Abstract
A variety of recent data demonstrate that vasoactive intestinal polypeptide (VIP) and VPAC receptors (which bind VIP, and to a lesser extent, pituitary adenylatecyclase activating peptide) are important for numerous social behaviors in songbirds, including grouping and aggression, although VIP relates to these behaviors in a site-specific manner. In order to determine the global effects of central VPAC receptor activation on social behavior, we here infused a VPAC receptor antagonist or vehicle twice daily into the lateral ventricle of colony-housed male and female zebra finches and quantified a wide range of behaviors. Aggressive behaviors were not altered by ventricular infusions, consistent with known opposing, site-specific relationships of VIP innervation to aggression. Courtship and self-maintenance behaviors were likewise not altered. However, VPAC antagonism produced significant deficits in pair bonding. Antagonist subjects took longer to form a pair bond and were paired for significantly fewer observation sessions relative to control subjects (median 1.5 of 6 observation sessions for antagonist subjects versus 4 for control subjects). Antagonist subjects were also significantly less likely to be paired in the final observation session. Based on the known distribution of VPAC receptors in finches and other vertebrates, we propose that VPAC receptors may mediate pair bonding via a variety of brain areas that are known to be important for the establishment of partner preferences in voles, including the lateral septum, ventral tegmental area, nucleus accumbens and ventral pallidum.
Keywords: vasoactive intestinal polypeptide, monogamy, bird
Vasoactive intestinal polypeptide (VIP) cells, fibers and/or binding sites (VPAC receptors) are found at high densities in virtually every brain area that contributes to the regulation of social behavior. These include the medial amygdala, hippocampus, lateral septum (LS), preoptic area, anterior hypothalamus (AH), bed nuclei of the stria terminalis, ventral tegmental area (VTA), and nucleus accumbens (NAcc)[1-3]. Consistent with these distributions, VIP signaling plays important roles in many social behaviors, including aggression in birds[4], sociability and social novelty in rodents[5], and social contact and gregariousness in zebra finches [6]. Notably, relationships between VPAC receptors/VIP and behavior in birds tend to be site-specific and sometimes sex-specific. In the present experiment, we quantified the behavioral effects of chronic, central VPAC antagonism in male and female zebra finches (Taeniopygiaguttata) housed in a colony environment, with a specific focus on determining the role of VIP signaling in the establishment of pair bonds.
The ability to form a selective social attachment with a mate, defined as a pair bond, is the central defining feature of a socially monogamous mating system. Despite the ecological and translational relevance of pair bonding, the underlying neural mechanisms have been well described for only a single species, the prairie vole (Microtusochrogaster)[7]. To date, most of the relevant work in voles has focused on the effects of oxytocin (OT)and vasopressin (VP) in the NAcc and ventral pallidum, and on their interactions with the mesolimbic dopamine system (reviewed in[7, 8]). However, other neurochemical systems and brain areas are also involved in pair bonding[8] and relevant mechanisms likely vary somewhat across species[9-12].
Although activation of OT (VT3) receptors is also necessary for pair bond formation in zebra finches[10, 11], the NAcc does not express detectable mRNA for any of the VP-OT receptor types[13], suggesting that relevant sites of action are at least partially different from those in voles. Hence, to the extent that socially relevant neuromodulators interact with mesolimbic dopamine to influence pair bonding in finches, we must look beyond the VP-OT peptide family. Of the possible candidates, VIP stands out based on its extensive association with the mesolimbic dopamine system, which includes strong expression of VIP mRNA in the VTA[3] and a high density of 125I-VIP binding sites in the NAcc[1]. Thus, based on this close association of VIP elements with the mesolimbic dopamine system, and the importance of VPAC receptors for affiliation behaviors[5, 6], we here test the hypothesis that endogenous VIP signaling promotes pair bonding in the socially monogamous zebra finch.
A total of 32 female and 32 male cannulatedzebra finches were tested, of which 26 females (14 control, 12 antagonist-treated) and 23 males (13 control, 10 antagonist-treated) exhibited accurate cannula placement and were retained for analyses. Subjects were housed in groups of 6–10 same-sex individuals prior to experimentation, and were maintained on a 14L:10D photoperiod (full spectrum), with food and water provided ad libitum. Experiments were conducted in a humane manner and were approved by the Bloomington Institutional Animal Care and Use Committee at Indiana University.
Birds were anesthetized with isoflurane vapor and stereotaxically fitted with a 26-gauge cannula directed at the right lateral ventricle(see [14]) and given a minimum of 5 days recovery. We initiated twice-daily infusions of either 0.5 μl saline vehicle or 0.5 μl containing 250 ng of a VPAC receptor antagonist with known selectivity for VIP binding sites in chickens (neurotens in 6-11–mouse VIP 7-28)[15] in a between-subjects design. This dose is based on previous studies and yields behavioral effects that are consistent with antisense knockdown of VIP production[4, 6]. The first round of infusions started at 1700 h on the day before colony testing and subsequent infusion rounds were initiated at 0800 h (lights-on) and 1700 h daily. Each round of infusions required approximately 30 min, based on an average of 8 subjects being testing concurrently. Behavioral observations were conducted in the order of infusion and approximately 30 minutes post infusion at 0800 h while afternoon observations were conducted just prior to afternoon infusions at 1700 h. At the completion of testing, subjects were euthanized by isoflurane vapor and perfused with 10% formaldehyde for histological verification of cannula placement [14].
Behavioral quantification followed standard lab protocols[9, 10]. Colony cages were 1.3 m W × 0.43 m H × 0.36 m D and each contained 4 nest cups (one per corner) and shredded burlap nesting material. In order to exclude subjects who were not behaviorally robust, subjects were prescreened in colonies containing 5 individuals of each sex, with a single 3 min observation immediately after colony establishment, and a single 5 min observation on the following day. No rigid criterion was used for exclusion; we simply excluded the least socially active bird of each sex, which is typically an individual that exhibits little quantifiable behavior. Pre-screenings also allowed us to counterbalance treatment groups based on aggression, which may exert confounding effects on pairing. Surgeries were conducted following prescreening and subjects were returned to their same-sex housing cages until the initiation of testing.
Each testing colony (16 total) contained 4 individuals of the focal sex and 5 opposite-sex individuals. The 4:5 sex ratio is intended to decrease the impact of aggression on mate acquisition, allowing us to quantify pair bonding without a strong confound of competitive ability. Each cage contained 2 control and 2 antagonist subjects of a single sex. Focal observations were conducted 6 times over 3 days. Session 1 observations were 3 min per subject (12 min per colony) and began 10 min after the establishment of colonies. Session 2-6 observations were 5 min per subject (20 min per colony). The shorter observation period for Session 1 allows for the quantification of all behavior during the initial burst of courtship and competitive aggression. Aggressive behaviors quantified were displacements, displacements received from other birds, threats, beak fences, and pecks. Aggression data were analyzed separately for behaviors directed toward same-sex and opposite-sex animals. Other social and nesting behaviors quantified were directed song, undirected song, pick up nest item, carry nest item to nest, time spent on nest, and latency to pair bond. Latency to pair bond is defined as the first session (of 6) in which the subject was observed to be paired. Subjects that did not pair were assigned a latency of 7. Maintenance (preens, feeding and drinking), general affiliation (greets) and arousal (beak wipes) behaviors were also recorded. Pairing status in zebra finches was determined by selective affiliation between a male and female.
Because colony tests are not amenable to video recording, behaviors must be recorded for quantification in real time. Thus, several aspects of selective association that are not quantified independently are considered when making the determination of pairing status, including the maintenance of close proximity, side-by-side perching (“clumping”), feeding together, allopreening, and exclusive occupation of a nest cup by a male and female. Of these, only allopreening was quantified independently, but was not exhibited at sufficient frequencies for strong analysis. Nest occupancy (“time in nest”) was also quantified independently. However, although exclusive co-occupancy is a major contributor to the determination of pairing, we simply used exclusive occupancy by a male-female pair as evidence for pairing without attempting numerical quantification of co-occupancy. Finally, subjects will often displace same-sex birds that are near their partner (mate guarding) or near their nest cup (nest defense). This is simply recorded as a “displacement,” but the context of the displacement contributes to the determination of pair bonding.
Data for the measures of latency to pair and number of sessions paired were not normally distributed and were therefore analyzed using Mann-Whitney tests. χ2 tests were used to analyze pair bond status at the end of testing. With the exception of time on the nest (or time in a nest cup), the numbers of all other behaviors were converted to frequencies by dividing them by the number of minutes not spent on the nest[9, 10] and were analyzed by ANOVA or t-tests. We have previously found that behavior is modulated differently in the contexts of mate competition (as in the first colony session) and nest defense (as in later colony sessions)[9]. We therefore conducted an initial set of aggression analyses using mixed-model ANOVAs with Session as a repeated measure (Session 1 vs. Sessions 2-6) and Sex and Treatment as between-subject variables. No differential effects were observed across sessions, and thus the data were collapsed across sessions for presentation in Table 1. With the exception of male song, all other behaviors were analyzed by ANOVA with Sex and Treatment as between-subject variables. Directed and undirected song were analyzed using unpaired t-tests.
Table 1.
Treatment and Sex × Treatment effects of VPAC antagonist infusions for behavior measures1 in all subjects with i.c.v. cannula placements.
| Behavior | Control female (mean ± s.e.m.) | OTA female (mean ± s.e.m.) | Control male (mean ± s.e.m.) | OTA male (mean ± s.e.m.) | Treatment | Sex × Treatment | ||
|---|---|---|---|---|---|---|---|---|
| F (1,1,45) 2 | P | F (1,1,45) | P | |||||
| Same-sex displacements | 0.61±0.13 | 0.33±0.13 | 0.80±0.33 | 0.94±0.22 | 0.11 | 0.75 | 0.89 | 0.35 |
| Total same-sex aggression | 1.18±0.22 | 0.78±0.22 | 1.50±0.43 | 1.64±0.29 | 0.18 | 0.67 | 0.73 | 0.40 |
| Opposite-sex displacements | 0.33±0.07 | 0.30±0.10 | 0.23±0.08 | 0.15±0.03 | 0.53 | 0.47 | 0.12 | 0.73 |
| Total opposite-sex aggression | 0.97±0.19 | 0.83±0.21 | 0.62±0.13 | 0.66±0.18 | 0.08 | 0.78 | 0.26 | 0.61 |
| Displacements rec'd (same-sex) | 0.59±0.62 | 0.66±0.23 | 1.20±0.48 | 0.69±0.24 | 0.43 | 0.51 | 0.74 | 0.40 |
| Displacements rec'd (opposite-sex) | 0.12±0.04 | 0.34±0.15 | 0.12±0.04 | 0.03±0.01 | 0.74 | 0.39 | 3.65 | 0.06 |
| Nest items picked up | 0.05±0.03 | 0.06±0.02 | 0.15±0.04 | 0.11±0.04 | 0.24 | 0.63 | 0.43 | 0.52 |
| Nest items carried to nest | 0.05±0.05 | 0.01±0.01 | 0.08±0.03 | 0.06±0.03 | 0.66 | 0.42 | 0.14 | 0.71 |
| Feeding movements | 1.03±0.21 | 0.77±0.22 | 1.10±0.19 | 0.96±0.26 | 0.84 | 0.36 | 0.08 | 0.79 |
| Drinks | 0.10±0.05 | 0.03±0.02 | 0.15±0.06 | 0.13±0.03 | 1.23 | 0.27 | 0.28 | 0.60 |
| Beak wipes | 0.16±0.04 | 0.16±0.06 | 0.36±0.09 | 0.26±0.11 | 0.56 | 0.46 | 0.41 | 0.53 |
| Greets | 0.07±0.03 | 0.04±0.01 | 0.12±0.03 | 0.09±0.02 | 1.44 | 0.24 | 3.4E-4 | 0.99 |
| Preens | 0.69±0.11 | 0.45±0.09 | 0.53±0.07 | 0.46±0.09 | 2.79 | 0.10 | 0.90 | 0.35 |
| Total nest time | 530.50±75.12 | 396.42±138.44 | 375.92±71.87 | 303.80±78.76 | 1.17 | 0.28 | 0.11 | 0.75 |
| Directed song (males only) | - | - | 0.63±0.22 | 0.32±0.12 | 1.29 | 0.27 | - | - |
| Undirected song (males only) | - | - | 0.11±0.08 | 0.02±0.01 | 1.20 | 0.29 | - | - |
| Total songs (males only) | - | - | 0.75±0.26 | 0.34±0.11 | 1.68 | 0.21 | - | - |
Data are presented as the number of behaviors exhibited per min not on the nest with the exception of total nest time.
Degrees of freedom exclude male specific courtship behaviors, which were analyzed by t-tests.
Data for aggression, song, nesting, maintenance (preens, feeding and drinking), general affiliation (greets) and arousal (beak wipes) are shown in Table 1. No significant Treatment effects or Sex × Treatment effects are observed (all P> 0.05), nor are there any statistical trends (P>0.07), with the exception of the Sex × Treatment interaction for displacements received from opposite sex individuals (P=0.06). This interaction reflects a trend for antagonist-treated females to be displaced more by males relative to control females and for antagonist-treated males to be displaced less by females compared to control males.
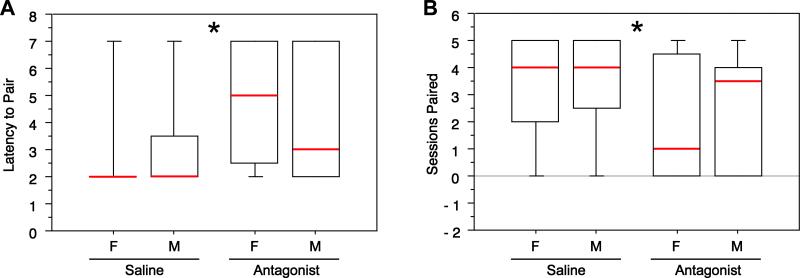
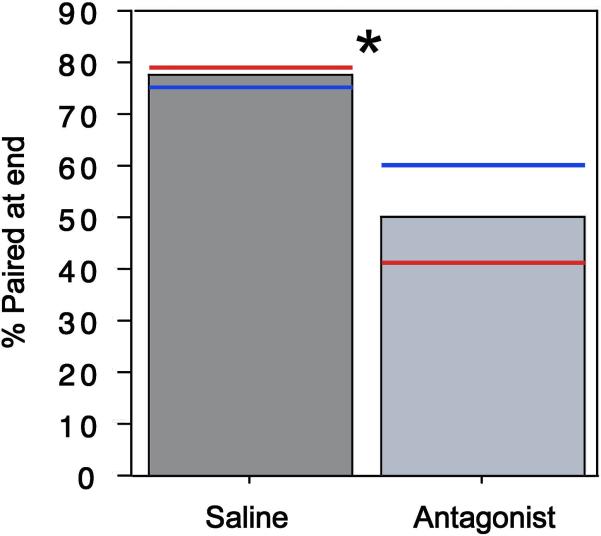
As shown in multiple measures, central VPAC antagonism significantly impaired pair bonding in both sexes. Antagonist-treated subjects showed a longer latency to pair compared to subjects administered saline (Mann-Whitney tied P = 0.016, Fig. 1A). In addition, antagonist-treated birds were paired for a fewer number of sessions compared to control birds (Mann-Whitney tied P = 0.042, Fig. 1B). However, antagonist-treated subjects were not significantly more likely to “divorce” (i.e. split after being scored as paired) despite a very weak numerical tendency in that direction (χ2P= 0.183). Finally, VPAC antagonism significantly reduced the percentage of birds that were paired at the end of the behavioral study, compared to control birds (χ2P = 0.042; Fig. 2). Allopreening between partners was observed at low rates and was not significantly affected by VPAC antagonism (χ2P = 0.367). Most sex-specific analyses were not significant (P> 0.05), with the exception of latency to pair (P = 0.016 for females), indicating that for the most part, pairing is not differentially affected by VPAC antagonism in males and females.
Fig. 1.
Intracerebroventricular infusions of a VPAC antagonist significantly increase the latency to pair (A) and the total number of behavioral sessions paired (B) in antagonist-treated subjects relative to saline infusions in control animals. Latency to pair bond is defined as the first session (of 6) in which the subject was observed to be paired. Subjects that did not pair were assigned a latency of 7. Box plots show the median (red line), 75th and 25th percentile (box) and 95% confidence interval (whiskers).*Mann-Whitney tied P<0.05 for effect of Treatment (sexes pooled).
Fig. 2.
VPAC receptor antagonism significantly decreases the percentage of zebra finches that are paired in the final behavioral session as compared to control animals. *χ2P< 0.042. Red and blue bars denote values for females and males, respectively.
VIP and VPAC receptors are implicated in numerous behavioral processes. However, the neural distributions of peptide neurons, axons and receptors are broad, and it is clear that VIP and VPAC receptors exert behavioral effects that can vary substantially across brain areas. For instance, whereas VIP cell numbers and fiber densities in the mediobasal hypothalamus of sparrows and waxbills are negatively related to aggression[4, 16], antisense-mediated knockdown of VIP production in the AH virtually abolishes aggression in finches and waxbills[4], consistent with the observations that VIP immunolabeling in the AH and caudocentral septum correlates positively with aggression in sparrows[16] and VIP cell numbers in the AH correlate positively with aggression in waxbills [4],. Not surprisingly then, relative to the profound effect of VIP antisense infusions into the AH, intracerebroventricular infusions of VPAC antagonist exert very weak effects on aggression in waxbills[4], and produced no detectable effect on aggression here. VPAC antagonist infusions also exert site-specific effects on gregariousness in zebra finches[6]. Given these site-specific and sometimes opposing relationships of VIP and VPAC receptors to behavior, it is perhaps not surprising that we here observe very limited effects of ventricular infusions on behavior. However, the impairments of pair bonding observed here are quite robust, suggesting that VPAC receptors do not exert opposing effects on pair bonding across different brain areas.
Because VPAC receptors are widely distributed in the brain, including within components of the mesolimbic dopamine system and all areas of the so-called “social behavior network” [1], VPAC effects on pairing potentially occur in numerous sites of action. Nonetheless, based on work in prairie voles, a few sites stand out as likely candidates, including the VTA and NAcc. Dopamine release in NAcc is necessary for bonding in prairie voles[17], and pair bonding is induced in the absence of mating by antagonism of GABA and AMPA receptors in the VTA of males[18]. Hence, both “ends” of the mesolimbic dopamine system are known to influence bonding. In zebra finches, VPAC binding is higher in the NAcc than in the surrounding striatum[1], and notably, sites of VIP production in both birds and mammals extensively overlap those of dopamine. These sites include the preoptic area, tuberal hypothalamus, posterior hypothalamic area, basal (ventrolateral) periaqueductal gray, and VTA[3, 19, 20]. Furthermore, a variety of findings in rodents demonstrate that VIP promotes catecholamine synthesis and neurosecretion[21]. Hence, VIP and VPAC receptors may promote pair bonding through modulatory interactions with mesolimbic dopamine.
VP infusions into the LS of male prairie voles also induce partner preferences in the absence of mating[22], and indirect evidence suggests that the LS is likely important for pair bonding in zebra finches, as well[10]. Notably, VIP binding densities in the zebra finch LS are high[1]. Because the dorsal LS is essential for linking context to reward in mice, via projections to the VTA[23], bonding-related processes of the LS may be upstream of processes regulated by mesolimbic dopamine.
The considerations above are based on the assumption that VPAC receptor activation is part of the process that induces the formation of a bond. However, we must also consider that VPAC activation is necessary for more basic functions that are likely prerequisite to bonding, such as social recognition. In rodents, social recognition is regulated by neural circuits that include the medial amygdala and LS[24], both of which exhibit VPAC receptors[2]. The medial amygdala is additionally a site of VIP mRNA expression[3]. Indeed, embryonic antagonism of VIP in mice produces adult deficits in both social recognition and sociability[5, 25], although in vivo manipulations in adults have not been performed. Similar to the sociability results in mice, infusions of a VPAC antagonist into the zebra finch medial telencephalon (a possible homologue of the medial prefrontal cortex) reduce gregariousness in both males and females[6]. Interestingly, VP and OT likewise modulate social recognition and general affiliation[7, 8, 24], and thus the evolution of pair bonding may reliably involve natural selection on neural systems that already perform these basic functions -- systems such as VP, OT and VIP. Notably, VPAC receptors appear to consistently present in areas that regulate pair bonding across vertebrates, suggesting that these receptors may be central to the evolution of monogamy across a wide range of species.
Research Highlights.
VPAC receptor antagonism increases the latency to pair bond in zebra finches.
VPAC receptor antagonism reduces the duration of a pair bond in zebra finches.
VIP signaling mediates pair bonding in the socially monogamous zebra finch.
Acknowledgments
We thank Sara E. Schrock for assistance with surgeries and behavioral observations. Support provided by National Institutes of Health grant RO1 MH092331.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goodson JL, Evans AK, Wang Y. Neuropeptide binding reflects convergent and divergent evolution in species-typical group sizes. Horm Behav. 2006;50:223–36. doi: 10.1016/j.yhbeh.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, et al. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- 3.Kuenzel WJ, McCune SK, Talbot RT, Sharp PJ, Hill JM. Sites of gene expression for vasoactive intestinal polypeptide throughout the brain of the chick (Gallus domesticus). J Comp Neurol. 1997;381:101–18. doi: 10.1002/(sici)1096-9861(19970428)381:1<101::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Goodson JL, Kelly AM, Kingsbury MA, Thompson RR. An aggression-specific cell type in the anterior hypothalamus of finches. Proc Natl Acad Sci U S A. 2012;109:13847–52. doi: 10.1073/pnas.1207995109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill JM, Cuasay K, Abebe DT. Vasoactive intestinal peptide antagonist treatment during mouse embryogenesis impairs social behavior and cognitive function of adult male offspring. Exp Neurol. 2007;206:101–13. doi: 10.1016/j.expneurol.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Kingsbury MA, Miller KM, Goodson JL. VPAC receptor signaling modulates grouping behavior and social responses to contextual novelty in a gregarious finch: A role for a putative prefrontal cortex homologue. Horm Behav. 2013;64:511–8. doi: 10.1016/j.yhbeh.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 8.Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabelik D, Klatt JD, Kingsbury MA, Goodson JL. Endogenous vasotocin exerts context-dependent behavioral effects in a semi-naturalistic colony environment. Horm Behav. 2009;56:101–7. doi: 10.1016/j.yhbeh.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klatt JD, Goodson JL. Oxytocin-like receptors mediate pair bonding in a socially monogamous songbird. Proc R Soc Lond B. 2013;280:2012–396. doi: 10.1098/rspb.2012.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pedersen A, Tomaszycki ML. Oxytocin antagonist treatments alter the formation of pair relationships in zebra finches of both sexes. Horm Behav. 2012;62:113–9. doi: 10.1016/j.yhbeh.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Turner LM, Young AR, Rompler H, Schoneberg T, Phelps SM, Hoekstra HE. Monogamy evolves through multiple mechanisms: evidence from V1aR in deer mice. Mol Biol Evol. 2010;27:1269–78. doi: 10.1093/molbev/msq013. [DOI] [PubMed] [Google Scholar]
- 13.Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, et al. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology. 2011;152:4865–81. doi: 10.1210/en.2011-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodson JL, Lindberg L, Johnson P. Effects of central vasotocin and mesotocin manipulations on social behavior in male and female zebra finches. Horm Behav. 2004;45:136–43. doi: 10.1016/j.yhbeh.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Nowak JZ, Sedkowska P, Zawilska JB, Gozes I, Brenneman DE. Antagonism of VIP-stimulated cyclic AMP formation in chick brain. J Mol Neurosci. 2003;20:163–72. doi: 10.1385/JMN:20:2:163. [DOI] [PubMed] [Google Scholar]
- 16.Goodson JL, Wilson LC, Schrock SE. To flock or fight: neurochemical signatures of divergent life histories in sparrows. Proc Natl Acad Sci U S A. 2012;109(Suppl 1):10685–92. doi: 10.1073/pnas.1203394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 18.Curtis JT, Wang Z. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol Behav. 2005;86:338–46. doi: 10.1016/j.physbeh.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 19.Kingsbury MA, Kelly AM, Schrock SE, Goodson JL. Mammal-Like Organization of the Avian Midbrain Central Gray and a Reappraisal of the Intercollicular Nucleus. Plos One. 2011:6. doi: 10.1371/journal.pone.0020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seroogy K, Tsuruo Y, Hokfelt T, Walsh J, Fahrenkrug J, Emson PC, et al. Further analysis of presence of peptides in dopamine neurons. Cholecystokinin, peptide histidine-isoleucine/vasoactive intestinal polypeptide and substance P in rat supramammillary region and mesencephalon. Exp Brain Res. 1988;72:523–34. doi: 10.1007/BF00250598. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra RK, Wakade AR. Vasoactive Intestinal Polypeptide Stimulates the Secretion of Catecholamines from the Rat Adrenal-Gland. J Physiol. 1987;388:285–94. doi: 10.1113/jphysiol.1987.sp016615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav Neurosci. 2001;115:910–9. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- 23.Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–7. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- 25.Hill JM, Hauser JM, Sheppard LM, Abebe D, Spivak-Pohis I, Kushnir M, et al. Blockage of VIP during mouse embryogenesis modifies adult behavior and results in permanent changes in brain chemistry. J Mol Neurosci. 2007;31:183–200. doi: 10.1385/jmn:31:03:185. [DOI] [PubMed] [Google Scholar]