Abstract
We examined the rat and mouse epididymis using helium ion microscopy (HIM), a novel imaging technology that uses a scanning beam of He+ ions to produce nanometer resolution images of uncoated biological samples. Various tissue fixation, sectioning and dehydration methods were evaluated for their ability to preserve tissue architecture. The cauda epididymidis was luminally perfused in vivo to remove most spermatozoa and the apical surface of the epithelial lining was exposed. Fixed epididymis samples were then subjected to critical point drying (CPD) and HIM. Apical stereocilia in principal cells and smaller apical membrane extensions in clear cells were clearly distinguishable in both rat and mouse epididymis using this technology. After perfusion with an activating solution containing CPT-cAMP, a permeant analog of cAMP, clear cells exhibited an increase in the number and size of membrane ruffles or microplicae. In contrast, principal cells did not exhibit detectable structural modifications. High-resolution HIM imaging clearly showed the ultrastructure of residual sperm cells, including the presence of concentric rings on the midpiece, and of cytoplasmic droplets in some spermatozoa. Close epithelium–sperm interactions were also detected. We found a number of sperm cells whose heads were anchored within the epididymal epithelium. In certain cases, the surface of the sperm cytoplasmic droplet was covered with vesicle-like structures whose size is consistent with that of epididymosomes. In conclusion, we describe here the first application of HIM technology to the study of the structure and morphology of the rodent epididymis. HIM technology represents a major imaging breakthrough that can be successfully applied to study the epididymis and spermatozoa, with the goal of advancing our understanding of their structure and function.
Keywords: epididymis, spermatozoa, helium ion microscopy, principal and clear cells, epididymosomes
Introduction
The epididymis is a long and highly convoluted tubule connecting the efferent ducts to the vas deferens (Jones, 1998). It is divided into four segments with distinct anatomical properties and gene expression patterns: the initial segment (IS), the caput (head), the corpus and the cauda epididymidis (for review see Cooper, 1998; Robaire et al., 2006; Cornwall, 2009; Shum et al., 2009; Belleannee et al., 2012). Four different cell types are present in the epididymal epithelium: principal, clear (in all regions except the IS), narrow (restricted to the IS) and basal cells (Shum et al., 2009).
Functionally, clear cells are responsible for the acidification of the epididymal lumen via vacuolar proton-pumping ATPase (V-ATPase)-mediated H+ secretion (Brown et al., 1992; Breton et al., 1996), whereas principal cells are mainly implicated in protein secretion, and ion and water transepithelial transport (Da Silva et al., 2006; Belleannee et al., 2012). Apocrine secretion of proteins in this tissue involves membranous microvesicles named epididymosomes (Sullivan and Saez, 2013), which were first reported almost three decades ago in the Chinese hamster (Yanagimachi et al., 1985). Generally, microvesicles include exosomes that are secreted following fusion of multivesicular bodies with the plasma membrane, and larger vesicles that are shed directly from the cell plasma membrane (Cocucci et al., 2009). They are found in various biological fluids, including blood (Caby et al., 2005), urine (Pisitkun et al. 2004; Keller et al., 2007; Miranda et al., 2010), saliva (Palanisamy et al., 2010), amniotic fluid (Keller et al., 2007) and cerebrospinal fluid (Harrington et al., 2009). Epididymosomes are vesicles measuring 20–500 nm in diameter (Krapf et al., 2012; Belleannee et al. 2013; Sullivan and Saez, 2013) that can be isolated from the epididymal fluid (Frenette et al., 2005; Rejraji et al., 2006). They were shown to be the mediators of the protein transfer between the epididymal epithelium and the sperm cells, which underlines the post-testicular maturation of spermatozoa (Saez et al., 2003; Sullivan et al., 2007; Thimon et al., 2008; Oh et al., 2009; Frenette et al., 2010; Sullivan and Saez, 2013).
Imaging the epididymis in various mammalian species has a long history, starting with light and electron microscopy (EM) studies (Horstmann, 1962; Holstein, 1964; Leeson and Leeson, 1964; Nicander, 1964; Schmidt, 1964; Fahrmann and Schuchardt, 1966; Horstmann et al., 1966; Murakami et al., 1976; Hamilton et al., 1977). Pioneering studies using EM to investigate the ultrastructure of sperm cells are even older (Reed and Reed, 1947, 1948; Sanders, 1948; Anberg, 1957). To this day, bright field immunocytochemistry and immunofluorescence microscopy (Da Silva et al., 2006; Robaire et al., 2006; Cyr et al., 2007; Cornwall, 2009; Joseph et al., 2011; Shum et al., 2011b; Belleannee et al., 2012), as well as scanning and transmission EM (Zhou et al., 1987; Fornes and De Rosas, 1991; Rajalakshmi et al., 1993; Stoffel and Friess, 1994; Villalpando et al., 2000; Hermo and Jacks, 2002; Koga and Ushiki, 2006; Takahashi et al., 2006; Lorenzana et al., 2007; Parent et al., 2011; Lin et al., 2013) remain the methods of choice for investigation of the epididymal epithelium and spermatozoa.
In the present study we used for the first time helium ion microscopy (HIM) to investigate the structure and morphology of epididymal epithelial cells and sperm. HIM is a novel technology that can yield high-resolution (nanometer or potentially even sub-nanometer) images by scanning the samples with a beam of He+ ions. The technical characteristics of the He+ beam allow it to focus to <0.75 nm and possibly down to ∼0.25 nm (Ward et al., 2007; Bell, 2009). For a review of the advantages of the HIM imaging technology compared with established scanning or transmission EM see (Bell, 2009). Traditionally used in materials science, HIM has been applied successfully in recent years to the study of various types of biological samples, including cancer cells (Bazou et al., 2011a), platelets (Bazou et al., 2011b) and kidney glomeruli and tubule cells (Rice et al., 2013).
We demonstrate here that when applied to the study of rodent epididymis and spermatozoa, HIM allows for the high-resolution imaging of stereocilia (the long microvilli of principal cells in all regions of the epididymis) in principal cells and reveals the presence of membrane ruffles or microplicae in clear cells that increase in number and size under activated conditions, as well as the ultrastructure of sperm cells, including their physical interaction with the epithelium. HIM also reveals that the surface of the sperm cytoplasmic droplet is covered with vesicle-like structures whose size and shape is consistent with that of epididymosomes.
Methods
Ethics approval
Animal experiments were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care, in accordance with the National Institutes of Health, Department of Agriculture, and AAALAC requirements.
Tissue harvesting
Adult (>10 week old) male Sprague–Dawley rats (Charles River Laboratories International, Wilmington, MA, USA), adult (>12 week old) male C57BL6 mice (Jackson Laboratory, Bar Harbor, ME, USA) and B1-EGFP transgenic mice expressing enhanced green fluorescent protein (EGFP) in clear and narrow cells (Miller et al., 2005) were housed under standard conditions, had free access to water and were maintained on a standard rodent diet.
The animals were anesthetized with pentobarbital sodium (60 mg/kg body wt i.p., Nembutal, Abbott Laboratories, Abbott Park, IL, USA) and the cauda epididymidis was luminally perfused under a dissecting microscope with a small catheter connected to a syringe infusion pump (model 100, Kd Scientific, Holliston, MA, USA) at a rate of 0.25 ml/h as previously described (Shum et al., 2008, 2011a; Belleannee et al., 2010). The lumen of the mouse epididymis was flushed free of spermatozoa for 5 min using a physiological solution based on previous reports (Levine and Marsh, 1971; Jenkins et al., 1980; Clulow et al., 1994), containing (in mM) 50 NaCl, 50 K gluconate, 1.2 MgSO4, 0.6 CaCl2, 4 Na acetate, 1 Na3 citrate, 6.4 NaH2PO4, 3.6 Na2HPO4 (pH 6.6). The osmolality was adjusted to 360 mmol/kg with raffinose (Shum et al., 2011a). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). Experimental mice were subsequently perfused for 20 min with an alkaline solution (pH 7.8) containing 1 mM 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (CPT-cAMP, Sigma-Aldrich), 1.0 mM NaH2PO4 and 9.6 mM Na2HPO4. The rat epididymal lumen was perfused for 15 min with a similar buffered perfusion solution, except for NaH2PO4 (8.8 mM) and Na2HPO4 (1.29 mM) (pH 6.0, 330 mmol/kg). Control rats were subsequently perfused with the same solution for another 30 min, whereas experimental rats were perfused for the same duration with an activating solution containing 1 mM CPT-cAMP and 15 mM NaHCO3. The pH was adjusted to 7.2 by modifying the concentration of NaH2PO4 (2.67 mM) and Na2HPO4 (7.33 mM). Osmolality was adjusted to 330 mmol/kg with raffinose to preserve the morphology of the epithelium.
For HIM imaging, all control and CPT-cAMP-treated tissues were fixed by luminal perfusion with 4% glutaraldehyde (GA) in 0.1 M sodium cacodylate buffer, and subsequently post-fixed in the same fixative overnight at 4°C. The following day, the tissues were washed extensively in phosphate-buffered saline (PBS, 0.9% NaCl in 10 mM phosphate buffer, pH 7.4), cut with a razor blade to expose the apical surface of the epididymal epithelium and stored at 4°C in PBS containing 0.02% NaN3 until further processing.
For conventional fluorescence microscopy, some tissues were fixed in paraformaldehyde-lysine-periodate (PLP) fixative containing 4% paraformaldehyde, 10 mM sodium periodate, 75 mM lysine and 5% sucrose in 0.1 M sodium phosphate buffer, as described previously (Shum et al., 2008). After three to five washes in PBS, the cauda epididymidis was cut using a TPI PELCO 101 series 1000 vibratome (Technical Products International, Inc., St. Louis, MO, USA). Some tissues were immunofluorescently labeled for the V-ATPase, using an affinity purified chicken antibody against the E subunit of the V-ATPase as we described previously (Breton et al., 2000; Herak-Kramberger et al., 2001; Pietrement et al., 2006).
Tissue processing for HIM
To preserve tissue morphology, critical point drying (CPD) requires a pretreatment with a suitable solvent (such as methanol, acetone or ethanol) to replace tissue water (Bazou et al., 2011a). We previously reported that dehydration in a series of graded methanol solutions allows for good quality post-CPD tissue morphology (Rice et al., 2013). Epididymal tissues were subjected to a similar dehydration schedule: 25% (v/v) MeOH in PBS for 60 min, 40% (v/v) MeOH in PBS for 45 min and 60% (v/v) MeOH in double-distilled water (ddH2O) for 45 min, all at room temperature. The MeOH solution was refreshed halfway through each incubation step. The tissues were then incubated in 80% (v/v) MeOH in ddH2O for 45 min at room temperature, followed by another 80% (v/v) MeOH overnight at 4°C. The following day, the tissues were placed in 100% MeOH for at least 1 h, and the pure MeOH was replaced once again before performing CPD.
Epididymal tissues were placed in metal baskets and subjected to CPD in a Samdri-795 CPD apparatus (Tousimis Research Corporation, Rockville, MD, USA) as previously described (Rice et al., 2013). During this process, the tissues were maintained at supercritical parameter values (1200 psi, 42°C) for at least 4 min, and subsequently the pressure was reduced slowly (<100 psi/min).
The protocols described here were selected after evaluating various fixatives, including GA and modified PLP (Paunescu et al., 2004; Rice et al., 2013), fixative concentrations (2 or 4% v/v GA), fixation methods, i.e. luminal perfusion of the epididymis versus whole animal transcardial perfusion (Paunescu et al., 2004), post-fixation and dehydration protocols, for their ability to preserve tissue architecture (Rice et al., 2013).
Helium ion microscopy
HIM was performed as previously reported (Rice et al., 2013) using an Orion helium ion microscope (Carl Zeiss Microscopy, Peabody, MA, USA) at a 35 keV beam energy, with a probe current of 0.1–1.5 pA and with no conductive coating of the samples. HIM images of uncoated samples, acquired by collecting the secondary electrons produced by the He+ beam/sample interaction, feature unmasked and unobscured surface morphological details (reviewed in Bell, 2009). A low-energy electron flood gun was used after each individual line pass of the imaging beam in order to achieve charge neutralization.
Digital images were not post-processed, except for brightness and contrast adjustment (Vanden Berg-Foels et al., 2011; Rice et al., 2013) performed using Adobe Photoshop version 9.0.2 software (Adobe Systems, San Jose, CA, USA).
Results
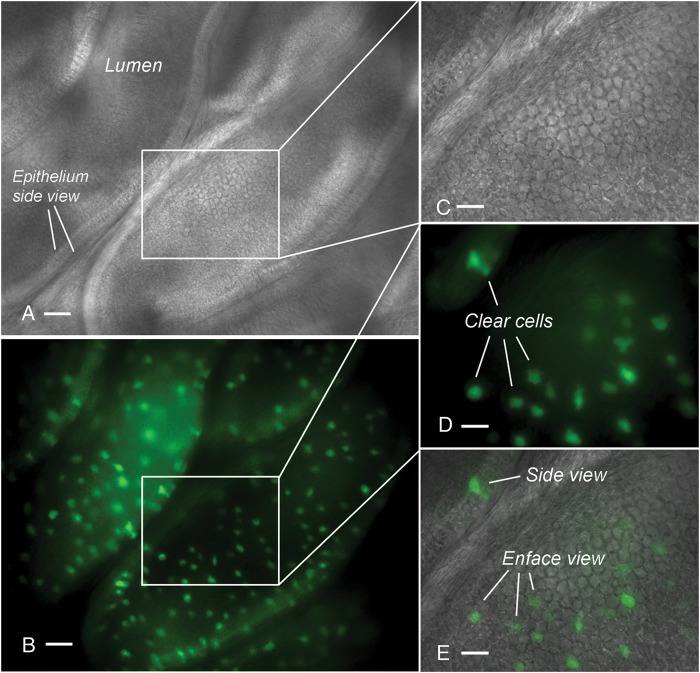
We first examined the apical surface of the epididymal epithelium using conventional microscopy. Many EGFP-expressing clear cells that are dispersed among non-fluorescent principal cells can be seen in vibratome sections of the cauda epididymidis of B1-EGFP mice (Fig. 1).
Figure 1.
Vibratome sections of the cauda epididymidis of B1-EGFP mouse, expressing EGFP specifically in clear cells, visualized by light (A) and epifluorescence (B) microscopy. The cauda tubule was cut longitudinally to expose the apical surface of the epithelium. Several EGFP-positive clear cells are seen. (C–E) Higher magnification of the area shown in the box (A and B). Scale bars: A, B: 100 µm; C, D, E: 50 µm.
Figure 2 shows an HIM image of a control rat cauda epididymidis. The high resolution, sharpness and depth of field of HIM images are remarkable even when taken at low magnification. In most orientations, the field of view reveals epididymal clear cells as darker spots of irregular shape and variable size ranging from 5 to 30 µm (arrows), interspersed among principal cells that appear brighter, due to their numerous stereocilia. The inset in Fig. 2 shows an enface view of the epithelium lining the rat cauda epididymidis, immunostained for the vacuolar proton-pumping ATPase, V-ATPase (red), which is expressed in the apical membrane of clear cells.
Figure 2.
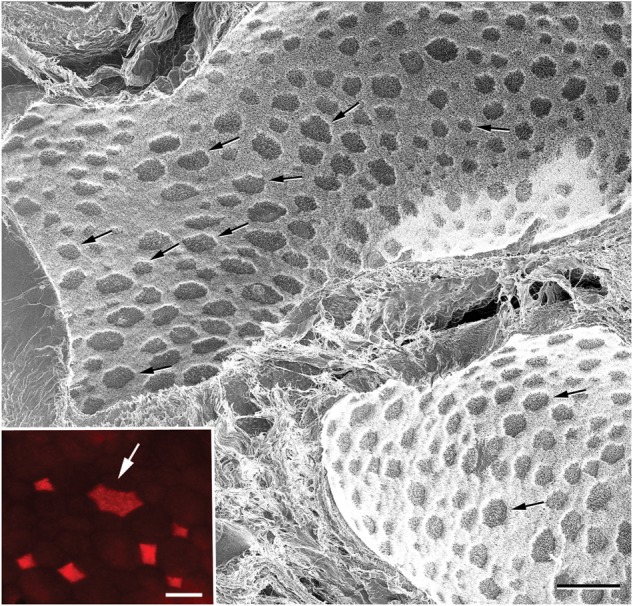
Low-magnification HIM image of a control rat cauda epididymidis. Epididymal spermatozoa were removed by perfusing the lumen with a physiological solution and the apical surface of the epithelium was exposed. Clear cells of various sizes are visible as darker spots (arrows) interspersed among principal cells that appear brighter due to their stereocilia. The inset shows the apical surface of a rat cauda epididymidis immunolabeled for the V-ATPase in red. Several clear cells expressing the V-ATPase are detected. The arrow indicates a large clear cell. Scale bar: 50 µm, Inset: 15 µm.
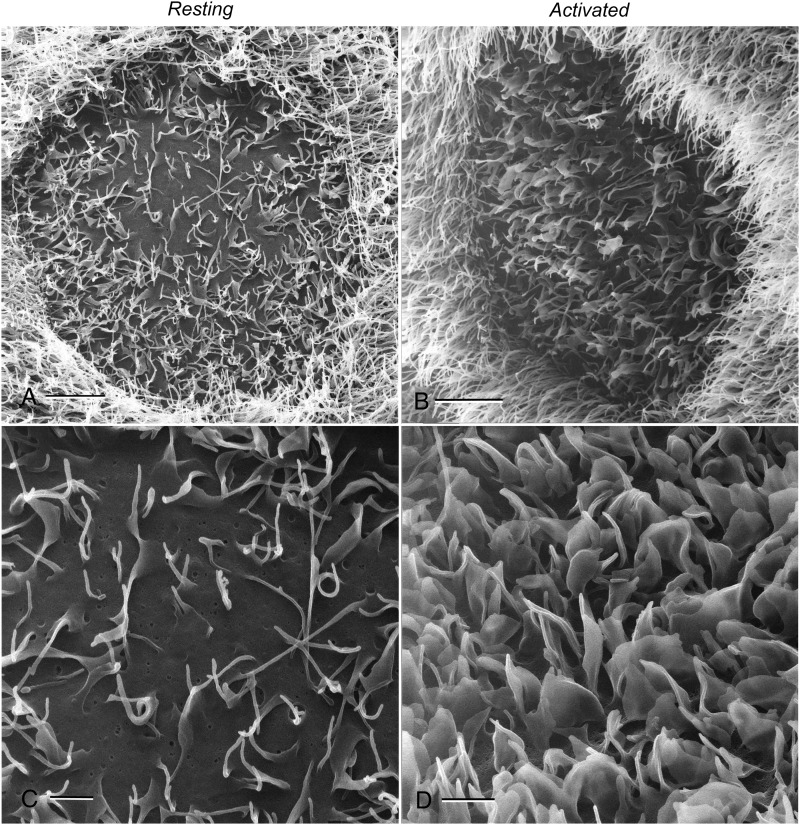
Higher magnification images (Fig. 3) further emphasize the distinction between clear cells and the surrounding principal cells. Under control conditions (Fig. 3A and C), the clear cell apical membrane features microvilli and a few, relatively short microplicae, in contrast to the longer stereocilia of the principal cells. The majority of the clear cell apical membrane is, however, clearly visible and allows for the visualization of indentations. Several factors, including cAMP, alkaline pH and bicarbonate are known to induce an increase in the number and length of apical membrane protrusions. High-magnification HIM imaging of activated clear cells from rat epididymis perfused with a solution containing 1 mM CPT-cAMP and 15 mM bicarbonate shows that these protrusions appear to be microplicae or small membrane ruffles (Fig. 3B and D). The density and size of these microplicae is increased in activated cells to such an extent that the flatter areas of the plasma membrane between the microplicae are virtually no longer visible (Fig. 3D). Similar apical microplicae were detected following cAMP treatment in clear cells of the mouse cauda epididymidis (Fig. 4). Unlike in the rat epididymis, where most clear cells are surrounded exclusively by principal cells, mouse clear cells can be seen clustered together. Interestingly, several membrane protrusions with morphological characteristics of membrane budding during apocrine secretion were detected in clear cells (arrows).
Figure 3.
Characteristic high-magnification images of rat epididymal clear cells under control (A and C) and 8-(4-chlorophenylthio)adenosine 3′,5′-cyclic monophosphate (CPT-cAMP)-activated conditions (B and D). Long stereocilia of principal cells surrounding the clear cell are evident, making the two cell types clearly distinguishable. As also emphasized by the higher magnification image shown in C, the apical membrane of control clear cells contains microvilli and relatively short microplicae, while a large part of the flatter apical membrane domain is devoid of such formations and, thus, remains visible. Conversely, the flat membrane region of the activated clear cell (D) is no longer visible due to the increased density and size of microplicae. Scale bars: A, B: 4 µm; C, D: 1 µm.
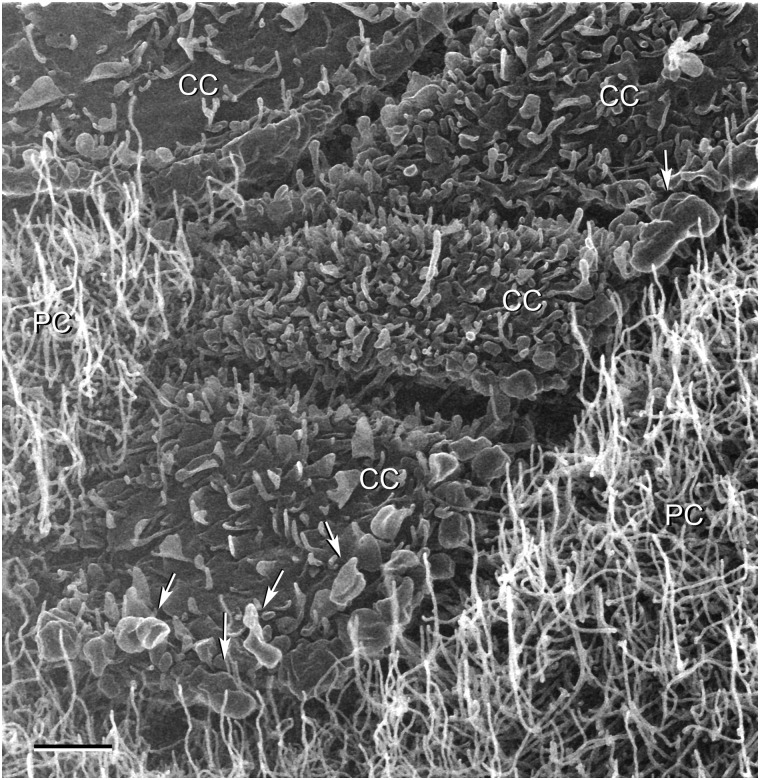
Figure 4.
HIM imaging of the luminal surface of the epididymal epithelium from a mouse cauda epididymidis perfused with an activating solution. The clear cells (CC) can be detected in rows and clusters, and the borders between neighboring clear cells appear dark by HIM. Their apical membranes show various levels of activation (increased size and density of microplicae and microvilli) in response to cAMP and alkaline pH. Some structures compatible with vesicles in the process of budding off the plasma membrane are also detected (arrows). PC: principal cells. Scale bar: 2 µm.
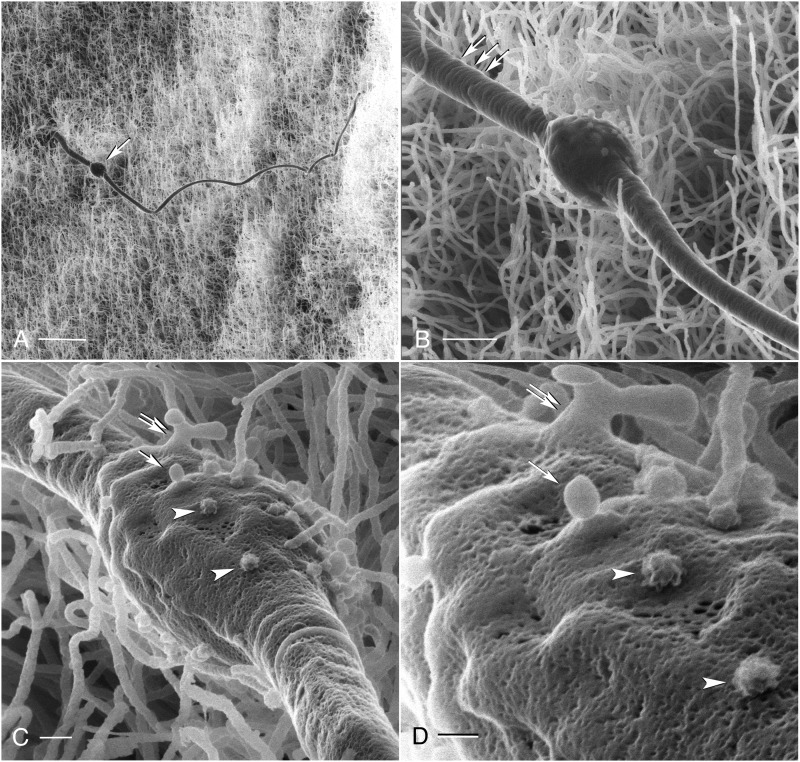
Despite extensive luminal perfusion of the cauda epididymidis before the fixation step, we made the surprising observation that not all sperm cells had been flushed from the lumen. Most of these remaining spermatozoa had their heads anchored within the epididymal epithelium (Fig. 5; arrows), indicating a close physical interaction between spermatozoa and the epithelial cells. The morphology of certain sperm cells suggests a distinct kind of interaction with the epididymal epithelium. Figure 6 shows a mouse spermatozoon whose head is embedded within the layer of principal cell stereocilia (panel A), as also illustrated in Fig. 5. Increasing the magnification reveals concentric rings on the sperm midpiece (Fig. 6B; arrows), as well as small, vesicle-like structures on the surface of the cytosolic droplet located at the mid-principal piece junction (Fig. 6B). The latter can be seen more clearly at an even higher magnification (Fig. 6C and D), where numerous surface depressions also become visible. The vesicle-like structures are round and measure ∼90 nm in diameter. A number of these structures feature a rough surface morphology (arrowheads in C and D), although smooth membranous vesicles can also be detected (arrows and double arrow in C and D). These structures might represent epididymosomes in the process of fusing with the spermatozoon membrane and/or vesicles in the process of budding off the cytoplasmic droplet.
Figure 5.
Sperm cells detected in the lumen of a mouse cauda epididymidis have their heads embedded within the layer of principal cell stereocilia (arrows), suggesting that they physically interact with the epididymal epithelium. These spermatozoa do not have a discernable cytoplasmic droplet. Scale bar: 5 µm.
Figure 6.
(A) A low-magnification HIM image showing a mouse cauda spermatozoon, whose head is embedded within the epididymal epithelium. This spermatozoon has a prominent cytoplasmic droplet located at the mid-principal piece junction (arrow). (B) An intermediate magnification image of the same sperm cell reveals concentric rings on its midpiece (arrows) and vesicle-like structures on the surface of the cytoplasmic droplet. (C and D) High-magnification HIM imaging of the cytoplasmic droplet shows these vesicle-like membranous structures more clearly, alongside a number of surface depressions. Some vesicles have a rough appearance (arrowheads), while other have a smooth surface (arrows). A more complex structure (double arrow) compatible with a membrane protrusion that is budding from the cytoplasmic droplet is also visible. Scale bars: A: 5 µm; B:1 µm; C: 200 nm; D: 100 nm.
Discussion
We describe in this report the first application of HIM technology to the study of the structure and morphology of the rodent epididymis. HIM allows high-resolution imaging of epididymal epithelial cells and spermatozoa. The distinction between epididymal clear and principal cells is obvious in HIM images, based on the brightness of the signal. The dense apical stereocilia of principal cells result in a brighter appearance, as we reported previously for the brush border of the renal proximal tubule, in which the apical microvilli are even more densely packed (Rice et al., 2013). The architecture of the epididymal epithelium, as determined by HIM, confirms previous results obtained using scanning EM (Murakami et al., 1976; Hamilton et al., 1977; Zhou et al., 1987; Fornes and De Rosas, 1991; Susheela and Kumar, 1991; Stoffel and Friess, 1994; Aire and Soley, 2000; Lorenzana et al., 2007), but the images in the current study demonstrate the significant improvement in resolution and overall clarity offered by this technology over previous imaging methods.
We have previously shown that basal cells send a narrow body projection that can extend all the way to the luminal side of the epithelium of rat and mouse epididymis (Shum et al., 2008, 2013, 2014). These luminal-reaching projections are present only in the IS of the mouse epididymis, and in the distal corpus and very proximal cauda epididymidis in the rat. We visualized here a more distal region (mostly the distal cauda), where these projections are very rare, and we therefore attributed the different appearance of the epithelial apical surface to two cell types: principal cells and clear cells. HIM imaging confirmed that clear cells possess a surface morphology that is quite distinct from principal cells. Under resting conditions, the majority of the clear cell membrane is visible and shows the presence of several indentations, reminiscent of renal collecting duct cell membrane features, which are thought to represent various configurations of endo- and/or exocytotic events (Rice et al., 2013). We have previously shown that several factors, including a permeant analog of cAMP (CPT-cAMP) and luminal bicarbonate, stimulate proton secretion in clear cells, via elongation of membrane protrusions that contain a high density of the proton pumping V-ATPase (Pastor-Soler et al., 2003; Beaulieu et al., 2005; Shum et al., 2009). Based on their appearance on 5 µm cryostat sections, we initially labeled these protrusions as ‘microvilli’ (Brown et al., 1992; Breton et al., 1996). HIM imaging now shows that these protrusions are in fact microplicae or small membrane ruffles, in agreement with an earlier study by the Cooper group, who used scanning EM to show the presence of ‘leaf-like’ microvilli on the surface of clear cells (Hamilton et al., 1977). A similar response to cAMP treatment has been detected when using HIM to image intercalated cells of the renal collecting duct (our unpublished observations), which extended the characterization of the previously reported effect of cAMP on the length of apical microvilli in this cell type (Paunescu et al., 2010). In contrast to the dramatic effect of CPT-cAMP and bicarbonate on clear cells, we did not detect morphological changes in epididymal principal cells after perfusion under these conditions in either rat or mouse epididymis.
In the mouse epididymis, in contrast to the rat epididymis, clusters of adjacent clear cells were detected. In previous studies, these clusters appeared as rows of clear cells in 5 µm cryostat sections (Miller et al., 2005). In mouse clear cells, in addition to microplicae we also detected the presence of large membrane protrusions. These might reflect budding of vesicles from the apical plasma membrane. While the process of vesicle shedding via plasma membrane budding was originally thought to represent an artifact caused by poor fixation, these vesicles are now considered to play significant roles in the communication between neighboring cells (Cocucci et al., 2009). Epididymosomes are important contributors to sperm maturation in the epididymis, allowing the transfer of new proteins from epithelial cells to the sperm cells (Belleannee et al., 2013; Sullivan and Saez, 2013). They are composed of different types of extracellular vesicles, originating either from the fusion of multivesicular bodies with the apical membrane followed by the release of exosomes, or from the budding of larger vesicles from the plasma membrane. While principal cells are currently thought to be the main contributors of epididymosomes to the luminal fluid, our data suggest that clear cells might also participate in the formation of at least one type of microvesicle—via budding of their plasma membrane. Interestingly, we have recently shown that cSrc, which is abundantly expressed in clear cells, is incorporated into spermatozoa during their transit through the mouse cauda epididymidis (Krapf et al., 2012). cSrc might, therefore, be delivered to spermatozoa via vesicles that budded off the plasma membrane of clear cells. The presence of a very dense array of stereocilia in principal cells precluded the detection of any exocytotic or fusion event that would take place at the level of the plasma membrane.
The ultrastructure of sperm cells and their interaction with the epithelium were also investigated using HIM technology. High-resolution images confirmed structural features of spermatozoa, such as the presence of concentric rings on the sperm midpiece, which have been previously described using scanning EM (Villalpando et al., 2000). In addition, in agreement with previous studies (Cooper, 2011; Xu et al., 2013), some spermatozoa had a cytoplasmic droplet located at the mid-principal piece junction (Fig. 6), while others did not have a discernable cytoplasmic droplet (Fig. 5). We describe here two aspects of the interaction between the spermatozoa and the epididymal epithelium. The concept of a direct physical interaction is supported by the presence of isolated sperm cells that remained attached to the epididymal tubule even after a lengthy period of luminal perfusion. These spermatozoa had their heads firmly anchored within the epithelium, indicating a very close interaction with epithelial cells. In a scanning EM study, a close interaction between spermatozoa and epididymal epithelium has also been previously reported in the rat epididymis (Fornes and De Rosas, 1991). Whether this interaction is indicative of the growing (albeit controversial) notion that active selection, removal and degradation of spermatozoa occur during epididymal transit (Sutovsky, 2003; Axner, 2006; Jrad-Lamine et al., 2011) will require further investigation. Interestingly, a different, indirect type of interaction is suggested by the fact that the surface of the sperm cytoplasmic droplet is covered with vesicle-like structures. The roughly spherical shape and the size (∼90 nm in diameter) of some of these structures suggest that they are epididymosomes imaged while fusing with the spermatozoon membrane, to deliver proteins originating from the epithelium during epididymal maturation (Saez et al., 2003; Sullivan et al., 2007; Thimon et al., 2008; Oh et al., 2009; Frenette et al., 2010; Sullivan and Saez, 2013). In addition, we also detected some structures that appeared to be in the process of budding from the cytoplasmic droplet.
In conclusion, our results confirm that the HIM technology advances the imaging of biological samples significantly and can be applied successfully to study the male reproductive tract and spermatozoa, thus enhancing our understanding of their structure, function and intercellular communication.
Authors' roles
T.G.P., D.B. and S.B. conceived and designed the study. T.G.P., W.W.C.S., C.H., L.L., B.G. and S.B. performed the experiments and collected the data. T.G.P., D.B. and S.B. performed the data analysis. T.G.P. and S.B. wrote the draft manuscript. All authors contributed to the final version of the manuscript.
Funding
This work was supported by NIH grants DK073266 to T.G.P., DK042956 to D.B., HD040793, DK097124 and DK085715 to S.B. Additional funding was provided by a sponsored research agreement to D.B. from the Zeiss Corporation.
Conflict of interest
T.G.P. and D.B. received partial funding from a sponsored research agreement with the Zeiss Corporation. The co-authors Chuong Huynh, Lorenz Lechner and Bernhard Goetze are employees of the Zeiss Corporation.
Acknowledgements
We are grateful to Dr Paul Kelly of Salem State University, Salem, MA, USA and to Ms. Ann Tisdale of the Schepens Eye Research Institute, Boston, MA, USA for providing access to their critical point drying apparatus.
References
- Aire TA, Soley JT. The surface features of the epithelial lining of the ducts of the epididymis of the ostrich (Struthio camelus) Anat Histol Embryol. 2000;29:119–126. doi: 10.1046/j.1439-0264.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- Anberg A. The ultrastructure of the human spermatozoon; an electronmicroscopic study of spermatozoa from sperm samples and the epididymis including some observations of the spermatid. Acta Obstet Gynecol Scand Suppl. 1957;36(Suppl 2):1–133. [PubMed] [Google Scholar]
- Axner E. Sperm maturation in the domestic cat. Theriogenology. 2006;66:14–24. doi: 10.1016/j.theriogenology.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Bazou D, Behan G, Reid C, Boland JJ, Zhang HZ. Imaging of human colon cancer cells using He-Ion scanning microscopy. J Microsc. 2011a;242:290–294. doi: 10.1111/j.1365-2818.2010.03467.x. [DOI] [PubMed] [Google Scholar]
- Bazou D, Santos-Martinez MJ, Medina C, Radomski MW. Elucidation of flow-mediated tumour cell-induced platelet aggregation using an ultrasound standing wave trap. Br J Pharmacol. 2011b;162:1577–1589. doi: 10.1111/j.1476-5381.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu V, Da Silva N, Pastor-Soler N, Brown CR, Smith PJ, Brown D, Breton S. Modulation of the actin cytoskeleton via gelsolin regulates vacuolar H+-ATPase recycling. J Biol Chem. 2005;280:8452–8463. doi: 10.1074/jbc.M412750200. [DOI] [PubMed] [Google Scholar]
- Bell DC. Contrast mechanisms and image formation in helium ion microscopy. Microsc Microanal. 2009;15:147–153. doi: 10.1017/S1431927609090138. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Da Silva N, Shum WW, Brown D, Breton S. Role of purinergic signaling pathways in V-ATPase recruitment to apical membrane of acidifying epididymal clear cells. Am J Physiol Cell Physiol. 2010;298:C817–C830. doi: 10.1152/ajpcell.00460.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleannee C, Thimon V, Sullivan R. Region-specific gene expression in the epididymis. Cell Tissue Res. 2012;349:717–731. doi: 10.1007/s00441-012-1381-0. [DOI] [PubMed] [Google Scholar]
- Belleannee C, Calvo E, Caballero J, Sullivan R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol Reprod. 2013;89:30. doi: 10.1095/biolreprod.113.110486. [DOI] [PubMed] [Google Scholar]
- Breton S, Smith PJ, Lui B, Brown D. Acidification of the male reproductive tract by a proton pumping (H+)-ATPase. Nat Med. 1996;2:470–472. doi: 10.1038/nm0496-470. [DOI] [PubMed] [Google Scholar]
- Breton S, Wiederhold T, Marshansky V, Nsumu NN, Ramesh V, Brown D. The B1 subunit of the H+ATPase is a PDZ domain-binding protein. Colocalization with NHE-RF in renal B-intercalated cells. J Biol Chem. 2000;275:18219–18224. doi: 10.1074/jbc.M909857199. [DOI] [PubMed] [Google Scholar]
- Brown D, Lui B, Gluck S, Sabolic I. A plasma membrane proton ATPase in specialized cells of rat epididymis. Am J Physiol. 1992;263(4 Pt 1):C913–C916. doi: 10.1152/ajpcell.1992.263.4.C913. [DOI] [PubMed] [Google Scholar]
- Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC, Hansen LA. Micropuncture and cannulation studies of fluid composition and transport in the ductuli efferentes testis of the rat: comparisons with the homologous metanephric proximal tubule. Exp Physiol. 1994;79:915–928. doi: 10.1113/expphysiol.1994.sp003817. [DOI] [PubMed] [Google Scholar]
- Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Cooper TG. Epididymis. In: Neill JD, Knobil E, editors. Encyclopedia of Reproduction. San Diego: Academic Press; 1998. pp. 1–17. [Google Scholar]
- Cooper TG. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl. 2011;13:130–138. doi: 10.1038/aja.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–227. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr DG, Gregory M, Dube E, Dufresne J, Chan PT, Hermo L. Orchestration of occludins, claudins, catenins and cadherins as players involved in maintenance of the blood–epididymal barrier in animals and humans. Asian J Androl. 2007;9:463–475. doi: 10.1111/j.1745-7262.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Da Silva N, Pietrement C, Brown D, Breton S. Segmental and cellular expression of aquaporins in the male excurrent duct. Biochim Biophys Acta. 2006;1758:1025–1033. doi: 10.1016/j.bbamem.2006.06.026. [DOI] [PubMed] [Google Scholar]
- Fahrmann W, Schuchardt E. [Light and electron microscopic studies on epididymis epithelium of rats before and after puberty] Z Mikrosk Anat Forsch. 1966;74:337–362. [PubMed] [Google Scholar]
- Fornes MW, De Rosas JC. Interactions between rat epididymal epithelium and spermatozoa. Anat Rec. 1991;231:193–200. doi: 10.1002/ar.1092310207. [DOI] [PubMed] [Google Scholar]
- Frenette G, Legare C, Saez F, Sullivan R. Macrophage migration inhibitory factor in the human epididymis and semen. Mol Hum Reprod. 2005;11:575–582. doi: 10.1093/molehr/gah197. [DOI] [PubMed] [Google Scholar]
- Frenette G, Girouard J, D'Amours O, Allard N, Tessier L, Sullivan R. Characterization of two distinct populations of epididymosomes collected in the intraluminal compartment of the bovine cauda epididymis. Biol Reprod. 2010;83:473–480. doi: 10.1095/biolreprod.109.082438. [DOI] [PubMed] [Google Scholar]
- Hamilton DW, Olson GE, Cooper TG. Regional variation in the surface morphology of the epithelium of the rat ductuli efferentes, ductus epididymidis and vas deferens. Anat Rec. 1977;188:13–27. doi: 10.1002/ar.1091880103. [DOI] [PubMed] [Google Scholar]
- Harrington MG, Fonteh AN, Oborina E, Liao P, Cowan RP, McComb G, Chavez JN, Rush J, Biringer RG, Huhmer AF. The morphology and biochemistry of nanostructures provide evidence for synthesis and signaling functions in human cerebrospinal fluid. Cerebrospinal Fluid Res. 2009;6:10. doi: 10.1186/1743-8454-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herak-Kramberger CM, Breton S, Brown D, Kraus O, Sabolic I. Distribution of the vacuolar H+ atpase along the rat and human male reproductive tract. Biol Reprod. 2001;64:1699–1707. doi: 10.1095/biolreprod64.6.1699. [DOI] [PubMed] [Google Scholar]
- Hermo L, Jacks D. Nature's ingenuity: bypassing the classical secretory route via apocrine secretion. Mol Reprod Dev. 2002;63:394–410. doi: 10.1002/mrd.90023. [DOI] [PubMed] [Google Scholar]
- Holstein AF. Electron microscopic studies on the epididymis in rabbits. Verh Anat Ges. 1964;59:53–61. [PubMed] [Google Scholar]
- Horstmann E. Electron microscopy of human epididymis epithelium. Z Zellforsch Mikrosk Anat. 1962;57:692–718. [PubMed] [Google Scholar]
- Horstmann E, Richter R, Roosen-Runge E. On electron microscopy of nuclear inclusions in human epididymal epithelium. Z Zellforsch Mikrosk Anat. 1966;69:69–79. [PubMed] [Google Scholar]
- Jenkins AD, Lechene CP, Howards SS. Concentrations of seven elements in the intraluminal fluids of the rat seminiferous tubules, rate testis, and epididymis. Biol Reprod. 1980;23:981–987. doi: 10.1095/biolreprod23.5.981. [DOI] [PubMed] [Google Scholar]
- Jones RC. Evolution of the vertebrate epididymis. J Reprod Fertil Suppl. 1998;53:163–181. [PubMed] [Google Scholar]
- Joseph A, Shur BD, Hess RA. Estrogen, efferent ductules, and the epididymis. Biol Reprod. 2011;84:207–217. doi: 10.1095/biolreprod.110.087353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jrad-Lamine A, Henry-Berger J, Gourbeyre P, Damon-Soubeyrand C, Lenoir A, Combaret L, Saez F, Kocer A, Tone S, Fuchs D, et al. Deficient tryptophan catabolism along the kynurenine pathway reveals that the epididymis is in a unique tolerogenic state. J Biol Chem. 2011;286:8030–8042. doi: 10.1074/jbc.M110.172114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Rupp C, Stoeck A, Runz S, Fogel M, Lugert S, Hager HD, Abdel-Bakky MS, Gutwein P, Altevogt P. CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 2007;72:1095–1102. doi: 10.1038/sj.ki.5002486. [DOI] [PubMed] [Google Scholar]
- Koga D, Ushiki T. Three-dimensional ultrastructure of the Golgi apparatus in different cells: high-resolution scanning electron microscopy of osmium-macerated tissues. Arch Histol Cytol. 2006;69:357–374. doi: 10.1679/aohc.69.357. [DOI] [PubMed] [Google Scholar]
- Krapf D, Ruan YC, Wertheimer EV, Battistone MA, Pawlak JB, Sanjay A, Pilder SH, Cuasnicu P, Breton S, Visconti PE. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev Biol. 2012;369:43–53. doi: 10.1016/j.ydbio.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeson TS, Leeson CR. An electron microscope study of the postnatal development of the ductus epididymis in the rat. Anat Anz. 1964;114:168–180. [PubMed] [Google Scholar]
- Levine N, Marsh DJ. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971;213:557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YW, Hsu TH, Yen PH. Mouse sperm acquire a new structure on the apical hook during epididymal maturation. Asian J Androl. 2013;15:523–528. doi: 10.1038/aja.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzana MG, Lopez-Wilchis R, Gomez CS, Aranzabal MC. A light and scanning electron microscopic study of the epididymis active state of the endemic Mexican rodent Peromyscus winkelmanni (Carleton) (Rodentia: Muridae) Anat Histol Embryol. 2007;36:230–240. doi: 10.1111/j.1439-0264.2006.00752.x. [DOI] [PubMed] [Google Scholar]
- Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD. V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol. 2005;288:C1134–C1144. doi: 10.1152/ajpcell.00084.2004. [DOI] [PubMed] [Google Scholar]
- Miranda KC, Bond DT, McKee M, Skog J, Paunescu TG, Da Silva N, Brown D, Russo LM. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int. 2010;78:191–199. doi: 10.1038/ki.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Shimada T, Noda S, Kawano T. Effects of castration and androgen-substitution on the morphology of the epididymal epithelium of the Japanese monkey, Macacus fuscatus, as revealed by scanning electron microscopy. Cell Tissue Res. 1976;170:515–521. doi: 10.1007/BF00361709. [DOI] [PubMed] [Google Scholar]
- Nicander L. Fine structure and cytochemistry of nuclear inclusions in the dog epididymis. Exp Cell Res. 1964;34:533–541. doi: 10.1016/0014-4827(64)90239-3. [DOI] [PubMed] [Google Scholar]
- Oh JS, Han C, Cho C. ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol Cells. 2009;28:441–446. doi: 10.1007/s10059-009-0140-x. [DOI] [PubMed] [Google Scholar]
- Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AD, Cornwall GA, Liu LY, Smith CE, Hermo L. Alterations in the testis and epididymis associated with loss of function of the cystatin-related epididymal spermatogenic (CRES) protein. J Androl. 2011;32:444–463. doi: 10.2164/jandrol.110.010694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, Buck J, Levin LR, Breton S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–49529. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu TG, Da Silva N, Marshansky V, McKee M, Breton S, Brown D. Expression of the 56-kDa B2 subunit isoform of the vacuolar H(+)-ATPase in proton-secreting cells of the kidney and epididymis. Am J Physiol Cell Physiol. 2004;287:C149–C162. doi: 10.1152/ajpcell.00464.2003. [DOI] [PubMed] [Google Scholar]
- Paunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, Breton S, Brown D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol. 2010;298:F643–F654. doi: 10.1152/ajprenal.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrement C, Sun-Wada GH, Silva ND, McKee M, Marshansky V, Brown D, Futai M, Breton S. Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod. 2006;74:185–194. doi: 10.1095/biolreprod.105.043752. [DOI] [PubMed] [Google Scholar]
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajalakshmi M, Kumar BV, Kapur MM, Pal PC. Ultrastructural changes in the efferent duct and epididymis of men with obstructive infertility. Anat Rec. 1993;237:199–207. doi: 10.1002/ar.1092370207. [DOI] [PubMed] [Google Scholar]
- Reed CI, Reed BP. Electron microscopy of bull sperm. Fed Proc. 1947;6(1 Pt 2):186. [PubMed] [Google Scholar]
- Reed CI, Reed BP. Comparative study of human and bovine sperm by electron microscopy. Anat Rec. 1948;100:1–7. doi: 10.1002/ar.1091000102. [DOI] [PubMed] [Google Scholar]
- Rejraji H, Sion B, Prensier G, Carreras M, Motta C, Frenoux JM, Vericel E, Grizard G, Vernet P, Drevet JR. Lipid remodeling of murine epididymosomes and spermatozoa during epididymal maturation. Biol Reprod. 2006;74:1104–1113. doi: 10.1095/biolreprod.105.049304. [DOI] [PubMed] [Google Scholar]
- Rice WL, Van Hoek AN, Paunescu TG, Huynh C, Goetze B, Singh B, Scipioni L, Stern LA, Brown D. High resolution helium ion scanning microscopy of the rat kidney. PLoS One. 2013;8:e57051. doi: 10.1371/journal.pone.0057051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Hinton B, Orgebin-Crist M. The epididymis. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 3rd edn. New York: Elsevier; 2006. pp. 1071–1148. [Google Scholar]
- Saez F, Frenette G, Sullivan R. Epididymosomes and prostasomes: their roles in posttesticular maturation of the sperm cells. J Androl. 2003;24:149–154. doi: 10.1002/j.1939-4640.2003.tb02653.x. [DOI] [PubMed] [Google Scholar]
- Sanders JH. Electron microscope studies of normal human spermatozoa. West J Surg Obstet Gynecol. 1948;56:306–308. [PubMed] [Google Scholar]
- Schmidt FC. Light and electron microscopic studies on the human testis and epididymis. Z Zellforsch Mikrosk Anat. 1964;63:707–727. [PubMed] [Google Scholar]
- Shum WW, Da Silva N, McKee M, Smith PJ, Brown D, Breton S. Transepithelial projections from basal cells are luminal sensors in pseudostratified epithelia. Cell. 2008;135:1108–1117. doi: 10.1016/j.cell.2008.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, Brown D, Breton S. Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol. 2009;212(Pt 11):1753–1761. doi: 10.1242/jeb.027284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Da Silva N, Belleannee C, McKee M, Brown D, Breton S. Regulation of V-ATPase recycling via a RhoA- and ROCKII-dependent pathway in epididymal clear cells. Am J Physiol Cell Physiol. 2011a;301:C31–C43. doi: 10.1152/ajpcell.00198.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Ruan YC, Da Silva N, Breton S. Establishment of cell-cell cross talk in the epididymis: control of luminal acidification. J Androl. 2011b;32:576–586. doi: 10.2164/jandrol.111.012971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Hill E, Brown D, Breton S. Plasticity of basal cells during postnatal development in the rat epididymis. Reproduction. 2013;146:455–469. doi: 10.1530/REP-12-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum WW, Smith TB, Cortez-Retamozo V, Grigoryeva LS, Roy JW, Hill E, Pittet MJ, Breton S, Da Silva N. Epithelial basal cells are distinct from dendritic cells and macrophages in the mouse epididymis. Biol Reprod. 2014;90:90. doi: 10.1095/biolreprod.113.116681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel MH, Friess AE. Morphological characteristics of boar efferent ductules and epididymal duct. Microsc Res Tech. 1994;29:411–431. doi: 10.1002/jemt.1070290603. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Saez F. Epididymosomes, prostasomes, and liposomes: their roles in mammalian male reproductive physiology. Reproduction. 2013;146:R21–R35. doi: 10.1530/REP-13-0058. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Frenette G, Girouard J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl. 2007;9:483–491. doi: 10.1111/j.1745-7262.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Susheela AK, Kumar A. A study of the effect of high concentrations of fluoride on the reproductive organs of male rabbits, using light and scanning electron microscopy. J Reprod Fertil. 1991;92:353–360. doi: 10.1530/jrf.0.0920353. [DOI] [PubMed] [Google Scholar]
- Sutovsky P. Ubiquitin-dependent proteolysis in mammalian spermatogenesis, fertilization, and sperm quality control: killing three birds with one stone. Microsc Res Tech. 2003;61:88–102. doi: 10.1002/jemt.10319. [DOI] [PubMed] [Google Scholar]
- Takahashi KL, Takahashi N, Hojo H, Kuwahara M, Aoyama H, Teramoto S. Pathogenetic transition in the morphology of abnormal sperm in the testes and the caput, corpus, and cauda epididymides of male rats after treatment with 4,6-dinitro-o-cresol. Reprod Toxicol. 2006;22:501–507. doi: 10.1016/j.reprotox.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Thimon V, Frenette G, Saez F, Thabet M, Sullivan R. Protein composition of human epididymosomes collected during surgical vasectomy reversal: a proteomic and genomic approach. Hum Reprod. 2008;23:1698–1707. doi: 10.1093/humrep/den181. [DOI] [PubMed] [Google Scholar]
- Vanden Berg-Foels W, Scipioni L, Huynh C, Wen X. High resolution visualization of the articular cartilage collagen network by helium ion microscopy. Microsc Microanal. 2011;17:282–283. doi: 10.1111/j.1365-2818.2012.03606.x. [DOI] [PubMed] [Google Scholar]
- Villalpando I, Villafan-Monroy H, Aguayo D, Zepeda-Rodriguez A, Espitia HG, Chavez-Olivares A. Ultrastructure and motility of the caudal epididymis spermatozoa from the volcano mouse (Neotomodon alstoni alstoni Merriam, 1898) J Exp Zool. 2000;287:316–326. [PubMed] [Google Scholar]
- Ward B, Notte JA, Economou NP. Helium-ion microscopy. Photonic Spectra. 2007;41:68–70. [Google Scholar]
- Xu H, Yuan SQ, Zheng ZH, Yan W. The cytoplasmic droplet may be indicative of sperm motility and normal spermiogenesis. Asian J Androl. 2013;15:799–805. doi: 10.1038/aja.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R, Kamiguchi Y, Mikamo K, Suzuki F, Yanagimachi H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am J Anat. 1985;172:317–330. doi: 10.1002/aja.1001720406. [DOI] [PubMed] [Google Scholar]
- Zhou LF, Qi SQ, Lei HP. Effect of gossypol acetic acid on the epididymis: histochemical and scanning electron microscope studies. J Ethnopharmacol. 1987;20:39–43. doi: 10.1016/0378-8741(87)90117-6. [DOI] [PubMed] [Google Scholar]