Abstract
Background
In this study, we evaluated changes in functioning and caregiver burden in Alzheimer’s disease (AD) patients after a dosage increase that was made based on pharmacists’ evaluation of AD patients’ behavior in daily life.
Methods
Pharmacists used a checklist, a questionnaire, and the Repetitive Saliva Swallowing Test (RSST) to gather data on the daily life of AD patients taking donepezil 5 mg/day and their caregivers. In 27 cases, pharmacists suggested a dosage change to 10 mg/day to AD patients’ physicians. Pharmacists then evaluated these patients for 16 weeks after the increase to determine changes in functional assessment staging, caregiver burden, and swallowing function.
Results
During the 16-week study, 20 of the 27 patients showed at least one-stage improvement in relation to the five assessed aspects of daily life (time/place, speech, bathing, dressing, and toileting). The mean score for caregiver burden due to personal strain was significantly lower after the dosage increase than before (5.15±3.76 at baseline; from 3.89±3.42 at week 4 to 3.59±3.90 at week 16; P<0.05), as was the mean score due to role strain (2.19±2.80 at baseline; 1.56±2.64 at week 8; P<0.05). After the dosage increase, the impaired swallowing function that accompanies AD was improved in the patients with swallowing problems, as indicated by a higher mean RSST score (1.22±0.67 at baseline; from 2.78±1.72 at week 4 to 2.78±1.79 at week 16; P<0.05).
Conclusion
The dosage increase not only decreased caregiver burden, but also appeared to improve impaired swallowing function. Medication therapy management by pharmacists of AD patients, including the use of a checklist, contributed to the correct use of donepezil and improved quality of life for caregivers.
Keywords: Alzheimer’s disease, caregiver burden, behavior in daily life, swallowing function, checklist, pharmacists
Video abstract
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease in which serious cognitive dysfunction develops, and no curative treatment for AD has been established. At present, medications that delay the progression of symptoms are used in clinical settings. While the mortality rates for many leading causes of death have declined over the past decade, deaths from dementia have steadily increased.1 Dementia patients, their families, and health care providers must understand and be prepared to confront the end stage of this disease, which is estimated to affect more than 5 million Americans currently and is expected to affect more than 13 million by 2050.2 Similarly, the number of dementia patients is expected to grow in countries worldwide, including Japan, as the elderly population increases.
The increasing number of dementia patients accompanying the growing aging population is a serious social issue, especially for caregivers who support AD patients. As the disease progresses, AD patients often develop behavioral and psychosocial symptoms, such as wandering, agitation, sexually inappropriate behavior, depression, anxiety, and delusions. Loitering, delusions, and aggressive behavior, in particular, place a large burden on caregivers, both physically and psychologically. It can also be difficult for family members to immediately accept that a family member has become demented. As such, the burden on caregivers is due not only to frequent changes in the AD patient’s medical condition, but also to physical and psychological stress due to financial anxiety.
At present, pharmacological treatment for AD includes the acetylcholinesterase inhibitors donepezil hydrochloride (donepezil), galantamine hydrobromide, rivastigmine tartrate, and the N-methyl-D-aspartate receptor agonist memantine hydrochloride. Long-term administration of donepezil was reported to delay the progression of cognitive dysfunction over a period of 54 weeks.3 In addition to improving cognitive dysfunction, many studies have reported a positive relationship between donepezil and caregiver burden; for example, it can improve reduced movement in daily life,4 decrease the time needed for caregivers to watch AD patients,5 and improve scores on the Japanese version of the Zarit Caregiver Burden Interview (J-ZBI_8).6–8
However, since AD is a progressive neurodegenerative disease, the effect of donepezil gradually lessens over time. The dosage of donepezil must therefore be increased, at an appropriate time, from 5 mg/day to 10 mg/day as the severity of dementia progresses.9 In addition, since the prognosis of advanced dementia is unfavorable, as indicated by a 6-month survival rate of 25% and average life expectancy of 1.3 years from onset,10 it is desirable to increase the dosage as early as possible when AD progresses to advanced dementia. Increasing the dosage of donepezil is expected to improve not only decreased cognitive function but also improve the impaired swallowing function caused by refusal of some patients to eat due to dysphagia,11,12 and at the same time help to reduce caregiver burden.
In this study, with the overall aims of improving quality of life for AD patients and reducing caregiver burden by pharmacists recommending timely dosage increases in donepezil, pharmacists interviewed AD patients and their caregivers during medication therapy management and used a checklist for AD patients’ behavior in daily life to collect information on how patients were coping on a day-to-day basis. If the pharmacist judged it to be an appropriate time for a dosage increase, he or she proposed an increase to 10 mg/day to the AD patient’s physician. This increase may result in improved quality of life for both AD patients and their caregivers. Therefore, once the dosage was increased, the pharmacist examined the effectiveness of this increase on caregiver burden and on the changes in dementia severity and swallowing function.
Materials and methods
Participants
Among the patients diagnosed with AD between September 2012 and January 2014 at Ohkawa Clinic (Niwa, Japan), Kume Clinic (Nagoya, Japan), Funabiki Clinic (Inuyama, Japan), Total Support Clinic (Nagoya, Japan), and Andoh Clinic (Inuyama, Japan), we selected those who had taken donepezil 5 mg/day for more than 4 weeks and who agreed along with their caregivers to participate in this study. Pharmacists recommended a dosage increase of donepezil to 10 mg/day to AD patients’ physicians. Pharmacists then evaluated the AD patients for 16 weeks after the dosage increase to determine changes in functional assessment staging, caregiver burden, and swallowing function compared with baseline.
Research methods
Dementia severity
Pharmacists interviewed AD patients and caregivers and collected information on the patients’ behavior in daily life by means of a checklist (Table 1) based on functional assessment staging.13 AD stage was evaluated for the following five aspects of daily life: Q1) time and place, Q2) speech, Q3) bathing, Q4) dressing, and Q5) toileting. We evaluated items Q1 to Q4 in three stages (stages 4–6: from moderate to severe) and item 5 in two stages (stages 4 and 6). Improvement was defined as a one-stage improvement in AD stage compared with week 0, where week 0 was set as the time when donepezil (Aricept®) was first increased from 5 mg/day (baseline) to 10 mg/day. When AD patients were assessed to be at AD stage 5 or 6, the pharmacists suggested to the AD patients’ physicians that the dosage of donepezil be increased to 10 mg/day.
Table 1.
Checklist of patient behavior in daily life
| Please choose one option to answer each question about the patient’s daily life
| ||
|---|---|---|
| Question | FAST stage | |
| Q1 | Does the patient sometimes seem unsure about dates, seasons, or places? (Time and place) | |
| Some uncertainty about dates (year, month, day) (understands mostly) | 4 | |
| Sometimes does not know the season or place, but can answer if given a hint | 5 | |
| Does not know where places are, such as the toilet, inside the house | 6 | |
| Q2 | Does the patient need help communicating, such as during conversation? (Speech) | |
| No help is required for daily conversations and knowledge is retained | 4 | |
| Can communicate what is necessary, understands simple conversations | 5 | |
| Understands simple conversations, but does not request anything on his/her own | 5 | |
| Does not understand simple conversations, but is able to express his/her emotions to some degree | 6 | |
| Q3 | Does the patient bathe on his/her own? (Bathing) | |
| He/she can bathe on his/her own | 4 | |
| Sometimes forgets to bathe but is able to wash himself/herself and adjust the water temperature | 5 | |
| Unable to bathe properly without help (unable to adjust the water temperature or amount, unable to wash own body well) | 6 | |
| Q4 | Does the patient select his/her clothing and dress him/herself? (Dressing) | |
| Able to choose own clothing and dress without help | 4 | |
| If assisted and appropriate clothing is presented, then he/she can dress him/herself | 5 | |
| Unable to choose appropriate clothing and unable to dress him/herself without assistance | 6 | |
| Q5 | Does the patient have accidents in the toilet? (Toileting) | |
| Able to use toilet on his/her own | 4 | |
| Forgets to flush the toilet or to wipe him/herself | 6 | |
Abbreviation: FAST, functional assessment staging.
Caregiver burden
Caregiver burden was evaluated using the J-ZBI_8,6,7 which consists of eight items scored on a 5-point scale (0–4 points) that ask about personal strain (five items) and role strain (three items). The personal strain items are used to evaluate negative emotions regarding situations that require care, while role strain items are used to evaluate the severity of obstacles in the caregivers’ social life due to the care they provide for their family members with AD. The evaluation method entailed dividing the total score by the highest score to calculate a rate, which was set as 100% when caregiver burden was at maximum. The maximum caregiver burden score for personal strain was 20 points (five items × four points) and that for role strain was 12 points (three items × four points). Each score was evaluated as a rate in relation to the mean score divided by the maximum score (Table 2).
Table 2.
Japanese version of the Zarit Caregiver Burden Interview
| Never | Rarely | Sometimes | Frequently | Almost always | |||
|---|---|---|---|---|---|---|---|
| A | Q1 | Do you feel embarrassed by the patient’s behavior? | 0 | 1 | 2 | 3 | 4 |
| A | Q2 | Do you feel angry when you are around the patient? | 0 | 1 | 2 | 3 | 4 |
| B | Q3 | Do you feel that the patient currently affects your relationships with family members or friends in a negative way? | 0 | 1 | 2 | 3 | 4 |
| A | Q4 | Do you feel any strain when you are around the patient? | 0 | 1 | 2 | 3 | 4 |
| B | Q5 | Do you feel that your social life has suffered because you are caring for the patient? | 0 | 1 | 2 | 3 | 4 |
| B | Q6 | Do you feel uncomfortable about having friends over because of the patient? | 0 | 1 | 2 | 3 | 4 |
| A | Q7 | Do you wish you could leave the care of the patient to someone else? | 0 | 1 | 2 | 3 | 4 |
| A | Q8 | Do you feel uncertain about what to do about the patient? | 0 | 1 | 2 | 3 | 4 |
Notes: Japanese version of the Zarit Caregiver Burden Interview consists of eight question items (Q), each scored on a five-point scale (0–4). Five items address personal strain (indicated by A) and three address role strain (indicated by B).
Swallowing function
Changes in the AD patients’ swallowing function were assessed using the Repetitive Saliva Swallowing Test (RSST) by comparing function determined at week 0 with that determined at week 4, 8, 12, and 16. Swallowing three or more times in 30 seconds was regarded as normal, while swallowing twice or less in 30 seconds was considered impaired.14,15
Statistical analysis
Improvement in patients’ dementia severity was analyzed using the Fisher’s exact test. Changes in caregiver burden and swallowing function were analyzed using the Wilcoxon signed rank test in KaleidaGraph version 4.1.1 (Hulinks Inc., Tokyo, Japan). Statistical significance was determined at a probability of less than 5%.
Ethical considerations
This study was conducted after obtaining approval from the clinical study ethics committee at the School of Pharmacy, Aichi Gakuin University.
Results
Patient and caregiver background
During the study, of the 35 patients who had their donepezil dosage increased to 10 mg/day, 27 (six men and 21 women, mean age 86.5±5.9 years) were included in the analysis. The remaining eight patients were excluded because they could not be followed continuously for 16 weeks; there were two deaths, two cases of decreased dosage due to side effects, and one transfer to another hospital, two showed poor adherence, and one was diagnosed with Parkinson’s disease as a underlying illness. Each of the 27 patients’ caregivers (20 family members, seven non-family members; two men and 25 women) were included in the analysis.
Improvements in dementia severity
Figure 1 shows the improvements in dementia severity seen in relation to time/place and speech compared with week 0. During the 16-week study, 20 of the 27 patients showed at least a one-stage improvement in severity. Improvement in severity in relation to time/place was seen in more than six patients during the 16-week study. Dementia severity was improved in relation to both aspects of time/place and speech in more than three patients during the 16-week study. However, the number of patients who showed improvement was not significantly different in relation to these two aspects during the 16-week study.
Figure 1.
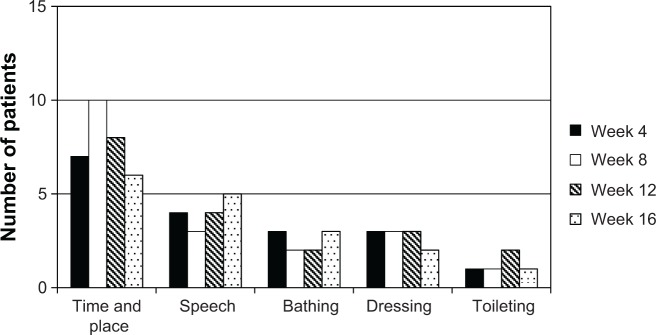
Improvement in dementia severity stage following the increase in donepezil to 10 mg/day.
Note: Values are expressed as the number of patients with Alzheimer’s disease evaluated as showing improvement (n=27).
Changes in caregiver burden
Table 3 shows changes in caregiver burden score compared with week 0. The mean J-ZBI_8 score for personal strain at week 0 was significantly decreased at all successive time points, from week 4 (P<0.05) to week 16 (P<0.01). The J-ZBI_8 score for role strain at week 0 was significantly decreased at week 8 (P<0.05).
Table 3.
Changes in scores on the Japanese version of the Zarit Caregiver Burden Interview
| Patients (n) | J-ZBI_8 score
|
Change at week 16 from week 0 | |||||
|---|---|---|---|---|---|---|---|
| Week 0 | Week 4 | Week 8 | Week 12 | Week 16 | |||
| J-ZBI_8 (personal strain) | 27 | 5.15±3.76 (25.8%) |
3.89±3.42* (19.4%) |
3.22±3.42** (16.1%) |
3.41±3.65** (17.1%) |
3.59±3.90** (18.0%) |
−1.56±2.59 (−7.8%) |
| J-ZBI_8 (role strain) | 27 | 2.19±2.80 (18.3%) |
1.85±2.88 (15.4%) |
1.56±2.64* (13.0%) |
1.81±2.83 (15.1%) |
2.07±3.16 (17.3%) |
−0.11±1.55 (−0.9%) |
Notes: Data are expressed as the mean ± standard deviation.
P<0.05,
P<0.01 (versus week 0), Wilcoxon signed rank test. The evaluation method of the J-ZBI_8 entails adding scores from each time point. The higher the total score, the heavier the caregiver burden. The maximum score for caregiver burden is 20 points (four points × five items) for personal strain and 12 points (four points × three items) for role strain. Values in parentheses indicate the rate in relation to the highest score for each item.
Abbreviation: J-ZBI_8, the Japanese version of the Zarit Caregiver Burden Interview.
Changes in swallowing function
Table 4 shows changes in swallowing function for 17 patients in whom the RSST could be performed on all five occasions. No significant changes were observed in AD patients with normal swallowing function. However, in AD patients with impaired swallowing function, the mean RSST score at week 0 was significantly improved at all successive time points (all P<0.05).
Table 4.
Changes in swallowing function as assessed by the RSST
| Swallowing function | Patients (n) | Week 0 | Week 4 | Week 8 | Week 12 | Week 16 |
|---|---|---|---|---|---|---|
| Normal (RSST score ≥3) (count/30 seconds) | 8 | 4.38±1.30 | 4.50±0.93 | 4.25±0.71 | 4.50±1.69 | 4.25±1.58 |
| Impaired (RSST score ≤2) (count/30 seconds) | 9 | 1.22±0.67 | 2.78±1.72* | 3.22±1.48* | 2.33±1.32* | 2.78±1.79* |
Notes: Data are expressed as the mean ± standard deviation.
P<0.05 (versus week 0), Wilcoxon signed-rank test. Subjects with RSST score ≥3 (three or more swallows in 30 seconds) were classified into the normal swallowing group and those with a score ≤2 (two or fewer swallows in 30 seconds) into the impaired swallowing group.
Abbreviation: RSST, Repetitive Saliva Swallowing Test.
Discussion
In this study, the increase in donepezil dosage from 5 mg/day to 10 mg/day not only improved the severity of dementia and decreased caregiver burden, but also appeared to improve the impaired swallowing function that can accompany AD. Following the dosage increase to 10 mg/day, AD stage was improved for two aspects of daily life assessed by the checklist (ie, time/place and speech), and in particular was significantly improved in relation to time and place. Kotani et al16 reported that donepezil acts in the rat brain at an early stage and the number of new neurons is increased 4 weeks after starting donepezil. The improvement seen in the cognitive dysfunction of AD by increasing the dosage of donepezil in this study indicates that this pharmacological action can be enhanced in a dose-dependent manner.
Caregiver burden decreased from week 4 to 16 following the increase in dosage to 10 mg/day. Specifically, personal strain was significantly decreased at week 16 compared with that at week 0; role strain was also decreased at week 16, although not significantly. The decreased burden on caregivers after increasing donepezil to 10 mg/day was likely the result of reducing the personal strain of caregivers, which in turn was probably the result of the decreased severity of dementia in the AD patients. Therefore, this improvement in caregiver burden was largely influenced by the amelioration of dementia severity in relation to time/place and speech, which are both factors that can lead to increased psychological strain in caregivers.
The improvements seen in AD stage for bathing, dressing, and toileting would also contribute to decreasing the burden of time that caregivers spent caring for AD patients. In this study, the scores were improved in 20 patients by at least one stage in the 16 weeks after the dosage increase. The checklist of AD patients’ behavior in daily life, based on functional assessment staging, was selected for use in this study because it is a simple tool encompassing the basic items that can indicate how AD patients are coping with daily life, and because it was expected to be an efficient measure for pharmacists and caregivers to complete. As a result of carrying out, we have confirmed the checklist useful for a better communication between pharmacists and AD patients’ physicians.
Since a relationship between dementia and dysphagia has been reported,11 the RSST was used in this study to determine if changes in swallowing function occurred after increasing the donepezil dosage. Swallowing problems are generally considered to be caused by esophageal retention17 and suppression of the cough reflex due to decreases in smooth and skeletal muscle function caused by anticholinergic medication.18 Since the acetylcholine concentration in AD patients is decreased, creating a condition similar to that where anticholinergic medications are needed, the increase in donepezil dosage would increase the acetylcholine concentration, thus improving esophageal smooth muscle function and thereby contributing to the improvement in swallowing problems. Our results show that the dosage increase significantly improved swallowing function at week 4, 8, 12, and 16 in AD patients with swallowing problems (RSST score ≤2) at baseline. These findings suggest that swallowing problems in AD patients will be improved after increasing the dosage of donepezil.
A limitation of this study was that the participating AD patients and caregivers did not receive any specialized counseling or training. In Japan, AD patients are typically cared for in their homes; therefore, in these situations, caregivers are usually family members and not professional caregivers. Family members of AD patients, including the patient’s spouse, children, and/or children-in-law, have varying levels and types of education. Greater variation in those parameters would be remained even if caregivers received any specialized counseling or training.
Dementia is a progressive disease, so it is difficult to stop its progression simply by increasing the donepezil dosage, despite its ability to temporarily improve symptoms. Similarly, caregiver burden may be temporarily reduced by increasing the dosage, but the burden will increase again as the AD patient’s symptoms progress. Therefore, it is necessary to provide continuous long-term support for AD patients and their caregivers. Part of the role of pharmacists is to provide support on medication adherence to AD patients and their caregivers as part of management of medication.19 Multidisciplinary teams composed of professionals from different disciplines, such as local public health, medicine, and social welfare, are also needed to provide such support. Based on the results of this study, a checklist of AD patients’ behavior in daily life is a convenient tool for pharmacists and caregivers to determine when the dosage of donepezil should be increased. A measure of caregiver burden is also valuable for ensuring safe use of medication and to monitor quality of life in both AD patients and their families.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hoyert DL, Kung HC, Smith BL. Deaths: preliminary data for 2003. Natl Vital Stat Rep. 2005;53:1–48. [PubMed] [Google Scholar]
- 2.Hebert LE, Scherr PA, Bienias JL. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 3.Mohs RC, Doody RS, Morris JC, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57:481–488. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 4.Winblad B, Black SE, Homma A, et al. Donepezil treatment in severe Alzheimer’s disease: a pooled analysis of three clinical trials. Curr Med Res Opin. 2009;25:2577–2587. doi: 10.1185/03007990903236731. [DOI] [PubMed] [Google Scholar]
- 5.Wimo A, Winblad B, Shah SN, et al. Impact of donepezil treatment for Alzheimer’s disease on caregiver time. Curr Med Res Opin. 2004;20:1221–1225. doi: 10.1185/030079902125004349. [DOI] [PubMed] [Google Scholar]
- 6.Arai Y, Tamiya N, Yano E. The short version of the Japanese version of the Zarit Caregiver Burden Interview (J-ZBI_8): its reliability and validity. Nihon Ronen Igakkai Zasshi. 2003;40:497–503. doi: 10.3143/geriatrics.40.497. Japanese. [DOI] [PubMed] [Google Scholar]
- 7.Arai Y, Kudo K, Hosokawa T, et al. Reliability and validity of the Japanese version of the Zarit Caregiver Burden interview. Psychitry Clin Neurosci. 1997;51:281–287. doi: 10.1111/j.1440-1819.1997.tb03199.x. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto M, Yatabe Y, Kaneda K, et al. Impact of donepezil hydrochloride on the care burden of family caregivers of patients with Alzheimer’s disease. Psychogeriatrics. 2009;9:196–203. doi: 10.1111/j.1479-8301.2009.00302.x. [DOI] [PubMed] [Google Scholar]
- 9.Robert H, Rupert M, James L, et al. Donepezil and memantine for moderate-to-severe Alzheimer’s disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- 10.Susan LM, Joan MT, Dan KK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361:1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuzuya M, Enoki H, Izawa S, et al. Prevalence of oral intake difficulty and associated factors in disabled elderly. The Journal of Japanese Society for Parenteral and Enteral Nutrition. 2011;26:1265–1270. [Google Scholar]
- 12.Wasson K, Tate H, Hayes C. Food refusal and dysphagia in older people with dementia: ethical and practical issues. Int J Palliat Nurs. 2001;7:465–71. doi: 10.12968/ijpn.2001.7.10.9902. [DOI] [PubMed] [Google Scholar]
- 13.Reisberg B. Functional assessment staging (FAST) Psychopharmacol Bull. 1988;24:653–659. [PubMed] [Google Scholar]
- 14.Oguchi K, Saitoh E, Mizuno M, et al. The Repetitive Saliva Swallowing Test (RSST) as a screening test of functional dysphagia. (1) Normal values of RSST. Jpn J Rehabil Med. 2000;37:375–382. [Google Scholar]
- 15.Oguchi K, Saitoh E, Baba M, et al. The Repetitive Saliva Swallowing Test (RSST) as a screening test of functional dysphagia (2) Validity of RSST. Jpn J Rehabil Med. 2000;37:383–388. [Google Scholar]
- 16.Kotani S, Yamauchi T, Teramoto T, et al. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–514. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 17.Tsubouchi T, Tsujimoto S, Sugimoto S, et al. Swallowing disorder and inhibition of cough reflex induced by atropine sulfate in conscious dogs. J Pharmacol Sci. 2008;106:452–459. doi: 10.1254/jphs.fp0071553. [DOI] [PubMed] [Google Scholar]
- 18.Stoschus B, Allescher D. Drug-induced dysphagia. Dysphagia. 1993;8:154–159. doi: 10.1007/BF02266997. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, Yamamura K, Suzuki Y, et al. Pharmacist-based donepezil outpatient consultation service to improve medication persistence. Patient Prefer Adherence. 2012;6:605–611. doi: 10.2147/PPA.S34984. [DOI] [PMC free article] [PubMed] [Google Scholar]


