Abstract
Myeloid dendritic cells (mDC) are key mediators of innate and adaptive immunity to virus infection, but the impact of HIV infection on the mDC response, particularly early in acute infection, is ill-defined. We studied acute pathogenic simian immunodeficiency virus (SIV) infection of rhesus macaques to address this question. The mDC in blood and bone marrow were depleted within 12 days of intravenous infection with SIVmac251, associated with a marked proliferative response. In lymph nodes, mDC were apoptotic, activated and proliferating, despite normal mDC numbers, reflecting a regenerative response that compensated for mDC loss. Blood mDC had increased expression of MHC class II, CCR7 and CD40, whereas in lymph nodes these markers were significantly decreased, indicating that acute infection induced maturation of mDC in blood but resulted in accumulation of immature mDC in lymph nodes. Following SIV infection, lymph node mDC had an increased capacity to secrete tumour necrosis factor-α upon engagement with a Toll-like receptor 7/8 ligand that mimics exposure to viral RNA, and this was inversely correlated with MHC class II and CCR7 expression. Lymph node mDC had an increased ability to capture and cleave soluble antigen, confirming their functionally immature state. These data indicate that acute SIV infection results in increased mDC turnover, leading to accumulation in lymph nodes of immature mDC with an increased responsiveness to virus stimulation.
Keywords: AIDS, apoptosis, cell proliferation, viral
Introduction
Myeloid dendritic cells (mDC) play an essential role in innate and adaptive immunity and are known to be significantly impacted by HIV and simian immunodeficiency virus (SIV) infections.1,2 In chronic infection, mDC are lost from the circulation3–9 through a process that involves caspase-mediated apoptosis10,11 and increased recruitment to lymph nodes.12–14 There is evidence that lymph node mDC in chronic HIV and SIV infection have an immature phenotype with reduced levels of co-stimulatory molecules,12–15 a paradoxical finding given the abundance of inflammatory factors that are known to induce mDC maturation.16–18 Acute infection with HIV and SIV also results in loss of circulating mDC14,19 but the impact of early infection on mDC kinetics and function is poorly understood.
To address these gaps in knowledge, we studied the mDC response during acute infection of rhesus macaques with the biological isolate SIVmac251, a pathological model in which AIDS-like disease develops that is similar to HIV-1 infection of humans but with an accelerated time frame.20 We find that acute infection results in marked apoptosis and proliferation of mDC and accumulation within lymph nodes of mDC with an increased capacity to process antigen and produce tumour necrosis factor-α (TNF-α) that is inversely correlated with MHC class II and CCR7 expression. These data suggest that acute SIV infection leads to a regenerative mDC response resulting in a predominance of functionally immature mDC in lymph nodes.
Materials and methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. All sample collections and surgeries were performed under appropriate levels of anaesthesia and all efforts were made to minimize pain and distress.
Animals, virus infection, sample collection and processing
Adult captive-bred Indian rhesus macaques (Macaca mulatta) of both sexes were used. Six animals were intravenously inoculated with 1000 TCID50 of SIVmac251 (provided by Preston A. Marx, Tulane National Primate Research Center, Covington, LA) and followed during the first 14 days of infection. This group of rhesus macaques was previously used to define the dynamics of the plasmacytoid DC response to acute SIV infection.21 Bone marrow aspirates were obtained at least 30 days before infection and on day 12 post-infection and cell suspensions were prepared by passing aspirates through a 70-μm nylon cell strainer, as previously described.21 Blood was collected before infection, on days 3 and 6 post-infection and daily from days 10 to 14 when animals were killed. Blood was processed either as peripheral blood leucocytes after red blood cell lysis or as peripheral blood mononuclear cells following density gradient centrifugation. The thymidine analogue 5-bromo-2′-deoxyuridine (BrdU) was administered by intravenous injection at 30 mg/kg at 24-hr intervals for four doses beginning at day 10 post-infection in the six infected macaques and two mock-infected macaques to label dividing cells.21 All animals underwent transcardiac perfusion with saline before necropsy and collection of peripheral lymph nodes (inguinal and axillary), which were weighed and processed into single cell suspensions using DNAse and collagenase as described previously.5 Plasma viral loads were determined by real-time PCR as described previously.22 In addition to the above animals, blood and lymph node samples from six SIV-naive macaques were used as controls in certain experiments.
Flow cytometry, functional analysis and cell enumeration
Monoclonal antibodies were purchased from BD Pharmingen or BD Biosciences (San Jose, CA) unless otherwise noted. Cells were incubated with the following antibodies to label cell surface markers: CD3 (clone SP34-2), CD20 (2H7) and CD14 (M5E2), combined as lineage markers using the same fluorochrome, and HLA-DR (G46-6), CD11c (S-HCL-3), CCR7 (150503 R&D Systems, Minneapolis, MN), CD40 (5C3), CD80 (L307.4), and CD86 (FUN-1). Dead cells were excluded based on labelling with the amine-reactive Live/Dead viability dye (Invitrogen, Carlsbad, CA). To measure responses to stimulation, peripheral blood mononuclear cells or lymph node cell suspensions were cultured for 5 hr with 10 μm of the Toll-like receptor 7/8 (TLR7/8) agonist 3M-007 (3M Pharmaceuticals, St Paul, MN) with and without the addition of 10 μg/ml brefeldin A (Sigma, St Louis, MO) after 1 hr, before cell surface labelling. Cells were then fixed and permeabilized and labelled with antibodies to TNF-α (MAb11) and interleukin-12 (IL-12) (8.6; Miltenyi Biotec, Bergisch Gladbach, Germany).14 To measure the capacity for antigen capture and processing, lymph node cells were incubated with 3M-007 at 4 or 37° for 30 min before the addition of 10 μg/ml DQ Green BSA (Molecular Probes, Eugene, OR) for an additional 30 min. Cells were then washed twice in ice-cold buffer before antibody staining for cell surface markers. For analysis of apoptosis, cells were cultured for 3 hr with and without incubation with 10 μm 3M-007 or aldrithiol-2-inactivated SIV particles23 (provided by Jeffrey D. Lifson, AIDS and Cancer Virus Program, SAIC-Frederick) at a capsid concentration of 400 ng/ml before fixation and permeabilization and incubation with antibody to active caspase-3 (C92-605). As a positive control for apoptosis, cells were exposed to 610 μW/cm2 UV-B light for 5 min.24 Absolute numbers of mDC were determined using a flow cytometry-based method combining CD45 labelling of whole blood in TruCOUNT tubes (BD Biosciences, San Jose, CA) with mDC identification in peripheral blood leucocytes as previously described.25 Some analyses were performed on a subset of animals based on sample availability. Samples were collected using a four-laser BD LSR II flow cytometer and data were analysed with facsdiva software (BD Bioscience) or flowjo software (TreeStar, Ashland, OR).
Detection of chemokine mRNA expression
Detection of chemokine mRNA expression in lymph nodes was carried out as previously described.14 Briefly, total RNA was extracted and purified from cell suspensions using RNAeasy (Qiagen, Hilden, Germany) after treatment with DNAse I (Invitrogen). The cDNA was synthesized using random primers and Superscript II reverse transcriptase (Invitrogen). Real-time PCR analysis was performed for expression of CCL19 and CCL21 using primers and probes from Taqman human gene expression arrays (Applied Biosystems, Foster City, CA). Messenger RNA expression levels were calculated with the 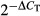 method using β-glucuronidase as the endogenous control.
method using β-glucuronidase as the endogenous control.
Statistical analysis
Comparison between groups used the Mann–Whitney U-test. Comparison of DC numbers across different time-points was performed using the Wilcoxon signed-rank test. Correlations were determined using the non-parametric Spearman rank test. For statistical analysis, graphpad prism 5 (Graphpad Software, San Diego, CA) was used. All P-values are two-sided with significance considered to be P < 0·05.
Results
Loss and proliferation of mDC in blood and bone marrow in acute SIV infection
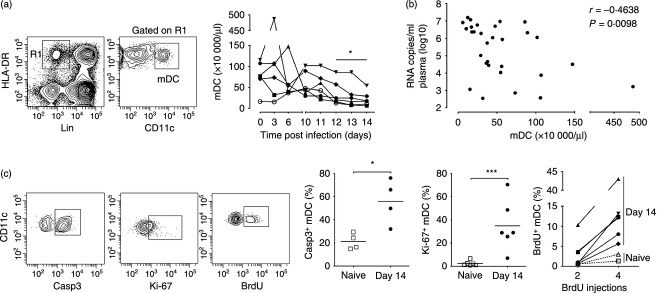
To monitor mDC numbers in circulation following SIVmac251 infection we performed repeated sampling over short-time intervals and identified and enumerated mDC in peripheral blood leucocytes by gating on the Lineage− HLA-DR+ CD11c+ population (Fig. 1a).25 Although mDC numbers varied in the first week after infection, by day 12 post-infection there was significant mDC loss from peripheral blood relative to pre-infection levels (Fig. 1a). The number of mDC in blood over the 14-day period was inversely correlated with plasma virus load (Fig. 1b). At day 14 after infection there was a significant increase in caspase-3+ mDC in blood relative to pre-infection time points, reflecting increased cell death by apoptosis. Associated with cell loss was a 12-fold increase in mDC expressing the proliferation marker Ki-67, indicating a compensatory proliferative response (Fig. 1c). This proliferation was confirmed by BrdU incorporation, as after four injections only 2–3% of circulating mDC in naive animals had incorporated BrdU, whereas in acutely infected macaques from 5% to as many as 43% of mDC were BrdU+ (Fig. 1c). We next evaluated bone marrow aspirates to determine if SIV infection also impacted mDC in this compartment. Bone marrow mDC were identified as Lineage− MHC class II+ cells expressing CD11c (Fig. 2a).21 Similar to blood, the absolute number of mDC in aspirates was significantly reduced at day 12 post-infection relative to pre-infection time-points, and this was accompanied by a 10-fold increase in the frequency of mDC expressing Ki-67 (Fig. 2a,b).
Figure 1.
Loss of blood myeloid dendritic cells (mDC) in acute simian immunodeficiency virus (SIV) infection associated with increased apoptosis and proliferation (a) Left: Flow cytometry plots demonstrating the gating strategy used to define mDC within the CD45+ gate. Right: Absolute number of mDC in blood following intravenous SIVmac251 infection for the six rhesus macaques on study. *P < 0·05 by Wilcoxon signed-rank test. (b) Correlation between number of mDC and plasma virus load over the 14-day period. (c) Left: Representative flow cytometry plots showing staining of mDC with antibodies to active caspase-3, Ki-67 and BrdU. Gating to define positive cells was based on fluorescence with an isotype control antibody. Right: Percentage of mDC in blood expressing active caspase-3 or Ki67 and percentage of mDC that incorporated BrdU after two and four injections in SIV-naive macaques and macaques 14 days after SIV infection. Symbols represent individual animals and horizontal bars represent means. *P < 0·05 and ***P < 0·005 by Mann–Whitney U-test.
Figure 2.
Increased turnover of myeloid dendritic cells (mDC) in bone marrow during acute simian immunodeficiency virus (SIV) infection (a) Left: Representative flow cytometry plots demonstrating the gating strategy used to define mDC within the CD45+ fraction of bone marrow aspirates. Right: Absolute number of mDC in bone marrow aspirates taken before and at day 12 after intravenous SIVmac251 infection. (b) Left: Representative flow cytometry plot showing staining of bone marrow mDC with antibody to Ki-67. Right: Percentage of mDC expressing Ki-67 in bone marrow aspirates taken before and at 12 days after SIV infection. Symbols represent individual animals and horizontal bars represent means.*P < 0·05 by Mann–Whitney U-test.
Concurrent apoptosis and proliferation underlie steady-state levels of mDC in acutely infected lymph nodes
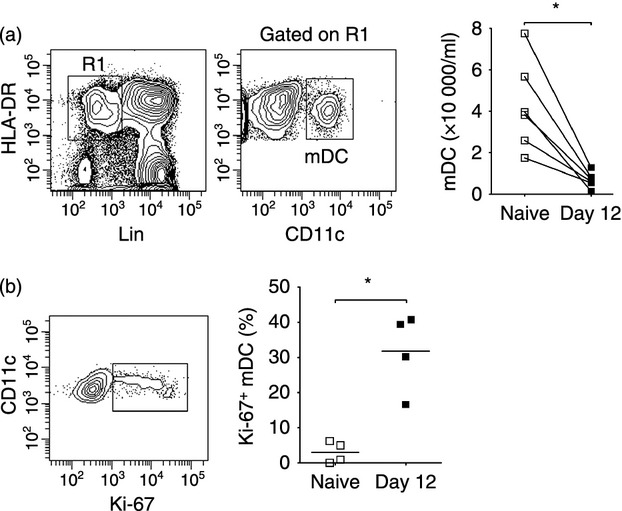
We next analysed mDC in peripheral lymph nodes from rhesus macaques at day 14 post-SIV infection and compared these with cells from uninfected animals. The mDC were identified within the live fraction of lymph node cell suspensions as Lineage− HLA-DRhigh CD11c+ cells with relatively high levels of autofluorescence, as previously defined (Fig. 3a).5 In contrast to blood and bone marrow there was no difference in the frequency of mDC in lymph nodes between uninfected macaques and macaques with acute SIV infection, when measured either as number of cells per unit weight or as a percentage of Lineage− MHC class II+ cells (Fig. 3a and data not shown). However, there was a fourfold increase in the frequency of lymph node mDC expressing active caspase-3, and a doubling of cells expressing CD95, indicative of activation and apoptosis (Fig. 3b). The mean percentage of mDC expressing Ki-67 also increased fourfold as a result of infection, from < 10% to about 40%. This increase in Ki-67 expression was paralleled by an increase in mDC incorporating BrdU in vivo relative to naive animals (Fig. 3b). There was a strong positive correlation between expression of active caspase-3 and Ki-67 by lymph node mDC, suggesting that cell proliferation compensated for increased apoptosis and accounted for the steady-state numbers of mDC (Fig. 3c), although the intra-group correlation was not statistically significant because of the small group size. To begin to address the mechanism of mDC apoptosis following SIV infection we incubated lymph node cells taken from macaques either before or 14 days after infection with inactivated SIV, which activates mDC through viral RNA engagement of TLR8 within endosomes.26,27 Exposure to inactivated SIV or the TLR7/8 agonist 3M-007 did not induce apoptosis of mDC from naive animals, although significant cell death was noted following UV-B irradiation. Similarly, the already high level of apoptosis detected in lymph node mDC taken from animals at day 14 after SIV infection was only increased by exposure to UV-B but not inactivated SIV particles or 3M-007 (Fig. 3d).
Figure 3.
Increased apoptosis and proliferation of myeloid dendritic cells (mDC) in acutely infected lymph nodes (a) Left: Representative flow cytometry plots showing staining of the gating strategy to define CD11c+ mDC within the Lineage− HLA-DR+ fraction of lymph node cell suspensions. Right: Number of mDC in lymph nodes per unit weight in simian immunodeficiency virus (SIV)-naive macaques and macaques at 14 days after intravenous SIVmac251 infection. (b) Top: Representative flow cytometry plots showing staining of lymph node mDC with antibodies to active caspase-3, CD95, Ki-67 and BrdU. Bottom: Percentage of mDC in lymph nodes of SIV-naive macaques and macaques 14 days after SIV infection that expressed active caspase-3, CD95 or Ki-67, and the percentage of mDC that incorporated BrdU after four injections. Symbols represent individual animals and horizontal bars represent means. *P < 0·05, **P < 0·01 by Mann–Whitney U-test. (c) Correlation between percentage of mDC expressing active caspase-3 and Ki-67 in lymph nodes of SIV-naive macaques and macaques 14 days after SIV infection. (d) Percentage of mDC expressing active caspase-3 following 3-hr incubation of lymph node cells taken either before or 14 days after SIV infection with the Toll-like receptor 7/8 (TLR7/8) agonist 3M-007 or inactivated SIV or after irradiation with UV-B light.
Acute SIV infection induces maturation of blood mDC but results in accumulation of immature mDC in lymph nodes
Next, we determined if the phenotype of blood and lymph node mDC was affected by SIV infection, using expression of MHC class II, CCR7 and co-stimulatory molecules CD40, CD80 and CD86 as indicators of maturation. In health, expression of each of these markers is significantly greater on lymph node mDC relative to blood mDC, reflecting the increased maturation state of mDC in the lymph node compartment (Fig. 4a,b).5 Following SIV infection, expression on blood mDC of MHC class II and CD86 was significantly up-regulated and expression of CCR7 and CD40 was induced relative to mDC at pre-infection time points. In contrast, expression of MHC class II, CCR7 and CD40 on lymph node mDC was significantly reduced as a consequence of SIV infection, whereas expression of CD80 was increased (Fig. 4a,b). We next measured the relative expression of the CCR7 ligands CCL19 and CCL21 in lymph node tissues by real-time PCR. While expression of CCL19 mRNA did not differ between uninfected and acutely infected lymph nodes, expression of CCL21 was reduced to 20% of pre-infection levels as a result of infection (Fig. 4c).
Figure 4.
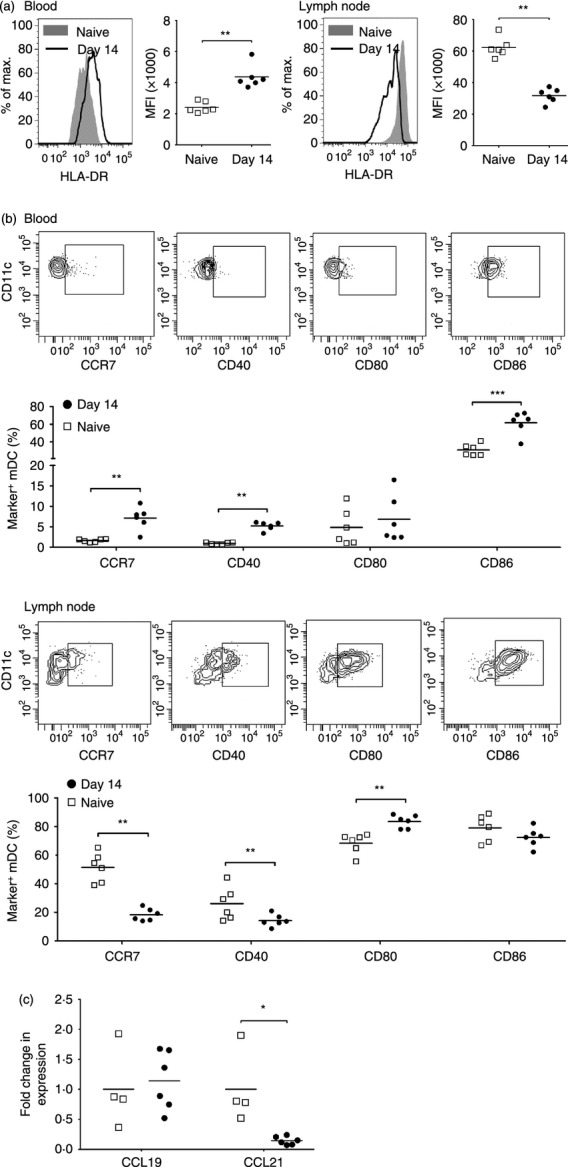
Acute simian immunodeficiency virus (SIV) infection results in maturation of blood myeloid dendritic cells (mDC) but accumulation of immature mDC in lymph nodes. (a) Representative histograms and mean fluorescence intensity (MFI) of HLA-DR expression on blood (left) and lymph node (right) mDC in SIV-naive macaques and macaques at 14 days after SIV infection. (b) Representative flow cytometry plots and percentage of mDC expressing CCR7, CD40, CD80 and CD86 in blood (top) and lymph node (bottom) of SIV-naive macaques and macaques at 14 days after SIV infection. (c) Fold change in mRNA expression of CCL19 and CCL21 in lymph node cell suspensions in SIV-naive macaques and macaques at 14 days after SIV infection. The fold change in expression was calculated by normalizing to the mean  of pre-infection lymph nodes. Symbols represent individual animals and horizontal bars represent means. *P < 0·05, **P < 0·01, ***P < 0·005 by Mann–Whitney U-test.
of pre-infection lymph nodes. Symbols represent individual animals and horizontal bars represent means. *P < 0·05, **P < 0·01, ***P < 0·005 by Mann–Whitney U-test.
Lymph node mDC in acutely infected macaques are functionally immature
To determine if acute SIV infection was associated with functional changes in mDC, we measured cytokine production by blood and lymph node cells in response to stimulation with the TLR7/8 agonist 3M-007. Blood mDC produced significant amounts of TNF-α and to a lesser extent IL-12 in response to TLR7/8 stimulation, and this was not affected by acute SIV infection (Fig. 5a). However, while lymph node mDC from SIV-naive macaques produced relatively low amounts of TNF-α and IL-12 in response to TLR7/8 stimulation compared with blood mDC, the proportion of stimulated mDC making TNF-α increased by 50% following SIV infection, a statistically significant change (Fig. 5a). There was a strong negative correlation between mDC production of TNF-α and expression of both HLA-DR and CCR7 in lymph nodes when naive and SIV-infected animals were analysed together (Fig. 5b), although significance was lost when infected animals alone were compared. To further assess mDC function we incubated lymph node cell suspensions following TLR7/8 stimulation with DQ Green BSA, a quenched dye conjugate of bovine serum albumin that fluoresces upon proteolytic cleavage. The capacity for rhesus macaque mDC to internalize and process DQ Green BSA is inversely correlated with maturation.28 In naive macaques, TLR7/8-stimulated mDC that were exposed to DQ Green BSA at 37° had essentially no capacity to internalize and cleave protein, based on overlapping fluorescence with cells incubated at 4°. However, in macaques with acute SIV infection fluorescence of mDC incubated at 37° increased twofold on average relative to the control, and this change was statistically different from naive macaques (Fig. 5c). Collectively these data suggest that acute SIV infection renders lymph node mDC phenotypically and functionally immature.
Figure 5.
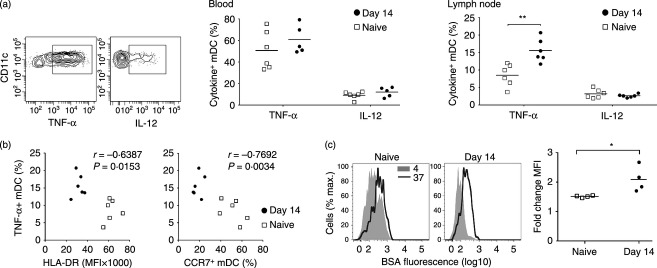
Increased cytokine production and antigen processing in lymph node myeloid dendritic cells (mDC) during acute simian immunodeficiency virus (SIV) infection (a) Left: Representative flow cytometry plots showing labelling of stimulated blood mDC with antibodies to tumour necrosis factor-α (TNF-α) and interleukin-12 (IL-12). Right: Percentage of mDC expressing TNF-α or IL-12 following stimulation with the Toll-like receptor 7/8 (TLR7/8) agonist 3M-007 in blood and lymph node from SIV-naive macaques and macaques at 14 days after SIV infection. Symbols represent individual animals and horizontal bars represent means. (b) Correlation between TNF-α production and HLA-DR and CCR7 expression on mDC in lymph nodes of SIV naive macaques and macaques at 14 days after SIV infection. (c) Left: Representative histograms of BSA fluorescence in stimulated lymph node mDC after 30 min incubation with DQ Green BSA at 4° and 37°. Right: Fold change in BSA fluorescence of mDC incubated with DQ Green BSA at 37° relative to 4° in SIV naive macaques and macaques at 14 days after SIV infection. Symbols represent individual animals and horizontal bars represent means. *P < 0·05, **P < 0·01 by Mann–Whitney U-test.
Discussion
Our findings reveal that acute SIV infection of rhesus macaques results in a coordinated switch in the phenotype and function of mDC, especially in lymph nodes, where expression of surface markers associated with maturation is reduced while the capacity to process soluble antigen and produce pro-inflammatory cytokine is enhanced. These are properties of immature mDC29 that do not predominate in lymphoid tissues under normal homeostatic conditions.30 Our findings extend previous reports indicating increased mDC reactivity to stimulation in SIV and HIV infection.14,19,27,31 The data are also consistent with findings of immature or ‘semi-mature’ mDC in lymph nodes and tonsil in individuals with acute and chronic HIV infection12,13,15 that have the capacity to drive expansion of regulatory T cells.15 Similarly, a recent study in rhesus macaques chronically infected with SIVmac251 showed that splenic and mesenteric lymph node mDC induced regulatory T cells more efficiently than did mDC from naive animals, although unlike our present findings, this was associated with an increase in mature mDC based on expression of CD83.32 It is possible that the different stage of SIV infection or type of lymphoid tissue analysed as well as criteria used to determine maturation state contribute to this discrepancy.
The reasons for the accumulation of immature mDC in lymph nodes in acute SIV infection may be multiple, including the reduced expression of CCL21 seen in this study and by others33 that is required for mDC maturation within lymph nodes34 and contact with viral proteins such as Vpr that have been shown to prevent DC maturation.35,36 The potential for increased migration of immature mDC from blood is also a possibility given the up-regulation of CCR7 by blood mDC; however, the marked reduction in CCL21, the dominant CCR7 ligand over CCL19,34 suggests that CCR7-mediated recruitment may be suboptimal. Local proliferation is a significant source of replenishment of mDC in lymph nodes under homeostatic conditions,37 and increased proliferation in acute SIV infection could conceivably contribute to accumulation of immature cells that have suppressed cues for maturation.
Our findings build on an expanding body of literature5,14,21,27,38–40 showing that mDC and other mononuclear phagocytes succumb to apoptosis in pathogenic SIV infection and respond to this apoptosis with marked proliferation. In the case of mDC, cell death is thought to occur through direct effects of virus that change the balance of pro-apoptotic and anti-apoptotic molecules in favour of caspase- and death receptor-dependent apoptosis.10,14,40 The lack of any effect of inactivated SIV particles or TLR7/8 agonist on mDC apoptosis in our study suggests that mechanisms other than TLR engagement may be involved in this process, although short-term exposure to these agonists in vitro does not recapitulate the prolonged and continuous exposure to virus in vivo. Virus infection of mDC leading to cell death is not likely to be a significant factor in vitro40 or in vivo, as at the peak of viraemia the proportion of mDC harbouring pro-viral DNA in lymph nodes of SIV-infected macaques is < 0·5%,21 and in animals with AIDS direct infection of mDC in lymph nodes appears to be rare.41
The consequences of increased antigen capture and production of pro-inflammatory cytokines by mDC in infected hosts remain to be determined and are likely to be complex. On the one hand, it is possible that increased production of TNF-α could contribute to tissue inflammation and chronic immune activation, which are critical factors in driving disease progression.19,31,42 Alternatively, increased mDC responsiveness may be beneficial to the host through promotion of innate immune responses and viral clearance. In support of the latter scenario, mDC taken at virus set-point from SIV-infected rhesus macaques that had long-term stable infection had increased TNF-α production in response to virus-encoded TLR7/8 ligands, whereas set-point mDC from macaques that subsequently progressed rapidly to AIDS had reduced TNF-α production.27 Ultimately, determining whether the change in mDC biology in HIV and SIV infection is beneficial or detrimental to the host may require antibody-mediated depletion or the use of TLR antagonists to block the in vivo response to virus. However, this latter approach may be complicated by redundancy in the TLR-dependent and -independent mediated response to virus infections.43
Acknowledgments
The authors thank Anita M. Trichel and Christopher Janssen for excellent veterinary care, Preston A. Marx for the SIVmac251 inoculum, and Jeffrey D. Lifson for the inactivated SIV preparation. VW, ALB, KB and XL performed the experiments, and VW and SMBB designed the study and wrote the paper. This work was funded by NIH grant AI071777 to SMBB.
Glossary
- BrdU
5-bromo-2′-deoxyuridine
- IL-12
interleukin-12
- mDC
myeloid dendritic cell
- SIV
simian immunodeficiency virus
- TLR
Toll-like receptor
- TNF-α
tumour necrosis factor-α
Disclosures
The authors have no competing financial or commercial interests to disclose.
References
- 1.Wonderlich ER, Barratt-Boyes SM. A dendrite in every pie: myeloid dendritic cells in HIV and SIV infection. Virulence. 2012;3:1–7. doi: 10.4161/viru.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derby N, Martinelli E, Robbiani M. Myeloid dendritic cells in HIV-1 infection. Curr Opin HIV AIDS. 2011;6:379–84. doi: 10.1097/COH.0b013e3283499d63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–71. [PubMed] [Google Scholar]
- 4.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 5.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–67. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 6.Donaghy H, Pozniak A, Gazzard B, Qazi N, Gilmour J, Gotch F, Patterson S. Loss of blood CD11c+ myeloid and CD11c– plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–6. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 7.Grassi F, Hosmalin A, McIlroy D, Calvez V, Debre P, Autran B. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS. 1999;13:759–66. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 8.Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 9.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–21. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 10.Dillon SM, Friedlander LJ, Rogers LM, Meditz AL, Folkvord JM, Connick E, McCarter MD, Wilson CC. Blood myeloid dendritic cells from HIV-1-infected individuals display a proapoptotic profile characterized by decreased Bcl-2 levels and by caspase-3+ frequencies that are associated with levels of plasma viremia and T cell activation in an exploratory study. J Virol. 2011;85:397–409. doi: 10.1128/JVI.01118-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meera S, Madhuri T, Manisha G, Ramesh P. Irreversible loss of pDCs by apoptosis during early HIV infection may be a critical determinant of immune dysfunction. Viral Immunol. 2010;23:241–9. doi: 10.1089/vim.2009.0112. [DOI] [PubMed] [Google Scholar]
- 12.Dillon SM, Robertson KB, Pan SC, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lore K, Sonnerborg A, Brostrom C, et al. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–92. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 14.Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6:e1001235. doi: 10.1371/journal.ppat.1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 16.Abel K, La Franco-Scheuch L, Rourke T, et al. Gamma interferon-mediated inflammation is associated with lack of protection from intravaginal simian immunodeficiency virus SIVmac239 challenge in simian-human immunodeficiency virus 89.6-immunized rhesus macaques. J Virol. 2004;78:841–54. doi: 10.1128/JVI.78.2.841-854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–9. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornfeld C, Ploquin MJ, Pandrea I, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest. 2005;115:1082–91. doi: 10.1172/JCI23006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabado RL, O'Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–52. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shedlock DJ, Silvestri G, Weiner DB. Monkeying around with HIV vaccines: using rhesus macaques to define ‘gatekeepers’ for clinical trials. Nat Rev Immunol. 2009;9:717–28. doi: 10.1038/nri2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5:e1000413. doi: 10.1371/journal.ppat.1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barratt-Boyes SM, Soloff AC, Gao W, et al. Broad cellular immunity with robust memory responses to simian immunodeficiency virus following serial vaccination with adenovirus 5- and 35-based vectors. J Gen Virol. 2006;87:139–49. doi: 10.1099/vir.0.81445-0. [DOI] [PubMed] [Google Scholar]
- 23.Kader M, Smith AP, Guiducci C, Wonderlich ER, Normolle D, Watkins SC, Barrat FJ, Barratt-Boyes SM. Blocking TLR7- and TLR9-mediated IFN-α production by plasmacytoid dendritic cells does not diminish immune activation in early SIV infection. PLoS Pathog. 2013;9:e1003530. doi: 10.1371/journal.ppat.1003530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 25.Brown KN, Barratt-Boyes SM. Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol. 2009;38:272–8. doi: 10.1111/j.1600-0684.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–91. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wonderlich ER, Wijewardana V, Liu X, Barratt-Boyes SM. Virus-encoded TLR ligands reveal divergent functional responses of mononuclear phagocytes in pathogenic simian immunodeficiency virus infection. J Immunol. 2013;190:2188–98. doi: 10.4049/jimmunol.1201645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barratt-Boyes SM, Zimmer MI, Harshyne LA, et al. Maturation and trafficking of monocyte-derived dendritic cells in monkeys: implications for dendritic cell-based vaccines. J Immunol. 2000;164:2487–95. doi: 10.4049/jimmunol.164.5.2487. [DOI] [PubMed] [Google Scholar]
- 29.Tschoep K, Manning TC, Harlin H, George C, Johnson M, Gajewski TF. Disparate functions of immature and mature human myeloid dendritic cells: implications for dendritic cell-based vaccines. J Leukoc Biol. 2003;74:69–80. doi: 10.1189/jlb.0702352. [DOI] [PubMed] [Google Scholar]
- 30.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 31.Chang JJ, Lacas A, Lindsay RJ, et al. Differential regulation of toll-like receptor pathways in acute and chronic HIV-1 infection. AIDS. 2012;26:533–41. doi: 10.1097/QAD.0b013e32834f3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Presicce P, Shaw JM, Miller CJ, Shacklett BL, Chougnet CA. Myeloid dendritic cells isolated from tissues of SIV-infected Rhesus macaques promote the induction of regulatory T cells. AIDS. 2011;26:263–73. doi: 10.1097/QAD.0b013e32834ed8df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi YK, Fallert BA, Murphey-Corb MA, Reinhart TA. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood. 2003;101:1684–91. doi: 10.1182/blood-2002-08-2653. [DOI] [PubMed] [Google Scholar]
- 34.Britschgi MR, Favre S, Luther SA. CCL21 is sufficient to mediate DC migration, maturation and function in the absence of CCL19. Eur J Immunol. 2010;40:1266–71. doi: 10.1002/eji.200939921. [DOI] [PubMed] [Google Scholar]
- 35.Majumder B, Janket ML, Schafer EA, Schaubert K, Huang XL, Kan-Mitchell J, Rinaldo CR, Jr, Ayyavoo V. Human immunodeficiency virus type 1 Vpr impairs dendritic cell maturation and T-cell activation: implications for viral immune escape. J Virol. 2005;79:7990–8003. doi: 10.1128/JVI.79.13.7990-8003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muthumani K, Hwang DS, Choo AY, Mayilvahanan S, Dayes NS, Thieu KP, Weiner DB. HIV-1 Vpr inhibits the maturation and activation of macrophages and dendritic cells in vitro. Int Immunol. 2005;17:103–16. doi: 10.1093/intimm/dxh190. [DOI] [PubMed] [Google Scholar]
- 37.Kabashima K, Banks TA, Ansel KM, Lu TT, Ware CF, Cyster JG. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–50. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Hasegawa A, Liu H, Ling B, et al. The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood. 2009;114:2917–25. doi: 10.1182/blood-2009-02-204263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burdo TH, Soulas C, Orzechowski K, et al. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS Pathog. 2010;6:e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laforge M, Campillo-Gimenez L, Monceaux V, et al. HIV/SIV infection primes monocytes and dendritic cells for apoptosis. PLoS Pathog. 2011;7:e1002087. doi: 10.1371/journal.ppat.1002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zimmer MI, Larregina AT, Castillo CM, Capuano S, 3rd, Falo LD, Jr, Murphey-Corb M, Reinhart TA, Barratt-Boyes SM. Disrupted homeostasis of Langerhans cells and interdigitating dendritic cells in monkeys with AIDS. Blood. 2002;99:2859–68. doi: 10.1182/blood.v99.8.2859. [DOI] [PubMed] [Google Scholar]
- 42.Wijewardana V, Kristoff J, Xu C, et al. Kinetics of myeloid dendritic cell trafficking and activation: impact on progressive, nonprogressive and controlled SIV infections. PLoS Pathog. 2013;9:e1003600. doi: 10.1371/journal.ppat.1003600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang K, Puel A, Zhang S, et al. Human TLR-7-, -8-, and -9-mediated induction of IFN-α/β and -λ is IRAK-4 dependent and redundant for protective immunity to viruses. Immunity. 2005;23:465–78. doi: 10.1016/j.immuni.2005.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


