Abstract
Background
There is evidence that patients with bipolar disorder (BD) score higher on affective temperament ratings compared to healthy controls (HCs). Moreover, unaffected relatives demonstrate similar patterns as BD patients suggesting that such temperaments are related to the genetic risk for BD and may serve as endophenotypes for the disorder. It is unknown whether affective temperaments are associated with other core features of BD, such as impairments in neurocognition. This study examined the relationship between affective temperaments and neurocognition in patients with BD and in HCs.
Methods
Temperaments were evaluated using the Temperament Evaluation of Memphis, Pisa, Paris, and San Diego, Auto-questionnaire version (TEMPS-A) in 64 patients with BD and 109 HCs. Neurocognitive functioning was evaluated using the MATRICS Consensus Cognitive Battery (MCCB). Correlational analyses between temperaments and cognition were conducted in BD and HC subjects.
Results
Data suggest that affective temperaments and neurocognition are correlated. In BD higher ratings of cyclothymia and irritability were associated with better processing speed, working memory, reasoning and problem-solving. In the HC group, increased irritability was related to worse performance on measures of attention and social cognition.
Limitations
Lack of functional outcome measures to evaluate the impact of temperaments and cognition on psychosocial functioning. It would be useful to test these findings on unaffected relatives of BD patients.
Conclusions
Cyclothymic and irritable temperaments are correlated with specific aspects of neurocognition in BD. This study is among the few exploring the dimensional relationship of temperaments and cognition in BD, and provides preliminary evidence for future studies investigating the neural and genetic mechanisms underlying the association between these variables.
Keywords: Temperament, Neurocognition, Bipolar Disorder, Personality Traits
Introduction
Bipolar disorder (BD) is a chronic psychiatric disorder characterized by an oscillation of depressive and (hypo)manic episodes, interspersed with periods of affective remission (DSM-V). Despite a general remission of overt affective symptoms during periods of euthymia, recent evidence suggests that illness features such as neurocognitive deficits persist beyond mood episodes and contribute to potentially persistent functional impairment (Arts et al., 2008; Bora et al., 2009; Robinson et al., 2006; Torres et al., 2007; Martinez-Aran et al., 2004). Cognitive deficits not only occur beyond the acute phase of the illness, but they are also present in unaffected relatives of patients with BD (Balanza-Martinez et al., 2008; Bora et al., 2009). This evidence supports the idea that neurocognitive deficits are potential endophenotypes for the disorder (Goldberg and Burdick, 2008; Gottesman and Gould, 2003; Glahn et al., 2004; Arts et al., 2008).
Identifying endophenotypes and investigating their relationship to other vulnerability factors is critical in gaining a better understanding of the complex architecture of BD. Within the framework of a dimensional conception of BD, in which core illness features are viewed as quantitative traits with a continuous distribution, it is important to understand how these dimensions may be interrelated.
In this study, we focused our attention on the relationship between neurocognitive functioning and affective temperaments. Temperamental factors are components of personality which are relatively stable over time (Goldsmith et al., 1987), specific to each individual, and reflect characteristics such as interpersonal style, energy level, and sensitivity and reactivity to internal and external stimuli. Since the beginning of the 20th century, Kraepelin (1921) recognized temperaments as steady personality characteristics out of which abnormal affective states may arise, potentially leading to the expression of a full-blown affective illness. Several researchers have developed this hypothesis into the concept of affective temperaments; that is, temperamental styles characterized by one or more of five main affective dimensions: anxious, irritable, cyclothymic, hyperthymic, and depressive (Akiskal, 1998; Akiskal and Mallya, 1987; Placidi et al., 1998). Conceptualized as quantitative dimensions, affective temperaments lie on a continuum from normality to pathology. In the last several decades, many studies have measured affective temperaments in different psychiatric samples, leading to the development of the Temperamental Evaluation of Memphis, Pisa, Paris, and San Diego-Autoquestionnaire (TEMPS-A) (Akiskal et al., 2005). Using this instrument, research suggests that patients with BD have higher ratings on several affective temperaments compared to non-clinical samples (Chiaroni et al., 2005; Evans et al., 2005; Mendlowicz et al., 2005) and that some temperaments might serve as markers of vulnerability for the disorder due to their over-representation in unaffected relatives of BD patients compared to healthy controls (Savitz et al., 2006; Evans et al., 2005; Mendlowicz et al., 2005). A very recent study from our group showed that unaffected siblings of patients with BD present with affective temperament ratings that fall intermediate to affected BD probands and an unrelated healthy control sample (Mahon et al., 2013).
Taken together, this evidence suggests that affective temperament and neurocognitive functioning may each represent dimensional endophenotypes in BD. Recent work suggests that, when affective temperament is measured categorically (i.e. when participants are determined to have a predominant affective temperament with subscale scores greater than or equal to one standard deviation above the mean), depressed patients with BD who had a predominantly hyperthymic temperament scored lower on measures of set-shifting and verbal working memory than depressed patients with BD with non-predominant affective temperaments (Xu et al., 2014). This work is the first to suggest that affective temperament may be associated with neurocognition in BD. However, no research has yet been conducted on the association between temperamental factors as a continuous, rather than a categorical measure, and neurocognitive functioning in BD. In the present work, we investigate the association between neurocognition and dimensionally-conceptualized affective temperaments during the euthymic phase. We first examined the levels of affective temperaments in patients with BD compared to a healthy control sample. We then explored potential relationships between affective temperaments and neurocognition in both the BD and healthy samples.
METHODS
Participants
The sample was composed of a total of 173 participants: 64 patients with BD and 109 HCs. Participants were recruited at two different sites: the Icahn School of Medicine at Mount Sinai and the Zucker Hillside Hospital (ZHH) – North Shore Long Island Jewish Health System.
BD sample: Inclusion criteria for patients included: 1) Diagnosis of BD I or BD II or BD Not Otherwise Specified (NOS) ascertained using the Structured Clinical Interview for DSM-IV (SCID-IV) (First et al., 2002) and 2) Current affective stability as measured by a score of < 15 on the Hamilton Rating Scale for Depression (HRSD) (Hamilton, 1960) and by a score of < 8 on the Clinician Administered Rating Scale for Mania (CARS-M) (Altman et al., 1994). HC sample: healthy controls with no evidence of Axis I disorders as determined by the SCID-NP were recruited through advertisements at ZHH. All participants were between the ages of 18 and 65 years old.
Exclusion criteria for all participants included: 1) History of CNS trauma, neurological disorder, and attention deficit hyperactivity disorder (ADHD) or a Learning Disability diagnosed in childhood; 2) Diagnosis of recent substance abuse/dependence (past 3 months); 3) Active, unstable medical problem; and 4) ECT in the past 12 months. In addition, healthy controls were excluded if they met criteria for an Axis I disorder as determined by the SCID-NP or if they reported a history of a diagnosed Axis I disorder in any first degree relatives. All procedures were approved by the local IRB and written informed consent was obtained from all participants.
Materials
Affective temperaments were assessed using the TEMPS-A (Akiskal et al., 2005), a 143-item self-report questionnaire that results in scores on five temperamental subscales: cyclothymic, depressive, anxious, hyperthymic, and irritable.
Neurocognitive performance was evaluated using the MATRICS Consensus Cognitive Battery (MCCB) (Nuechterlein and Green, 2006). The MCCB is composed of tests that give rise to the following 7 cognitive domains: 1) Processing Speed (assessed by the Brief Assessment of Cognition in Schizophrenia (BACS) and Trail Making Test part A); 2) Attention (assessed by the Continuous Performance Test—Identical Pairs (CPT-IP); 3) Working Memory (measured by the Wechsler Memory Scale [spatial and letter-number span]); 4) Verbal Learning (using the Hopkins Verbal Learning Test—Revised [HVLT-R]); 5) Visual Learning (as assessed using the Brief Visuospatial Memory Test—Revised [BVMT-R]); 6) Reasoning and Problem Solving (as assessed by the Neuropsychological Assessment Battery [NAB] Mazes subtest); and 7) Social Cognition (as measured by the Mayer–Salovey–Caruso Emotional Intelligence Test [MSCEIT[).
Analytic Approach
Patients with BD and HCs were first compared in terms of demographic characteristics (age, sex and race), clinical features (manic and depressive symptoms as measured by the CARS-M and by the HRSD), affective temperaments, and neurocognitive functioning (as measured by the cognitive domains from the MCCB, as well as premorbid IQ) using Chi-Square and independent sample t-tests as appropriate.
To evaluate whether affective temperaments were related to current sub-threshold mood symptoms, bivariate correlations were calculated between the five TEMPS-A subscales and depressive and manic symptoms (HRSD and CARS-M scores, respectively). Partial correlation analyses were used to test the association between the TEMPS-A subscales and cognitive domains in the whole sample using HRSD, CARS-M and WRAT-3 (premorbid IQ) scores as covariates; the same analysis was then conducted in the two samples of BD and HC subjects separately. The False Discovery Rate (FDR) was applied to control for type I error due to multiple comparisons.
RESULTS
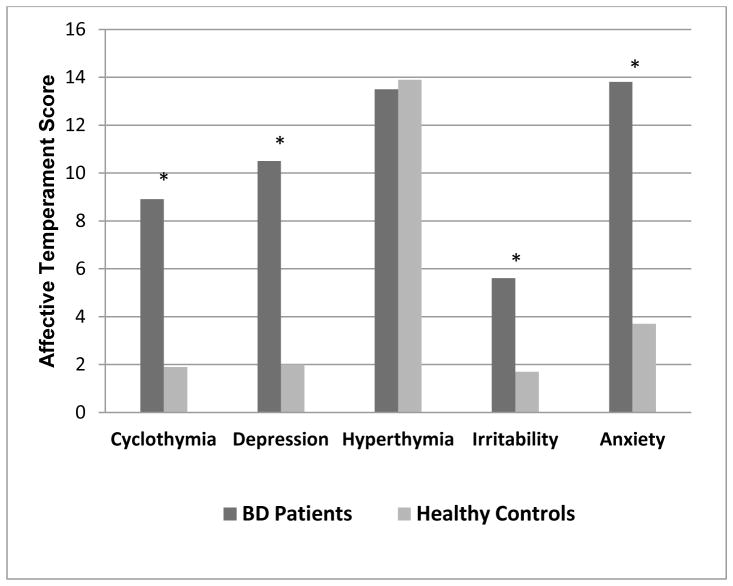
The sample was comprised of 64 patients with BD and 109 HCs. Among BD patients, the majority (77%; n=49) had a diagnosis of BD I, 14% (n=9) had a diagnosis of BPD II and 9.4% (n=6) had a diagnosis of BD NOS. No statistically significant differences were detected between patients and healthy controls in terms of sex, race, age and premorbid IQ (Table 1). Although affectively stable at the time of assessment, BD patients scored significantly higher than HCs on depressive and manic symptoms (Table 1) and demonstrated significantly higher scores on all of the affective temperaments except for hyperthymia, wherein patients and controls did not differ (Figure 1). With respect to cognitive performance, statistically significant differences emerged across MCCB cognitive domains; specifically, patients performed worse than controls in all domains except for visual memory and (p=.178) and reasoning and problem solving (p=.505; Table 1).
Table 1.
Comparison between Bipolar Disorder (BD) Patients and Healthy Control (HC) Subjects in Demographic, Clinical and Cognitive Characteristics
| BD Patients (N=64) | HC Subjects (N=109) | Significance
|
|||||
|---|---|---|---|---|---|---|---|
| df | t-test or χ2 | p value | |||||
|
| |||||||
| % | N | % | N | ||||
|
|
|||||||
| Sex | |||||||
| Males | 50.0 | 32 | 53.2 | 58 | |||
| Females | 50.0 | 32 | 46.8 | 51 | 1 | .17 | .683 |
|
| |||||||
| Race | |||||||
| Caucasian | 33.9 | 21 | 34.9 | 38 | 1 | .02 | .896 |
| Non Caucasian | 66.1 | 41 | 65.1 | 71 | |||
|
| |||||||
| Mean | SD | Mean | SD | ||||
|
| |||||||
| Age (in years) | 41.2 | 10.5 | 37.9 | 11.6 | 171 | −1.89 | .060 |
|
| |||||||
| Premorbid IQ (WRAT-3) | 94.7 | 12.1 | 98.0 | 9.9 | 157 | 1.73 | .087 |
|
| |||||||
| Depressive symptoms (HRSD) | 12.0 | 9.8 | 1.8 | 2.9 | 165 | −7.98 | <.001 |
|
| |||||||
| Manic symptoms (CARS-M) | 5.2 | 6.7 | 0.84 | 1.4 | 164 | −4.98 | <.001 |
|
| |||||||
| Diagnosis |
N (%)
|
||||||
| Bipolar Disorder I | 49 (76.6) | --- | --- | ||||
| Bipolar Disorder II | 9 (14.0) | --- | --- | ||||
| Bipolar NOS | 6 (9.4) | --- | --- | ||||
|
| |||||||
| TEMPS-A | Mean | SD | Mean | SD | |||
| Cyclothymia | 8.9 | 4.3 | 1.9 | 2.3 | 169 | 65.17 | <.001 |
| Depression | 10.5 | 4.4 | 2.0 | 2.4 | 169 | 51.61 | <.001 |
| Hyperthymia | 13.5 | 6.3 | 13.9 | 5.1 | 167 | .469 | .640 |
| Irritability | 5.6 | 3.8 | 1.7 | 1.8 | 170 | 19.74 | <.001 |
| Anxiety | 13.8 | 6.2 | 3.7 | 3.3 | 166 | 29.48 | <.001 |
|
| |||||||
| Cognitive Domains-Mean (T-score) | Mean | SD | Mean | SD | |||
|
| |||||||
| Processing Speed | 41.3 | 10.2 | 50.0 | 8.9 | 104 | 4.63 | <.001 |
| Attention | 40.4 | 11.3 | 48.0 | 9.3 | 102 | 3.70 | <.001 |
| Working Memory | 40.4 | 10.4 | 46.2 | 10.2 | 104 | 2.90 | .005 |
| Verbal Learning | 39.4 | 7.6 | 44.2 | 8.4 | 102 | 3.04 | .003 |
| Visual Learning | 40.2 | 13.1 | 43.3 | 9.9 | 104 | 1.36 | .178 |
| Reasoning/Problem Solving | 41.7 | 10.0 | 43.0 | 10.0 | 104 | .67 | .505 |
| Social Cognition | 41.8 | 13.0 | 48.6 | 10.7 | 103 | 2.89 | .005 |
| Composite Score | 35.0 | 10.9 | 43.7 | 9.5 | 100 | 17.94 | <.001 |
Figure 1. Comparison between Bipolar Disorder (BD) patients and Healthy Control (HC) Subjects in Temperamental Mean Scores.
Bars describe the mean scores of Bipolar Disorder (BD) patients and Healthy Control (HC) subjects across the five affective temperaments. The asterisk is showed for those comparisons that resulted statistically significant (all p<.001).
* p<.001
Pearson correlations showed that in the BD group, all of the temperamental factors were positively associated with subthreshold symptoms of mania, with effect sizes ranging from r=.297, for the depressed temperament, up to r=.544, for the irritable temperament (Supplemental Material 1). Symptoms of depression were significantly positively correlated with the depressive (r=.475) and anxious temperaments (r=.492). In the HC group there were no significant associations between mania ratings (CARS-M) and any of the affective temperaments; however, there were significant positive correlations between depression ratings (HRSD) and all temperamental subscales except for hyperthymia (Supplemental Material 1). However, it should be noted that in both the patient and control groups, scores on both the mania and depression symptom rating scales fell within a relatively restricted range.
Association between Affective Temperaments and Cognition
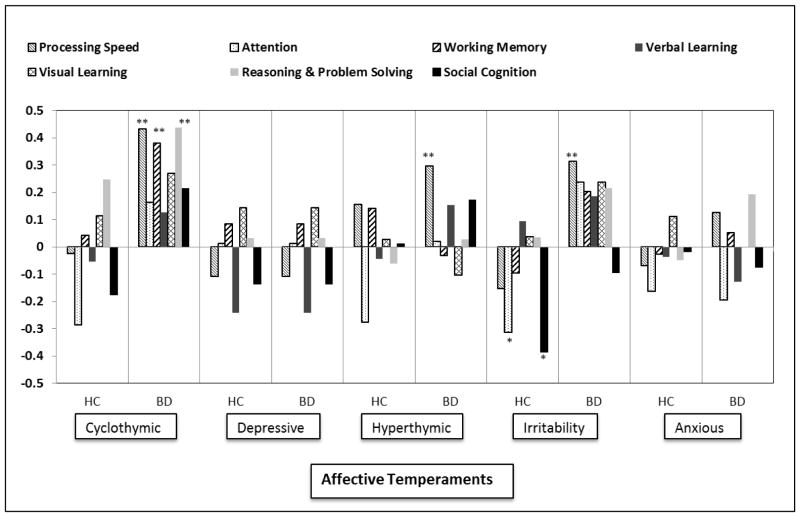
In the full sample, partial correlation analysis controlling for affective symptoms and premorbid IQ, revealed an association between affective temperaments and cognitive performance. A significant positive relationship emerged between cyclothymic temperament and the reasoning and problem solving domain (r=.226, p=.028), as well as between hyperthymic temperament and processing speed (r=.238, p=.022). A significant negative association characterized the relationship between depressive temperament and processing speed (r=−.338, p=.001) and the relationship between anxious temperament and attention (r=−.283, p=.007).
When the associations were inspected in BD patients and HC subjects separately (shown in Figure 2), we found that in the HC sample the irritable temperament was significantly negatively correlated with both attention (r=−.315, p=.035) and social cognition (r=−.386, p=.009). In the BD sample, there were many more significant associations between temperament and cognition which followed a different pattern. In particular, higher ratings on the cyclothymic scale were associated with better processing speed (r=.432, p=.002), working memory (r=.379, p=.009), reasoning and problem solving (r=.438, p=.002) and global cognition as measured by an overall composite score (r=.467, p=.001). In addition, higher scores on the hyperthymic scale and the irritability scale were associated with better processing speed [r=.295 (p=.046) and r=.312 [p=.032] respectively). After controlling for type I error using the FDR method, all results remained statistically significant with the exception of the association between hyperthymia and processing speed in the BD sample.
Figure 2. Correlations between Neurocognitive Domains and Affective Temperaments in Healthy Control (HC) Subjects and Bipolar Disorder (BD) Patients.
Bars describe the r (Pearson) resulting from the correlation analysis between the seven MCCB neurocognitive domains and the five affective temperaments in Healthy Control (HC) Subjects and Bipolar Disorder (BD) Patients. Statistically significant differences are reported with asterisks (* p<.05; ** p<.01).
* p<.05; ** p<.01
CONCLUSIONS
Our results replicate previous findings of increased levels of affective temperament in patients with BD compared to controls (Chiaroni et al., 2005; Evans et al., 2005; Mendlowicz et al., 2005) and extend these findings to demonstrate associations between affective temperament and neurocognitive performance. Moreover, the nature of the association between temperament and neurocognition differed between the two groups.
We found that in the full sample, higher scores on the cyclothymic and hyperthymic subscales were positively correlated with cognitive performance while higher scores on the depressive and anxious subscales were negatively associated with cognition. The strongest correlation demonstrated across the entire sample was that of the depressive affective temperament and processing speed; this strong negative association is consistent with previous evidence that depressive symptomatology negatively impacts this particular domain with a moderate to large effect size (Burdick et al., 2009).
When relationships between affective temperament and neurocognition were examined in each group individually, positive associations emerged in the BD sample, whereas negative associations were revealed within the HC sample. Results indicate that in HCs, higher scores on irritability negatively affected performance in the attention and social cognition domains. Conversely, in the BD sample, irritability was positively correlated with processing speed and there were no relationships between this subscale and any other cognitive domain. The affective temperament that was the most strongly associated with neurocognition in the BD sample was the cyclothymic temperament where higher scores were associated with better performance on processing speed, working memory, reasoning and problem solving and global cognition.
Taken together, these findings support and expand upon previous research on affective temperaments in BD. They appear to confirm that most affective temperaments are significantly higher in patients with BD compared to healthy controls, with the exception of the hyperthymic subscale (Chiaroni et al., 2005; Evans et al., 2005; Mendlowicz et al., 2005). Our finding that the hyperthymic temperament was not significantly different between BD and HCs in this and several other studies is likely due to the intrinsic characteristics of the subscale; in particular, the items of this subscale are perceived as positive and socially adaptive compared to those of the other subscales (Akiskal et al., 2005; Evans et al., 2005) such that healthy controls, as well as patients with BD, may be more socially and culturally prone to adhere to them. This is also supported by a recent study which showed that the hyperthymic temperament has a strong positive correlation with extraversion and is inversely correlated with neuroticism, which in turn was positively associated with cyclothymic and irritable temperaments (Kwapil et al., 2013).
Our findings expand upon recent data suggesting the presence of a relationship between affective temperament and cognition in BD depression (Xu et al., 2014). Our study appears to support this recent work by providing preliminary evidence that affective temperament, when analyzed as a continuous measure, is significantly associated with several neurocognitive domains in euthymic BD. Moreover, our findings suggest a differential pattern of the relationship between the irritable temperament and neurocognition in healthy controls versus patients with BD. Such a discrepancy in the association between temperaments and cognition in patients with BD and in healthy controls is perhaps relevant to the question of whether affective temperaments are dimensions of normality or pathology (Rovai et al., 2013). Based on our results, it appears that in absence of an affective disorder, higher levels of trait irritability may be associated with worse neurocognitive functioning within the domains of attention and social cognition. In contrast, higher levels of trait irritability and cyclothymia in patients with BD do not appear to have a negative effect on cognitive functioning, and in fact, may be associated with improved functioning across multiple domains relative to patients with lower levels of these traits. This perhaps counterintuitive finding may be consistent with previous work suggesting that, among patients with affective psychosis, there is an inverted U-shaped relationship between levels of mania and cognitive functioning (Kravariti et al., 2012). One possible explanation for this is that it may be that, up to a certain point, increased levels of trait irritability and cyclothymia in individuals with BD are somewhat beneficial for cognition. However, it should be kept in mind that the evidence presented in the current work is preliminary; replication in a larger sample is required to draw more firm conclusions.
This study has some limitations. First, the inclusion of functional outcome measures could have provided additional information regarding the impact of the investigated relationship between affective temperaments and cognition on psychosocial functioning and quality of life in BD. Previous work suggests that both neurocognitive impairment and higher levels of affective temperament are associated with decreased quality of life (Jaeger and Vieta, 2007; Martinez-Aran et al., 2004; Vazquez et al., 2007), but more work is needed to explore the relationship among these variables in greater detail in BD. Second, the restricted range of symptom rating scores, especially in the healthy control sample limited our ability to detect relationships among subthreshold affective traits and cognitive performance. As unaffected siblings have been shown to have intermediate levels of most of the affective temperaments, an examination of the association between temperaments and neurocognition in this population may help to clarify the relationship between these illness dimensions in affected and unaffected samples. Finally, although we attempted to minimize Type I error by using the FDR method, it remains possible that multiple comparisons could have accounted for at least some of our results.
In spite of these limitations, this study is among the few exploring the relationship of affective temperaments and cognitive functioning in euthymic BD patients and HCs. It provides preliminary insights for potential future studies investigating the brain mechanisms underlying the associations between these variables. Further investigations are warranted in order to elucidate the role of personality traits in the development of BD as well as subclinical manifestations of the disorder.
Supplementary Material
Acknowledgments
ROLE OF FUNDING SOURCE
This study was funded by grants from the National Institute of Mental Health (NIMH) to KEB (K23077807, R03079995 and R01 MH100125).
Footnotes
CONTRIBUTORS
Dr Russo contributed to the design, statistical analyses and interpretation of data, as well as the drafting and revision of the manuscript. Dr Burdick contributed to the conception and study design, as well of interpretation of the data and revision of the manuscript. Dr Mahon contributed to the interpretation of data and the critical revision of the manuscript. Ms Shanahan, Solon and Ramjas contributed to the critical revision of the manuscript. Dr Braga made substantial contributions to the conception and design of the study. All the authors approved the final manuscript.
CONFLICT OF INTEREST
The authors affirm that they have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiskal HS. Toward a definition of generalized anxiety disorder as an anxious temperament type. Acta Psychiatr Scand Suppl. 1998;393:66–73. doi: 10.1111/j.1600-0447.1998.tb05969.x. [DOI] [PubMed] [Google Scholar]
- Akiskal HS, Mallya G. Criteria for the “soft” bipolar spectrum: treatment implications. Psychopharmacol Bull. 1987;23:68–73. [PubMed] [Google Scholar]
- Akiskal HS, Akiskal KK, Haykal RF, Manning JS, Connor PD. TEMPS-A: progress towards validation of a self-rated clinical version of the Temperament Evaluation of the Memphis, Pisa, Paris, and San Diego Autoquestionnaire. J Affect Disord. 2005;85:3–16. doi: 10.1016/j.jad.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36:124–134. doi: 10.1016/0006-3223(94)91193-2. [DOI] [PubMed] [Google Scholar]
- Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Rubio C, Selva-Vera G, Martinez-Aran A, Sanchez-Moreno J, Salazar-Fraile J, Vieta E, Tabares-Seisdedos R. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neurosci Biobehav Rev. 2008;32:1426–1438. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Gunawardane N, Goldberg JF, Helperin JM, Garno JL, Malhotra AK. Attention and psychomotor functioning in bipolar depression. Psychiatry Res. 2009;166:192–200. doi: 10.1016/j.psychres.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Chiaroni P, Hantouche EG, Gouvernet J, Azorin JM, Akiskal HS. The cyclothymic temperament in healthy controls and familially at risk individuals for mood disorder: endophenotype for genetic studies? J Affect Disord. 2005;85:135–145. doi: 10.1016/j.jad.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Evans L, Akiskal HS, Keck PE, Jr, McElroy SL, Sadovnick AD, Remick RA, Kelsoe JR. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J Affec Disord. 2005;85:153–168. doi: 10.1016/j.jad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Willians JBW. Structured Clinical Interview for DSM IV TR Axis I Disorders, Patient Edition (SCID-I/P) NewYork: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Burdick KE. Cognitive dysfunction in bipolar disorder: a guide for clinicians. American Psychiatric Publishing, Inc; Arlington, VA: 2008. [Google Scholar]
- Goldsmith HH, Buss AH, Plomin R, Rothbart MK, Thomas A, Chess S, Hinde RA, McCall RB. Roundtable: what is temperament? Four approaches Child Dev. 1987;58:505–529. [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Vieta E. Functional outcome and disability in bipolar disorders: ongoing research and future directions. Bipolar Disord. 2007;9:1–2. doi: 10.1111/j.1399-5618.2007.00441.x. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Manic-depressive insanity and paranoia. E. & S. Livingstone; Edinburgh, SCT: 1921. [Google Scholar]
- Kravariti E, Russo M, Vassos E, Morgan K, Fearon P, Zanelli JW, Demjaha A, Lappin JM, Tsakanikos E, Dazzan P, Morgan C, Doody GA, Harrison G, Jones PB, Murray RM, Reichenberg A. Linear and non-linear associations of symptom dimensions and cognitive function in first-onset psychosis. Schizophr Res. 2012;140:221–231. doi: 10.1016/j.schres.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Kwapil TR, DeGeorge D, Walsh MA, Burgin CJ, Silvia PJ, Barrantes-Vidal N. Affective temperaments: unique constructs or dimensions of normal personality by another name? J Affect Disord. 2013;151:882–890. doi: 10.1016/j.jad.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Mahon K, Perez-Rodriguez MM, Gunawardane N, Burdick KE. Dimensional endophenotypes in bipolar disorder: Affective dysregulation and psychosis proneness. J Affect Disord. 2013;151:695–701. doi: 10.1016/j.jad.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Colom F, Torrent C, Sánchez-Moreno J, Reinares M, Benabarre A, Goikolea JM, Brugué E, Daban C, Salamero M. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6:224–232. doi: 10.1111/j.1399-5618.2004.00111.x. [DOI] [PubMed] [Google Scholar]
- Mendlowicz MV, Jean-Louis G, Kelsoe JR, Akiskal HS. A comparison of recovered bipolar patients, healthy relatives of bipolar probands, and normal controls using the short TEMPS-A. J Affect Disord. 2005;131:37–44. doi: 10.1016/j.jad.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF. MATRICS Consensus Cognitive Battery. MATRICS Assessment, Inc; Los Angeles, CA: 2006. [Google Scholar]
- Placidi GF, Signoretta S, Liguori A, Gervasi R, Maremmani I, Akiskal HS. The semi-structured affective temperament interview (TEMPS-I): Reliability and psychometric properties in 1010 14–26-year-old students. J Affect Disord. 1998;47:1–10. doi: 10.1016/s0165-0327(97)00122-5. [DOI] [PubMed] [Google Scholar]
- Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, Moore PB. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Rovai L, Maremmani AG, Rugani F, Bacciardi S, Pacini M, Dell’Osso L, Akiskal HS, Maremmani I. Do Akiskal & Mallya’s affective temperaments belong to the domain of pathology or to that of normality? Eur Rev Med Pharmacol Sci. 2013;17:2065–2079. [PubMed] [Google Scholar]
- Savits JB, Ramesar RS. Personality: is it a viable endophenotype for genetic studies of bipolar affective disorder? Bipolar Disord. 2006;8:322–337. doi: 10.1111/j.1399-5618.2006.00309.x. [DOI] [PubMed] [Google Scholar]
- Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl. 2007;434:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- Vázquez GH, Kahn C, Schiavo CE, Goldchluk A, Herbst L, Piccione M, Saidman N, Ruggeri H, Silva A, Leal J, Bonetto GG, Zaratiegui R, Padilla E, Vilapriño JJ, Calvó M, Guerrero G, Strejilevich SA, Cetkovich-Bakmas MG, Akiskal KK, Akiskal HS. Bipolar disorders and affective temperaments: A national family study testing the “endophenotype” and “subaffective” theses using the TEMPS-A Buenos Aires. J Affect Disord. 2008;108:25–32. doi: 10.1016/j.jad.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Xu G, Lu W, Ouyang H, Dang Y, Guo Y, Miao G, Bessonov D, Akiskal KK, Akiskal HS, Lin K. Association of affective temperaments measured by TEMPS-A with cognitive deficits in patients with bipolar disorder. J Affect Disord. 2014;161:109–115. doi: 10.1016/j.jad.2014.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.