Cultivating crops under saline conditions is of high importance due to global fresh water shortage for irrigation. Crithmum maritimum is a halophytic plant that has a long history of human consumption and was suggested as a cash crop for biosaline agriculture. Our results highlight variations existing among Crithmum maritimum genotypes from different geographic origins regarding salt-induced changes in plant growth, flowering behavior and leaf metabolites with nutritional value. Our results indicate that genotypic characteristics should be taken into account when evaluating wild plant species for future crop cultivation.
Keywords: Antioxidant compounds, Crithmum maritimum, flowering, germination, omega-3 polyunsaturated fatty acids, salinity.
Abstract
The fresh water shortage in agriculture is an increasing problem worldwide, therefore the possibility of cultivating crops under saline conditions is of high importance. Crithmum maritimum, a halophytic plant naturally found on the rocky coastlines of the Atlantic Ocean and the Mediterranean Sea, has a long history of human consumption and was recently suggested as a cash crop for biosaline agriculture. In the present study, we compared the responses of different genotypes originating from France, Portugal and Israel to moderate saline irrigation (up to 100 mM NaCl). The genotypes varied greatly in the onset of flowering, their leaf appearance, growth habits and leaf metabolite content. Both Atlantic genotypes (from France and Portugal) flowered earlier than those from the Mediterranean, but the number of inflorescences decreased with salinity. Irrigation with 50 and 100 mM NaCl led to a reduction in biomass production in both the Israeli and the Portuguese genotypes, while the French genotype was found to produce maximum leaf yield at 50 mM NaCl. With increasing salinity, salt was accumulated by the plants, as indicated by increasing electrical conductivities of the leaf extracts. Concomitantly, antioxidant compounds (such as ascorbic acid), total polyphenols and ureides responded to salinity in a genotype-dependent manner; either they increased, decreased or were unaffected. Notably, the total fatty acid concentration increased with salinity in both Mediterranean genotypes, reaching 2.7 and 2.4 % total fatty acids (on a dry weight basis) at 100 mM NaCl. Moreover, the proportion assigned to omega-3 fatty acids in these genotypes was higher than in their Atlantic counterparts at the highest salinity tested. Our results highlight the variations existing among C. maritimum genotypes from different origins regarding salt-induced changes in plant growth, flowering behaviour and leaf metabolites with nutritional value. Thus, genotypic characteristics should be taken into account when evaluating a wild plant species for future crop cultivation.
Introduction
Salinization is one of the major environmental constraints limiting crop production (Maggio et al. 2011). This phenomenon is particularly expressed in arid and semiarid regions due to high evaporation and low precipitation rates. Paradoxically, irrigation in these extreme environments has led to the accumulation of salts in the uppermost soil layers of arable lands. Thus, to date, large areas of freshwater-irrigated lands have suffered from salt and water build-up in the root zone (Yensen 2006; Rozema and Flowers 2008). Halophytes are equipped with well-defined adaptive mechanisms that enable them not only to withstand periodical high salinity, but also to complete their entire lifecycles at high salinities (Flowers et al. 2010). The tolerance of halophytes to salinity relies mainly on the controlled uptake of ions and the vacuolar compartmentalization of Na+, K+ and Cl− with the achievement of an osmotic balance between vacuoles and cytoplasm by synthesis of osmotically active metabolites (Sagi et al. 1997; Flowers and Colmer 2008). Conversely, the increased synthesis of these organic compounds and the active transport of toxic ions across the vacuolar membrane have a considerable energetic cost, resulting in growth retardation and ultimately in the reduction of productivity (Greenway and Munns 1980; Zhu 2001). Nevertheless, halophytes are able to generate economic yields, although exposed to salt stress conditions (Koyro et al. 2011; Ventura et al. 2011a).
When plants are subjected to environmental stresses, including salinity, the production of reactive oxygen species may disrupt the integrity of cellular membranes and the activities of various enzymes resulting, eventually, in plant damage (Zhu 2001). To counteract the salt-mediated oxidative stress, plants up-regulate antioxidative enzymes and production of small non-enzymatic molecules [e.g. ascorbate (ASC), flavenoids and polyphenolic compounds]. High levels of antioxidants were found to be associated with oxidative stress tolerance in glycophytes and halophytes (Türkan and Demiral 2009). In addition, ureides, which are nitrogen-rich compounds with antioxidant activity, may play an important role during nitrogen transport under stressful conditions (Brychkova et al. 2008; Ventura et al. 2010). Enhanced tolerance to salt stress can be further achieved by changes in the saturation of fatty acids in the chloroplast membrane (Allakhverdiev et al. 1999), which mainly comprises α-linolenic acid (18 : 3ω3) and linoleic acid (18 : 2) in many plants (Simopoulos 2004).
Crithmum maritimum (Apiaceae) is a wild halophyte growing on the rocky coast lines of Western Europe and the Mediterranean. This plant species is well known for its high content of antioxidant compounds, and its leaves have been consumed, fresh or pickled, by humans for many years (Meot-Duros and Magné 2009; Atia et al. 2011). Crithmum maritimum was listed as a salt-tolerant species with the potential to be developed into a cash crop halophyte (Yensen 2006; Atia et al. 2011). To date, halophytes have had a broad field of utilization, ranging from consumption, through ornamental applications and renewable energy sources, to environmental protection (Koyro et al. 2011). An initial step in the development of a halophytic species as a cash crop includes the primary screening for its agronomic potential (Koyro et al. 2006).
Most publications investigating C. maritimum have used plant material collected from the wild, referring to specific sites, environments and seasons of sampling and therefore comparing only a narrow range of local genotypes (Cunsolo et al. 1993; Guil-Guerrero and Rodríguez-García 1999; Ben Amor et al. 2005; Özcan et al. 2006; Meot-Duros and Magné 2009). In the current study, we investigated the potential of four genotypes of C. maritimum for dual application: as an ornamental crop and as a leafy vegetable crop, both for saline agriculture. We determined leaf and flower appearance, biomass production and changes in nutritional metabolites in response to increasing salinity.
Methods
Sample collection and experimental setup
Seeds of two Crithmum genotypes were collected at two locations along the Mediterranean Sea shore in Israel and designated HB and IS. Two additional genotypes, originating from the Atlantic coasts of southern Portugal (PO) and Brittany in France (FR), were compared with their Mediterranean counterparts. The collected seeds were stored in a cold room at 4 °C for ∼2 months until sowing. All experiments were carried out in a temperature-controlled greenhouse located at Ben-Gurion University in Beer Sheva, Israel. Summer temperatures were kept <33 °C, using a cooling system, while in the winter the greenhouse was heated when temperatures dropped <20 °C. Midday photosynthetic photon flux density in the greenhouse was 300–500 μmol m−2 s−1. Seeds were germinated in 9-cm diameter Petri dishes on a double layer of filter paper (Whatman No. 1, Maidstone, Kent, UK) at room temperature (25 °C ± 3) using distilled water. Germinated seedlings of similar size were transferred to 12-cm diameter plastic pots filled with commercial potting mixture HR-1, composed of peat, tuff and synthetic sponge, including slow release fertilizer (Shacham Givat Ada, Ltd, Israel) and grown in the same greenhouse. Two weeks after plant transfer salt treatments (0, 50 and 100 mM NaCl) were applied by gradually (over 2 weeks) increasing the salt concentrations until the final concentration was reached. Plants were irrigated three times a week with the respective salt solution supplemented with commercial N–P–K fertilizer (20–20–20+microelements; Haifa Chemicals Ltd, Haifa, Israel) at a final concentration of 40 ppm nitrogen. Each salt concentration comprised five plants per genotype.
Growth parameters
Plants were grown for ∼2.5 months and then harvested by cutting the green biomass ∼2 cm above the pot. Immediately after harvest, total shoot biomass per harvested pot unit was recorded. The dry weight was obtained after drying the plant material at 70 °C for 48 h and per cent dry weight was calculated. Leaves were photographed to document their morphology. Complete plant re-growth occurred approximately within a 1-month time period after the harvest (data not shown).
Flower appearance
Appearance of inflorescences was recorded for the first time ∼2.5 months after the leaf harvest (months after harvest, MAH) and their number was recorded. The number of flowering branches was confirmed at two additional time points, 3.5 and 4.5 MAH. The data represent one of two similar experiments conducted during the same season.
Evaluation of leaf constituents
Fresh leaf samples were collected before flower initiation, immediately snap-frozen in liquid nitrogen and stored at −80 °C until further evaluation. Extraction was performed as described previously by Ventura et al. (2011a). Briefly, the frozen samples were homogenized on ice at a 1 : 4 (w/v) ratio with doubly distilled water, centrifuged at 17 500 g for 20 min at 4 °C and filtered through Whatman filter paper No.1. Electrical conductivity (EC) was measured in the filtered water extract with a conductivity meter (CyberSan 500 Con, Eutech Instruments, Singapore), and total soluble solids (TSS [%]) were determined using a digital refractometer (Atago PR-1, Atago Co. Ltd, Japan).
Determination of reduced ascorbate and dehydroascorbate
Ascorbate determination followed the procedure described by Law et al. (1983). Frozen plant tissue was homogenized with 5 % meta-phosphoric acid at a ratio of 1 : 5 (w/v). The extract was centrifuged at 14 000 g for 20 min at 4 °C (Z 233 MK-2, HERMLE) and the supernatant was collected. Reduced ASC was assayed in 5 mM ethylenediaminetetraacetic acid in 150 mM sodium-phosphate buffer (pH 7.4). Dehydroascorbate (DHA) was reduced to ASC prior to determination by the addition of 10 mM dithiothreitol (DTT), the excess of which was oxidized after 15-min incubation by the addition of 0.5 % N-ethylmalemide. The colorimetric assay was carried out at 37 °C for 1 h after the addition of a mixture containing 10 % trichloroacetic acid, 44 % o-phosphoric acid, 4 % α,α-dipyridil in ethanol and 3 % aqueous ferric chloride solution at a ratio of 2 : 2 : 1 : 1 (v/v). Light absorbance of the resultant samples was measured at 525 nm. Dehydroascorbate was calculated by the difference between total ASC and reduced ASC.
Total polyphenol determination
Total polyphenols were determined according to Singleton and Rossi (1965). Frozen leaf samples were extracted with 100 mM sodium-phosphate buffer (pH 7) at a 1 : 4 (w/v) ratio using a mortar and pestle. Resulting extracts were centrifuged for 20 min, 14 000 g at 4 °C (Z 233 MK-2, HERMLE). Aliquots of the collected supernatant were assayed with 2 N Folin–Cioclateur reagent (Sigma) and 35 % Na2CO3, by 60-min incubation at 30 °C in a water bath. The absorbance of the resulting colour was measured at 730 nm (JASCO, model V-530) against a known standard of gallic acid. Total polyphenols were expressed in mg 100 g−1 FW as gallic acid equivalents (GAE).
Determination of the ureides, allantoin and allantoate
Frozen leaf material was ground with 80 % ethanol at a ratio of 1 : 4 (w/v). The plant extract was centrifuged as described above. The collected supernatant was used to determine ureides according to Vogel and van der Drift (1970). For allantoin (ALN), the sample was boiled at 100 °C in 0.5 M NaOH for 8 min, then neutralized with 0.65 N HCl and boiled again for 4 min. Subsequently, 0.4 M P-buffer (Na2HPO4–KH2PO4) pH 7.0 and 18 mM phenyl hydrazine were added. Finally, the assay was mixed with concentrated ice-cold HCl and 50.6 mM potassium ferricyanide. Absorbance was determined at 535 nm. For allantoate (ALT) determination, 0.15 N HCl was added to the ethanolic extract and the assay mixture was boiled for 4 min at 100 °C. After cooling down, the assay was continued from the step of P-buffer addition as described for ALN.
Fatty acid profile
To determine the fatty acid profile and content, young shoot tips and leaves were dipped for 30 s in chloroform to remove cuticular waxes prior to lyophilization. The lyophilized plant material was then ground into powder and transmethylated with 2 % sulfuric acid in dry methanol at 80 °C over 1.5 h in an argon atmosphere. Heptadecanoic acid (C17) (Sigma Chemical Co., St Louis, MO) was added as an internal standard. Fatty acid methyl esters (FAMEs) were then extracted with n-hexane, and their profiles were identified as previously described on a Thermo Ultra gas chromatograph equipped with autosampler, PTV injector, FID detector and a Zebron GC column (ZB-WAXplus 30 mL × 0.32 mm ID × 0.25 µm df), using authentic standards (Sigma Chemical Co.) (Ventura et al. 2011a).
Statistical analysis
Statistical analysis was performed by two-factor analysis of variance (ANOVA) for all parameters by comparing salinity and genotype as main factors using the JMP In 5.0.1a software package (SAS Institute, Inc., Campus Drive, Cary, NC, USA). Subsequently, mean comparisons were performed according to Student's t-test with the same software.
Results
Growth parameters and plant appearance
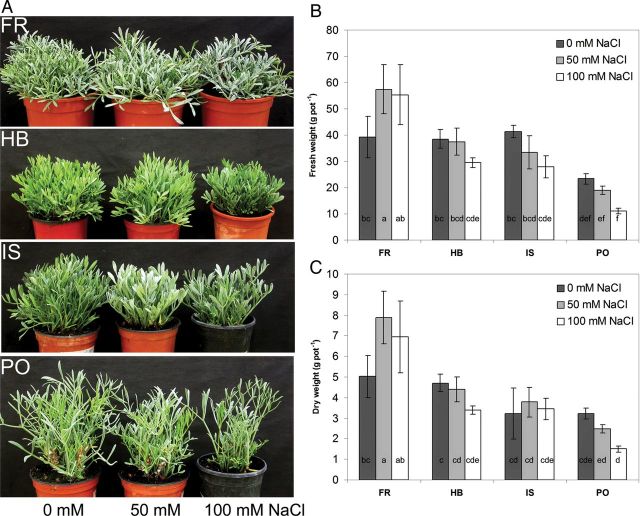
Four genotypes of C. maritimum were investigated for their potential as cash crop halophytes with a dual function, as ornamental flowering plants for landscaping and as leafy vegetables with nutritional value. All genotypes could be easily distinguished by their leaf appearance; PO had a very narrow leaf lamina, while HB could be identified by its broad lamina of single leaflets (Fig. 1). IS and FR were intermediate, with FR being closer to its narrow-leaved Atlantic counterpart.
Figure 1.
Leaf appearance of four C. maritimum genotypes: FR, HB, PO and IS.
The highest biomass was produced by FR, followed by HB, IS and PO, which produced significantly less fresh weight than all other genotypes. Salinity significantly increased fresh and dry biomass only in the FR genotype at 50 mM NaCl, indicating its salt tolerance (Fig. 2B and C). Genotypes FR, HB and IS exhibited a compact growth habit, while PO presented a loose appearance (Fig. 2C), probably due to its long petioles and narrow leaf lamina.
Figure 2.
Effect of salinity on plant morphology (A), fresh weight (B) and dry weight (C) in four C. maritimum genotypes. Values denoted by different letters are significantly different, P < 0.05.
Flowering pattern
In order to test the multi-potential of C. maritimum as a leafy vegetable and ornamental crop, leaf metabolites were determined in leaves sampled before the onset of flowering. The fast re-growth, during ∼1 month, to the plant size before the harvest (data not shown) enabled the investigation of the continuous plant development and subsequent flower appearance.
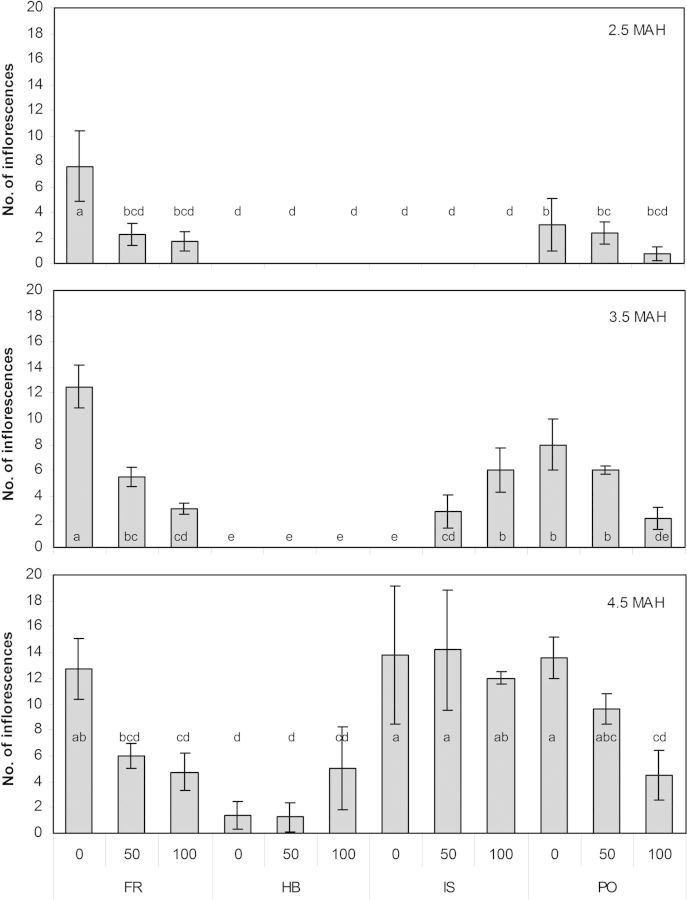
The influence of salinity on the flowering pattern was investigated in the four genotypes. The number of inflorescences was counted at three time points. Both Atlantic genotypes, FR and PO, preceded the Mediterranean genotypes at flowering onset time (Fig. 3), determined 2.5 MAH. Salinity significantly reduced the number of inflorescences in FR at this time point. Similarly, a reduction in the number of flowering branches with increasing salinity was observed for FR and PO.
Figure 3.
Effect of salinity on the flowering pattern of four C. maritimum genotypes. Number of inflorescences was counted at three time points after the onset of flowering (2.5, 3.5 and 4.5 MAH). Values denoted by different letters are significantly different, P < 0.05.
One month later (3.5 MAH), genotype IS initiated flowering branches first in the saline treatments, while HB had still not flowered. After an additional 2 months (4.5 MAH), the IS genotype exhibited the maximum and its Mediterranean counterpart the minimum number of inflorescences, both regardless of the salt treatment.
Evaluation of leaf constituents
Total soluble solids, which represent an estimate of the accumulation of compatible organic solutes and EC, a parameter indicative of mineral accumulation, were determined in leaf extracts (Table 1). Electrical conductivity values increased significantly with increasing salinity in all genotypes without major differences between them, pointing to general salt accumulation in the leaves. On the other hand, TSS significantly increased only in the Atlantic genotypes PO and FR; FR accumulated the highest TSS at the 100 mM salt treatment of all the genotypes.
Table 1.
Effect of salinity on EC and TSS in four C. maritimum genotypes. Values denoted by different letters are significantly different, P < 0.05.
| Leaf constituents | Salinity (mM NaCl) | FR | HB | IS | PO |
|---|---|---|---|---|---|
| EC (dS m−1) | 0 | 19.8e | 14.7f | 14.8f | 16.5f |
| 50 | 22.3d | 28.0a | 25.3bc | 23.3d | |
| 100 | 25.3bc | 27.0ab | 26.3ab | 24.0cd | |
| TSS (%) | 0 | 4.5e | 4.7de | 5.2bcd | 5.0cde |
| 50 | 5.5bc | 5.3bc | 5.0cde | 5.7ab | |
| 100 | 6.2a | 5.0cde | 5.2bcd | 5.7ab |
Determination of ASC
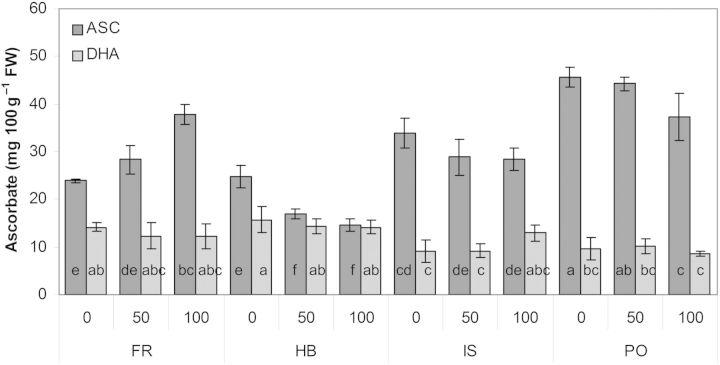
Total ASC concentration in the leaves was determined by two components, reduced ASC and DHA. Dehydroascorbate was not influenced by genotype or by salinity (Fig. 4). Ascorbate significantly increased in FR and decreased in HB, with the increase in salinity. Salinity had no effect on ASC in the genotypes IS and PO. The highest ASC concentration was found in PO at all salinity levels, reaching almost double that in HB.
Figure 4.
Effect of salinity level on ASC and DHA in four C. maritimum genotypes. Values denoted by different letters are significantly different, P < 0.05.
Total polyphenol determination
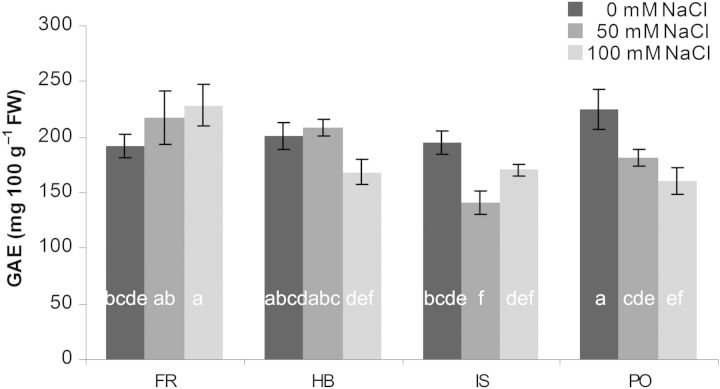
Leaf polyphenol concentration decreased with increasing salinity in all genotypes, except FR, where a significant increase was observed (Fig. 5). The most remarkable reduction was evident in genotype PO at 100 mM NaCl—30 % lower polyphenol concentration than the non-saline control. In the control treatment (no added salinity), there were no differences in polyphenol concentration between genotypes, but when grown under salinity polyphenol concentration in genotype FR exceeded that of all the others, particularly at the highest salt treatment.
Figure 5.
Effect of salinity level on total polyphenols, expressed as gallic acid equivalents (GAE), in leaves of four C. maritimum genotypes. Values denoted by different letters are significantly different, P < 0.05.
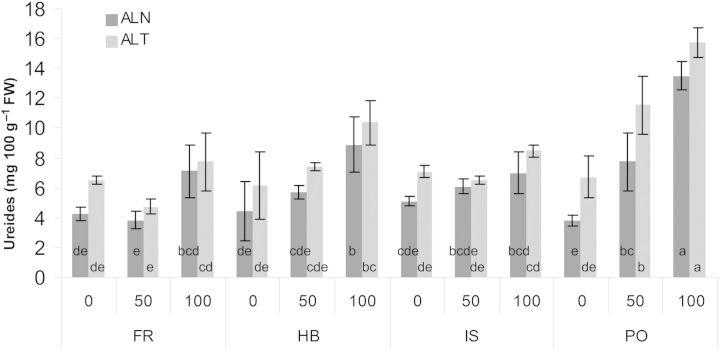
Determination of ureides
During purine degradation, uric acid is catabolized into ureides ALN and ALT. In C. maritimum leaves, the ratio between ALN and ALT is ∼1 : 1 (Fig. 6). When plants were grown without salt there were no differences between the genotypes, but with salt application genotype PO differed significantly from its counterparts for both ureides, ALN and ALT (Fig. 6). Saline irrigation of 100 mM NaCl induced a 3.5-fold enhancement of ALN and a 2.3-fold enhancement of ALT, in genotype PO. In genotype HB, there was a significant increase of ALT, the more degraded ureide, at 100 mM NaCl, while in FR and IS leaf ureide concentration was not influenced by salinity.
Figure 6.
Effect of salinity on allantoin (ALN) and allantoate (ALT) levels in leaves of four C. maritimum genotypes. Values denoted by different letters are significantly different, P < 0.05.
Fatty acid profile
A leaf fatty acid profile for the four Crithmum genotypes at three salinity levels was composed (Table 2). Total fatty acid concentration increased significantly with increasing salinity in HB and IS, but was unaffected in genotypes FR and PO. Most fatty acids were not influenced by salinity. Differences between genotypes, not related to salinity, were found in the content of omega-3 fatty acid 16 : 3ω3, which was significantly lower in genotype FR, while linoleic acid (18 : 2) was highest in FR when compared with all other genotypes.
Table 2.
Effect of salinity on FAME profile in leaves of four C. maritimum genotypes. Values in the table are mean fatty acid (as % of total fatty acids) (n = 3). Values denoted by different letters are significantly different, P < 0.05.
| FAME | Genotype |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FR |
HB |
IS |
PO |
|||||||||
| Salinity (mM NaCl) | ||||||||||||
| 0 | 50 | 100 | 0 | 50 | 100 | 0 | 50 | 100 | 0 | 50 | 100 | |
| 16 : 0 | 19.9bc | 19.2bc | 18.4c | 22.3a | 20.2bc | 21.1ab | 19.0c | 18.8c | 19.0c | 19.3bc | 19.0c | 18.9c |
| 16 : 1 | 3.2bc | 2.9bc | 3.0bc | 2.5c | 3.0bc | 2.6bc | 4.1a | 3.4ab | 3.2bc | 2.5c | 2.6bc | 2.9bc |
| 16 : 1 pg | 2.5e | 2.4e | 2.2e | 3.3a | 3.1ab | 3.4a | 2.7cd | 2.8cd | 2.9c | 2.9bc | 2.8cd | 2.8c |
| 16 : 2 | 0.5b | 0.5Ab | 0.6b | 0.0e | 0.3cd | 0.5b | 0.1de | 0.5bc | 0.7b | 0.5b | 0.6b | 0.9a |
| 16 : 3ω3 | 6.4b | 6.4b | 6.2b | 8.6a | 9.3a | 9.9a | 9.1a | 9.5a | 10.0a | 9.8a | 9.5a | 9.5a |
| 18 : 0 | 2.5ab | 2.5ab | 2.6a | 2.4abc | 2.0d | 2.1cd | 2.4abc | 2.2bcd | 2.3bcd | 2.1cd | 2.2bcd | 2.4abc |
| 18 : 1ω9 | 1.7bcd | 1.6bcde | 1.8bc | 1.2cde | 1.1e | 1.2de | 1.5bcde | 1.8bc | 2.0ab | 2.0ab | 1.8bc | 2.4a |
| 18 : 2 | 32.3a | 33.2ab | 33.9a | 29.9cd | 28.1ef | 26.9f | 29.1cde | 29.4cde | 28.8de | 29.3cde | 30.4c | 30.0cd |
| 18 : 3ω3 | 30.7a | 31.3a | 31.1a | 29.2a | 32.2a | 31.4a | 31.Aa | 31.2a | 30.7a | 31.0a | 30.2a | 29.3a |
| Others | 0.4cdef | 0.0f | 0.2def | 0.6abcd | 0.6abcd | 1.0a | 0.1ef | 0.5bcde | 0.6abcde | 0.6abcd | 0.9ab | 0.7abc |
| Total FAME (%DW) | 2.3bcd | 2.2bcd | 2.3bcd | 1.9d | 2.4abc | 2.4abc | 2.1cd | 2.5ab | 2.7a | 2.7a | 2.5ab | 2.5abc |
| Total ω3 | 37.1c | 37.7bc | 37.3c | 37.9bc | 41.5a | 41.2a | 40.9ab | 40.6ab | 40.7ab | 40.8ab | 39.7abc | 38.8abc |
Discussion
The comparison between four C. maritimum genotypes revealed differences in leaf appearance, plant habit and flowering pattern (Figs 1–3). Crithmum maritimum leaves were described by Atia et al. (2011) as fleshy and succulent, which was confirmed for all genotypes. The appearance of a broad or narrow leaf lamina was determined by the origin of the genotype and influenced its overall plant morphology. When used as ground cover for landscaping, plants with a compact growth habit and broad leaf appearance (in our case FR, HB, IS) may be of advantage, particularly because their biomass production was not negatively affected by the salt treatment (Fig. 2). Interestingly, in the Atlantic genotypes, FR and PO, the onset of flowering was early in the season regardless of salinity, but the number of inflorescences significantly decreased with the increase of salt concentration, indicating that those genotypes are salt sensitive during their reproductive phase. On the contrary, the late-season Mediterranean genotype IS showed a salt-induced onset of flowering (Fig. 3, middle) and no reduction in the number of inflorescences, thus demonstrating the long-term salt tolerance of this genotype, a characteristic that would be advantageous as a flowering ground cover suitable to ‘greenify’ beach-site areas where mainly saline irrigation is available (Lieth 2000). Growing halophytic species with attractive flowers, several leaf forms or growth patterns (e.g. Aster tripolium, Atriplex species, Batis maritima) also increases the interest in halophyte gardening and the use of new resources by florists (Lieth 2000). Mixing of different leaf types and seasonal variation in flowering may further increase the interest in combining Crithmum genotypes for saline landscaping.
Crithmum maritimum is a ‘salt-including’ halophyte. These types of halophytes compartmentalize Na+ and Cl− in vacuoles after these ions are inevitably taken up by the roots (Ben Amor et al. 2005). All genotypes accumulated the salt in a similar manner, as indicated by increasing EC values (Table 1), but an adjustment of the osmotic balance by increasing sugars as an osmo-protective mechanism (Türkan and Demiral 2009), indicated here by TSS values, was observed only in the Atlantic genotypes FR and PO.
Plants exposed to abiotic stresses prevent oxidative damage by synthesizing antioxidant compounds, such as ASC, carotenoids, glutathione and tocopherol as well as antioxidant enzymes (Ashraf and Harris 2004; Ben Hamed et al. 2007). Ksouri et al. (2007) reported that salt-tolerant accessions of Cakile maritima were found to possess an enhanced antioxidant production capacity, associated with higher ASC and polyphenol content than in less tolerant accessions. Similarly, we found an increase in ASC in genotype FR, the most salt tolerant in respect to fresh and dry biomass production, with increasing salinity (Figs 2 and 4). Crithmum maritimum has previously been evaluated as a rich source of phenolic compounds, which was influenced by location, season and flowering stage (Maleš et al. 2003; Meot-Duros and Magné 2009). Similar absolute values of phenolics to those reported by Meot-Duros and Magné (2009) for leaves sampled in spring were determined for genotype FR, while genotype PO, representing the smallest phenotype with low biomass production, exhibited a significant lower total polyphenol and ASC concentration (Figs 2, 4 and 5).
The ureides ALN and ALT are well known to be important N storage and transport compounds in legumes (Smith and Atkins 2002; Zrenner et al. 2006). However, the metabolic role of ureides in non-legumes is not well known (Mazzafera et al. 2008). Nevertheless, it was recently demonstrated that ureides accumulate in non-leguminous plants exposed to stress such as salinity, high NH4+ concentrations, dark-induced senescence and normal senescence (Sagi et al. 1998; Brychkova et al. 2008; Ventura et al. 2010). Surprisingly, our results demonstrate an enhancement of ureide concentrations with increasing salinity only in genotypes PO and HB. Nevertheless, the leaf ureide concentration in the halophyte C. maritimum under control conditions (without salinity) was more than 20-fold higher than that found in Salicornia spp., highlighting the possible use of ureide for efficient N-storage, under elevated salt conditions (Ventura et al. 2011a, b).
Omega-3 fatty acids, which are a major constituent of plant lipids located in the chloroplast membrane, are known for their beneficial effects on human health (Simopoulos 2004). The presence of these essential fatty acids differed greatly among six tested plants with edible leaves, including four halophytes and two non-halophytic species; C. maritimum was found to have the highest concentration in leaves after Portulaca oleracea (Guil-Guerrero and Rodríguez-García 1999). Ben Hamed et al. (2005) demonstrated an increase in total lipids as response to irrigation with up to 100 mM NaCl in a Tunisian C. maritimum variant and pointed out the important role of sulfolipids in the salt tolerance of C. maritimum, probably through stabilization of photosynthetic processes.
The current comparison revealed differences in fatty acid concentration between the four genotypes within the tested salt range: FR and PO were not influenced by salinity, while HB and IS exhibited an increase in fatty acid concentration with increasing salinity (Table 2). Although the fatty acid concentration did not change with salinity in genotype FR, this genotype had the highest 18 : 2 and lowest 16 : 3ω3 values. The characteristic of restructuring membranes with less polyunsaturated acids during salt stress is suggested to protect against the oxidative effects of salt ions (Qureshi et al. 2013). The absolute fatty acid concentrations of all tested genotypes exceeded most of those reported by Ben Hamed et al. (2005), but were below those found by Guil-Guerrero and Rodríguez-García (1999) for plant materials collected in Spain, highlighting the importance of comparative studies of plant material from different origins.
The four genotypes differed in most of the parameters we investigated, including onset of flowering and leaf metabolites. Environmental adaptations at the natural saline habitats were probably the main reason for the differences; particularly, the characteristic of flower appearance could be classified according to the geographic origin (Mediterranean and Atlantic) of the plants. We can speculate that the early onset of flowering in the Atlantic plant group (FR and PO) was due to an effect of latitude, resembling the early flowering observed in Salicornia spp. from northern geographical regions (Ventura et al. 2011b). The differences in leaf appearance and growth habits of the genotypes (Figs 1 and 2) may indicate adaptation to the environmental conditions (climate, sea water salinity, soil type, distance to sea, tides) existing in their natural habitats that may be followed by the synthesis of their particular leaf metabolites. The combination of both plant morphology and leaf metabolites likely endowed the genotypes with their overall salt tolerance.
Conclusions
Four C. maritimum genotypes from different origins (Mediterranean and Atlantic) were compared for their dual potential as ornamental plant and leafy vegetable under saline irrigation. Flowering onset seems to be dependent on parameters (e.g. origin) other than salinity, while a higher salt tolerance, determined by biomass enhancement, of the Atlantic genotype FR was correlated with increasing TSS, polyphenols and ASC. The exact role of ureides and fatty acids in C. maritimum genotypes in response to salinity still needs further investigation.
The parameters we evaluated are important criteria for genotype selection and development towards a multi-purpose halophytic crop for ornamental landscaping and as a leafy vegetable with nutritional potential.
Sources of Funding
The research was supported by a grant from the Chief Scientist, Ministry of Agriculture and Rural Development, Israel, grant no. 857-0548-08.
Contributions by the Authors
The research was conducted in M.S. laboratory. Y.V. and M.S. contributed to the main idea, data analysis and writing of the manuscript, M.M. carried out the experiments; Z.A. and R.O. supervised M.M. and I.K.-G. was responsible for the FAME evaluation.
Conflicts of Interest Statement
None declared.
Acknowledgements
The authors thank Prof. T.J. Flowers for his critical comments on the manuscript and Dr T. Brunner for her English editing.
Literature Cited
- Allakhverdiev SI, Nishiyama Y, Suzuki I, Tasaka Y, Murata N. Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proceedings of the National Academy of Sciences of the USA. 1999;96:5862–5867. doi: 10.1073/pnas.96.10.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Science. 2004;166:3–16. [Google Scholar]
- Atia A, Barhoumi Z, Mokded R, Abdelly C, Smaoui A. Environmental eco-physiology and economical potential of the halophyte Crithmum maritimum L. (Apiaceae) Journal of Medicinal Plants Research. 2011;5:3564–3571. [Google Scholar]
- Ben Amor N, Ben Hamed K, Debez A, Grignon C, Abdelly C. Physiological and antioxidant responses of the perennial halophyte Crithmum maritimum to salinity. Plant Science. 2005;168:889–899. [Google Scholar]
- Ben Hamed K, Ben Youssef N, Ranieri A, Zarrouk M, Abdelly C. Changes in content and fatty acid profiles of total lipids and sulfolipids in the halophyte Crithmum maritimum under salt stress. Journal of Plant Physiology. 2005;162:599–602. doi: 10.1016/j.jplph.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Ben Hamed K, Castagna A, Salem E, Ranieri A, Abdelly C. Sea fennel (Crithmum maritimum L.) under salinity conditions: a comparison of leaf and root antioxidant responses. Plant Growth Regulation. 2007;53:185–194. [Google Scholar]
- Brychkova G, Fluhr R, Sagi M. Formation of xanthine and the use of purine metabolites as a nitrogen source in Arabidopsis plants. Plant Signaling & Behavior. 2008;3:12. doi: 10.4161/psb.6304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunsolo F, Ruberto G, Amico V, Piatelli M. Bioactive metabolites from Sicilian marine fennel, Crithmum maritimum. Journal of Natural Products. 1993;56:1598–1600. doi: 10.1021/np50099a022. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Colmer TD. Salinity tolerance in halophytes. New Phytologist. 2008;179:945–963. doi: 10.1111/j.1469-8137.2008.02531.x. [DOI] [PubMed] [Google Scholar]
- Flowers TJ, Galal HK, Bromham L. Evolution of halophytes: multiple origins of salt tolerance in land plants. Functional Plant Biology. 2010;37:604–612. [Google Scholar]
- Greenway H, Munns R. Mechanisms of salt tolerance in non-halophytes. Annual Review of Plant Physiology. 1980;31:149–190. [Google Scholar]
- Guil-Guerrero JL, Rodríguez-García I. Lipids classes, fatty acids and carotenes of the leaves of six edible wild plants. European Food Research and Technology. 1999;209:313–316. [Google Scholar]
- Koyro HW, Geissler N, Hussin S, Huchzermeyer B. Mechanisms of cash crop halophytes to maintain yields and reclaim saline soils in arid areas. In: Khan MA, Weber DJ, editors. Ecophysiology of high salinity tolerant plants. Dordrecht: Springer; 2006. pp. 345–366. [Google Scholar]
- Koyro HW, Ajmal Khan M, Lieth H. Halophytic crops: a source for the future to reduce the water crisis? Emirates Journal of Food and Agriculture. 2011;23:1–6. [Google Scholar]
- Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritime. Plant Physiology and Biochemistry. 2007;45:244–249. doi: 10.1016/j.plaphy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Law MY, Charles SA, Halliwell B. Glutathione and ascorbic acid in spinach (Spinacia oleracea) chloroplasts. Biochemical Journal. 1983;210:899–903. doi: 10.1042/bj2100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieth H. 2000. Cashcrop halophytes for future halophyte growers. EU concerted action project IC 18CT96–0055, final meeting at the beginning of the EXPO 2000. Institute of Environmental Systems Research, University of Osnabrück, Germany. ISSN 09336-3114 No. 20.
- Maggio A, De Pascale S, Fagnano M, Barbieri G. Saline agriculture in Mediterranean environments. Italian Journal of Agronomy. 2011;6:36–43. [Google Scholar]
- Maleš E, Žuntar I, Nigović B, Plazibat M, Bilušić Vundać V. Quantitative analysis of the polyphenols of the aerial parts of rock samphire—Crithmum maritimum L. Acta Pharmaceutica. 2003;53:139–144. [PubMed] [Google Scholar]
- Mazzafera P, Katia VG, Milton MS. Control of allantoin accumulation in comfrey. Natural Product Communications. 2008;3:1411–1422. [Google Scholar]
- Meot-Duros L, Magné C. Antioxidant activity and phenol content of Crithmum maritimum L. leaves. Plant Physiology and Biochemistry. 2009;47:37–41. doi: 10.1016/j.plaphy.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Özcan M, Pedro LG, Figueiredo AC, Barroso JG. Constituents of the essential oil of sea fennel (Crithmum maritimum L.) growing wild in Turkey. Journal of Medicinal Food. 2006;9:128–130. doi: 10.1089/jmf.2006.9.128. [DOI] [PubMed] [Google Scholar]
- Qureshi MI, Abdin MZ, Ahmad J, Iqbal M. Effect of long-term salinity on cellular antioxidants, compatible solute and fatty acid profile of sweet Annie (Artemisia annua L.) Phytochemistry. 2013;95:215–223. doi: 10.1016/j.phytochem.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Rozema J, Flowers TJ. Crops for a salinized world. Science. 2008;322:1478–1480. doi: 10.1126/science.1168572. [DOI] [PubMed] [Google Scholar]
- Sagi M, Savidov NA, L'vov NP, Lips SH. Nitrate reductase and molybdenum cofactor in annual ryegrass as affected by salinity and nitrogen source. Physiologia Plantarum. 1997;99:546–553. [Google Scholar]
- Sagi M, Omarov RT, Lips SH. The Mo-hydroxylases xanthine dehydrogenase and aldehyde oxidase in ryegrass as affected by nitrogen and salinity. Plant Science. 1998;135:125–135. [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids and antioxidants in edible wild plants. Biological Research. 2004;37:263–277. doi: 10.4067/s0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16:144–158. [Google Scholar]
- Smith PMC, Atkins CA. Purine biosynthesis. Big in cell division, even bigger in nitrogen assimilation. Plant Physiology. 2002;128:793–802. doi: 10.1104/pp.010912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türkan I, Demiral T. Recent developments in understanding salinity tolerance. Environmental and Experimental Botany. 2009;67:2–9. [Google Scholar]
- Ventura Y, Wuddineh WA, Ephrath Y, Shpigel M, Sagi M. Molybdenum as an essential element for improving total yield in seawater-grown Salicornia europaea L. Scientia Horticulturae. 2010;126:395–401. [Google Scholar]
- Ventura Y, Wuddineh WA, Myrzabayeva M, Alikulov Z, Khozin-Goldberg I, Shpigel M, Samocha TM, Sagi M. Effect of seawater concentration on the productivity and nutritional value of annual Salicornia and perennial Sarcocornia halophytes as leafy vegetable crops. Scientia Horticulturae. 2011a;128:189–196. [Google Scholar]
- Ventura Y, Wuddineh WA, Shpigel M, Samocha TM, Klim BC, Cohen S, Shemer Z, Santos R, Sagi M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Scientia Horticulturae. 2011b;130:510–516. [Google Scholar]
- Vogels GD, van der Drift C. Differential analyses of glyoxylate derivatives. Analytical Biochemistry. 1970;33:143–157. doi: 10.1016/0003-2697(70)90448-3. [DOI] [PubMed] [Google Scholar]
- Yensen NP. Halophyte uses for the twenty-first century. In: Khan MA, Weber DJ, editors. Ecophysiology of high salinity tolerant plants. Dordrecht: Springer; 2006. pp. 367–397. [Google Scholar]
- Zhu JK. Plant salt tolerance. Trends in Plant Science. 2001;6:66–71. doi: 10.1016/s1360-1385(00)01838-0. [DOI] [PubMed] [Google Scholar]
- Zrenner R, Stitt M, Sonnewald U, Boldt R. Pyrimidine and purine biosynthesis and degradation in plants. Annual Review of Plant Biology. 2006;57:805–836. doi: 10.1146/annurev.arplant.57.032905.105421. [DOI] [PubMed] [Google Scholar]