Common variable immunodeficiency (CVID) is characterized by abnormally low levels of antibodies in the blood and dysfunctional immune cells called CD4+ T cells. Perreau et al. now show evidence that bacteria-fighting CD4+ T cells in these patients are in a state of exhaustion due to a constant leakage of normal gut bacteria into the bloodstream, possibly due to insufficient antibody levels.
Abstract
In the present study, we have investigated the functional profile of CD4 T cells from patients with common variable immunodeficiency (CVID), including production of cytokines and proliferation in response to bacteria and virus-derived antigens. We show that the functional impairment of CD4 T cells, including the reduced capacity to proliferate and to produce IFN-γ and IL-2, was restricted to bacteria-specific and not virus-specific CD4 T cells. High levels of endotoxins were found in the plasma of patients with CVID, suggesting that CD4 T cell dysfunction might be caused by bacterial translocation. Of note, endotoxemia was associated with significantly higher expression of programmed death 1 (PD-1) on CD4 T cells. The blockade of the PD-1–PD-L1/2 axis in vitro restored CD4 T cell proliferation capacity, thus indicating that PD-1 signaling negatively regulates CD4 T cell functions. Finally, we showed that intravenous immunoglobulin G (IVIG) treatment significantly reduced endotoxemia and the percentage of PD-1+ CD4 T cells, and restored bacteria-specific CD4 T cell cytokine production and proliferation. In conclusion, the present study demonstrates that the CD4 T cell exhaustion and functional impairment observed in CVID patients is associated with bacterial translocation and that IVIG treatment resolves bacterial translocation and restores CD4 T cell functions.
Common variable immunodeficiency (CVID), is a heterogeneous group of disorders characterized by hypogammaglobulinemia associated with B cell, T cell, and dendritic cell defects (De Gast et al., 1980; Reinherz et al., 1981; Levy et al., 1998; Cunningham-Rundles and Bodian, 1999; Bonhomme et al., 2000; Cunningham-Rundles et al., 2001; Bayry et al., 2004; Park et al., 2008; Paquin-Proulx et al., 2013b). The clinical picture is characterized by recurrent bacterial infections predominantly caused by Streptococcus pneumoniae, Klebsiella pneumoniae, and Haemophilus influenzae (Van der Hilst et al., 2002; Park et al., 2008; Hong et al., 2010). Several genetic mutations associated with CVID have been identified only in 15–20% of CVID cases (Park et al., 2008). In particular, mutations in the TNFRSF13B (TACI; Castigli et al., 2005), ICOS (Grimbacher et al., 2003), CD19 (van Zelm et al., 2006), CD20 (Kuijpers et al., 2010), and CD81 (van Zelm et al., 2010) genes have been previously described.
Hypogammaglobulinemia is defined by the plasmatic concentration of IgG <4.9 mg/ml, and the current treatment consists of intravenous IgG (IVIG) replacement every 3–4 wk (Cunningham-Rundles, 2010) with the goal of protecting the patients against extracellular pathogen infections. Although protection against extracellular bacteria is commonly assigned to B cell responses with the production of high affinity antibodies, adequate CD4 T cell function is essential for optimal B cell maturation and antibody production, activation of macrophages, and/or recruitment of effector cells to the site of infection (Bloom and Bennett, 1970; David, 1973; Nathan et al., 1983; Ishihara et al., 1986; Parker, 1993; Ye et al., 2001; McHeyzer-Williams and McHeyzer-Williams, 2005). Several CD4 T cell abnormalities have been documented in CVID patients (Sneller and Strober, 1990; Aukrust et al., 1994; Cunningham-Rundles and Bodian, 1999; Giovannetti et al., 2007) and include the reduction of CD4 T cell count, inversion of CD4/CD8 ratio, and functional alterations such as reduced proliferation capacity and/or impaired production of cytokines (Sneller and Strober, 1990; Aukrust et al., 1994; Cunningham-Rundles and Bodian, 1999; Giovannetti et al., 2007). However, the causes of the CD4 T cell functional impairment remains unknown.
In the present study, we hypothesized that the recurrent bacterial infections occurring in CVID patients may lead to secondary CD4 T cell deficiency. To test this hypothesis, we have performed a comprehensive investigation of the functional profile of CD4 T cells including the capacity to produce cytokines, such as TNF, IFN-γ, IL-2, and IL-17A, and/or to proliferate in response to bacteria- and virus-derived antigens.
We demonstrate that bacteria-specific but not virus-specific CD4 T cells were impaired in both their capacity to produce IFN-γ and IL-2 and to proliferate. Interestingly, bacteria-specific but not virus-specific CD4 T cells expressed higher levels of programmed death 1 (PD-1) molecule. In addition, the blockade of the PD-1–PD ligand 1/2 (PDL-1/2) pathway was associated with the restoration of bacteria-specific CD4 T cell proliferation, thus demonstrating that the functional impairment of bacteria-specific CD4 T cells was caused by PD-1–associated cell exhaustion. Of note, we also showed that all untreated CVID patients have detectable levels of endotoxins, i.e., a marker of bacterial translocation, and that endotoxemia inversely correlated with IgG concentration. Finally, longitudinal analyses of CVID patients demonstrated that IVIG treatment significantly reduced endotoxemia and PD-1 expression on CD4 T cells, and restored bacteria-specific CD4 T cell cytokine production and proliferation. The present study provides new insights in the mechanisms responsible for the CD4 T cell functional impairment in CVID patients and indicates that IVIG treatment results in resolution of bacterial translocation and restoration of CD4 T cell functions.
RESULTS
Bacteria-specific CD4 T cells from CVID patients are functionally impaired
In the present study, 26 CVID patients and 30 healthy individuals have been enrolled (Tables 1 and 2). It is important to underscore that none of the CVID patients investigated in the present study for phenotypic and functional analyses and for the measures of endotoxins in plasma had documented active bacterial infections at the time of blood collection. In this regard, C reactive protein (CRP) was measured in 18 patients at the time of the study. CRP levels were <10 mg/liter (cut-off of the method in the central laboratory of the hospital) in 17 out of 18 patients and slightly positive, i.e., 11.5 mg/liter, in one patient.
Table 1.
Characteristics of CVID patients
| Subjects | Gender | Year of birth | Baseline IgG | Baseline IgA | Baseline IgM | CD4 count | CD8 count | CD19 cell count | CD4/CD8 ratio | |||
| mg/ml | mg/ml | mg/ml | % | cells/µl | % | cells/µl | % | cells/µl | ||||
| CVID-1 | F | 1962 | 4.9 | <0.06 | 0.19 | 31.9 | 559 | 33.2 | 581 | 12.1 | 221 | 0.96 |
| CVID-2 | F | 1971 | 1.34 | <0.06 | <0.17 | 60 | nd | 15 | nd | 10 | nd | 4.00 |
| CVID-3 | M | 1949 | 1.04 | 0.1 | 1.63 | 36.6 | 1096 | 21.2 | 710 | 12 | 411 | 1.73 |
| CVID-4 | F | 1976 | 1.75 | 0.09 | 0.56 | 48 | 941 | 28 | 552 | 11 | 221 | 1.70 |
| CVID-5 | M | 1973 | 3.63 | <0.06 | 0.66 | 37 | 1463 | 23.2 | 917 | 27.7 | 1180 | 1.60 |
| CVID-6 | M | 1949 | 3.46 | 0.24 | 0.43 | nd | nd | nd | nd | 4.2 | 33 | nd |
| CVID-7 | F | 1965 | 0.15 | <0.06 | <0.17 | 32 | 793 | 46 | 1141 | 6 | 175 | 0.70 |
| CVID-8 | M | 1972 | 0.84 | <0.06 | <0.17 | 19.6 | 624 | 51.4 | 1636 | 6.5 | 203 | 0.38 |
| CVID-9 | F | 1977 | 1.12 | <0.06 | 1.03 | 50.1 | 723 | 19.6 | 283 | 9.3 | 138 | 2.55 |
| CVID-10 | F | 1948 | <0.5 | <0.06 | <0.17 | nd | nd | nd | nd | nd | nd | nd |
| CVID-11 | F | 1992 | 1.94 | <0.06 | 0.43 | 23.3 | 505 | 24.2 | 524 | 15.4 | 332 | 0.96 |
| CVID-12 | F | 1953 | 1.57 | 0.19 | 0.18 | 32.9 | 389 | 24.5 | 289 | 4.4 | 52 | 1.35 |
| CVID-13 | M | 1982 | 3.67 | 0.17 | 0.21 | 49.5 | 746 | 40 | 603 | 4.2 | 91 | 1.24 |
| CVID-14 | M | 1958 | 0.83 | <0.06 | 0.19 | nd | nd | nd | nd | nd | nd | nd |
| CVID-15 | M | 1941 | 2.6 | <0.06 | <0.17 | 28 | 632 | 43 | 994 | 11 | 253 | 0.64 |
| CVID-16 | M | 1992 | 1.3 | <0.25 | <0.17 | 33 | 355 | 31 | 334 | 13 | 139 | 1.06 |
| CVID-17 | F | 1980 | <0.5 | <0.06 | <0.17 | nd | nd | nd | nd | nd | nd | nd |
| CVID-18 | F | 1983 | 4.4 | <0.06 | <0.17 | 54 | 1091 | 26 | 532 | 11 | 221 | 2.05 |
| CVID-19 | M | 1958 | 1.54 | <0.06 | <0.17 | 27 | 380 | 50 | 700 | 4 | 56 | 0.54 |
| CVID-20 | M | 1981 | 0.6 | <0.25 | <0.17 | 42 | 686 | 35 | 579 | 10 | 165 | 1.18 |
| CVID-21 | F | 1946 | 1.95 | <0.06 | <0.17 | 41 | nd | 37 | nd | <1 | nd | nd |
| CVID-22 | M | 1975 | 1.9 | <0.06 | 0.2 | 36 | 366 | 22 | 221 | 15 | 148 | 1.66 |
| CVID-23 | F | 1986 | 4.4 | 0.55 | 0.76 | 49 | 1107 | 30 | 683 | 9.3 | 207 | 1.62 |
| CVID-24 | F | 1973 | 0.8 | <0.25 | <0.17 | 38 | 296 | 40 | 316 | 10 | 82 | 0.94 |
| CVID-25 | M | 1980 | 1.9 | 0.8 | 0.7 | 37 | nd | 36 | nd | 9 | nd | 1.02 |
| CVID-26 | M | 1957 | 4.9 | 0.77 | 1.22 | 47 | 789 | 35 | 585 | 10 | 169 | 1.35 |
nd, not determined.
Table 2.
Clinical manifestation of CVID patients
| Subjects | LPS | Autoimmunity | IBD | Lymphoid hyperplasia | Granulomatous disease |
| CVID-1 | No | No | No | Yes (intestinal) | No |
| CVID-2 | Yes | No | No | No | No |
| CVID-3 | Yes | No | No | No | No |
| CVID-4 | Yes | No | No | No | No |
| CVID-5 | Yes | Yes | No | Yes (intestinal) | Yes |
| CVID-6 | No | No | No | No | No |
| CVID-7 | No | No | No | No | No |
| CVID-8 | No | No | No | No | No |
| CVID-9 | Yes | Yes | No | No | Yes |
| CVID-10 | No | No | No | No | No |
| CVID-11 | Yes | No | No | Yes (intestinal) | No |
| CVID-12 | Yes | Yes | No | No | No |
| CVID-13 | No | Yes | Yes | Yes (intestinal) | Yes |
| CVID-14 | Yes | Yes | No | No | Yes |
| CVID-15 | Yes | Yes (diabetes) | No | Yes (intestinal) | No |
| CVID-16 | Yes | No | No | Yes (intestinal) | No |
| CVID-17 | No | No | No | Yes (intestinal) | No |
| CVID-18 | No | No | No | Yes (intestinal) | No |
| CVID-19 | Yes | No | No | Yes (intestinal) | No |
| CVID-20 | Yes | No | No | Yes (intestinal) | No |
| CVID-21 | Yes | No | No | Yes (intestinal) | No |
| CVID-22 | No | No | No | Yes (intestinal) | No |
| CVID-23 | No | No | No | Yes (intestinal) | No |
| CVID-24 | No | No | No | Yes (intestinal) | No |
| CVID-25 | No | No | No | Yes (intestinal) | No |
| CVID-26 | No | Yes (diabetes) | No | Yes (intestinal) | No |
To evaluate the functional profile of bacteria-specific and virus-specific CD4 T cells of CVID patients, blood mononuclear cells of CVID patients (n = 12) and healthy individuals (used as control; n = 30) were stimulated with bacteria-derived antigens from Staphylococcus aureus, S. pneumoniae, Streptococcus agalactiae (i.e., group B Streptococcus), Pseudomonas aeruginosa, K. pneumoniae, and two serotypes of Escherichia coli or virus-derived antigens (Ad5 and CMV), and cytokine production and proliferation capacity were analyzed by multiparametric flow cytometry.
Representative flow cytometry profiles for S. pneumoniae and K. pneumoniae in one healthy donor and one CVID patient are shown in Fig. 1 (A and B). The cumulative data for all the bacteria- and virus-specific CD4 T cell responses mentioned above showed that the frequencies of IFN-γ– and IL-2–producing bacteria-specific but not virus-specific CD4 T cells were significantly reduced in CVID patients as compared with healthy individuals (P = 0.0006 and P = 0.003, respectively; Fig. 1 C and not depicted). However, the frequencies of both bacteria-specific and virus-specific CD4 T cells producing TNF were not significantly different between the two groups (P = 0.6841 and P = 0.8202, respectively; Fig. 1 C), and only a trend toward lower frequency of bacteria-specific CD4 T cells producing IL-17A was observed in CVID patients (P = 0.2413; Fig. 1 C). Of note, the capacity of bacteria-specific CD4 T cells to produce IL-4 or IL-21 was also evaluated by flow cytometry. The frequencies of IL-4– or IL-21–producing bacteria-specific CD4 T cells were very low and not significantly different between CVID patients and healthy subjects (P = 0.3950 and P = 0.8714, respectively; data not shown). The proliferation capacity of bacteria-specific but not virus-specific CD4 T cells was also significantly reduced (P < 0.0001 and P = 0.8745, respectively) in CVID patients as compared with healthy subjects (Fig. 1, D and E), thus demonstrating that bacteria-specific CD4 T cells of CVID patients are selectively dysfunctional.
Figure 1.
Functional impairment of bacteria-specific CD4 T cells in CVID patients. CD4 T cells from healthy subjects (HS) or CVID patients were stimulated with S. pneumoniae and K. pneumoniae bacteria antigen preparations and expression of IL-17A, IL-2, TNF, and IFN-γ were measured by flow cytometry. CD4 T cell responses to α-CD3/α-CD28 (polyclonal stimulation) or unstimulated (negative control) are also shown (n = 30 healthy individuals and 12 CVID patients). (A and B) Representative flow plots from one healthy subject (#882; A) and one representative CVID patient (CVID-11; B) are shown. (C) Frequencies of bacteria-specific and virus-specific CD4 T cells producing IFN-γ, IL-2, TNF, or IL-17A were calculated. (D) CD4 T cells from healthy subjects (n = 10) and CVID patients (n = 12) were stimulated with S. pneumoniae and K. pneumoniae and proliferation was measured by CFSE dilution. Unstimulated cell cultures (negative control) and cell cultures stimulated with SEB (positive control) are also shown. (E) Percentage of bacteria- and virus-specific CD4 T cell proliferation. P-values were calculated using Student’s t test. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
It is also important to mention that no differences between the cytokine profile or the proliferation profile of S. pneumoniae/K. pneumoniae and S. aureus and E. coli were observed (unpublished data). The absence of difference in the virus-specific T cell responses between CVID patients and healthy controls is consistent with the lack of clinical evidence of increased reactivation of chronic virus infections in CVID patients (Cunningham-Rundles and Bodian, 1999; Raeiszadeh et al., 2006).
Detection of endotoxins in plasma of CVID patients
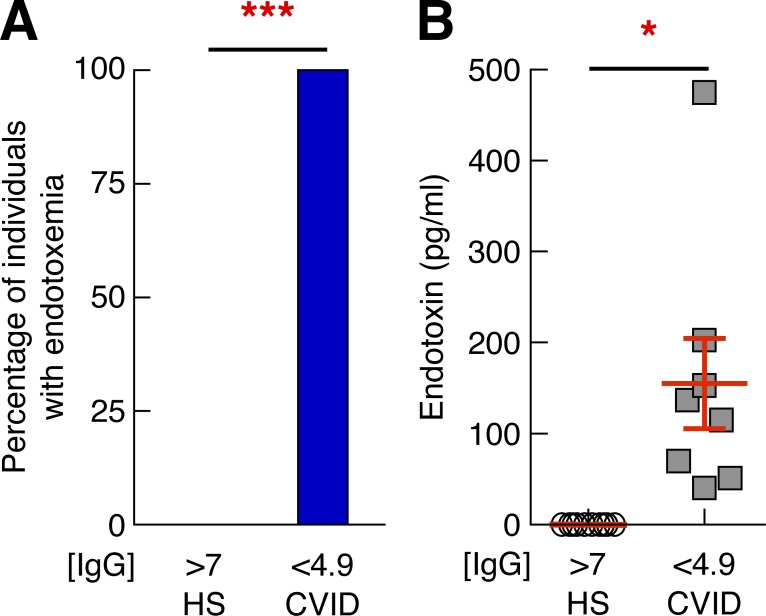
We postulated that the CD4 T cell dysfunction observed in CVID patients could be related to bacterial translocation. To test this hypothesis, bacterial translocation was evaluated using an enzymatic reaction detecting the presence of endotoxins in both the plasma of CVID patients with serum levels of IgGs <4.9 mg/ml (n = 8) and in healthy individuals (n = 10; Roger et al., 2009). All CVID patients with serum IgG levels <4.9 mg/ml had measurable levels of endotoxin, whereas endotoxin was not detected in healthy controls (0%; P < 0.0001; Fig. 2 A). Consistent with this, the levels of endotoxin were significantly higher in CVID patients with IgG levels <4.9 mg/ml as compared with healthy subjects (P = 0.0027; Fig. 2 B). Therefore, these results demonstrate the occurrence of bacterial translocation in CVID patients with low serum IgG levels.
Figure 2.
Endotoxin levels in CVID patients correlate with IgG levels. (A) Proportion of healthy subjects (n = 10) and CVID patients (n = 9) with endotoxemia as measured by limulus assay test. (B) Levels of endotoxin in plasma of healthy individuals and CVID patients were measured by limulus assay test. P-values were calculated using either χ2 analysis for comparison of positive proportions or Student’s t test for multiple comparisons. *, P < 0.05; ***, P < 0.0001. [IgG], concentration of IgG in mg/ml.
Increased PD-1 expression on CD4 T cells of CVID patients
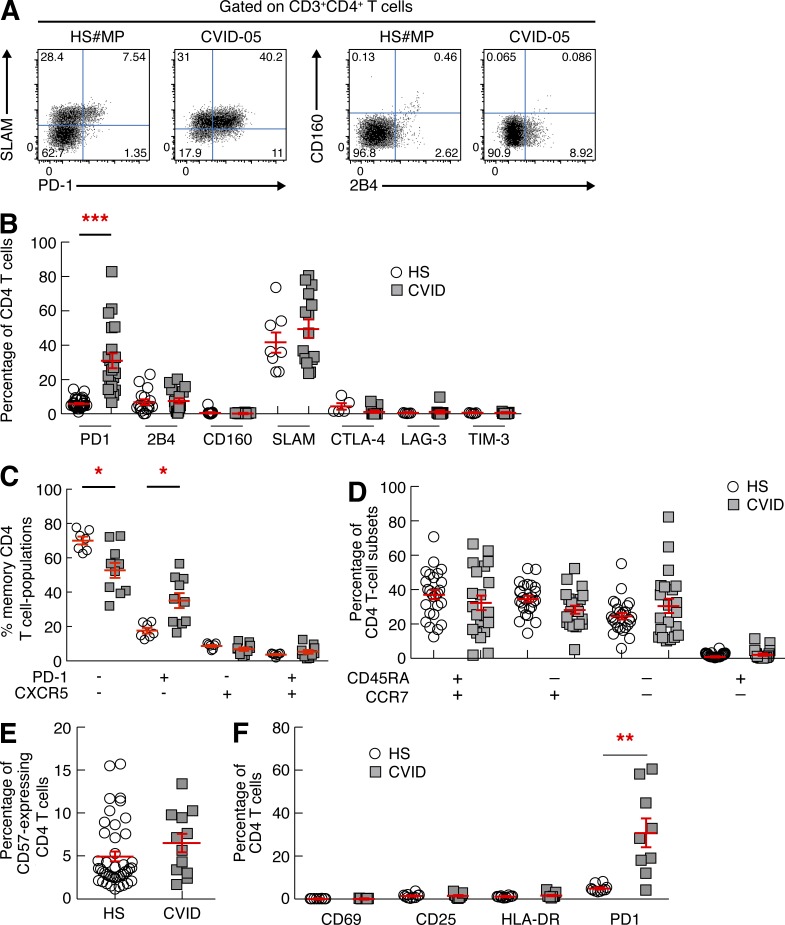
On the basis of the occurrence of microbial translocation and the observed selective dysfunction of bacteria-specific CD4 T cells, we speculated that the functional impairment of bacteria-specific CD4 T cells might result from the modulation of the expression of co-inhibitory molecules (Barber et al., 2006; Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006; McMahan et al., 2010). We therefore evaluated the expression of PD-1, 2B4, CD160, signaling lymphocytic activation molecule (SLAM), CTLA-4, LAG-3, and TIM-3 on CD4 T cells of CVID patients (n = 21) and healthy subjects (n = 30) by flow cytometry. CD4 T cells of CVID patients expressed significantly higher levels of PD-1 as compared with healthy subjects (P < 0.0001; Fig. 3, A and B). No significant differences were observed in the expression of 2B4, CD160, SLAM, CTLA-4, LAG-3, and TIM-3 (P > 0.05; Fig. 3, A and B).
Figure 3.
CD4 T cells of CVID patients express high levels of PD-1. CD4 T cells from healthy subjects (n = 30) and CVID patients (n = 21) were analyzed for the expression of PD-1, SLAM, CD160, 2B4, CTLA-4, LAG-3, and TIM-3 by flow cytometry. (A) Representative flow plots showing expression of PD-1 and SLAM or CD160 and 2B4 in one representative healthy subject (#MP) and one CVID patient (CVID-05). (B) Cumulative data showing co-inhibitory molecule expression (healthy subjects [HS], n = 30; CVID patients, n = 21). (C) Percentages of PD-1−CXCR5−, PD-1+CXCR5−, PD-1−CXCR5+, and PD-1+CXCR5+ (blood Tfh-like cells) CD4 T cell populations (HS, n = 10; CVID patients, n = 10). (D) Percentages of naive (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), and terminally differentiated effector memory (CD45RA+CCR7−) CD4 T cells among total CD4 T cells (HS, n = 26; CVID patients, n = 21). (E) Percentage of CD57-expressing CD4 T cells. (F) Percentage of CD69-, CD25-, HLA-DR–, and PD-1–expressing CD4 T cells (HS, n = 10; CVID patients, n = 9). P-values were calculated using either one-way ANOVA (Kruskal-Wallis test) or Student’s t test for multiple comparisons. *, P < 0.05; **, P < 0.001; ***, P < 0.0001.
Morita et al. (2011) showed that blood PD-1+CXCR5+ CD4 T cells shared functional properties with germinal center T follicular helper (Tfh) cells, i.e., IL-21 production and induction of Ig production from autologous B cells. Therefore, the expression of CXCR5 and PD-1 was evaluated in memory (CD45RA−) CD4 T cell populations of CVID patients and healthy subjects to determine whether increased PD-1 expression was associated with CXCR5 expression. As shown in the cumulative data (Fig. 3 C), the frequencies of PD-1+CXCR5+ CD4 T cell populations were not significantly different between CVID patients and healthy individuals (P = 0.2704). These data demonstrate that the increased expression of PD-1 in memory CD4 T cells observed in CVID patients is not associated with CXCR5 expression, thus indicating that blood Tfh-like cell populations are not increased in CVID patients.
T cell dysfunction may also result from skewed T cell differentiation or immunosenescence (Champagne et al., 2001; Deeks et al., 2012). T cell differentiation (based on CCR7 and CD45RA expression; Sallusto et al., 1999) and immunosenescence (based on CD57 expression; Petrovas et al., 2006) markers were then investigated. No significant differences were observed in the frequency of central memory (CD45RA−CCR7+), effector memory (CD45RA−CCR7−), and terminally differentiated effector memory (CD45RA+CCR7−) CD4 T cells (Fig. 3 D), and in that of CD57-expressing CD4 T cells between CVID patients and healthy subjects (P > 0.05; Fig. 3 E). A trend toward a skewed effector memory phenotype was observed only in three CVID patients. Because increased PD-1 expression may also be associated with T cell activation (Cellerai et al., 2010), the expression of activation markers, such as CD69, CD25, and HLA-DR, was assessed by flow cytometry but no significant differences were observed in the expression of these markers between CVID patients and healthy subjects (P > 0.05; Fig. 3 F).
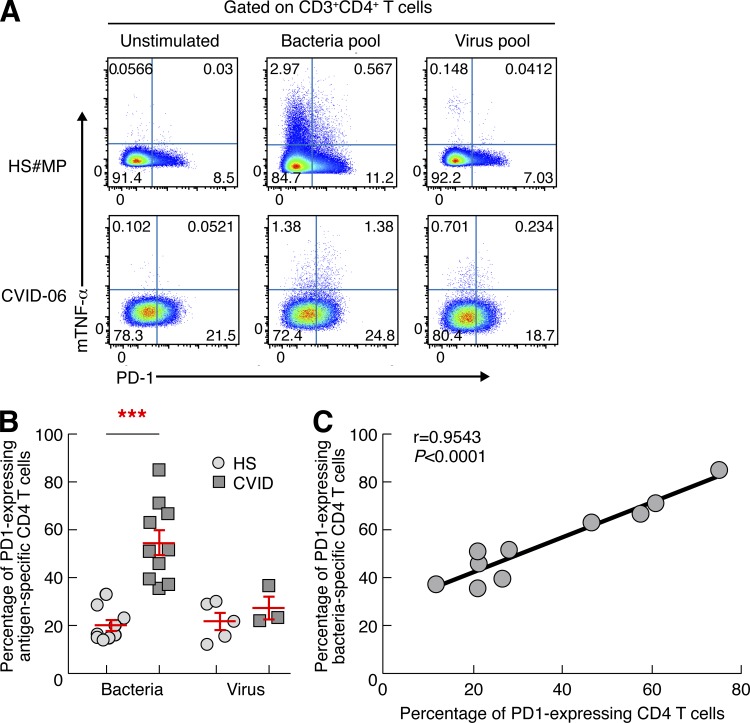
As shown above, CD4 T cell dysfunction was found to be restricted to bacteria-specific CD4 T cells. It was then important to determine whether the increased PD-1 expression was also predominantly restricted to bacteria-specific CD4 T cells. To assess and to compare the expression of PD-1 in bacteria- and virus-specific CD4 T cells, we took advantage of a recently described flow cytometry–based technique by Haney et al. (2011) which allows the identification and characterization of antigen-specific CD4 T cells on the basis of the expression of membrane TNF (mTNF). Levels of PD-1 were significantly higher in bacteria-specific but not virus-specific CD4 T cells in CVID patients as compared with healthy subjects (P < 0.0001; Fig. 4, A and B). In addition, the percentage of PD-1–expressing total CD4 T cells directly correlated with the percentage of PD-1–expressing bacteria-specific CD4 T cells (r = 0.9543, P < 0.0001; Fig. 4 C). Collectively, these results indicate that the selective dysfunction of bacteria-specific CD4 T cells results from the increased expression of the negative regulatory receptor PD-1 which is likely driven by microbial translocation.
Figure 4.
Bacteria-specific CD4 T cells of CVID patients express high levels of PD-1. Percentages of bacteria-specific and virus-specific CD4 T cells expressing PD-1 were analyzed by flow cytometry. (A) Representative flow plots from one healthy subject (#MP) and one CVID patient (CVID-06). (B) Proportion of antigen-specific CD4 T cells expressing PD-1 in healthy subjects (n = 9) and CVID patients (n = 10). (C) Correlation between the percentage of PD-1–expressing total CD4 T cells and the percentage of PD-1–expressing bacteria-specific CD4 T cells. Statistical significance (p-values) in B were calculated using Student’s t test and in C using Spearman’s rank correlations. ***, P < 0.0001.
PD-1 blockade restores bacteria-specific CD4 T cell proliferation
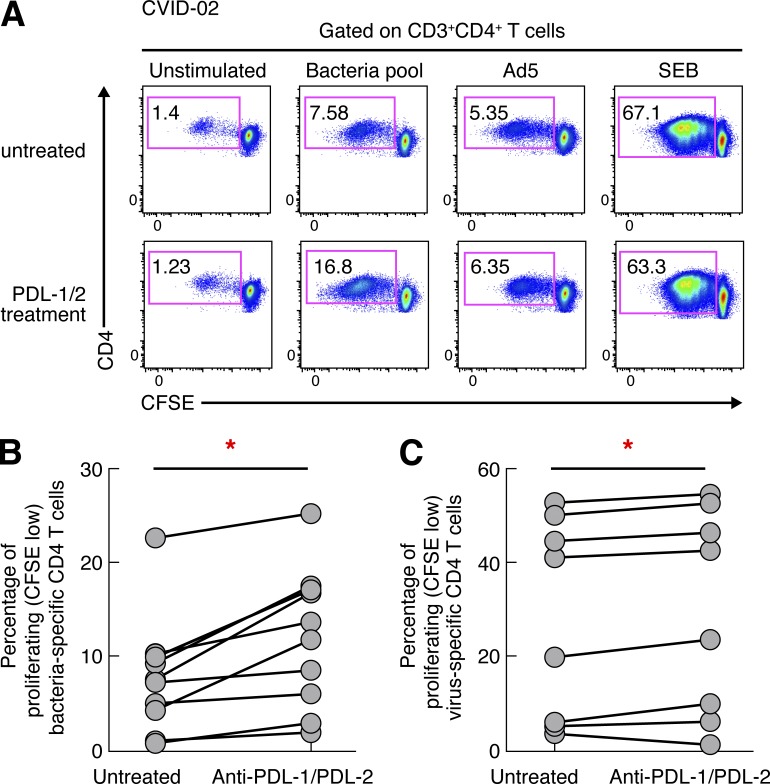
PD-L1 and PD-L2 are the ligands to PD-1 and are expressed on different cell types (Ito et al., 2007). PD-L1 is expressed on hematopoietic and nonhematopoietic cells, whereas PD-L2 is expressed on DCs and macrophages (Ito et al., 2007). To delineate the involvement of PD-1–PD-L1/2 signaling in the impaired proliferation of bacteria-specific CD4 T cells, blood mononuclear cells of CVID patients (n = 10) were labeled with CFSE and stimulated with bacteria- or virus-derived antigens in the presence or in the absence of anti–PD-L1/2 blocking antibodies or isotype controls (Petrovas et al., 2006; Trautmann et al., 2006; Yamamoto et al., 2011). The frequency of proliferating CD4 T cells (CD3+CD4+ CFSElow cells) was assessed at day 6. Representative flow cytometry profiles (Fig. 5 A) and cumulative data (Fig. 5 B) showed that the blockade of PD-1–PD-L1/2 signaling significantly increased bacteria-specific—and also, to a lower extent, virus-specific—CD4 T cell proliferation (1.83-fold increase, P = 0.0024, and 1.07-fold increase, P = 0.0396, respectively; Fig. 5, B and C), supporting the involvement of PD-1–PD-L1/2 signaling as the primary cause of the impaired bacteria-specific CD4 T cell proliferation. Isotype control treatment did not have any effect on virus-specific CD4 T cell proliferation capacity. Of note, the blockade of PD-1–PD-L1/2 signaling restored the proliferation of bacteria-specific CD4 T cells in CVID patients at levels comparable to healthy subjects (P > 0.05).
Figure 5.
PD-1 blockade restores bacteria-specific CD4 T cell proliferation in CVID patients. Blood mononuclear cells were labeled with CFSE and stimulated with bacteria- or virus-derived antigens in the presence or in the absence of α–PDL-1/2 Abs and proliferation was measured in CD4-positive T cells at day 6. (A) Representative flow cytometry profiles of bacteria-specific and virus-specific CD4 T cell proliferation in one representative CVID patient (CVID-02). Unstimulated cell cultures (negative control) and cell cultures stimulated with SEB (positive control) are also shown. (B and C) Percentage of bacteria-specific (B) or virus-specific (C) proliferating CD4 T cells in the presence or absence of α–PD-L1/2 Abs in CVID patients (n = 10). P-values were calculated using paired Student’s t test. *, P < 0.05.
PD-1 expression influences CD4 T cell cytokine profile
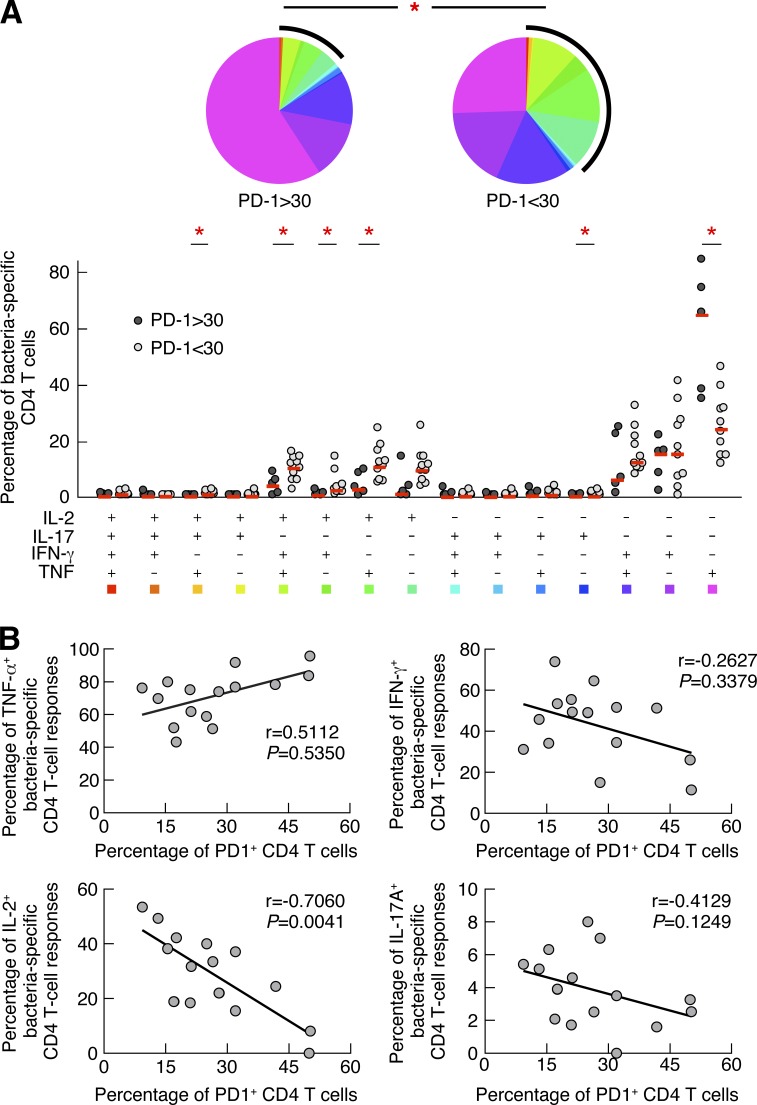
We then tested whether PD-1 expression on CD4 T cells may influence the CD4 T cell cytokine profile in CVID patients. In this regard, the cytokine profile of bacteria-specific CD4 T cells was correlated with PD-1 expression. The qualitative differences in the cytokine profile of bacteria-specific CD4 T cells on the basis of PD-1 expression are shown in Fig. 6. The cumulative data showed that the cytokine profile of bacteria-specific CD4 T cells expressing higher levels of PD-1 (>30%) was skewed toward single TNF-producing bacteria-specific CD4 T cells, whereas bacteria-specific CD4 T cells expressing lower levels of PD-1 (<30%) were significantly more polyfunctional (produced TNF, IFN-γ, and IL-2; P = 0.003; Fig. 6 A). Interestingly, the proportion of triple TNF/IFN-γ/IL-2, dual IFN-γ/IL-2, and dual TNF/IL-2 was significantly increased at the expense of single TNF-producing CD4 T cell populations in bacteria-specific CD4 T cell responses of CVID patients with PD-1–expressing CD4 T cells <30% as compared with those with PD1 expression >30% (P < 0.05; Fig. 6 A). Finally, the proportion of IL-2–producing bacteria-specific CD4 T cells negatively correlated with the frequency of PD-1–expressing CD4 T cells (r = −0.7060, P = 0.0041; Fig. 6 B).
Figure 6.
PD-1 expression directly influences CD4 T cell cytokine profile in CVID patients. Blood mononuclear cells from CVID patients (n = 12) were stimulated with bacteria antigen preparations and cytokine production (IL-17A, IL-2, TNF, and IFN-γ) by CD4 T cells from CVID patients with low frequency (<30%; white circles) or high frequency (>30%; black circles) of PD-1 expression was assessed by polychromatic flow cytometry. (A) Proportion of bacteria-specific CD4 T cells producing TNF, IFN-γ, IL-2, and/or IL-17A. All the possible combinations of the responses are shown on the x axis and the percentage of the functionally distinct cell populations within the bacteria-specific CD4 T cell populations are shown on the y axis. The pie chart summarizes the data, and each slice corresponds to the fraction of CD4 T cells with a given number of functions within the responding CD4 T cell population. Statistical analyses of the global cytokine profiles (pie charts) were performed by partial permutation tests using the SPICE software as previously described (Roederer et al., 2011). Bars correspond to the fractions of different functionally distinct T cell populations within the total CD4 T cells. Red stars indicate statistical significance (*, P < 0.05) calculated using a Student’s t test. (B) Correlations between the percentage of PD-1–expressing CD4 T cells and the percentage of TNF-, IFN-γ–, IL-2–, or IL-17A–producing bacteria-specific CD4 T cells (n = 12). Statistical significance (p-values) in B was calculated using Spearman’s rank correlations.
Effective IVIG treatment restores the CD4 T cell functional impairment
As shown above, the detection of endotoxemia was observed in CVID patients with hypogammaglobulinemia. On the basis of these findings, we have determined the correlation between endotoxemia and IgG serum levels, and the effects of Ig replacement therapy on endotoxemia and, in turn, on CD4 T cell functions. We show that endotoxemia inversely correlated with the serum levels of IgG (r = −0.5988, P < 0.0001; Fig. 7 A). With regard to serum IgA levels, we did not find any correlation between serum IgA levels and endotoxemia (r = 0.066 and P = 0.6930). Furthermore, with regard to the frequencies of B cell populations in the CVID patients (we have used the Euro class definition; Wehr et al., 2008), no association was observed between the proportion of CD27+ B cells (or any other B cell population) and endotoxemia (unpublished data). Finally, endotoxemia did not correlate with inflammatory bowel diseases (Table 2).
Figure 7.
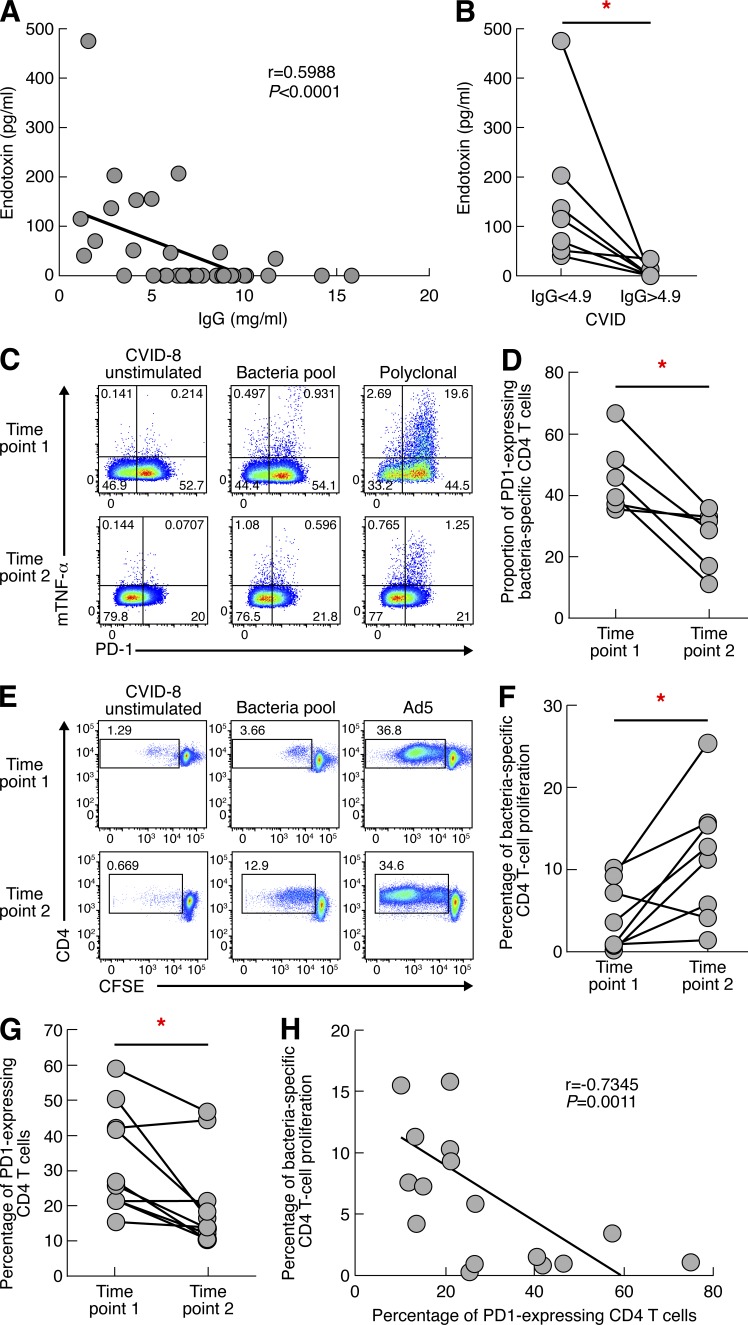
Efficient IVIG treatment restores CD4 T cell functions. IgG concentration, endotoxin level, PD-1 expression, and bacteria-specific CD4 T cell proliferation were assessed on sera and blood mononuclear cells from CVID patients. (A) Correlation of endotoxin levels with IgG levels (n = 40). (B) Reduction of endotoxin levels after Ig substitution. (C) Representative flow cytometry profiles of bacteria-specific CD4 T cells expressing PD-1 in one representative CVID patient (CVID-08) at two time points. (D) Proportion of antigen-specific CD4 T cells expressing PD-1. (E) Representative flow cytometry profiles of bacteria-specific and virus-specific proliferating CD4 T cells in one representative CVID patient (CVID-8) at two time points. Unstimulated cell cultures (negative control) and cell cultures stimulated with SEB (positive control) are also shown. (F) Cumulative data of bacteria-specific CD4 T cell proliferation in treated CVID patients (n = 8). (G) Cumulative data of PD-1 expression in CD4 T cells in treated CVID patients (n = 8). (H) Correlation between bacteria-specific CD4 T cell proliferation and PD-1 expression. P-values were calculated using a paired Student’s t test for multiple comparisons in B, D, and F or Spearman Rank test for correlation in A and G. *, P < 0.05.
Furthermore, the presence of endotoxin was assessed in CVID patients (n = 8) before and after IVIG therapy. The IgG titers consistently increased >4.9 mg/ml after IVIG therapy (Fig. 7 B). Endotoxemia levels consistently and significantly decreased after initiation of therapy, suggesting that IVIG therapy was associated with efficient reduction of bacterial translocation in CVID patients (P = 0.0437; Fig. 7 B). IVIG therapy might potentially influence endotoxemia levels through the presence of anti-LPS IgG antibodies. To exclude the potential impact of anti-LPS antibodies in the detection/concentration of LPS, we determined the effects of treating in vitro the serum of an untreated CVID patient positive for the detection of LPS with IVIG at a final IgG concentration of 10 mg/ml. A CVID patient with low LPS levels, i.e., 53 pg/ml, was selected to have the best experimental conditions to detect any potential interference of the IVIG treatment. Of note, serum treatment with IVIGs did not significantly reduce LPS concentration (P = 0.7802; unpublished data). These data indicate that IVIG treatment is unlikely to influence the detection of LPS in the sera of CVID patients.
We then investigated the effects of continuous IVIG therapy in restoring normal CD4 T cell function. To address this issue, PD-1 expression on bacteria-specific CD4 T cells was assessed in blood mononuclear cells of IVIG-treated CVID patients (n = 6) at two time points (spaced by 8.8 mo on average). Representative flow cytometry profiles and cumulative data showed that IVIG treatment significantly reduced PD-1 expression on bacteria-specific CD4 T cells (P = 0.0120; Fig. 7, C and D). In addition, the proliferation capacity of bacteria-specific CD4 T cells was assessed in blood mononuclear cells of IVIG-treated CVID patients (n = 8) at two time points (spaced by 11.2 mo on average). Representative flow cytometry profiles and cumulative data showed that IVIG treatment significantly restored bacteria-specific CD4 T cell proliferation (P = 0.0165; Fig. 7, E and F). Of note, IVIG therapy restored the proliferation of bacteria-specific CD4 T cells in CVID patients at levels comparable to healthy subjects (P > 0.05; unpublished data). In addition, the percentage of PD-1 expression on CD4 T cells was also significantly reduced after 11 mo of IVIG treatment (P = 0.008; Fig. 7 G). Finally, we showed that the PD-1 expression level in CD4 T cells inversely correlated with the percentage of bacteria-specific CD4 T cell proliferation (r = −0.7345, P = 0.0011; Fig. 7 D). Collectively these data demonstrate that IVIG treatment induces resolution of bacterial translocations in the large majority of patients and restores CD4 T cell proliferation.
DISCUSSION
In the present study, we have hypothesized that recurrent bacterial infections observed in CVID patients were responsible for the CD4 T cell impairment and we postulated that the CD4 T cell dysfunction was predominantly restricted to bacteria-specific CD4 T cells. In this regard, the clinical picture of CVID patients is complicated by recurrent bacterial infections (Van der Hilst et al., 2002; Park et al., 2008; Hong et al., 2010), whereas no increase in the frequencies of acute (i.e., influenza) or reactivation of chronic viral infections (i.e., EBV or CMV; Raeiszadeh et al., 2006) has been reported.
Our results indicate that the impairment of CD4 T cell functions was selective to bacteria-specific but not virus-specific T cell responses. CD4 T cells from CVID patients had reduced capacities to produce cytokines (IL-2 and IFN-γ) and to proliferate. The defect in the production of IL-2 may be responsible for the defects of T cell proliferation described in CVID patients together with the exhaustion associated with the PD-1 expression. The defect in the production of IFN-γ may not necessarily be responsible for the direct impairment of CD4 T cells but for the defective bactericidal activity of macrophages through the defective activation of macrophages by impaired Th1 CD4 T cells from CVID patients. Of note, no significant differences were observed in CD4 T cell differentiation, immunosenescence, and activation markers between CVID patients and healthy individuals in our cohort of patients. An increase in the proportion of activation markers has been recently reported in CD4 T cells of CVID patients (Paquin-Proulx et al., 2013a).
The severe impairment in CD4 T cell function found in CVID patients was reminiscent of that observed in patients with HIV infection (Day et al., 2006; Petrovas et al., 2006; Trautmann et al., 2006). The CD4 T cell dysfunction in HIV infection has been at least partially attributed to the chronic immune stimulation resulting from microbial translocation (Brenchley et al., 2004; Brenchley and Douek, 2008). We therefore investigated the presence of microbial translocation in CVID patients through the determination of endotoxemia levels. Interestingly, we found increased endotoxemia levels in patients with CVID which inversely correlated with IgG serum levels. The continuous leak of bacteria in the gut and bronchial mucosa might be responsible for the exhaustion of bacteria-specific CD4 T cells. In support of our hypothesis, CD4 T cells of CVID patients showed significant increased expression of PD-1 as compared with healthy individuals. We also provided evidence that PD-1 was selectively increased in bacteria-specific CD4 T cells, as indicated by the increased expression of PD-1 on mTNF-positive CD4 T cells. Further demonstration that the expression of PD-1 was the primary cause of the exhaustion and of the associated functional impairment of bacteria-specific CD4 T cells was obtained by the finding that restoration of proliferation occurred after the blockade of PD-1–PD-L1 axis.
Levels of endotoxin inversely correlated with IgG serum levels, thus indicating that hypogammaglobulinemia was potentially responsible for microbial translocation (Brenchley et al., 2004; Brenchley and Douek, 2008) and increased endotoxemia in CVID patients. This hypothesis was supported by the finding that IVIG treatment induced the reduction of endotoxemia.
CVID patients were also longitudinally followed up to determine whether IVIG treatment had any impact on the restoration of CD4 T cell functions. Interestingly, IVIG treatment was associated with lower PD-1 expression and restoration of polyfunctional cytokine responses, and increased proliferation in response to stimulation with bacteria-derived antigens. No more differences in the percentage of proliferating bacteria-specific CD4 T cells were observed between healthy subjects and CVID patients after treatment with anti-PDL antibodies in vitro and IVIG therapy.
Collectively, the results on the relationship between IgG levels and endotoxemia and the impact of IVIG therapy on the levels of endotoxemia indicate that endotoxemia may be instrumental to monitor the response to therapy in CVID patients. Because suppression of endotoxemia is also associated with the improvement of bacteria-specific CD4 T cell functions, the measure of endotoxemia may also serve as an indirect marker of restoration of bacteria-specific CD4 T cell functions.
Our results favor the following model that is not exclusive of CVID but likely applicable to any pathological condition causing Ig deficiency. Recurrent bacterial infections in CVID patients result from defective levels of IgA in the mucosa and of IgG that circulates and passively diffuses through the oropharyngeal or gut mucosa (Butcher et al., 1975; Lieberman et al., 1979; Coruh and Mason, 1980; Morris et al., 1980; Brandtzaeg et al., 1985). Ig deficiency is associated with microbial translocation, which causes selective exhaustion of bacteria-specific CD4 T cells as indicated by the up-regulation of the co-inhibitory receptor PD-1 in these cells. In turn, PD-1–expressing bacteria-specific CD4 T cells show reduced proliferation capacity and defective production of cytokines (IL-2 and IFN-γ). The treatment with IVIG restores bacteria-specific CD4 T cell responses, likely due to the reduction of bacteria-derived antigen load which, in turn, results in reduction of the magnitude of chronic stimulation and PD-1 expression on bacteria-specific CD4 T cells. The restoration of CD4 T cell functions is consistent with the similar effects observed in chronic viral infections, such as HIV or hepatitis C virus, after suppression of viral load with antiviral therapy (Day et al., 2006; Trautmann et al., 2006).
The current recommendation of IgG trans-complementation is to reach plasmatic IgG serum levels of 6 mg/ml by administering 400 mg/kg Igs every 3–4 wk (Orange et al., 2006). More than 95% of healthy individuals have IgG serum levels >7 mg/ml. Whether the dose of Ig replacement should be increased to achieve higher IgG serum levels in CVID patients has been debated (Cunningham-Rundles, 2010). One could speculate that higher IgG concentration may be beneficial to CVID patients by reducing the bacterial infections and likely by reducing the general degree of inflammation. In support of this assumption, it has been recently shown that high-dose therapy of IVIG (range 1–3 g/kg) leads to antiinflammatory effects via FcγRII-mediated IL-33 production by dendritic cells that induces IL-4 secretion by basophils and promotes antiinflammatory (Th2) responses (Anthony et al., 2011). In conclusion, the present study provides new insights in the mechanisms responsible for the CD4 T cell impairment present in patients with CVID and has identified endotoxemia as a novel marker to monitor the therapeutic response to Igs trans-complementation.
MATERIALS AND METHODS
Study groups.
In the present study, 26 CVID patients (Table 1) and 30 healthy individuals have been enrolled. Blood samples from the healthy subjects were obtained from the Blood Bank of Lausanne, Switzerland. CVID patients were either recruited at the Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland) or at the Hôpital Henri-Mondor Albert-Chenevier (Paris, France). The Institutional Review Boards of both institutions approved this study and informed consent was obtained from each individual enrolled. The diagnosis of CVID was formulated according to the European Society for ImmunoDeficiencies (ESID) and Pan-American Group for Immunodeficiency (PAGID) criteria. The clinical manifestations of CVID include predominantly recurrent bacterial infections, noninfectious lung and digestive pathologies, autoimmune diseases, and increased susceptibility to cancers (Table 2). The medical records of the patients studied were analyzed to determine the clinical symptoms before the diagnosis of CVID. Most patients suffered from recurrent bacterial infections several years before the diagnosis of CVID was confirmed. All patients suffered from recurrent sinusitis and antibiotic therapy was administered several times per year. Recurrent lower respiratory tract infections (bronchitis and pneumonia) were present in 19 out of 26 patients. One patient experienced bacterial meningitis. Gastrointestinal complaints were identified in 3 patients, mainly recurrent diffuse abdominal pain and diarrhea. An intestinal parasite (Strongyloides stercoralis) was found in 1 patient. 7 out of 26 patients had autoimmune manifestations, 16 out 26 patients had intestinal lymphoid hyperplasia, and 4 out of 26 granulomatosis disease.
Cell isolation.
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient (GE Healthcare). After centrifugation (300 g, 20 min), PBMC ring was harvested in PBS-EDTA 2 mM (Laboratorium Dr Bichsel AG, and Ambion for PBS and EDTA, respectively). Cells were either used directly or cryopreserved in liquid nitrogen for future experiments.
Antibodies.
The following antibodies were used: allophycocyanin (APC)-H7–conjugated anti-CD3 (clone SK7), FITC-conjugated anti-CD4 (clone RPA-T4), APC-conjugated anti-CD4 (clone RPA-T4), Pacific blue (PB)–conjugated anti-CD4 (clone RPA-T4), APC-H7–conjugated anti-CD8 (clone SK1), PerCP-Cy5.5–conjugated anti-CD8 (clone SK1), PerCP-conjugated anti-CD69 (clone L78), PECy7-conjugated anti-CD279 (PD-1; clone EH12.1), APC-conjugated anti-TNF (MAbII), FITC-conjugated anti-CD25 (OX-39), PECy-conjugated anti-TNF (clone MabII; BD); energy coupled dye (ECD)–conjugated anti-CD3 (clone UCHT1), ECD-conjugated anti-CD45RA (clone 2H4), ECD-conjugated anti-CD4 (clone T4; Beckman Coulter); PECy5.5-conjugated anti-2B4 (CD244; clone C1.7), PE-conjugated anti-SLAM (CD150; clone A12), PB-conjugated anti-CD57 (clone HCD57), Alexa Fluor 647–conjugated anti-CD160 (clone BY55; BioLegend); EFluor 625NC-conjugated anti-CD8 (clone RPA-T8; eBioscience); and FITC-conjugated anti-CCR7 (clone 150503) and Alexa Fluor 700–conjugated anti–HLA-DR (clone LN3; R&D Systems).
Flow cytometry.
Data were acquired on an LSR SORP with four lasers (405, 488, 532, and 633 nm; BD), analyzed using FlowJo (version 9.4.11; Tree Star). When required SPICE software (version 5.21; developed by M. Roederer, National Institutes of Health) was used (Roederer et al., 2011). At least 100,000 events were acquired for each sample.
Antigens preparation.
S. aureus, S. pneumoniae, S. agalactiae, P. aeruginosa, K. pneumoniae, and the two serotypes of E. coli were grown in tryptic soy broth (BD) at 37°C, washed, and heat-inactivated by incubation for 2 h at 56°C as previously described (Perreau et al., 2012).
Assessment of CD4 T cell cytokine profile.
Freshly isolated blood mononuclear cells (106 cells) were stimulated overnight as previously described (Perreau et al., 2012) in complete RPMI medium (10% FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin [Bioconcept]). Blood mononuclear cells were stimulated with 1 µg/ml Ad5 vector or CMV lysat (Advanced Biotechnologies INC) or 5 × 107 CFU/ml of individual bacteria or bacteria pool depending on the experiment. As a positive control, cells were stimulated with anti-CD3/anti-CD28 beads (Invitrogen). Cells were incubated for 18 h in 1 ml of complete media containing 1 µl/ml brefeldin A (Golgiplug; BD). At the end of the stimulation period, dead cells were stained (4°C, 15 min) using the violet LIVE/DEAD stain kit (Invitrogen). Cells were washed, permeabilized (Cytofix/Cytoperm solution; BD), and stained (4°C, 15 min) with anti–CD3-APC-H7, anti–CD4-ECD, anti–CD8-PerCP, anti–IFN-γ-AF700, anti–IL-17A-AF647, anti–TNF-PE-CY7, and anti–IL-2-PE as previously described (Perreau et al., 2012). Frequencies of cytokine-producing CD4 T cells were assessed by flow cytometry.
Assessment of CD4 T cell proliferation.
Mononuclear cells were resuspended at 106/ml in PBS and incubated for 7 min at 37°C with 0.25 µM 5, 6-CFSE (Invitrogen) as previously described (Perreau et al., 2011). The reaction was quenched with one volume of fetal calf serum (FBS; Institut de Biotechnologies Jacques Boy). Subsequently, cells were washed and cultured in 4% human AB serum (Institut de Biotechnologies Jacques Boy) RPMI (Gibco; Life Technologies). Cells were stimulated with virus-derived antigens (Ad5 or CMV) or bacteria-derived antigens (either individual bacteria or bacteria pool depending on the experiment), with Staphylococcus enterotoxin B (SEB, Sigma-Aldrich) as positive control, or remained unstimulated (negative control). After 6 d of in vitro T cell expansion, dead cells were stained (4°C, 15 min) using the violet LIVE/DEAD stain kit (Invitrogen), and cells were stained (4°C, 15 min) with anti–CD3-APC-H7, anti–CD4-ECD, and anti–CD8-PerCP. Frequencies of proliferating CD4 T cells were assessed by flow cytometry.
Restoration of CD4 T cells proliferation.
CFSE-labeled PBMCs were stimulated with virus-derived antigens (Ad5 or CMV; 1 µg/ml), bacteria pool (5 × 107 CFU/ml), SEB as positive control, or unstimulated cells as negative control, in the presence or in the absence of 10 µg/ml PD-L1/2 (eBioscience) as previously described (Trautmann et al., 2006). After 6 d of stimulation, cells were washed and stained with violet LIVE/DEAD stain kit (4°C, 15 min). Cells were stained (4°C, 15 min) with anti–CD3-APC-H7, anti–CD4-ECD, and anti–CD8-PerCP-Cy5.5 and analyzed by flow cytometry.
Assessment of co-inhibitory molecule expression, T cell activation, T cell differentiation, and immunosenescence.
PBMCs were resuspended (106 cells/ml and per condition) in complete RPMI medium. Dead cells were stained using the amcyan LIVE/DEAD stain kit (Life Technologies; 4°C, 15 min). Cells were washed and stained (4°C, 15 min) with panels of monoclonal antibodies to measure the expression of co-inhibitory molecules (anti–CD3-APC H7, anti–CD4-PB, anti–CD8-Efluor625NC, anti–2B4-PeCY5.5, anti–CD160-APC, anti–PD-1-PeCy7, and anti–SLAM-PE), T cell differentiation (anti–CD3-APC H7, anti–CD4-PB, anti–CD8-Efluor625NC, anti–CD45RA-ECD, and anti–CCR7-FITC), T cell activation, and immunosenescence (anti–CD3-ECD, anti–CD4-APC, anti–CD8-APC-H7, anti–CD69-PerCP, anti–CD25-FITC, anti–PD-1-PeCy7, anti–HLADR–Alexa Fluor 700, and anti–CD57-PB). Data were acquired by flow cytometry.
mTNF production.
Blood mononuclear cells (106 cells/ml) were stimulated (18 h) with heat-inactivated bacteria pool (5 × 107 CFU/ml) or Ad5 (1 µg/ml) in complete RPMI containing TAPI-0 (10 µM; EMD Millipore) and APC-conjugated anti-TNF (0.2 mg/ml) as previously described (Haney et al., 2011). PHA (Sigma-Aldrich) was used as positive control and unstimulated cells as negative control. At the end of the stimulation period, dead cells were stained using the violet LIVE/DEAD stain kit (4°C, 15 min). Cells were stained (4°C, 15 min) with anti–CD3-ECD, anti–CD4-FITC, anti–CD8-APC-H7, and anti–PD-1 PeCY7. Data were analyzed by flow cytometry.
Endotoxin measurement.
Endotoxins were quantified in plasma or sera using limulus assay test (Charles River). In brief, plasma were diluted (1/50) in sterile water (Laboratorium Dr Bichsel AG) and LPS was assessed using Endosafe-Point Test system (Charles River).
Ig quantification.
Total IgG quantification was performed in the Immunology and Allergy diagnostic laboratory by nephelometry.
Statistical analyses.
Statistical significance (p-values) was derived from χ2 analyses for comparison of positive proportions. P-values were derived using one-way ANOVA (Kruskal-Wallis test), followed by Student’s t test for multiple comparisons. Spearman’s rank test was used for correlations. The analyses of multiple comparisons were taken into account for the calculation of statistical significance.
Acknowledgments
We are grateful to Nicole Grandchamp, Aurore Crétignier, Dao Thi Ngoc Dung, and Celine Crausaz for technical assistance. We thank the study managers Nils Rettby and Delphine Gani. We thank Dr. Stephanie Petitpierre for helpful discussions. We also thank the students Hugh C. Welles, Elisa Leuthold, Riddhima Banga, and Olivia Hall for their help.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- APC
- allophycocyanin
- CVID
- common variable immunodeficiency
- ECD
- energy coupled dye
- IVIG
- intravenous IgG
- mTNF
- membrane TNF
- PB
- Pacific blue
- PBMC
- peripheral blood mononuclear cell
- PD-1
- programmed death 1
- PDL-1/2
- PD ligand 1/2
- SEB
- Staphylococcus enterotoxin B
- SLAM
- signaling lymphocytic activation molecule
References
- Anthony, R.M., Kobayashi T., Wermeling F., and Ravetch J.V.. 2011. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature. 475:110–113 10.1038/nature10134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust, P., Müller F., and Frøland S.S.. 1994. Elevated serum levels of interleukin-4 and interleukin-6 in patients with common variable immunodeficiency (CVI) are associated with chronic immune activation and low numbers of CD4+ lymphocytes. Clin. Immunol. Immunopathol. 70:217–224 10.1006/clin.1994.1032 [DOI] [PubMed] [Google Scholar]
- Barber, D.L., Wherry E.J., Masopust D., Zhu B., Allison J.P., Sharpe A.H., Freeman G.J., and Ahmed R.. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- Bayry, J., Lacroix-Desmazes S., Kazatchkine M.D., Galicier L., Lepelletier Y., Webster D., Lévy Y., Eibl M.M., Oksenhendler E., Hermine O., and Kaveri S.V.. 2004. Common variable immunodeficiency is associated with defective functions of dendritic cells. Blood. 104:2441–2443 10.1182/blood-2004-04-1325 [DOI] [PubMed] [Google Scholar]
- Bloom, B.R., and Bennett B.. 1970. Macrophages and delayed-type hypersensitivity. Semin. Hematol. 7:215–224 [PubMed] [Google Scholar]
- Bonhomme, D., Hammarström L., Webster D., Chapel H., Hermine O., Le Deist F., Lepage E., Romeo P.H., and Levy Y.. 2000. Impaired antibody affinity maturation process characterizes a subset of patients with common variable immunodeficiency. J. Immunol. 165:4725–4730 10.4049/jimmunol.165.8.4725 [DOI] [PubMed] [Google Scholar]
- Brandtzaeg, P., Valnes K., Scott H., Rognum T.O., Bjerke K., and Baklien K.. 1985. The human gastrointestinal secretory immune system in health and disease. Scand. J. Gastroenterol. Suppl. 20:17–38 10.3109/00365528509093765 [DOI] [PubMed] [Google Scholar]
- Brenchley, J.M., and Douek D.C.. 2008. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 1:23–30 10.1038/mi.2007.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley, J.M., Schacker T.W., Ruff L.E., Price D.A., Taylor J.H., Beilman G.J., Nguyen P.L., Khoruts A., Larson M., Haase A.T., and Douek D.C.. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 10.1084/jem.20040874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher, B.T., Salvaggio J.E., and Leslie G.A.. 1975. Secretory and humoral immunologic response of atopic and non-atopic individuals to intranasally administered antigen. Clin. Allergy. 5:33–42 10.1111/j.1365-2222.1975.tb01834.x [DOI] [PubMed] [Google Scholar]
- Castigli, E., Wilson S.A., Garibyan L., Rachid R., Bonilla F., Schneider L., and Geha R.S.. 2005. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat. Genet. 37:829–834 10.1038/ng1601 [DOI] [PubMed] [Google Scholar]
- Cellerai, C., Perreau M., Rozot V., Bellutti Enders F., Pantaleo G., and Harari A.. 2010. Proliferation capacity and cytotoxic activity are mediated by functionally and phenotypically distinct virus-specific CD8 T cells defined by interleukin-7Rα (CD127) and perforin expression. J. Virol. 84:3868–3878 10.1128/JVI.02565-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne, P., Ogg G.S., King A.S., Knabenhans C., Ellefsen K., Nobile M., Appay V., Rizzardi G.P., Fleury S., Lipp M., et al. 2001. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 410:106–111 10.1038/35065118 [DOI] [PubMed] [Google Scholar]
- Coruh, G., and Mason D.Y.. 1980. Serum proteins in human squamous epithelium. Br. J. Dermatol. 102:497–505 10.1111/j.1365-2133.1980.tb07646.x [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles, C.2010. How I treat common variable immune deficiency. Blood. 116:7–15 10.1182/blood-2010-01-254417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham-Rundles, C., and Bodian C.. 1999. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin. Immunol. 92:34–48 10.1006/clim.1999.4725 [DOI] [PubMed] [Google Scholar]
- Cunningham-Rundles, C., Bodian C., Ochs H.D., Martin S., Reiter-Wong M., and Zhuo Z.. 2001. Long-term low-dose IL-2 enhances immune function in common variable immunodeficiency. Clin. Immunol. 100:181–190 10.1006/clim.2001.5052 [DOI] [PubMed] [Google Scholar]
- David, J.R.1973. Lymphocyte mediators and cellular hypersensitivity. N. Engl. J. Med. 288:143–149 10.1056/NEJM197301182880311 [DOI] [PubMed] [Google Scholar]
- Day, C.L., Kaufmann D.E., Kiepiela P., Brown J.A., Moodley E.S., Reddy S., Mackey E.W., Miller J.D., Leslie A.J., DePierres C., et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 443:350–354 10.1038/nature05115 [DOI] [PubMed] [Google Scholar]
- De Gast, G.C., Wilkins S.R., Webster A.D., Rickinson A., and Platts-Mills T.A.. 1980. Functional ‘immaturity’ of isolated B cells from patients with hypogammaglobulinaemia. Clin. Exp. Immunol. 42:535–544 [PMC free article] [PubMed] [Google Scholar]
- Deeks, S.G., Verdin E., and McCune J.M.. 2012. Immunosenescence and HIV. Curr. Opin. Immunol. 24:501–506 10.1016/j.coi.2012.05.004 [DOI] [PubMed] [Google Scholar]
- Giovannetti, A., Pierdominici M., Mazzetta F., Marziali M., Renzi C., Mileo A.M., De Felice M., Mora B., Esposito A., Carello R., et al. 2007. Unravelling the complexity of T cell abnormalities in common variable immunodeficiency. J. Immunol. 178:3932–3943 10.4049/jimmunol.178.6.3932 [DOI] [PubMed] [Google Scholar]
- Grimbacher, B., Hutloff A., Schlesier M., Glocker E., Warnatz K., Dräger R., Eibel H., Fischer B., Schäffer A.A., Mages H.W., et al. 2003. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat. Immunol. 4:261–268 10.1038/ni902 [DOI] [PubMed] [Google Scholar]
- Haney, D., Quigley M.F., Asher T.E., Ambrozak D.R., Gostick E., Price D.A., Douek D.C., and Betts M.R.. 2011. Isolation of viable antigen-specific CD8+ T cells based on membrane-bound tumor necrosis factor (TNF)-α expression. J. Immunol. Methods. 369:33–41 10.1016/j.jim.2011.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, R., Agrawal S., Gollapudi S., and Gupta S.. 2010. Impaired pneumovax-23-induced monocyte-derived cytokine production in patients with common variable immunodeficiency. J. Clin. Immunol. 30:435–441 10.1007/s10875-010-9371-z [DOI] [PubMed] [Google Scholar]
- Ishihara, T., Takahara S., and Fathman C.G.. 1986. IL 2 restores memory B cell activation by antigen-specific T cell clone variants. J. Immunol. 136:39–43 [PubMed] [Google Scholar]
- Ito, T., Yang M., Wang Y.H., Lande R., Gregorio J., Perng O.A., Qin X.F., Liu Y.J., and Gilliet M.. 2007. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 204:105–115 10.1084/jem.20061660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers, T.W., Bende R.J., Baars P.A., Grummels A., Derks I.A., Dolman K.M., Beaumont T., Tedder T.F., van Noesel C.J., Eldering E., and van Lier R.A.. 2010. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J. Clin. Invest. 120:214–222 10.1172/JCI40231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, Y., Gupta N., Le Deist F., Garcia C., Fischer A., Weill J.C., and Reynaud C.A.. 1998. Defect in IgV gene somatic hypermutation in common variable immuno-deficiency syndrome. Proc. Natl. Acad. Sci. USA. 95:13135–13140 10.1073/pnas.95.22.13135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman, M.M., McKissock D.C., and Wright G.L.. 1979. Passive immunization against Pseudomonas with a ribosomal vaccine-induced immune serum and immunoglobulin fractions. Infect. Immun. 23:509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams, L.J., and McHeyzer-Williams M.G.. 2005. Antigen-specific memory B cell development. Annu. Rev. Immunol. 23:487–513 10.1146/annurev.immunol.23.021704.115732 [DOI] [PubMed] [Google Scholar]
- McMahan, R.H., Golden-Mason L., Nishimura M.I., McMahon B.J., Kemper M., Allen T.M., Gretch D.R., and Rosen H.R.. 2010. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J. Clin. Invest. 120:4546–4557 10.1172/JCI43127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita, R., Schmitt N., Bentebibel S.E., Ranganathan R., Bourdery L., Zurawski G., Foucat E., Dullaers M., Oh S., Sabzghabaei N., et al. 2011. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 34:108–121 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, J.A., Wray C., and Sojka W.J.. 1980. Passive protection of lambs against enteropathogenic Escherichia coli: role of antibodies in serum and colostrum of dams vaccinated with K99 antigen. J. Med. Microbiol. 13:265–272 10.1099/00222615-13-2-265 [DOI] [PubMed] [Google Scholar]
- Nathan, C.F., Murray H.W., Wiebe M.E., and Rubin B.Y.. 1983. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J. Exp. Med. 158:670–689 10.1084/jem.158.3.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange, J.S., Hossny E.M., Weiler C.R., Ballow M., Berger M., Bonilla F.A., Buckley R., Chinen J., El-Gamal Y., Mazer B.D., et al. Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. 2006. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J. Allergy Clin. Immunol. 117:S525–S553 10.1016/j.jaci.2006.01.015 [DOI] [PubMed] [Google Scholar]
- Paquin-Proulx, D., Santos B.A., Carvalho K.I., Toledo-Barros M., Barreto de Oliveira A.K., Kokron C.M., Kalil J., Moll M., Kallas E.G., and Sandberg J.K.. 2013a. IVIg immune reconstitution treatment alleviates the state of persistent immune activation and suppressed CD4 T cell counts in CVID. PLoS ONE. 8:e75199 10.1371/journal.pone.0075199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin-Proulx, D., Santos B.A., Carvalho K.I., Toledo-Barros M., Oliveira A.K., Kokron C.M., Kalil J., Moll M., Kallas E.G., and Sandberg J.K.. 2013b. Dysregulated CD1 profile in myeloid dendritic cells in CVID is normalized by IVIg treatment. Blood. 121:4963–4964 10.1182/blood-2013-04-499442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, M.A., Li J.T., Hagan J.B., Maddox D.E., and Abraham R.S.. 2008. Common variable immunodeficiency: a new look at an old disease. Lancet. 372:489–502 10.1016/S0140-6736(08)61199-X [DOI] [PubMed] [Google Scholar]
- Parker, D.C.1993. T cell-dependent B cell activation. Annu. Rev. Immunol. 11:331–360 10.1146/annurev.iy.11.040193.001555 [DOI] [PubMed] [Google Scholar]
- Perreau, M., Welles H.C., Harari A., Hall O., Martin R., Maillard M., Dorta G., Bart P.A., Kremer E.J., Tartaglia J., et al. 2011. DNA/NYVAC vaccine regimen induces HIV-specific CD4 and CD8 T-cell responses in intestinal mucosa. J. Virol. 85:9854–9862 10.1128/JVI.00788-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreau, M., Welles H.C., Harari A., Calandra T., Roger T., and Pantaleo G.. 2012. Modulation of human memory T-cell function by different antigen-presenting cells. Eur. J. Immunol. 42:799–802 10.1002/eji.201142094 [DOI] [PubMed] [Google Scholar]
- Petrovas, C., Casazza J.P., Brenchley J.M., Price D.A., Gostick E., Adams W.C., Precopio M.L., Schacker T., Roederer M., Douek D.C., and Koup R.A.. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281–2292 10.1084/jem.20061496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeiszadeh, M., Kopycinski J., Paston S.J., Diss T., Lowdell M., Hardy G.A., Hislop A.D., Workman S., Dodi A., Emery V., and Webster A.D.. 2006. The T cell response to persistent herpes virus infections in common variable immunodeficiency. Clin. Exp. Immunol. 146:234–242 10.1111/j.1365-2249.2006.03209.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz, E.L., Geha R., Wohl M.E., Morimoto C., Rosen F.S., and Schlossman S.F.. 1981. Immunodeficiency associated with loss of T4+ inducer T-cell function. N. Engl. J. Med. 304:811–816 10.1056/NEJM198104023041403 [DOI] [PubMed] [Google Scholar]
- Roederer, M., Nozzi J.L., and Nason M.C.. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A. 79A:167–174 10.1002/cyto.a.21015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger, T., Froidevaux C., Le Roy D., Reymond M.K., Chanson A.L., Mauri D., Burns K., Riederer B.M., Akira S., and Calandra T.. 2009. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. USA. 106:2348–2352 10.1073/pnas.0808146106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto, F., Lenig D., Förster R., Lipp M., and Lanzavecchia A.. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712 10.1038/44385 [DOI] [PubMed] [Google Scholar]
- Sneller, M.C., and Strober W.. 1990. Abnormalities of lymphokine gene expression in patients with common variable immunodeficiency. J. Immunol. 144:3762–3769 [PubMed] [Google Scholar]
- Trautmann, L., Janbazian L., Chomont N., Said E.A., Gimmig S., Bessette B., Boulassel M.R., Delwart E., Sepulveda H., Balderas R.S., et al. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198–1202 10.1038/nm1482 [DOI] [PubMed] [Google Scholar]
- Van der Hilst, J.C., Smits B.W., and van der Meer J.W.. 2002. Hypogammaglobulinaemia: cumulative experience in 49 patients in a tertiary care institution. Neth. J. Med. 60:140–147 [PubMed] [Google Scholar]
- van Zelm, M.C., Reisli I., van der Burg M., Castaño D., van Noesel C.J., van Tol M.J., Woellner C., Grimbacher B., Patiño P.J., van Dongen J.J., and Franco J.L.. 2006. An antibody-deficiency syndrome due to mutations in the CD19 gene. N. Engl. J. Med. 354:1901–1912 10.1056/NEJMoa051568 [DOI] [PubMed] [Google Scholar]
- van Zelm, M.C., Smet J., Adams B., Mascart F., Schandené L., Janssen F., Ferster A., Kuo C.C., Levy S., van Dongen J.J., and van der Burg M.. 2010. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J. Clin. Invest. 120:1265–1274 10.1172/JCI39748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr, C., Kivioja T., Schmitt C., Ferry B., Witte T., Eren E., Vlkova M., Hernandez M., Detkova D., Bos P.R., et al. 2008. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 111:77–85 10.1182/blood-2007-06-091744 [DOI] [PubMed] [Google Scholar]
- Yamamoto, T., Price D.A., Casazza J.P., Ferrari G., Nason M., Chattopadhyay P.K., Roederer M., Gostick E., Katsikis P.D., Douek D.C., et al. 2011. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 117:4805–4815 10.1182/blood-2010-11-317297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–528 10.1084/jem.194.4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]