Abstract
In cases of arginine depletion, lymphocyte proliferation, cytokine production and CD3ζ chain expression are all diminished. In addition to myeloid suppressor cells, polymorphonuclear cells (PMN) also exert T-cell immune suppressive effects through arginase-induced l-arginine depletion, especially during pregnancy. In this study, we investigated how arginase/l-arginine modulates neonatal lymphocyte proliferation. Results showed that the neonatal plasma l-arginine level was lower than in adults (48·1 ± 11·3 versus 86·5 ± 14·6 μm; P = 0·003). Neonatal PMN had a greater abundance of arginase I protein than adult PMN. Both transcriptional regulation and post-transcriptional regulation were responsible for the higher arginase I expression of neonatal PMN. Exogenous l-arginine enhanced neonate lymphocyte proliferation but not that of adult cells. The RNA-binding protein HuR was important but was not the only modulation factor in l-arginine-regulated neonatal T-cell proliferation. l-Arginine-mediated neonatal lymphocyte proliferation could not be blocked by interleukin-2 receptor blocking antibodies. These results suggest that the altered arginase/l-arginine cascade may be one of the mechanisms that contribute to altered neonatal immune responses. Exogenous l-arginine could enhance neonate lymphocyte proliferation through an interleukin-2-independent pathway.
Keywords: arginase, interleukin-2, l-arginine, lymphocyte proliferation, neonate
Introduction
l-Arginine is known to play an important role in both innate and adaptive immunity. It regulates T-cell proliferation and B-cell development.1,2 Arginase is the key hydrolytic enzyme for catalysing the conversion of l-arginine to ornithine and urea, so eliminating excess nitrogen from the body.3 Arginase activation may limit the availability of l-arginine as a substrate for inducible nitric oxide synthase, thereby inhibiting nitric oxide secretion.4 There are two arginase isozymes (arginase I and II), which exist in mammals, both hydrolysing arginine to ornithine and urea. These two arginase isoenzymes differ in sub-cellular localization and function.3 Arginase I is a cytosolic enzyme, which is localized in the cytosol of hepatocytes and the azurophil granules of polymorphonuclear cells (PMN), whereas arginase II is localized in the mitochondria and is found in a variety of tissues.3,5 Arginase has now emerged as a key player in the mammalian immune system.6 Arginase-I-expressing myeloid suppressor cells have been reported to exert a systemic effect and cause a state of arginine deficiency in certain infectious and tumour conditions. This leads to the inhibition of T-lymphocyte proliferation, cytokine production and CD3ζ chain down-regulation.7,8 In a state of arginine depletion, the proliferation of natural killer cells and their interleukin-12 (IL-12)/IL-18-induced secretion of interferon-γ were also diminished.9 Both PMN and myeloid suppressor cells exert T-cell immune suppressive effects through arginase-induced l-arginine depletion during activation.7 During pregnancy, the arginase activity of placental PMN and macrophages is also enhanced and this has been identified as one of the mechanisms for temporary T-cell hypo-responsiveness and maintenance of allogeneic pregnancy.10 Taken together, this evidence addresses the important role of l-arginine with regards to immune regulation.
Human newborns are known to be susceptible to microbial infections.11,12 Both innate and adaptive immunity are distinct at birth relative to adulthood.13,14 T helper type 1 immune responses in newborns compromise several steps including deficient production of T helper type 1 cytokines.13 l-Arginine is a semi-essential amino acid. Although it can be synthesized by adult humans, l-arginine must be supplemented by diet for the fetus and neonates.15 In our previous study, we found more abundant arginase I in cord blood mononuclear cells (MNC) and this might partially account for the impaired immune response in newborns.16 Although some animal experiments suggested that l-arginine could have some beneficial effects in restoring T-lymphocyte counts under certain stress-related conditions,17,18 information about the regulatory effects of l-arginine in human neonatal leucocytes is still lacking. In this study, the mechanism by which neonatal leucocytes had higher arginase I expression was explored. The modulation effects of exogenous l-arginine on neonatal lymphocyte proliferation were also investigated.
Materials and methods
Collection of human umbilical cord blood and adult peripheral blood and cell separation
Human umbilical cord blood was collected in heparinized tubes (10 U/ml) by cordocentesis at the time of elective caesarean section or normal spontaneous delivery in healthy mothers, after informed consent was obtained from the women. The peripheral blood samples were obtained from healthy adult volunteers aged 20–40 years. Heparinized blood samples were collected, and the plasma was stored at −80° before analysis. The leucocyte separation protocol was as previously described.19,20 In brief, the whole blood was mixed with 4·5% (w/v) dextran sedimentation to separate leucocytes from the red blood cells. Leucocytes were further separated into PMN and MNC by density gradient centrifugation in the Ficoll-Paque™ (Amersham Pharmacia, Uppsala, Sweden) at a ratio of 2 : 1. After centrifugation over a Ficoll cushion, MNC were washed and counted on a haemocytometer by trypan blue staining. The PMN fraction in MNC was < 1% both in adult or neonate. The study protocol was approved by the Institutional Review Board of the study hospital.
Detection of l-arginine by high-performance liquid chromatography
Plasma l-arginine levels were measured using HPLC (HP series 1100; Agilent Technologies, Inc., Santa Clara, CA) using the OPA-3MPA derivatization reagent as previously described.21
Preparation of different cell populations
CD3-, CD4-, CD14- and CD19-positive T cells, monocytes and B cells were isolated from adult peripheral blood and cord blood MNC by using CD3, CD4, CD14, and CD19 isolation kits (Miltenyi Biotec, Bergisch Gladbach, Germany), respectively; the cells were then subjected to positive magnetic sorting using autoMACS (Miltenyi Biotec) according to the manufacturer's protocol. The purity of isolated cells was further confirmed by flow cytometry, and all the cells isolated were > 90% purity.20
Western blot and arginase activity assay
Leucocytes were washed and lysed in cold radioimmunoprecipitation assay lysis buffer (Sigma, St Louis, MO) containing protease inhibitors (Complete Mini™; Roche Diagnostics, Indianapolis, IN), and the protein concentration was determined using a protein assay kit (Bio-Rad, Hercules, CA) according to the manufacturer's instructions. For Western blotting assay, protein samples were incubated with SDS loading buffer (10 mm Tris–HCl, pH 6·8, 1% SDS, 25% glycerol, 0·1 mm β-mercaptoethanol and 0·03% bromophenol blue), boiled for 5 min, and subjected to 12% (w/v) SDS–PAGE. After being transferred to a PVDF membrane (Millipore Corporation, Billerica, MA) and blocked with Tris Buffered Saline with Tween 20 containing 5% dry milk, specific proteins in the SDS–PAGE gel were detected by first antibodies: arginase I (Millipore Corporation), arginase II (Gentex, Irvine, CA), HuR (Millipore, Bedford, MA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotech, Santa Cruz, CA) or cytochrome C oxidase IV (Cell Signaling Technology, Danvers, MA), and then horseradish peroxidase-conjugated second antibody (Santa Cruz Biotech) for 2 hr. Finally, the blots were developed using an enhanced chemiluminescence (ECL) Plus kit (Amersham Biosciences, Piscataway, NJ), exposed to Kodak X-ray film (Kodak, Rochester, NY) and images were scanned using a Personal Densitometer SI (Amersham Biosciences). Images were analysed using an LAS-3000 (Fujifilm, Tokyo, Japan) and multi gauge version 3.0 (Fujifilm).20 All Western blotting was performed in at least three separate experiments.
Arginase activity was determined with an arginase activity assay kit (BioAssay Systems, Hayward, CA) according to the manufacturer's protocols.
Cell culture and drug treatment
Aliquots of PMN (1× 106 cells/ml) in RPMI-1640 medium containing 10% heat-inactivated fetal bovine serum were treated with actinomycin D (1 μg/ml) or cycloheximide (Sigma-Aldrich), which had been prepared in stock solutions made in water or DMSO as indicated. Cells were harvested by centrifugation for total RNA or protein collection.
Reverse transcription-polymerase chain reaction of arginase I and GAPDH mRNAs
Total RNA from cells was extracted using an RNeasy kit (Qiagen, Valencia, CA). The RNA pellets were dissolved in diethypyrocarbonate-treated water and stored at −80° until use. A total of 200 ng of RNA was mixed with 1 μl dNTP and 5 μl oligo-dT for 5 min at 65°, and was subsequently mixed with 4 μl 5 × buffer, 2 μl 0·1 m dithiothreitol, and 1 μl RNaseOUT in a total volume of 20 μl before being subjected to reverse transcription for 60 min at 42° using reverse transcriptase (Invitrogen, San Diego, CA). The reverse-transcribed cDNA products were subjected to PCR amplification with specific primers and SYBR GREEN quantification for amplifying three different transcripts, as follows:
arginase I: forward primer 5′-GCTCAAGTGCAGCAAAGAGA-3′ and reverse primer 3′-CCGACCAGACGAACTCTTTG-5′.
GAPDH: forward primer 5′-CATGAGAAGTATGACAACAGCCT-3′ and reverse primer 3′-AGTCCTTCCACGATACCAAAGT-5′.
The PCR steps were activated by heating for 10 min at 95°, then for 60 seconds at 60°, and finally for 15 seconds at 95° for 40 cycles in a PCR mix containing 2 μl of the cDNA template, 1 × qPCR Mastermix (RT-QP2X-03; Eurogentec, Seraing, Belgium), 100 nm of each primer, and 1 μl of SYBR GREEN in a total volume of 30 μl. The system generated a kinetic amplification plot based on the normalized fluorescence. All reactions were performed in an ABI-Prism 7500 sequence detection system (Applied Biosystems, Foster City, CA) as previously described.13
Proliferation assay
Proliferation of MNC was assessed by a BrdU assay. Adult or cord blood MNC were suspended to 5 × 105 cells/ml in a 96-well plate and were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, 1 mm glutamine, 100 IE/ml penicillin and 100 μg/ml streptomycin. Adult or cord blood MNC were then stimulated with or without 10 μg/ml phytohaemagglutinin (PHA; Sigma) or 10 μg/ml anti-IL-2 receptor (IL-2R) monoclonal antibody (Basiliximab; Novartis International AG, Basel, Switzerland) where indicated. Read-out of the proliferation was carried out using the BrdU-Assay obtained from Millipore according to the manufacturer's protocols.
Induction of secretory IL-2 and cell surface IL-2R expression determination
Adult or cord blood MNC were suspended to 2 × 106 cells/ml in a 24-well plate and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1 mm glutamine, 100 IE/ml penicillin and 100 μg/ml streptomycin. Adult or cord blood MNC were then stimulated with or without 10 μg/ml PHA or 10 μg/ml Basiliximab where indicated. The cell pellets and culture supernatants were collected at the times indicated and detected for cell surface IL-2R expression using phycoerythrin-conjugated anti-CD25 (Beckman Coulter, Fullerton, CA) and secretory IL-2 protein production with ELISA (Biolegend, San Diego, CA) as previously described.22
Statistics
Data were expressed as mean ± standard error of the mean. The statistical calculation was performed with an analysis of variance test for comparison among groups. The Mann–Whitney U-test was used when two groups were analysed. Results with a P-value < 0·05 were considered to be statistically significant. All statistical tests were performed using spss 15.0 for Windows XP (SPSS, Inc., Chicago, IL).
Results
Cord blood plasma has a lower l-arginine level than adult peripheral blood plasma
At first the plasma l-arginine levels of cord blood and adult blood were determined. As shown in Fig. 1, cord blood plasma has a lower l-arginine level than healthy adult plasma (48·1 ± 11·3 versus 86·5 ± 14·6 μm; P = 0·003).
Figure 1.
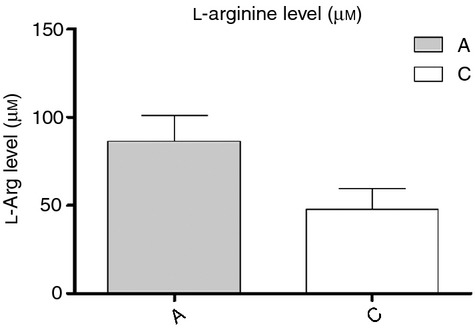
The plasma l-arginine levels of adults and neonates. To compare the plasma l-arginine levels of adults and neonates, we collected six pairs of adult peripheral blood versus cord blood plasma for determination of the l-arginine levels by HPLC. Results showed that plasma l-arginine levels of cord blood were significantly lower than those of adults (48·14 ± 11·32 versus 86·52 ± 14·63 μm; P = 0·003). Data presented were calculated from six replicate experiments. l-Arg, l-arginine.
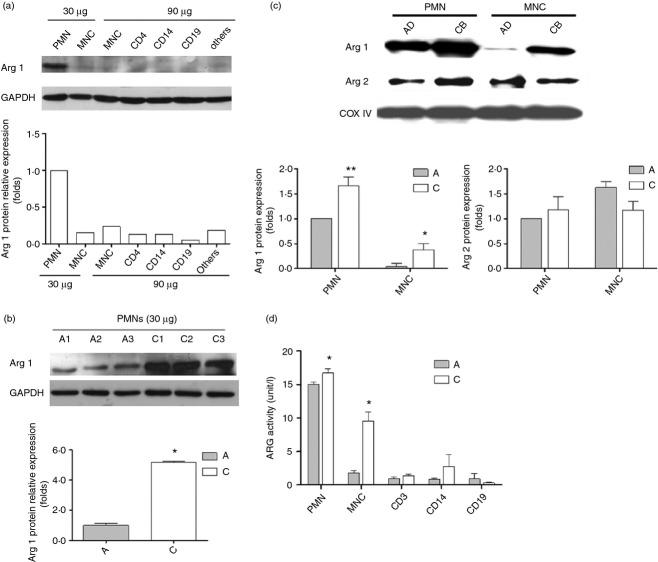
Among human leucocytes, arginase I is selectively expressed in PMN fraction. Neonatal PMN have more abundant arginase I protein abundance and activity than adult PMN
Then the cellular proteins of different sub-populations of adult leucocytes were extracted and indicated for arginase I expression by Western blot. As shown in Fig. 2(a), among the different leucocyte populations, PMN has more abundant arginase I protein. Our results supported the findings of previous reports that arginase I protein was selectively expressed in PMN more than other sub-populations, such as T cells (CD4+), monocytes (CD14+), or B cells (CD19+).5 Then equal amounts of cellular proteins from cord blood and adult peripheral blood PMN were subjected to Western blot to compare their arginase I contents. As compared with adult PMN, neonatal PMN show greater arginase I protein expression (Fig. 2b). The greater levels of arginase I in neonatal PMN offered a likely explanation for the lower l-arginine levels of cord blood plasma when compared with adult plasma. Recently, neonatal CD71+ cells were reported to express arginase II, which is essential for the immunosuppressive properties of these cells.23 Hence the arginase II of adult and neonatal PMN/MNC was also determined with Western blotting. As shown in Fig. 2(c), neonatal PMN and MNC showed more abundant arginase I protein than adults. There was no significant difference between adult and neonatal PMN/MNC in arginase II proteins.
Figure 2.
The arginase I (Arg I) expression among different sub-populations of leucocyte. (a) Different leucocyte lysates from adult peripheral blood were analysed by Western blotting with anti-arginase I or anti-GAPDH antibodies. Adult polymorphonuclear cells (PMN) had more abundant arginase I protein expression than mononuclear cells (MNC), helper T lymphocytes (CD4+), monocytes (CD14+), B cells (CD19+), and other cells (Others: the residual cells after CD4+, CD14+ and CD+ cells were removed from MNC). Results presented were derived from three replicate experiments. (b) The arginase I expression between adult PMN and neonatal PMN in Western blot analysis. Thirty micrograms of PMN lysates samples were separated by 12% SDS–PAGE and analysed by Western blotting with anti-arginase I antibodies. Lanes A1–A3 were samples of adult PMN and lanes C1–C3 were samples of cord blood PMN from different individuals. Results presented were derived from three replicate experiments. *P < 0·05 as compared with adult PMN (c) The arginase I and arginase II expressions between adult and neonatal leucocytes in Western blot analysis. Results presented were derived from six replicate experiments. **P < 0·01 as compared with adult PMN; *P < 0·05 as compared with adult MNC. (d) The arginase activities between adult and neonatal leucocytes. Results presented were derived from six replicate experiments. *P < 0·05 as compared with adult.
Thereafter, the arginase activities of different leucocyte sub-populations were determined with an assay kit. Compatible with protein expressions, the neonatal PMN showed higher arginase activity than other leucocyte subpopulations. The neonatal PMN and MNC had higher arginase activity than adult PMN and MNC, respectively (Fig. 2d).
Both transcriptional and post-transcriptional regulations were responsible for the greater arginase I protein levels of neonatal PMN compared with adult PMN
To clarify the mechanism responsible for abundant arginase I protein in neonatal PMN, we compared the arginase I mRNA expressions of cord blood and adult peripheral blood PMN by RT-PCR. Compatible with protein expression, neonatal PMN also showed higher arginase I mRNA expression than adult PMN (Fig. 3a). Then the PMN from cord blood and adult blood were treated with actinomycin D (1 μg/ml; Act-D) to block mRNA synthesis for mRNA half-life determination. As shown in Fig. 3(b), the arginase I mRNA half-life value of neonatal PMN was longer than that of adult PMN. The arginase I mRNA half-life values for neonatal PMN and adult PMN were 226·2 and 176·8 min, respectively (P = 0·038). Hence, the differential expressions of PMN arginase I proteins between neonate and adult were both due to transcriptional and post-transcriptional regulations.
Figure 3.
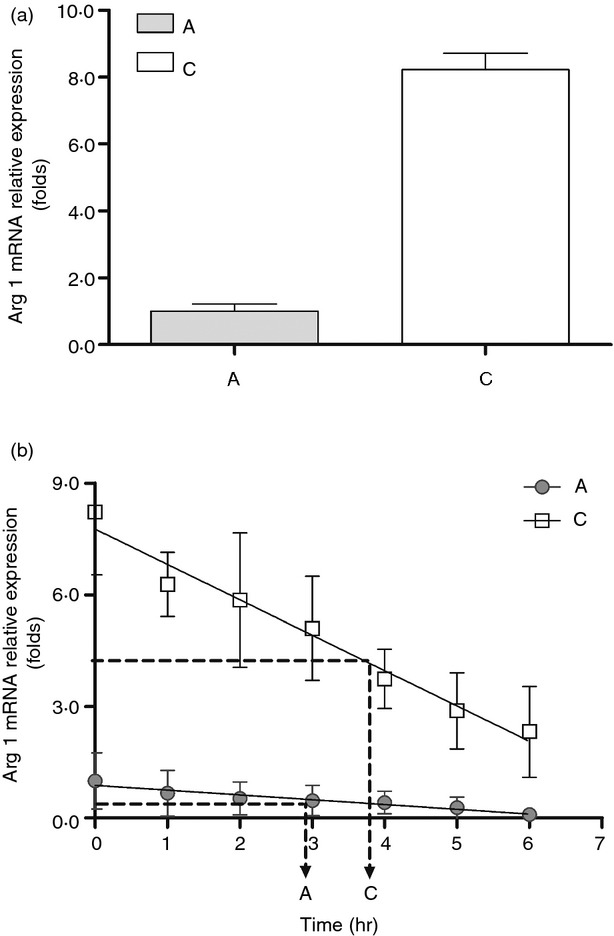
Quantitative RT-PCR analysis of arginase I and GAPDH mRNA expression in cord and adult blood polymorphonuclear cells (PMN). (a) Representative RT-PCR analysis of arginase I (Arg I) and GAPDH mRNA levels at resting status. ‘C’ indicates cord blood PMN, and ‘A’ indicates adult blood PMN. Comparison of the mRNA levels of arginase I/GAPDH in cord and adult blood PMN is shown in the bar graphs. There was significant difference about the mRNA levels of arginase I/GAPDH between adult and cord blood PMN (1·00 ± 0·22 versus 8·23 ± 0·49; P < 0·001). Data presented were calculated from six replicate experiments. (b) Half-life of arginase mRNA between adult and neonate PMN. PMN from adult blood and cord blood were treated with actinomycin D (1 μg/ml) to block mRNA synthesis for studying mRNA half-life. ‘C’ indicates cord blood PMN, and ‘A’ indicates adult blood PMN. Data are presented by mean ± SE. Triplicate determinations were averaged at each data-point. There was a significant difference about the arginase I mRNA half-life values between adult and cord blood PMN (176·81 ± 12·62 versus 226·27 ± 15·16; P = 0·038).
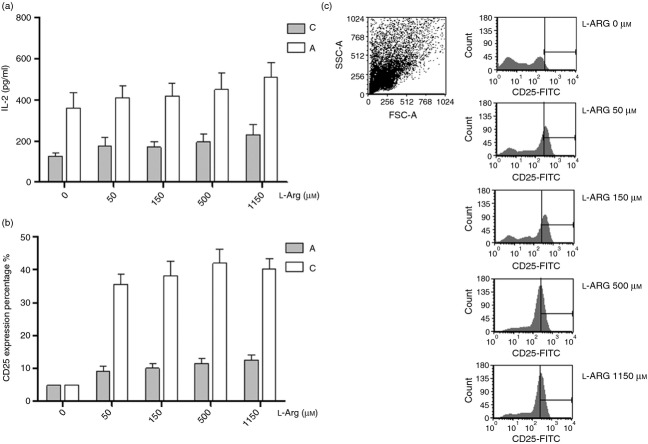
Exogenous l-arginine improves neonatal T-cell proliferation
To test the modulation effects of l-arginine on T-cell proliferation, adult peripheral blood MNC and cord blood MNC were stimulated with PHA in the medium with indicated l-arginine levels. T-cell proliferation was determined by BrdU assay at 72 hr. As shown in Fig. 4, the proliferation of cord blood MNC, but not adult MNC, was impaired in the state of l-arginine depletion. The proliferation of cord MNC had the trend of being enhanced as l-arginine levels increased and peaked at an l-arginine level at 150 μm.
Figure 4.
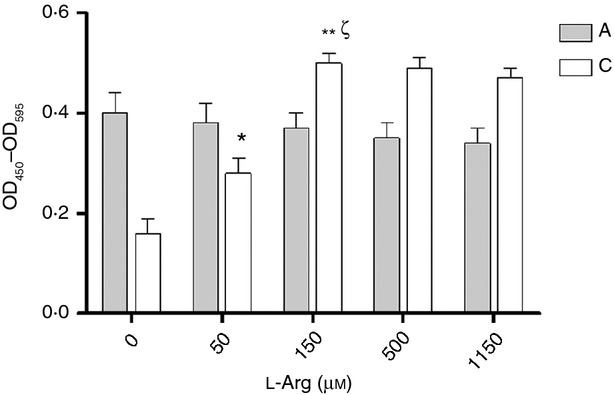
Effects of l-arginine on cell proliferation for adult and neonatal mononuclear cells (MNC). Adult or cord blood MNC were treated with phytohaemagglutinin (PHA) under the indicated l-arginine condition mediums. Lymphocyte proliferation was assayed using BrdU assays as described in Materials and methods. The results were normalized to those obtained in pair-controlled studies of adult blood MNC. ‘C’ indicates cord blood MNC, and ‘A’ indicates adult blood MNC. The data shown are mean ± SE from 10 replicate experiments (*P = 0·014 compared with cord blood MNC at l-arginine 0 μm, **P = 0·003 compared with cord blood MNC at l-arginine 0 μm, P = 0·017 compared with cord blood MNC at l-arginine 50 μm). Arg 1, arginase I.
The RNA-binding protein HuR is an important but not the only modulating factor in l-arginine-regulated neonatal T-cell proliferation
It has been reported that l-arginine deprivation impairs expression of the RNA-binding protein HuR and leads to cell cycle arrest through a mechanism that arrests global protein synthesis.24,25 To investigate the role of HuR in l-arginine-mediated neonatal lymphocyte proliferation, we compared the HuR expression between adult and neonate MNC with indicated l-arginine conditions. As shown in Fig. 5, the HuR protein expression was enhanced as l-arginine level increased in a dose-dependent manner in neonatal MNC. However, the HuR protein expression of neonatal MNC was always lower than that of adults.
Figure 5.
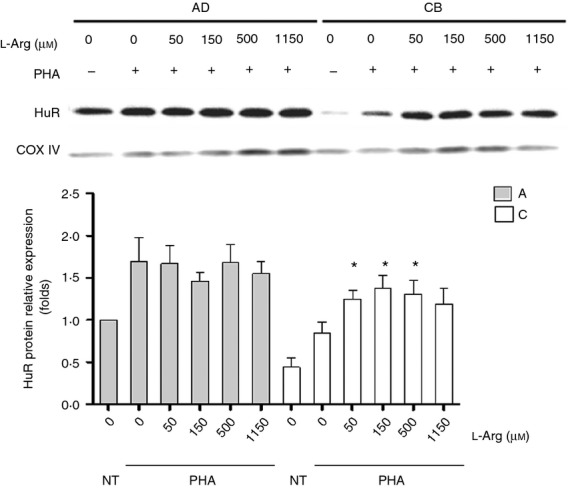
The HuR expressions of adult and neonatal mononuclear cells (MNC) under various l-arginine condition. Thirty micrograms of MNC lysates from adult peripheral blood or cord blood was analysed by Western blotting with anti-HuR or anti-GAPDH antibodies. The ‘A’ indicates adult MNC, and ‘C’ indicates cord blood MNC. Adult MNC had more abundant HuR protein expression than neonatal MNC. The HuR protein expression was enhanced as l-arginine level increased. Results presented were derived from six replicate experiments. *P < 0·05 compared with cord blood MNC at l-arginine 0 μm.
Neonatal MNC have higher IL-2 production and cellular IL-2R expression than adult MNC
To determine whether the IL-2 signalling pathway was involved in the proliferation mechanism modulated by l-arginine, the secretary IL-2 and surface IL-2R (CD25) were determined at a series of l-arginine levels. At first, we detected the soluble IL-2 secreted by mononuclear cells. We found that the secretory IL-2 depleted rapidly (data not shown). Then, we used an anti-IL-2R monoclonal antibody, Basiliximab, to block the consumption of secretory IL-2. As shown in Fig. 6(a), cord blood MNC always produced more secretory IL-2 than adult MNC after PHA stimulation. Secretory IL-2 production was independent of l-arginine levels, in both cord blood MNC and adult MNC.
Figure 6.
Effects of l-arginine on interleukin-2 (IL-2) production and IL-2 receptor (IL-2R) expression for adult and neonatal mononuclear cells (MNC). Adult or cord blood MNC were suspended to 2 × 106/ml in 24-well plates then treated with 10 μg/ml phytohaemagglutinin (PHA) and 10 μg/ml anti-IL-2R monoclonal antibody. (a) The culture supernatants were collected at 24 hr and then indicated for secretory IL-2 detection with ELISA. (b) In other experiments, after culture for 48 hr, cell pellets were collected for surface IL-2R (CD25) expression with flow cytometry. The lymphocytes shown in the dot plots were gated on MNC by forward sideways scatter characteristics. l-Arginine enhanced the cellular IL-2R expressions of lymphocytes. The results are representative of six duplicate experiments. (c) Plots illustrate the cellular IL-2R expressions (expressed as % gated in the ordinate) of adult and cord blood lymphocytes under different l-arginine conditions. Data presented were calculated from 10 replicate experiments.
The cell surface IL-2R expression by adult and cord blood MNC were then determined. We found that l-arginine was necessary for adequate expression of IL-2R. Cord blood MNC and adult MNC have equal IL-2R expression when l-arginine is depleted. Cord blood MNC and adult MNC exhibited significantly increased surface IL-2R expression upon PHA stimulation after introduction of l-arginine to cultures (Fig. 6b); and the response of cord blood MNC was significantly greater than that of MNC from adults (Fig. 6c).
l-Arginine improves neonatal T-cell proliferation through an IL-2-independent pathway
To see the role of IL-2 in l-arginine-regulated neonatal lymphocyte proliferation, Basiliximab was used to block the IL-2 signal pathway. As shown in Fig. 6(a), anti-IL-2R antibody inhibited adult MNC proliferation slightly at low l-arginine but not high l-arginine levels (Fig. 7a). However, anti-IL-2R antibodies did not inhibit cord blood MNC proliferation modulated by l-arginine (Fig. 7b). Hence, l-arginine improves neonatal T-cell proliferation through an IL-2-independent pathway.
Figure 7.
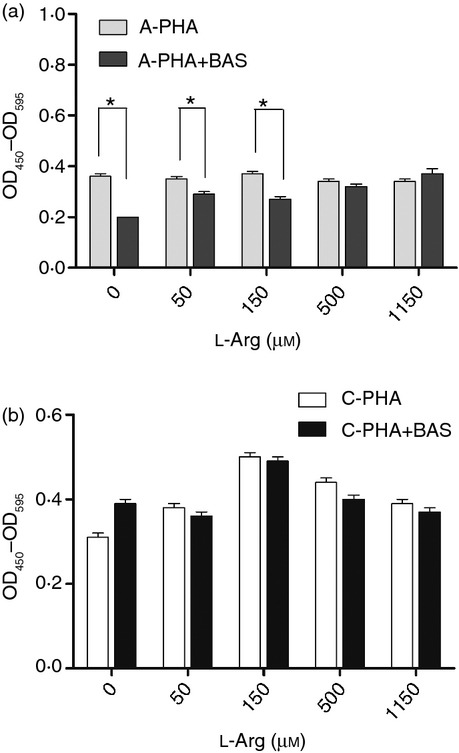
Effects of anti-interleukin-2 receptor (IL-2R) monoclonal antibody on lymphocyte proliferation modulated by l-arginine. Adult or cord blood mononuclear cells (MNC) were suspended to 5 × 105/ml in 72-well plates and treated with or without 10 μg/ml anti-IL-2R monoclonal antibody. Upon stimulation with phytohaemagglutinin (PHA; 10 μg/ml) for 72 hr, lymphocyte proliferation was assayed using BrdU assays. Anti-IL-2R antibody inhibited peripheral blood MNC proliferation at low l-arginine levels (a) but had no influence on cord blood MNC proliferation (b). The results were normalized to those obtained in pair-controlled studies of adult blood MNC. ‘C’ indicates cord blood MNC, and ‘A’ indicates adult blood MNC. The data shown are from 10 replicate experiments *P < 0·05.
Discussion
In this study, we found that cord blood plasma has a lower l-arginine level. The neonatal PMN have a greater abundance of arginase I protein expression than adult PMN. Both transcriptional and post-transcriptional regulations were responsible for the higher arginase I expression of neonatal PMN. In further studies, we showed that exogenous l-arginine enhances neonate lymphocyte proliferation through an IL-2-independent pathway.
Modulation of T-cell responses by arginase-related l-arginine depletion is emerging as an important immunoregulatory mechanism in certain tumour conditions, such as renal cell carcinoma, prostate cancer, breast cancer and lung cancer, and in certain infections, like pulmonary tuberculosis.26–30 In patients with sickle cell anaemia, a correlation between T-cell hypo-responsiveness and arginase-mediated l-arginine depletion has also been described.31 Enhanced arginase activity in normal term pregnancy is recognized as one of the mechanisms contributing to the suppression of maternal immune responses by reducing the bioavailability of l-arginine and leading to down-regulation of CD3ζ expression and induction of functional T-cell hypo-responsiveness.10 Here we demonstrated that this arginase-mediated T-cell hypo-responsiveness regulatory mechanism extended from pregnant mother to newborn. The imbalanced arginase/l-arginine cascade may be one of the mechanisms that contribute to the altered neonatal immune responses.
Early in vitro studies have identified that 200 μm l-arginine is required for the maximum proliferation of murine lymphocytes in response to mitogens.32,33 In our study, as stimulated with PHA, adult lymphocyte proliferation was not influenced by l-arginine. In contrast, neonatal lymphocyte proliferation was considerably enhanced by l-arginine in a dose-dependent manner and reached a maximum proliferation at 150 μm l-arginine. Clinical studies have shown that the enteral or parenteral provision of arginine improves immune functions and clinical outcomes in patients with burn injury, cancer, major traumas and gastrointestinal surgical operations.34,35 The impact of l-arginine intake on newborns has not been reported in the related literature. Since exogenous l-arginine could reverse the arginase-mediated T-cell suppression of neonates, l-arginine supplementation may support the use of an immune-enhanced infant formula for newborns with associated arginase-mediated T-cell dysfunction.
Although more abundant, arginase I in cord blood leucocytes might contribute to the decrease of plasma l-arginine in newborns. The depletion of plasma l-arginine is not only the result of an increase of arginase I in leucocytes. Arginase I is liberated during activation.7 Besides this, the liver and other sources of arginase may contribute to the decreased levels of plasma concentration of l-arginine.
Through the control of the RNA-binding protein HuR, l-arginine depletion causes mRNA stability to decrease and T-cell cycle arrest.25 From our study, although enhanced as l-arginine level increased in a dose-dependent manner, the HuR protein expression of neonatal MNC was always lower than that of adults. The HuR expression cannot totally explain the T-cell proliferation modulated by l-arginine. Hence, HuR was important but not the only modulation factor in l-arginine-regulated neonatal T-cell proliferation.
Loss of CD3ζ chain has been reported to be the only l-arginine starvation-triggered mechanism that is proven to have a direct relevance to T-cell function. Other T-cell functions, such as up-regulation of IL-2R and production of IL-2, were maintained even in the absence of l-arginine.36 Interleukin-2 is produced by T cells and is necessary for the proliferation, differentiation and survival of effector T cells.37 In our study we found that l-arginine enhances neonatal lymphocyte proliferation in a dose-dependent manner but not adult lymphocytes under PHA stimulation. The IL-2R of neonatal lymphocytes was decreased in situations where l-arginine was depleted and could be restored by exogenous l-arginine supplementation. Anti-IL-2R blocking antibody could partially block the proliferation of adult lymphocytes at low l-arginine levels but not neonatal lymphocytes. This suggests that l-arginine improves neonatal T-cell proliferation through an IL-2-independent pathway.
However, there are some limitations regarding this study that are worth noting. First, although exogenous l-arginine can improve neonatal lymphocyte proliferation in vitro, further in vivo studies are needed to confirm its clinical effects. Second, only the lymphocyte proliferation and IL-2 signal pathway modulation of l-arginine were studied there. In addition to proliferation, T-cell polarization and regulation are also important cellular immune functions. Arginase I has been reported as being induced in murine myeloid cells by T helper type 2 cytokines, such as IL-4 and IL-13,4,38 resulting in l-arginine depletion. However, the modulation effects of l-arginine towards T-cell immune polarization and regulation are understood at this time. Hence, further studies are needed to evaluate the modulation effects of l-arginine in relation to T-cell immune polarization and regulation, especially for neonates.
In conclusion, this is the first report to highlight lower plasma l-arginine levels and higher leucocyte arginase I protein expression in neonates compared with adults. These physiological differences and l-arginine bioavailability reduction may partially account for altered immune responses in newborns. Since exogenous l-arginine could reverse the arginase-mediated T-cell suppression of neonates, l-arginine supplementation may provide us with an immune-enhanced infant formula for newborns associated with arginase-mediated T-cell dysfunction.
Acknowledgments
This study was supported in part by grants CMRPG8B0781 (H. R. Yu) and NSC 102-2314-B-182A-042-MY3 (H. R. Yu) from the National Science Council, Taiwan.
Disclosures
The authors declare that there are no commercial or financial conflicts of interest.
References
- 1.Wu G, Bazer FW, Davis TA, et al. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–68. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. Br J Nutr. 2007;98:237–52. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 3.Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–32. doi: 10.1016/0305-0491(95)02138-8. [DOI] [PubMed] [Google Scholar]
- 4.Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–7. [PubMed] [Google Scholar]
- 5.Munder M, Mollinedo F, Calafat J, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–56. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 6.Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–51. doi: 10.1111/j.1476-5381.2009.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munder M, Schneider H, Luckner C, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108:1627–34. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 8.Singer K, Gottfried E, Kreutz M, Mackensen A. Suppression of T-cell responses by tumor metabolites. Cancer Immunol Immunother. 2011;60:425–31. doi: 10.1007/s00262-010-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oberlies J, Watzl C, Giese T, et al. Regulation of NK cell function by human granulocyte arginase. J Immunol. 2009;182:5259–67. doi: 10.4049/jimmunol.0803523. [DOI] [PubMed] [Google Scholar]
- 10.Kropf P, Baud D, Marshall SE, et al. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–45. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang KD, Hill HR. Immune responses to infectious diseases: an evolutionary perspective. Pediatr Infect Dis J. 1996;15:355–64. doi: 10.1097/00006454-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Goldmann DA. Prevention and management of neonatal infections. Infect Dis Clin North Am. 1989;3:779–813. [PubMed] [Google Scholar]
- 13.Yu HR, Chang JC, Chen RF, et al. Different antigens trigger different Th1/Th2 reactions in neonatal mononuclear cells (MNCs) relating to T-bet/GATA-3 expression. J Leukoc Biol. 2003;74:952–8. doi: 10.1189/jlb.0902474. [DOI] [PubMed] [Google Scholar]
- 14.Hou PC, Yu HR, Kuo HC, et al. Different modulating effects of adenosine on neonatal and adult polymorphonuclear leukocytes. ScientificWorldJournal. 2012;2012:387923. doi: 10.1100/2012/387923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu G, Jaeger LA, Bazer FW, Rhoads JM. Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. J Nutr Biochem. 2004;15:442–51. doi: 10.1016/j.jnutbio.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Yu HR, Kuo HC, Huang HC, et al. Identification of immunodeficient molecules in neonatal mononuclear cells by proteomic differential displays. Proteomics. 2011;11:3491–500. doi: 10.1002/pmic.201100123. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Liu YL, Fan W, et al. Dietary l-arginine supplementation alleviates immunosuppression induced by cyclophosphamide in weaned pigs. Amino Acids. 2009;37:643–51. doi: 10.1007/s00726-008-0184-9. [DOI] [PubMed] [Google Scholar]
- 18.Tan B, Li XG, Kong X, et al. Dietary l-arginine supplementation enhances the immune status in early-weaned piglets. Amino Acids. 2009;37:323–31. doi: 10.1007/s00726-008-0155-1. [DOI] [PubMed] [Google Scholar]
- 19.Yu HR, Chen RF, Hong KC, et al. IL-12-independent Th1 polarization in human mononuclear cells infected with varicella-zoster virus. Eur J Immunol. 2005;35:3664–72. doi: 10.1002/eji.200526258. [DOI] [PubMed] [Google Scholar]
- 20.Yu HR, Kuo HC, Huang HC, et al. Glyceraldehyde-3-phosphate dehydrogenase is a reliable internal control in Western blot analysis of leukocyte subpopulations from children. Anal Biochem. 2011;413:24–9. doi: 10.1016/j.ab.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Tain YL, Hsieh CS, Chen CC, Sheen JM, Lee CT, Huang LT. Melatonin prevents increased asymmetric dimethylarginine in young rats with bile duct ligation. J Pineal Res. 2010;48:212–21. doi: 10.1111/j.1600-079X.2010.00745.x. [DOI] [PubMed] [Google Scholar]
- 22.Yu HR, Huang HC, Kuo HC, et al. IFN-α production by human mononuclear cells infected with varicella-zoster virus through TLR9-dependent and -independent pathways. Cell Mol Immunol. 2011;8:181–8. doi: 10.1038/cmi.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–62. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez PC, Quiceno DG, Ochoa AC. l-Arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–73. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez PC, Hernandez CP, Morrow K, et al. l-Arginine deprivation regulates cyclin D3 mRNA stability in human T cells by controlling HuR expression. J Immunol. 2010;185:5198–204. doi: 10.4049/jimmunol.1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zea AH, Rodriguez PC, Atkins MB, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 27.Bronte V, Kasic T, Gri G, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–68. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteside TL. Down-regulation of ζ-chain expression in T cells: a biomarker of prognosis in cancer? Cancer Immunol Immunother. 2004;53:865–78. doi: 10.1007/s00262-004-0521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez PC, Quiceno DG, Zabaleta J, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 30.Zea AH, Culotta KS, Ali J, et al. Decreased expression of CD3ζ and nuclear transcription factor κB in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006;194:1385–93. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- 31.Morris CR, Kato GJ, Poljakovic M, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–6. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 33.Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E. Effects of l-arginine on the proliferation of T lymphocyte subpopulations. JPEN J Parenter Enteral Nutr. 2001;25:23–9. doi: 10.1177/014860710102500123. [DOI] [PubMed] [Google Scholar]
- 34.Suchner U, Heyland DK, Peter K. Immune-modulatory actions of arginine in the critically ill. Br J Nutr. 2002;87(Suppl. 1):S121–32. doi: 10.1079/bjn2001465. [DOI] [PubMed] [Google Scholar]
- 35.Marin VB, Rodriguez-Osiac L, Schlessinger L, Villegas J, Lopez M, Castillo-Duran C. Controlled study of enteral arginine supplementation in burned children: impact on immunologic and metabolic status. Nutrition. 2006;22:705–12. doi: 10.1016/j.nut.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Zea AH, Rodriguez PC, Culotta KS, et al. l-Arginine modulates CD3ζ expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 37.Cantrell DA, Smith KA. The interleukin-2 T-cell system: a new cell growth model. Science. 1984;224:1312–6. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 38.Barksdale AR, Bernard AC, Maley ME, et al. Regulation of arginase expression by T-helper II cytokines and isoproterenol. Surgery. 2004;135:527–35. doi: 10.1016/j.surg.2003.10.007. [DOI] [PubMed] [Google Scholar]