Abstract
Glycoproteins expressing the Lutheran blood group antigens were isolated from human erythrocyte membranes and from human fetal liver. Amino acid sequence analyses allowed the design of redundant oligonucleotides that were used to generate a 459-bp, sequence-specific probe by PCR. A cDNA clone of 2400 bp was isolated from a human placental lambda gt 11 library and sequenced, and the deduced amino acid sequence was studied. The predicted mature protein is a type I membrane protein of 597 amino acids with five potential N-glycosylation sites. There are five disulfide-bonded, extracellular, immunoglobulin superfamily domains (two variable-region set and three constant-region set), a single hydrophobic, membrane-spanning domain, and a cytoplasmic domain of 59 residues. The overall structure is similar to that of the human tumor marker MUC 18 and the chicken neural adhesion molecule SC1. The extracellular domains and cytoplasmic domain contain consensus motifs for the binding of integrin and Src homology 3 domains, respectively, suggesting possible receptor and signal-transduction function. Immunostaining of human tissues demonstrated a wide distribution and provided evidence that the glycoprotein is under developmental control in liver and may also be regulated during differentiation in other tissues.
Full text
PDF

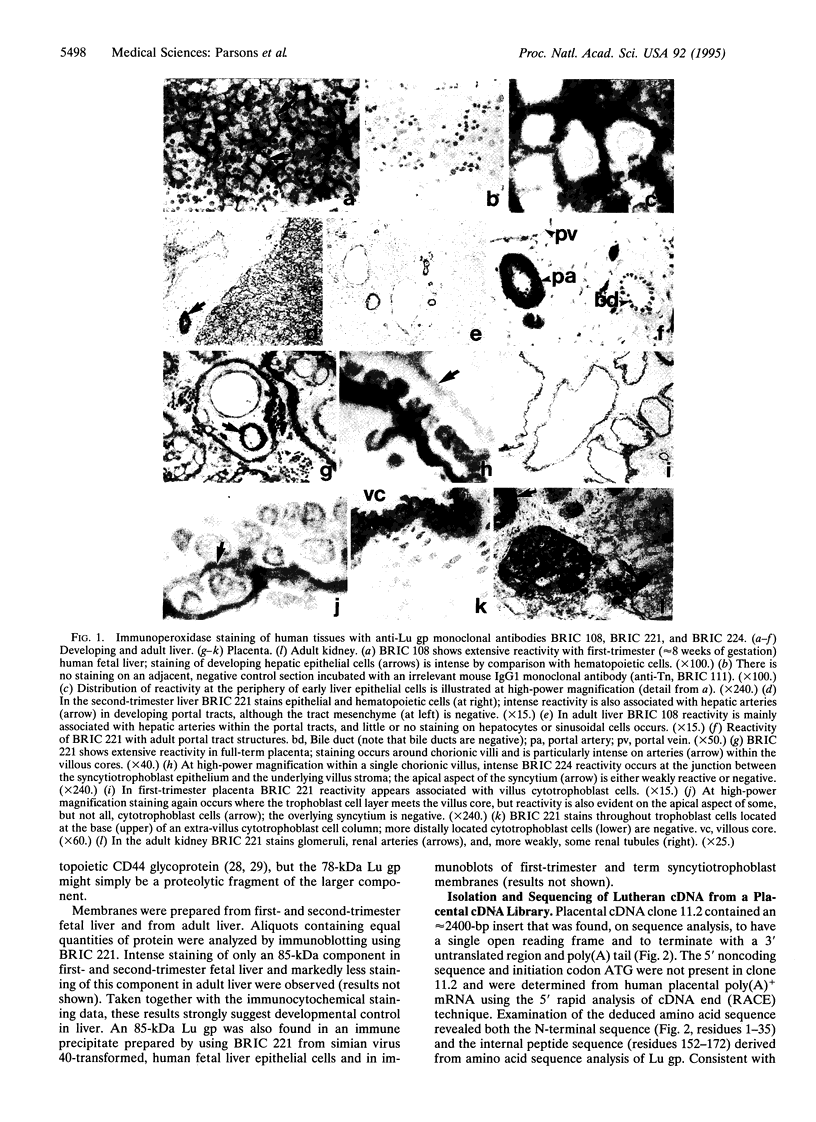


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford D. A., Alafi C. D., Gamble V. M., Mackay D. J., Rademacher T. W., Williams P. J., Dwek R. A., Barclay A. N., Davis S. J., Somoza C. Site-specific glycosylation of recombinant rat and human soluble CD4 variants expressed in Chinese hamster ovary cells. J Biol Chem. 1993 Feb 15;268(5):3260–3267. [PubMed] [Google Scholar]
- Avent N., Judson P. A., Parsons S. F., Mallinson G., Anstee D. J., Tanner M. J., Evans P. R., Hodges E., Maciver A. G., Holmes C. Monoclonal antibodies that recognize different membrane proteins that are deficient in Rhnull human erythrocytes. One group of antibodies reacts with a variety of cells and tissues whereas the other group is erythroid-specific. Biochem J. 1988 Apr 15;251(2):499–505. doi: 10.1042/bj2510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly P., Hermand P., Callebaut I., Sonneborn H. H., Khamlichi S., Mornon J. P., Cartron J. P. The LW blood group glycoprotein is homologous to intercellular adhesion molecules. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5306–5310. doi: 10.1073/pnas.91.12.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. G., Foulkes W. D., Senger G., Trowsdale J., Garin-Chesa P., Rettig W. J. Molecular cloning of the B-CAM cell surface glycoprotein of epithelial cancers: a novel member of the immunoglobulin superfamily. Cancer Res. 1994 Nov 15;54(22):5761–5765. [PubMed] [Google Scholar]
- Daniels G. Evidence that the Auberger blood group antigens are located on the Lutheran glycoproteins. Vox Sang. 1990;58(1):56–60. doi: 10.1111/j.1423-0410.1990.tb02056.x. [DOI] [PubMed] [Google Scholar]
- Daniels G., Khalid G. Identification, by immunoblotting, of the structures carrying Lutheran and para-Lutheran blood group antigens. Vox Sang. 1989;57(2):137–141. doi: 10.1111/j.1423-0410.1989.tb01151.x. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Eiberg H., Mohr J., Nielsen L. S., Simonsen N. Genetics and linkage relationships of the C3 polymorphism: discovery of C3-Se linkage and assignment of LES-C3-DM-Se-PEPD-Lu synteny to chromosome 19. Clin Genet. 1983 Sep;24(3):159–170. doi: 10.1111/j.1399-0004.1983.tb02233.x. [DOI] [PubMed] [Google Scholar]
- Goldstein L. A., Zhou D. F., Picker L. J., Minty C. N., Bargatze R. F., Ding J. F., Butcher E. C. A human lymphocyte homing receptor, the hermes antigen, is related to cartilage proteoglycan core and link proteins. Cell. 1989 Mar 24;56(6):1063–1072. doi: 10.1016/0092-8674(89)90639-9. [DOI] [PubMed] [Google Scholar]
- Holmes C. H., Simpson K. L., Wainwright S. D., Tate C. G., Houlihan J. M., Sawyer I. H., Rogers I. P., Spring F. A., Anstee D. J., Tanner M. J. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990 Apr 15;144(8):3099–3105. [PubMed] [Google Scholar]
- Holness C. L., Simmons D. L. Structural motifs for recognition and adhesion in members of the immunoglobulin superfamily. J Cell Sci. 1994 Aug;107(Pt 8):2065–2070. doi: 10.1242/jcs.107.8.2065. [DOI] [PubMed] [Google Scholar]
- Houlihan J. M., Biro P. A., Fergar-Payne A., Simpson K. L., Holmes C. H. Evidence for the expression of non-HLA-A,-B,-C class I genes in the human fetal liver. J Immunol. 1992 Jul 15;149(2):668–675. [PubMed] [Google Scholar]
- Judson P. A., Spring F. A., Parsons S. F., Anstee D. J., Mallinson G. Report on group 8 (Lutheran) antibodies. Rev Fr Transfus Immunohematol. 1988 Apr;31(2):433–440. doi: 10.1016/s0338-4535(88)80135-1. [DOI] [PubMed] [Google Scholar]
- King M. J., Parsons S. F., Wu A. M., Jones N. Immunochemical studies on the differential binding properties of two monoclonal antibodies reacting with Tn red cells. Transfusion. 1991 Feb;31(2):142–149. doi: 10.1046/j.1537-2995.1991.31291142945.x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Riethmüller G., Johnson J. P. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F. P., Gresham H. D., Schwarz E., Brown E. J. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993 Oct;123(2):485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mallinson G., Martin P. G., Anstee D. J., Tanner M. J., Merry A. H., Tills D., Sonneborn H. H. Identification and partial characterization of the human erythrocyte membrane component(s) that express the antigens of the LW blood-group system. Biochem J. 1986 Mar 15;234(3):649–652. doi: 10.1042/bj2340649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawby W. J., Holmes C. H., Anstee D. J., Spring F. A., Tanner M. J. Isolation and characterization of CD47 glycoprotein: a multispanning membrane protein which is the same as integrin-associated protein (IAP) and the ovarian tumour marker OA3. Biochem J. 1994 Dec 1;304(Pt 2):525–530. doi: 10.1042/bj3040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry A. H., Gardner B., Parsons S. F., Anstee D. J. Estimation of the number of binding sites for a murine monoclonal anti-Lub on human erythrocytes. Vox Sang. 1987;53(1):57–60. doi: 10.1111/j.1423-0410.1987.tb04915.x. [DOI] [PubMed] [Google Scholar]
- Parsons S. F., Jones J., Anstee D. J., Judson P. A., Gardner B., Wiener E., Poole J., Illum N., Wickramasinghe S. N. A novel form of congenital dyserythropoietic anemia associated with deficiency of erythroid CD44 and a unique blood group phenotype [In(a-b-), Co(a-b-)]. Blood. 1994 Feb 1;83(3):860–868. [PubMed] [Google Scholar]
- Parsons S. F., Judson P. A., Anstee D. J. BRIC 18: a monoclonal antibody with a specificity related to the kell blood group system. J Immunogenet. 1982 Dec;9(6):377–380. doi: 10.1111/j.1744-313x.1982.tb00998.x. [DOI] [PubMed] [Google Scholar]
- Parsons S. F., Mallinson G., Judson P. A., Anstee D. J., Tanner M. J., Daniels G. L. Evidence that the Lub blood group antigen is located on red cell membrane glycoproteins of 85 and 78 kd. Transfusion. 1987 Jan-Feb;27(1):61–63. doi: 10.1046/j.1537-2995.1987.27187121477.x. [DOI] [PubMed] [Google Scholar]
- Shih I. M., Elder D. E., Speicher D., Johnson J. P., Herlyn M. Isolation and functional characterization of the A32 melanoma-associated antigen. Cancer Res. 1994 May 1;54(9):2514–2520. [PubMed] [Google Scholar]
- Simpson K. L., Houlihan J. M., Holmes C. H. Complement regulatory proteins in early human fetal life: CD59, membrane co-factor protein (MCP) and decay-accelerating factor (DAF) are differentially expressed in the developing liver. Immunology. 1993 Oct;80(2):183–190. [PMC free article] [PubMed] [Google Scholar]
- Smith N. C., Brush M. G., Luckett S. Preparation of human placental villous surface membrane. Nature. 1974 Nov 22;252(5481):302–303. doi: 10.1038/252302b0. [DOI] [PubMed] [Google Scholar]
- Stamenkovic I., Amiot M., Pesando J. M., Seed B. A lymphocyte molecule implicated in lymph node homing is a member of the cartilage link protein family. Cell. 1989 Mar 24;56(6):1057–1062. doi: 10.1016/0092-8674(89)90638-7. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Matsui T., Agata A., Tomura M., Kubota I., McFarland K. C., Kohr B., Lee A., Phillips H. S., Shelton D. L. Molecular cloning and expression of a novel adhesion molecule, SC1. Neuron. 1991 Oct;7(4):535–545. doi: 10.1016/0896-6273(91)90366-8. [DOI] [PubMed] [Google Scholar]
- Tate C. G., Uchikawa M., Tanner M. J., Judson P. A., Parsons S. F., Mallinson G., Anstee D. J. Studies on the defect which causes absence of decay accelerating factor (DAF) from the peripheral blood cells of an individual with the Inab phenotype. Biochem J. 1989 Jul 15;261(2):489–493. doi: 10.1042/bj2610489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippett P. Regulator genes affecting red cell antigens. Transfus Med Rev. 1990 Jan;4(1):56–68. doi: 10.1016/s0887-7963(90)70248-9. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N. The immunoglobulin superfamily--domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]
- Zelinski T., Coghlan G., Greenberg C. R., McAlpine P. J., Lewis M. Evidence that SE is distal to LU on chromosome 19q. Transfusion. 1989 May;29(4):304–305. doi: 10.1046/j.1537-2995.1989.29489242794.x. [DOI] [PubMed] [Google Scholar]




