Abstract
Staphylococcus aureus is a major pathogen that can cause a broad spectrum of serious infections including skin infections, pneumonia and sepsis. Peritoneal mast cells have been implicated in the host response towards various bacterial insults and to provide mechanistic insight into the role of mast cells in intraperitoneal bacterial infection we here studied the global effects of S. aureus on mast cell gene expression. After co-culture of peritoneal mast cells with live S. aureus we found by gene array analysis that they up-regulate a number of genes. Many of these corresponded to pro-inflammatory cytokines, including interleukin-3, interleukin-13 and tumour necrosis factor-α. The cytokine induction in response to S. aureus was confirmed by ELISA. To study the role of peritoneal mast cells during in vivo infection with S. aureus we used newly developed Mcpt5-Cre+ × R-DTA mice in which mast cell deficiency is independent of c-Kit. This is in contrast to previous studies in which an impact of mast cells on bacterial infection has been proposed based on the use of mice whose mast cell deficiency is a consequence of defective c-Kit signalling. Staphylococcus aureus was injected intraperitoneally into mast-cell-deficient Mcpt5-Cre+ × R-DTA mice using littermate mast-cell-sufficient mice as controls. We did not observe any difference between mast-cell-deficient and control mice with regard to weight loss, bacterial clearance, inflammation or cytokine production. We conclude that, despite peritoneal mast cells being activated by S. aureus in vitro, they do not influence the in vivo manifestations of intraperitoneal S. aureus infection.
Keywords: bacterial infection, inflammation, mast cells, Staphylococcus aureus
Introduction
Mast cells are situated at the host environment interface and they are equipped with a multitude of pathogen-recognition receptors. As such they are well equipped to respond promptly to invading pathogens.1 Staphylococcus aureus is generally a commensal bacteria that persistently colonizes the anterior nares of 20% of the human population.2 Invasive S. aureus infection is generally a result of a break in the epithelial barriers, which allows the pathogen to invade underlying tissue.3 After invasion of the organism, S. aureus can cause serious and life threatening infections including skin infections, pneumonia and sepsis.4
Previous studies have shown that S. aureus can internalize and persist within bone marrow-derived mast cells (BMMCs)5 and that BMMCs exert anti-microbial activity against S. aureus by releasing extracellular traps and anti-microbial compounds.5 Moreover, mast cells degranulate and release tumour necrosis factor-α (TNF-α) in response to S. aureus and it has been demonstrated that S. aureus-infected BMMCs have antimicrobial activity.5 Staphylococcus aureus can also invade human cord blood-derived mast cells, causing TNF-α and interleukin-8 (IL-8) release.6 However, although the above-mentioned studies have shown that mast cells respond to S. aureus infection, there is a lack of knowledge of the global impact of S. aureus on mast cell function. Moreover, previous studies have mainly been performed using relatively immature mast cells, e.g. BMMCs, whereas the effect of S. aureus on terminally differentiated mast cells has been less studied. To provide a deeper understanding of how mast cells respond to infection by S. aureus we here chose to study the global effects of S. aureus on gene expression in peritoneal cell-derived mast cells (PCMCs), which is an emerging model for studies of terminally differentiated mast cells.7 This analysis revealed extensive induction of a large number of genes in mast cells co-cultured with live S. aureus, many of which corresponded to cytokines such as IL-3, IL-13 and TNF-α.
Using mice that lack mast cells due to defective c-Kit signalling, e.g. KitW/W−v and KitW−sh/W−sh mice, mast cells have been shown to be protective against a number of different bacterial infections. In most of these studies, a role for the peritoneal mast cell population in combating bacterial infection has been suggested, in particular using the caecal ligation and puncture model.8–15 Recently, mice in which mast cell deficiency is independent of defective c-Kit signalling have been developed.16,17 In studies on these mice some of the findings based on using c-Kit-defective mice have been confirmed, including the role of mast cells in allergic airway hyper-responsiveness. However, there are also some conflicting data between the c-Kit-dependent and -independent mast-cell-deficient mice, e.g. with regard to the proposed role of mast cells in autoimmunity, leading to a need to also re-evaluate the role of mast cells in bacterial infections.16,17 To provide new insight into the role of mast cells in intraperitoneal bacterial infection, we here used Mcpt5-Cre+ × R-DTA mice in which mast cells are lacking due to mast cell-specific expression of diphtheria toxin.18 We demonstrate that there was no difference in the course of intraperitoneal S. aureus [strain 8325-4 (Φ11)] infection in mast-cell-deficient compared with littermate mast-cell-sufficient control animals. This indicates that, despite peritoneal mast cells being activated in vitro by S. aureus, they do not influence the course of peritoneal S. aureus infection in vivo.
Materials and methods
Peritoneal cell-derived mast cells
The PCMCs were established according to a protocol described by Malbec et al.7
Mice
Mast cell-deficient Mcpt5-Cre+ × R-DTA mice were used, as described.18 Littermate Mcpt5-Cre− × R-DTA mice were used as controls. All animal experiments were approved by the local ethics committee (no C118/11).
Staphylococcus aureus culture
Mice (strain C57BL/6) were infected intraperitoneally with S. aureus 8325-4 (Φ11).19 After post mortem examination the strain was re-isolated and frozen. The S. aureus strain was streaked on a horse blood agar plate, incubated 37° overnight and then inoculated in 20 ml tryptone soya broth (TSB) at 37°. After overnight incubation, 200 μl was transferred to 20 ml fresh TSB and the culture was grown at 37° until the optical density at 600 nm (OD600) reached 0·5.
In vitro co-culture of PCMCs and S. aureus
The PCMCs were washed twice in PBS and resuspended in antibiotic-free medium at a density of 1 × 106 cells/ml and plated in 24-well tissue plates. The bacteria were washed twice in PBS and added to a final concentration of ∼2·5 × 107 cells/ml; multiplicity of infection (MOI) 1 : 25. Four hours after infection, cells were collected by centrifugation. Media and cell fractions were frozen and stored at −20°.
In vivo infection
Mice were injected intraperitoneally with 100 μl TSB medium containing ∼5 × 107 S. aureus. Controls were injected with 100 μl TSB medium. Body weight was monitored every day. After 4 hr, 1 day or 3 days, the mice were killed and peritoneal lavage was performed with 5-ml Tyrodes buffer. To determine the colony-forming units (CFU) in the peritoneal lavage fluid, the fluid was plated onto Baird–Parker agar medium plates (Merck, White House Station, NJ) supplemented with Egg Yolk Tellurite emulsion (Oxoid, Basingstoke, UK). The cells in the peritoneal wash were counted. Cytospins of the peritoneal cells were stained with May–Grünwald/Giemsa and differential counts were performed. The cells in the peritoneal wash were pelleted by centrifugation; the supernatant was collected and frozen at −20°.
RNA preparation and Affymetix microarray
Total RNA from 1 × 106 cultured cells was isolated by using NucleoSpin RNA II (Macherey-Nagel, Düren, Germany). The RNA quality was evaluated with the Agilent 2100 Bioanalyzer system. The microarray analysis was performed at the Uppsala Array Platform (Uppsala, Sweden) as previously described.20
Cytokine array and ELISAs
Secretion of cytokines was determined using RayBio® Mouse Cytokine Antibody Array 3 (RayBiotech, Inc., Norcross, GA) according to the manufacturer's instructions. ELISAs for IL-3, IL-13, TNF-α (Peprotech, Rocky Hill, NJ), monocyte chemoattractant protein-1 (MCP-1) and IL-6 (eBioscience, San Diego, CA) were performed according to the manufacturer's instructions.
Statistical analysis
Data are shown as means ± standard error of the mean. Statistical analyses were performed by using GraphPad Prism 4.0c (GraphPad Software, La Jolla, CA) and unpaired Student's t-test for two-tailed distributions (*P < 0·05; **P < 0·01; ***P < 0·001).
Results
Live S. aureus induces a strong pro-inflammatory response in cultured peritoneal mast cells
Most of the previous studies addressing the role of mast cells in anti-bacterial responses have mainly used relatively immature mast cells, such as BMMCs or various mast-cell-like cell lines of murine or human origin.1,5,6 Moreover, many previous studies have investigated the effects of purified bacterial products on mast cells, rather than investigating the effects of live bacteria. Here we sought to clarify the impact of bacteria on mast cells in a physiologically more relevant setting, by examining the effect of live S. aureus on mature mast cells of peritoneal origin, i.e. PCMCs.
To investigate the global effect of S. aureus on mast cell gene expression, live S. aureus were co-cultured with PCMCs. After 4 hr the cells were collected, followed by RNA extraction and Affymetrix microarray analysis. As displayed in Table 1, 52 genes were significantly up-regulated with a higher than 2 log2-fold change (Table 1). Of these, several corresponded with cytokines and chemokines, indicating that S. aureus induces a strong pro-inflammatory response in mast cells (Table 1). The cytokine IL-3 was the gene that was induced to the highest extent of all genes, with a 5·35 log2-fold change (Fig. 1). To confirm the induction of pro-inflammatory cytokines at the protein level, ELISA was performed. As shown in Fig. 1, the expression and secretion of IL-3, IL-13 and TNF-α were confirmed by ELISA; all of these cytokines were undetectable in the medium of control cells but clearly detectable after co-culture of PCMCs with S. aureus (Fig. 1). Together, these findings reveal a profound induction of pro-inflammatory genes in mature mast cells exposed to live S. aureus.
Table 1.
Genes showing significant (P < 0·05) up-regulation after co-culture of peritoneal cell-derived mast cells and Staphylococcus aureus for 4 hr
| Probe set ID | Gene title | Gene symbol | log2-fold change | P-value |
|---|---|---|---|---|
| 10385918 | Interleukin 3 | Il3 | 5·35 | 9·78E–06 |
| 10427035 | Nuclear receptor subfamily 4, group A, member 1 | Nr4a1 | 5·28 | 3·56E–07 |
| 10560481 | FBJ osteosarcoma oncogene B | Fosb | 4·93 | 1·26E–05 |
| 10450369 | Heat-shock protein 1A | Hspa1a | 4·62 | 1·01E–05 |
| 10397346 | FBJ osteosarcoma oncogene | Fos | 4·27 | 3·34E–06 |
| 10504838 | Nuclear receptor subfamily 4, group A, member 3 | Nr4a3 | 4·15 | 3·80E–06 |
| 10450367 | Heat-shock protein 1B/heat-shock protein 1A | Hspa1b/1a | 3·99 | 1·02E–05 |
| 10385837 | Interleukin 13 | Il13 | 3·78 | 4·30E–05 |
| 10449284 | Dual specificity phosphatase 1 | Dusp1 | 3·57 | 6·49E–05 |
| 10565819 | Solute carrier organic anion transporter family, member 2b1 | Slco2b1 | 3·54 | 1·28E–05 |
| 10482772 | Nuclear receptor subfamily 4, group A, member 2 | Nr4a2 | 3·48 | 2·17E–06 |
| 10545588 | Hexokinase 2 | Hk2 | 3·34 | 0·00059 |
| 10545130 | Growth arrest and DNA-damage-inducible 45α | Gadd45a | 3·33 | 6·13E–05 |
| 10584580 | Small nucleolar RNA, C/D box 14E | Snord14e | 3·28 | 2·75E–05 |
| 10536794 | RIKEN cDNA 2310016C08 gene | 2310016C08Rik | 3·23 | 0·00032 |
| 10520862 | Fos-like antigen 2 | Fosl2 | 3·21 | 2·01E–05 |
| 10451198 | Vascular endothelial growth factor A | Vegfa | 3·17 | 0·00026 |
| 10584576 | Heat-shock protein 8/small nucleolar RNA, C/D box 14D/small nucleolar RNA, C/D box 14C | Hspa8/Snord14d/14c | 3·14 | 1·55E–05 |
| 10352448 | Dual specificity phosphatase 10 | Dusp10 | 3·08 | 0·00021 |
| 10580282 | Jun-B oncogene | Junb | 3·08 | 0·00062 |
| 10449741 | Salt-inducible kinase 1 | Sik1 | 2·98 | 0·00015 |
| 10551891 | Nuclear factor of κ light polypeptide gene enhancer in B-cells inhibitor, delta | Nfkbid | 2·91 | 0·00051 |
| 10373912 | Oncostatin M | Osm | 2·85 | 0·00031 |
| 10531073 | UDP glucuronosyltransferase 2 family, polypeptide B38 | Ugt2b38 | 2·78 | 0·04599 |
| 10515399 | Polo-like kinase 3 (Drosophila) | Plk3 | 2·76 | 2·43E–05 |
| 10350516 | Prostaglandin-endoperoxide synthase 2 | Ptgs2 | 2·71 | 2·41E–05 |
| 10550906 | Plasminogen activator, urokinase receptor | Plaur | 2·62 | 9·61E–05 |
| 10508723 | Small nucleolar RNA, H/ACA box 61 | Snora61 | 2·60 | 0·00229 |
| 10489204 | Transglutaminase 2, C polypeptide | Tgm2 | 2·56 | 0·00024 |
| 10377439 | Period homologue 1 (Drosophila) | Per1 | 2·55 | 0·00012 |
| 10545200 | Similar to Igk-C protein | LOC100046894 | 2·55 | 0·02605 |
| 10597758 | Cysteine-serine-rich nuclear protein 1 | Csrnp1 | 2·53 | 2·82E–05 |
| 10546450 | A disintegrin-like and metallopeptidase (reprolysin type) with thrombospondin type 1 motif, 9 | Adamts9 | 2·53 | 0·00519 |
| 10361091 | Activating transcription factor 3 | Atf3 | 2·52 | 0·00011 |
| 10389231 | Chemokine (C-C motif) ligand 3 | Ccl3 | 2·49 | 0·00015 |
| 10450501 | Tumour necrosis factor | Tnf | 2·48 | 0·00044 |
| 10472923 | Adenylate kinase 3-like 1 | Ak3 l1 | 2·48 | 6·94E–05 |
| 10358408 | Regulator of G-protein signalling 1 | Rgs1 | 2·38 | 1·37E–05 |
| 10374197 | Receptor (calcitonin) activity modifying protein 3 | Ramp3 | 2·35 | 0·00067 |
| 10531057 | UDP glucuronosyltransferase 2 family, polypeptide B5 | Ugt2b5 | 2·31 | 0·04142 |
| 10379518 | Chemokine (C-C motif) ligand 7 | Ccl7 | 2·30 | 1·29E–05 |
| 10364030 | Adenosine A2a receptor | Adora2a | 2·30 | 1·66E–05 |
| 10429926 | Diacylglycerol O-acyltransferase 1 | Dgat1 | 2·25 | 0·02014 |
| 10520452 | Interleukin 6 | Il6 | 2·21 | 0·00863 |
| 10523156 | Chemokine (C-X-C motif) ligand 2 | Cxcl2 | 2·20 | 0·00217 |
| 10373918 | Leukaemia inhibitory factor | Lif | 2·19 | 0·00071 |
| 10563659 | SPT2, Suppressor of Ty, domain containing 1 (Saccharomyces cerevisiae) | Spty2d1 | 2·13 | 9·43E–05 |
| 10368277 | Ribosomal protein S12 | Rps12 | 2·07 | 0·00381 |
| 10503334 | GTP-binding protein (gene over-expressed in skeletal muscle) | Gem | 2·06 | 0·00154 |
| 10523547 | 1-acylglycerol-3-phosphate O-acyltransferase 9 | Agpat9 | 2·05 | 0·04722 |
| 10478890 | CCAAT/enhancer binding protein (C/EBP), β | Cebpb | 2·05 | 0·00246 |
Figure 1.
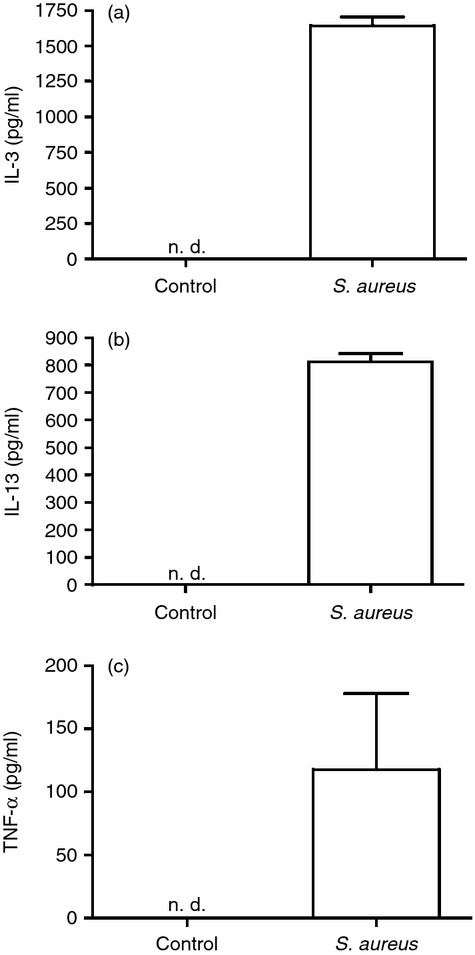
Co-culture of peritoneal cell-derived mast cells (PCMCs) and Staphylococcus aureus induces release of cytokines. PCMCs (1 × 106 cells/ml) were cultured either alone (control) or together with S. aureus (MOI = 25) At 4 hr, medium samples were collected and analysed for content of interleukin-3 (IL-3) (a), IL-13 (b) or tumour necrosis factor-α (TNF-α) (c) by ELISA. Mean ± SEM (n = 3).
Mast cells do not influence the clearance of S. aureus in vivo
To investigate the role of mast cells in peritoneal S. aureus infection in vivo, Mcpt5-Cre+ × R-DTA were used, with littermate Mcpt5-Cre− × R-DTA mice as mast-cell-sufficient control animals. Mast-cell-deficient and control mice were injected i.p. with S. aureus. To follow the course of infection, the change in body weight was monitored. As shown in Fig. 2(a), the infection with S. aureus caused an initial weight drop up to day 2. However, after this initial weight loss, the mice regained normal weight, suggesting recovery from infection. Notably, there was no significant difference in the weight loss or time of recovery when comparing mast-cell-sufficient and -deficient mice. To monitor the effect of mast cells on efficiency of bacterial clearance, infected mice were killed after either 4 hr, 1 day or 3 days. Clearance of S. aureus in the peritoneum was examined after performing peritoneal lavage, followed by plating of the fluid and quantification of CFU. This analysis showed the presence of bacteria in the peritoneum 4 hr and 1 day after infection, but there were no significant differences in the amounts of bacteria when comparing mast cell-sufficient and -deficient mice. After 3 days, bacteria were not detected in the peritoneum of either mast cell-sufficient- or deficient mice (Fig. 2b). Together, these data indicate that mast cells are dispensable for clearance of peritoneal S. aureus infection.
Figure 2.
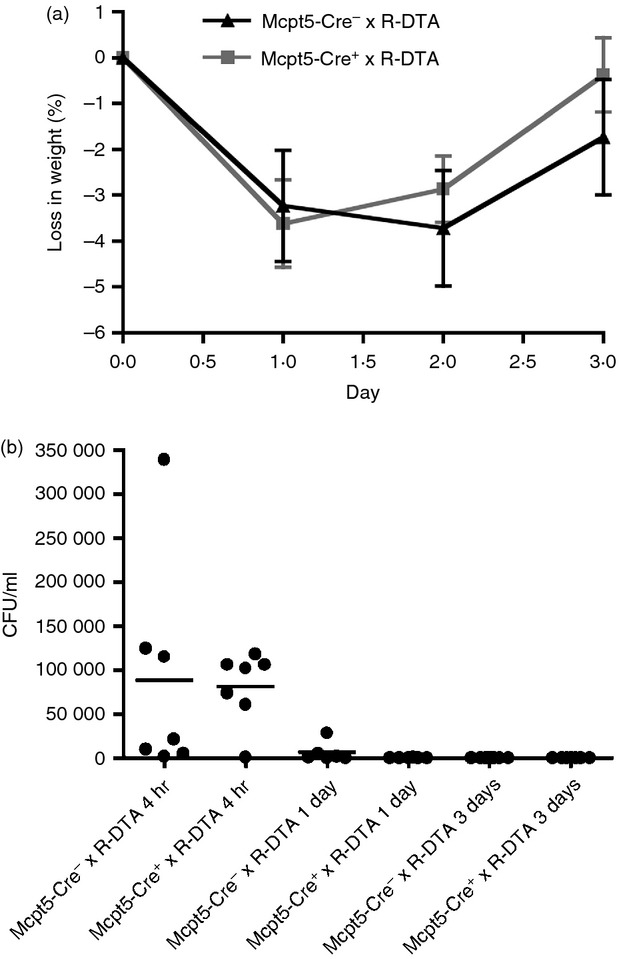
Mast cells do not influence the weight loss of Staphylococcus aureus-infected mice or the clearance of S. aureus. (a) Mcpt5-Cre+ × R-DTA and Mcpt5-Cre− × R-DTA were injected intraperitoneally with ∼5 × 107 CFU of S. aureus and monitored for change in weight. As a control, TSB medium only (bacterial growth medium) was injected. (b) After 4 hr, 1 day or 3 days, the mice were killed and peritoneal lavage was performed with subsequent determination of S. aureus counts (CFU) in the lavage fluid. Mean ± SEM (n = 7 to n = 11). Results shown are a representative of four independent experiments.
Mast cells do not influence the inflammation in response to peritoneal S. aureus infection in vivo
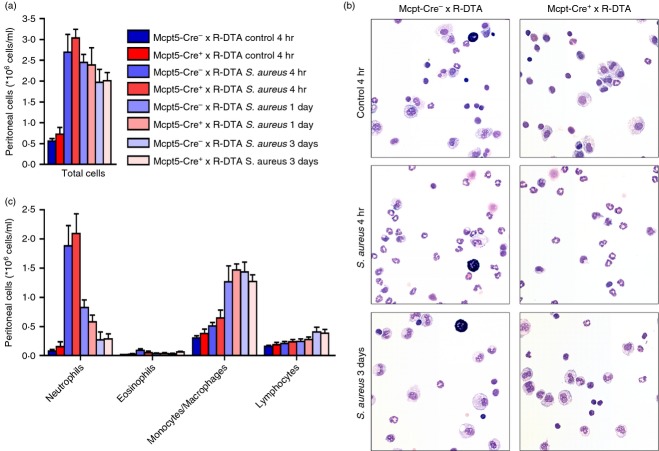
Although the data above indicated that mast cells are dispensable for clearance of peritoneal S. aureus infection, it cannot be excluded that mast cells affect the inflammatory response at levels not necessarily manifested by differences in efficiency of bacterial clearance. To investigate the impact of mast cells on the inflammatory response, peritoneal cell populations from S. aureus-infected mice were recovered, followed by counting of the total number of cells, staining with May–Grünwald/Giemsa and differential counting (Fig. 3). Four hours after S. aureus infection, a profound increase in the number of peritoneal cells was observed compared with non-infected control animals. This was due to a significant increase in the number of neutrophils. However, there were no significant differences in the numbers of recruited neutrophils when comparing mast cell sufficient- and deficient mice. After 3 days, the number of neutrophils had declined to baseline levels and this decline was accompanied by an increase in peritoneal monocytes/macrophages. No differences in numbers of monocytes/macrophages or other peritoneal cell populations were observed at this time-point when comparing the mast-cell-sufficient and -deficient mice (Fig. 3). As expected, mast cells were readily detected in the peritoneal exudate of the Mcpt5-Cre− × R-DTA mice but were undetectable in peritoneum of Mcpt5-Cre+ × R-DTA littermates (Fig. 3b).
Figure 3.
Mast cells do not influence the inflammation after Staphylococcus aureus infection. Mcpt5-Cre+ × R-DTA and Mcpt5-Cre− × R-DTA were infected intraperitoneally with S. aureus. As a control, TSB medium only (bacterial growth medium) was injected. After either 4 hr, 1 day or 3 days, peritoneal lavage was performed. The cells in the peritoneal lavage were counted (a), stained with May–Grünwald/Giemsa (b) and differential counts were performed (c). Mean ± SEM (n = 5 to n = 11). Results shown are a representative of four independent experiments.
Mast cells do not influence the cytokine induction in response to peritoneal S. aureus infection in vivo
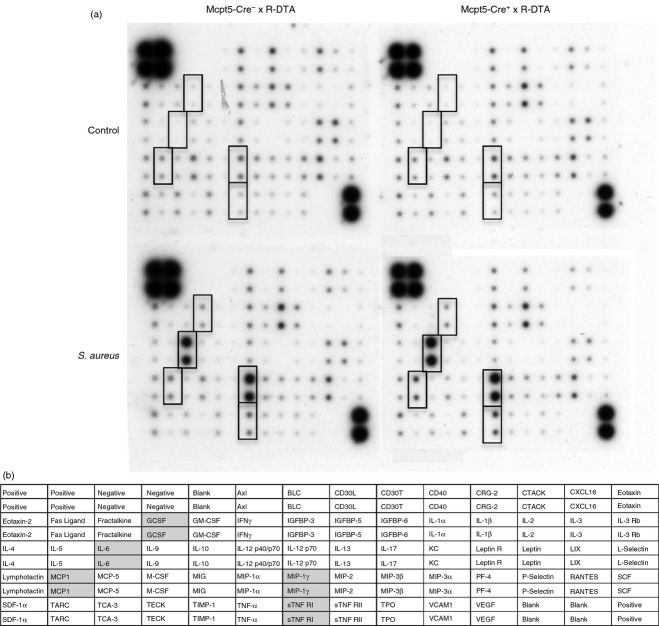
Since the co-culture of peritoneal mast cells with S. aureus was shown to induce a robust cytokine response (see Fig. 1 and Table 1), it was also of interest to investigate whether the peritoneal mast cell population contributes to the total cytokine output in response to infection of mice with S. aureus. For this purpose we used cytokine/chemokine membrane arrays to obtain an unbiased view of the cytokine profile induced by the bacterial infection. As seen in Fig. 4, S. aureus infection caused elevated levels of various cytokines in the peritoneum, including IL-6, macrophage inflammatory protein-1γ, granulocyte colony-stimulating factor, MCP-1 and sTNF RI but no difference was seen between the mast-cell-sufficient and -deficient mice. To verify these results, ELISA for IL-6 and MCP-1 was performed. This analysis demonstrated a significant up-regulation of these cytokines at 4 hr after infection and confirmed that there were no differences in the levels of IL-6 or MCP-1 as a consequence on mast cell presence or absence (Fig. 5a,b). In addition, we analysed for possible effects of mast cells on the levels of IL-3 and IL-13, i.e. two of the cytokines that were profoundly induced upon co-culture of mast cells with S. aureus in vitro (see Table 1, Fig. 1). However, as displayed in Fig. 5(c,d), no significant differences in the levels of these cytokines were seen due to the absence or presence of mast cells.
Figure 4.
Mast cells do not influence the production of cytokines in the peritoneum after Staphylococcus aureus intraperitoneal infection. Mcpt5-Cre+ × R-DTA and Mcpt5-Cre− × R-DTA were infected intraperiteonally with S. aureus. As a control, TSB medium only (bacterial growth medium) was injected. After either 4 hr, peritoneal lavage was performed. Peritoneal lavage fluid from three different animals in each group was pooled and analysed using cytokine membranes. Cytokines that were up-regulated in the infected mice are marked with rectangles (a). A scheme of the cytokine membrane; cytokines that were up-regulated in the infected mice are marked with grey (b).
Figure 5.
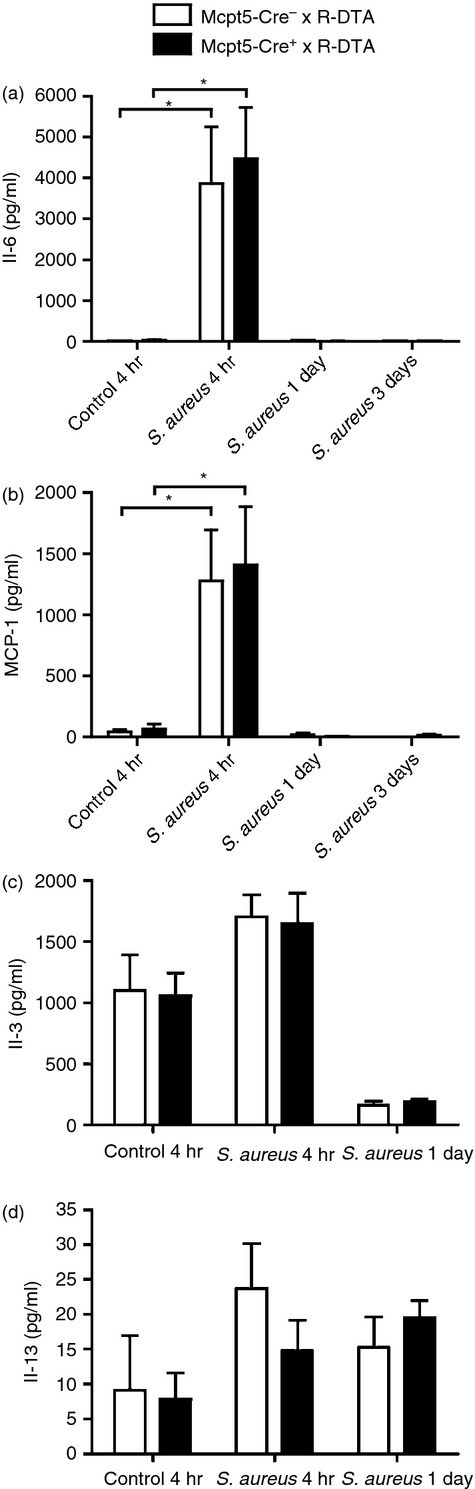
Mast cells do not influence the production of interleukin-6 (IL-6), monocyte chemoattractant protein 1 (MCP-1), IL-3 and IL-13 in the peritoneum after Staphylococcus aureus infection. Mcpt5-Cre+ × R-DTA and Mcpt5-Cre− × R-DTA were infected intraperitoneally with S. aureus. As a control, TSB medium only (bacterial growth medium) was injected. After 4 hr or 1 day, peritoneal lavage was performed. Peritoneal lavage fluids were analysed for levels of IL-6 (a), MCP-1 (b), IL-3 (c) and IL-13 (d) by ELISA. Mean ± SEM (n = 5 to n = 11). *P < 0.05.
Discussion
It has been shown that mast cells produce certain cytokines, e.g. TNF-α and IL-6, in response to S. aureus.5,6,21 Here we extend these findings by unbiased technology and show that peritoneal mast cells also up-regulate a number of additional pro-inflammatory compounds when encountering S. aureus, including IL-3, IL-13, Oncostatin M, Lif, and chemokines Ccl3, Ccl7 and Cxcl2. Among other genes, we note a profound up-regulation of all members of the Nr4a family of nuclear receptors, i.e. Nr4a1, -2 and -3 in response to live S. aureus. We previously showed that all of these genes were strongly up-regulated in more immature mast cells, i.e. BMMCs, which were exposed to either group C streptococci or to IgE receptor cross-linking.22 Hence, the findings presented here indicate that a profound up-regulation of the Nr4a family members is a general consequence of mast cell activation in response to a broad range of activating stimuli, and in mast cells of different states of maturity. We also note, similarly to our previous findings on BMMCs activated by either IgE receptor cross-linking or by group C streptococci,23 that PCMCs exposed to S. aureus display a strong up-regulation of the gene for ADAMTS9, a metalloprotease implicated in extracellular matrix remodelling.
Based on the strong induction of numerous cytokines, e.g. IL-3 and IL-13, in PCMCs co-cultured with S. aureus in vitro, we anticipated that the absence of mast cells may cause a reduction in the levels of these cytokines following intraperitoneal infection of mice with S. aureus. In particular, because the mast cells used for the in vitro experiments were mature and of peritoneal origin, i.e. having a phenotype closely resembling that of the mast cells of the peritoneal cavity in vivo,7 we anticipated that the i.p. infection with S. aureus would affect the peritoneal mast cells in a fashion similar to that observed in the in vitro co-culture setting. Intriguingly though, we did not see any effects of mast cell deficiency on the levels of these cytokines. Furthermore, the absence of mast cells did not affect the levels of a range of additional cytokines, including IL-6 and MCP-1. Hence, mast cells do not contribute significantly to the total pool of these pro-inflammatory cytokines during intraperitoneal S. aureus infection in vivo. One potential explanation for these findings may be that although peritoneal mast cells may express these cytokines in response to S. aureus in vivo, their total output is negligible in comparison with contributions from other cells, e.g. monocytes/macrophages. In this context it is important to point out that mast cells represent a relatively minor population of the peritoneum, resident mast cells accounting for approximately 2% of the total peritoneal cells. Hence, even though mast cells may in fact express significant amounts of the respective cytokines, their relative contribution is too low to be detectable by the methods used here. Another potential explanation for the apparent discrepancy between the cytokine responses seen in vivo versus in vitro is that the mechanism of mast cell activation may differ between the in vitro situation and in vivo, such that genes that are up-regulated after contact with S. aureus in vitro may not necessarily be induced during the in vivo conditions.
Mast cells have been shown to be protective to a number of different bacterial infections, but we failed to see any significant contribution of mast cells in the course of peritoneal S. aureus infection. One potential explanation for this seeming discrepancy could be related to the fact that previous studies have been performed on KitW/W−v and KitW−sh/W−sh mice, in which the mast cell absence is caused by defective signalling through c-Kit. These mice have, in addition to being mast cell-deficient, a number of different abnormalities including complex alterations of many haematopoietic compartments. To prove that any effects seen in KitW/W−v and KitW−sh/W−sh mice are in fact the result of their lack of mast cells rather than other effects of defective c-Kit, it has therefore been essential to reconstitute the mast cell niche of these mice and to show that this reverses the phenotype to that of mast-cell-sufficient mice. However, it is not certain that the distribution and function of the reconstituted mast cells will be a reflection of mast cells in wild-type mice.17,24 Moreover, the recent development of c-Kit-independent mast-cell-deficient mice has provided some conflicting data in comparison with those generated based on KitW/W−v and KitW−sh/W−sh mice. For example, the role of mast-cell-derived IL-10 in contact hypersensitivity18,25 and the role of mast cells in autoimmunity26–28 has been questioned (reviewed in Rodewald et al.17 and Reber et al.16). This has led to a need to re-evaluate a range of proposed mast cell functions derived from the c-Kit-defective mice, such as their protective role in bacterial infections. The data reported here may therefore question the importance of mast cells in the host defence towards bacterial infections. On the other hand, even though the present data did not reveal any significant role of mast cells in intraperitoneal S. aureus infection, we cannot exclude that mast cells can influence the host defence towards other pathogenic bacteria, or to bacterial infection occurring through routes of administration other than the peritoneum. Neither can we exclude that mast cells may have an impact on the host defence towards other strains of S. aureus than the one used here, for example strains of higher virulence. It is also important to emphasize that much of the previous work on this topic has been based on the caecal ligation and puncture model, a model of severe sepsis. In contrast, the model used in this study represents a milder course of disease, so we cannot exclude that a protective role of mast cells is more evident in severe than in mild infections.
In conclusion, we have shown that peritoneal mast cells are activated by S. aureus in vitro whereas they do not play a role in intraperitoneal S. aureus infection in vivo. However, to make more general conclusions about the bona fide role of mast cells in bacterial infection, it will be imperative to perform more extensive studies on the novel c-Kit-independent mast-cell-deficient mice, using different bacterial strains, different administration routes for bacteria, and different experimental setups.
Acknowledgments
This work was supported by grants from Formas (GP) and from The Swedish Research Council (GP).
Disclosures
The authors declare that they have no conflict of interests.
References
- 1.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–20. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wertheim HF, Vos MC, Ott A, et al. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet. 2004;364:703–5. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 4.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 5.Abel J, Goldmann O, Ziegler C, et al. Staphylococcus aureus evades the extracellular antimicrobial activity of mast cells by promoting its own uptake. J Innate Immun. 2011;3:495–507. doi: 10.1159/000327714. [DOI] [PubMed] [Google Scholar]
- 6.Rocha-de-Souza CM, Berent-Maoz B, Mankuta D, Moses AE, Levi-Schaffer F. Human mast cell activation by Staphylococcus aureus: interleukin-8 and tumor necrosis factor α release and the role of Toll-like receptor 2 and CD48 molecules. Infect Immun. 2008;76:4489–97. doi: 10.1128/IAI.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malbec O, Roget K, Schiffer C, Iannascoli B, Dumas AR, Arock M, Daeron M. Peritoneal cell-derived mast cells: an in vitro model of mature serosal-type mouse mast cells. J Immunol. 2007;178:6465–75. doi: 10.4049/jimmunol.178.10.6465. [DOI] [PubMed] [Google Scholar]
- 8.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–7. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 9.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Zhang D, Lyubynska N, et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–25. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malaviya R, Ikeda T, Abraham SN. Contribution of mast cells to bacterial clearance and their proliferation during experimental cystitis induced by type 1 fimbriated E. coli. Immunol Lett. 2004;91:103–11. doi: 10.1016/j.imlet.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Wei OL, Hilliard A, Kalman D, Sherman M. Mast cells limit systemic bacterial dissemination but not colitis in response to Citrobacter rodentium. Infect Immun. 2005;73:1978–85. doi: 10.1128/IAI.73.4.1978-1985.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siebenhaar F, Syska W, Weller K, Magerl M, Zuberbier T, Metz M, Maurer M. Control of Pseudomonas aeruginosa skin infections in mice is mast cell-dependent. Am J Pathol. 2007;170:1910–6. doi: 10.2353/ajpath.2007.060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piliponsky AM, Chen CC, Grimbaldeston MA, et al. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW−sh/W−sh mice. Am J Pathol. 2010;176:926–38. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–15. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 16.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–25. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Dudeck A, Dudeck J, Scholten J, et al. Mast cells are key promoters of contact allergy that mediate the adjuvant effects of haptens. Immunity. 2011;34:973–84. doi: 10.1016/j.immuni.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Sjostrom JE, Lofdahl S, Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975;123:905–15. doi: 10.1128/jb.123.3.905-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ronnberg E, Guss B, Pejler G. Infection of mast cells with live streptococci causes a toll-like receptor 2- and cell–cell contact-dependent cytokine and chemokine response. Infect Immun. 2010;78:854–64. doi: 10.1128/IAI.01004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma YJ, Kim CH, Ryu KH, Kim MS, So YI, Lee KJ, Garred P, Lee BL. Adenosine derived from Staphylococcus aureus-engulfed macrophages functions as a potent stimulant for the induction of inflammatory cytokines in mast cells. BMB Rep. 2011;44:335–40. doi: 10.5483/BMBRep.2011.44.5.335. [DOI] [PubMed] [Google Scholar]
- 22.Lundequist A, Calounova G, Wensman H, Ronnberg E, Pejler G. Differential regulation of Nr4a subfamily nuclear receptors following mast cell activation. Mol Immunol. 2011;48:1753–61. doi: 10.1016/j.molimm.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Faroldi G, Ronnberg E, Orro A, Calounova G, Guss B, Lundequist A, Pejler G. ADAMTS: novel proteases expressed by activated mast cells. Biol Chem. 2012;394:291–305. doi: 10.1515/hsz-2012-0270. [DOI] [PubMed] [Google Scholar]
- 24.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant KitW−sh/W−sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimbaldeston MA, Nakae S, Kalesnikoff J, Tsai M, Galli SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. 2007;8:1095–104. doi: 10.1038/ni1503. [DOI] [PubMed] [Google Scholar]
- 26.Feyerabend TB, Weiser A, Tietz A, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–44. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–92. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 28.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–22. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]