Abstract
The identification of the HVCN1 gene, encoding the only mammalian voltage-gated proton channel, prompted a number of studies on how proton channels affect cellular functions. As their expression is mainly restricted to immune cells, it is not surprising that proton channels regulate different aspects of immune responses. In this review, I will examine the current knowledge of voltage-gated proton channels in both innate and adaptive responses and assess the remaining outstanding questions.
Keywords: Hv1, HVCN1, leucocytes, lymphocyte activation, proton channel
Introduction
HVCN1, or hydrogen voltage-gated channel 1, is the gene coding for the only mammalian voltage-gated proton channel (also called Hv1 and VSOP).1,2 This highly proton-selective channel is a small four-transmembrane domain protein, similar to the voltage-sensor domain of other voltage-gated cation channels but lacking a pore-forming domain. HVCN1 is expressed at the plasma membrane and physiologically exists as a dimer,3,4 with each monomer possessing a separate permeation pathway. Although the dimerization is not essential for its function, it influences the opening of the two pathways, which is highly cooperative.5,6 Interestingly, proton channels can be inhibited by divalent cations such as Zn2+ but more so when they are in their dimeric form.5 As its name suggests, HVCN1 opening is voltage-dependent, therefore changes in membrane potential activate the channel and result in the generation of a proton current out of the cell. Channel opening is highly pH dependent, such that under physiological conditions, HVCN1 always mediates currents out of the cell that help to relieve intracellular acidification.1,7 In addition, proton channels can be regulated by protein kinase C-dependent phosphorylation, which results in the so-called enhanced-gating: channels open at more negative voltages, open faster and close more slowly, producing larger proton currents.8 Cells have many ways to relieve intracellular acidification through exchangers and co-transporters,9 two advantages conferred by proton channels are that they do not require ATP and they do not depend on (nor affect) the concentration of other electrolytes.
Proton currents were described long before the gene coding for a proton channel was identified.10 They have been most thoroughly studied in innate immune cells such as phagocytic cells, in association with the activity of the NADPH oxidase (Fig. 1). This enzymatic complex can generate reactive oxygen species (ROS) such as superoxide anion (O2•–) and is expressed in different cell types. It assembles on the plasma membrane (or phagosomes) of granulocytes, macrophages and B lymphocytes upon stimulation.11–13 It has also been described in T cells.14
Figure 1.
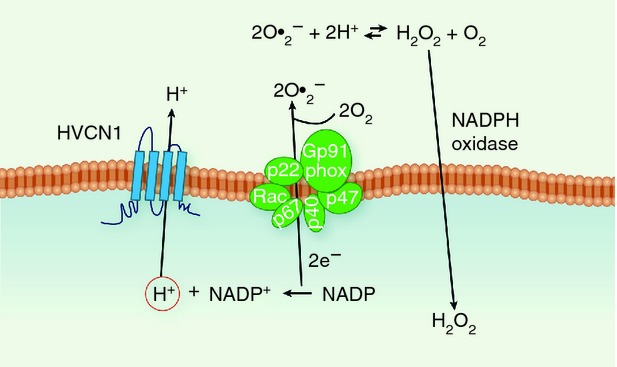
Schematic representation of the cooperation between the enzymatic complex NADPH oxidase and proton channels. The NADPH oxidase is assembled at the plasma membrane or on the membrane of phagosomes following a number of stimuli in phagocytic cells, B cells and T cells.11–14 The enzyme takes electrons (e−) from NADPH and transfers them to molecules of oxygen (O2), generating the radical superoxide anion (O2•−). This highly unstable reactive oxygen species (ROS) is then neutralized by conversion to more stable ROS, such as hydrogen peroxide (H2O2). The activity of the NADPH oxidase results in an accumulation of protons, H+, in the cytosol and an increase in membrane potential, because negative charges are transferred across the membrane. Proton channels can promptly rebalance charges and pH across the membrane.16,18
Superoxide anion, O2•–, is a highly unstable precursor to other ROS, with the most abundant being hydrogen peroxide, H2O2.
NADPH oxidase activity is electrogenic, i.e. it depolarizes the plasma membrane, because the electrons extracted from cytoplasmic NADPH are translocated to extracellular or intraphagosomal O2, and thereby reduced to O2•–.15 Without charge compensation, the membrane would depolarize to very positive voltages, at which NADPH oxidase ceases to function.16 Proton currents provide most of this charge compensation,17 and alleviate the cytosolic acidification that results from NADPH utilization, which also inhibits NADPH oxidase.18 ROS produced extracellularly and in the phagosome are required to clear engulfed bacteria by phagocytic cells, as shown by impaired immune responses in patients with chronic granulomatous disease,19 who have mutations in components of the NADPH oxidase.
Despite the ample evidence on co-expression of HVCN1 and NADPH oxidase and their co-activity, proton channels are also present in cells not expressing the NADPH oxidase (such as basophils,20 human spermatozoa21 and airway epithelial cells22) and can therefore mediate additional functions.
With the exception of human spermatozoa, where proton channels regulate spermatozoa motility,23 capacitation and subsequent activation,21 and airway epithelial cells, where, among other transporters, they contribute to acidification of the airway mucosa,22 the expression of HVCN1 appears restricted to leucocytes. To date, proton channels have been described in granulocytes (neutrophils, eosinophils and basophils),24 monocytes,25 macrophages,26 microglia,27,28 B lymphocytes,24,29 T lymphocytes29 and dendritic cells (Table 1).30 Although the availability of an HVCN1 knockout (KO) mouse line has significantly increased our understanding of proton channels in each cell type, many questions remain open, especially regarding their role in immune responses in vivo.
Table 1.
Summary of cell types expressing HVCN1 and its relative function
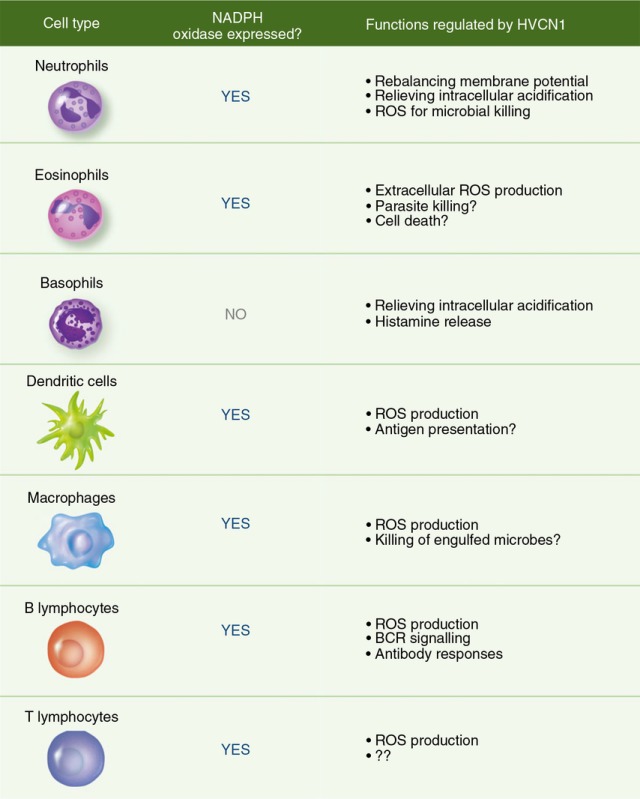 |
Proton channels in innate immune cells
Neutrophils
The best described role of proton channels in neutrophils is sustaining NADPH oxidase-dependent ROS production, one of the weapons required to kill engulfed bacteria. Indeed, in vitro studies on neutrophils from HVCN1 KO mice showed that their killing of phagocytosed bacteria was impaired31 and this was accompanied by diminished NADPH oxidase-dependent ROS production. When challenged with Staphylococcus aureus injected intraperitoneally, however, HVCN1 KO mice recovered as well as their wild-type counterpart to the infection. Furthermore, HVCN1 KO mice appeared to have normal responses to Pseudomonas aeruginosa and Burkholderia cepacia administered by intranasal inoculation. Why the in vitro defect did not seem to translate into an in vivo one remains to be established. One major question is whether additional cell types can compensate for the neutrophil impairment. Furthermore, we do not know if infections with other bacterial strains, which might be more susceptible to ROS killing, or infections at different sites, would give similar results.
A subsequent article by Demaurex and colleagues showed that HVCN1 KO neutrophils are also impaired in their migration in vitro, which correlated with a defect in Ca2+ influx after activation.32 This was due to the loss of a driving force for Ca2+ entry into the cell, because the absence of HVCN1 would result in an accumulation of protons, and hence of positive charges in the cytoplasm. The defective Ca2+ entry should result in impaired activation of downstream signalling pathways, which could impact on cytokine production as well as migration. However, this aspect was not investigated. Along these lines, it would be interesting to study also how additional inflammatory processes in which neutrophils are involved, such as formation of atherosclerotic plaques or tumour responses, might be impaired in HVCN1 KO neutrophils.
A more recent publication from the same group has described a role for proton channels in the acidification of neutrophil phagosomes.33 The authors confirmed the presence of HVCN1 on the membrane of phagosomes and found that, in HVCN1 KO neutrophils, phagosomal pH was more alkaline than in wild-type cells. In phagosomes from HVCN1 KO macrophages, on the other hand, they did not observe an alkalinization but rather a reduction in the rate of acidification. Their results corroborate evidence that proton channels are expressed on phagosomes and help to regulate their pH.
Eosinophils
Eosinophils are granulocytic cells known for their role in allergic diseases and helminth infections.34 Similarly to neutrophils, eosinophils also express the NADPH oxidase enzyme, and their oxidative burst is greater than in neutrophils.35 Interestingly, the cellular distribution of NADPH oxidase in the two cell types appears to be different, mainly at the plasma membrane for eosinophils and mainly in phagosomes for neutrophils,36 which suggests a more prominent role for eosinophil-derived ROS in the extracellular environment. Eosinophils were shown to possess proton currents almost two decades ago,37 and their activation was linked to the activity of the NADPH oxidase.38,39 More recently, the protein coding for proton channels, HVCN1, was shown to be expressed in human40 and mouse41 eosinophils. In the latter study, the authors confirmed that HVCN1 KO eosinophils had impaired NADPH oxidase-dependent ROS production.41 Unlike in neutrophils, however, the authors did not observe a defect in Ca2+ mobilization. This is probably due to the presence of additional channels that can mediate charge compensation and maintain a driving force for Ca2+ entry, such as the Ca2+ activated K+ channels.42 Interestingly, however, the authors noted an increased susceptibility to cell death in HVCN1 KO eosinophils upon PMA stimulation, which was NADPH oxidase dependent. The sensitivity of HVCN1 KO cells appeared to be specific to PMA stimulation, because alternative apoptotic insults did not result in differences in cell death. As PMA stimulation resulted in increased membrane depolarization and intracellular acidification in eosinophils lacking proton channels, the authors proposed that these were both responsible for the increased susceptibility. Their results suggested that in vivo responses, involving NADPH oxidase activation in HVCN1 KO eosinophils, should be affected by both a reduction in ROS production and a degree of eosinophil cell death. It would be interesting to see how this would affect immune responses where eosinophils play a significant role, such as allergic reactions and infections with parasitic helminths.
Basophils
While in other granulocytes the activity of proton channels is linked to the NADPH oxidase, this is not the case for basophils, which do not express the ROS-generating enzyme.20 Similarly to eosinophils, basophils are also mainly involved in allergic reactions and parasitic infections. Furthermore, they share some characteristics with mast cells, such as storage of histamine, as well as newly synthesized leukotriene C in their granules, which is released upon activation with different stimuli.43 Interestingly, the DeCoursey laboratory showed that stimulation of basophils with PMA or agonists of histamine release such as N-formyl-methionyl-leucyl-phenylalanine and anti-IgE, resulted in a strong increase in proton currents. This suggested a requirement for proton channels in basophil activation. Furthermore, the group showed that when proton channels were blocked with the divalent cation Zn2+, there was impaired histamine release after both N-formyl-methionyl-leucyl-phenylalanine or anti-IgE cross-linking, an observation that correlated with a significant increase in intracellular acidification. The result indicated that basophils undergo significant acidification upon activation, which is normally compensated by proton channels. Evidently, additional regulators of pH, such as the Na+/H+ exchanger, are not able to compensate for the inhibition of proton channels. How the altered intracellular pH affects degranulation and histamine release remains to be established. Furthermore, it remains to be defined if the inhibition of proton channels would affect allergic reactions or responses to parasitic infections. In this respect, it would be interesting to investigate if mast cells also express proton channels and if their histamine release is equally affected by proton channel inhibition.
Dendritic cells
We still have a limited understanding of HVCN1's role in dendritic cells, the main cell type responsible for antigen presentation and activation of T cells. Szteyn et al.30 have shown that HVCN1 is expressed in bone marrow-derived dendritic cells and that lipopolysaccharide stimulation can enhance proton currents, an observation that correlated with increased ROS production. When immature, dendritic cells undergo extensive endocytosis and macropinocytosis, which stops once they become mature, whereas the number of dendrites increases and MHC molecules on their surface are significantly up-regulated. In dendritic cells, ROS production has been shown to be required for optimal antigen processing and presentation. According to a study conducted by the Amigorena group, ROS help to maintain an alkaline pH in the antigen-containing endosomes by consuming protons in the lumen (which react with O2•– to generate H2O2). The alkaline pH inhibits proteolytic enzymes and preserves antigen integrity until fusion of endosomes with MHC-containing compartments. More recently, however, another study has shown that the phagosomes of dendritic cells do acidify, and ROS do not affect pH but are required to inhibit proteolysis through a direct effect on lytic enzymes such as cysteine cathepsins.44 Given the role of ROS in oxidizing cysteines (see paragraph on B cells), this effect is not surprising. The authors also investigated the role played by proton channels in ROS production in dendritic cells. HVCN1 KO dendritic cells showed diminished ROS production in their phagosomes, an effect that was exacerbated by inhibition of the proton pump V-ATPase, suggesting that charge compensation can be partly compensated by this protein. The authors, however, did not investigate the effect that HVCN1 loss would have on antigen presentation. Interestingly, in the study by Szteyn et al.,30 24-hr lipopolysaccharide stimulation resulted in a reduction of proton currents, an effect that was not mediated by overall diminished expression of HVCN1. Maybe this is due to proton channel internalization in activated cells and localization along the endolysosomal pathway, rather than at the plasma membrane. It would be interesting to investigate for how long the expression of proton channels is maintained in fully activated, antigen-presenting dendritic cells. Given the central role of dendritic cells in initiating adaptive immune responses, it remains to be addressed if proton channel inhibition in these cells would have functional consequences on their ability to activate T cells in a range of in vivo responses.
Macrophages
Proton channels are expressed in phagosomes45 and contribute to the regulation of their pH,33 nonetheless, relatively little is known about their functional role in macrophages. A recent publication on the role played by granulocyte–macrophage colony-stimulating factor (GM-CSF) in macrophages during infection with the yeast Histoplasma capsulatum, a strain that infects the lungs of mammals and replicates in the phagosomes of alveolar macrophages, also described a role for HVCN1 in this infection. The authors demonstrated that the reason why GM-CSF treatment inhibits yeast replication is the induction of a transcriptional reprogramming that results in Zn2+ sequestration away from the phagosome and into the Golgi, through the up-regulation of Zn2+-chelating proteins. The authors proposed that Zn2+ sequestration acts in two ways, on one hand it deprives H. capsulatum of Zn2+, essential for its replication, and on the other hand it removes Zn2+ from the phagosome, thereby removing its inhibitory effect on proton channels. Therefore, Zn2+ relocation to the Golgi increases ROS production in phagosomes, because proton channels are not inhibited and can therefore sustain NADPH oxidase activity. This latest point, however, is not completely clarified: the authors mention that the Zn2+-scavenger proteins up-regulated by GM-CSF are also able to scavenge ROS, hence their observation that cells that are KO for these proteins have diminished ROS production could be due to this mechanism, rather than one that involves the effect of Zn2+ on proton channels. In addition, pH and charge compensation in the phagosome are regulated by a number of different exchangers, transporter and, most importantly, the proton pump. As indicated by Rybicka et al.,44 the proton pump is able to compensate at least partially for the loss of proton channels, therefore it is possible that the strong impairment in ROS production and yeast growth inhibition observed in HVCN1 KO macrophages is not simply due to an effect on sustaining the NADPH oxidase, as the authors propose. Nonetheless these results highlight a potential new role for proton channels in macrophages and clearance of phagocytosed microbes that should be investigated further in the future.
Proton channels in adaptive immune responses
B lymphocytes
HVCN1 in B cells was initially identified in a proteomic screen of plasma membrane proteins from mantle cell lymphoma primary cells.46 Immunoblot of naive and memory B cells with anti-HVCN1 antibodies showed that protein expression levels were similar and comparable to granulocytes; however, in proliferating B cells, such as germinal centre cells or primary B cells stimulated in vitro with agonistic anti-CD40 and interleukin-4, HVCN1 was down-regulated.24 This pattern of expression suggested a requirement for HVCN1 in the initial phase of B-cell activation and B-cell receptor (BCR) stimulation. When the BCR recognizes an antigen, the binding leads to internalization of the BCR–antigen complex, and eventually to presentation of antigen-derived peptides on MHC class II molecules. Antigen internalization resembles phagocytosis of invading pathogens by phagocytes; similarly, it happens simultaneously to activation of the NADPH oxidase, in phagocytic cells as well as in B cells. B cells, however, use the 10 times smaller ROS production not for killing bacteria but for signalling. The hypothesis that ROS were necessary for BCR signalling was first proposed by Michael Reth, who suggested that ROS were necessary to inhibit phosphatases and allow activation of signalling pathways.47 As the activity of phosphatases is higher than that of kinases (removal of a phosphate group is less energy-demanding and faster than the addition of one), in the absence of a temporary inhibition of phosphatases there would be no activation of signalling pathways. Phosphatases have a cysteine residue in their catalytic site: due to its low pKa, the sulfhydryl group of the cysteine side chain, -SH, is deprotonated at physiological pH to a thiolate anion -S–, necessary for its catalytic activity. The thiolate anion, however, is susceptible to oxidation, which can be reversible in the presence of weak oxidants. The oxidation of phosphatases does not occur normally, because the cytosol is rich in reducing agents. Nonetheless, the intracellular environment can become oxidizing, at least locally, following production of oxidants by the cells, as happens upon NADPH oxidase activation or by ROS release by mitochondria.47 With the discovery of HVCN1 in B cells it was possible to assess (i) if proton channels were required to sustain the NADPH oxidase, and therefore ROS production, in cells other than phagocytic cells; and (ii) if an effect on ROS production was linked to defective signalling.
B cells from HVCN1 KO mice appeared to develop normally, an observation that correlated with the absence of HVCN1 expression in B-cell precursor cells.24 However, when mice were challenged with both T-independent and T-dependent antigens, they showed impaired antibody responses, indicating that B cells were receiving a weaker stimulation in response to antigen recognition.48,49 This was confirmed by diminished proliferation in response to BCR stimulation in vitro. The activation of signalling pathways appeared unaffected at early time-points but was not sustained at later time-points, as was the case for spleen tyrosine kinase (Syk). Syk controls many downstream pathways such as mitogen-activated protein kinase activation, Ca2+ mobilization (from endoplasmic reticulum stores as well as entry from the extracellular milieu) and phosphoinositide 3-kinase (PI3K) activation.50,51 Surprisingly, not all Syk downstream pathways were affected equally, as neither extracellular signal-regulated kinase (ERK) activation nor Ca2+ mobilization were impaired. On the other hand, the protein kinase Akt, which is downstream of PI3K,52 showed diminished activation in HVCN1-deficient cells and this resulted in decreased cell metabolism, as both mitochondrial respiration and glycolysis were reduced following BCR stimulation. The impaired Syk and Akt activation was accompanied by diminished oxidation of a key protein tyrosine phosphatase, src homology 2 domain-containing tyrosine phosphatase SHP-1. SHP-1 is recruited to the BCR complex upon activation, where it can be oxidized by locally generated ROS. In HVCN1 KO B cells, BCR-dependent ROS production was not sustained, therefore SHP-1 oxidation was diminished, and the more active phosphatase could dephosphorylate its substrates such as Syk. Indeed, the defect in Syk activation could be ‘rescued’ by treating HVCN1 KO B cells with low doses of an SHP-1 inhibitor, sodium stibogluconate.53 It is interesting to note that ROS production was not completely absent in HVCN1-deficient B cells, but rather was not sustained. This result is in agreement with the observation that the initial activation of multiple BCR-dependent signalling pathways took place normally in HVCN1 KO B cells; however, it could not be sustained at later time-points. This result might also explain why different signalling pathways were not affected in the same way by the impaired ROS production; for example, Ca2+ mobilization and ERK activation were unaffected by loss of HVCN1. It is possible that different kinetics of activation explain this difference: the activation of BCR signalling pathways is spatially regulated, with ERK being activated mainly while the BCR is at the plasma membrane and Akt being activated at the endolysosomes, once the BCR has been internalized.54 In light of these results, it is possible to speculate that ROS production takes place only on the membrane of endolysosomes after the BCR has been internalized; therefore a defect in their production will affect signal initiated here (Akt); however, it would not affect signal initiated at the membrane (Ca2+, Erk). Further experiments to clarify where the NADPH oxidase is assembled and where it generates ROS within B cells would be required to clarify this point.
The defects observed in HVCN1-deficient B cells were specific for BCR stimulation, as signalling pathways activated by Toll-like receptor 4 and CD40 stimulation were unimpaired.
Consistent with the BCR-specific defect in signalling, HVCN1 was found to be associated with the BCR complex and to co-localize with the receptor upon stimulation. This raises the possibility that close proximity of H+ transport to the BCR might be important. Whether proximity is necessary to support NADPH oxidase activity or for other reasons is unknown and will require further investigation.
The defects observed in HVCN1 KO B cells can be recapitulated by the diminished ROS production and downstream consequences on BCR signalling. Nonetheless, impaired ROS production is accompanied also by increased intracellular acidification upon BCR stimulation in HVCN1 KO cells (M. Capasso and T. DeCoursey, unpublished observation), highlighting how proton channels play a similar role in B cells and phagocytic cells, that is to rebalance pH and charges across the plasma membrane. It remains to be defined, however, whether the altered intracellular pH has additional consequences on B-cell function in general and signal transduction in particular, which might contribute to the defective phenotype observed in HVCN1 KO B cells.
T lymphocytes
Proton currents in T lymphocytes were first reported by Claudia Eder and co-workers, who described small currents in resting human T cells that increased after 24 hr of stimulation with PMA.29 It is not clear if the increase in proton currents would coincide with an up-regulation of NADPH oxidase in T cells. What we do know is that NADPH oxidase components are expressed in T cells, to a lesser extent compared with B cells, and mediate ROS production after T-cell receptor stimulation.14 Based on the limited data on proton channels in T cells, it is difficult to speculate what role their loss or inhibition would have on T-cell responses. A recent report by the Okamura laboratory described an increase in memory T cells after lymphocytic choriomeningitis virus infection in HVCN1 KO mice. These authors also showed reduced ROS production in splenic T cells, especially in a second wave of ROS, which peaked at 40 min after PMA stimulation.55 Given that the studies were conducted in germline HVCN1 KO mice, it is difficult to ascertain whether the increase in memory T cells is due to an intrinsic phenotype of T or additional cells. Furthermore, the study goes on to describe an autoimmune phenotype in a proportion of aged KO mice (10% of mice on C57BL/6J background and half of mice of mixed C57BL/6J-129 background). Mice presented splenomegaly at 6 months, accompanied by an increase in circulating antibodies and immunoglobulin deposition in kidneys. The authors do not explain the nature of the splenomegaly, as B and T cells numbers were unaffected, nor what was responsible for the increased production of autoantibodies. We would like to note that in a small cohort of aged germline HVCN1 KO mice (> 12 months, on C57BL/6J-129 background), we failed to notice splenomegaly (M. Capasso, unpublished observation), so it is possible that housing conditions or differences in the substrains of C57BL/6J mice might play a role in the autoimmune phenotype.
Given the limited data, a more thorough investigation with cell-specific HVCN1 KO mice (floxed HVCN1) is required to discover more about the overall alterations of immune responses and the potential susceptibility to an autoimmune phenotype in the absence of HVCN1.
Conclusions
The discovery in recent years of the gene coding for the only mammalian proton channel, HVCN1, accompanied by the generation of HVCN1 KO mice, has ignited a renewed interest in the function of voltage-gated proton channels in leucocytes. Although we know relatively well how proton channels cooperate with the NADPH oxidase in the generation of ROS, little is known about what phenotypic consequences this has in different immune cell types. More importantly, we know even less about the role played by HVCN1 in in vivo immune responses. The existence of a floxed HVCN1 line, which allows its conditional deletion, will advance our understanding of proton channels and their role in a range of immune responses. Further knowledge of their function will provide the rationale for exploring HVCN1's full potential as a drug target (see Seredenina et al.56 for a full review of this topic).
Glossary
- BCR
B-cell receptor
- ERK
extracellular signal-regulated kinase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HVCN1
hydrogen voltage-gated channel 1
- KO
knockout
- PI3K
phosphoinositide 3-kinase
- ROS
reactive oxygen species
- Syk
spleen tyrosine kinase
- SHP-1
src homology 2 domain-containing tyrosine phosphatase
Disclosures
The authors have no competing interests to declare.
References
- 1.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–6. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–92. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 3.Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–5. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–6. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musset B, Smith SM, Rajan S, Cherny VV, Sujai S, Morgan D, DeCoursey TE. Zinc inhibition of monomeric and dimeric proton channels suggests cooperative gating. J Physiol. 2010;588:1435–49. doi: 10.1113/jphysiol.2010.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tombola F, Ulbrich MH, Kohout SC, Isacoff EY. The opening of the two pores of the Hv1 voltage-gated proton channel is tuned by cooperativity. Nat Struct Mol Biol. 2010;17:44–50. doi: 10.1038/nsmb.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 8.Musset B, Capasso M, Cherny VV, Morgan D, Bhamrah M, Dyer MJ, DeCoursey TE. Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J Biol Chem. 2010;285:5117–21. doi: 10.1074/jbc.C109.082727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 10.Thomas RC, Meech RW. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 1982;299:826–8. doi: 10.1038/299826a0. [DOI] [PubMed] [Google Scholar]
- 11.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 12.Volkman DJ, Buescher ES, Gallin JI, Fauci AS. B cell lines as models for inherited phagocytic diseases: abnormal superoxide generation in chronic granulomatous disease and giant granules in Chediak–Higashi syndrome. J Immunol. 1984;133:3006–9. [PubMed] [Google Scholar]
- 13.Jones OTG, Jones SA, Wood JD. Expression of components of the superoxide generating NADPH oxidase by human leucocytes and other cells. Protoplasma. 1995;184:79–85. [Google Scholar]
- 14.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol. 2004;5:818–27. doi: 10.1038/ni1096. [DOI] [PubMed] [Google Scholar]
- 15.Henderson LM, Chappell JB, Jones OT. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J. 1987;246:325–9. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCoursey TE, Morgan D, Cherny VV. The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature. 2003;422:531–4. doi: 10.1038/nature01523. [DOI] [PubMed] [Google Scholar]
- 17.Murphy R, DeCoursey TE. Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta. 2006;1757:996–1011. doi: 10.1016/j.bbabio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Morgan D, Capasso M, Musset B, Cherny VV, Rios E, Dyer MJ, DeCoursey TE. Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc Natl Acad Sci USA. 2009;106:18022–7. doi: 10.1073/pnas.0905565106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland SM. Chronic granulomatous disease. Clin Rev Allergy Immunol. 2010;38:3–10. doi: 10.1007/s12016-009-8136-z. [DOI] [PubMed] [Google Scholar]
- 20.Musset B, Morgan D, Cherny VV, MacGlashan DW, Jr, Thomas LL, Rios E, DeCoursey TE. A pH-stabilizing role of voltage-gated proton channels in IgE-mediated activation of human basophils. Proc Natl Acad Sci USA. 2008;105:11020–5. doi: 10.1073/pnas.0800886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–37. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Iovannisci D, Illek B, Fischer H. Function of the HVCN1 proton channel in airway epithelia and a naturally occurring mutation, M91T. J Gen Physiol. 2010;136:35–46. doi: 10.1085/jgp.200910379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musset B, Clark RA, DeCoursey TE, et al. NOX5 in human spermatozoa: expression, function, and regulation. J Biol Chem. 2012;287:9376–88. doi: 10.1074/jbc.M111.314955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capasso M, Bhamrah MK, Henley T, et al. HVCN1 modulates BCR signal strength via regulation of BCR-dependent generation of reactive oxygen species. Nat Immunol. 2010;11:265–72. doi: 10.1038/ni.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musset B, Cherny VV, DeCoursey TE. Strong glucose dependence of electron current in human monocytes. Am J Physiol Cell Physiol. 2012;302:C286–95. doi: 10.1152/ajpcell.00335.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS., Jr Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity. 2013;39:697–710. doi: 10.1016/j.immuni.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu LJ, Wu G, Akhavan Sharif MR, et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15:565–73. doi: 10.1038/nn.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eder C, Fischer HG, Hadding U, Heinemann U. Properties of voltage-gated potassium currents of microglia differentiated with granulocyte/macrophage colony-stimulating factor. J Membr Biol. 1995;147:137–46. doi: 10.1007/BF00233542. [DOI] [PubMed] [Google Scholar]
- 29.Schilling T, Gratopp A, DeCoursey TE, Eder C. Voltage-activated proton currents in human lymphocytes. J Physiol. 2002;545:93–105. doi: 10.1113/jphysiol.2002.028878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szteyn K, Yang W, Schmid E, Lang F, Shumilina E. Lipopolysaccharide-sensitive H+ current in dendritic cells. Am J Physiol Cell Physiol. 2012;303:C204–12. doi: 10.1152/ajpcell.00059.2012. [DOI] [PubMed] [Google Scholar]
- 31.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA. 2009;106:7642–7. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N. VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J Exp Med. 2010;207:129–39. doi: 10.1084/jem.20091837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El Chemaly A, Nunes P, Jimaja W, Castelbou C, Demaurex N. Hv1 proton channels differentially regulate the pH of neutrophil and macrophage phagosomes by sustaining the production of phagosomal ROS that inhibit the delivery of vacuolar ATPases. J Leukoc Biol. 2014;95:827–39. doi: 10.1189/jlb.0513251. [DOI] [PubMed] [Google Scholar]
- 34.Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74. doi: 10.1146/annurev.immunol.24.021605.090720. [DOI] [PubMed] [Google Scholar]
- 35.Yagisawa M, Yuo A, Yonemaru M, Imajoh-Ohmi S, Kanegasaki S, Yazaki Y, Takaku F. Superoxide release and NADPH oxidase components in mature human phagocytes: correlation between functional capacity and amount of functional proteins. Biochem Biophys Res Commun. 1996;228:510–6. doi: 10.1006/bbrc.1996.1691. [DOI] [PubMed] [Google Scholar]
- 36.Lacy P, Abdel-Latif D, Steward M, Musat-Marcu S, Man SF, Moqbel R. Divergence of mechanisms regulating respiratory burst in blood and sputum eosinophils and neutrophils from atopic subjects. J Immunol. 2003;170:2670–9. doi: 10.4049/jimmunol.170.5.2670. [DOI] [PubMed] [Google Scholar]
- 37.Gordienko DV, Tare M, Parveen S, Fenech CJ, Robinson C, Bolton TB. Voltage-activated proton current in eosinophils from human blood. J Physiol. 1996;496(Pt 2):299–316. doi: 10.1113/jphysiol.1996.sp021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banfi B, Schrenzel J, Nusse O, Lew DP, Ligeti E, Krause KH, Demaurex N. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med. 1999;190:183–94. doi: 10.1084/jem.190.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeCoursey TE, Cherny VV, DeCoursey AG, Xu W, Thomas LL. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J Physiol. 2001;535:767–81. doi: 10.1111/j.1469-7793.2001.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petheo GL, Orient A, Barath M, et al. Molecular and functional characterization of Hv1 proton channel in human granulocytes. PLoS ONE. 2010;5:e14081. doi: 10.1371/journal.pone.0014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X, Mose E, Zimmermann N. Proton channel HVCN1 is required for effector functions of mouse eosinophils. BMC Immunol. 2013;14:24. doi: 10.1186/1471-2172-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito M, Sato R, Hisatome I, Narahashi T. RANTES and platelet-activating factor open Ca2+-activated K+ channels in eosinophils. FASEB J. 1996;10:792–8. doi: 10.1096/fasebj.10.7.8635697. [DOI] [PubMed] [Google Scholar]
- 43.Voehringer D. Regulation of type 2 immunity by basophils. Adv Exp Med Biol. 2013;785:37–41. doi: 10.1007/978-1-4614-6217-0_4. [DOI] [PubMed] [Google Scholar]
- 44.Rybicka JM, Balce DR, Chaudhuri S, Allan ER, Yates RM. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J. 2012;31:932–44. doi: 10.1038/emboj.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okochi Y, Sasaki M, Iwasaki H, Okamura Y. Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009;382:274–9. doi: 10.1016/j.bbrc.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 46.Boyd RS, Jukes-Jones R, Walewska R, Brown D, Dyer MJ, Cain K. Protein profiling of plasma membranes defines aberrant signaling pathways in mantle cell lymphoma. Mol Cell Proteomics. 2009;8:1501–15. doi: 10.1074/mcp.M800515-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reth M. Hydrogen peroxide as second messenger in lymphocyte activation. Nat Immunol. 2002;3:1129–34. doi: 10.1038/ni1202-1129. [DOI] [PubMed] [Google Scholar]
- 48.Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med. 2006;203:1081–91. doi: 10.1084/jem.20060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–49. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 50.Kurosaki T. Molecular mechanisms in B cell antigen receptor signaling. Curr Opin Immunol. 1997;9:309–18. doi: 10.1016/s0952-7915(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 51.Beitz LO, Fruman DA, Kurosaki T, Cantley LC, Scharenberg AM. SYK is upstream of phosphoinositide 3-kinase in B cell receptor signaling. J Biol Chem. 1999;274:32662–6. doi: 10.1074/jbc.274.46.32662. [DOI] [PubMed] [Google Scholar]
- 52.Franke TF, Kaplan DR, Cantley LC. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–7. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 53.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol. 2001;167:3391–7. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- 54.Chaturvedi A, Martz R, Dorward D, Waisberg M, Pierce SK. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nat Immunol. 2011;12:1119–26. doi: 10.1038/ni.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sasaki M, Tojo A, Okochi Y, Miyawaki N, Kamimura D, Yamaguchi A, Murakami M, Okamura Y. Autoimmune disorder phenotypes in Hvcn1-deficient mice. Biochem J. 2013;450:295–301. doi: 10.1042/BJ20121188. [DOI] [PubMed] [Google Scholar]
- 56.Seredenina T, Demaurex N, Krause KH. Voltage-gated proton channels as novel drug targets: from NADPH oxidase regulation to sperm biology. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5806. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]


