Abstract
Prostaglandin E2 (PGE2) is an important inducer of inflammation, which is also closely linked to the progress of tumours. In macrophages, PGE2 production is regulated by arachidonic acid release and cyclooxygenase-2 (COX-2) expression. In the present study, we found that COX-2 expression can be achieved by activating Ca2+/Calmodulin (CaM)-dependent protein kinase II (CaMKII) and cAMP-response element-binding protein (CREB) in rat peritoneal macrophages. Our results indicated that lipopolysaccharide and PMA could elicit the transient increase of the concentration of intracellular free calcium ions ([Ca2+]i), which induced activation of CaMKs with the presence of CaM. The subtype of CaMKs, CaMKII, then triggered the activation of CREB, which elevated COX-2 expression and PGE2 production in a chronological order. These results suggested that Ca2+/CaM-dependent CaMKII plays an important role in mediating COX-2 expression and PGE2 production by activating CREB in macrophages. The study also provides more useful information to clarify the mechanism of calcium regulation of PGE2 production, which plays an essential role in inflammation and cancers.
Keywords: calcium, calmodulin, calmodulin-dependent kinase, cyclooxygenase-2, cAMP-response element-binding protein, prostaglandin E2
Introduction
Macrophages play an important role in innate and adaptive immune responses, which serve as the first line of host defence against pathogenic or inflammatory challenge. In response to stimulants, macrophages produce numerous pro-inflammatory cytokines and secondary mediators, which are pivotal for the innate and adaptive immune responses.1,2 Uncontrolled expression of the substances can initiate septic shock syndrome, which is characterized as fever, hypotension, disseminated intravascular coagulation and multiple organ failure.3,4 Among these factors, prostaglandin E2 (PGE2) is an essential mediator that contributes to vasodilatation, pain and fever.5,6 Additionally, PGE2 is also overproduced in many tumours, where it aids cancer progression by promoting angiogenesis and metastasis.7,8
In macrophages, PGE2 production is tightly controlled by arachidonic acid (AA) release and cyclooxygenase-2 (COX-2) expression.9,10 For AA release, the activation of calcium-dependent cytosolic phospholipase A2 (cPLA2) plays an essential role, which catalyses the hydrolysis of membrane phospholipid into AA.11,12 In turn, COX-2 converts AA into PGE2.13,14 To achieve its full activation, cPLA2 needs to binds to Ca2+ and be phosphorylated at the serine residues. Usually, the phosphorylation of cPLA2 at its serine residues is mediated by protein kinases, including mitogen-activated protein kinase (MAPK) and MAPK-interacting kinase. The binding of Ca2+ to the N-terminal C2 domain of cPLA2 induces the translocation of cPLA2 from the cytosol to the plasma membrane, where it catalyses the conversion of AA into PGE2.15–17 On the other hand, COX-2 expression is associated with the activation of transcription factors, such as nuclear factor-κB, nuclear factor of activated T-cell, cAMP-response element binding protein (CREB) and CCAAT/enhancer binding protein β (c/EBPβ).18–20 Among these transcription factors, CREB and c/EBPβ are essential for both basal transcription and the induction of COX-2.20 It has been reported that c/EBPβ is phosphorylated and activated by calmodulin (CaM)-dependent protein kinases (CaMK) in response to the increase of intracellular free calcium ion concentration ([Ca2+]i). The phosphorylation at serine 276 within the leucine zipper of c/EBPβ appears to confer calcium-regulated transcriptional activation of promoters containing binding sites for c/EBPβ.21 Different from c/EBPβ activation, the activation of CREB is achieved through its phosphorylation at serine 133, which might be regulated by cAMP-dependent protein kinase A and protein kinase C.22–,26 However, to our knowledge, no report shows that calcium-dependent CaMK mediates CREB activation and thereby regulates COX-2 expression and PGE2 production in primary macrophages.
In the present study, we sought to investigate whether calcium-dependent CaMK activation was involved in regulating COX-2 expression and PGE2 production by activating CREB in primary rat peritoneal macrophages. The results indicated that lipopolysaccharide (LPS) or PMA induced the transient increase of [Ca2+]i, activated CaMKII, CaMKIV and CREB, and increased COX-2 expression and PGE2 production in chronological order. Inhibitor treatment or small interfering RNA (siRNA) knocking-down of CREB blocked LPS- or PMA-induced increase of COX-2 expression and PGE2 production. When intracellular or intercellular free calcium ion was chelated with 1,2-bis (2-amino-5-fluorophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl) ester (BAPTA-AM) or ethylene glycol-bis-(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), LPS- or PMA-induced activation of CaMKs and CREB was blocked. Meanwhile, increased COX-2 expression and PGE2 production were also inhibited. Furthermore, CaM inhibitor or siRNA treatment had a similar effect to BAPTA-AM and EGTA pre-treatment. Additionally, CaMKII inhibitor or siRNA treatment blocked LPS- or PMA-induced CREB activation, which led to the inhibition of the increase of COX-2 expression and PGE2 production. Hence, these results demonstrate that Ca2+/CaM-dependent CaMKII regulates COX-2 expression and PGE2 production by activating CREB in primary rat peritoneal macrophages.
Materials and methods
Regents
Lipopolysaccharide (Escherichia coli serotype 0127:B8 prepared by phenol extraction), PMA, EGTA and Fura-2 acetoxymethyl ester (Fura-2) were purchased from Sigma (St Louis, MO). KN93 (CaMK inhibitor) and K92 (KN93 analogue; as a control of KN93) were obtained from Calbiochem (San Diego, CA). BAPTA-AM (intracellular Ca2+ chelator) was produced by Molecular Probes (Eugene, OR). The 20–25-nucleotide siRNAs of CREB, CaM, CaMKIV and CaMKII and the antagonist of CaM, A7 hydrochloride, were purchased from Santa Cruz Biotechnology (Santa Crus, CA).
Isolation and culture of rat peritoneal macrophages
Male Wistar rats (∼250 g) were treated humanely in compliance with institutional guidelines. The rats were killed by anaesthesia and PBS was then injected into the abdomen. PBS-containing macrophages were then collected and centrifuged at 500 g for 5 min. Next, the cell pellet was resuspended in RPMI-1640 with HEPES buffer (20 mm); non-essential amino-acid solution (1×); l-glutamine (2 mm); 10% heat-inactivated, defined fetal bovine serum and antibiotics. After 6 hr of incubation at 37° with 5% CO2, adherent cells were collected and incubated for another 48 hr for further use. Non-specific esterase staining showed that > 95% of the adherent cells were macrophages.
Measurement of [Ca2+]i in macrophages
[Ca2+]i was detected by the ratiometric fluorescent Ca2+ indicator dye Fura-2 and microspectrofluorometer as described before.27 Macrophages were incubated with 3 μm Fura-2/AM for 50 min at room temperature and then washed twice with PBS. Changes in fluorescence intensity of Fura-2 at excitation wavelengths of 340 and 380 nm and the emission wavelength of 510 nm were monitored in an individual macrophage. The concentration of [Ca2+]i was calculated by:
Kd was the constant for Fura-2 chelating Ca2+. Its value was 135 nm when the temperature was 22°.
Measurement of PGE2 production
Macrophages (106 cells/ml) were incubated in a 24-well plate at 37° with 5% CO2. The supernatant was collected by centrifugation at 500 g for 5 min at 4°. PGE2 concentrations in the supernatant were detected using a high sensitivity PGE2 Enzyme Immunoassay Kit (Assay Designs Inc., Farmingdale, NY) following the manufacturer-provided protocol.
Whole cell protein extraction
Whole cell protein was isolated from macrophages as described previously.27–29 Briefly, cultured macrophages (107 cells/sample) were collected and washed three times with ice-cold PBS. The cell pellet was then resuspended in 200 μl ice-cold lysis buffer [20 mm 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid, 1% (v/v) Triton X-100, 0·4% (w/v) SDS, 1 mm EDTA, 50 mm sodium fluoride, 2·5 mm p-nitrophenyl phosphate, 1 mm sodium orthovanadate, 10 μg/ml leupeptin, 10% (v/v) glycerol, 1 mm PMSF, and a protease and phosphatase inhibitor mixture containing 1 μg/ml anti-pain, 1 μg/ml leupeptin, 1 μg/ml pepstatin and 10 μg/ml soybean trypsin inhibitor, pH 7·3] and passed through a 200-μl pipette tip. After incubation on ice for 15 min, the cells were centrifuged at 12 000 g for 10 min at 4°. The supernatant (whole cell protein) was collected and stored at −80° for further use.
Immunoblotting
The whole cell proteins were separated by SDS–PAGE (12%) and transferred to a nitrocellulose membrane, which was then blocked in Tris-buffered saline with 0·1% Tween-20 (TBST) with 5% non-fat milk for 2 hr at room temperature. Next, the membrane was incubated with the primary antibody [rabbit anti-rat CaMKI (Abcam, Cambridge, MA), rabbit anti-rat phosphorylated CaMKI (Thr-177) (Abcam), rabbit anti-rat activated phosphothreonine CaMKII (Thr-286) (Abcam), rabbit anti-rat CaMKII (Abcam), rabbit anti-rat activated phosphothreonine CaMKIV (Thr-196 and Thr-200) (Abcam), rabbit anti-rat CaMKIV (Abcam), rabbit anti-rat CaMKV (Abcam), rabbit anti-rat CREB (Abcam), rabbit anti-rat activated phosphoserine CREB (Ser-133) (Abcam), rabbit anti-rat CaM (Abcam), or rabbit anti-rat COX-2 (Abcam), rabbit anti-rat C/EBPβ (Abcam), rabbit anti-rat activated phosphothreonine C/EBPβ (Thr-188 and Thr-235) (Abcam)] in TBST with 5% non-fat milk at 4° overnight. As the loading control, β-actin was detected by using rabbit anti-rat β-actin (Abcam) as the primary antibody. After washing three times with TBST with 5% non-fat milk, the horseradish peroxidase-conjugated secondary antibody specific for rabbit immunoglobulin (Amersham Biosciences, Pittsburgh, PA) was added to the membrane and incubated at room temperature for 2 hr. Next, the membrane was washed with TBST with 5% non-fat milk and developed using a Novex® ECL chemiluminescence substrate reagent kit (Invitrogen, Grand Island, NY). The immunoblot was semi-quantified using a ChemiDoc XRS camera in the chemiluminescence mode.
RNA interference assay
Macrophages were seeded in 10 ml of antibiotic-free medium with 10% fetal bovine serum and incubated for 24 hr at 37° with 5% CO2. An siRNA duplex solution [6 μl of non-targeting siRNA, CREB siRNA, CaM siRNA, CaMKIV siRNA or CaMKII siRNA in 100 μl siRNA transfection medium (Santa Cruz Biotechnology)] was directly added to the diluted transfection reagent [6 μl of siRNA transfection reagent (Santa Cruz Biotechnology) in 100 μl siRNA transfection medium], mixed and incubated for 30 min at room temperature to obtain the work solution. Next, 4 ml of siRNA transfection medium was added to each tube of work solution and mixed gently. After washing the cells with siRNA transfection medium, the mixture was added to the washed cells and incubated for 8 hr at 37° with 5% CO2. Then, 5 ml of growth medium containing serum and antibiotics was added without removing the transfection medium and incubated for 24 hr. At last, the medium was aspirated and replaced with fresh normal growth medium. The macrophages were incubated to check the expression of mRNA and protein or for further usage.
Real-time quantitative reverse transcription PCR assay for mRNA expression analysis
Macrophages were collected to isolate total RNA using RNA-STAT60 (Tel-Test, Friendswood, TX) according to the manufacturer's protocol, except that following precipitation of the RNA with isopropanol, the centrifugation time was increased to 45 min. The resulting RNA preparation was treated with DNase I (DNA-free; Ambion, Inc., Grand Island, NY) to remove contaminating DNA. Purified RNA was reverse-transcribed into cDNA using oligo(dT) (Invitrogen) and SuperScript III reverse transcriptase as directed. Subsequently, the cDNA was used as template to run real-time quantitative PCR (qPCR) for COX-2 (Applied Biosystems, Grand Island, NY; FAM-labelled; Lot no.: Rn01483828_m1), CaM (Applied Biosystem; FAM-labelled; Lot no.: Rn01487166_s1), CaMKI (Applied Biosystem; FAM-labelled; Lot no.: Rn00593272_m1), CaMKII (Applied Biosystem; FAM-labelled; Lot no.: Rn00572627_m1), CaMKIV (Applied Biosystem; FAM-labelled; Lot no.: Rn01405585_m1), CaMKV (Applied Biosystem; FAM-labelled; Lot no.: Rn00577017_m1), CREB (Applied Biosystem; FAM-labelled; Lot no.: Rn00578829_g1), and GAPDH (Applied Biosystem; VIC-TAMRA-labelled; Lot no.: 4352340E) with TaqMan Universal PCR Master Mix. PCR conditions were as follows: one 2-min cycle at 50°, one 10-min cycle at 95°; followed by 40 cycles of 15 seconds at 95°, and 1 min at 60° in an ABI 7500 thermocycler. The primers were used at 900 nm and the probe at 250 nm. The mRNA expression of specific gene was normalized to the mRNA expression of GAPDH and compared with the control group, which was arbitrarily set as a value ‘1’.
Statistical analysis
Analysis of data was performed using a two-tailed Student's t-test. Values of P < 0·05 were considered significant. All assays were repeated at least five times, and representative results were shown. Some results were demonstrated as the mean ± standard error of mean (SEM). Unless specifically stated, the error bars indicate the SEM.
Results
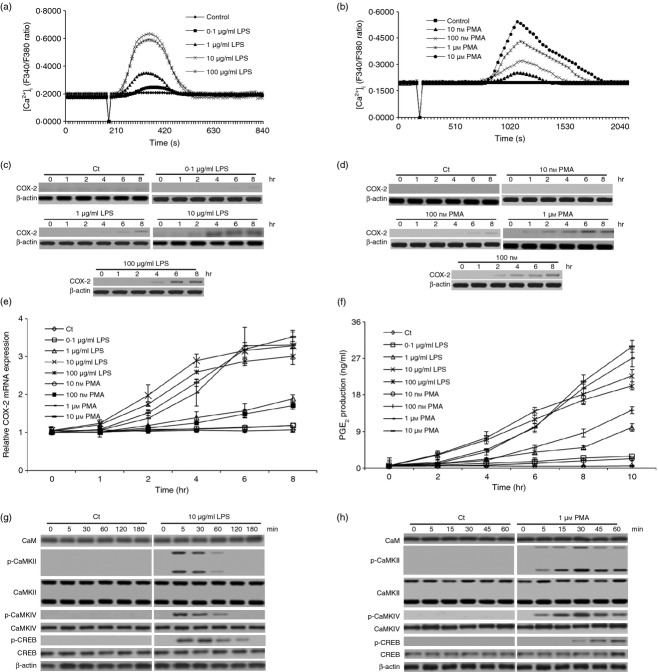
At the inflammation site, macrophages produce numerous factors, which play essential roles in triggering the initiation of septic shock syndrome.30 Among these factors, PGE2 is one of the most important, which is also linked to the progress of tumours.31 Because COX-2 expression is controlled by the c/EBPβ and CREB signalling pathways in macrophage cell line20 and Ca2+-dependent CaMKs play an important role in mediating c/EBPβ activation in pituitary cells,21 in the present study, we sought to investigate whether Ca2+-dependent CaMKs also played an essential role in regulating CREB activation, which mediated COX-2 expression and PGE2 production in primary macrophages. Primary rat peritoneal macrophages were therefore isolated and treated with LPS or PMA to detect [Ca2+]i changes, COX-2 expression and PGE2 production. Meanwhile, we also measured the expression of CaM and analysed the expression and activation of CaMKII, CaMKIV and CREB. As expected, both LPS (0·1, 1, 10 or 100 μg/ml) and PMA (0·01, 0·1, 1 or 10 μm) elicited the transient increase of [Ca2+]i without affecting the viability of these macrophages (Fig. 1a,b; see Supporting information, Fig. S1a). LPS induced a transient increase of [Ca2+]i within 2 min while PMA did so after 10 min of treatment. Moreover, LPS steadily increased COX-2 expression and PGE2 production after 2 hr of treatment (P < 0·001). PMA also increased COX-2 expression and PGE2 production (P < 0·001), which was slightly later than that induced by LPS (Fig. 1c–f). Because 10 μg/ml LPS and 1 μm PMA efficiently induced the transient increase of [Ca2+]i and increased COX-2 expression and PGE2 production, and they also had no obvious effect on the viability of the macrophages, we selected these concentrations for the remainder of our experiments. Our results also indicated that LPS elicited CaMKII, CaMKIV and CREB phosphorylation within 5 min, which reached their peak around 5, 5 and 30 min, respectively (Fig. 1g,h). Slightly different from LPS, PMA triggered the phosphorylation of CaMKII and CaMKIV within 5 min and regulated the phosphorylation of CREB within 30 min, which reached their peak around 30, 30 and 60 min, respectively (Fig. 1g,h). Additionally, LPS and PMA had no obvious effect on the expression of CaM, CaMKII, CaMKIV and CREB (Fig. 1g,h, Fig. S1b–e). Notably, the protein and mRNA expression of the other two CaMKs, CaMKI and CaMV, were not detected in the rat peritoneal macrophages in our experiment (data not shown). Taken together, these results suggested that LPS- or PMA-induced [Ca2+]i transient increase, CaMKs activation, CREB activation, COX-2 expression and PGE2 production happened in a chronological order, which might suggest that COX-2 expression and PGE2 production are regulated through the calcium-dependent CaMK–CREB signalling pathway.
Figure 1.
Lipopolysaccharide (LPS) and PMA elicited transient [Ca2+]i increase, induced calmodulin-dependent protein kinases (CaMKs) and cAMP-response element binding protein (CREB) activation, and increased cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production in rat peritoneal macrophages. (a, b) the change of [Ca2+]i was measured in rat peritoneal macrophages stimulated with LPS or PMA. The arbitrary zero of F340/F380 ratio indicated the time-point when LPS or PMA was applied. (c, d) the expression of COX-2 was detected by immunoblotting in these macrophages. (e, f) COX-2 mRNA expression and PGE2 production were also detected in these cells by quantitative PCR and ELISA respectively. (g, h) the expression and phosphorylation of CaM, CaMKII, CaMKIV and CREB were also detected by immunoblotting in these macrophages. The antibodies were specific for CaM, CaMKII, phoshporylated CaMKII, CaMKIV, phoshporylated CaMKIV, CREB, phoshporylated CREB and COX-2. β-actin was measured as a loading control.
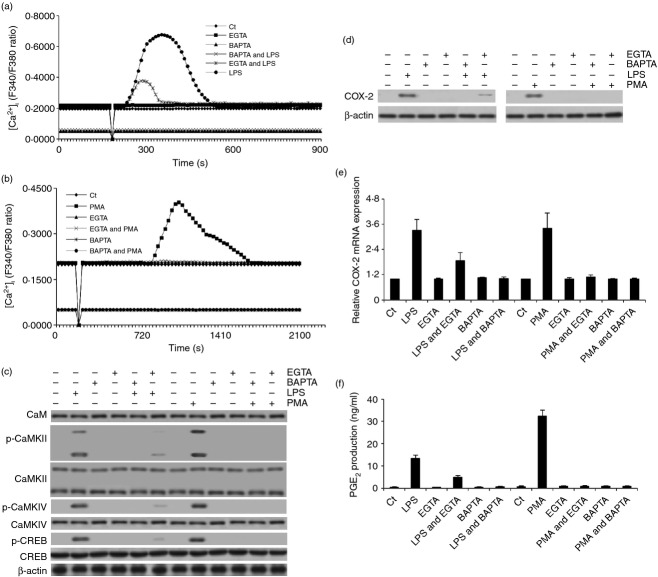
To further clarify the essential role of Ca2+ in this process, the macrophages were pre-treated with 5 mm EGTA (intercellular Ca2+ chelator; enough to eliminate the intercellular Ca2+, which is < 3 mm in the culture media) for 5 min or 5 μm BAPTA-AM (intracellular Ca2+ chelator; enough to eliminate the intracellular Ca2+, which is < 0·5 μm in the cytosol) for 30 min to remove intercellular or intracellular free calcium ions and then LPS or PMA was added to assess [Ca2+]i change, COX-2 expression and PGE2 production. The results demonstrated that EGTA pre-treatment blocked LPS-induced transient increase of [Ca2+]i partially and PMA-induced transient increase of [Ca2+]i completely. BAPTA-AM pre-treatment completely inhibited LPS- and PMA-elicited transient increase of [Ca2+]i and lowered the absolute concentration of [Ca2+]i (Fig. 2a,b). Because LPS- and PMA-induced CaMKs activation, CREB activation, COX-2 expression and PGE2 production reached their peaks at different time-points (5 and 30 min for CaMK activation, 30 and 60 min for CREB activation, 6 and 8 hr for COX-2 expression, and 6 and 8 hr for PGE2 production in response to the stimulation of LPS and PMA, respectively), we selected these time-points to detect CaMKs and CREB activation, COX-2 expression and PGE2 production for the remainder of our experiments. Consistently with the change of [Ca2+]i, EGTA eliminated LPS- or PMA-induced increased COX-2 expression and PGE2 production partially or completely, respectively, while BAPTA-AM pre-treatment completely blocked these two events (P < 0·001) (Fig. 2d–f). Moreover, BAPTA-AM pre-treatment also completely blocked LPS- or PMA-induced CaMKII, CaMKIV and CREB phosphorylation (Fig. 2c). In slight contrast to BAPTA-AM, EGTA completely inhibited PMA-induced CaMKII, CaMKIV and CREB phosphorylation whereas it partially blocked these events induced by LPS (Fig. 2c). Meanwhile, we did not detect an obvious effect of EGTA or BAPTA-AM on the expression of CaM, CaMKII, CaMKIV and CREB (Fig. 2c; see Supporting information, Fig. S2b,c). EGTA and BAPTA-AM pre-treatment also did not change the viability of these macrophages (Fig. S2a). Hence, these results suggested that the transient increase of [Ca2+]i was the upstream event of CaMK activation, CREB activation, COX-2 expression and PGE2 production.
Figure 2.
EGTA and BAPTA blocked lipopolysaccharide (LPS) or PMA-induced transient [Ca2+]i increase, calmodulin-dependent protein kinases (CaMKs) and cAMP-response element binding protein (CREB) activation, and cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production. (a, b) the effect of LPS and PMA on the change of [Ca2+]i was analysed in EGTA- or BAPTA-pre-treated rat peritoneal macrophage. The arbitrary zero of F340/F380 ratio indicated the time-point when LPS or PMA was added. (c, d) the effect of LPS or PMA on the expression or phosphorylation of CaM, CaMKII, CaMKIV, CREB and COX-2 was also detected by immunoblotting in EGTA- or BAPTA-pre-treated macrophages. (e, f) COX-2 mRNA expression and PGE2 production were also measured in these macrophages.
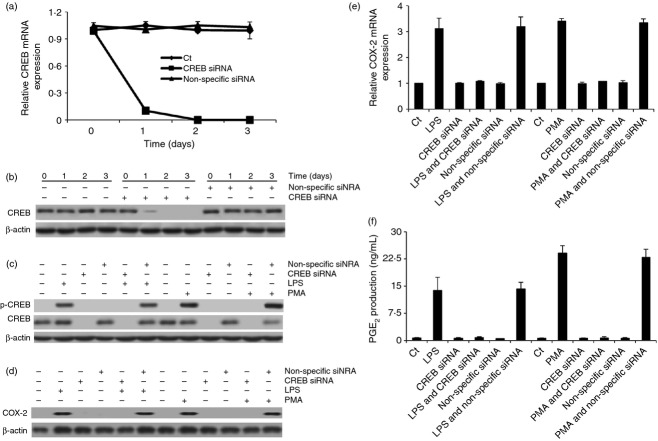
Next, we sought to investigate the relationship among CaMKs and CREB activation, COX-2 expression and PGE2 production. As CREB is a transcript factor that regulates protein expression,22 we knocked-down CREB by using specific siRNA to study whether CREB also plays an essential role in regulating COX-2 expression in our study. The results demonstrated that CREB siRNA specifically knocked down CREB expression within 24 hr, which did not obviously change the viability of the macrophages (Fig. 3a,b; see Supporting information, Fig. S3e and S4). After 48 hr of treatment with CREB siRNA, LPS or PMA was added to detect COX-2 expression and PGE2 production in these macrophages. The results showed that LPS and PMA could not elevate COX-2 expression and PGE2 production in these CREB siRNA-treated macrophages (P < 0·001) (Fig. 3d–f). However, CREB siRNA treatment did not eliminate the effect of LPS and PMA in inducing [Ca2+]i transient increase and CaMKII and CaMKIV phosphorylation (Fig. S3a–d,f). Moreover, CREB siRNA also had no obvious effect in altering the expression of CaM, CaMKII and CaMKIV (Fig. S3f–h). Combined together, these results suggested that the transient increase of [Ca2+]i regulated CREB activation to control COX-2 expression and PGE2 production, possibly through the activation of CaMKs.
Figure 3.
cAMP-response element binding protein (CREB) small interfering RNA (siRNA) treatment blocked lipopolysaccharide (LPS) or PMA-elicited CREB activation, cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production. (a, b) CREB mRNA and protein expression were analysed in siRNA-pre-treated rat peritoneal macrophage by quantitative PCR and immunoblotting. (c) The effect of LPS or PMA on the expression and phosphorylation of CREB was also measured by immunoblotting in siRNA-pre-treated macrophages. Meanwhile, the protein (d) and mRNA (e) expression of COX-2 and the production of PGE2 (f) were also assessed in these macrophages.
Next, we sought to study whether CaMKs directly regulated CREB activation to increase COX-2 expression and PGE2 production. The rat peritoneal macrophages were therefore pre-treated with 5 μm KN93 (CaMK inhibitor; the IC50 of KN93 is around 1 μm) for 30 min and then stimulated with LPS or PMA to detect the activation of CREB. As a specific control of KN93, KN92 (5 μm) was also included in the experiment. The results showed that KN93 efficiently inhibited LPS- or PMA-induced CaMKII and CaMKIV phosphorylation while KN92 had no obvious effect on these events. KN93, not KN92, also specifically blocked CREB phosphorylation and eliminated the increase of COX-2 expression and PGE2 production (P < 0·001) without affecting the transient increase of [Ca2+]i in LPS- or PMA-stimulated macrophages (Fig. 4d–i; Fig. S3a–d). Moreover, KN93 and KN92 pre-treatment did not obviously alter the viability of these macrophages and had no obvious effect in changing the expression of CaM, CaMKII, CaMKIV and CREB (Fig. 4d,e; see Supporting information, Fig. S5a–c).
Figure 4.
Calmodulin-dependent protein kinase (CaMK) inhibitor and CaMKII small interfering RNA (siRNA) treatment blocked lipopolysaccharide (LPS) or PMA-induced cAMP-response element binding protein (CREB) activation, cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production. (a–c) CaMKII and CaMKIV mRNA and protein expression were detected in siRNA-pre-treated rat peritoneal macrophage by quantitative PCR and immunoblotting assay. (d, e) The effect of CaMK inhibitor or siRNA treatment on the expression or phosphorylation of CaM, CaMKII, CaMKIV and CREB was measured by immunoblotting in LPS- or PMA-stimulated macrophages. Meanwhile, COX-2 protein (f and g) and mRNA (h) expression and PGE2 production (i) were also assessed in these macrophages.
Since KN93 is an inhibitor that targets several CaMK subtypes, we knocked-down CaMK subtypes by treating the macrophages with specific siRNAs to investigate whether a specific subtype of CaMKs exerts its effect on eliciting the activation of CREB. As expected, CaMKII and CaMKIV siRNA treatment specifically knocked down the expression of CaMKII and CaMKIV within 24 hr, which had no obvious effect in changing the viability of the macrophages (Fig. 4a–c; Fig. S4). After 48 hr of treatment with CaMKII or CaMKIV siRNA, LPS or PMA was added to detect whether these stimuli could still induce CREB activation and elevate COX-2 expression and PGE2 production. The results demonstrated that LPS and PMA could not induce the phosphorylation of CREB and the increase of COX-2 expression and PGE2 production (P < 0·001) in CaMKII, not CaMKIV, siRNA-treated macrophages (Fig. 4d–i). Moreover, neither CaMKII nor CaMKIV siRNA treatment had any obvious effect on LPS- or PMA-induced transient increase of [Ca2+]i (Fig. S3a–d). CaMKII and CaMKIV siRNA treatment also had no obvious effect in altering the expression of CaM and CREB (Fig. 4d,e; Fig. S5a–c). Hence, these results suggested that, in macrophage, the transient increase of [Ca2+]i was the initiative step to mediate the activation of CaMKII, which in turn regulated the activation of CREB to mediate COX-2 expression and PGE2 production.
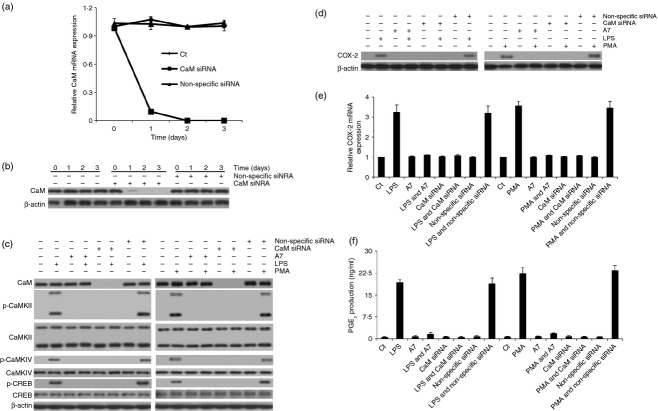
Since the activation of CaMKII was regulated by the Ca2+/calmodulin complex,32 we also sought to study whether CaM also plays an essential role in eliciting CaMKII and CREB activation and increasing COX-2 expression and PGE2 production. To achieve this goal, we pre-treated the rat peritoneal macrophages with 15 μm A7 (CaM inhibitor; the IC50 of A7 is around 3 μm) for 30 min and then LPS or PMA were added. The results showed that A7 pre-treatment efficiently blocked LPS- or PMA-induced phosphorylation of CaMKII, CaMKIV and CREB, which led to the elimination of LPS- or PMA-elevated COX-2 expression and PGE2 production (P < 0·001) (Fig. 5c–f). However, A7 pre-treatment did not inhibit LPS- or PMA-induced transient increase of [Ca2+]i. Nor did it alter the expression of CaM, CaMKII, CaMKIV and CREB. Moreover, A7 pre-treatment did not obviously change the viability of these macrophages (see Supporting information, Fig. S3a–d and S6a–c). These results suggested that CaM also played an essential role in mediating CaMK activation and CaMKII in turn regulated the activation of CREB and increased the expression of COX-2. To confirm the conclusion, we also knocked down CaM in the macrophages with CaM-specific siRNA. As expected, CaM siRNA treatment specifically knocked down the expression of CaM within 24 hr, which had no obvious effect on the viability of the macrophages (Fig. 5a,b; Fig. S4). After 48 hr treatment with CaM siRNA, we also added LPS or PMA to the macrophages to detect whether these stimuli could still induce CaMK and CREB activation and increase COX-2 expression and PGE2 production. The results indicated that LPS and PMA could not induce CaMKII, CaMKIV and CREB phosphorylation in CaM siRNA-treated macrophages. LPS and PMA also could not increase COX-2 expression and PGE2 production in these macrophages (P < 0·001) (Fig. 5c–f). However, CaM siRNA treatment neither altered the expression of CaMKII, CaMKIV and CREB nor inhibited LPS- or PMA-induced transient increase of [Ca2+]i (Fig. 5c–f; Fig. S3a–d, S4 and S6a–c). Therefore, these results together suggested that Ca2+ and CaM together mediated CaMK activation and CaMKII then triggered CREB activation and induced the increase of COX-2 expression and PGE2 expression in LPS- or PMA-stimulated rat peritoneal macrophages.
Figure 5.
Calmodulin (CaM) inhibitor or small interfering RNA (siRNA) treatment blocked lipopolysaccharide (LPS) or PMA-induced calmodulin-dependent protein kinase (CaMK) and cAMP-response element binding protein (CREB) activation, cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production. (a, b) CaM mRNA and protein expression were measured in siRNA-pre-treated rat peritoneal macrophage by quantitative PCR and immunoblotting assay. (c) the effect of CaM inhibitor or siRNA treatment on the expression or phosphorylation of CaM, CaMKII, CaMKIV and CREB was assessed by immunoblotting in LPS- or PMA-stimulated macrophages. Meanwhile, COX-2 protein (d) and mRNA (e) expression and the PGE2 production (f) were also assessed in these macrophages.
Discussion
Prostaglandin E2 is an essential mediator for inflammation, which contributes to vasodilation, pain and fever and plays an important role in initiating the septic shock syndrome. In inflammation sites, PGE2 acts in an autocrine or paracrine manner to induce and amplify the host responses.5 In many cancer patients, PGE2 is also overproduced and aids the progression of tumours by promoting angiogenesis and metastasis.7,8 For the production of PGE2, AA release and COX-2 expression are two pivotal events.9,10 As an important second messenger in cells, Ca2+ plays a pivotal role in cell physiology and pathology, including mediating the production of PGE2.33 In macrophages, Ca2+ regulates PGE2 production by activating cPLA2 to promote the release of AA.11,12 In pituitary cells, Ca2+ also exerts its role on COX-2 expression by activating CaMKs to induce the activation of c/EBPβ, which is one of the two most important transcript factors that are essential for both basal transcription and the induction of COX-2.20,21 In the present study, we found that Ca2+ could also regulate COX-2 expression and PGE2 production by activating CaMKII to induce the activation of CREB, the other important transcript factor that is essential for both basal transcription and the induction of COX-2, in primary rat peritoneal macrophages. Specifically, LPS and PMA induced the transient [Ca2+]i increase, CaMKII and CaMKIV activation and CREB activation and increased COX-2 expression and PGE2 production in a chronological order. The pre-treatment with BAPTA chelated the intracellular Ca2+, which blocked the effect of LPS and PMA on these events. Moreover, EGTA pre-treatment completely eliminated the effect of PMA while it partially blocked the effect of LPS. Additionally, although LPS and PMA still elicited the transient increase of [Ca2+]i and triggered CaMKII and CaMKIV activation in CREB-knocked down macrophages, the increase of COX-2 expression and PGE2 production was blocked completely. After the macrophages were treated with CaM inhibitor or siRNA, LPS and PMA also could induce transient increase of [Ca2+]i. However, the activation of CaMKII, CaMKIV and CREB were blocked, which also resulted in the inhibition of the increase of COX-2 expression and PGE2 production. When we treated the macrophages with CaMK inhibitor or CaMKII and CaMKIV siRNA, the results showed that LPS and PMA still elicited the transient [Ca2+]i increase. However, the activation of CREB and the following increase of COX-2 expression and PGE2 production were blocked completely in the macrophages pre-treated with CaMK inhibitor or CaMKII siRNA. Together, these results suggest that calcium/calmodulin-dependent CaMKII directly mediates the activation of CREB, which in turn increases COX-2 expression and PGE2 production. To our knowledge, there is no report showing that the calcium/calmodulin-dependent CaMKII-CREB signalling pathway regulates COX-2 expression and PGE2 production in primary rat peritoneal macrophages.
In cells, a different pattern of [Ca2+]i increase regulates different cellular functions.34,35 In the present study, although both LPS and PMA induced the transient increase of [Ca2+]i, which could be blocked completely by the pre-treatment with BAPTA, the increase pattern of [Ca2+]i was slightly different. LPS triggered the transient increase of [Ca2+]i, within 2 min, which was partially blocked by the pre-treatment with EGTA. However, PMA-induced transient increase of [Ca2+]i appeared only after 10 min treatment, which was eliminated completely by the pre-treatment with EGTA. Moreover, even with EGTA pre-treatment, the pattern of LPS-induced transient increase of [Ca2+]i was similar to that without EGTA, except that the peak was much lower and it also faded back to the basal level much faster. Together, these results indicated that LPS-induced transient [Ca2+]i increase was composed of the initial Ca2+ release from the intracellular Ca2+ pool and the following Ca2+ influx from the intercellular space. In contrast to LPS, PMA induced the transient increase of [Ca2+]i only by triggering the intercellular Ca2+ influx. In macrophages, LPS binding with LPS-binding protein interacts with a complex that consists of CD14 and Toll-like receptor 4 to activate protein tyrosine kinases.36,37 Activated protein tyrosine kinase induces the activation of phosphatidylinositol-specific phospholipase Cγ, which catalyses the hydrolysis of phosphatidylinositol 4,5-biphosphate into inositol 1,4,5-triphosphate and 1,2-diacylglycerol.27,38 In the cytoplasm, inositol 1,4,5-triphosphate mediates the opening of the inositol 1,4,5-triphosphate receptor in the endoplasmic reticulum, which results in the release of Ca2+.39 Following the release of Ca2+ from the intracellular Ca2+ pool is the Ca2+ influx from the intracellular space, which is accomplished through a process called store-operated calcium entry. The influx of Ca2+ from intercellular space is regulated by Ca2+ release-activated Ca2+ channels including STIM, Orai and TRCP.40–46 In contrast to LPS, PMA induced the transient increase of [Ca2+]i, which appeared after 10 min of treatment. So far, we still cannot fully explain this; it would be a good project to work on in the future. Full understanding of the mechanism will also shed new light on our future work.
In cells, the activation of CREB is based on its phosphorylation on Ser133 by some kinases.47 In the present study, we found that the activation of CaMKII was a downstream effector of Ca2+ and CaM in LPS- or PMA-stimulated rat peritoneal macrophages. Usually, CaMKII is made up of 8–12 subunits and self-inhibits its phosphorylating function. After binding to Ca2+-calmodulin complex, CaMKII is autophosphorylated at Thr286 and activated in macrophages.48,49 When the macrophages were treated with BAPTA or EGTA, LPS-induced CaMKII activation was completely blocked or partly blocked, respectively. Moreover, BAPTA or EGTA pre-treatment completely eliminated PMA-elicited CaMKII activation. These results confirm that Ca2+-dependent CaMKII activation is tightly regulated by the transient [Ca2+]i increase. When the macrophages were pre-treated with CaMK inhibitor, the activation of CREB was completely inhibited, which also resulted in the inhibition of the increase of COX-2 expression and PGE2 production. These results provide a clue that it might be CaMK that mediates the activation of CREB. When CaMKII or CaMKIV expression was knocked-down in the macrophages, LPS and PMA could not induce CREB activation in CaMKII-defective, not CaMKIV-defective, macrophages. Therefore, it is Ca2+-dependent CaMKII that regulates the activation of CREB in LPS- or PMA-stimulated rat peritoneal macrophages. Therefore, the data together highlight that the transient [Ca2+]i increase and CaM control CaMKII activation, which regulates CREB actvation to mediate COX-2 expression and PGE2 production.
In cells, COX-2 expression is regulated by nuclear factor-κB, nuclear factor of activated T-cell, CREB and c/EBPβ.18–20 Among these transcription factors, CREB and c/EBPβ are essential for both basal transcription and the induction of COX-2.20 For the activation of c/EBPβ in pituitary cells, CaMKs play an essential role in response to the increase of intracellular free calcium ion concentration ([Ca2+]i).21 In the present study, our results also demonstrated that LPS and PMA could induce the activation of c/EBPβ in primary rat peritoneal macrophages, which was efficiently blocked by the treatment with EGTA or BAPTA. However, CREB, CaM, CaMKII and CaMKIV knocking-down had no obvious effect on LPS- or PMA-induced c/EBPβ activation (see Supporting information, Fig. S7). These results further prove that the calcium–CaMKII–CREB signalling pathway is a new pathway that is independent of the activation of c/EBPβ. It also suggests that c/EBPβ activation is related to calcium signal. However, differing from the signalling pathway in pituitary cells that links c/EBPβ activation with CaMKs,21 we found that calcium-dependent c/EBPβ activation did not rely on the activation of CaMKII or CaMKIV in primary rat peritoneal macrophages. As CaMKI and CaMKV were not detected in primary rat peritoneal macrophages, it is highly possible that calcium signal exerts its effect on c/EBPβ activation through an unknown signalling pathway, which needs future investigations.
In general, the present study demonstrates that calcium/calmodulin-dependent CaMKII plays an important role in regulating COX-2 expression and PGE2 production by activating CREB; i.e., stimuli-induced transient increase in [Ca2+]i can activate CaMKII, which regulates the phosphorylation and activation of CREB. Next, activated CREB promotes the expression of COX-2, which triggers the production of PGE2. Hence, Ca2+ mediates PGE2 production, not only by regulating the release of AA through Ca2+-cPLA2 signalling pathway, but also by inducing the expression of COX-2 through Ca2+-CaM-CaMKII-CREB and Ca2+-c/EBPβ signalling pathway. Therefore, Ca2+ plays an important role in regulating PGE2 production. It also suggests that Ca2+ may play an important role in the occurrence of inflammation or multiple organ failure during septic shock and it is also linked to the progress of tumours.
Acknowledgments
We thank Dr Changyan Li and Dr Fuchu He for providing help in conducting the experiments. We also thank Dr Greg F. Burton and Dr Barry M. Willardson for reading through the manuscript and giving us valuable suggestions.
Disclosures
This research complied with all relevant federal and institutional policies. We acknowledge that we have no financial or other relationships that would pose a conflict of competing interests relating to the research present in this study.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Lipopolysaccharide (LPS) and PMA treatment had no obvious effect on the viability of macrophage and the mRNA expression of calmodulin (CaM), calmodulin-dependent protein kinase II (CaMKII), CaMKIV and cAMP-response element binding protein (CREB). (a) the viability of the LPS- or PMA-stimulated rat peritoneal macrophage was assessed by trypan blue staining. (b–e) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S2. The viability of macrophage and the mRNA expression of calmodulin (CaM), calmodulin-dependent protein kinase II (CaMKII), CaMKIV and cAMP-response element binding protein (CREB) were not inhibited by the pre-treatment with EGTA and BAPTA. (a) the viability of EGTA- or BAPTA-pre-treated rat peritoneal macrophages was measured by trypan blue staining, which were stimulated with lipopolysaccharide (LPS) or PMA. (b, c) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S3. Effect of inhibitor or small interfering RNA (siRNA) treatment on macrophage viability and lipopolysaccharide (LPS) or PMA-induced transient [Ca2+]i increase and calmodulin (CaM), calmodulin-dependent protein kinase II (CaMKII), and CaMKIV expression and phosphorylation. (a–d) the effect of A7, KN93, KN92, CaM siRNA, CaMKII siRNA, CaMKIV siRNA and cAMP-response element binding protein (CREB) siRNA treatment on the transient [Ca2+]i increase was measured in LPS- or PMA-stimulated rat peritoneal macrophages. (e) Effect of CREB siRNA treatment on the viability of these macrophages was also assessed by trypan blue staining. (f–h) The mRNA and protein expression of CaM, CaMKIV, CaMKII and CREB was also detected in CREB siRNA-treated macrophages by qPCR and Immunoblotting, respectively.
Figure S4. The treatment with small interfering RNA (siRNA) had no obvious effect on the viability of rat peritoneal macrophage. The viability of the rat peritoneal macrophages was detected by trypan blue staining after the treatment with calmodulin (CaM) siRNA, calmodulin-dependent protein kinase II (CaMKII) siRNA, CaMKIV siRNA, cAMP-response element binding protein (CREB) siRNA or non-specific siRNA.
Figure S5. Calmodulin-dependent protein kinase (CaMK) inhibitor and CaMKII small interfering RNA (siRNA) treatment had no obvious effect on the viability of macrophage and the mRNA expression of calmodulin (CaM) and cAMP-response element binding protein (CREB). (a) the viability of CaMK inhibitor- or siRNA-pre-treated rat peritoneal macrophages was measured by trypan blue staining. (b, c) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S6. Calmodulin (CaM) inhibitor and small interfering RNA (siRNA) treatment had no obvious effect on the viability of macrophage and the mRNA expression of calmodulin-dependent protein kinase II (CaMKII), CaMKIV and cAMP-response element binding protein (CREB). (a) the viability of CaMK inhibitor- or siRNA-pre-treated rat peritoneal macrophages was measured by trypan blue staining. (b, c) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S7. EGTA and BAPTA pre-treatment blocked lipopolysaccharide (LPS) and PMA-induced C/EBPβ activation. (a) the expression of C/EBPβ and phosphorylated C/EBPβ was detected by immunoblotting in LPS-stimulated macrophages. (b) the expression of C/EBPβ and phosphorylated C/EBPβ was also accessed in PMA-stimulated macrophages. (c) after 60 min treatment of LPS, the expression of C/EBPβ and phosphorylated C/EBPβ was also analysed in macrophages pre-treated with EGTA or BAPTA for 30 min or with specific or non-specific siRNAs for 2 days. (d) after 60 min treatment of PMA, the expression of C/EBPβ and phosphorylated C/EBPβ was also analysed in macrophages pre-treated with EGTA or BAPTA for 30 min or with specific or non-specific siRNAs for 2 days.
References
- 1.Park YM, Won JH, Yun KJ, Ryu JH, Han YN, Choi SK, Lee KT. Preventive effect of Ginkgo biloba extract (GBB) on the lipopolysaccharide-induced expressions of inducible nitric oxide synthase and cyclooxygenase-2 via suppression of nuclear factor-κB in RAW 264.7 cells. Biol Pharm Bull. 2006;29:985–90. doi: 10.1248/bpb.29.985. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Liu S, Liu J, Zhang T, Shen Q, Yu Y, Cao X. Immune complex/Ig negatively regulate TLR4-triggered inflammatory response in macrophages through Fcγ RIIb-dependent PGE2 production. J Immunol. 2009;182:554–62. doi: 10.4049/jimmunol.182.1.554. [DOI] [PubMed] [Google Scholar]
- 3.Bulger EM, Arbabi S, Garcia I, Maier RV. The macrophage response to endotoxin requires platelet activating factor. Shock. 2002;17:173–9. doi: 10.1097/00024382-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Parillo JE. Pathogenic mechanisms of septic shock. N Engl J Med. 1993;328:1471–7. doi: 10.1056/NEJM199305203282008. [DOI] [PubMed] [Google Scholar]
- 5.Astiz M, Saha D, Lustbader D, Lin R, Rackow E. Monocyte response to bacterial toxins, expression of cell surface receptors, and release of anti-inflammatory cytokines during sepsis. J Lab Clin Med. 1996;128:594–600. doi: 10.1016/s0022-2143(96)90132-8. [DOI] [PubMed] [Google Scholar]
- 6.Jegerschöld C, Pawelzik SC, Purhonen P, et al. Structural basis for induced formation of the inflammatory mediator prostaglandin E2. Proc Natl Acad Sci USA. 2008;105:11110–5. doi: 10.1073/pnas.0802894105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res. 2004;10:4266s–9s. doi: 10.1158/1078-0432.CCR-040014. [DOI] [PubMed] [Google Scholar]
- 8.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Haidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumor microenvironment. Carcinogenesis. 2009;30:377–86. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 9.Arias-Negrete S, Keller K, Chadee K. Proinflammatory cytokines regulate cyclooxygenase-2 mRNA expression in human macrophages. Biochem Biophys Res Commun. 1995;208:582–9. doi: 10.1006/bbrc.1995.1378. [DOI] [PubMed] [Google Scholar]
- 10.Percival MD, Ouellet M, Vincent CJ, Yergey JA, Kennedy BP, O'Neill GP. Purification and characterization of recombinant human cyclooxygenase-2. Arch Biochem Biophys. 1994;315:111–8. doi: 10.1006/abbi.1994.1478. [DOI] [PubMed] [Google Scholar]
- 11.Ambs P, Baccarini M, Fitzke E, Dieter P. Role of cytosolic phospholipase A2 in arachidonic acid release of rat-liver macrophages: regulation by Ca2+ and phosphorylation. Biochem J. 1995;311:189–95. doi: 10.1042/bj3110189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakraborti S. Phospholipase A2 isoforms: a perspective. Cell Signal. 2003;15:637–65. doi: 10.1016/s0898-6568(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 13.Kozak W, Fraifeld V. Non-prostaglandin eicosanoids in fever and anapyrexia. Front Biosci. 2004;9:3339–55. doi: 10.2741/1486. [DOI] [PubMed] [Google Scholar]
- 14.Rouzer CA, Marnett LJ. Structural and functional difference between cyclooxygenases: fatty acid oxygenase with a critical role in cell signaling. Biochem Biophys Res Commun. 2005;338:34–44. doi: 10.1016/j.bbrc.2005.07.198. [DOI] [PubMed] [Google Scholar]
- 15.Caivano M, Cohen P. Role of mitogen-activated protein kinase cascades in mediating lipopolysaccharide-stimulated induction of cyclooxygenase-2 and IL-1β in RAW264 macrophages. J Immunol. 2000;164:3018–25. doi: 10.4049/jimmunol.164.6.3018. [DOI] [PubMed] [Google Scholar]
- 16.Gijón MA, Spencer DM, Siddiqi AR, Bonventre JV, Leslie CC. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J Biol Chem. 2000;275:20146–56. doi: 10.1074/jbc.M908941199. [DOI] [PubMed] [Google Scholar]
- 17.Hirabayashi T, Murayama T, Shimizu T. Regulatory mechanism and physiological role of cytosolic phospholipase A2. Biol Pharm Bull. 2004;27:1168–73. doi: 10.1248/bpb.27.1168. [DOI] [PubMed] [Google Scholar]
- 18.Chen CC, Sun YT, Chen JJ, Chang YJ. Tumor necrosis factor-α-induced cyclooxygenase-2 expression via sequential activation of ceramide-dependent mitogen-activated protein kinases, and IκB kinase 1/2 in human alveolar epithelial cells. Mol Pharmacol. 2001;59:493–500. doi: 10.1124/mol.59.3.493. [DOI] [PubMed] [Google Scholar]
- 19.de Gregorio R, Iniguez MA, Fresno M, Alemany S. Cot kinase induces cyclooxygenase-2 expression in T cells through activation of the nuclear factor of activated T cells. J Biol Chem. 2001;276:27003–9. doi: 10.1074/jbc.M100885200. [DOI] [PubMed] [Google Scholar]
- 20.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–66. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 21.Wegner M, Cao Z, Rosenfeld MG. Calcium-regulated phosphorylation within the leucine zipper of C/EBPβ. Science. 1992;256:370–3. doi: 10.1126/science.256.5055.370. [DOI] [PubMed] [Google Scholar]
- 22.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–9. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto KM, Frank DA. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res. 2009;15:2583–7. doi: 10.1158/1078-0432.CCR-08-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayr B, Montminy M. Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol. 2001;2:599–609. doi: 10.1038/35085068. [DOI] [PubMed] [Google Scholar]
- 25.Brindle P, Linke S, Montminy M. Protein-kinase-A-dependent activator in transcription factor CREB reveals new role for CREM repressers. Nature. 1993;364:821–4. doi: 10.1038/364821a0. [DOI] [PubMed] [Google Scholar]
- 26.Gubina E, Luo X, Kwon E, Sakamoto K, Shi YF, Mufson RA. βc cytokine receptor-induced stimulation of cAMP response element binding protein phosphorylation requires protein kinase C in myeloid cells: a novel cytokine signal transduction cascade. J Immunol. 2001;167:4303–10. doi: 10.4049/jimmunol.167.8.4303. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X, Yang W, Li J. Ca2+- and protein kinase C-dependent signaling pathway for nuclear factor-κB activation, inducible nitric-oxide synthase expression, and tumor necrosis factor-α production in lipopolysaccharide-stimulated rat peritoneal macrophages. J Biol Chem. 2006;281:31337–47. doi: 10.1074/jbc.M602739200. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Shapiro L, Fellingham G, Willardson BM, Burton GF. HIV replication in CD4+ T lymphocytes in the presence and absence of follicular dendritic cells: inhibition of replication mediated by α-1-antitrypsin through altered IκBα ubiquitination. J Immunol. 2011;186:3148–55. doi: 10.4049/jimmunol.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thacker TC, Zhou X, Estes JD, Jiang Y, Keele BF, Elton TS, Burton GF. Follicular dendritic cells and human immunodeficiency virus type 1 transcription in CD4+ T cells. J Virol. 2009;83:150–8. doi: 10.1128/JVI.01652-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harizi H, Gualde N. Pivotal role of PGE2 and IL-10 in the cross-regulation of dendritic cell-derived inflammatory mediators. Cell Mol Immunol. 2006;3:271–7. [PubMed] [Google Scholar]
- 31.Wilborn J, DeWitt DL, Peters-Golden M. Expression and role of cyclooxygenase isoforms in alveolar and peritoneal macrophages. Am J Physiol. 1995;268:L294–301. doi: 10.1152/ajplung.1995.268.2.L294. [DOI] [PubMed] [Google Scholar]
- 32.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin trapping by calcium-calmodulin-dependent protein kinase. Science. 1992;256:1199–202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 33.Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR. Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol. 2003;111:S18–36. doi: 10.1067/mai.2003.25. [DOI] [PubMed] [Google Scholar]
- 34.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol. 1993;465:359–86. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 36.Mancuso G, Midiri A, Biondo C, et al. Bacteroides fragilis-derived lipopolysaccharide produces cell activation and lethal toxicity via toll-like receptor 4. Infect Immun. 2005;73:5620–7. doi: 10.1128/IAI.73.9.5620-5627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuguchi T, Masuda A, Sugimoto K, Nagai Y, Yoshikai Y. JNK-interacting protein 3 associates with Toll-like receptor 4 and is involved in LPS-mediated JNK activation. EMBO J. 2003;22:4455–64. doi: 10.1093/emboj/cdg438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinji H, Akagawa KS, Tsuji M, Maeda M, Yamada R, Matsuura K, Yamamoto S, Yoshida T. Lipopolysaccharide-induced biphasic inositol 1,4,5-trisphosphate response and tyrosine phosphorylation of 140-kilodalton protein in mouse peritoneal macrophages. J Immunol. 1997;158:1370–6. [PubMed] [Google Scholar]
- 39.Letari O, Nicosia S, Chiavaroli C, Vacher P, Schlegel W. Activation by bacterial lipopolysaccharide causes changes in the cytosolic free calcium concentration in single peritoneal macrophages. J Immunol. 1991;147:980–3. [PubMed] [Google Scholar]
- 40.Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E. SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell. 2012;149:425–38. doi: 10.1016/j.cell.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Cheng KT, Bandyopadhyay BC, et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1−/− mice. Proc Natl Acad Sci USA. 2007;104:17542–7. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–41. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–3. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 44.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–45. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stathopulos PB, Zheng L, Ikura M. Stromal interaction molecule (STIM) 1 and STIM2 calcium sensing regions exhibit distinct unfolding and oligomerization kinetics. J Biol Chem. 2009;284:728–32. doi: 10.1074/jbc.C800178200. [DOI] [PubMed] [Google Scholar]
- 46.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–3. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siu YT, Jin DY. CREB – a real culprit in oncogenesis. FEBS J. 2007;274:3224–32. doi: 10.1111/j.1742-4658.2007.05884.x. [DOI] [PubMed] [Google Scholar]
- 48.Misra UK, Akabani G, Pizzo SV. The role of cAMP-dependent signaling in receptor-recognized forms of α2-macroglobulin-induced cellular proliferation. J Biol Chem. 2002;277:36509–20. doi: 10.1074/jbc.M203543200. [DOI] [PubMed] [Google Scholar]
- 49.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: modulators of neuronal development and plasticity. Neuron. 2008;59:914–31. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Lipopolysaccharide (LPS) and PMA treatment had no obvious effect on the viability of macrophage and the mRNA expression of calmodulin (CaM), calmodulin-dependent protein kinase II (CaMKII), CaMKIV and cAMP-response element binding protein (CREB). (a) the viability of the LPS- or PMA-stimulated rat peritoneal macrophage was assessed by trypan blue staining. (b–e) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S2. The viability of macrophage and the mRNA expression of calmodulin (CaM), calmodulin-dependent protein kinase II (CaMKII), CaMKIV and cAMP-response element binding protein (CREB) were not inhibited by the pre-treatment with EGTA and BAPTA. (a) the viability of EGTA- or BAPTA-pre-treated rat peritoneal macrophages was measured by trypan blue staining, which were stimulated with lipopolysaccharide (LPS) or PMA. (b, c) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S3. Effect of inhibitor or small interfering RNA (siRNA) treatment on macrophage viability and lipopolysaccharide (LPS) or PMA-induced transient [Ca2+]i increase and calmodulin (CaM), calmodulin-dependent protein kinase II (CaMKII), and CaMKIV expression and phosphorylation. (a–d) the effect of A7, KN93, KN92, CaM siRNA, CaMKII siRNA, CaMKIV siRNA and cAMP-response element binding protein (CREB) siRNA treatment on the transient [Ca2+]i increase was measured in LPS- or PMA-stimulated rat peritoneal macrophages. (e) Effect of CREB siRNA treatment on the viability of these macrophages was also assessed by trypan blue staining. (f–h) The mRNA and protein expression of CaM, CaMKIV, CaMKII and CREB was also detected in CREB siRNA-treated macrophages by qPCR and Immunoblotting, respectively.
Figure S4. The treatment with small interfering RNA (siRNA) had no obvious effect on the viability of rat peritoneal macrophage. The viability of the rat peritoneal macrophages was detected by trypan blue staining after the treatment with calmodulin (CaM) siRNA, calmodulin-dependent protein kinase II (CaMKII) siRNA, CaMKIV siRNA, cAMP-response element binding protein (CREB) siRNA or non-specific siRNA.
Figure S5. Calmodulin-dependent protein kinase (CaMK) inhibitor and CaMKII small interfering RNA (siRNA) treatment had no obvious effect on the viability of macrophage and the mRNA expression of calmodulin (CaM) and cAMP-response element binding protein (CREB). (a) the viability of CaMK inhibitor- or siRNA-pre-treated rat peritoneal macrophages was measured by trypan blue staining. (b, c) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S6. Calmodulin (CaM) inhibitor and small interfering RNA (siRNA) treatment had no obvious effect on the viability of macrophage and the mRNA expression of calmodulin-dependent protein kinase II (CaMKII), CaMKIV and cAMP-response element binding protein (CREB). (a) the viability of CaMK inhibitor- or siRNA-pre-treated rat peritoneal macrophages was measured by trypan blue staining. (b, c) the mRNA expression of CaM, CaMKIV, CaMKII and CREB was also detected in these macrophages by quantitative PCR.
Figure S7. EGTA and BAPTA pre-treatment blocked lipopolysaccharide (LPS) and PMA-induced C/EBPβ activation. (a) the expression of C/EBPβ and phosphorylated C/EBPβ was detected by immunoblotting in LPS-stimulated macrophages. (b) the expression of C/EBPβ and phosphorylated C/EBPβ was also accessed in PMA-stimulated macrophages. (c) after 60 min treatment of LPS, the expression of C/EBPβ and phosphorylated C/EBPβ was also analysed in macrophages pre-treated with EGTA or BAPTA for 30 min or with specific or non-specific siRNAs for 2 days. (d) after 60 min treatment of PMA, the expression of C/EBPβ and phosphorylated C/EBPβ was also analysed in macrophages pre-treated with EGTA or BAPTA for 30 min or with specific or non-specific siRNAs for 2 days.