Abstract
Nod-like receptors are a family of innate immune receptors that link cytosolic sensing of microbial and danger stimuli to the activation of immune responses. Two Nod-like receptor family members, Nod1 and Nod2, recognize bacterial peptidoglycan and activate immune responses via nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK). The function of Nod1 and Nod2 has been largely studied in macrophages, but the role of these receptors in other innate immune cells remains unclear. In this study, we examined the function of Nod1 and Nod2 in innate immune responses of neutrophils. Mice were injected intraperitoneally with thioglycollate, and then peritoneal neutrophils were isolated 4 hr after injection. Tri-DAP and muramyl-dipeptide (MDP) were used as Nod1 and Nod2 agonists, respectively. The level of cytokines [interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α)] and chemokines (CXCL1 and CCL2) was increased by MDP, but not Tri-DAP in wild-type (WT) neutrophils. Increased production of cytokines and chemokines with MDP was abolished in Nod2- and Rip2-deficient neutrophils. MDP also induced the activation of NF-κB and MAPK in WT neutrophils, but not in Nod2- and Rip2-deficient cells. Flow cytometry analysis showed that l-selectin shedding was induced by MDP in WT neutrophils, but not in Nod2- and Rip2-deficient cells. MDP and Toll-like receptor (TLR) agonists (Pam3CSK4 and lipopolysaccharide) exerted synergistic effects on the production of IL-6 and CXCL1 in neutrophils. Moreover, Nod2 and TLR4 cooperated to produce IL-6, TNF-α, CXCL1 and CCL2 in neutrophils in response to Gram-negative bacteria. Our findings suggest that the Nod2–Rip2 axis may contribute to the innate immune response of neutrophils against bacterial infection.
Keywords: immune response, muramyl dipeptide, neutrophils, Nod2, Rip2
Introduction
Neutrophils are the most abundant leucocytes, comprising about 50–70% of all leucocytes.1 These cells play important roles in inflammation and innate immunity against most bacterial and fungal pathogens before the adaptive immunity and cell-mediated immunity are triggered.2 Neutrophils are rapidly recruited in large numbers to sites of acute injury or infection and act in a variety of antimicrobial effector functions such as phagocytosis and oxidant mechanisms against pathogens.3 Furthermore, neutrophils produce cytokines to initiate inflammatory responses and chemokines to induce trafficking of immune cells.4,5 These functions of neutrophils are initiated by pattern-recognition receptor-mediated recognition of pathogen-associated molecular patterns at cell surface or cytosol of host innate immune cells.
Toll-like receptors (TLRs) recognize various microbial molecules such as lipopolysaccharide (LPS), lipoprotein, flagellin and nucleic acids at the cell surface and endosomal membranes.6 In contrast, Nod (nucleotide-binding oligomerization domain) -like receptors (NLRs) mediate cytosolic recognition of microbial molecules. Nod1 and Nod2, the first members of the NLR family to be identified, recognize distinct sub-structures from bacterial peptidoglycan.7 Nod1 recognizes meso-diaminopomelic acid (meso-DAP), which is uniquely found in peptidoglycan from all Gram-negative bacteria and several Gram-positive bacteria such as Listeria and Bacillus spp. In contrast, Nod2 senses muramyl dipeptide (MDP), which is a conserved structure in peptidoglycan from all Gram-negative and Gram-positive bacteria.8–11 After recognition of their ligands, Nod1 and Nod2 directly recruit receptor interacting protein 2 (Rip2/RICK/CARDIAK), a caspase recruitment domain (CARD) -containing serine/threonine kinase through CARD–CARD interactions.12 Subsequently, Rip2 is conjugated with K63-linked polyubiquitin chains. This process leads to linking of transforming growth factor-β-activated kinase-1 to IκB kinase complex, which was essential for nuclear factor-κB (NF-κB) activation, resulting in the production of pro-inflammatory cytokines and chemokines.12 In addition, Nod1/Nod2–Rip2 signalling leads to the activation of mitogen-activated protein kinases (MAPKs) in host cells such as macrophages and mesothelial cells.13,14
The role of TLRs in the immune response of neutrophils has been widely studied, whereas the function of NLRs in neutrophils is not well-understood. Nod1 regulates the migration and phagocytic capacity of neutrophils isolated from pooled blood or bone marrow of mice.15,16 Nod1 ligation also leads to the activation of NF-κB and MAPKs in neutrophils.16 In addition, previous studies revealed that Nod2 is expressed on both human and mouse neutrophils17,18 and the Nod2 agonist MDP induces interleukin-8 (IL-8) production, CD62 ligand shedding, and CD11b up-regulation.17 However, it remains to be elucidated whether Nod2 and Rip2 are required for the MDP-induced immune response of neutrophils and whether Nod1 or Nod2 are involved in neutrophil immune responses in response to bacterial infection. We demonstrate here that Nod2 and its adaptor molecule Rip2 are essential for MDP-mediated immune responses in neutrophils, including the production of cytokines and chemokines and the activation of NF-κB and MAPKs. Moreover, Nod2 cooperates with TLRs to produce cytokines in neutrophils against live bacterial infection.
Materials and methods
Mice
Rip2-, Nod2-, and TLR4-deficient mice on C57BL/6 background were purchased from the Jackson Laboratories (Bar Harbor, ME). Mice with a combined deficiency of Nod2 and TLR4 were generated by interbreeding Nod2- and TLR4-deficient animals in our animal facility. Wild-type C57BL/6 mice were obtained from Koatech (Pyeongtaek, Korea). All animal studies were approved and followed by the regulations of the Institutional Animal Care and Use Committee in Konyang University (Daejeon, Korea).
Reagents and bacterial culture
The MDP was purchased from Bachem (Torrance, CA). Tri-DAP and Ultrapure LPS from Escherichia coli O111:B4 were purchased from InvivoGen (San Diego, CA). Single colonies of bacteria were inoculated into 5 ml of Luria–Bertani broth and grown overnight at 28° in the shaking incubator. A 1/5 dilution of the overnight culture was prepared and allowed to grow at 37° with shaking to A600 = 0·6, which corresponds to ∼ 109 colony-forming units/ml. After washing twice with PBS (pH 7·4), bacteria were diluted to the desired media concentrations and used in a further experiment.
Preparation of thioglycollate-elicited and bone marrow-derived neutrophils
Mouse peritoneal exudate neutrophils were isolated from the mouse peritoneal cavity as previously described.19 Mice were injected intraperitoneally with 2 ml of 4% thioglycollate broth (Sigma Aldrich, St Louis, MO) and peritoneal lavage was performed with 5 ml PBS 4 hr later. Lysis of red blood cells was carried out using red blood cell lysis buffer and total cell numbers were counted with a haemocytometer. Cell morphology was determined on cell monolayers prepared by Cytospin (Cellspin; Hanil Science Industrial, Incheon, Korea) and stained with Diff-Quik (Thermo Fisher Scientific, Pittsburgh, PA). For the preparation of neutrophils derived from bone marrow, mice were killed and their femur and tibia from both back legs were flushed with PBS supplemented with BSA and glucose (PBS-BG) to extract bone marrow. The bone marrow cells suspended with the 45% Percoll solution (GE Healthcare Life Science, Uppsala, Sweden) were overlaid on a four-layer Percoll gradient of 81%, 62%, 55% and 50% Percoll solution, respectively, diluted in PBS-BG, and centrifuged (600 g, 30 min, 10°) without braking. The neutrophils were between the 81% and 62% layer. The cells resuspended in PBS-BG solution were laid on top of the Histopaque-1119 (Sigma) again and centrifuged (500 g, 30 min, 10°) without braking. The neutrophils in the interface were used for bacterial infection.
Intracellular staining for Nod1, Nod2 and Rip2
For intracellular staining of Nod1, Nod2 and Rip2, neutrophils were washed with PBS containing 0·5% BSA, fixed and permeabilized with BD Cytofix/Cytoperm solution (BD Biosciences, San Jose, CA) for 30 min at 4°. Then the cells were stained with anti-mouse Nod1, Rip2 (Cell Signaling Technology, Beverly, MA), Nod2 (Santa Cruz Biotechnology, Santa Cruz, CA) or control IgG (Imgenex, San Jose, CA) for 30 min at 4°, followed by FITC-conjugated secondary antibody at 4° for another 30 min. The expression of Nod1, Nod2 and Rip2 was assessed by flow cytometry (BD FACSCalibur flow cytometer).
Bacterial infection
The cells were seeded in six-well or 48-well plates at a concentration of 4 × 105 or 8 × 105 cells and incubated in a 5% CO2 incubator at 37°. Subsequently, the cells were left uninfected or infected with bacteria at the indicated multiplicity of infection for 60 min and extracellular bacterial growth was inhibited by gentamicin (50 μg/ml) treatment. Culture supernatants were collected 24 hr after infection for cytokine measurement.
Immunoblotting
The cells were stimulated with MDP, harvested, and lysed in a buffer containing 1% Nonidet-P40 supplemented with a complete protease inhibitor ‘cocktail’ (Roche, Mannheim, Germany) and 2 mm dithiothreitol. Lysates were separated by 10% SDS–PAGE and were transferred to nitrocellulose (NC) membranes by electroblotting. The membranes were immunoblotted with primary antibodies, such as regular- and phospho-IκB-α, phospho-Jun N-terminal kinase (JNK) (Cell Signaling Technology,), phospho-p38, regular- and phospho-extracellular signal-regulated kinase (ERK) (Santa Cruz Biotechnology). After immunoblotting with secondary antibodies, proteins were detected with enhanced chemiluminescence reagent (Intron Biotechnology, Seong-Nam, Korea).
Cytokines measurement
The concentration of IL-6, tumour necrosis factor-α (TNF-α), IL-1β, CXCL1 and CCL2 in culture supernatants was determined using a commercial ELISA kit from R&D Systems (Minneapolis, MN).
l-Selectin shedding
To determine l-selectin shedding on neutrophils by MDP stimulation, neutrophils (4 × 106 cells) were treated with MDP (1 μg/ml) for 24 hr at 37°. After stimulation for 24 hr, collected cells were washed three times with PBS, and then resuspended in 0·5% BSA. The l-selectin shedding of neutrophils was analysed by flow cytometry (BD FACSCalibur flow cytometer).
Statistical analysis
The differences in mean values among different groups were tested, and the values were expressed as mean ± SD. All of the statistical calculations were performed by one or two-way analysis of variance with Bonferroni post-tests using graphpad prism version 5.00. Values of P < 0·05 were considered significant.
Results
Nod2 and Rip2, but not Nod1, are strongly expressed in thioglycollate-elicited neutrophils
We first determined the characteristic of thioglycollate-elicited mouse neutrophils used in this study. Flow cytometry analysis revealed that over 90% of cells were both CD11b+ and Ly6G+, known as markers expressed on neutrophils (see Supporting information, Fig. S1a). When stained with Diff-Quick solution after cytospin, most cells were multi-nucleated, which is a typical morphology of neutrophils (Fig. S1b). We also determined the intracellular protein expression of Nod1, Nod2 and their adaptor molecule Rip2 in mouse neutrophils. Nod1 expression was very low in the cells, whereas Nod2 and Rip2 proteins were strongly expressed (Fig. S1c,d).
MDP, but not Tri-DAP, induces the production of cytokines and chemokines in mouse neutrophils through Nod2- and Rip2-dependent manner
The activation of neutrophils with TLR agonists leads to the production of various cytokines and chemokines.20 We therefore examine whether Nod1 and Nod2 stimulation induces cytokine and chemokine production in peritoneal mouse neutrophils elicited by thioglycollate. Peritoneal neutrophils were treated with Tri-DAP (a Nod1 agonist), MDP (a Nod2 agonist) or LPS as a positive control for 24 hr and the level of IL-6, TNF-α, CXCL1 and CCL2 in culture supernatant was measured by ELISA. MDP treatment increased the production of all cytokines and chemokines tested in mouse neutrophils, whereas Tri-DAP did not (Fig. 1a–d), suggesting that Nod2 acts as a major cytosolic receptor to initiate immune response in neutrophils against bacterial infection. We next explored the contribution of Nod2 and Rip2 to the production of cytokines and chemokines by neutrophils in response to MDP. Consistent with results shown in Fig. 1, MDP induced the production of IL-6, TNF-α, CXCL1 and CCL2 in wild-type (WT) neutrophils which was abolished in Nod2- and Rip2-deficient neutrophils (Fig. 2a–d). In contrast, LPS produced a similar level of those cytokines and chemokines in WT, Nod2- and Rip2-deficient neutrophils (Fig. 2a–c). These findings indicate that Nod2 and Rip2 are required for cytokine and chemokine production elicited by MDP stimulation in neutrophils.
Figure 1.
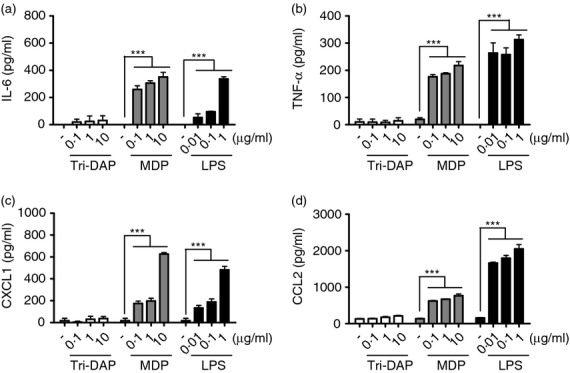
Muramyl-dipeptide (MDP), but not Tri-DAP, induces the production of cytokines and chemokines in mouse neutrophils. Thioglycollate-elicited neutrophils from wild-type (WT) mice were stimulated with Tri-DAP, MDP, and lipopolysaccharide (LPS) at the indicated doses (a–d). At 24 hr after stimulation, the concentrations of interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), CXCL1 and CCL2 in culture supernatant were determined by ELISA. The results are from one representative experiment of three independent experiments (***P < 0·001).
Figure 2.
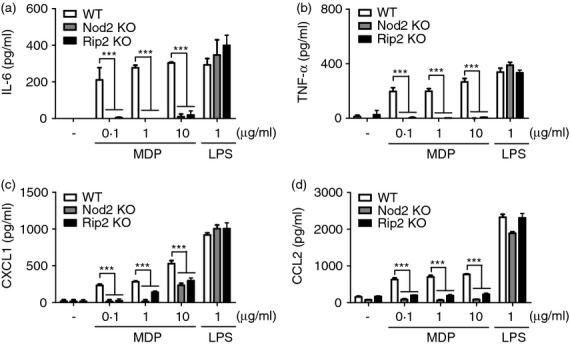
Nod2 and Rip2 are required for muramyl-dipeptide (MDP) -induced production of interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), CXCL1 and CCL2 in mouse neutrophils. Wild-type (WT), Nod2-, and Rip2-deficient neutrophils were stimulated with MDP and lipopolysaccharide (LPS) at the indicated doses. At 24 hr after stimulation, the concentrations of IL-6 (a), TNF-α (b), CXCL1 (c) and CCL2 (d) in culture supernatant were determined by ELISA. The results are from one representative experiment of three independent experiments (***P < 0·001).
Nod2 and Rip2 are required for MDP-induced activation of NF-κB and MAPKs in neutrophils
Stimulation with MDP leads to the activation of NF-κB and MAPKs in macrophages.13,21,22 To determine whether Nod2 and Rip2 contribute to NF-κB and MAPK activation by MDP in neutrophils, WT, Nod2- and Rip2-deficient neutrophils were stimulated with MDP and phosphorylation of IκB-α, p38, ERK and JNK was assessed by immunoblotting. MDP stimulation led to IκB-α degradation and phosphorylation in WT neutrophils at 30 and 60 min, respectively, but which was not observed in Nod2- and Rip2-deficient cells (Fig. 3). P38, JNK, and ERK MAPK phosphorylation was enhanced at 30 min after MDP stimulation in WT neutrophils, but not in Nod2- and Rip2-deficient cells (Fig. 3). These findings indicate that MDP leads to the activation of NF-κB and MAPKs in neutrophils via Nod2 and Rip2 signalling.
Figure 3.
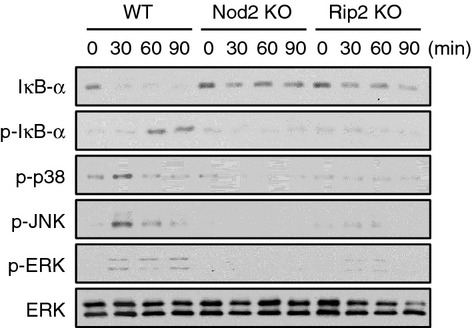
Muramyl-dipeptide (MDP) stimulates the activation of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) in wild-type (WT) neutrophils, but not in Nod2- and Rip2-deficient cells. WT, Nod2-, and Rip2-deficient neutrophils were stimulated with MDP (10 μg/ml) and cellular proteins from stimulated neutrophils were extracted at the indicated time-points. IκB-α degradation and the phosphorylation of IκB-α, p38, Jun N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) were examined by Western blotting. Primary antibody against total ERK was used to verify equal loading. The results are from one representative experiment of two independent experiments.
Nod2 and Rip2 mediate l-selectin shedding for MDP stimulation in mouse neutrophils
l-Selectin is rapidly shed from the surface of leucocytes upon activation and contributes to physiological leucocyte rolling.23,24 Accordingly, we examined whether MDP induces l-selectin shedding in mouse neutrophils and Nod2 and Rip are critical for this process. Flow cytometry analysis showed that l-selectin shedding was induced by MDP at 1 hr post stimulation in WT neutrophils (Fig. 4a, b). In contrast, MDP-induced L-selectin shedding was observed in neither Nod2-deficient nor Rip2-deficient neutrophils (Fig. 4a, b). Hence, MDP-induced l-selectin shedding is mediated via Nod2 and Rip2 in neutrophils.
Figure 4.
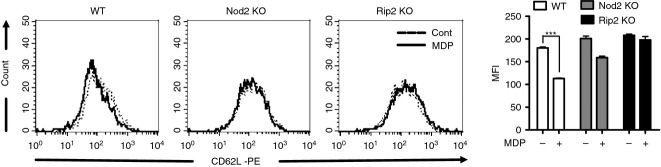
L-Selectin shedding is induced by muramyl-dipeptide (MDP) in wild-type (WT) neutrophils, but not in Nod2- and Rip2-deficient cells. For the experiment of L-selectin shedding, WT, Nod2-, and Rip2-deficient neutrophils were stimulated with MDP (10 μg/ml) for 1 hr. The cells were then collected in PBS and labelled with phycoerythrin-conjugated anti-CD62L antibody. MDP-treated (solid line) and untreated neutrophils (dotted line) were fixed and analysed by flow cytometry (a). The results in triplicates are expressed as mean ± SD (b).
MDP cooperates with TLR agonists to produce cytokines and chemokines in mouse neutrophils
Co-stimulation of TLR agonists and Nod1/Nod2 agonists synergistically increases cytokine production in immune cells.13,25 To determine whether MDP enhances TLR agonist-induced production of cytokines/chemokines in neutrophils, WT cells were stimulated with Pam3CSK4 (TLR2 agonist) and LPS (TLR4 agonist) in the absence or presence of MDP. At this time, IL-6 and CXCL1 were measured as representative cytokine and chemokine, respectively. Compared with treatment with each agonist alone, co-stimulation of MDP and TLR agonists (Pam3CSK4 or LPS) enhanced the secretion of IL-6 and CXCL1 in mouse neutrophils (Fig. 5a–d). These phenomena were confirmed in bone marrow-derived neutrophils. Unlike thioglycollate-elicited neutrophils, MDP alone did not induce any cytokine and chemokine production in bone marrow-derived neutrophils (see Supporting information, Fig. S2a–c). However, the production of IL-6, TNF-α and CCL2 induced by Pam3CSK4 or LPS was enhanced by MDP treatment (Fig. S2a–c). CXCL1 was below the detectable level (data not shown). In addition, the enhanced production of IL-6 by co-stimulation of MDP and TLR agonists was abolished in Nod2- and Rip2-deficient neutrophils (Fig. 5e, f). These findings indicate that Nod2–Rip2 signalling cooperates with TLRs to produce cytokines/chemokines in neutrophils.
Figure 5.
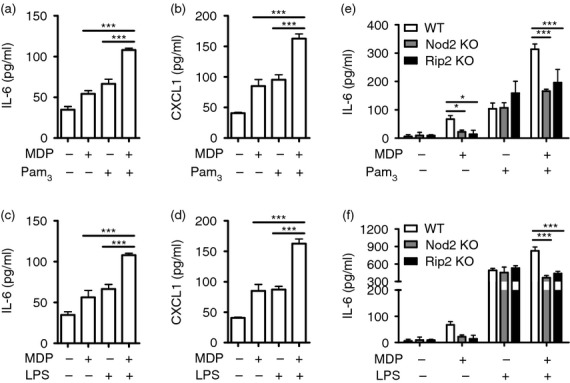
Muramyl-dipeptide (MDP) synergizes with Toll-like receptor (TLR) agonists to produce interleukin-6 (IL-6) and CXCL1 in wild-type (WT) neutrophils, but not Nod2- and Rip2-deficient cells. WT neutrophils were stimulated with MDP (1 μg/ml) and TLR agonists [Pam3CSK4 or lipopolysaccharide (LPS); 10 ng/ml] alone or in combination. At 24 hr after stimulation, the concentration of IL-6 and CXCL1 in culture supernatant was determined by ELISA. Likewise, IL-6 production by WT neutrophils was compared with that elicited in Nod2- and Rip2-deficient cells in response to MDP (e, f). The results are from one representative experiment of three independent experiments (*P < 0·05, ***P < 0·001).
Both Nod2 and TLR4 are required for optimal production of cytokines and chemokines in mouse neutrophils in response to Gram-negative bacteria
We next investigated whether Nod2 and Rip2 are involved in cytokine/chemokine production in neutrophils in response to infection with bacteria. To clarify this, neutrophils from WT and Rip2-deficient mice were infected with Yersinia pseudotuberculosis (Gram-negative bacteria) and Listeria monocytogenes (Gram-positive bacteria) and the amounts of IL-6 and CXCL1 were measured in culture supernatant. The results showed that Rip2 single deficiency did not alter the production of IL-6, TNF-α, CXCL1 and CCL2 in neutrophils in response to Y. pseudotuberculosis and L. monocytogenes (Fig. S3a–d). Subsequently, we sought to determine whether Nod2 cooperates with TLR4 to produce optimal cytokines and chemokines in neutrophils in response to Gram-negative bacteria. As expected, the levels of IL-6, TNF-α, CXCL1 and CCL2 produced by Gram-negative bacteria including Y. pseudotuberculosis, E. coli and Pseudomonas aeruginosa were not significantly different between WT and Nod2-deficient neutrophils (Fig. 6a–i). In contrast, at specific multiplicities of infection, TNF-α and CCL2 production were significantly decreased in Nod2/TLR4 double-deficient neutrophils in response to Y. pseudotuberculosis, compared with TLR4 singly deficient cells (Fig. 6b, d). Moreover, the production of IL-6, TNF-α, CXCL1 and CCL2 by E. coli and P. aeruginosa were significantly decreased in Nod2/TLR4 double-deficient neutrophils, compared with TLR4 singly deficient cells (Fig. 6a–i). These results indicate that Nod2 is required for optimal production of cytokines and chemokines in response to Gram-negative bacteria.
Figure 6.
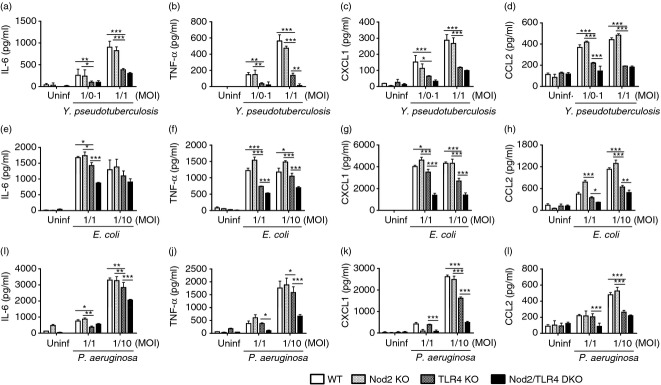
Nod2 and Toll-like receptor 4 (TLR4) cooperate to produce interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), CXCL1, and CCL2 by neutrophils in response to Gram-negative bacteria. For in vitro experimental infection, wild-type (WT), Nod2- and TLR4-, and Nod2/TLR4 double-deficient neutrophils were infected with Yersinia pseudotuberculosis (a–d), Escherichia coli (e–h) and Pseudomonas aeruginosa (i–l) at the indicated multiplicities of infection (MOI) and treated with gentamicin to inhibit extracellular bacterial growth 60 min after infection. At 6 hr (Y. pseudotuberculosis and P. aeruginosa) or 12 hr (E. coli) after infection, the concentration of IL-6, TNF-α, CXCL1 and CCL2 in culture supernatant was determined by ELISA. The results are from one representative experiment of three independent experiments (*P < 0·05, **P < 0·01 and *** P < 0·001).
Discussion
Although TLR signalling and function have been well-studied in neutrophils, only a few studies have examined the role of Nod1 and Nod2 in innate immune responses in these cells. In the present study, Nod2 was strongly expressed in thioglycollate-elicited mouse neutrophils and its agonist MDP induced the production of cytokines and chemokines, whereas Nod1 expression was very low and its stimulation with Tri-DAP did not produce any cytokines and chemokines tested. These phenomena are similarly found in human neutrophils.17 Nevertheless, the function of Nod1 in neutrophils should not be overlooked. Clarke et al.15 showed that Nod1 is essential for bacterial killing by neutrophils. They concluded that Nod1 recognizes microbiota-derived peptidoglycan in neutrophils, which enhances the ability of neutrophils to kill bacteria. Indeed, bacterial killing ability was reduced in neutrophils from germ-free mice or antibiotic-treated mice, as compared with specific pathogen-free or untreated WT mice.15 Dharancy et al.16 also showed that Nod1 regulates the migration and phagocytic capacity of neutrophils isolated from pooled blood or bone marrow of mice. Although Nod1 was not expressed in human neutrophils and its agonist (iE-DAP) did not induce IL-8 production,17 further studies should be performed to determine whether Nod1 is functional in human neutrophils or not. It has been reported that Nod1 and Nod2 are functional in various cells, although their agonists alone could not induce the production of cytokines or chemokines. Muramyl-dipeptide alone does not lead to the production of cytokines in murine bone marrow-derived macrophages, it can activate NF-κB and MAPKs in the same cells and synergizes with TLR agonists to produce cytokines.13,26 Likewise, Nod1, Nod2 and Rip2 are expressed in hepatocytes, but only iE-DAP could induce chemokine (KC and RANTES) production and NF-κB activation.27 Interestingly, although MDP alone could not induce any cytokine/chemokine responses in hepatocytes, MDP induced NF-κB activation in the cells pre-treated with TLR agonists such as LPS and Poly I:C.27 These phenomena were similarly observed in our previous study using prostate epithelial cells.28 These findings suggest that even though Nod1 or Nod2 agonists alone do not produce cytokines and chemokines in some cell types, they can induce the activation of downstream signals or mediate other functions such as phagocytosis. In the present study, we revealed that MDP alone up-regulates the production of cytokines or chemokines in mouse neutrophils via the Nod2–Rip2 pathway. In addition, a recent study by Oh et al.29 showed that MDP induces secretion of IL-1β and IL-10 in mouse peritoneal neutrophils via a Nod2-dependent pathway. Therefore, Nod2–Rip2 signalling may contribute to immune responses such as cytokine/chemokine production in neutrophils against bacterial infection.
Initial studies suggested that Rip2/RICK was essential for TLR-mediated innate and adaptive immune responses.30,31 Furthermore, LPS stimulation enhanced the protein expression of Rip2 in macrophages.26,32 Moreover, cytokine production by TLR agonists and LPS-induced activation of NF-κB and MAPKs were impaired in Rip2-deficient macrophages and survival from endotoxin shock was improved in Rip2-deficient mice.30 However, it was subsequently revealed that LPS preparations used to examine the role of Rip2 in mice were contaminated with peptidoglycan and specifically Nod1 and Nod2 agonists.8,13 Careful studies demonstrated that Rip2 is required for Nod1- and Nod2-mediated signalling in various cells such as macrophages and mesothelial cells, but not TLR signalling.13,14 In this study, cytokine/chemokine production and activation of NF-κB and MAPKs by MDP was abolished in Rip2-deficient neutrophils, whereas LPS responses were comparable to that observed in WT neutrophils, indicating that Rip2 is a critical molecule in the MDP-induced immune response in neutrophils, but is not involved in LPS-mediated signalling.
Rip2 appears to differentially mediate the immune response depending on bacterial and host cell types. In macrophages, Nod1 and Nod2 exert a redundant function in the production of cytokines in response to L. monocytogenes.13 Although IL-6 and TNF-α production induced by bacterial infection was not different between WT and Nod1- or Nod2-deficient macrophages, the cytokine production was down-regulated in Rip2- and Nod1/2 double-deficient macrophages.13 However, in the present study, both WT and Rip2-deficient neutrophils secreted comparable levels of IL-6 and CXCL1 in response to Gram-positive (Listeria) as well as Gram-negative (Yersinia) bacteria. Because Rip2 is dispensable for cytokine production in macrophages infected with Gram-negative bacteria,33 the results suggest that the role of TLRs in inducing cytokines/chemokines is dominant in neutrophils and therefore no redundant effect of Nod1 and Nod2 can be observed. In addition, Kim et al.26 showed that Nod1/Nod2 signalling plays an important role in host immune responses in macrophages tolerized to TLR stimulation or in mice pre-treated with LPS. Consistently, a role of Rip2 in the cytokine response elicited by Gram-negative bacterial infection could be revealed in macrophages tolerized to TLR stimulation.33 Furthermore, Rip2 inhibitors (SB203580, PP2, and Gefitinib) reduced IL-6 production in TLR4-deficient macrophages, but not in WT cells, in response to Gram-negative Y. enterocolitica.34 These findings suggest that Nod2 and Rip2 may play an important, but redundant, role in immune response against Gram-negative bacteria. In this study, we clearly revealed that Nod2 cooperates with TLR4 to produce cytokines/chemokines in neutrophils in response to Gram-negative bacteria.
In conclusion, our study showed that MDP, a bacterial peptidoglycan molecule, induces an immune response in mouse neutrophils via a Nod2–Rip2-dependent pathway. In addition, Nod2 plays an important role for optimal production of cytokines in neutrophils in response to live bacteria, which is revealed in TLR-deficient neutrophils. Further studies are needed to clarify the role of Nod2 and Rip2 within neutrophils in the regulation of the immune response in vivo against pathogenic bacteria.
Acknowledgments
This study was supported by grants from Agency for Defense Development (No. ADD-12-70-06-01), National Research Foundation of Korea (NRF-2012R1A1A2041944), and DK61707 from the National Institutes of Health.
Conflicts of interest
The authors declare no financial or commercial conflict of interest.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Characterization of thioglycollate-elicited neutrophils from the peritoneal cavity of mice.
Figure S2. Muramyl-dipeptide (MDP) synergizes with Pam3CSK4 or lipopolysaccharide (LPS) to produce interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and CCL2 in neutrophils isolated from bone marrow of mice.
Figure S3. Yersinia enterocolitica and Listeria monocytogenes-induced cytokine and chemokine production is unimpaired in Rip2-deficient neutrophils.
References
- 1.Freitas M, Lima JL, Fernandes E. Optical probes for detection and quantification of neutrophils' oxidative burst. A review. Anal Chim Acta. 2009;649:8–23. doi: 10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 2.Malech HL. The role of neutrophils in the immune system: an overview. Methods Mol Biol. 2007;412:3–11. doi: 10.1007/978-1-59745-467-4_1. [DOI] [PubMed] [Google Scholar]
- 3.Yamashiro S, Kamohara H, Wang JM, Yang D, Gong WH, Yoshimura T. Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses. J Leukoc Biol. 2001;69:698–704. [PubMed] [Google Scholar]
- 4.Thomas CJ, Schroder K. Pattern recognition receptor function in neutrophils. Trends Immunol. 2013;34:317–28. doi: 10.1016/j.it.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Inohara C, McDonald C, Nunez G. NOD-LRR proteins: role in host–microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–83. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 8.Inohara N, Ogura Y, Fontalba A, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–12. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 9.Chamaillard M, Hashimoto M, Horie Y, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4:702–7. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 10.Girardin SE, Boneca IG, Carneiro LA, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300:1584–7. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 11.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–72. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa M, Fujimoto Y, Lucas PC, Nakano H, Fukase K, Nunez G, Inohara N. A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J. 2008;27:373–83. doi: 10.1038/sj.emboj.7601962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Núñez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–6. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Kim YG, Shaw M, Kanneganti TD, Fujimoto Y, Fukase K, Inohara N, Núñez G. Nod1/RICK and TLR signaling regulate chemokine and antimicrobial innate immune responses in mesothelial cells. J Immunol. 2007;179:514–21. doi: 10.4049/jimmunol.179.1.514. [DOI] [PubMed] [Google Scholar]
- 15.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–31. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dharancy S, Body-Malapel M, Louvet A, et al. Neutrophil migration during liver injury is under nucleotide-binding oligomerization domain 1 control. Gastroenterology. 2010;138:1546–56. doi: 10.1053/j.gastro.2009.12.008. 56 e1-5. [DOI] [PubMed] [Google Scholar]
- 17.Ekman AK, Cardell LO. The expression and function of Nod-like receptors in neutrophils. Immunology. 2010;130:55–63. doi: 10.1111/j.1365-2567.2009.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho JS, Guo Y, Ramos RI, et al. Neutrophil-derived IL-1β is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 2012;8:e1003047. doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forlow SB, Ley K. Selectin-independent leukocyte rolling and adhesion in mice deficient in E-, P-, and L-selectin and ICAM-1. Am J Physiol Heart Circ Physiol. 2001;280:H634–41. doi: 10.1152/ajpheart.2001.280.2.H634. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Moran T, Swanson E, et al. Regulation of IL-8 and IL-1β expression in Crohn's disease associated NOD2/CARD15 mutations. Hum Mol Genet. 2004;13:1715–25. doi: 10.1093/hmg/ddh182. [DOI] [PubMed] [Google Scholar]
- 22.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1β processing. Science. 2005;307:734–8. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 23.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–75. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–41. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 25.Fritz JH, Girardin SE, Fitting C, et al. Synergistic stimulation of human monocytes and dendritic cells by Toll-like receptor 4 and NOD1- and NOD2-activating agonists. Eur J Immunol. 2005;35:2459–70. doi: 10.1002/eji.200526286. [DOI] [PubMed] [Google Scholar]
- 26.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Nunez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–57. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Scott MJ, Chen C, Sun Q, Billiar TR. Hepatocytes express functional NOD1 and NOD2 receptors: a role for NOD1 in hepatocyte CC and CXC chemokine production. J Hepatol. 2010;53:693–701. doi: 10.1016/j.jhep.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang MJ, Heo SK, Song EJ, Kim DJ, Han SY, Han JH, Kim BY, Park JH. Activation of Nod1 and Nod2 induces innate immune responses of prostate epithelial cells. Prostate. 2012;72:1351–8. doi: 10.1002/pros.22483. [DOI] [PubMed] [Google Scholar]
- 29.Oh SJ, Kim JH, Chung DH. NOD2-mediated suppression of CD55 on neutrophils enhances C5a generation during polymicrobial sepsis. PLoS Pathog. 2013;9:e1003351. doi: 10.1371/journal.ppat.1003351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–9. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 31.Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y, Cheng G. Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature. 2002;416:190–4. doi: 10.1038/416190a. [DOI] [PubMed] [Google Scholar]
- 32.Lu C, Wang A, Dorsch M, et al. Participation of Rip2 in lipopolysaccharide signaling is independent of its kinase activity. J Biol Chem. 2005;280:16278–83. doi: 10.1074/jbc.M410114200. [DOI] [PubMed] [Google Scholar]
- 33.Park JH, Kim YG, Nunez G. RICK promotes inflammation and lethality after gram-negative bacterial infection in mice stimulated with lipopolysaccharide. Infect Immun. 2009;77:1569–78. doi: 10.1128/IAI.01505-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong YJ, Kim CH, Kim JC, Oh SM, Lee KB, Park JH, Kim DJ. RIP2/RICK-dependent cytokine production upon Yersinia enterocolitica infection in macrophages with TLR4 deficiency. Scand J Immunol. 2013;78:401–7. doi: 10.1111/sji.12100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of thioglycollate-elicited neutrophils from the peritoneal cavity of mice.
Figure S2. Muramyl-dipeptide (MDP) synergizes with Pam3CSK4 or lipopolysaccharide (LPS) to produce interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and CCL2 in neutrophils isolated from bone marrow of mice.
Figure S3. Yersinia enterocolitica and Listeria monocytogenes-induced cytokine and chemokine production is unimpaired in Rip2-deficient neutrophils.


