Abstract
Serum samples from 150 NS1-negative (Platelia ELISA) patients presumptively diagnosed with dengue were analyzed by the TaqMan probed real-time reverse transcription PCR (TaqMan qRT-PCR) method. The qRT-PCR positive samples were tested for serotype by semi-nested RT-PCR and a qualitative immunochromatographic assay for IgG and IgM. Molecular detection methods showed 33 (22%) positive samples out of 150 NS1-antigen negative samples. Of these, 72% were collected up to day 2 after the onset of symptoms, when diagnostic sensitivity of NS1-antigen test assays is significantly enhanced. Most of the cases were not characterized as secondary infection. Twenty-eight samples were successfully serotyped, 75% of which for DENV-4, 14% for DENV-2, 7% for DENV-3 and 4% for DENV-1. These findings reaffirm the hyperendemic situation of the state of Roraima and suggest a lower sensitivity of the NS1 test, mainly when DENV-4 is the predominant serotype. Health care providers should therefore be aware of samples tested negative by NS1 antigen assays, especially when clinical symptoms and other laboratory data results show evidence of dengue infection.
Keywords: Dengue, Serotypes, Diagnosis, NS1 antigen, qRT-PCR
Abstract
Amostras séricas de 150 pacientes, com diagnóstico presuntivo de dengue e resultado negativo para dengue por ELISA-NS1-Antígeno do kit Platelia™ (NS1-Ag), foram analisadas pela técnica de TaqMan Transcrição Reversa seguida da Reação em Cadeia da Polimerase em Tempo Real (qRT-PCR). As amostras positivas por qRT-PCR, foram submetidas a identificação dos sorotipos por RT-Hemi nested-PCR e a ensaio imunocromatográfico para detecção qualitativa dos anticorpos IgG e IgM. A técnica molecular apresentou como resultado 33 (22%) amostras positivas entre as 150 negativas pela detecção do NS1-Ag, destas o 72% foram coletadas até o segundo dia de início dos sintomas da doença, período de maior sensibilidade para pesquisas de NS1-Ag. A maioria dos casos não evidenciou infecção secundária. Dessas amostras, 28 foram satisfatoriamente sorotipadas sendo 75% de DENV-4, 14% de DENV-2, 7% de DENV-3 e 4% de DENV-1. Os resultados reafirmam a situação hiperendêmica do Estado de Roraima e sugerem baixa sensibilidade do NS1 test, especialmente quando o sorotipo predominante é DENV-4. Sugerimos assim, que a comunidade médica deve ser alertada no sentido de ser cautelosa com resultados de NS1-Ag negativo, principalmente quando sintomas clínicos e outros resultados laboratoriais sejam indicativos de provável infecção por dengue.
Dengue is currently regarded as the most important mosquito-borne viral disease, in terms of both geographical distribution and number of cases reported annually. Recent studies have estimated a risk population of 3.97 billion people, and of the major vector-borne diseases the cases of dengue have been prevailing over those of malaria in the Americas, Asia and much of Africa5,6.
The etiological agent, the Dengue Virus (DENV), belongs to the genus Flavivirus of the family Flaviviridae, and the main mosquito vector to transmit the disease to humans is the predominantly urban species Aedes aegypti 11.
The disease has a wide clinical spectrum - ranging from a flu-like illness to severe dengue - and, therefore, it is not possible to determine the clinical manifestations that can ensue at the time of dengue infection. Although, there is no specific treatment for dengue, early diagnosis may guide appropriate clinical management and, thereby, prevent severe complications and/or death24.
The state of Roraima, located in the Brazilian Amazon region, has shown a high incidence of infection over the past decade1 and is currently recognized as a hyperendemic area, as well as a port-of-entry for serotypes and genotypes of dengue viruses into Brazil. DENV-4 reemerged in Roraima in 20102,19, 28 years after it was last detected in the state, and now all four dengue serotypes are circulating1. In recent years, the state has seen an increase in severe forms of the disease, probably due to secondary infections and/or higher virulence of circulating strains1. The World Health Organization (WHO) currently recommends early laboratory tests for the diagnosis of the dengue virus infection using NS1 antigen detection in human serum24, and this is mostly adopted in Roraima. The highly immunogenic non-structural glycoprotein NS1 contains approximately 353 amino acids (46 kDa) and is more conserved among the flaviviruses. Although, NS1 does not form part of the virus structure, it is required for viral RNA replication. It is also displayed on the surface of infected cells and is secreted into the bloodstream, during the acute phase of the disease. This latter characteristic makes it sensitive enough to diagnose a patient on the very first days of infection4,17.
In 2012, 998 serum samples from presumptive diagnosis of dengue were tested with the use of the Bio-Rad Platelia™ Dengue NS1 antigen capture kit at the Central Public Health Laboratory in Roraima (LACEN/RR). Of these samples, 778 (78%) tested negative, i.e. they were ruled out for dengue infection through laboratory diagnosis. Based on this, two questions arise: have these patients been infected by other arboviruses? Does the dengue NS1 antigen test give rise to false-negative results?
To find answers to these questions, it was first decided to assess the possibility of false-negative results for the samples ruled out by the dengue virus NS1 antigen detection. In order to carry out this evaluation, the most sensitive TaqMan - MGB probed real-time quantitative reverse transcription PCR (TaqMan qRT-PCR) technique was applied.
Of the 778 cases initially discarded in 2012 as not being dengue, 150 samples were randomly selected from patients who, in addition to fever, had in their clinical records one or more common signs of dengue such as arthralgia, headache, retro-orbital pain, chills, myalgia, and rash. The average time of sample collection was 1-5 days after the onset of the symptoms.
Viral RNA was extracted directly from serum by the Axygen® AxyPrep™ Body Fluid Viral DNA/RNA Miniprep kit, and subsequently subjected to the qRT-PCR assay (GURUKUMAR et al., 2009)12. This technique is able to detect the genome of any of the four dengue serotypes, but unable to identify specific serotype DENV infection. Fluorescence reading was performed on a StepOne™ Real-Time PCR system. All reactions were performed under the same conditions with the use of ROX as a passive reference dye for normalization of data and VIC as reporter. Samples with Ct < 39 were considered to be dengue positive and underwent serotyping by semi-nested RT-PCR, as described by LANCIOTTI et al. (1992)15 and an immunochromatographic assay that allows qualitative and differential detection for IgG and IgM antibodies (Dengue Test Bioeasy)8. IgG positive samples, collected until the 4th day from the onset of symptoms, were characterized as secondary infection7.
Of the 150 samples tested, 33 (22%) were positive by qRT-PCR. The samples with the false-negative NS1 antigen were collected between 1-4 days after the onset of symptoms (Table 1) within this period, according to the literature and the manufacturer's instructions, dengue NS1 antigen tests are highly sensitive3,20.
Table 1. Results description of used samples in this study.
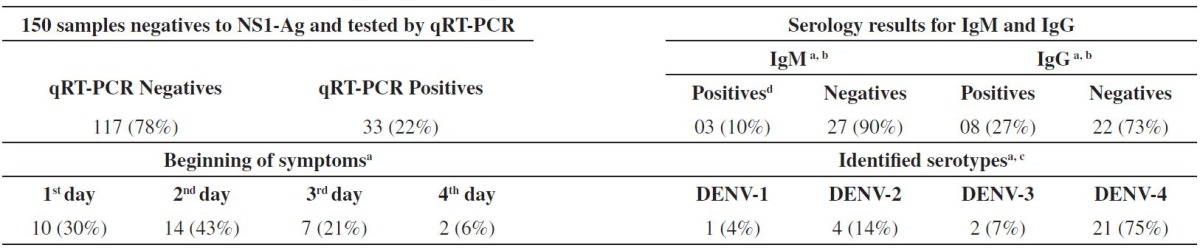
Symptom onset results, serology and identified serotypes refer to samples positive by qRT-PCR;
03 samples tested by qRT-PCR were insufficient for serology;
Five samples positive by qRT-PCR could not be detected by semi-nested RT-PCR;
All samples positives to IgM were also positive to IgG.
Studies from different countries indicate a low sensitivity on the NS1 test in secondary infection[9,14,16,18.23], and as Roraima is a hyperendemic State to dengue, at first, it was considered that the main cause for this high number of false negative samples was due to secondary infection. Nevertheless, only eight (28%), of the 30 analyzed samples, showed positive IgG (Table 1).
In 37% of the IgG positive samples IgM antibodies were also detected. FELIX et al., on a dengue outbreak in 2010 in Santos-Brazil, found 64.2% IgM positive among a total of 260 samples with secondary infection10.
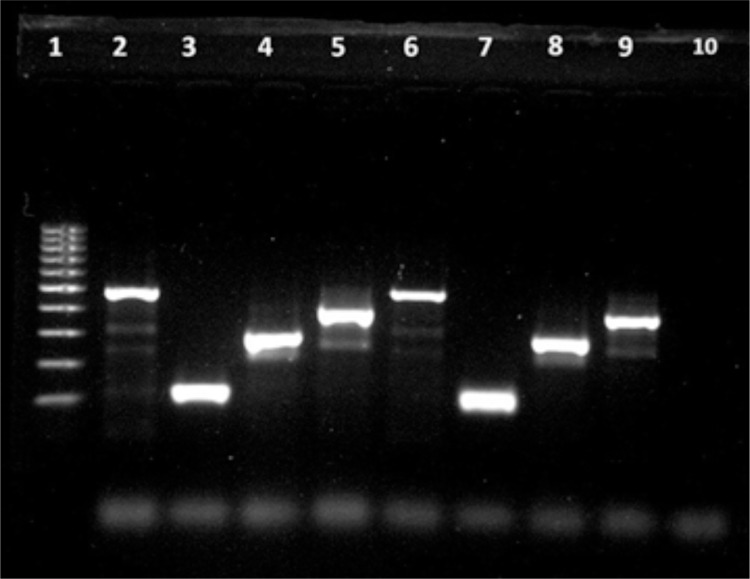
All four dengue virus serotypes were reported among the positive cases (Fig.1), and these findings are sufficient to classify the state of Roraima as a hyperendemic area. The most common serotype DENV-4 accounted for 75% of the tested serum samples. These data are consistent with that of the Roraima State Epidemiology and Surveillance Branch for this period, which shows that a further 90% of the samples sent for viral identification in 2012 were DENV-421. According to the manufacturer's instructions the sensitivity of the assay does not differentiate between dengue serotypes20, however, a study conducted in the state of Sergipe, in Northeastern Brazil, found 48.7% false negative, among 119 samples tested for the NS1 antigen. All the negative samples were identified as DENV-4, and authors point to a low sensitivity of the Platelia dengue NS1Ag test in regions where this serotype is predominant22. The DENV-4 strains tested in this study belong to genotype II, the same one that is circulating in the state of Roraima19, and considering that the Bio-Rad kit uses a monoclonal antibody, it was suggested that this antibody is likely to have low-affinity to epitopes of NS1 from the DENV-4 genotype II, however, there are studies that did not find any difference in the NS1 amino acid sequence in DENV-2 false negative and positive samples for the NS1 Ag detection10.
Fig 1. Agarose gel analysis of Hemi nested RT-PCR products from Dengue virus. Lane 1: 100pb ladder; Lane 2: positive control DENV-1 (482pb); Lane 3: positive control DENV-2 (119pb); Lane 4: positive control DENV-3 (290pb); Lane 5: positive control DENV-4 (392pb); Lane 6: sample patient A05 (482pb); Lane 7: sample patient A16 (119pb); Lane 8: sample patient A63 (290pb); Lane 9: sample patient A44 (392pb); Lane 10 negative control.
After interrelating the NS1 test sensitivity to dengue serotypes, GUZMAN et al. 's study13 evaluated the four dengue serotypes in samples from Southeast Asia and Latin America, which described the lower sensitivities by the Platelia Dengue NS1 Ag kit in DENV-2. Studies performed in Brazil, before DENV-4 reemerged, found the lowest sensitivity to serotypes DENV-2 and DENV-310,18.
The analysis presented here reinforces the importance of increasing the awareness of healthcare providers regarding the fact that patients tested negative by the NS1 antigen detection may be infected with the dengue virus, especially when the clinical symptoms and other laboratory data indicate such infection.
ACKNOWLEDGMENTS
The authors would like to thank the Federal University of Roraima. This work was supported by grants from Instituto Leônidas e Maria Deane and Santander Universidades.
REFERENCES
- 1.Acosta POA, Cordeiro JS, Granja F, Siqueira TCS, Brito FEG, Freitas AG, et al. Dengue in the northernmost part of Brazil from 1999 to 2011: characterization of circulating DENV strains. Dengue Bull. 2012;36:50–63. [Google Scholar]
- 2.Acosta POA, Maito RM, Granja F, Cordeiro JS, Siqueira T, Cardoso MN, et al. Dengue virus serotype 4, Roraima State, Brazil. Emerg Infect Dis. 2011;17:1979–80. doi: 10.3201/eid1710.110776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40:376–81. doi: 10.1128/JCM.40.2.376-381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K, et al. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193:1078–88. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496((7446)):504–7. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6: doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza VAUF, Tateno AF, Oliveira RR, Domingues RB, Araújo ES, Kuster GW, et al. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J Clin Virol. 2007;39:230–3. doi: 10.1016/j.jcv.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Dengue IgG/IgM test Bioeasy Available from: http://trisul.com.br/arquivos/dengue_(bula).pdf.
- 9.Duong V, Ly S, Lorn Try P, Tuiskunen A, Ong S, Chroeung N, et al. Clinical and virological factors influencing the performance of a NS1 antigen-capture assay and potential use as a marker of dengue disease severity. PLoS Negl Trop Dis. 2011;5: doi: 10.1371/journal.pntd.0001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix AC, Romano CM, Centrone CC, Rodrigues CL, Villas-Boas L, Araujo ES, et al. Low sensitivity of NS1 protein tests evidenced during a dengue type 2 virus outbreak in Santos, Brazil, in 2010. Clin Vaccine Immunol. 2012;19:1972–6. doi: 10.1128/CVI.00535-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubler DJ. Epidemic dengue and dengue hemorrhagic fever: a global public health problem in the 21st Century. In: Scheld WM, Armstrong D, Hughes JM, editors. Emerging Infections 1. Washington: ASM Press; 1998. pp. 1–14. [Google Scholar]
- 12.Gurukumar KR, Priyadarshini D, Patil JA, Bhagat A, Singh PS, Shah PS, et al. Development of real time PCR for detection and quantitation of dengue viruses. Virol J. 2009;6:10:1–8. doi: 10.1186/1743-422X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman MG, Jaenisch T, Gaczkowski R, Ty Hang VT, Sekaran SD, Kroeger A, et al. Multi-country evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hang VT, Nguyet NM, Trung DT, Tricou V, Yoksan S, Dung NM, et al. Diagnostic accuracy of NS1 ELISA and lateral flow rapid tests for dengue sensivity, specificity and relationship to viraemia and antibody responses. PLoS Negl Trop Dis. 2009;3: doi: 10.1371/journal.pntd.0000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lanciotti RS, Calisher CH, Gubler DJ, Chang G-J, Vorndam V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lapphra K, Sangcharaswichai A, Chokephaibulkit K, Tiengrim S, Piriyakarnsakul W, Chakorn T, et al. Evaluation of an NS1 antigen detection for diagnosis of acute dengue infection in patients with acute febrile illness. Diagn Microbiol Infect Dis. 2008;60:387–91. doi: 10.1016/j.diagmicrobio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S, et al. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis. 2002;186:1165–8. doi: 10.1086/343813. [DOI] [PubMed] [Google Scholar]
- 18.Lima MRQ, Nogueira RMR, Schatzmayr HG, Santos FB. Comparison of three commercially available dengue NS1 antigen capture assays for acute diagnosis of dengue in Brazil. PLos Negl Trop Dis. 2010;4: doi: 10.1371/journal.pntd.0000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naveca FG, Souza VC, Silva GAV, Maito RM, Granja F, Siqueira T, et al. Complete genome sequence of a dengue virus serotype 4 strain isolated in Roraima, Brazil. J Virol. 2012;86:1897. doi: 10.1128/JVI.06731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PlateliaTM Dengue NS1 Ag (Bio-Rad): detecção qualitativa ou semi-quantitativa do antígeno NS1 do vírus da dengue no soro ou plasma humano, pelo método imunoenzimático. Available from: https://www.bio-rad.com/webroot/web/pdf/inserts/CDG/pt/72830_12-2008.pdf.
- 21.Roraima Secretaria Estadual de Saúde. Departamento Vigilância Epidemiológica. Relatório: casos de dengue notificados no ano 2012. Sistema de Informação de Agravos de Notificação (SINAN) Boa Vista: SINAN. 2012 [Google Scholar]
- 22.Sea VRF, Cruz ACR, Gurgel RQ, Nunes BTD, Silva EVP, Dolabella SS, et al. Underreporting of dengue-4 in Brazil due to low sensitivity of the NS1 Ag test in routine control programs. PLoS One. 2013;8: doi: 10.1371/journal.pone.0064056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekaran SD, Ew CL, Subramaniam G, Kanthesh BM. Sensitivity of dengue virus NS1 detection in primary and secondary infections. Afr J Microbiol Res. 2009;3:105–110. [Google Scholar]
- 24.World Health Organization . Geneva: World Health Organization; 2009. Dengue: guidelines for diagnosis, treatment, prevention and control. Available from: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. [PubMed] [Google Scholar]