormone and low levels of 25-OH and 1–25(OH)2 vitamin D and hypercalciuria is consistent with this line of thinking.
Conclusions
It is crucial to remember that patients with rhabdomyolysis are not out of danger when serum creatinine and urine output start to improve. One must be aware of and watch for severe hypercalcemia, which may occur during the diuretic phase of rhabdomyolysis-induced acute injury. Close follow-up and meticulous management of fluids and electrolytes is of utmost importance to the eventual recovery of kidney function.
References:
- 1.Khan FY. Rhabdomyolysis: a review of the literature. Neth J Med. 2009;67:272–83. [PubMed] [Google Scholar]
- 2.Lerma EV, Nissenson AR. Rhabdomyolysis. In: Lerma EV, Nissenson AR, editors. Nephrology Secrets. 3rd ed. St. Louis, MO: Elsevier; 2012. pp. 90–95. [Google Scholar]
- 3.Holt SG, Moore KP. Pathogenesis and treatment of renal dysfunction in rhabdomyolysis. Intensive Care Med. 2001;27(5):803–11. doi: 10.1007/s001340100878. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez E, Soler MJ, Rap O, et al. Risk factors for acute kidney injury in severe rhabdomyolysis. PLoS One. 2013;8(12):e82992. doi: 10.1371/journal.pone.0082992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaney KA, Givens ML, Vohra RB. Use of RIFLE criteria to predict the severity and prognosis of acute kidney injury in emergency department patients with rhabdomyolysis. J Emerg Med. 2012;42(5):521–28. doi: 10.1016/j.jemermed.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Morris JA, Mucha P, Ross SE, et al. Acute posttraumatic renal failure: a multicenter perspective. J Trauma. 1991;31:1584–90. doi: 10.1097/00005373-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Melli G, Ghaudhry V, Cornblath DR. Rhabdomyolysis: An evaluation of 475 hospitalized patients. Medicine (Baltimore) 2005;84(6):377–85. doi: 10.1097/01.md.0000188565.48918.41. [DOI] [PubMed] [Google Scholar]
- 8.De Meijer AR, Fikkers BG, de Keijzer MH, et al. Serum creatine kinase as a predictor of clinical course in rhabdomyolysis: a 5-year intensive care survey. Intensive Care Med. 2003;29(7):1121–25. doi: 10.1007/s00134-003-1800-5. [DOI] [PubMed] [Google Scholar]
- 9.Brown CV, Rhee P, Chan L, et al. Preventing renal failure in patients with rhabdomyolysis: Do bicarbonate and mannitol make a difference? J Trauma. 2004;56(6):1191–96. doi: 10.1097/01.ta.0000130761.78627.10. [DOI] [PubMed] [Google Scholar]
- 10.Bosch X, Poch E, Grau JM. Rhabdomyolysis and Acute Kidney Injury. N Engl J Med. 2009;361:62–72. doi: 10.1056/NEJMra0801327. [DOI] [PubMed] [Google Scholar]
- 11.Shrestha SM, Berry JL, Davies M, et al. Biphasic hypercalcemia in severe rhabdomyolysis: serial analysis of PTH and vitamin D metabolites. A case report and literature review. Am J Kidney Dis. 2004;43(3):e31–35. doi: 10.1053/j.ajkd.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Graziani G, Calvetta A, Cucchiari D, et al. Life-threatening hypercalcemia in patients with rhabdomyolysis-induced oliguric acute renal failure. J Nephrol. 2011;24:128–31. doi: 10.5301/jn.2010.5794. [DOI] [PubMed] [Google Scholar]
- 13.Giannoglou GD, Chatzizisis YS, Misirli G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur J Intern Med. 2007;18(2):90–100. doi: 10.1016/j.ejim.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman JL, Shen MC. Rhabdomyolysis. Chest. 2013;144(3):1058–65. doi: 10.1378/chest.12-2016. [DOI] [PubMed] [Google Scholar]
- 15.Vanholder R, Sever MS, Erek E, et al. Rhabdomyolysis. J Am Soc Nehprol. 2000;11(8):1553–61. doi: 10.1681/ASN.V1181553. [DOI] [PubMed] [Google Scholar]
- 16.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis – an overview for clinicians. Crit Care. 2005;9(2):158–69. doi: 10.1186/cc2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frohlich G, Grawe A, Schmidt N, et al. Difficult course of rhabdomyolysis in influenza A/H1N1. Dtsch Med Wochenschr. 2013;138(46):2351–54. doi: 10.1055/s-0033-1349642. [DOI] [PubMed] [Google Scholar]
- 18.Wen Z, Chuanwei L, Chunyu Z, et al. Rhabdomyolysis presenting with severe hypokalemia in hypertensive patients: A case series. BMC Res Notes. 2013;6:155. doi: 10.1186/1756-0500-6-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grifoni E, Fabbri A, Ciuti G, et al. Hypokalemia-induced rhabdomyolysis. Intern Emerg Med. 2014;9(4):487–88. doi: 10.1007/s11739-013-1033-8. [DOI] [PubMed] [Google Scholar]
- 20.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int. 1996;49(2):314–26. doi: 10.1038/ki.1996.48. [DOI] [PubMed] [Google Scholar]
- 21.Weiner ID, Linas SL, Wingo CS. Disorders of Potassium Metabolism. In: Floege J, Johnson RJ, Feehally J, editors. Comprehensive Clinical Nephrology. 4th ed. St. Louis, Missouri: Elsevier; 2010. pp. 118–19. [Google Scholar]
- 22.Uchida K, Kondo J, Imoto K, et al. Dissecting aortic aneurysm associated with myonephropathic metabolic syndrome and hypercalcemia. Nihon Kyobu Geka Gakkai Zasshi. 1991;39(1):86–89. [PubMed] [Google Scholar]
- 23.Sperling LS, Tumlin JA. Case report: delayed hypercalcemia after rhabdomyolysis-induced acute renal failure. Am J Med Sci. 1996;311(4):186–88. doi: 10.1097/00000441-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Olazo E, Sanchez-De La Nieta MD, Rivera F, et al. Massive hypercalcaemia in rhabdomyolysis associated with acute renal failure. Nefrologia. 2012;32(5):690–92. doi: 10.3265/Nefrologia.pre2012.Jun.11577. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein EI, Akmal M, Telfer N, et al. Delayed hypercalcemia with acute renal failure associated with nontraumatic rhabdomyolysis. Arch Intern Med. 1981;141(6):753–55. [PubMed] [Google Scholar]
- 26.Koffler A, Friedler RM, Massry SG. Acute Renal Failure Due to Nontraumatic Rhabdomyolysis. Ann Intern Med. 1976;85(1):23–28. doi: 10.7326/0003-4819-85-1-23. [DOI] [PubMed] [Google Scholar]