Five potent and broadly anti-HIV neutralizing monoclonal antibodies are able to block infection by two different SHIVs in monkeys. The authors show that antibodies targeting the outer glycan coat were the most effective and determined that titers of roughly 1:100 protected half the animals.
Abstract
It is widely appreciated that effective human vaccines directed against viral pathogens elicit neutralizing antibodies (NAbs). The passive transfer of anti–HIV-1 NAbs conferring sterilizing immunity to macaques has been used to determine the plasma neutralization titers, which must be present at the time of exposure, to prevent acquisition of SIV/HIV chimeric virus (SHIV) infections. We administered five recently isolated potent and broadly acting anti-HIV neutralizing monoclonal antibodies (mAbs) to rhesus macaques and challenged them intrarectally 24 h later with either of two different R5-tropic SHIVs. By combining the results obtained from 60 challenged animals, we determined that the protective neutralization titer in plasma preventing virus infection in 50% of the exposed monkeys was relatively modest (∼1:100) and potentially achievable by vaccination.
A major challenge in HIV research since the onset of the AIDS epidemic more than 30 years ago has been the development of an effective prophylactic vaccine. Most effective prophylactic vaccines directed against human pathogens elicit neutralizing antibodies (NAbs; Amanna and Slifka, 2011). Historically, monoclonal and polyclonal NAbs have been passively administered to susceptible humans and animals to prevent virus-induced disease (Keller and Stiehm, 2000; Buchwald and Pirofski, 2003). However, because HIV-1 infections, once established, nearly invariably lead to fatal outcomes, effective passively transferred antibodies must block virus acquisition to prevent disease. The passive transfer of early generations of anti–HIV-1 mAbs demonstrated that they could confer sterilizing protection in macaques against challenges with SHIVs (Mascola et al., 1999; Baba et al., 2000; Parren et al., 2001). However, the amounts of antibody required to prevent virus acquisition were so high that it was believed that this type of protection was not achievable by vaccination.
During the past four to five years, a new generation of potent, broadly acting, neutralizing mAbs have been isolated from HIV-1–infected individuals (Burton et al., 2012; Kwong and Mascola, 2012; Klein et al., 2013b). These mAbs target the CD4 binding site (CD4bs), protein-glycan epitopes associated with the gp120 V1/V2, V3, and V4 regions, and the membrane proximal external region of gp41 (Walker et al., 2009, 2010, 2011a; Wu et al., 2010; McLellan et al., 2011; Scheid et al., 2011; Huang et al., 2012; Kong et al., 2013) and typically exhibit great breadth and potency against heterologous HIV-1 isolates when assayed for neutralization in vitro. In this study, five of the new neutralizing mAbs were individually administered to groups of rhesus macaques, which were subsequently separately challenged intrarectally with either of two different R5 SHIVs. Levels of HIV-1 NAbs in the blood and tissues were measured at the time of virus challenge. By combining the results obtained from 60 animals challenged with two different SHIVs, we determined that the plasma neutralization titer preventing virus acquisition was relatively modest (∼1:100) and potentially achievable by vaccination. These findings provide guidance for determining the levels of neutralizing activity in plasma that an effective HIV vaccine should elicit.
RESULTS
In vitro characterization of 11 broadly reactive anti-HIV NAbs against two R5-tropic SHIVs
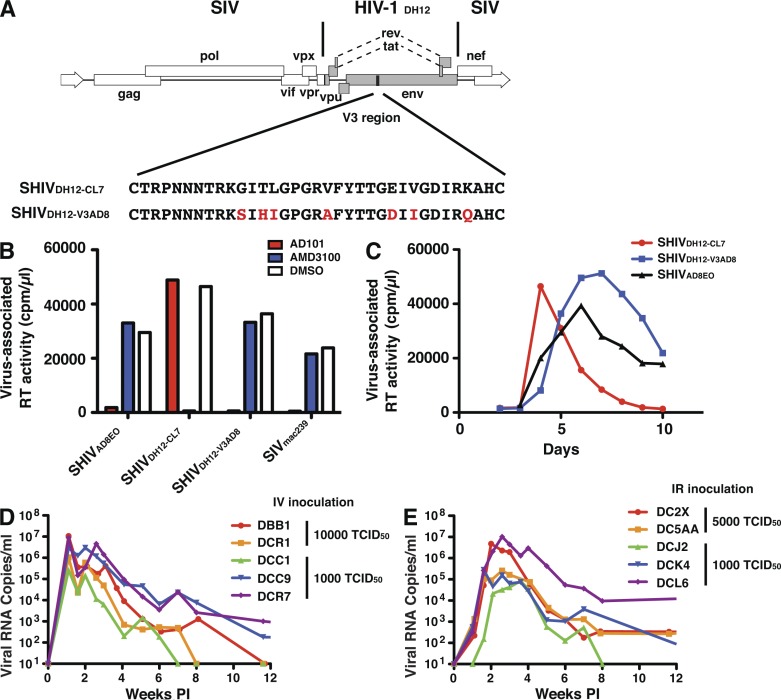
The neutralization sensitivities of two different R5-tropic SHIVs, to be subsequently used as challenge viruses in the preexposure passive antibody experiments described below, were assessed using the TZM-bl cell neutralization assay. One of the R5 SHIVs evaluated, SHIVAD8EO (Shingai et al., 2012), is a molecularly cloned derivative of SHIVAD8 (Nishimura et al., 2010), replicates to high levels in rhesus macaque PBMCs, exhibits a Tier 2 neutralization sensitivity phenotype (Gautam et al., 2012), and generates sustained levels of plasma viremia and depletion of CD4+ T cells leading to symptomatic immunodeficiency in inoculated monkeys. The second R5-tropic SHIV, SHIVDH12-V3AD8, was newly constructed by inserting the entire 33 amino acid gp120 V3 coding region of SHIVAD8EO, which confers the capacity to use the CCR5 coreceptor for cell entry, into the genetic background of the previously described X4-tropic SHIVDH12-CL-7 (Fig. 1 A; Sadjadpour et al., 2004). SHIVDH12-V3AD8 exhibits robust replication kinetics during infection of rhesus monkey PBMC and exclusively utilizes CCR5 to enter these cells (Fig. 1, B and C). The gp120s of SHIVDH12-V3AD8 and SHIVAD8EO differ by 10% at the nucleotide level. Their sensitivities to a panel of sera from HIV-1–infected individuals exhibiting a wide range of neutralizing activity indicates that both possess a Tier 2 anti–HIV-1 neutralization phenotype (Table 1). Rhesus macaques inoculated intravenously or intrarectally with SHIVDH12-V3AD8 exhibited peak levels of plasma viremia ranging from 105 to 107 viral RNA copes/ml of plasma at weeks 2 to 3 post infection (PI; Fig. 1, D and E).
Figure 1.
Construction and characterization of the R5-tropic SHIVDH12-V3AD8. (A) The entire gp120 V3 region of the X4-tropic SHIVDH12-CL7 (Sadjadpour et al., 2004) was replaced with the gp120 V3 region from the R5-tropic SHIVAD8EO (Shingai et al., 2012) by PCR-mediated mutagenesis. The red letters indicate the amino acid changes from the SHIVAD8EO V3 loop conferring R5 tropism to SHIVDH12-V3AD8. Virus stocks of the resultant SHIVDH12-V3AD8 were prepared in Concanavalin A–stimulated macaque PBMC using 293T cell transfection supernatants. (B) The coreceptor utilization of SHIVDH12-V3AD8 was compared with that of the previously described X4-tropic SHIVDH12-CL7 (Sadjadpour et al., 2004) and the R5-tropic SHIVAD8EO (Shingai et al., 2012) during entry to rhesus PBMC in the presence of the CXCR4 (AMD3100)- or the CCR5 (AD101)-specific inhibitors. Data are representative of two independent experiments. (C) Replication kinetics of the X4-tropic SHIVDH12-CL7 or the R5-tropic SHIVAD8EO and SHIVDH12-V3AD8 in rhesus PBMC were determined by measuring the 32P reverse transcription activity released into the tissue culture medium. Data are representative of two independent experiments. (D and E) SHIVDH12-V3AD8 replication in rhesus macaques after inoculation by intravenous (n = 5; D) or IR (n = 5; E) routes. Plasma viral RNA levels were measured by RT-PCR as previously described (Endo et al., 2000).
Table 1.
Neutralization phenotypes of primate lentiviruses.
| Patient serum ID | IC50 in TZM-bl cells | Tier phenotype | |||||||
| S321 | C500 | B520 | G435 | T520b | M263 | M600c | HIVIG | ||
| mg/ml | |||||||||
| R5 SHIVDH12-V3AD8 | 321 | 289 | 77 | 172 | 168 | 429 | 134 | 132 | 2 |
| R5 SHIVAD8EO | 48 | 36 | 39 | 31 | 41 | 44 | 48 | 1,768 | 2 |
| X4 SHIVDH12-CL7 | 110 | 94 | 50 | 65 | 109 | 115 | 65 | 530 | 2 |
| HIV-1CAAN5342.A2 | 84 | <20 | 27 | <20 | <20 | 77 | 185 | 638 | 2 |
| HIV-1MN.3 | 13,944 | 9,152 | 822 | 8,432 | 3,968 | 43,722 | 1,709 | 1.81 | 1 |
IC50 values are the serum dilution at which relative luminescence units (RLUs) were reduced 50% compared with virus control wells (no test sample).
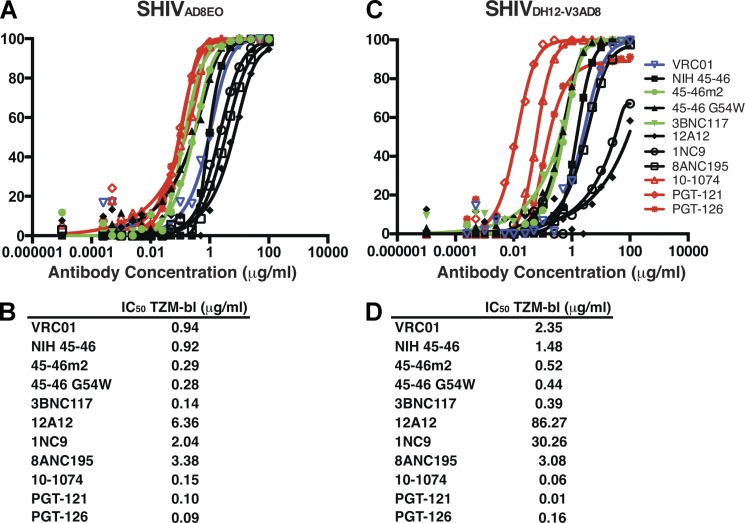
The neutralization sensitivity of SHIVAD8EO to 11 recently reported broadly reactive anti–HIV-1 mAbs was initially determined in the TZM-bl assay system. Eight of these antibodies (VRC01 [Zhou et al., 2010], NIH45-46 [Diskin et al., 2011], 45–46G54W [Diskin et al., 2011], 45-46m2 [Diskin et al., 2013], 3BNC117 [Scheid et al., 2011], 12A12 [Scheid et al., 2011], 1NC9 [Scheid et al., 2011], and 8ANC195 [Scheid et al., 2011]) target the gp120 CD4bs and three (10–1074, PGT121, and PGT126; Walker et al., 2011a; Mouquet et al., 2012) are dependent on the presence of the HIV-1 gp120 N332 glycan, located immediately downstream of the V3 loop. When tested against SHIVAD8EO, all three glycan-dependent mAbs exhibited greater potency than the CD4bs mAbs (Fig. 2 A). The IC50 values for the three mAbs targeting the gp120 N332 glycan ranged from 0.09 to 0.15 µg/ml (Fig. 2 B). The CD4bs mAbs exhibited a much broader range (0.14 to 6.36 µg/ml) of IC50 neutralizing activities with 3BNC117 being the most potent. A similar hierarchy (glycan dependent > CD4bs dependent) of neutralizing mAb potency was also observed against SHIVDH12-V3AD8 (Fig. 2 C), but the neutralizing activity was distributed across a much wider (>800-fold) range compared with the IC50 values observed for SHIVAD8EO (Fig. 2 D). SHIVDH12-V3AD8 was somewhat more sensitive to the glycan-targeting mAbs and more resistant to the CD4bs neutralizing mAbs than SHIVAD8EO.
Figure 2.
Neutralization of two R5-tropic SHIVs with a panel of 11 broadly acting anti–HIV-1 mAbs. (A and C) Neutralization was performed with SHIVAD8EO (A) or SHIVDH12-V3AD8 (C) pseudovirions and the indicated mAbs using TZM-bl target cells. The titration curves for the glycan-dependent neutralizing mAbs are red; for VRC01 in blue; and two potent CD4bs mAbs (45-46m2 and 3BNC117) in green. (B and D) The calculated IC50 values for neutralizing SHIVAD8EO (B) or SHIVDH12-V3AD8 (D) are shown below. The individual assays were repeated three times.
Pre-exposure passive transfer of five mAbs to rhesus macaques and challenge with two different R5 SHIVs
Although in vitro neutralization assays can provide important information about antibody potency and breadth, and guide the development of new mAbs with improved entry inhibiting properties, a more relevant test of antiviral efficacy is protection against an in vivo challenge in passive transfer experiments. As noted earlier, the critical goal of vaccination, in the case of primate lentiviruses, is sterilizing protection. Based on the results shown in Fig. 2, five neutralizing mAbs were selected for a preexposure passive transfer study: VRC01, because it was the first CD4bs NAb of the newly isolated broadly acting NAbs to be characterized; the CD4bs mAbs 45-46m2 and 3BNC117, both of which exhibited strong neutralizing activity against both SHIVAD8EO and SHIVDH12-V3AD8; and the gp120 N332 glycan-dependent mAbs PGT121 and 10–1074. Comparable but higher IC50 values for these five mAbs were obtained in a 14-d PBMC-based neutralization assay, using replication-competent SHIVAD8EO or SHIVDH12-V3AD8 (Table S1).
The protocol used for passive transfer experiments was to administer decreasing amounts of neutralizing mAbs intravenously and challenge animals intrarectally 24 h later. Because our goal was to block virus acquisition, coupled with the knowledge that repeated administrations of human anti-HIV mAbs to individual macaques could reduce their potency and/or possibly induce anaphylactic responses, we elected to use a SHIV challenge dose of sufficient size to establish an in vivo infection after a single virus inoculation. In this regard, we had previously conducted intrarectal (IR) titrations of SHIVAD8 in rhesus monkeys and reported that the inoculation of 103 TCID50, determined by endpoint dilution in rhesus macaque PBMC, was equivalent to administering ∼3–5 animal infectious doses50 (AID50; Gautam et al., 2012). In fact, single IR inoculations of 3 to 5 AID50 have resulted in the successful establishment of infection in 20 of 20 rhesus macaques with SHIVAD8EO or SHIVDH12-V3AD8. Pathogenic molecularly cloned viruses rather than uncloned SHIV stocks were used as inocula to facilitate the identification of neutralization resistant viral variants emerging after challenge.
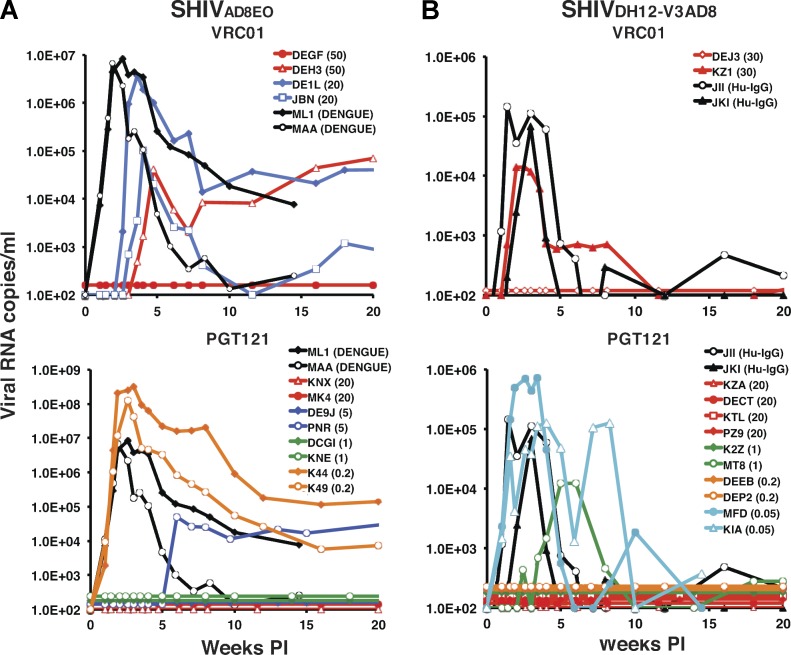
As a control for the first passive transfer experiment, an anti–dengue virus NS1 IgG1 mAb was administered intravenously to two animals, which were challenged by the IR route with SHIVAD8EO 24 h later. Both monkeys (ML1 and MAA) rapidly became infected, generating peak levels of plasma viremia 14 to 17 d PI (Fig. 3 A). VRC01 was the first anti–HIV-1 neutralizing mAb tested for protection against SHIVAD8EO acquisition and was administered to two macaques at a dose of 50 mg/kg. One (DEGF) of the two inoculated macaques was completely protected from the SHIVAD8EO challenge, with no evidence of plasma viremia or cell-associated viral DNA over a 45-wk observation period. The other recipient of 50 mg/kg VRC01 (DEH3) became infected, but peak plasma viremia was delayed until week 5 PI. Two additional macaques administered lower amounts (20 mg/kg) of VRC01 were not protected from the SHIVAD8EO challenge (Fig. 3 A). These results are summarized in Table 2.
Figure 3.
Passive transfer of VRC01 or PGT121 mAbs and subsequent challenge with two different R5-tropic SHIVs. (A and B) Plasma viral loads in rhesus monkeys, administered VRC01 or PGT121 and challenged by the IR route 24 h later with SHIVAD8EO (n = 14; A) or SHIVDH12-V3AD8 (n = 14; B), were determined by RT-PCR. The amounts of mAb injected intravenously (mg/kg) are indicated in parentheses for each animal. Macaques ML1 and MAA received 20 mg/kg of control anti–dengue virus NS1 IgG1 mAb. Human IgG from uninfected individuals was also transferred to macaques JII and JKI as a negative control.
Table 2.
Plasma neutralizing antibody concentrations and titers at the time of virus challenge
| Animal ID | Abs | Dosage | PROTECTED | Ab concentration at day 0 | Titer (TZM-bl) at day 0 |
| mg/kg | µg/ml | ||||
| SHIVAD8EO Challenged Animals | |||||
| DEGF | VRC01 | 50 | Yes | 586.9 | 1:162 |
| DEH3 | No | 711.0 | 1:176 | ||
| DE1L | 20 | No | 206.5 | 1:65 | |
| JBN | No | 188.1 | 1:80 | ||
| KNX | PGT121 | 20 | Yes | 267.9 | 1:2,495 |
| MK4 | Yes | 253.6 | 1:2,773 | ||
| DE9J | 5 | Yes | 55.7 | 1:563 | |
| PNR | No | 47.2 | 1:618 | ||
| DCGI | 1 | Yes | 24.0 | 1:116 | |
| KNE | Yes | 19.7 | 1:55 | ||
| K44 | 0.2 | No | 1.8 | <1:20 | |
| K49 | No | 1.8 | 1:17 | ||
| DEEM | 10-1074 | 20 | Yes | 289.8 | 1:2,004 |
| KIL | Yes | 257.7 | 1:2,075 | ||
| ME1 | 5 | Yes | 112.9 | 1:633 | |
| PNV | Yes | 117.5 | 1:384 | ||
| PID | 1 | No | 19.9 | 1:56 | |
| DCHX | No | 24.8 | 1:53 | ||
| PZE | 3BNC117 | 5 | Yes | 105.4 | 1:272 |
| PM5 | Yes | 76.1 | 1:372 | ||
| KMH | 1 | No | 39.6 | 1:55 | |
| MJ5 | No | 15.1 | 1:75 | ||
| PLD | 45-46m2 | 20 | No | 15.0 | 1:27 |
| MA9 | No | 17.6 | <1:20 | ||
| MC6 | 5 | No | 2.3 | <1:20 | |
| DE0CA | No | 2.2 | <1:20 | ||
| ML1 | DEN3 | 20 | No | ND | <1:20 |
| MAA | No | ND | <1:20 | ||
| SHIVDH12-V3AD8 Challenged Animals | |||||
| DEJ3 | VRC01 | 30 | Yes | 395.8 | 1:52 |
| KZ1 | No | 306.0 | 1:70 | ||
| KZA | PGT121 | 20 | Yes | 215.1 | 1: 13,120 |
| DECT | Yes | 200.7 | 1: 13,805 | ||
| KTL | Yes | 282.7 | 1: 12,669 | ||
| PZ9 | Yes | 133.1 | 1: 12,055 | ||
| K2Z | 1 | Yes | 15.1 | 1:422 | |
| MT8 | No | 29.3 | 1:539 | ||
| DEEB | 0.2 | Yes | 3.1 | 1:159 | |
| DEP2 | Yes | 1.6 | 1:101 | ||
| MFD | 0.05 | No | 1.0 | <1:20 | |
| KIA | No | 1.3 | <1:20 | ||
| KIM | 10-1074 | 20 | Yes | 290.3 | 1:1,972 |
| KWM | Yes | 173.3 | 1:2,282 | ||
| MJW | 5 | Yes | 96.6 | 1:420 | |
| MJT | Yes | 95.3 | 1:376 | ||
| DENI | 1 | Yes | 28.4 | 1:106 | |
| JHZ | No | 18.6 | 1:136 | ||
| HE8 | 0.2 | No | 19.4 | 1:39 | |
| KCZ | No | 19.7 | 1:35 | ||
| MFBA | 3BNC117 | 20 | Yes | 294.9 | 1:143 |
| MER | Yes | 272.7 | 1:142 | ||
| KIV | 5 | Yes | 114.6 | 1:80 | |
| KPI | No | 133.1 | 1:90 | ||
| DE9D | 1 | No | 23.3 | 1:20 | |
| DEW7 | Yes | 29.6 | 1:18 | ||
| MEV | 0.2 | No | 3.9 | <1:20 | |
| MF9 | No | 5.7 | <1:20 | ||
| KZMA | 45-46m2 | 5 | No | 2.1 | ND |
| KNP | No | 4.0 | ND | ||
| JII | hu-IgG | 100 | No | ND | ND |
| JK1 | No | ND | ND | ||
ND, not done.
We next examined the protective properties of PGT121 against a SHIVAD8EO challenge. PGT121 was one of the most potent glycan-targeting neutralizing mAbs measured in the TZM-bl assay (Fig. 2). Based on the results obtained with VRC01 and the fact that PGT 121 was more potent in the in vitro assay, we began the in vivo PGT121 mAb titration at 20 mg/kg. As shown in Fig. 3 A, both monkeys (KNX and MK4) receiving 20 mg/kg PGT121 resisted the SHIVAD8EO challenge. When lower amounts (5 mg/kg, 1 mg/kg, or 0.2 mg/kg) of PGT121 were administered, one of two, two of two, and zero of two animals, respectively, were protected (Fig. 3 A and Table 2).
The capacity of VRC01 and PGT121 mAbs to block SHIVDH12-V3AD8 acquisition was similarly evaluated (Fig. 3 B; Table 2). The results obtained with VRC01 were comparable to those observed with the SHIVAD8EO challenge: one of two recipients of 30 mg/kg was protected from the establishment of a SHIVDH12-V3AD8 infection. The PGT121 mAb was considerably more potent than VRC01 in preventing SHIVDH12-V3AD8 acquisition; pairs of recipients of 20, 1.0, and 0.2 mg/kg PGT121 resisted infection, but 0.05 mg/kg did not. Thus, PGT121 was more effective in preventing SHIVDH12-V3AD8 versus SHIVAD8EO in vivo infections (Table 2), a result consistent with the 10-fold difference in IC50 values for PGT121 for neutralizing the two SHIVs in in vitro assays (Fig. 2, B and D).
The results of passively transferring 10–1074, 3BNC117, and 45-46m2 neutralizing mAbs to rhesus monkeys, followed by a challenge with either SHIVAD8EO or SHIVDH12-V3AD8, are summarized in Table 2. We had previously reported that the 10–1074 and 3BNC117 mAbs prevented the acquisition of SHIVAD8EO infection when administered at a dose of 5 mg/kg, but not at 1 mg/kg (Shingai et al., 2013). In the case of SHIVDH12-V3AD8 challenges, the N332-dependent 10–1074 mAb potently blocked infection at 5 mg/kg and protected one of two monkeys at a dose of 1 mg/kg. 3BNC117 successfully prevented the acquisition of a SHIVDH12-V3AD8 infection in one of two monkeys at doses of 5 mg/kg and 1 mg/kg (Table 2). Surprisingly, despite exhibiting potent neutralizing activities against both viruses in vitro (Fig. 2), the 45-46m2 CD4bs mAb failed to block the acquisition of either SHIV at any dose administered (Table 2).
Plasma concentrations at the time of challenge and pharmacokinetics of mAbs after passive transfer
The amount of mAb administered to this cohort of macaques was selected based primarily on the results of the in vitro neutralization assays shown in Fig. 2. One of our goals was to identify a plasma mAb concentration conferring sterilizing protection. With the exception of the 45-46m2 mAb, the plasma concentrations for a given dose (e.g., 20 mg/kg) of each of the anti-HIV mAbs at the time of challenge (24 h after antibody administration) were similar in all animals (Table 2). However, the plasma concentrations of different mAbs needed to prevent SHIVAD8EO or SHIVDH12-V3AD8 acquisition varied greatly. PGT121 was clearly the most effective against both viruses, with SHIVDH12-V3AD8 exhibiting somewhat greater sensitivity to this mAb (two of two monkeys protected at a plasma concentration of 0.2 µg/ml). In contrast, a plasma concentration of nearly 400 µg/ml of VRC01 was required to protect one of two animals against the same SHIVDH12-V3AD8 challenge virus (Table 2). The most potent CD4bs mAb administered to macaques in this study, 3BNC117, was ∼6–10-fold more effective than VRC01 in preventing the acquisition of either SHIV.
Neutralizing mAb levels were also measured in several tissues from two rhesus macaques (A11E039 and MIB), sacrificed 24 h after the administration of 20 mg/kg of 3BNC117. This time point was chosen because it corresponds to the time of SHIV challenge in the preexposure passive transfer experiments. Replicate samples were obtained from 11 different tissues of each animal at the time of necropsy. Triplicate T-PER cell lysates or PBS cell suspensions were independently prepared and analyzed for the presence of gp120-bound 3BNC117 by ELISA, using a specific anti–human IgG antibody. As shown in Table 3, 3BNC117 was not only measurable in plasma but was readily detectable at concentrations ranging from 10 to 75× lower than in plasma in replicate cell suspensions and lysates from multiple lymphoid tissues, GI tract mucosal preparations, vaginal specimens, and liver from both animals 24 h after administration. Although significantly lower than plasma levels, the levels in tissues are higher than the levels required for neutralization in vitro. Comparable levels of mAb were also detected in the same replicate tissue suspensions and lysates from monkey A11E039 when an anti-idiotype, specific for the 3BNC117, was used in ELISA (unpublished data). No anti-HIV antibody was detectable in tissue suspensions or lysates prepared from a control animal (ELB) which did not receive 3BNC117.
Table 3.
NAb concentrations in tissues 24 h after passive transfer
| Tissue | A11E039 (20 mg/kg 3BNC117) | MIB (20 mg/kg 3BNC117) | ELB (control) | ||||||
| Plasma | Plasma | CSF | Plasma | Plasma | CSF | Plasma | CSF | ||
| T = 0 | T = 24 h | T = 24 h | T = 0 | T = 24 h | T = 24 h | ||||
| 1.29 µg/ml | 137.22 µg/ml | <1 µg/ml | <0.5 µg/ml | 158.53 µg/ml | <0.5 µg/ml | 1.28 µg/ml | <0.5 µg/ml | ||
| Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | Sample 1 | Sample 2 | Sample 3 | |
| µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | |
| Cell Lysates | |||||||||
| Axillary LN | 9.26 | 8.46 | 7.53 | 5.04 | 5.27 | 5.09 | <0.5 | <0.5 | <0.5 |
| Inguinal LN | 5.91 | 13.07 | 7.19 | 6.30 | 6.35 | 5.89 | <0.5 | <0.5 | <0.5 |
| Mesenteric LN | 12.03 | 11.65 | 13.01 | 9.05 | 10.94 | 10.57 | <0.5 | <0.5 | <0.5 |
| Colonic LN | 8.92 | 10.01 | 7.11 | 6.34 | 7.03 | 6.86 | <0.5 | <0.5 | <0.5 |
| Spleen | 7.04 | 6.34 | 6.20 | 4.74 | 5.87 | 10.45 | <0.5 | <0.5 | <0.5 |
| Liver | 8.50 | 7.74 | 13.01 | 6.74 | 7.36 | 4.97 | <0.5 | <0.5 | <0.5 |
| Jejunum | 5.20 | 9.06 | 7.26 | 0.60 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Colon | 5.22 | 5.54 | 4.97 | 1.40 | 1.30 | 1.42 | <0.5 | <0.5 | <0.5 |
| Rectum | 7.39 | 4.93 | 5.42 | 1.95 | 2.39 | 2.59 | <0.5 | <0.5 | <0.5 |
| Ileum | 4.26 | 5.11 | 4.58 | 0.69 | 0.72 | 0.66 | <0.5 | <0.5 | <0.5 |
| Vagina | 1.34 | 3.67 | 2.20 | 3.29 | 4.01 | 7.40 | <0.5 | <0.5 | <0.5 |
| Cell Suspensions | |||||||||
| Axillary LN | 12.07 | 17.60 | 13.23 | 6.00 | 5.77 | 4.24 | <0.5 | <0.5 | <0.5 |
| Inguinal LN | 13.16 | 11.62 | 6.38 | 3.89 | 4.06 | 4.48 | <0.5 | <0.5 | <0.5 |
| Mesenteric LN | 12.42 | 13.45 | 12.14 | 8.06 | 8.27 | 9.14 | <0.5 | <0.5 | <0.5 |
| Colonic LN | 10.31 | 8.37 | 5.66 | 6.70 | 4.55 | 5.61 | <0.5 | <0.5 | <0.5 |
| Spleen | 6.39 | 6.42 | 4.65 | 4.69 | 5.18 | 4.76 | <0.5 | <0.5 | <0.5 |
| Liver | 10.44 | 10.69 | 13.56 | 4.84 | 4.80 | 5.39 | <0.5 | <0.5 | <0.5 |
| Jejunum | 6.17 | 3.34 | 3.86 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 |
| Colon | 8.81 | 9.12 | 10.50 | 2.08 | 1.53 | 1.32 | <0.5 | <0.5 | <0.5 |
| Rectum | 3.57 | 5.52 | 3.44 | 2.45 | 2.53 | 4.10 | <0.5 | <0.5 | <0.5 |
| Ileum | 2.37 | <1 | 1.33 | 0.75 | 0.93 | 0.59 | <0.5 | <0.5 | <0.5 |
| Vagina | 2.76 | 4.19 | 3.39 | 6.78 | 3.14 | 3.37 | <0.5 | <0.5 | <0.5 |
| Wick Filtrates | |||||||||
| Spleen | 14.65 | 11.40 | ND | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 |
| Liver | 57.83 | 50.04 | 57.71 | 57.47 | 38.60 | 36.35 | 1.33 | 1.20 | 0.80 |
| Jejunum | <1.5 | <1.5 | <1.5 | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 |
| Colon | <1.5 | <1.5 | ND | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 |
| Rectum | 1.55 | <1.5 | <1.5 | 1.27 | <0.75 | 2.73 | <0.75 | <0.75 | <0.75 |
| Ileum | <1.5 | <1.5 | <1.5 | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 | <0.75 |
| Vagina | 4.51 | 3.52 | 5.53 | 4.61 | 1.61 | <0.75 | <0.75 | <0.75 | <0.75 |
ND, not done. T, sample collection time after NAb administration
We also attempted to measure anti-HIV antibody on the moist surfaces of nonlymphoid tissues immediately after their removal at necropsy. Cellulose wicks were applied in triplicate to these specimens for 5 min to absorb surface secretions, weighed, centrifuged in cellulose acetate filter columns, and assayed for HIV antibody in the filtrate by ELISA. As indicated in Table 3, 3BNC117 was found on the surface of vaginal mucosa samples from both monkeys but was below detectable levels in assays of GI tract specimens. The high levels of HIV antibody detected in liver samples with the wick system very likely reflect 3BNC117 in blood on the cut surfaces of this organ. Collectively, the results presented in Table 3 demonstrate that the intravenous administration of anti-HIV neutralizing mAb is rapidly and systemically disseminated to tissues, and present at levels capable of blocking the establishment of a SHIV infection at the site of virus inoculation.
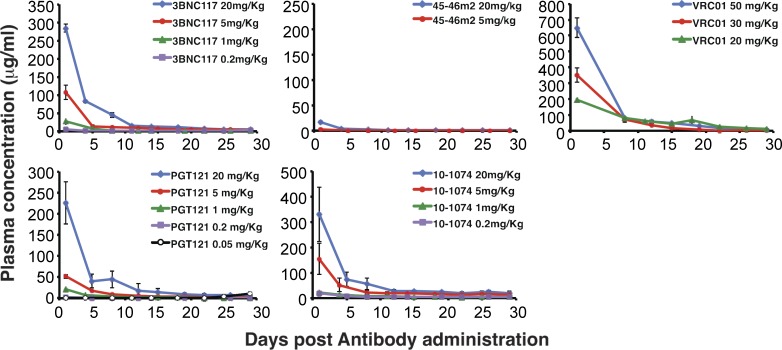
The ineffectiveness and unexpectedly low plasma levels of the 45-46m2 mAb (Table 2) led us to determine the decay rates of all five neutralizing mAbs administered to macaques (Fig. 4). The calculated half-lives of PGT121, 10–1074, 3BNC117, and VRC01 mAbs were quite similar: 3.5, 3.5, 3.3, and 3.1 d, respectively. In contrast, the half-life of 45-46m2 was not directly determinable. Based on the plasma mAb concentrations in several macaques 24 h after the administration of 20 mg/kg of the VRC101, PGT121, 10–1074, and 3BNC117 neutralizing mAbs (∼250 µg/ml; Table 2), the two monkeys receiving 20 mg/kg 45-46m2 had plasma mAb concentrations of only 15.0 and 17.6 µg/ml, representing a decay of >95% relative to other neutralizing mAbs within 24 h of administration (Fig. 4). Thus, despite its high potency as measured in vitro (Fig. 2), 45-46m2 was ineffective in macaques.
Figure 4.
Levels of neutralizing mAbs in the plasma of passively transferred macaques. The concentrations of the indicated mAbs in plasma at various times after administration were determined by ELISA using recombinant HIV-1 gp120. Plasma antibody levels were measured in the animals described in Table 2. Means and SDs are shown.
Neutralizing titers are predictive of protection
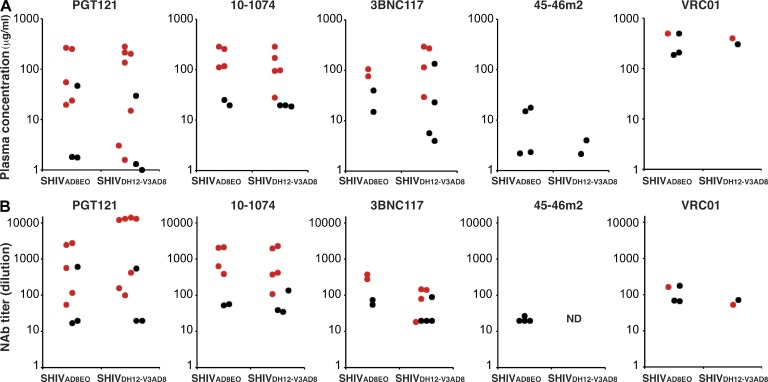
The plasma antibody concentrations for each of the five anti-HIV monoclonal NAbs conferring sterilizing protection against SHIVAD8EO or SHIVDH12-V3AD8 are shown in Fig. 5 A. These protective concentrations ranged from 587 to 1.6 µg/ml (Table 2). The titers of neutralizing activity in the plasma of monkeys at the time of SHIV challenge are a more informative and functional metric of circulating anti–HIV-1 NAbs than their IgG concentrations. Presentation of data in this format would clearly be relevant for vaccine development where titers of circulating antivirus neutralizing activity, not plasma antibody concentrations, could be correlated with in vivo protection. Neutralization titers were therefore measured on plasma samples collected 24 h after mAb administration when the macaques were challenged with SHIVAD8EO or SHIVDH12-V3AD8 (Table 2). When this is done, a relatively weak mAb such as VRC01, when administered at a dose of 30 or 50 mg/kg, generates protective plasma neutralization titers in the 1:100 range, whereas the more potent PGT121 mAb can achieve this same level of activity after the administration of only 0.2 mg/kg. The neutralization titers in the entire cohort of macaques at the time of virus challenge as well as in those protected from infection are shown graphically in Fig. 5 B. This presentation of plasma neutralizing activity suggests that a neutralization titer of ∼1:100 may be a possible threshold required to prevent virus acquisition.
Figure 5.
Levels of neutralizing mAbs in the plasma of passively transferred macaques at the time of challenge. (A) The concentrations (µg/ml) of the indicated mAbs in plasma 24 h after passive transfer of mAbs were determined by ELISA using recombinant HIV-1 gp120. (B) The plasma IC50 titers of the indicated mAbs 24 h after passive transfer of mAbs were determined using the TZM-bl cell assay. Red circles indicate protected (no acquisition) monkeys; black circles denote infected animals. Plasma antibody levels and NAb titers were measured in the 60 animals described in Table 2.
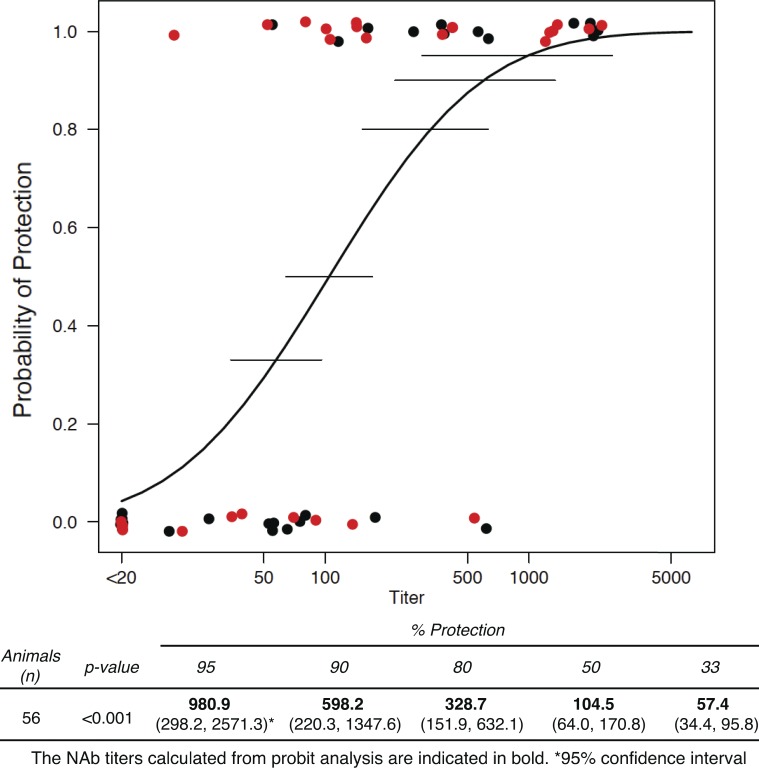
The method described by Reed and Muench (1938) was next used to calculate the neutralization titers, measured in plasma, needed to prevent virus acquisition in 50% of challenged monkeys. These protective titers for the 28 monkeys, challenged with SHIVAD8EO, or the 32 monkeys, challenged with SHIVDH12-V3AD8, were separately deduced (Tables S2 and S3). The plasma neutralization titers required for protecting 50% of the SHIVAD8EO or SHIVDH12-V3AD8 challenged animals were calculated to be 1:115 and 1:96, respectively. Because these two titers were quite similar and were obtained after the administration of the same ensemble of neutralizing mAbs to 60 animals, challenged by identical routes and inoculum size but with two SHIVs bearing genetically distinct envelope glycoproteins, the neutralization data from all of the monkeys were combined and subjected to probit regression to determine the relationship between plasma neutralization titers and in vivo protection (Finley, 2007). As a further check, when a term for the SHIV virus was included in the probit regression model on all 60 macaques, there was no evidence of a difference between the two SHIV viruses (P = 0.16). When applied to the entire group of 60 macaques, probit regression estimated that a plasma neutralization titer of 1:104.5 would prevent virus acquisition in 50% of animals (Fig. 6). The inset at the bottom of Fig. 6 predicts the neutralization titers, determined by TZM-bl assay and calculated by probit regression, needed to protect different proportions of a SHIV-exposed monkey population. For example, a neutralization titer of 1:328.7 would be required for protection of 80% of challenged animals, with the caveat that the 90% confidence interval of titers to achieve this outcome is quite large (1:152 to 1:632).
Figure 6.
Probit regression analysis relating NAb titers at the time of virus challenge and probability of protection. The black circles denote SHIVAD8EO challenged monkeys; the red circles denote SHIVDH12-V3AD8 challenged monkeys. Horizontal lines indicate 90% confidence intervals of plasma neutralization titers for 33, 50, 80, 90, and 95% probability of protection. The estimated neutralization titers in plasma conferring different levels of protection and their corresponding 90% confidence intervals are shown at the bottom of the figure.
DISCUSSION
This study has broader implications beyond simply being another preexposure passive transfer experiment using recently developed broad and potent anti-HIV neutralizing mAbs. It reaches the optimistic conclusion that if an immunogen can be identified/designed to elicit the breadth of anti-HIV neutralizing activity possessed by some of the new generation of mAbs, a vaccine containing such an immunogen may only have to generate modest protective titers to prevent the establishment of infection. It should be noted that the challenge virus inoculum size (3–5 AID50) used in this study was selected to ensure that all of the monkeys would be infected after a single IR inoculation. This challenge dose is orders of magnitude higher than that estimated to establish an HIV-1 infection after vaginal exposure in humans (4–8 per 10,000 exposures; Patel et al., 2014). If, in fact, the calculated 50% protective titer of ∼1:100 against a virus challenge of 3–5 AID represents a gross overestimate, then the true protective titer might be tempered by recent studies reporting that 10–20-fold higher levels of neutralizing activity are required to block spread by cell-associated virus in tissue culture systems (Abela et al., 2012; Malbec et al., 2013).
The identification and development of immunogens capable of inducing neutralizing activities possessing the breadth of the newest generation of antiviral mAbs and generating plasma titers in the 1:100 range is a major challenge facing the HIV vaccine field. Several vaccine studies have immunized macaques with the SHIVSF162P4 envelope glycoprotein or its gp120V2del derivative (Barnett et al., 2008, 2010; Bogers et al., 2008; Page et al., 2012). Others have used HIV-1YU-2 gp120 trimers (Sundling et al., 2010a,b) or trimerized consensus HIV-1 gp140 proteins (Eugene et al., 2013). Protective efficacy was evaluated using easy-to-neutralize challenge viruses such as the closely related SHIVSF162P4 or SHIV89.6P, and SHIVsbg. In some instances, modest levels of antivirus neutralizing activity against some Tier 1 HIV-1 isolates were induced before virus challenge and, in a few cases, homologous virus acquisition appeared to be blocked (Bogers et al., 2008; Barnett et al., 2010). However, the levels of NAbs conferring sterilizing protection could not be readily determined from these studies. For example, in one study using a homologous challenge, virus was undetectable in five of eight vaccinated monkeys with preexposure anti-SHIVSF162P4 NAb titers ranging from 1:80 to 1:700 (Bogers et al., 2008). In another study, heterologous SHIVSF162P4 acquisition was blocked in 2 of 4 animals vaccinated with HIV-1 gp120 consensus envelope trimers, which had induced anti–HIV-1SF162 neutralizing titers of 1:40 and 1:320 (Eugene et al., 2013). However, one of the two protected macaques carried the Mamu B*08 MHC allele and the other animal released virus after the administration of the depleting anti-CD8 mAb, M-T807R1, indicating that SHIV acquisition had not been blocked. A recent study using adenovirus and poxvirus vector-based vaccines, which expressed mosaic HIV-1 Env/Gag/Pol immunogens, induced NAb titers in the 1:69 to 1:153 range against Tier 1 viral strains in rhesus macaques (Barouch et al., 2013a). This latter vaccine regimen, followed by repeated low-dose challenges with the difficult-to-neutralize SHIVSF162P3, reduced the per-exposure virus acquisition risk by 90%.
Earlier passive transfer studies have estimated protective plasma neutralizing concentrations and/or neutralization titers using a variety of less potent and more narrowly focused mAbs, X4- or R5-tropic SHIVs, and different routes of inoculation (Mascola et al., 1999; Shibata et al., 1999; Parren et al., 2001; Hessell et al., 2010). For example, it was recently reported that plasma neutralizing titers ranging from 1:200 to 1:495, achieved after the administration of a single potent anti-HIV mAb (PGT121), protected 3 of 5 macaques from a vaginal challenge with the R5-tropic SHIVSF162P3 (Moldt et al., 2012). The 1:100 protective neutralization titer reported in our study was calculated for a cohort of 60 macaque recipients of 5 different potent anti-HIV mAbs and challenged with either of two R5-tropic SHIVs by the IR route. It is also worth noting that neutralizing titers in the 1:100 range are attainable by the human immune system, based on a recent study showing that 34% of 463 sera from chronically HIV-infected individuals had neutralizing titers >1:100 when tested against viral strains from 4 or more HIV-1 clades (Simek et al., 2009). Given the caveats regarding the 3–5 AID50 inoculum size used in this study, the reported 1:100 value should not be regarded as a rigid and fixed number, as indicated by the titers measured in some of the unprotected macaques. It was calculated from the results obtained from a large number of animals and should be considered to be a minimal number to be achieved by a new generation of immunogens.
With respect to generating the modest protective neutralizing titers preventing virus acquisition, it is now appreciated that the very large number of genetic changes (40–100 nucleotides) attending the maturation of B cells, capable of producing potent and broadly acting anti-HIV NAbs, poses a major impediment for developing an effective viral vaccine (Scheid et al., 2009; Walker et al., 2009, 2011a; Xiao et al., 2009; Wu et al., 2010; Scheid et al., 2011; Mouquet et al., 2012; Klein et al., 2013a). This number far exceeds the nucleotide changes required to produce protective antibodies against most other viral pathogens and makes the design of an efficacious immunogen a most difficult challenge. Several possible solutions have been proposed to address this issue. One, suggested from natural HIV infections of humans and SHIV infections of macaques, involves the identification and use of unique HIV-1 envelope glycoproteins, able to guide the development of potent neutralizing activity after the initial encounter with the immune system (Walker et al., 2011b; Shingai et al., 2012; Klein et al., 2013b; Doria-Rose et al., 2014). Another utilizes epitope-focused and scaffold-based immunogens, similar to those recently reported for an anti-respiratory syncytial virus vaccine (Correia et al., 2014), that specifically target structurally defined epitopes for known protective NAbs, and which are also able to interact with germline IgG (Jardine et al., 2013; Klein et al., 2013b).
Two interesting findings relevant to the selection and clinical use of antiviral neutralizing mAbs emerged from this study. First, the intravenous administration of such antibodies resulted in their rapid dissemination systemically. They became detectable in several lymphoid and mucosal tissues within 24 h, reaching levels of activity sufficient to block virus acquisition even when administered at a dosage as low as 0.2 mg/kg. Second, virus neutralization measured in vitro is not absolutely predictive of neutralizing activity achieved in vivo. Although the results obtained using the TZM-bl cell assay correlated well with the in vivo protective effects for four (PGT121, 10–1074, 3BNC117, and VRC01) of the anti-HIV mAbs (Fig. 2 and Table 2), the in vitro engineered 45-46m2 CD4bs mAb, which exhibited very high potency in vitro, was a complete failure in vivo. It exhibited an extremely short half-life in plasma, with only ∼5% activity remaining in the circulation by 24 h after administration.
The in vivo decay of the broadly acting anti-HIV mAbs used in this study was relatively short (half-life = 3.5 d), most likely reflecting their human rather than macaque origin, which very likely contributed to this rapid decline. Nonetheless, the administration of high-dose combination neutralizing mAb treatment, including three of the antibodies assessed here (3BNC117, 10–1074, and PGT121), has recently been reported to suppress plasma viremia to the limits of detection in chronically SHIVAD8- or SHIVSF162-infected macaques (Barouch et al., 2013b; Shingai et al., 2013). In this regard, before the development of an effective vaccine, preexposure immunoprophylaxis against hepatitis A virus infection by administering 0.06 ml/kg of immune globulin intramuscularly was common practice, and conferred protection for 3–5 mo (Advisory Committee on Immunization Practices et al., 2006). It is therefore not unreasonable to envisage the future development and use of reengineered derivatives of anti-HIV mAbs with greatly extended half-lives. Genetic modification of the existing anti-HIV mAbs could prolong their neutralizing activities in vivo for long periods of time, allowing semiannual or annual immunizations as an alternative to vaccination.
MATERIALS AND METHODS
Animal experiments.
All animals were housed and cared for in accordance with the American Association for Accreditation of Laboratory Animal Care standards in American Association for Accreditation of Laboratory Animal Care–accredited facilities, and all animal procedures and experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). 60 male and female rhesus macaques (Macaca mulatta) of Indian genetic origin ranging from 2 to 10 yr of age were maintained in accordance with NIH guidelines (Committee, 1985) and were housed in a biosafety level 2 NIAID facility. Phlebotomies, euthanasia, and sample collection were performed as previously described (Endo et al., 2000). The macaques used in this study were negative for the MHC class I Mamu-A*01 allele. For SHIV inoculations, a pediatric speculum was used to gently open the rectum and a 1 ml suspension of virus in a tuberculin syringe was slowly infused into the rectal cavity.
Construction of the R5-tropic SHIVDH12-V3AD8.
PCR mutagenesis, with primers corresponding to the 5′ and 3′ halves of the SHIVAD8EO (Shingai et al., 2012) gp120 V3 coding region (forward primer: 5′-AGAGCATTTTATACAACAGGAGACATAATAGGAGATATAAGACAAGCACATTGCAACATTAGTAAAGTAAAATGGC-3′; and reverse primer: 5′-TCCTGGTCCTATATGTATACTTTTCCTTGTATTGTTGTTGGGTCTTGTACAATTAATTTCTACAGTTTCATTC-3′), was used to introduce these V3 sequences into the genetic background of the pSHIVDH12-CL7 molecular clone (Sadjadpour et al., 2004) using Platinum PFX DNA polymerase (Invitrogen). After gel purification, the PCR product was treated with T4 polynucleotide kinase (Gibco) and blunt-end ligated to create pSHIVDH12-V3AD8, which was used to transform competent cells.
Viruses.
Virus stocks were prepared by first transfecting 293T cells with the SHIVAD8EO (Shingai et al., 2012) or SHIVDH12-V3AD8 molecular clones using Lipofectamine 2000 (Invitrogen). Culture supernatants were collected 48 h later and aliquots stored at −80°C until use. Concanavalin A–stimulated rhesus PBMCs (2 × 106 cells in 500 µl) were infected with transfected cell supernatants by spinoculation (O’Doherty et al., 2000) for 1 h, mixed with the same number/volume of activated PBMC, and cultures were maintained for at least 12 d with daily replacement of culture medium. Samples of supernatant medium were pooled around the times of peak reverse transcriptase production to prepare individual virus stocks.
Antibodies.
11 mAbs (VRC01, NIH45-46, 45-46G54W, 45-46m2, 3BNC117, 12A12, 1NC9, and 8ANC195, 10–1074, PGT121, and PGT126) were isolated and produced as previously described (Zhou et al., 2010; Diskin et al., 2011, 2013; Scheid et al., 2011; Walker et al., 2011a; Mouquet et al., 2012). The 45-46G54W and 45-46m2 mAbs were provided by P. Marcovecchio and H. Gao (California Institute of Technology, Pasadena, CA). DEN3, a dengue virus NS1-specific human IgG1 mAb (Moldt et al., 2012) or control human IgG (NIH Nonhuman Primate Reagent Resource) were used as the negative control antibodies in this study. The mAbs selected for preexposure passive transfer were administered intravenously 24 h before virus challenge.
Quantitation of plasma viral RNA levels.
Viral RNA levels in plasma were determined by real-time reverse transcription PCR (Prism 7900HT sequence detection system; Applied Biosystems) as previously reported (Endo et al., 2000).
Antibody concentrations in plasma.
The concentrations of administered mAbs in monkey plasma were determined by ELISA, using recombinant HIV-1JRFL gp120 (Progenics Pharmaceuticals) or HIVIIIB (Advanced Biotechnology Inc.) as previously described (Parren et al., 2001). In brief, microtiter plates were coated with 2 µg/ml HIV-1 gp120 and incubated overnight at 4°C. The plates were washed with PBS/0.05% Tween-20 and blocked with 1% (vol/vol) BSA. After blocking, serial dilution of antibodies or plasma samples were added to the plate and incubated for 1 h at room temperature. Binding was detected with a goat anti–human IgG F(ab′)2 fragments coupled to alkaline phosphatase (Thermo Fisher Scientific) and visualized with SIGMAFAST OPD (Sigma-Aldrich). The decay half-lives of neutralizing mAbs were calculated by a single-exponential decay formula based on the plasma concentrations beginning on day 5 or day 7 after antibody administration (Hessell et al., 2010).
Antibody concentrations in tissue.
The following tissues were collected from two rhesus monkeys sacrificed 24 h after the administration of 20 mg/kg of the 3BNC117 mAb or from a single control animal receiving no antibody: axillary, inguinal, mesenteric, and colonic lymph nodes; or spleen, liver, jejunum, colon, ileum, rectum, and vagina. Several independent samples were collected from each tissue specimen and weighed (40–300 mg). To generate cell suspensions, three samples from each tissue were immediately homogenized in 4× volume (wt/vol) of PBS using disposable pestles (Axygen, Inc.) and centrifuged at 12,000 rpm for 10 min at 4°C. The collected supernatants were filtered through 0.45-µm–pore size Spin-X (Corning). Cell lysates were prepared from three other samples from the same tissue, suspended in 4× volume (wt/vol) of T-PER (Thermo Fisher Scientific), and homogenized using disposable pestles. The protein extracts obtained were centrifuged at 12,000 rpm for 10 min at 4°C and the middle protein layer was collected and recentrifuged under the same conditions. The recovered cell lysate was filtered through a 0.45-µm–pore size Spin-X column, and frozen until assay by ELISA.
Antibody on the surfaces of vaginal, intestinal, splenic, and hepatic tissues collected at necropsy was absorbed to individual cellulose wicks (WECK-CEL Cellulose; Beaver-Visitec International, Inc.) for 5 min and measured as previously described (Kozlowski et al., 2000; Moldt et al., 2012). Wicks were weighed before and after their application to individual tissue specimens and then placed in a 0.45-µm–pore size Spin-X column. After a 15-min incubation in 9× (wt/vol) of PBS at 4°C, filtrates were collected by centrifugation and frozen until assay by ELISA.
Neutralization assays.
The in vitro potency of each mAb and the neutralization activity present in plasma samples collected from rhesus macaques were assessed by two types of neutralization assays: (1) TZM-bl entry assay with pseudotyped challenge virus (Willey et al., 2010) or (2) a 14-d PBMC replication assay with replication competent virus (Shibata et al., 1999; Nishimura et al., 2002). For the TZM-bl assay, serially diluted mAb or plasma samples were incubated with pseudotyped viruses, expressing env gene derived from SHIVAD8EO or SHIVDH12-V3AD8 and prepared by co-transfecting 293T cells with pNLenv1 and pCMV vectors expressing the respective envelope proteins (Nishimura et al., 2010). The IC50 titer was calculated as the dilution causing a 50% reduction in relative luminescence units (RLU) compared with levels in virus control wells after subtraction of cell control RLU as previously described (Shu et al., 2007; Seaman et al., 2010). The neutralization phenotype (Tier levels) of the SHIVDH12-V3AD8 molecular clone was determined by TZM-bl cell assay (Shu et al., 2007; Wu et al., 2009; Seaman et al., 2010) using plasma samples from a cohort study, which exhibit a wide range of neutralizing activities against subtype B HIV-1 isolates (Dreja et al., 2010).
Determinations of animal protective titers and statistical analyses.
Calculation of the neutralizing titer in plasma against each R5 SHIV, resulting in the prevention of virus acquisition of 50 or 80% of the virus-challenged animals, was performed using the method of Reed and Muench (1938). One significant outlier animal (DEW7) was omitted from the calculation. Probit regression was used to model the relationship between the titers in plasma required to confer sterilizing immunity in vivo using all 60 passively immunized monkeys (Finley, 2007), with p-values from this model based on Likelihood ratio tests. Plasma titers needed for different levels of in vivo protection (33, 50, 80, 90, and 95%) were determined from the probit model estimates and the method of bootstrapping was used to construct 90% confidence intervals.
Online supplemental material.
Table S1 shows anti-HIV IC50 neutralization titers determined in rhesus PBMC. Table S2 shows determination of the 50% protection titer against SHIVAD8EO (Reed and Muench, 1938). Table S3 shows determination of the 50% protection titer against SHIVDH12-V3AD8 (Reed and Muench, 1938). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20132494/DC1.
Supplementary Material
Acknowledgments
We thank Keiko Tomioka and Robin Kruthers for determining plasma viral RNA loads and Boris Skopets, William Magnanelli, and Rahel Petros for diligently assisting in the maintenance of animals and assisting with procedures.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- AID50
- animal infectious dose50
- CD4bs
- CD4 binding site
- IR
- intrarectal
- NAb
- neutralizing antibody
- PI
- post infection
References
- Abela, I.A., Berlinger L., Schanz M., Reynell L., Günthard H.F., Rusert P., and Trkola A.. 2012. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 8:e1002634 10.1371/journal.ppat.1002634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advisory Committee on Immunization Practices (ACIP), Fiore A.E., Wasley A., and Bell B.P.. 2006. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm. Rep. 55:1–23 [PubMed] [Google Scholar]
- Amanna, I.J., and Slifka M.K.. 2011. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 411:206–215 10.1016/j.virol.2010.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba, T.W., Liska V., Hofmann-Lehmann R., Vlasak J., Xu W., Ayehunie S., Cavacini L.A., Posner M.R., Katinger H., Stiegler G., et al. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200–206 10.1038/72309 [DOI] [PubMed] [Google Scholar]
- Barnett, S.W., Srivastava I.K., Kan E., Zhou F., Goodsell A., Cristillo A.D., Ferrai M.G., Weiss D.E., Letvin N.L., Montefiori D., et al. 2008. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. AIDS. 22:339–348 10.1097/QAD.0b013e3282f3ca57 [DOI] [PubMed] [Google Scholar]
- Barnett, S.W., Burke B., Sun Y., Kan E., Legg H., Lian Y., Bost K., Zhou F., Goodsell A., Zur Megede J., et al. 2010. Antibody-mediated protection against mucosal simian-human immunodeficiency virus challenge of macaques immunized with alphavirus replicon particles and boosted with trimeric envelope glycoprotein in MF59 adjuvant. J. Virol. 84:5975–5985 10.1128/JVI.02533-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch, D.H., Stephenson K.E., Borducchi E.N., Smith K., Stanley K., McNally A.G., Liu J., Abbink P., Maxfield L.F., Seaman M.S., et al. 2013a. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 155:531–539 10.1016/j.cell.2013.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch, D.H., Whitney J.B., Moldt B., Klein F., Oliveira T.Y., Liu J., Stephenson K.E., Chang H.W., Shekhar K., Gupta S., et al. 2013b. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 503:224–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogers, W.M., Davis D., Baak I., Kan E., Hofman S., Sun Y., Mortier D., Lian Y., Oostermeijer H., Fagrouch Z., et al. 2008. Systemic neutralizing antibodies induced by long interval mucosally primed systemically boosted immunization correlate with protection from mucosal SHIV challenge. Virology. 382:217–225 10.1016/j.virol.2008.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald, U.K., and Pirofski L.. 2003. Immune therapy for infectious diseases at the dawn of the 21st century: the past, present and future role of antibody therapy, therapeutic vaccination and biological response modifiers. Curr. Pharm. Des. 9:945–968 10.2174/1381612033455189 [DOI] [PubMed] [Google Scholar]
- Burton, D.R., Poignard P., Stanfield R.L., and Wilson I.A.. 2012. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science. 337:183–186 10.1126/science.1225416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Committee. 1985. Guide for the care and use of laboratory animals. Department of Health and Human Services publication no. NIH 85-23. National Institutes of Health, Bethesda, MD [Google Scholar]
- Correia, B.E., Bates J.T., Loomis R.J., Baneyx G., Carrico C., Jardine J.G., Rupert P., Correnti C., Kalyuzhniy O., Vittal V., et al. 2014. Proof of principle for epitope-focused vaccine design. Nature. 507:201–206 10.1038/nature12966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin, R., Scheid J.F., Marcovecchio P.M., West A.P. Jr, Klein F., Gao H., Gnanapragasam P.N., Abadir A., Seaman M.S., Nussenzweig M.C., and Bjorkman P.J.. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science. 334:1289–1293 10.1126/science.1213782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin, R., Klein F., Horwitz J.A., Halper-Stromberg A., Sather D.N., Marcovecchio P.M., Lee T., West A.P. Jr, Gao H., Seaman M.S., et al. 2013. Restricting HIV-1 pathways for escape using rationally designed anti–HIV-1 antibodies. J. Exp. Med. 210:1235–1249 10.1084/jem.20130221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose, N.A., Schramm C.A., Gorman J., Moore P.L., Bhiman J.N., DeKosky B.J., Ernandes M.J., Georgiev I.S., Kim H.J., Pancera M., et al. NISC Comparative Sequencing Program. 2014. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. 509:55–62 10.1038/nature13036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreja, H., O’Sullivan E., Pade C., Greene K.M., Gao H., Aubin K., Hand J., Isaksen A., D’Souza C., Leber W., et al. 2010. Neutralization activity in a geographically diverse East London cohort of human immunodeficiency virus type 1-infected patients: clade C infection results in a stronger and broader humoral immune response than clade B infection. J. Gen. Virol. 91:2794–2803 10.1099/vir.0.024224-0 [DOI] [PubMed] [Google Scholar]
- Endo, Y., Igarashi T., Nishimura Y., Buckler C., Buckler-White A., Plishka R., Dimitrov D.S., and Martin M.A.. 2000. Short- and long-term clinical outcomes in rhesus monkeys inoculated with a highly pathogenic chimeric simian/human immunodeficiency virus. J. Virol. 74:6935–6945 10.1128/JVI.74.15.6935-6945.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugene, H.S., Pierce-Paul B.R., Cragio J.K., and Ross T.M.. 2013. Rhesus macaques vaccinated with consensus envelopes elicit partially protective immune responses against SHIV SF162p4 challenge. Virol. J. 10:102 10.1186/1743-422X-10-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley, D.J.2007. Probit Analysis. Cambridge University Press, Cambridge, England: 272 pp [Google Scholar]
- Gautam, R., Nishimura Y., Lee W.R., Donau O., Buckler-White A., Shingai M., Sadjadpour R., Schmidt S.D., LaBranche C.C., Keele B.F., et al. 2012. Pathogenicity and mucosal transmissibility of the R5-tropic simian/human immunodeficiency virus SHIV(AD8) in rhesus macaques: implications for use in vaccine studies. J. Virol. 86:8516–8526 10.1128/JVI.00644-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessell, A.J., Rakasz E.G., Tehrani D.M., Huber M., Weisgrau K.L., Landucci G., Forthal D.N., Koff W.C., Poignard P., Watkins D.I., and Burton D.R.. 2010. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J. Virol. 84:1302–1313 10.1128/JVI.01272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J., Ofek G., Laub L., Louder M.K., Doria-Rose N.A., Longo N.S., Imamichi H., Bailer R.T., Chakrabarti B., Sharma S.K., et al. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature. 491:406–412 10.1038/nature11544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine, J., Julien J.P., Menis S., Ota T., Kalyuzhniy O., McGuire A., Sok D., Huang P.S., MacPherson S., Jones M., et al. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science. 340:711–716 10.1126/science.1234150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M.A., and Stiehm E.R.. 2000. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 13:602–614 10.1128/CMR.13.4.602-614.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, F., Diskin R., Scheid J.F., Gaebler C., Mouquet H., Georgiev I.S., Pancera M., Zhou T., Incesu R.B., Fu B.Z., et al. 2013a. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell. 153:126–138 10.1016/j.cell.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein, F., Mouquet H., Dosenovic P., Scheid J.F., Scharf L., and Nussenzweig M.C.. 2013b. Antibodies in HIV-1 vaccine development and therapy. Science. 341:1199–1204 10.1126/science.1241144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, L., Lee J.H., Doores K.J., Murin C.D., Julien J.P., McBride R., Liu Y., Marozsan A., Cupo A., Klasse P.J., et al. 2013. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 20:796–803 10.1038/nsmb.2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski, P.A., Lynch R.M., Patterson R.R., Cu-Uvin S., Flanigan T.P., and Neutra M.R.. 2000. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J. Acquir. Immune Defic. Syndr. 24:297–309 10.1097/00126334-200008010-00001 [DOI] [PubMed] [Google Scholar]
- Kwong, P.D., and Mascola J.R.. 2012. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 37:412–425 10.1016/j.immuni.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbec, M., Porrot F., Rua R., Horwitz J., Klein F., Halper-Stromberg A., Scheid J.F., Eden C., Mouquet H., Nussenzweig M.C., and Schwartz O.. 2013. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J. Exp. Med. 210:2813–2821 10.1084/jem.20131244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascola, J.R., Lewis M.G., Stiegler G., Harris D., VanCott T.C., Hayes D., Louder M.K., Brown C.R., Sapan C.V., Frankel S.S., et al. 1999. Protection of Macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan, J.S., Pancera M., Carrico C., Gorman J., Julien J.P., Khayat R., Louder R., Pejchal R., Sastry M., Dai K., et al. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 480:336–343 10.1038/nature10696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldt, B., Rakasz E.G., Schultz N., Chan-Hui P.Y., Swiderek K., Weisgrau K.L., Piaskowski S.M., Bergman Z., Watkins D.I., Poignard P., and Burton D.R.. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. USA. 109:18921–18925 10.1073/pnas.1214785109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouquet, H., Scharf L., Euler Z., Liu Y., Eden C., Scheid J.F., Halper-Stromberg A., Gnanapragasam P.N., Spencer D.I., Seaman M.S., et al. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc. Natl. Acad. Sci. USA. 109:E3268–E3277 10.1073/pnas.1217207109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, Y., Igarashi T., Haigwood N., Sadjadpour R., Plishka R.J., Buckler-White A., Shibata R., and Martin M.A.. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123–2130 10.1128/jvi.76.5.2123-2130.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, Y., Shingai M., Willey R., Sadjadpour R., Lee W.R., Brown C.R., Brenchley J.M., Buckler-White A., Petros R., Eckhaus M., et al. 2010. Generation of the pathogenic R5-tropic simian/human immunodeficiency virus SHIVAD8 by serial passaging in rhesus macaques. J. Virol. 84:4769–4781 10.1128/JVI.02279-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty, U., Swiggard W.J., and Malim M.H.. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074–10080 10.1128/JVI.74.21.10074-10080.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, M., Stebbings R., Berry N., Hull R., Ferguson D., Davis L., Duffy L., Elsley W., Hall J., Ham C., et al. 2012. Heterologous protection elicited by candidate monomeric recombinant HIV-1 gp120 vaccine in the absence of cross neutralising antibodies in a macaque model. Retrovirology. 9:56 10.1186/1742-4690-9-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parren, P.W., Marx P.A., Hessell A.J., Luckay A., Harouse J., Cheng-Mayer C., Moore J.P., and Burton D.R.. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340–8347 10.1128/JVI.75.17.8340-8347.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, P, Borkowf C.B., Brooks J.T., Lasry A., Lansky A., and Mermin J.. 2014. Estimating per-act HIV transmission risk: a systematic review. AIDS. 28:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed, L.J., and Muench H.. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497 [Google Scholar]
- Sadjadpour, R., Theodore T.S., Igarashi T., Donau O.K., Plishka R.J., Buckler-White A., and Martin M.A.. 2004. Induction of disease by a molecularly cloned highly pathogenic simian immunodeficiency virus/human immunodeficiency virus chimera is multigenic. J. Virol. 78:5513–5519 10.1128/JVI.78.10.5513-5519.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, J.F., Mouquet H., Feldhahn N., Seaman M.S., Velinzon K., Pietzsch J., Ott R.G., Anthony R.M., Zebroski H., Hurley A., et al. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature. 458:636–640 10.1038/nature07930 [DOI] [PubMed] [Google Scholar]
- Scheid, J.F., Mouquet H., Ueberheide B., Diskin R., Klein F., Oliveira T.Y., Pietzsch J., Fenyo D., Abadir A., Velinzon K., et al. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science. 333:1633–1637 10.1126/science.1207227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.S., Janes H., Hawkins N., Grandpre L.E., Devoy C., Giri A., Coffey R.T., Harris L., Wood B., Daniels M.G., et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 10.1128/JVI.02108-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata, R., Igarashi T., Haigwood N., Buckler-White A., Ogert R., Ross W., Willey R., Cho M.W., and Martin M.A.. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204–210 10.1038/5568 [DOI] [PubMed] [Google Scholar]
- Shingai, M., Donau O.K., Schmidt S.D., Gautam R., Plishka R.J., Buckler-White A., Sadjadpour R., Lee W.R., LaBranche C.C., Montefiori D.C., et al. 2012. Most rhesus macaques infected with the CCR5-tropic SHIV(AD8) generate cross-reactive antibodies that neutralize multiple HIV-1 strains. Proc. Natl. Acad. Sci. USA. 109:19769–19774 10.1073/pnas.1217443109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingai, M., Nishimura Y., Klein F., Mouquet H., Donau O.K., Plishka R., Buckler-White A., Seaman M., Piatak M. Jr, Lifson J.D., et al. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 503:277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, Y., Winfrey S., Yang Z.Y., Xu L., Rao S.S., Srivastava I., Barnett S.W., Nabel G.J., and Mascola J.R.. 2007. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 25:1398–1408 10.1016/j.vaccine.2006.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek, M.D., Rida W., Priddy F.H., Pung P., Carrow E., Laufer D.S., Lehrman J.K., Boaz M., Tarragona-Fiol T., Miiro G., et al. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348 10.1128/JVI.00110-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundling, C., Forsell M.N., O’Dell S., Feng Y., Chakrabarti B., Rao S.S., Loré K., Mascola J.R., Wyatt R.T., Douagi I., and Karlsson Hedestam G.B.. 2010a. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J. Exp. Med. 207:2003–2017 10.1084/jem.20100025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundling, C., O’Dell S., Douagi I., Forsell M.N., Mörner A., Loré K., Mascola J.R., Wyatt R.T., and Karlsson Hedestam G.B.. 2010b. Immunization with wild-type or CD4-binding-defective HIV-1 Env trimers reduces viremia equivalently following heterologous challenge with simian-human immunodeficiency virus. J. Virol. 84:9086–9095 10.1128/JVI.01015-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, L.M., Phogat S.K., Chan-Hui P.Y., Wagner D., Phung P., Goss J.L., Wrin T., Simek M.D., Fling S., Mitcham J.L., et al. Protocol G Principal Investigators. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 326:285–289 10.1126/science.1178746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, L.M., Simek M.D., Priddy F., Gach J.S., Wagner D., Zwick M.B., Phogat S.K., Poignard P., and Burton D.R.. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog. 6:e1001028 10.1371/journal.ppat.1001028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, L.M., Huber M., Doores K.J., Falkowska E., Pejchal R., Julien J.P., Wang S.K., Ramos A., Chan-Hui P.Y., Moyle M., et al. Protocol G Principal Investigators. 2011a. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature. 477:466–470 10.1038/nature10373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, L.M., Sok D., Nishimura Y., Donau O., Sadjadpour R., Gautam R., Shingai M., Pejchal R., Ramos A., Simek M.D., et al. 2011b. Rapid development of glycan-specific, broad, and potent anti-HIV-1 gp120 neutralizing antibodies in an R5 SIV/HIV chimeric virus infected macaque. Proc. Natl. Acad. Sci. USA. 108:20125–20129 10.1073/pnas.1117531108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey, R., Nason M.C., Nishimura Y., Follmann D.A., and Martin M.A.. 2010. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res. Hum. Retroviruses. 26:89–98 10.1089/aid.2009.0144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Zhou T., O’Dell S., Wyatt R.T., Kwong P.D., and Mascola J.R.. 2009. Mechanism of human immunodeficiency virus type 1 resistance to monoclonal antibody B12 that effectively targets the site of CD4 attachment. J. Virol. 83:10892–10907 10.1128/JVI.01142-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X., Yang Z.Y., Li Y., Hogerkorp C.M., Schief W.R., Seaman M.S., Zhou T., Schmidt S.D., Wu L., Xu L., et al. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 329:856–861 10.1126/science.1187659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X., Chen W., Feng Y., Zhu Z., Prabakaran P., Wang Y., Zhang M.Y., Longo N.S., and Dimitrov D.S.. 2009. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem. Biophys. Res. Commun. 390:404–409 10.1016/j.bbrc.2009.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, T., Georgiev I., Wu X., Yang Z.Y., Dai K., Finzi A., Kwon Y.D., Scheid J.F., Shi W., Xu L., et al. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 329:811–817 10.1126/science.1192819 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.