Abstract
The nematode Calodium hepaticum (syn. Capillaria hepatica) is a zoonotic helminth found mainly infecting rats. It was studied the prevalence of C. hepaticum infection in Rattus norvegicus in an urban area of Rio de Janeiro (Brazil), with low urban planning and sanitation. The presence of C. hepaticum was identified through visible yellowish-white lesions in liver tissue and histological analyses. The total prevalence of infection was 45%, with no significant differences between sex and age. The presence of infected rodents near the peridomestic area poses substantial risk to human health.
Keywords: Rattus norvegicus, Calodium hepaticum, Prevalence, Rio de Janeiro
Abstract
O nematóide Calodium hepaticum (sin. Capillaria hepatica) é um helminto zoonótico encontrado infectando principalmente ratos. A prevalência da infecção de C. hepaticum em Rattus norvegicus foi investigada em área urbana do Rio de Janeiro (Brasil) com baixo planejamento e saneamento. A presença de C. hepaticum foi identificada através da presença de lesões macroscópicas caracterizadas por manchas extensas de coloração branco-amarelada difusa por toda superfície do tecido do fígado e através de análise histológica. A prevalência total da infecção foi de 45% sem diferença significativa entre o sexo e idade. A presença de roedores infectados próximos do peridomicílio representa um risco substancial para a saúde humana.
The zoonotic nematode Calodium hepaticum (Brancroft, 1893) Moravec, 1982 (syn. Capillaria hepatica, Tricocephalus hepaticus, Hepaticola hepatica) has global propagation and mainly infects rodents and other mammals, including humans3,9,10. Synanthropic rats of the genus Rattus are considered the most important hosts and reservoirs of this parasite, due to the high prevalence and low pathogenicity of the infection10.
Adult worms colonize the hepatic parenchyma of the host, where the eggs are released. For successful parasite transmission, host death is necessary, after which the infected tissue needs either to be consumed by a predator and released into the feces (spurious infection) or decomposed in the environment, allowing the eggs to become embryonated and infective. In this stage, humans can be infected ingesting the eggs in water, soil or contaminated fruits and vegetables8. The disseminator animals ingest the eggs, which pass through the gastrointestinal tract and are dispersed in the environment11.
In Brazil, this parasite has been reported infecting humans and various species of domestic and wild mammals2,12,13,14,15,18,20. In synanthropic rodents, C. hepaticum has been recorded in the states of São Paulo, Bahia and Pará5,7,13. In Rio de Janeiro, a previous survey has been performed to monitor Rattus norvegicus for detection of zoonotic parasitic diseases in a densely populated urban region19. The objective of this study was to determine the prevalence of C. hepaticum infection in R. norvegicus, in an urban area with low urban planning and sanitation.
In 2011, seventy-four specimens of R. norvegicus were captured in the city of São Gonçalo (22°48′26.7″S, 43°00′49.1″W), state of Rio de Janeiro, using Tomahawk® traps (Model 201; 40.6 x 12.7 x 12.7 cm). Rodent collection permits were obtained from the committee on animal research ethics (CEUA no. LW 24/10) of Oswaldo Cruz Foundation (Fiocruz). Body weight was used as a proxy for host age, according to WEBSTER & MACDONALD (1995)21. Prevalence was measured as per BUSH et al. 1 followed by a 95% confidence interval (CI). Chi-squared analyses were used to test significant differences in the prevalence of parasites between the sex and age of the rodents16. Macroscopic examination of the liver was conducted to screen for the presence of C. hepaticum, detected by the presence of yellowish-white lesions caused by adult worms and eggs. These findings were then confirmed by histology. Collected liver samples were fixed in Milloning and routinely processed for histological examination, then embedded in paraffin and sliced into 5-µm sections. The material was stained with hematoxylin-eosin (HE). The histological slides were observed under a Zeiss Observer Z1 light microscope, and images were acquired using a Zeiss Axio Cam HRc camera. The images were processed using Axio Vision Rel. 4.7 software. Two randomly chosen sections of each lesion were included for histological examination.
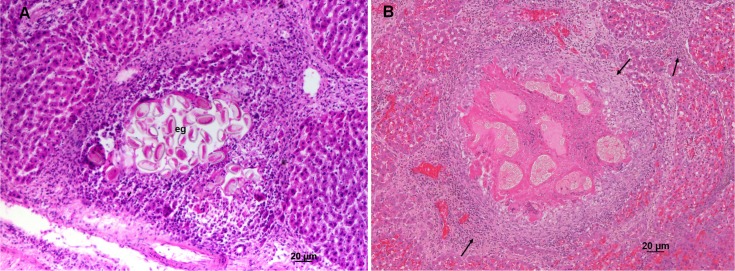
The overall prevalence of infection was 45% IC 33.8-55.9 (33/74); 52% IC 33.7-66.5 (22/42) in males and 34% IC 20.3-51.7 (11/32) in females. Among the rat ages, the prevalence rates were: 20% IC 4.5-52.1 (2/10) in juveniles (< 100g), 40% IC 19.7-64.3 (6/15) in sub-adults (100-200g) and 51% IC 37.6-68.2 (25/49) in adults (> 200g). There was no significant difference between sex and age (p > 0.05). The histological analysis of the infected livers revealed a granulomatous tissue reaction with different stages of fibrocellular tissue remodeling. Some granulomas with central necrosis were observed, and they contained intact eggs, with surrounding intense granulomatous inflammatory infiltration (monocytes and macrophages) and congestion in the infiltration area (Fig. 1A). Pronounced septal fibrosis remodeling of the parenchyma was also observed (Fig. 1B).
Fig. 1. Histological features of the liver of Rattus norvegicus infected with Calodium hepaticum. A) Hepatic parenchyma characterized by the presence of worm eggs (eg) and reaction of the cellular immune system of the host. B) Hepatic parenchyma with chronic infection characterized by the presence of fibrous tissue (arrows) and buildup of conjunctive tissue adjacent to the body of the parasite filled with eggs.
This is the first report on infection by C. hepaticum in R. norvegicus in Rio de Janeiro. The prevalence in this study is high and similar to that found in the states of São Paulo (59%)5, Bahia (56%)8 and Pará (42%)13. The short life cycle of rats causes rapid release of large numbers of eggs in the environment. Moreover, the high rat birth rate provides a sufficient number of hosts to maintain the parasite cycle6, contributing towards the high prevalence in this host.
The lack of influence of sex and age on the prevalent infection is in accordance with other studies of urban areas4,9. This probably occurs because of cannibalism, predation, presence of vector vertebrates and invertebrates, soil texture and social behavior, which are important factors in the propagation and maintenance of C. hepaticum infection4. Moreover, the study area has large presence of dogs and cats in the streets (both stray and those allowed to wander freely by their owners). These animals often prey on infected rats, perhaps contributing towards the dissemination of eggs and serving as a source of infection to other animals and humans.
The histopathological findings in the liver were according to the observations of other studies in naturally infected rats4,9,13. The most characteristic finding in rats infected with C. hepaticum is septal fibrosis of the liver, which is characterized by formation of long and thin fibrous septa along the acinar zone III, the connection of central veins between them and, later, the development of portal spaces, forming bridges17.
From these results, it is possible to conclude that the infected rodents are a potential source of parasite transmission to domestic animals in peridomestic areas, with substantial risk to human health, mainly to children18.
ACKNOWLEDGEMENTS
Arnaldo Maldonado Jr., José L. Luque and Raquel O. Simões received financial support from the National Council for Scientific and Technological Research (CNPq).
REFERENCES
- 1.Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol. 1997;83:575–83. [PubMed] [Google Scholar]
- 2.Camargo LMA, Camargo JSAA, Vera LJS, Barreto PTC, Tourinho EK, Souza MM. Capillariaisis (Trichurida, Trichinellidae, Capillaria hepatica) in the Brazilian Amazon: low pathogenicity, low infectivity and a novel mode of transmission. Parasit Vectors. 2010;3:11. doi: 10.1186/1756-3305-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carvalho-Costa FA, Silva AG, de Souza AH, Moreira CJC, Souza DL, Valverde JG, et al. Pseudoparasitism by Calodium hepaticum (syn. Capillaria hepatica; Hepaticola hepatica) in the Negro River, Brazilian Amazon. Trans R Soc Trop Med Hyg. 2009;103:1071–3. doi: 10.1016/j.trstmh.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Ceruti R, Sonzogni O, Origgi F, Vezzoli F, Cammarata S, Giusti AM, et al. Capillaria hepatica infection in wild brown rats (Rattus norvegicus) from the urban area of Milan, Italy. J Vet Med B Infect Dis Vet Public Health. 2001;48:235–40. doi: 10.1046/j.1439-0450.2001.00436.x. [DOI] [PubMed] [Google Scholar]
- 5.Chieffi PP, Dias RMDS, Mangini ACS, Grispino DMA, Pacheco MA Capillaria hepatica (Brancroft, 1893), em murídeos capturados no município de São Paulo, SP, Brasil. Rev Inst Med Trop Sao Paulo. 1981;23:143–6. [PubMed] [Google Scholar]
- 6.Farhang-Azad A. Ecology of Capillaria hepatica (Bancroft 1893) (Nematoda). II. Egg-releasing mechanisms and transmission. J Parasitol. 1977;63:701–6. [PubMed] [Google Scholar]
- 7.Galvão VA. Capillaria hepatica, estudo da incidência em ratos de Salvador, Bahia, e dados imunopatológicos preliminares. Rev Soc Bras Med Trop. 1976;10:333–8. [Google Scholar]
- 8.Galvão VA. Estudos sobre Capillaria hepatica: uma avaliação do seu papel patogênicos para o homem. Mem Inst Oswaldo Cruz. 1981;76:415–33. doi: 10.1590/s0074-02761981000400010. [DOI] [PubMed] [Google Scholar]
- 9.Kataranovski M, Zolotarevski L, Belij S, Mirkov I, Stosic J, Popov A, et al. First record of Calodium hepaticum and Taenia taeniaeformis liver infection in wild Norway rats (Rattus norvegicus) in Serbia. Arch Biol Sci. 2010;62:431–40. [Google Scholar]
- 10.Layne JN. Host and ecological relationship of the parasitic helminth Capillaria hepatica in Florida mammals. Zoologica. 1968;53:107–23. [Google Scholar]
- 11.Li C-D, Yang H-L, Wang Y. Capillaria hepatica in China. World J Gastroenterol. 2010;16:698–702. doi: 10.3748/wjg.v16.i6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilha MRS, Barros CSL. Capilariose hepática em cães e gatos: 15 casos. Cienc Rural. 2000;30:665–9. [Google Scholar]
- 13.Moreira VLC, Giese EG, Silva DCB, Melo FTV, Furtado AP, Maldonado A, et al. Calodium hepaticum (Nematoda: Capillariidae) in synanthropic rodents (Rattus norvegicus and Rattus rattus) in Eastern Amazonia. Rev Bras Parasitol Vet. 2013;22:265–9. doi: 10.1590/S1984-29612013000200046. [DOI] [PubMed] [Google Scholar]
- 14.Piazza R, Correa MO, Fleury RN. Sôbre um caso de infestação humana por Capillaria hepatica . Rev Inst Med Trop Sao Paulo. 1963;5:37–41. [PubMed] [Google Scholar]
- 15.Quadros RM, Pilati C, Marques SMT, Mazzolli M, Benedet RC. Capillaria hepatica in Puma concolor: first report in Brazil. J Zoo Wildl Med. 2009;40:586–7. doi: 10.1638/2008-0194.1. [DOI] [PubMed] [Google Scholar]
- 16.Reiczigel J, Rózsa L. Quantitative Parasitology 3.0. Budapest. 2005 Available from: http://www.zoologia.hu/qp.html. [Google Scholar]
- 17.Santos AB, Tolentino M Jr, Andrade ZA. Pathogenesis of hepatic septal fibrosis associated with Capillaria hepatica infection of rats. Rev Soc Bras Med Trop. 2001;34:503–6. doi: 10.1590/s0037-86822001000600001. [DOI] [PubMed] [Google Scholar]
- 18.Sawamura R, Fernandes MI, Peres LC, Galvão LC, Goldani HA, Jorge SM, et al. Hepatic capillariasis in children: report of 3 cases in Brazil. Am J Trop Med Hyg. 1999;61:642–7. doi: 10.4269/ajtmh.1999.61.642. [DOI] [PubMed] [Google Scholar]
- 19.Simões RO, Monteiro FA, Sanchez E, Thiengo SC, Garcia JS, Costa-Neto SF, et al. Endemic angiostrongyliasis, Rio de Janeiro, Brazil. Emerg Infect Dis. 2011;17:1331–3. doi: 10.3201/eid1707.101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soares MCP, Nunes HM, Silveira FAA, Alves MM, Souza AJS. Capillaria hepatica (Bancroft, 1893) (Nematoda) entre populações indígenas e mamíferos silvestres no noroeste do Estado do Mato Grosso, Brasil, 2000. Rev Pan-Amazônica Saúde. 2011;2:35–40. [Google Scholar]
- 21.Webster JP, Macdonald DW. Parasites of wild brown rats (Rattus norvegicus) on UK farms. Parasitology. 1995;111:247–55. doi: 10.1017/s0031182000081804. [DOI] [PubMed] [Google Scholar]